Abstract
NADPH oxidase has been implicated in ANG II-induced oxidative stress and hypertension in males; however, the contribution of oxidative stress to ANG II hypertension in females is unknown. In the present study, we tested the hypothesis that greater antioxidant capacity in female spontaneously hypertensive rats (SHR) blunts ANG II-induced oxidative stress and hypertension relative to males. Whole body and renal cortical oxidative stress levels were assessed in female and male SHR left untreated or following 2 wk of chronic ANG II infusion. Chronic ANG II infusion increased NADPH oxidase enzymatic activity in the renal cortex of both sexes; however, this increase only reached significance in female SHR. In contrast, male SHR demonstrated a greater increase in all measurements of reactive oxygen species production in response to chronic ANG II infusion. ANG II infusion increased plasma superoxide dismutase activity only in female SHR (76 ± 9 vs. 190 ± 7 Units·ml−1·mg−1, P < 0.05); however, cortical antioxidant capacity was unchanged by ANG II in either sex. To assess the functional implication of alterations in NADPH enzymatic activity and oxidative stress levels following ANG II infusion, additional experiments assessed the ability of the in vivo antioxidant apocynin to modulate ANG II hypertension. Apocynin significantly blunted ANG II hypertension in male SHR (174 ± 2 vs. 151 ± 1 mmHg, P < 0.05), with no effect in females (160 ± 11 vs. 163 ± 10 mmHg). These data suggest that ANG II hypertension in male SHR is more dependent on increases in oxidative stress than in female SHR.
Keywords: superoxide, kidney, apocynin, NADPH oxidase
the renin-angiotensin-aldosterone system (RAAS) is important in the regulation of body fluid and blood pressure homeostasis, and overactivation of the RAAS contributes to the development of numerous pathophysiological processes (20, 21, 40). There is an expanding literature regarding sex differences in the expression of RAAS components, as well as in functional responses to RAAS activation to both acute and chronic ANG II infusion (24, 25, 36, 38, 46). We recently published that greater levels of the vasodilatory peptide ANG (1–7) contribute to sex differences in the hypertensive response to chronic ANG II infusion in spontaneously hypertensive rats (SHR), although it is unknown whether there are additional molecular mechanism(s) contributing to sex differences in the blood pressure response to ANG II. Activation of angiotensin type 1 (AT1) receptors mediate most well-known biological functions of ANG II, including the stimulation of oxidative stress, and female SHR have fewer AT1 receptors than male SHR (43). However, little is known regarding the relative contribution of ANG II-mediated oxidative stress on blood pressure regulation in male vs. female rats.
Reactive oxygen species (ROS) are generated by all cell types and act as intercellular and intracellular signaling molecules. However, the highly reactive nature of ROS also poses potential deleterious effects on cellular structure and integrity, and increases in oxidative stress contribute to the development and progression of numerous cardiovascular diseases, including hypertension (11, 15, 35). In addition to known sex differences in the RAAS, there are also sex differences in oxidative stress levels. Both clinical and experimental studies have shown that oxidative stress levels are higher in males compared with females (10, 21, 23, 26), and sex differences in oxidative stress have been implicated in the sexual dimorphism in hypertension (10, 21, 26). Oxidative stress is determined by the balance of the production of free radicals and the ability of antioxidants to neutralize them. Deficiencies in antioxidant systems have been reported in hypertensive patients and in experimental models of hypertension (8, 30, 41, 42), and females tend to have greater antioxidant capacity compared with males (5, 58). Thus, the differential balance in the prooxidant and antioxidant status may play an important role in blunting oxidative stress-mediated cardiovascular pathologies in females.
Evidence in the literature suggests that there are sex differences in ANG II-induced increases in oxidative stress. An acute dose of ANG II (0.1 μmol/l) mediated greater superoxide (O2−) and hydrogen peroxide (H2O2) production in isolated cerebral arteries from male C57BL6/J mice than females (12), and chronic ANG II infusion increased plasma thiobarbituric acid reactive substances (TBARS), a marker of oxidative stress, in male, but not female, C57BL/6 mice (13). It is well established in male experimental animals that ANG II-mediated increases in oxidative stress contribute to the development of hypertension (28, 59), and increased NADPH oxidase activity has been reported as a source of the excess O2− levels following ANG II infusion (27, 29, 21, 13). Although studies have suggested that males are more susceptible to ANG II-mediated increases in oxidative stress, the contribution of increases in oxidative stress to the development of ANG II hypertension in both sexes has not been clearly examined.
We hypothesize that there is a sex difference in ANG II-mediated increases in ROS generation and in the compensatory activation of antioxidant capacity in SHR, which contributes to sex differences in ANG II-induced hypertension. Studying male and female SHR allows for the assessment of the effect of sex on oxidative stress and antioxidant systems in essential hypertension, as well as in response to chronic ANG II infusion. There are sex differences in renal cortical oxidative stress levels and in inner medullary antioxidant capacity in SHR (49, 50), and Fortepiani and Reckelhoff (15) showed that male SHR have a decrease in basal blood pressure in response to the antioxidant tempol, while female SHR do not. Thus, the first goal of this study was to assess whole body and renal cortical measures of oxidative stress and antioxidant capacity in male and female SHR. Animals were studied under basal conditions and following ANG II infusion to determine how direct stimulation of the RAAS alters oxidative stress. We found that following ANG II infusion, male SHR had a more robust increase in oxidative stress levels than female SHR. On the basis of these observations, additional studies were designed to test the hypothesis that ANG II-induced hypertension in male SHR is more dependent on increases in oxidative stress than in female SHR.
MATERIALS AND METHODS
Animals.
Male and female SHR were used in this study (Harlan Laboratories, Indianapolis, IN). All experiments were conducted in accordance with the National Institutes of Health's Guide for the Care and Use of Laboratory Animals and were approved and monitored by the Georgia Health Sciences University Institutional Animal Care and Use Committee. A subset of male and female SHR (n = 9) were implanted with telemetry transmitters (Data Sciences International, St. Paul, MN) at 11 wk of age, as previously described (46). Rats were allowed 1 wk to recover before they were placed on telemetry receivers for the measurement of baseline blood pressure. After 5 days, rats were randomized to receive one of the following treatments: chronic ANG II infusion (200 ng·kg−1·min−1 for 14 days; Phoenix Pharmaceuticals, Burlingame, CA) via subcutaneous osmotic minipump (Alzet, Cupertino, CA; n = 5) or the in vivo antioxidant apocynin (1.5 mmol/l) in the drinking water for 7 days prior to ANG II infusion, and throughout the infusion period (n = 4).
Additional male and female SHR (11–12 wk, respectively) were left untreated (n = 5–8) or received chronic ANG II infusion as described above (n = 5). Rats were placed in metabolic cages weekly for 24-h urine collection. Following 2 wk of ANG II infusion, rats (13–14 wk) were anesthetized with ketamine/xylazine (48 mg/kg and 6.4 mg/kg ip, respectively; Phoenix Pharmaceuticals, St. Joseph, MO), a terminal blood sample was taken, and plasma was separated by centrifugation. Kidneys were removed, and the renal cortexes were isolated and snap frozen in liquid nitrogen.
Lucigenin chemiluminescence assay.
Renal cortexes were homogenized for determination of NADPH oxidase enzymatic activity and basal O2− levels via lucigenin chemiluminescence, as previously described (47). NADPH oxidase activity was measured in the presence of 100 μmol/l NADPH (Sigma, St. Louis, MO) and incubated with lucigenin (Sigma; 5 μmol/l). All counts were normalized to total protein: 35 μg/well for the determination of NADPH oxidase activity; 350 μg/well for basal determination of O2−. The data were expressed as counts per minute per milligram protein. Specificity of the assay was confirmed by the inclusion of the superoxide scavenger, tempol (Sigma; 10 mmol/l).
Dihydroethidine staining.
Glomeruli were isolated from the renal cortex (n = 5) and stained with dihydroethidine (DHE; Invitrogen, Carlsbad, CA) for the detection of O2− generation, as previously described with slight modifications (14). Briefly, the kidney cortexes were minced and filtered through successive mesh filters (200–50 μm) in ice-cold 0.9% NaCl solution. Glomeruli retained on the mesh were washed (4°C, 2,000 g) and suspended in 50 mM Tris-HCl (pH 7.4). The purity of the preparation was assessed by light microscopy. One-hundred microliters of the isolated glomeruli suspension was fixed on slides, and slides were incubated in 2 μM DHE for 30 min at 37°C. Images were obtained with a LSM 510 META confocal microscope, with an excitation of 488 nm and emission of 574 to 595 nm or a 560-nm long pass filter. The slides were blind scored by three examiners, and the intensity of fluorescence was quantified in each group using MetaMorph software (Molecular Devices, Sunnyvale, CA).
Nitrotyrosine slot blot.
Slot-blot analysis was used as described previously (1). Briefly, 100 μg of renal cortical homogenate (n = 5 or 6) was immobilized onto a nitrocellulose membrane. After blocking with PBST containing 5% nonfat milk, membranes were reacted with antibody against nitrotyrosine (Calbiochem, San Diego, CA) using 1:500 dilutions in PBST containing 5% nonfat milk and applied overnight at 4°C. Primary antibody was detected by 1:5,000 dilutions of horseradish peroxidase-conjugated sheep anti-mouse antibody in PBST and enhanced-chemiluminescence (GE Healthcare, Piscataway, NJ). The optical density of the samples was quantified using densitometry software (Alpha Innotech, San Leandro, CA) and compared with that of controls and expressed as relative optical density.
Western blot analysis.
Renal cortical samples (n = 6/group) were homogenized, and Western blotting was performed as previously described (49). Following homogenization, protein concentrations were determined by standard Bradford assay (Bio-Rad, Hercules, CA) using BSA as the standard. Briefly, after proteins were transferred onto polyvinylidene difluoride membranes and blocked in 5% milk in Tris-buffered saline, two-color immunoblots were performed using polyclonal primary antibodies to CuZnSOD, MnSOD, EcSOD (polyclonal; Stressgen Bioreagents, Victoria, BC, Canada), and catalase (polyclonal; Abcam, Cambridge, MA). Specific bands were detected using the Odyssey infrared imager in conjunction with the appropriate IRDye secondary antibodies (LI-COR Biosciences, Lincoln, NE). β-actin (monoclonal; Sigma) was used to verify equal protein loading, and all densitometric results are reported normalized to β-actin.
Kits and assays.
All assays were performed according the manufacturer's instructions. Plasma 8-isoprostane levels were measured at a wavelength of 410 nm, and data were expressed as pg/ml (Cayman Chemicals, Ann Arbor, MI). For the detection of H2O2 levels and TBARS, urine samples were spun at 3,000 rpm for 10 min to remove particulate matter prior to assay, and levels were expressed as nanomoles excreted/day (H2O2; Invitrogen Amplex Red, Eugene, OR; and TBARS: Cayman Chemicals, Ann Arbor, MI). Total antioxidant potential was measured in the kidney cortex and urine samples, with the data expressed as millimoles copper-reducing equivalents per milligram protein (BIOXYTECH AOP-450, Oxis Research, Foster City, CA). Catalase and superoxide dismutase (SOD) activity were measured in plasma and renal cortical samples by ELISA kits, and the data were expressed as units per milliliter per milligram protein (Cayman Chemicals, Ann Arbor, MI).
Statistical analysis.
All data are presented as means ± SE. Mean arterial pressure (MAP) and urinary protein excretion data were analyzed using ANOVA for repeated measurements. Urinary TBARS excretion rates were compared using a one-way ANOVA. The rest of the data was analyzed using two-way ANOVA, factor 1 was sex of the animal, and factor 2 was ANG II treatment. Differences were considered statistically significant with P < 0.05. Analyses were performed using GraphPad Prism version 4.0 software (GraphPad Software, La Jolla, CA).
RESULTS
Total body oxidative stress measurements.
Plasma 8-isoprostane levels and urinary H2O2 excretion rates were measured in untreated and ANG II-infused male and female SHR, as measures of total body oxidative stress (Table 1). Basal 8-isoprostane levels were comparable in untreated male and female SHR. Chronic ANG II infusion increased plasma 8-isoprostane levels in male (105% increase), but not female, SHR (two-way ANOVA: effect of sex, P = 0.013; effect of ANG II, P = 0.0014; interaction, P = 0.05). Basal H2O2 excretion rates tended to be greater in males compared with females. H2O2 excretion increased with chronic ANG II infusion in both male (290% increase) and female SHR (189% increase); however, even after ANG II infusion, excretion rates remained greater in male SHR (two-way ANOVA: effect of sex, P = 0.0068; effect of ANG II, P = 0.0016; interaction, P = 0.07).
Table 1.
Total Body oxidative stress measurements in untreated and ANG II infused male and female SHR
| Oxidative Stress Parameters | Male | Male+ANG II | Female | Female+ANG II |
|---|---|---|---|---|
| Plasma 8-isoprostane, pg/ml | 80 ± 18 | 164 ± 26 | 81 ± 12 | 68 ± 3 |
| Urinary H2O2 excretion, nmol/day | 20 ± 2 | 78 ± 20 | 9 ± 2 | 26 ± 5 |
Values are presented as means ± SE using two-way ANOVA; n = 5–8. Plasma 8-isoprostane-sex, P = 0.013; ANG II, P = 0.0014; interaction, P = 0.05. Urinary H2O2 excretion-sex, P = 0.0068; ANG II, P = 0.0016; interaction, P = 0.07.
NADPH oxidase enzymatic activity.
NADPH oxidase enzymatic activity was assessed by adding exogenous NADPH to renal cortical homogenates; therefore, this assay measures the potential of the enzyme complex to produce O2− and is not a measure of NADPH oxidase-derived O2− production in vivo. Consistent with our previous data (50), NADPH oxidase enzymatic activity was comparable in untreated male and female SHR. Chronic ANG II infusion increased NADPH oxidase enzymatic activity in both male (43% increase) and female (154% increase) SHR; however, the increase was greater in female SHR (two-way ANOVA: effect of sex, P = 0.015; effect of ANG II, P = 0.014; interaction, P < 0.0001, Fig. 1A).
Fig. 1.
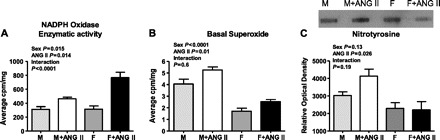
Renal cortical NADPH oxidase enzymatic activity (A) and basal cortical O2− production (B) in untreated and ANG II-treated male (M) and female (F) spontaneously hypertensive rats, as measured by lucigenin chemiluminescence. C: nitrotyrosine content was measured in the renal cortex as an oxidative stress marker by slot blot analysis; n = 5–6.
Renal cortical oxidative and nitrative stress measurements.
Additional experiments were performed to assess whether increases in renal cortical NADPH oxidase enzymatic activity translated into comparable increases in tissue oxidative stress levels. Renal cortical O2− levels were measured using lucigenin chemiluminescence, cortical nitrotyrosine levels were assessed using slot blot analysis, and glomerular O2− levels were measured using DHE staining. Consistent with our previous report (50), basal O2− levels were greater in the renal cortex of male SHR compared with females (Fig. 1B). Renal cortical O2− levels increased with chronic ANG II infusion in both male (30% increase) and female SHR (48% increase), however, even after ANG II infusion levels remained greater in male SHR (two-way ANOVA: effect of sex, P < 0.0001; effect of ANG II, P = 0.01; interaction, P = 0.6). Cortical nitrotyrosine content also tended to be greater in untreated male SHR compared with females. Chronic ANG II infusion increased nitrotyrosine content in the renal cortex of male SHR (36% increase) with no change in females (two-way ANOVA: effect of sex, P = 0.13; effect of ANG II, P = 0.026; interaction, P = 0.19; Fig. 1C). Sex differences in oxidative stress levels were also indicated in isolated glomeruli. DHE fluorescence was greater in glomeruli from untreated male SHR than females, and ANG II infusion increased DHE intensity only in males (24% increase; two-way ANOVA: effect of sex, P < 0.001; effect of ANG II, P = 0.09; interaction, P = 0.006; Fig. 2, A–E).
Fig. 2.
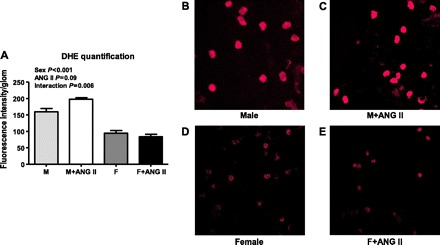
Dihydroethidine (DHE) staining for O2− generation in isolated glomeruli. Average intensity of fluorescence quantified by MetaMorph software (A) with representative images from an untreated male (M; B), a male treated with ANG II (M+ANG II; C), an untreated female (F; D) and a female treated with ANG II (F+ANG II; E) below. n = 5.
Total body antioxidant potential (AOP), SOD, and catalase activity.
Antioxidant capacity is an important determinant of oxidative stress levels. Urinary AOP tended to be less in untreated male SHR compared with female SHR. Infusion of ANG II decreased urinary AOP in both male (41% decrease) and female SHR (39% decrease); however, AOP levels tended to remain higher in females (two-way ANOVA: effect of sex, P = 0.18; effect of ANG II, P = 0.009; interaction, P = 0.54; Table 2). Plasma SOD activity was also less in male SHR compared with females, and chronic ANG II infusion increased SOD activity only in the female SHR (150% increase; two-way ANOVA: effect of sex, P < 0.0001; effect of ANG II, P < 0.0001; interaction, P < 0.0001). Plasma catalase activity was comparable in all groups (two-way ANOVA: effect of sex, P = 0.53; effect of ANG II, P = 0.39; interaction, P = 0.28).
Table 2.
Total body and renal cortical measurements for antioxidant potential, SOD, and catalase activity in untreated and ANG II-infused male and female spontaneously hypertensive rats
| Antioxidant Parameters | Male | Male+ANG II | Female | Female+ANG II |
|---|---|---|---|---|
| AOP, mM copper-reducing equivalents/mg protein | ||||
| Urine | 2.4 ± 0.1 | 1.4 ± 0.2 | 2.8 ± 0.7 | 1.7 ± 0.5 |
| Cortex | 0.16 ± 0.01 | 0.17 ± 0.01 | 0.16 ± 0.01 | 0.14 ± 0.01 |
| SOD activity, units/ml | ||||
| Plasma | 54 ± 1 | 56 ± 1 | 76 ± 9 | 190 ± 7 |
| Cortex | 3.8 ± 0.5 | 3.6 ± 0.4 | 3.2 ± 0.4 | 3.0 ± 0.2 |
| Catalase activity, μM·min−1·ml−1·mg protein−1 | ||||
| Plasma | 5 ± 2 | 6 ± 1 | 8 ± 1 | 7 ± 1 |
| Cortex | 11 ± 2 | 13 ± 1 | 4 ± 1 | 7 ± 1 |
Values are presented as means ± SE, using two-way ANOVA; n = 6–8. Urinary antioxidant potential (AOP)-sex, P = 0.18; ANG II, P = 0.009; interaction, P = 0.54; cortex AOP-sex, P = 0.54; ANG II, P = 0.83; interaction, P = 0.22. Plasma SOD activity-sex, P < 0.0001; ANG II, P < 0.0001; interaction, P < 0.0001; cortex SOD activity-sex, P = 0.18; ANG II, P = 0.56; interaction, P = 0.93. Plasma catalase activity-sex, P = 0.53; ANG II, P = 0.39; interaction, P = 0.28; cortex catalase activity-sex, P < 0.001; ANG II, P = 0.068; interaction, P = 0.67
Renal cortical antioxidant potential, SOD, and catalase activity.
Renal cortical AOP was comparable in untreated male and female SHR and unaffected by ANG II infusion (two-way ANOVA: effect of sex, P = 0.54; effect of ANG II, P = 0.83; interaction, P = 0.22; Table 2). Total SOD and catalase activity were also measured in renal cortical homogenates. Neither sex of the animal or ANG II infusion altered SOD activity (two-way ANOVA: effect of sex, P = 0.18; effect of ANG II, P = 0.56; interaction, P = 0.93; Table 2). Catalase activity was higher in the renal cortex of male SHR than females in untreated rats, and ANG II infusion tended to increase catalase activity in female SHR (75% increase; two-way ANOVA: effect of sex, P < 0.001; effect of ANG II, P = 0.068; interaction, P = 0.67).
CuZnSOD, MnSOD, and EcSOD protein expression levels were also determined and consistent with the activity data; protein expression levels were similar between groups (two-way ANOVA: CuZnSOD-effect of sex, P = 0.55; effect of ANG II, P = 0.49; interaction, P = 0.53; MnSOD-effect of sex, P = 0.89; effect of ANG II, P = 0.59; interaction, P = 0.56; EcSOD-effect of sex, P = 0.96; effect of ANG II, P = 0.85; interaction, P = 0.80; Fig. 3, A–C). Catalase protein expression was also comparable between all groups (two-way ANOVA: effect of sex P = 0.62; effect of ANG II P = 0.25; interaction P = 0.28; Fig. 3D).
Fig. 3.
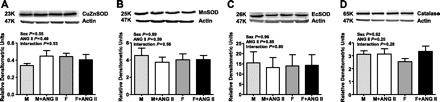
Antioxidant protein expression in the renal cortex of untreated and ANG II-treated male (M) and female (F) spontaneously hypertensive rats (SHR). Protein expression of CuZn SOD (A), MnSOD (B), EcSOD (C), and catalase (D) measured by Western blot analysis in the renal cortex normalized to actin; n = 6.
Mean arterial pressure measurements.
Consistent with our previous report, male SHR had higher blood pressures than females at baseline and a greater increase in blood pressure in response to ANG II infusion than female SHR at all time points (47). Compared with baseline, ANG II infusion significantly increased blood pressure in males after 5 days of ANG II infusion and after 8 days in females (percent increase in MAP: males, 19 ± 3%; females, 12 ± 2%; P < 0.05). To determine the contribution of oxidative stress to the increase in blood pressure in response to chronic ANG II infusion, additional male and female SHR were treated with the in vivo antioxidant apocynin. Treatment with apocynin alone for 1 wk did not alter basal blood pressure in either sex (Fig. 4, A and B). Infusion of ANG II in the presence of apocynin increased blood pressure in both sexes (percent increase in MAP: males, 9 ± 2%; females, 13 ± 5%). Apocynin attenuated an ANG II-induced increase in blood pressure in male SHR (Fig. 4A), with no effect on ANG II-mediated increases in blood pressure in female SHR (Fig. 4B), thereby abolishing the sex difference in the ANG II blood pressure response.
Fig. 4.
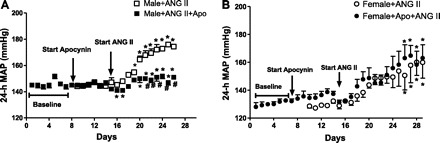
Effect of apocynin on ANG II hypertension. Twenty-four-hour mean arterial pressure (MAP) was measured by telemetry and in male and female SHR. Apocynin treatment was begun on day 8 with ANG II infusion initiated on day 15 in male (A) and female SHR (B). #Significant difference from untreated, P < 0.05. *Significant difference from same-sex baseline blood pressure, P < 0.05; n = 4 or 5.
To assess the effectiveness of apocynin to decrease markers of oxidative stress, urinary TBAR excretion rates were measured. In male SHR, ANG II infusion increased urinary TBAR excretion from 76 ± 5 to 112 ± 12 nmol/day (P < 0.05), and apocynin attenuated this increase (87 ± 9 nmol/day; P < 0.05; n = 4). TBAR excretion rates were comparable among all three groups of female SHR (untreated: 43 ± 3 nmol/day; ANG II treated: 47 ± 7 nmol/day; apocynin+ANG II: 48 ± 5 nmol/day; not significant, n = 4).
DISCUSSION
Although there is an ever expanding literature base supporting the existence of sex differences in chronic ANG II-mediated hypertension, the molecular mechanisms responsible have yet to be fully elucidated. Ample evidence suggests that ANG II-mediated increases in oxidative stress contribute to ANG II-driven pathologies in males (Fig. 5); the role of oxidative stress in ANG II hypertension in females is unknown. This becomes especially intriguing in light of recent reports of sex differences in ANG II-induced oxidative stress. As such, the primary novel finding of this study is that despite a robust increase in NADPH oxidase, enzymatic activity in the renal cortex and modest increases in indices of oxidative stress in female SHR, treatment with the antioxidant apocynin did not alter ANG II-induced hypertension. In contrast, apocynin significantly attenuated ANG II hypertension in male SHR, thereby abolishing the sex difference in blood pressure and verifying that differences in ANG II-induced oxidative stress in males vs. females contribute to sex differences in ANG II-mediated increases in blood pressure.
Fig. 5.
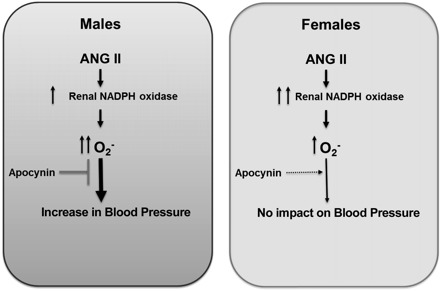
Schematic flow chart of the impact of ANG II-mediated increases in oxidative stress on blood pressure in male and female SHR.
Males are known to have higher levels of ROS compared with females under basal conditions, and there is sufficient evidence in the literature showing that ANG II infusion increases oxidative stress in males (10, 15, 44, 49). Consistent with our results, chronic ANG II infusion has been shown to induce increases in urinary H2O2 excretion and plasma 8-isoprostanes levels in male Sprague-Dawley rats (17, 33, 35, 38, 39, 45, 56). Although less is known regarding the effect of chronic ANG II infusion in female experimental animals, available data support our results that females are less susceptible to ANG II-induced increases in oxidative stress. ANG II infusion in C57BL/6J mice for 14 or 28 days resulted in increases in plasma TBARS and cardiac NADPH-driven generation of O2− in male, but not female, mice (13). Similarly, chronic ANG II infusion causes cerebral endothelial dysfunction in male MnSOD-deficient mice, but not in female mice, suggesting greater ANG II-mediated increases in oxidative stress in male mice (9). In the current study, we found that ANG II infusion resulted in modest increases in oxidative stress in females, although the absolute levels of oxidative stress attained remained less in females relative to their male counterparts. The increase in oxidative stress seen in females in the current study is likely related to the use of SHR as opposed to normotensive mice as the background for the ANG II infusion. Regardless, the modest increases in systemic and tissue levels of oxidative stress did not translate into an exaggerated increase in blood pressure following ANG II infusion in females.
NADPH oxidase has been implicated as the primary source of increases in O2·− production in the vasculature and kidneys of male experimental animals infused with ANG II (10, 12, 31, 55), and increased NADPH oxidase activity has been reported in isolated vascular smooth muscle cells from male SHR treated with ANG II (6). Sex differences in NADPH oxidase have also been reported, with male C57BL/6 mice having greater cardiac NADPH oxidase enzymatic activity following ANG II infusion than females (13). Surprisingly, in the present study, we found that renal cortical NADPH oxidase enzymatic activity was increased to a greater degree in female SHR following 2 wk of ANG II infusion than in males. Despite this finding, female SHR did not have correspondingly greater increases in tissue levels of oxidative stress following ANG II infusion compared with male SHR. We speculate that the disconnect between NADPH enzymatic activity and oxidative stress in female SHR may be related to lower levels of AT1 protein expression or lowered efficiency of AT1 receptor coupling to the NADPH oxidase activation compared with male SHR. Future studies will directly address this question. ANG II stimulation of NADPH oxidase has been shown to reduce nitric oxide (NO) bioavailability in kidneys of male SHR (2), and we have previously published that female SHR have greater NO bioavailability in the renal cortex than male SHR, independent of alterations in NO synthase activity or expression (48). We proposed that greater NO bioavailability in females was the result of less scavenging of NO by O2·− (50); therefore, we hypothesize that enhanced NO bioavailability may mitigate ANG II-induced increases in blood pressure (BP) to a greater extent in female SHR.
To begin to assess the molecular mechanism responsible for the observed sex differences in the degree of ANG II-induced oxidative stress, we assessed the primary antioxidant systems. It is anticipated that the ability of antioxidants to neutralize free radicals is of equal importance to the production of free radicals in the determination of oxidative stress levels (18, 22). We postulated that sex differences in oxidative stress following chronic ANG II infusion were due to sex differences in antioxidant capacity. There are clinical and experimental reports of greater antioxidant potential in females compared with males. Under basal conditions, female Wistar rats have greater liver mitochondrial MnSOD protein expression than males (5), and female imprinting control region mice have greater aortic Cu/Zn-SOD and EC-SOD protein expression than males (51). Female Wistar rats also have greater catalase activities in macrophages compared with males (3). Of particular relevance to the current study, increasing antioxidant capacity has been shown to be critical in mediating physiological responses to ANG II in experimental animals. Overexpression of human superoxide dismutase 1 or an infusion of an SOD mimetic both inhibit ANG II-mediated increases in oxidative stress in male mice (55, 58) and rats (33). In the present study, we found that plasma SOD activity was significantly increased following ANG II infusion in female SHR. This increase in plasma SOD activity was accompanied by a decrease in plasma 8-isoprostane levels; however, there was not a corresponding increase in catalase activity, which may account for the increase in H2O2 excretion in females following ANG II infusion. Although not directly tested in this study, EC-SOD has been reported to be the primary SOD isoform in plasma, interstitial fluid, lymph, and synovial fluid and has a long circulatory half-life (16). Therefore, we hypothesize that EC-SOD mediates the increase in plasma total SOD activity measured in female SHR. Consistent with other studies in female C57BL/6J mice and albino Wistar rats, there was no evidence of increased antioxidant capacity at the tissue level in either males or females following ANG II infusion (15, 53). Our findings suggest that enhanced antioxidant potential in females is not solely responsible for sex differences in ANG II-mediated oxidative stress levels in the renal cortex; however, we cannot rule out alternative tissue-specific alterations in antioxidant capacity. Although not examined in this study, it should be noted that sex differences in ANG II-induced oxidative stress may be related to female sex hormones. Chronic central administration of 17β-estradiol in conjunction with ANG II infusion blocks ROS generation in male C57BL/6J mice in the subfornical organ (58), and ovariectomy of female C57BL/6J mice results in greater ANG II-mediated increases in cardiac NADPH oxidase-derived O2·− production compared with intact females (13). Future studies will determine the role of sex steroids in regulating ANG II-mediated oxidative stress.
Similar to previous reports from our laboratory and others, males had a greater increase in blood pressure with chronic ANG II infusion than females (46, 53, 57). We have previously published that greater levels of ANG (1–7) contribute to the lower blood pressure maintained by female SHR during ANG II infusion (46). However, is the lower level of ANG (1–7) the only determining factor for greater increase in blood pressure in males? The direct relationship between ROS and the blood pressure response to ANG II in males was nicely demonstrated in BALB/c mice, in which the increase in systolic blood pressure induced by ANG II was prevented by apocynin (54). To examine the functional implications of the increase in NADPH oxidase activity and oxidative stress measurements following ANG II infusion in our current study, rats were treated with the in vivo antioxidant apocynin. Apocynin had no effect on baseline blood pressure in either male or female SHR, which is consistent with previous reports in male SHR rats when treating with 2.5 mM apocynin in drinking water (34). These data imply that the sex difference in basal blood pressure in SHR may not mediated by oxidative stress. Infusion of ANG II in the presence of apocynin, however, abolished the sex difference in the ANG II blood pressure response. Therefore, while oxidative stress contributes to ANG II-mediated increases in blood pressure in male SHR, blood pressure in female SHR is resistant to ANG II-mediated increases in oxidative stress, despite evidence of modest increases in oxidative stress.
The conclusion that oxidative stress does not mediate basal blood pressure in SHR may be related to the age of the rats when treatment began, the duration of treatment, the drug used, and the route of drug administration. Fortepiani and Reckelhoff (15) reported that treatment of male and female SHR with the antioxidant tempol (30 mg·kg−1·day−1) from 9 to 15 wk of age significantly decreased basal blood pressure only in male SHR, while treatment with tempol from birth to 15 wk of age blunted the age-related increase in blood pressure in both sexes, suggesting that oxidative stress may indeed contribute to basal blood pressure control in SHR. Treatment of SHR with apocynin (100 mg·kg−1·day−1 by subcutaneous injection for 7 days) has also been shown to selectively lower basal blood pressure in 12-wk-old male SHR, with no effect in females, when given by injection, and to decrease renal indices of oxidative stress and NADPH oxidase activity in male SHR (7, 19, 37). Therefore, it is possible that 1 wk of apocynin in the drinking water was insufficient to scavenge enough ROS to lower basal blood pressure. The question of what is modulating basal blood pressure in SHR, therefore, remains. Treatment of male and female SHR with the angiotensin-converting enzyme inhibitor enalapril reduced blood pressures to similar levels in both sexes, lending support to the idea that both the hypertension and the sex difference in blood pressure in SHR are RAAS mediated (40); however, the distinct pathways by which the RAAS modulates basal blood pressure are likely complex and have yet to be fully elucidated.
A potential limitation of this study is the use of apocynin; the use of apocynin as a selective inhibitor of NADPH oxidase has been questioned. However, a recent paper by Taye and Wind (52) shows comparable decreases in aortic NADPH oxidase activity in aged male SHR by the following NADPH oxidase inhibitors: DPI (10 μmol/l), VAS2870 (10 μmol/l), and apocynin (1 mmol/l), suggesting that apocynin is effective as an NADPH oxidase inhibitor. Additionally, the dose of apocynin (1.5 mmol/l) used in this paper is similar to a previously reported dose that lowered NADPH oxidase activity in male SHR (52). Therefore, although the data potentially support a specific role for NADPH oxidase as the source of the increase in ANG II-mediated oxidative stress in this study, we have referred to apocynin as an in vivo antioxidant throughout this article. In addition, we cannot attest to any other nonspecific effects of apocynin, as additional parameters were not examined in our study; however, indices of oxidative stress were reduced by apocynin in male SHR. Finally, all biochemical analyses were made in both plasma and urine as measures of whole body oxidative stress or directly in the kidney. Since apocynin was delivered systemically, we cannot differentiate the relative role of renal oxidative stress in mediating the blood pressure response to ANG II compared with increases in oxidative stress that may also occur in other tissues and organs that also modulate blood pressure.
Perspectives and Significance
We recently published that greater levels of the vasodilatory peptide ANG (1–7) in female SHR contributes to the lower BP response to chronic ANG II infusion in females; however, whether there were additional prohypertensive factors that contributed to the greater increase in blood pressure in males was unknown. As a result, the current study focused on oxidative stress, and indeed, we found that male SHR have greater increases in ANG II-mediated oxidative stress contributing to their hypertension. Although female SHR had lower levels of ANG II-mediated increases in renal cortical oxidative stress, there were only modest changes in antioxidant capacity, suggesting additional mechanisms may be mediating oxidative stress levels in female SHR. Of interest, ANG-(1–7) has been reported to attenuate ANG II-mediated NADPH oxidase activation and ROS production in glomeruli and mesangial cells from type 2 diabetic mice (32) and decrease renal NADPH oxidase activity in the diabetic SHR kidney (4), thus implicating a role for ANG (1–7) in suppressing oxidative stress. Therefore, we speculate that greater levels of ANG (1–7) in ANG II-infused female SHR antagonizes ANG II-mediated increases in oxidative stress, thereby limiting increases in blood pressure.
GRANTS
This study was funded by National Institutes of Health Grant 1R01 HL093271–01A1 to J. C. Sullivan.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: K.B., A.A.E., and A.E.-R. performed experiments; K.B. and J.C.S. analyzed data; K.B. and J.C.S. interpreted results of experiments; K.B. prepared figures; K.B. drafted manuscript; K.B., A.A.E., A.E.-R., and J.C.S. edited and revised manuscript; J.C.S. conception and design of research; J.C.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to Dr. David Pollock for assistance with telemetry studies. The authors would like to acknowledge the assistance of Krystal Brinson and Chet Joyner for glomeruli isolation and Western blot analysis.
REFERENCES
- 1. Abdelsaid MA, Pillai BA, Matragoon S, Prakash R, Al-Shabrawey M, El-Remessy AB. Early intervention of tyrosine nitration prevents vaso-obliteration and neovascularization in ischemic retinopathy. J Pharmacol Exp Ther 332: 125–134, 2010 [DOI] [PubMed] [Google Scholar]
- 2. Adler S, Huang H. Oxidant stress in kidneys of spontaneously hypertensive rats involves both oxidase overexpression and loss of extracellular superoxide dismutase. Am J Physiol Renal Physiol 287: F907–F913, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Azevedo RB, Lacava ZG, Miyasaka CK, Chaves SB, Curi R. Regulation of antioxidant enzyme activities in male and female rat macrophages by sex steroids. Braz J Med Biol Res 34: 683–687, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Benter IF, Yousif MH, Dhaunsi GS, Kaur J, Chappell MC, Diz DI. Angiotensin-(1–7) prevents activation of NADPH oxidase and renal vascular dysfunction in diabetic hypertensive rats. Am J Nephrol 28: 25–33, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Borrás C, Sastre J, García-Sala D, Lloret A, Pallardó FV, Viña J. Mitochondria from females exhibit higher antioxidant gene expression and lower oxidative damage than males. Free Radic Biol Med 34: 546–552, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Briones AM, Tabet F, Callera GE, Montezano AC, Yogi A, He Y, Quinn MT, Salaices M, Touyz RM. Differential regulation of Nox1, Nox2 and Nox4 in vascular smooth muscle cells from WKY and SHR. J Am Soc Hypertens 5: 137–153, 2011 [DOI] [PubMed] [Google Scholar]
- 7. Cao P, Ito O, Guo Q, Ito D, Muroya Y, Rong R, Mori T, Ito S, Kohzuki M. Endogenous hydrogen peroxide up-regulates the expression of nitric oxide synthase in the kidney of SHR. J Hypertens 29: 1167–1174, 2011 [DOI] [PubMed] [Google Scholar]
- 8. Chaves FJ, Mansego ML, Blesa S, Gonzalez-Albert V, Jiménez J, Tormos MC, Espinosa O, Giner V, Iradi A, Saez G, Redon J. Inadequate cytoplasmic antioxidant enzymes response contributes to the oxidative stress in human hypertension. Am J Hypertens 20: 62–69, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Chrissobolis S, Faraci FM. Sex differences in protection against angiotensin II-induced endothelial dysfunction by manganese superoxide dismutase in the cerebral circulation. Hypertension 55: 905–910, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dantas AP, Franco Mdo C, Silva-Antonialli MM, Tostes RC, Fortes ZB, Nigro D, Carvalho MH. Gender differences in superoxide generation in microvessels of hypertensive rats: role of NAD(P)H-oxidase. Cardiovasc Res 61: 22–29, 2004 [DOI] [PubMed] [Google Scholar]
- 11. de Champlain J, Wu R, Girouard H, Karas M, Midaoui A EL, Laplante MA, Wu L. Oxidative stress in hypertension. Clin Exp Hypertens 26: 593–601, 2004 [DOI] [PubMed] [Google Scholar]
- 12. De Silva TM, Broughton BR, Drummond GR, Sobey CG, Miller AA. Gender influences cerebral vascular responses to angiotensin II through Nox2-derived reactive oxygen species. Stroke 40: 1091–1097, 2009 [DOI] [PubMed] [Google Scholar]
- 13. Ebrahimian T, He Y, Schiffrin EL, Touyz RM. Differential regulation of thioredoxin and NAD(P)H oxidase by angiotensin II in male and female mice. J Hypertens 25: 1263–1271, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Elmarakby AA, Loomis ED, Pollock JS, Pollock DM. NADPH oxidase inhibition attenuates oxidative stress but not hypertension produced by chronic E.T-1. Hypertension 45: 283–287, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Fortepiani LA, Reckelhoff JF. Role of oxidative stress in the sex differences in blood pressure in spontaneously hypertensive rats. J Hypertens 23: 801–805, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Fukai T, Folz RJ, Landmesser U, Harrison DG. Extracellular superoxide dismutase and cardiovascular disease. Cardiovasc Res 55: 239–249, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Haugen EN, Croatt AJ, Nath KA. Angiotensin II induces renal oxidant stress in vivo and heme oxygenase-1 in vivo and in vitro. Kidney Int 58: 144–152, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Ide T, Tsutsui H, Ohashi N, Hayashidani S, Suematsu N, Tsuchihashi M, Tamai H, Takeshita A. Greater oxidative stress in healthy young men compared with premenopausal women. Arterioscler Thromb Vasc Biol 22: 438–442, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Iliescu R, Cucchiarelli VE, Yanes LL, Iles JW, Reckelhoff JF. Impact of androgen-induced oxidative stress on hypertension in male SHR. Am J Physiol Regul Integr Comp Physiol 292: R731–R735, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Ji H, Zheng W, Wu X, Liu J, Ecelbarger CM, Watkins R, Arnold AP, Sandberg K. Sex chromosome effects unmasked in angiotensin II-induced hypertension. Hypertension 55: 1275–1282, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jung O, Schreiber JG, Geiger H, Pedrazzini T, Busse R, Brandes RP. gp91phox-containing NADPH oxidase mediates endothelial dysfunction in renovascular hypertension. Circulation 109: 1795–1801, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Kashyap MK, Yadav V, Sherawat BS, Jain S, Kumari S, Khullar M, Sharma PC, Nath R. Different antioxidants status, total antioxidant power and free radicals in essential hypertension. Mol Cell Biochem 277: 89–99, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Kayali R, Cakatay U, Tekeli F. Male rats exhibit higher oxidative protein damage than females of the same chronological age. Mech Ageing Dev 128: 365–369, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev 59: 251–287, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Komukai K, Mochizuki S, Yoshimura M. Gender and the renin-angiotensin-aldosterone system. Fundam Clin Pharmacol 24: 687–698, 2010 [DOI] [PubMed] [Google Scholar]
- 26. Lacy F, Kailasam MT, O'Connor DT, Schmid-Schönbein GW, Parmer RJ. Plasma hydrogen peroxide production in human essential hypertension: role of heredity, gender, and ethnicity. Hypertension 36: 878–884, 2000 [DOI] [PubMed] [Google Scholar]
- 27. Landmesser U, Cai H, Dikalov S, McCann L, Hwang J, Jo H, Holland SM, Harrison DG. Role of p47(phox) in vascular oxidative stress and hypertension caused by angiotensin II. Hypertension 40: 511–515, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Laursen JB, Rajagopalan S, Galis Z, Tarpey M, Freeman BA, Harrison DG. Role of superoxide in angiotensin II-induced but not catecholamine-induced hypertension. Circulation 95: 588–593, 1997 [DOI] [PubMed] [Google Scholar]
- 29. Li JM, Wheatcroft S, Fan LM, Kearney MT, Shah AM. Opposing roles of p47phox in basal versus angiotensin II-stimulated alterations in vascular O2− production, vascular tone, and mitogen-activated protein kinase activation. Circulation 109: 1307–1313, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Lijnen PJ, van Pelt JF, Fagard RH. Downregulation of manganese superoxide dismutase by angiotensin II in cardiac fibroblasts of rats: Association with oxidative stress in myocardium. Am J Hypertens 23: 1128–1135, 2010 [DOI] [PubMed] [Google Scholar]
- 31. López B, Salom MG, Arregui B, Valero F, Fenoy FJ. Role of superoxide in modulating the renal effects of angiotensin II. Hypertension 42: 1150–1156, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Moon JY, Tanimoto M, Gohda T, Hagiwara S, Yamazaki T, Ohara I, Murakoshi M, Aoki T, Ishikawa Y, Lee SH, Jeong KH, Lee TW, Ihm CG, Lim SJ, Tomino Y. Attenuating effect of angiotensin-(1–7) on angiotensin II-mediated NAD(P)H oxidase activation in type 2 diabetic nephropathy of KK-A(y)/Ta mice. Am J Physiol Renal Physiol 300: F1271–F1282, 2011 [DOI] [PubMed] [Google Scholar]
- 33. Ortiz MC, Manriquez MC, Romero JC, Juncos LA. Antioxidants block Angiotensin II-induced increases in blood pressure and endothelin. Hypertension 38: 655–659, 2001 [DOI] [PubMed] [Google Scholar]
- 34. Paliege A, Pasumarthy A, Mizel D, Yang T, Schnermann J, Bachmann S. Effect of apocynin treatment on renal expression of COX-2, NOS1, and renin in Wistar-Kyoto and spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 290: R694–R700, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Pech V, Sikka SC, Sindhu RK, Vaziri ND, Majid DS. Oxidant stress and blood pressure responses to angiotensin II administration in rats fed varying salt diets. Am J Hypertens 19: 534–540, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Pendergrass KD, Pirro NT, Westwood BM, Ferrario CM, Brosnihan KB, Chappell MC. Sex differences in circulating and renal angiotensins of hypertensive mRen(2). Lewis but not normotensive Lewis rats. Am J Physiol Heart Circ Physiol 295: H10–H20, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pinto V, Pinho MJ, Hopfer U, Jose PA, Soares-da-Silva P. Oxidative stress and the genomic regulation of aldosterone-stimulated NHE1 activity in SHR renal proximal tubular cells. Mol Cell Biochem 310: 191–201, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Polichnowski AJ, Jin C, Yang C, Cowley AW., Jr Role of renal perfusion pressure versus angiotensin II on renal oxidative stress in angiotensin II-induced hypertensive rats. Hypertension 55: 1425–1430, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Reckelhoff JF, Zhang H, Srivastava K, Roberts LJ, 2nd, Morrow JD, Romero JC. Subpressor doses of angiotensin II increase plasma F(2)-isoprostanes in rats. Hypertension 35: 476–479, 2000 [DOI] [PubMed] [Google Scholar]
- 40. Reckelhoff JF, Zhang H, Srivastava K. Gender differences in development of hypertension in spontaneously hypertensive rats: role of the renin-angiotensin system. Hypertension 35: 480–483, 2000 [DOI] [PubMed] [Google Scholar]
- 41. Redon J, Oliva MR, Tormos C, Giner V, Chaves J, Iradi A, Saez GT. Antioxidant activities and oxidative stress byproducts in human hypertension. Hypertension 41: 1096–1101, 2003 [DOI] [PubMed] [Google Scholar]
- 42. Rodrigo R, Prat H, Passalacqua W, Araya J, Guichard C, Bächler JP. Relationship between oxidative stress and essential hypertension. Hypertens Res 30: 1159–1167, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Rogers JL, Mitchell AR, Maric C, Sandberg K, Myers A, Mulroney SE. Effect of sex hormones on renal estrogen and angiotensin type 1 receptors in female and male rats. Am J Physiol Regul Integr Comp Physiol 292: R794–R799, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Sartori-Valinotti JC, Iliescu R, Fortepiani LA, Yanes LL, Reckelhoff JF. Sex differences in oxidative stress and the impact on blood pressure control and cardiovascular disease. Clin Exp Pharmacol Physiol 34: 938–945, 2007 [DOI] [PubMed] [Google Scholar]
- 45. Sasser JM, Moningka NC, Cunningham MW, Jr, Croker B, Baylis C. Asymmetric dimethylarginine in angiotensin II-induced hypertension. Am J Physiol Regul Integr Comp Physiol 298: R740–R746, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sullivan JC, Bhatia K, Yamamoto T, Elmarakby AA. Angiotensin (1–7) receptor antagonism equalizes angiotensin II-induced hypertension in male and female spontaneously hypertensive rats. Hypertension 56: 658–666, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sullivan JC, Pardieck JL, Brinson K, Kang KT. Effects of estradiol on renal cyclic guanosine monophosphate and oxidative stress in spontaneously hypertensive rats. Gend Med 6: 498–510, 2009 [DOI] [PubMed] [Google Scholar]
- 48. Sullivan JC, Pardieck JL, Hyndman KA, Pollock JS. Renal NOS activity, expression, and localization in male and female spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 298: R61–R69, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sullivan JC, Sasser JM, Pollock JS. Sexual dimorphism in oxidant status in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 292: R764–R768, 2007 [DOI] [PubMed] [Google Scholar]
- 50. Sullivan JC, Semprun-Prieto L, Boesen EI, Pollock DM, Pollock JS. Sex and sex hormones influence the development of albuminuria and renal macrophage infiltration in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 293: R1573–R1579, 2007 [DOI] [PubMed] [Google Scholar]
- 51. Takenouchi Y, Kobayashi T, Taguchi K, Matsumoto T, Kamata K. Gender differences in endothelial function in murine aortas. Atherosclerosis 206: 397–404, 2009 [DOI] [PubMed] [Google Scholar]
- 52. Taye A, Wind S. Role of NADPH oxidase in the endothelial dysfunction and oxidative stress in aorta of aged spontaneous hypertensive rats. Iranian J Basic Med Sci 13: 48–56, 2010 [Google Scholar]
- 53. Venegas-Pont M, Sartori-Valinotti JC, Glover PH, Reckelhoff JF, Ryan MJ. Sexual dimorphism in the blood pressure response to angiotensin II in mice after angiotensin-converting enzyme blockade. Am J Hypertens 23: 92–96, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Virdis A, Neves MF, Amiri F, Touyz RM, Schiffrin EL. Role of NAD(P)H oxidase on vascular alterations in angiotensin II-infused mice. J Hypertens 22: 535–542, 2004 [DOI] [PubMed] [Google Scholar]
- 55. Wang HD, Johns DG, Xu S, Cohen RA. Role of superoxide anion in regulating pressor and vascular hypertrophic response to angiotensin II. Am J Physiol Heart Circ Physiol 282: H1697–H1702, 2002 [DOI] [PubMed] [Google Scholar]
- 56. Welch WJ, Blau J, Xie H, Chabrashvili T, Wilcox CS. Angiotensin-induced defects in renal oxygenation: role of oxidative stress. Am J Physiol Heart Circ Physiol 288: H22–H128, 2005 [DOI] [PubMed] [Google Scholar]
- 57. Xue B, Johnson AK, Hay M. Sex differences in angiotensin II- induced hypertension. Braz J Med Biol Res 40: 727–734, 2007 [DOI] [PubMed] [Google Scholar]
- 58. Xue B, Zhao Y, Johnson AK, Hay M. Central estrogen inhibition of angiotensin II-induced hypertension in male mice and the role of reactive oxygen species. Am J Physiol Heart Circ Physiol 295: H1025–H1032, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhou MS, Jaimes EA, Raij L. Inhibition of oxidative stress and improvement of endothelial function by amlodipine in angiotensin II-infused rats. Am J Hypertens 17: 167–171, 2004 [DOI] [PubMed] [Google Scholar]


