Abstract
Claudins are the major determinants of paracellular epithelial permeability in multicellular organisms. In Atlantic salmon (Salmo salar L.), we previously found that mRNA expression of the abundant gill-specific claudin 30 decreases during seawater (SW) acclimation, suggesting that this claudin is associated with remodeling of the epithelium during salinity change. This study investigated localization, protein expression, and function of claudin 30. Confocal microscopy showed that claudin 30 protein was located at cell-cell interfaces in the gill filament in SW- and fresh water (FW)-acclimated salmon, with the same distribution, overall, as the tight junction protein ZO-1. Claudin 30 was located at the apical tight junction interface and in cell membranes deeper in the epithelia. Colocalization with the α-subunit of the Na+-K+-ATPase was negligible, suggesting limited association with mitochondria-rich cells. Immunoblotting of gill samples showed lower claudin 30 protein expression in SW than FW fish. Retroviral transduction of claudin 30 into Madin-Darby canine kidney cells resulted in a decreased conductance of 19%. The decreased conductance correlated with a decreased permeability of the cell monolayer to monovalent cations, whereas permeability to chloride was unaffected. Confocal microscopy revealed that claudin 30 was expressed in the lateral membrane, as well as in tight junctions of Madin-Darby canine kidney cells, thereby paralleling the findings in the native gill. This study suggests that claudin 30 functions as a cation barrier between pavement cells in the gill and also has a general role in cell-cell adhesion in deeper layers of the epithelium.
Keywords: cation permeability, Madin-Darby canine kidney cells, Na+-K+-ATPase, osmoregulation, retroviral transduction, Salmo salar, tight junction, transepithelial resistance, ZO-1
euryhaline and anadromous fish, such as the Atlantic salmon, need to be able to regulate the permeability of their exposed epithelia when encountering changes in salinity. Since the surface area of the gill epithelium is very large and thin and directly exposed to the surrounding water, passive fluxes of solutes and water may be threatening homeostasis of the internal environment. In a given situation, the permeability of the gill epithelium is defined as a trade-off between respiratory, acid-base, and osmoregulatory needs (9) and may be modulated as the environmental conditions change. Transport of water and solutes across the gill epithelium can occur via transcellular or paracellular pathways, and multiple studies have shown that both processes are affected by changes in external salinity (9, 26, 37). In fresh water (FW), ion uptake is primarily driven by the basolateral Na+-K+-ATPase and apical V-type H+-ATPase, which together create an electrochemical gradient that is able to drive the apical uptake of sodium into mitochondria-rich cells (MRCs), despite a great opposing ionic gradient (9, 13, 24). Chloride uptake is facilitated apically by Cl−/HCO3− exchange and, possibly, NaCl cotransport and basolaterally by chloride-selective ion channels (16). The driving force for anion transport is still debated but is likely to be aided by the electronegative cytosolic compartment. The paracellular movement of monovalent ions in FW is minimal and governed by tight junctions (28, 30). In seawater (SW), the Na+-K+-ATPase transports sodium from the MRC into the lateral intercellular space, thereby facilitating the uptake of chloride from the interstitial fluid through the Na+-K+-2Cl− cotransporter (9, 34). Chloride exits passively across the apical membrane of the MRC via the cystic fibrosis transmembrane conductance regulator (14, 23, 25), while sodium is thought to be excreted through a paracellular route down its electrochemical gradient (25, 28, 30). It is believed that paracellular transport of sodium requires molecular junctional modifications to increase the specific sodium permeability of the epithelium while keeping the paracellular route impermeable to chloride. Tight junctions between MRCs or between MRCs and accessory cells in SW-acclimated fish are shallower than those between MRCs or MRCs and pavement cells in FW-acclimated fish or between pavement cells in the gill epithelium in general (17, 30).
Recently, tight junctions have been shown to be composed of multiple membrane-spanning proteins such as occludin, junctional adhesion molecules, and claudins (2, 6, 42). Claudins are by far the most diverse group of these proteins, with 24 isoforms identified in mammals and 56 isoforms in the genome of Takifugu rubripes (22). In the Atlantic salmon, a total of 26 claudin isoforms have been detected in the Expressed Sequence Tags (EST) database at the National Center for Biotechnology Information, and five of these were found almost exclusively in the gill (claudin 10e, 27a, 28a, 28b, and 30) (37). Claudins are proteins with four transmembrane-spanning domains with a short intracellular amino-terminal end and a long intracellular carboxy-terminal tail (2, 6, 42). The first extracellular domain of a claudin is responsible for creating the seal between cells by spanning the intercellular space and connecting with the first extracellular loop of another claudin anchored on the opposing cell (12, 42, 43). These interactions are not fully understood, but it is believed that multiple claudin isoforms oligomerize to form continuous strands in the tight junction, thereby effectively creating a seal and, in some cases, a pore to ions and larger solutes (43, 46). The first extracellular claudin domain bears differentially charged amino acids in the different claudin isoforms. Therefore, properties of tight junction pores formed in any epithelium are determined by its composition of specific claudin isoforms.
We previously identified gill-specific claudin isoforms in the transcriptome of the Atlantic salmon (37), but until now, the localization patterns of these in the gill have remained unknown. We therefore sought to investigate the localization and function of one of these claudin isoforms, claudin 30. On the basis of its relative abundance in gill EST libraries (http://www.ncbi.nlm.nih.gov/) and the tissue expression compared with other claudin isoforms of the gill, claudin 30 is likely the most abundant claudin expressed in the gill (37). Since we previously found that claudin 30 expression is elevated in FW and by cortisol (36, 37), we suspected that this claudin might be a barrier-forming tight junction protein that increases the tightness of the gill, as observed during FW acclimation and cortisol treatment of isolated pavement cells (44). A homologous polyclonal antibody against claudin 30 was developed and used in the present studies to estimate relative levels of claudin 30 protein expression in FW- and SW-acclimated Atlantic salmon and its cellular localization in the gill. As a new approach in the comparative field, the functional properties of claudin 30 were investigated by retroviral transduction into low-resistance Madin-Darby canine kidney (MDCK) cells. Functional assays employing Ussing chamber techniques (40) were used to determine the effect of claudin 30 expression on the paracellular transport properties of the MDCK cell monolayer, thereby increasing our knowledge of a putative function of claudin 30 in the salmon gill.
MATERIALS AND METHODS
Localization and Protein Expression of Claudin 30 in the Gill of Atlantic Salmon
Animals and tissue sampling.
FW-acclimated Atlantic salmon (+1 postsmolts, 40–50 g) were obtained from the Danish Center for Wild Salmon (Randers, Denmark) and acclimated to recirculated, biofiltered FW or to 25 ppt artificial SW (Red Sea Salt, Eliat, Israel) for ≥3 wk before sampling on the Odense Campus of the University of Southern Denmark. The fish were kept in a 12:12-h light-dark photoperiod at 14°C and were fed 1% body weight/day until sampling. Upon sampling, fish were stunned with a blow to the head, then cervical dislocation and pithing of the brain were performed. Gill arches were dissected for fixing in 4% paraformaldehyde (PFA)-0.9% NaCl in 5 mM NaH2PO4 (pH 7.8) or freezing at −80°C in SEI buffer (300 mM sucrose, 10 mM EDTA, and 50 mM imidazole, pH 7.3) until Western blot analysis of claudin protein abundance. The experimental protocols were approved by the Danish Animal Experiments Inspectorate and in accordance with the European convention for the protection of vertebrate animals used for experiments and other scientific purposes (no. 86/609/EØF).
Primary antibodies.
A polyclonal antibody against Salmo salar claudin 30 was produced and affinity-purified by BioGenes (Berlin, Germany). The antibody was raised in rabbits by immunization with a 15-amino acid synthetic peptide (CPPKEDNYPTKYSAA) corresponding to an amino acid sequence found in the carboxy terminus of the protein (GenBank entry DAA06169.1). The affinity-purified polyclonal ZO-1 antibody was raised in rabbits against a fusion protein corresponding to amino acids 463–1109 in human ZO-1 cDNA (Invitrogen, Carlsbad, CA). The α-5 antibody was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development and maintained by the Department of Biological Sciences at the University of Iowa (Iowa City, IA). It reacts with conserved regions in all isoforms of the Na+-K+-ATPase α-subunit. A mouse anti-β-actin IgG (Ab 8224, Abcam, Cambridge, UK) was used as loading control.
Immunofluorescence localization by light and confocal microscopy.
Trimmed gill arches from FW- and SW-acclimated salmon were fixed in PFA overnight at 4°C, dehydrated through graded series of ethanol and xylene, and finally embedded in paraffin. Sagittal and frontal sections (5 μm) were prepared, and the sections were deparaffinized and then demasked in boiling citrate buffer (10 mM trisodium citrate, pH 6.0) and blocked in 3% BSA in PBS (mM: 137 NaCl, 2.7 KCl, 4.3 Na2HPO4, and 1.4 KH2PO4, pH 7.3). The sections were dual-labeled overnight at 4°C with polyclonal rabbit claudin 30 antibody or polyclonal ZO-1 antibody in combination with monoclonal mouse α-5 pan Na+-K+-ATPase antibody diluted to 1.5, 2.5, or 0.2 μg/ml, respectively, in PBS-1% BSA. After they were rinsed in PBS, the sections were labeled for 1 h at 37°C with a cocktail of two secondary antibodies (both from Invitrogen): Alexa Fluor 568-conjugated goat anti-rabbit IgG (1:500 dilution) and Oregon Green-conjugated goat anti-mouse IgG (1:500 dilution), rinsed in PBS and Milli-Q water, and mounted with Vectashield antifade mounting medium (Vector Labs, Burlingame, CA). For the blocking experiment, the claudin 30 antibody was incubated overnight at 4°C with the immunization peptide (200 μg/ml) before it was applied to the sections. The sections were inspected with a Leica HC microscope (Leica Microsystems, Heidelberg, Germany), and digital images of representative areas were captured using a Leica DC 200 camera. In some cases, confocal images were taken using a Zeiss LSM510 META confocal microscope (Carl Zeiss, Jena, Germany) with a ×63 objective.
Immunoblotting.
Gill tissue was homogenized in chilled SEID buffer (SEI with 0.1% sodium deoxycholate) with a protease inhibitor cocktail (stock no. P8340, Sigma Aldrich) using a Polytron homogenizer (Brinkmann, Westbury, NY). The supernatant was isolated after centrifugation at 1,000 g for 10 min (4°C), and a microsomal fraction, for further analysis, was isolated from the supernatant by centrifugation at 50,000 g for 30 min (4°C). Proteins were resolved by SDS-PAGE under reducing conditions, as described previously (35), and with minor modifications according to the manufacturer's instructions (NuPAGE, Invitrogen). An equal quantity of gill protein (10 μg) was loaded in all lanes and separated in 4–12% Bis-Tris gels and MES-SDS buffer at 200 V (Xcell II SureLock, Invitrogen). Molecular mass was estimated by inclusion of a marker (Novex Sharp Pre-Stained Protein Standard; Invitrogen). After electrophoresis, the proteins were immunoblotted onto 0.45-μm nitrocellulose membranes (Invitrogen) by submerged blotting for 1 h at 30 V (XCell II, Invitrogen) with transfer buffer (25 mM Tris, 192 mM glycine, and 20% methanol). Membranes were blocked in Tris-buffered saline with Tween 20 (140 mM NaCl, 20 mM Tris, and 0.1% Tween 20, pH 7.6) with blocking buffer (stock no. B6429, Sigma Aldrich) and washed in Tris-buffered saline with Tween 20. Immunological detection was obtained by incubation overnight (4°C) with the rabbit anti-claudin 30 antibody (1 μg/ml). Claudin 30 was visualized, along with β-actin (0.5 μg/ml) as loading control, by double labeling. After they were washed, the membranes were incubated for 1 h with goat anti-mouse and goat anti-rabbit secondary antibodies conjugated to Cy3 and Cy5, respectively (Lab Vision and Neomarkers, Fremont, CA). Blotted proteins were detected and quantified using a Typhoon Trio scanner (GE Healthcare Amersham Biosciences, Uppsala, Sweden). To validate the specificity of the claudin antibody, the primary antibody was neutralized by incubation overnight at 4°C with the immunization peptide (200 μg/ml) before application to the blot.
Functional Characterization of Salmon Claudin 30
Generation of MDCK II Tet-Off claudin 30 cell lines.
Salmon cDNA was synthesized from mRNA extracted from Atlantic salmon gill homogenate, as described elsewhere (37). Primers for the amplification of the 627-bp salmon claudin 30 were designed with an amino-terminal FLAG tag inserted immediately after the start codon. Primers also contained a Kozak consensus sequence upstream of the starting codon and restriction sites HindIII or NotI for cloning into the retroviral vector pRevTREP (modified version of pRevTRE, Clontech, Mountain View, CA) downstream of the Tet-responsive element (Table 1). Correct insertion of the gene was evaluated by sequencing the claudin in both directions, thereby confirming the already published cDNA sequence of salmon claudin 30 (accession no. BK006405) (37). Retroviral transduction of the gene into the MDCK II Tet-Off cells (Clontech) was performed as described previously (45, 46). Briefly, Lipofectamine was used to transfect the pRevTREP vector containing the FLAG-tagged salmon claudin 30 into the viral packaging cell line PT67 (Clontech), and a population of positive clones were selected by hygromycin (Invitrogen). These clones produced viral supernatant, which was used to infect MDCK II Tet-Off cells, thereby introducing the salmon claudin 30 gene into their genome. Hygromycin-resistant clones of MDCK II Tet-Off cells were then selected and isolated using cloning cylinders and transferred to 96-well tissue culture plates (Corning Life Sciences, Lowell, MA). Passage of each clone was performed on 24-well, 60-mm, and 100-mm tissue culture plates (Corning Life Sciences) by trypsinization (0.05% trypsin and 0.5 mM EDTA in calcium- and magnesium-free PBS); then they were grown to confluence in each type of plate over 2–3 days. The clones were grown and maintained in DMEM (Invitrogen) containing 5% fetal bovine serum and 0.3 mg/ml hygromycin. For suppression of claudin 30 expression, doxycycline (Clontech) was added in a concentration of 20 ng/ml to the culture medium. Confluent clones from the 100-mm plate were trypsinized and seeded at confluence in Snapwell filters (Corning Life Sciences) or Transwell filters (Corning Life Sciences) and processed as described below.
Table 1.
Primer sequences for amplification of Atlantic salmon claudin 30
| Primer Name | Primer Sequence (5′ → 3′) | Accession No. |
|---|---|---|
| Salmo salar claudin 30 | BK006405 | |
| Forward | ACTGCGGCCGCGGCCACCATGGACTACAAGGACGACGACGACAAGGCATCTGCTGGGTTTCAGATG | |
| Reverse | CGCAAGCTTTCAAACATAGTCCCTGGGTGC |
Underlined letters mark restriction sites and FLAG tag, bold letters mark Kozak consensus sequence, and italic letters mark start and stop codons.
Immunoblotting.
Protein lysate from MDCK II Tet-Off cells expressing claudin 30 was made by suspension of a cell monolayer in ice-cold RIPA buffer containing 50 mM Tris·HCl, pH 8, 150 mM NaCl, 1% NP-40, 0.5% deoxycholate, 0.1% SDS, and a protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN). The suspension was agitated for 20 min in the tissue culture plates at 4°C. It was then centrifuged for 10 min at 16,000 g at 4°C, and the supernatant was saved at −80°C for further analysis. Protein concentration of the lysate was evaluated using the bicinchoninic acid protein assay kit according to the manufacturer's instructions (Pierce Protein Research Products, Rockford, IL). Twenty micrograms of protein lysate were suspended in 4 μl of 6× SDS sample buffer and water (final sample concentration: 58.3 mM Tris, 60 mM SDS, 0.55 M glycerol, 142 mM β-mercaptoethanol, and 30 mM bromophenol blue), heated for 5 min at 90°C, and separated by SDS-PAGE in a 10% acrylamide gel for 1 h 15 min at 100 V. Proteins were blotted on a polyvinylidene difluoride membrane using a wet transfer cell set-up (Bio-Rad, Hercules, CA). Transfer of proteins was performed at 100 V for 1 h 30 min. The membrane was blocked for 1 h at room temperature in 5% milk in Tris-buffered saline with Tween 20 (150 mM NaCl, 50 mM Tris, and 1% Tween 20, pH 7.6). Salmon claudin 30 was visualized using the rabbit polyclonal affinity-purified antibody described above in a concentration of 0.33 μg/ml. The other endogenous tight junction proteins (claudin 1, 2, 3, 4, and 7, occludin, and ZO-1) were detected using commercial antibodies at dilutions recommended by the manufacturer (Table 2). Antibody for the desired protein was diluted in a solution of Tris-buffered saline-Tween 20 with 5% milk and incubated overnight at 4°C. The membrane was then washed repeatedly, and secondary antibody was diluted in 5% milk in Tris-buffered saline-Tween 20 (goat anti-mouse- or goat anti-rabbit-horseradish peroxidase conjugate, 1:5,000 dilution; GE Healthcare Life Sciences, Piscataway, NJ), added to the membrane, and incubated for 1 h. Finally, the membrane was washed four times with Tris-buffered saline-Tween 20 and developed using the Amersham ECL Western blot detection reagents (GE Healthcare Life Sciences) and scanned in an imager (ImageQuant LAS 4000, GE Healthcare Life Sciences).
Table 2.
Antibodies used for characterization of MDCK II cells
| Antibody | Producer | Catalog No. | Working Dilution |
|---|---|---|---|
| Rabbit claudin 1 | Invitrogen | 71-7800 | 250× WB |
| Rabbit claudin 2 | Zymed | 51-6100 | 250× WB |
| Rabbit claudin 3 | Zymed | 34-1700 | 1,000× WB |
| Mouse claudin 4 | Invitrogen | 32-9400 | 500 WB |
| Rabbit claudin 7 | Zymed | 34-9100 | 1,000× WB |
| Mouse occludin | Zymed | 33-1500 | 1,000× WB |
| Mouse ZO-1 | Santa Cruz Biotechnology | N/A* | 250× WB, 200× IF |
| Mouse β-catenin | Zymed | 13-8400 | 100× IF |
MDCK, Madin-Darby canine kidney; WB, Western blot; IF, immunofluorescence.
No longer available from Santa Cruz Biotechnology.
Immunofluorescence and confocal microscopy.
Stable clones were grown on filter supports for 8 days in the presence or absence of doxycycline (Dox+ or Dox−). The monolayers were washed in PBS (mM: 155.17 NaCl, 2.97 Na2HPO4·7H2O, and 1.06 KH2PO4, pH 7.4) and fixed in 4% PFA in PBS for 20 min at 4°C. Then the monolayers were incubated with blocking solution containing PBS, 1% BSA, 5% goat serum, and 0.3% Triton X-100 for 1 h at room temperature. The blocking solution was aspirated, and the primary antibody was added in a solution containing PBS, 1% BSA, and 5% goat serum and incubated for 1 h at room temperature. Primary antibodies against β-catenin or ZO-1 (Table 2), together with the polyclonal antibody raised against the carboxy terminus of claudin 30 (6 μg/ml), were used. After incubation with primary antibody, the monolayers were washed twice in PBS for 5 min, and secondary antibody (Alexa Fluor 488 and 555, Molecular Probes, Eugene, OR) was added in a dilution of 1:1,000 in a solution of PBS, 1% BSA, and 5% goat serum. Monolayers were incubated in the dark for 1 h with the secondary antibody and then washed four times for 5 min each with PBS. The filters containing the monolayers were mounted on glass slides using ProLong Gold antifade reagent (Invitrogen) and examined on a confocal microscope (model TCS SP2, Leica Microsystems).
Transepithelial resistance measurements.
Time course measurements of the transepithelial resistance (TER) were performed with a chopstick-type Ag/AgCl electrode connected to a Millicell-ERS Voltohmeter (Millipore, Bedford, MA), as described elsewhere (45). Results are shown as conductance in millisiemens (1,000/Ω) for ease of comparison with permeability measurements.
Ussing chamber studies.
Experiments followed the methods described by Yu et al. (45, 46). Briefly, MDCK II Tet-Off cells expressing claudin 30 were plated at confluence in Snapwell filters (Corning Life Sciences) and allowed to grow for 8 days. Medium was changed on days 3 and 6. The medium was carefully aspirated, and the filter was immediately inserted into the Ussing chamber when ready for an experiment, as described above. The following solutions were made on the day before the experiment: 150 mM sodium Ringer (SR), 75 mM SR, 150 mM lithium Ringer, potassium Ringer, rubidium Ringer, cesium Ringer, and tretramethylammonium (TMA) Ringer solution. The Ringer solutions contained (in mM) 150 XCl (X = alkali or organic cation), 2 CaCl2, 1 MgCl2, 10 glucose, and 10 HEPES, pH 7.4. In 75 mM SR solution, osmolality was held constant by addition of 150 mM mannitol. A typical experiment began with 150 mM SR solution in the apical and basolateral chambers. The solution in the basolateral chamber was then exchanged with 75 mM SR or 150 mM XR solution, and the voltage was recorded during pulses of injected current until a stable reading was obtained. The solution in the basolateral chamber was exchanged for 150 mM SR solution again, and the voltage was read. The dilution potential (VT) was defined as
The experimentally measured dilution potential (VEM) was adjusted for the difference in pipette potentials (VLb − VLa). These liquid junction potentials for 3 M KCl pipettes (VLb − VLa) have previously been determined (46) and were between −5.44 and 2.67 mV, and the measured dilution and bi-ionic potentials (VEM) were between −47.94 and 3.49 mV, depending on the solution exchanged in the basolateral chamber. Conductance of the cell monolayer (GM) was also adjusted for the conductance of the blank filter (GF)
The Goldman-Hodgkin-Katz equation could then be applied to the measured transepithelial potential and rearranged to give the relative permeability of the monolayer to sodium and chloride
where x = e−VF/RT (F = 96485.339 C/mol, R = 8.314 J·K−1·mol−1, and T = 310.15 K at 37°C) and α is the activity ratio of NaCl in the apical compared with the basolateral compartment. The ion activity coefficients of sodium and chloride are assumed to be the same. The α was calculated based on the activity coefficients of NaCl at 75 and 150 mM. The potential measured when the basolateral solution is exchanged to 75 SR solution was inserted into Eq. 4, and the relative permeability of chloride to sodium was calculated. The absolute permeability of the monolayer to sodium was then calculated based on the equation developed by Kimizuka and Koketsu (20)
The absolute permeability of the monolayer to chloride was calculated from β. The permeability of the monolayer to other alkali metal ions was calculated by use of the Goldman-Hodgkin-Katz equation, where X is the cation in question and γ = PX/PNa
where bl and ap represent basolateral and apical, respectively. Under experimental conditions, the expression can be rewritten and γ can be isolated
The absolute permeability of the cell monolayer to the alkali or organic cation was then derived from γ.
Statistics
All statistical comparisons were done by comparing Dox− samples with Dox+ samples and gill samples from FW- and SW-acclimated salmon, respectively, with a two-tailed t-test using GraphPad Prism 5.0 software (La Jolla, CA). Data were transformed to fit a Gaussian distribution if necessary. If transformation did not produce a Gaussian distribution, a nonparametric Mann-Whitney test was used to compare Dox− with Dox+ groups. A significant difference between treatment groups was accepted when P < 0.05.
RESULTS
Claudin 30 Protein Localization and Expression in the Gill of Atlantic Salmon
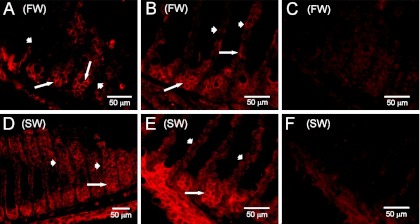
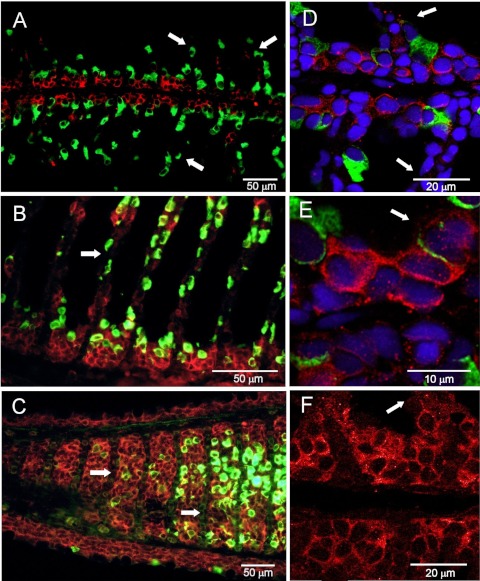
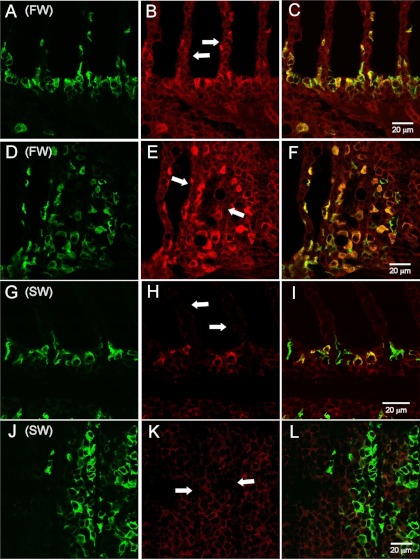
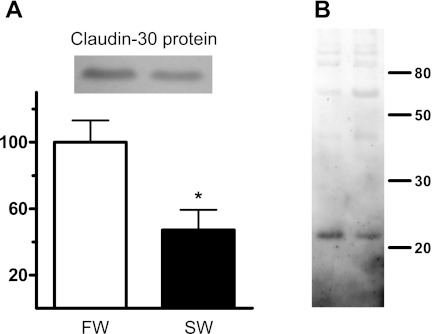
Immunofluorescence showed that claudin 30 was expressed at the cell-cell interface in all cell layers of the gill filament and only sparsely on the lamellae (Figs. 1 and 2). Control slides incubated with primary antibody blocked with the antigenic peptide showed only weak tissue autofluorescence (Fig. 1, C and F). ZO-1 staining was also abundant at cell junctions in the filament and on the lamellae but to a lesser extent deeper in the filament, thereby showing partly the same expression pattern as claudin 30 (Fig. 3). The staining pattern of claudin 30 protein in gill tissue was independent of osmotic environment, since SW- and FW-acclimated animals showed no difference in localization of claudin 30. Interestingly, ZO-1 colocalized with the membrane-bound α-subunit of the Na+-K+-ATPase in FW-acclimated salmon but only in a few cells in SW-acclimated salmon (Fig. 3, C and F vs. Fig. 3, I and L). This pattern was not observed for claudin 30, which generally did not colocalize with Na+-K+-ATPase (SW data not shown) and, thus, may not be associated with MRCs (Fig. 2). Claudin 30 protein was detected by Western blotting in gill homogenates from FW- and SW-acclimated salmon (Fig. 4). Expression of claudin 30 was significantly higher in FW- than SW-acclimated animals. When the claudin 30 antibody binding of the 22-kDa band was removed by preincubation with the blocking peptide, it still showed nonspecific binding with multiple high-molecular-mass (>50-kDa) proteins (not shown). However, as stated below, only clones with induced claudin 30 expression had the 22-kDa band, and specific immunofluorescence was abolished by the blocking peptide (Fig. 1, C and F).
Fig. 1.
Immunofluorescence microscopy with claudin 30 antibody (red) of gill tissue from Atlantic salmon. A–C: freshwater (FW)-acclimated gills. D–F: seawater (SW)-acclimated gills. A and D: frontal section. B and E: sagittal section. C and F: sections blocked with antigenic peptide. Short arrows indicate lamellae; long arrows point to base of lamellae.
Fig. 2.
Immunofluorescence microscopy with claudin 30 (red) and Na+-K+-ATPase α-subunit (α-5; green) antibodies of gill tissue from FW-acclimated Atlantic salmon. A–C: light-microscopic images. D–F: confocal microscopic images. A, B, D, E, and F: sagittal sections. C: frontal section. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (blue) in D and E. Arrows point to gill lamellae.
Fig. 3.
Immunofluorescence microscopy with ZO-1 (red) and Na+-K+-ATPase α-subunit (α-5; green) antibodies of gill tissue from Atlantic salmon. A–F: FW gills. G–L: SW gills. A–C and G–I: sagittal sections. D–F and J–L: frontal sections. Arrows point to gill lamellae (B and H) or base of gill lamellae (E and K).
Fig. 4.
A: expression of claudin 30 protein in gill tissue homogenates from FW- and SW-acclimated salmon. Inset: 22-kDa claudin 30 band on a representative Western blot with gill homogenates from 1 FW- and 1 SW-acclimated salmon. B: higher-molecular-mass bands. Size markers (in kDa) are shown at right. Claudin 30 protein content in gill homogenates was normalized to FW value (100%). β-Actin was used as loading control. Values are means ± SE; n = 5. *Significantly different from FW, P < 0.05.
Localization and Functional Characterization of Salmon Claudin 30 in MDCK II Tet-Off Cells
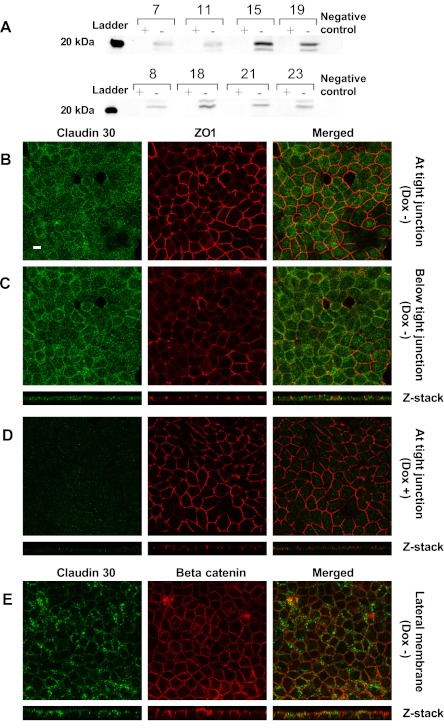
Claudin 30 was successfully transfected into MDCK II Tet-Off cells by retroviral transduction. The expression of claudin 30 was under control of the Tet-responsive element, allowing for strict control of transcription of the gene by the addition of doxycycline to the culture medium, which effectively suppressed expression of the salmon claudin. This was apparent when cell lysate collected from clones grown in the presence or absence of doxycycline (Dox+ or Dox−) was subjected to SDS-PAGE and Western blotting. All chosen hygromycin-resistant clones had detectable expression of claudin 30 in the absence of doxycycline, although there was some variability within the clones examined (Fig. 5A). The antibody directed against the carboxy terminus of claudin 30 showed nonspecific binding with multiple high-molecular-mass (>50-kDa) proteins in Dox+ and Dox− preparations and in negative control samples (not shown). Specific binding to a 22-kDa protein (claudin 30) was only present in Dox− samples, thereby confirming the specificity of the antibody.
Fig. 5.
Expression of claudin 30 in Madin-Darby canine kidney (MDCK) II Tet-Off cell lines created by retroviral transduction. A: Western blots of 8 clones in the presence (+) or absence (−) of doxycycline [suppression of claudin 30 expression (Dox+) or no suppression (Dox−)] probed with claudin 30 antibody. Band of claudin 30 was apparent at ∼22 kDa in Dox− samples only. Protein was loaded at 20 μg per lane, and claudin 30 antibody was used in a concentration of 0.33 μg/ml. Negative control was a MDCK I Tet-Off cell line expressing mouse wild-type claudin 2. B and C: confocal images of a MDCK II Tet-Off cell layer expressing claudin 30 (Dox−), taken as xy sections (large images) at or below the tight junction, as judged by localization of the tight junction-associated protein ZO-1. Z stack shows vertical distribution of claudin 30 and ZO-1 in cell monolayer. Apical side is at the top of the Z stack. D: confocal images of a MDCK II Tet-Off cell layer where expression of claudin 30 is suppressed by doxycycline, taken as xy sections at the tight junction where ZO-1 is most abundant. E: confocal images of a MDCK II Tet-Off cell layer expressing claudin 30 (Dox−), taken as xy sections (large images) at the level of the lateral membrane, as judged by localization of the lateral membrane-associated protein β-catenin. Z stack shows a vertical section of the cell monolayer where claudin 30 and β-catenin are found to colocalize along the lateral membrane. Scale bar, 10 μm.
Confocal microscopy of MDCK cell monolayers expressing claudin 30 revealed that the salmon claudin was only expressed in cells without doxycycline added to the growth medium (Fig. 5, B, C, and E vs. Fig. 5D). Claudin 30 was found throughout the cell cytoplasm, along the lateral membrane and at the tight junction. Colocalization was observed between claudin 30 and ZO-1 in isolated places, confirming that claudin 30 was present at the tight junction in small amounts (Fig. 5B). More intense staining of claudin 30 was seen along the lateral membrane in an area below the tight junction (Fig. 5, C and E), as shown by colocalization of claudin 30 and the adherens junction and lateral membrane-associated protein β-catenin (Fig. 5E).
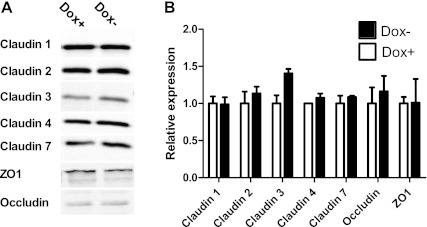
Claudin 30 expression did not affect the expression of most endogenous tight junction proteins, except for a nonsignificant increase in the expression of claudin 3 (n = 2; Fig. 6).
Fig. 6.
Effect of claudin 30 expression on expression of endogenous tight junction proteins of MDCK II Tet-Off cells. A: representative immunoblots of MDCK II Tet-Off cell lysate probed with antibodies against occludin, claudin 1, 2, 3, 4, and 7, and ZO-1. B: densitometric analysis of endogenous tight junction protein expression. Tight junction protein content in monolayers with expression of claudin 30 (Dox−) is normalized to tight junction protein content in monolayers with suppression of claudin 30 expression (Dox+). Values are means ± SE; n = 2.
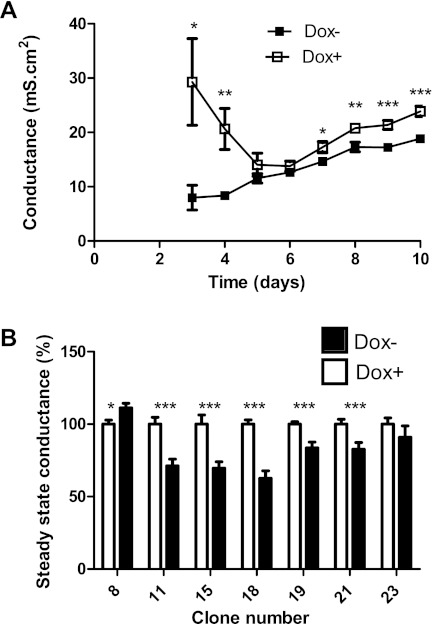
The conductance of MDCK II Tet-Off cell monolayers stably transfected with salmon claudin 30 varied during the first 8 days before reaching a steady state (Fig. 7A). After 3 days, conductance was higher in the monolayers without claudin 30 expression (Dox+) than in the monolayers with claudin 30 expression (Dox−; Fig. 7A). The conductance of cell monolayers with and without suppression of claudin 30 expression was at the same level at around day 5. After 8–10 days, the conductance of cell monolayers with expression of claudin 30 had stabilized at 17.81 ± 0.52 mS, whereas the cell monolayers with suppression of claudin 30 expression had stabilized at 22.02 ± 0.95 mS. When the clones were evaluated separately, the same tendency was observed. Six clones showed a significantly lower conductance when claudin 30 was expressed, and only one clone showed the opposite trend (Fig. 7B). The conductance of the cell monolayers expressing claudin 30 varied from 63% to 91% (not including clone 8) of the conductance in the corresponding cell monolayers where expression of the salmon claudin was suppressed.
Fig. 7.
Effect of claudin 30 expression on conductance of MDCK II Tet-Off monolayers. A: conductance of MDCK II Tet-Off monolayers expressing salmon claudin 30. Dox−, monolayers with expression of salmon claudin; Dox+, monolayers where expression of salmon claudin was suppressed by the presence of doxycycline. Seven clones were analyzed, and 6 replicates of each clone were plated in Transwell filters, giving a total of 42. Values are means ± SE of all clones on each day after plating starting with day 3. B: steady-state conductance of MDCK II Tet-Off clones expressing claudin 30. Steady-state conductance was calculated as mean conductance from day 8 to day 10. Values are means ± SE, expressed as percentage of conductance of monolayers from the same clone with suppressed expression of claudin 30 (Dox+); n = 6 for each clone of each treatment group on each day. Significant difference between groups: *P < 0.05, **P < 0.01, ***P < 0.001.
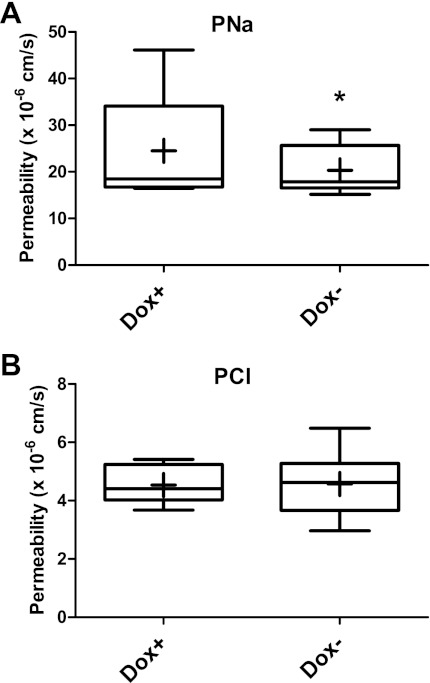
Ussing chamber experiments revealed a significant decrease in permeability of the MDCK II Tet-Off cell monolayer to sodium when claudin 30 was expressed (Fig. 8A). Sodium permeability decreased 17% and was not associated with a concurrent decrease in permeability of the cell monolayer to chloride, which was unaffected by the expression of claudin 30 (Fig. 8B). Furthermore, the permeability of the cell monolayer to all alkali metal ions and the organic cation TMA relative to sodium did not change when claudin 30 was expressed (Table 3). Thus an overall decrease in the permeability of the monolayer to monovalent cations was observed upon expression of claudin 30. The MDCK cell monolayer was almost equally permeable to cesium and sodium, whereas it was more permeable to potassium and rubidium and less permeable to lithium and TMA. The following order of permeability was observed: potassium > rubidium > sodium ∼ cesium > lithium >> TMA. Ions did not pass in direct relation to their nonhydrated or hydrated size but, rather, somewhere in between (Table 3).
Fig. 8.
Effect of claudin 30 expression on permeability of MDCK II Tet-Off monolayer to sodium (PNa) and chloride (PCl). In box-and-whiskers plots, whiskers show minimum and maximum values and mean is indicated with a cross. *Significantly different from Dox+, P < 0.05.
Table 3.
Relative permeability of monovalent cations
| Diameter* |
PX/PNa |
|||
|---|---|---|---|---|
| Ion | Unhydrated | Hydrated | Dox+ | Dox− |
| Li+ | 1.88 | 7.64 | 0.77 ± 0.014 | 0.77 ± 0.014 |
| Na+ | 2.34 | 7.16 | 1 | 1 |
| K+ | 2.98 | 6.62 | 1.34 ± 0.047 | 1.38 ± 0.045 |
| Rb+ | 3.26 | 6.58 | 1.19 ± 0.017 | 1.20 ± 0.037 |
| Cs+ | 3.72 | 6.58 | 0.97 + 0.051 | 0.97 ± 0.072 |
| TMA+ | 5.7 | 6.94 | 0.21 + 0.017 | 0.11 ± 0.094 |
Values are means ± SE (n = 3).
TMA, tetramethylammonium.
Mean diameters (in Å, 100 pm) of unhydrated and hydrated ions are calculated as twice the radius of the bare and hydrated ions, respectively [compiled by Volkov et al. (43a)]. No significant differences in relative permeability of the cations (PX/PNa) were found between monolayers with (Dox−) and without (Dox+) claudin 30 expression.
Alignment Analysis of Salmon Claudin 30
The first extracellular loop of salmon claudin 30 was aligned with similar vertebrate isoforms to deduce a putative functional role of this protein in vivo (Fig. 9). This loop is thought to be involved in intercellular dimer formation across the intermembrane space and determination of ion selectivity of the tight junction (42). Salmon claudin 30 shared the greatest homology with the other fish members of the alignment. The human claudins were included, because functional investigations of these claudins have been undertaken. Interestingly, salmon claudin 30 shares a negative residue with human claudin 7 at one position where none of the other teleost claudins bear a charged residue. Another interesting difference is the presence of a polar, but noncharged, residue (Q) in salmon claudin 30, where the other teleost claudins and human claudin 4 bear a negatively charged residue. Most of the charged residues in the first extracellular loop are conserved across all species tested, as well as the characteristic cysteines of all claudins, which are suspected to form a disulfide bond (42).
Fig. 9.
Alignment of the first extracellular loop of claudin isoforms similar to claudin 30 (clustalW2). Omo, Oreochromis mossambicus; Tru, Takifugu rubripes; Ssa, Salmo salar; Hs, Homo sapiens. Acidic residues are red, basic residues are green. Boxes mark residues that, when mutated, have been shown to alter or abolish claudin function. See Refs. 1 and 7 for further information. ∗, Identical residue; :, conserved residue; ., semiconserved residue.
DISCUSSION
This is the first study to investigate the functional properties of a teleost claudin. Claudin expression dynamics in teleost gills have been investigated extensively (3, 4, 31, 35, 36, 37) but generally on the mRNA level and in relatively few species. In this study, we investigated the protein expression and localization in the gill, as well as the functional properties of the claudin 30 protein from Atlantic salmon.
Claudin 30 Expression in the Native Gill
A carboxy-terminal antibody against claudin 30 was developed and validated in gill samples from Atlantic salmon. On Western blots, the antibody recognized a specific protein at ∼22 kDa that matches the expected molecular mass of claudin 30. The band was present in extracts from MDCK cell monolayers, where claudin 30 expression was activated (Dox−), but not in control (Dox+) cells. Claudin 30 expression was higher in FW- than SW-acclimated salmon, which matches our earlier observation of higher claudin 30 mRNA levels in gills from FW- than SW-acclimated salmon (37). These findings support our hypothesis that this claudin isoform is critical to maintaining tight junction structure in the FW gill. Visualization of claudin 30 by immunofluorescence supported a broader role of claudin 30 in the structural organization of the gill epithelia. Claudin 30 was generally not localized in association with the Na+-K+-ATPase immunostaining cells in FW- or SW-acclimated animals. This indicated that claudin 30 was not associated with MRCs and, thus, not associated with the tight junctions of MRCs known to change in depth with change in salinity (30). SW- and FW-acclimated salmon showed the same staining pattern of claudin 30 in the gill, and a qualitative change in distribution in SW-acclimated animals was therefore not evident by immunofluorescence. Some staining of claudin 30 at the outermost cell layers of the gill suggests that claudin 30 is part of tight junctions between pavement cells. The more intense staining of claudin 30 in the deeper-lying cell layers of the gill filament, however, suggests a role of claudin 30 other than participation in apical tight junctions. The staining pattern of the tight junction protein ZO-1 in the gill of Atlantic salmon also showed staining of the deeper cell layers, but because of the complex structural nature of the gill filament, it was difficult to determine whether all ZO-1 staining was located at tight junctions. Staining of another tight junction protein, occludin, in goldfish has been shown to locate at cell junctions throughout the gill filament and on the edges of the lamellae (5), showing that tight junctions or tight junction-associated proteins may not be limited to the outermost cell layer of the gill epithelia. Another interesting aspect of ZO-1 staining was evident in gills from FW-acclimated salmon, where this protein colocalized with the Na+-K+-ATPase staining of the membranous network of MRCs. The role of ZO-1 in these cells is, to our knowledge, unknown, but we propose that ZO-1 may have a role in organizing the highly folded basolateral membrane of MRCs. A recent study of gill lamellae ultrastructure reported that ZO-1 staining was associated with pillar cells and suggested that this protein might be involved in the formation of autocellular junctions around collagen columns (20) and, thus, located ZO-1 away from the surface epithelia. It is puzzling, however, that ZO-1 is not associated with all MRCs in SW-acclimated salmon, which may suggest that the architecture of these cells is organized by other proteins.
Claudin 30 Expression in MCDK Cell Monolayers
Confocal microscopy of MDCK cell layers expressing claudin 30 revealed a claudin that was expressed at the apical tight junction but also more laterally, as evaluated by its location relative to ZO-1 in these cells. There was also a certain amount of cytoplasmic staining, suggesting that not all claudin 30 was inserted into cell junctions. The lateral location was unexpected but matches well with the more general location of claudin 30 in the deeper cell layers of the native gill.
Even though the role and localization of claudins have mostly been investigated in the tight junction of various epithelia, claudin isoforms have also been found along the lateral membrane in different intestinal tissues, i.e., liver (29) and kidney (21) and even airway epithelia (8). Mammalian claudin isoforms were investigated in all these studies, but it was shown that claudin proteins occasionally are located away from the tight junction. This suggests that claudins participate in interactions other than occupying and defining the apical intercellular seal and, e.g., paracellular pores. Recent studies of claudin localization in MDCK cells have also shown that the lateral distribution of claudins may be caused by regulatory signals. In one study, the lateral distribution of claudins in MDCK cells was affected by epidermal growth factor, which at 100 ng/ml led to increased expression of claudin 4 and 7 along the lateral membrane (10). Another study showed that PKC activation of claudins also affected their intracellular position. In this study, claudin 1, 3, 4, and 5 were affected by PKC activation, which led to more claudins in an insoluble fraction of cell lysate suspected to contain tight junction complexes (33). The lateral distribution of claudins was also affected by PKC activation, with some claudins being more apparent at the most apical end of cell-cell contacts following 2 h of treatment (33). These studies show that claudin function and localization depend on activation by cellular signaling cascades, which may be specific to a certain species or even organs (6). We know from previous studies that transcription of claudin 30 in the salmon gill is enhanced by cortisol in vivo and in vitro, an activation that takes place through the glucocorticosteroid receptor (36). This dynamic regulation of salmon claudin expression and possibly localization might be important in maintaining the paracellular seal during acclimation to SW and FW.
Functional Analysis of Salmon Claudin 30 in MDCK Cells
MDCK cells have been widely used for overexpression studies of mammalian claudin isoforms (41, 42, 43, 45, 46) and for silencing studies as well (15). Therefore, we suspected that functional insight into salmon claudin function could be achieved by expressing claudin 30 in low-resistance MDCK cells. Expression of the salmon claudin led to a decreased conductance of the MDCK cell monolayer. The decrease in conductance was 19%, which correlates well with the decreased conductance seen in other overexpression experiments with mammalian claudins (41, 45). The results obtained in the present study also correlated well with the decrease in TER in an RNA interference study, where silencing of claudin 4 in MDCK cells led to a decrease in TER of 31% of the control value (15). The effect of claudin 30 expression on conductance must be seen in the light of possible changes in the expression of endogenous tight junction proteins. Only expression of claudin 3 seemed to increase slightly when claudin 30 was expressed. This may be a compensatory change due to introduction of an exogenous claudin isoform. Claudin 3 is known to participate in heterophilic interactions with other claudins, as shown by coimmunoprecipitation (8, 12), and a possible explanation of increased claudin 3 could be an association of this isoform with the salmon claudin, although more evidence is needed to support this hypothesis. Claudin 3 is by itself a barrier-forming claudin in MDCK cells (15), so it cannot be ruled out that the decreased conductance is, in part, due to an interaction of claudin 3 with claudin 30. This illustrates the difficulty of interpreting overexpression studies, as the tight junction is a dynamic and variable compartment that may undergo rapid changes by many different mechanisms (32).
Ussing chamber experiments showed that the decrease in conductance correlated well with a decrease in the permeability of the monolayer to sodium (17%) when claudin 30 was expressed. The finding that chloride permeability was not affected supports the hypothesis that claudin 30 forms a cation barrier, as inferred by the charge distribution in the first extracellular loop. When other monovalent cations were tested, the decrease in sodium permeability of the monolayer with claudin 30 expression was extended to include all alkali ions and an organic cation. Expression of claudin 30, therefore, led to a general decrease in the permeability of the monolayer to monovalent cations. Overexpression of claudin 30 led to the same results obtained when human claudin 4 (41) or mouse claudin 8 (45) was expressed in MDCK cells, suggesting a functional relationship between these isoforms. The first extracellular loop of salmon claudin 30 is greatly conserved in related fish species. It seems from these analyses that the key charged residues shared with human claudin 4 are those responsible for maintaining the function as a cation barrier, since the effects on electrophysiological parameters of both claudins are similar.
Significance and Perspectives
Immunofluorescence of claudin 30 in the gill of Atlantic salmon has revealed a claudin with a broad distribution in vivo. The experimental, as well as structural, evidence of this study strongly suggests that claudin 30 is a cation barrier when localized to tight junctions between pavement cells in the gill epithelia. Its expression may thus contribute to a tighter epithelium in the gill of FW salmon. A role of claudin 30 in cell-cell adhesion is also suspected on the basis of its localization in the deeper cell layers of the gill filament and is supported by the lateral distribution when claudin 30 is expressed in MDCK cell monolayers (8, 21, 29, 33). Future studies in this area should focus on the interactions between claudins and proteins linked to the extracellular matrix, such as EpCAM, which has been linked to claudins (27). Acute regulation of salmon claudins in the gill will also be an interesting area for future studies. Knowledge of phosphorylation and subcellular trafficking of teleost claudins is lacking but may prove especially important during acclimation of euryhaline fishes to different salinities and will naturally be a fruitful area for future research.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-062283 (to A. S. L. Yu). M. B. Engelund was supported by grants from the American Scandinavian Foundation and the Frimodt-Heineke Foundation. S. S. Madsen was supported by Danish Natural Research Council Grant 09-070689.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: M.B.E., A.S.L.Y., S.S.M., N.J.F., and C.K.T. are responsible for conception and design of the research; M.B.E., A.S.L.Y., J.L., S.S.M., N.J.F., and C.K.T. performed the experiments; M.B.E., J.L., S.S.M., and C.K.T. analyzed the data; M.B.E., A.S.L.Y., J.L., S.S.M., N.J.F., and C.K.T. interpreted the results of the experiments; M.B.E., S.S.M., and C.K.T. prepared the figures; M.B.E. drafted the manuscript; M.B.E., A.S.L.Y., S.S.M., and C.K.T. edited and revised the manuscript; M.B.E., A.S.L.Y., J.L., S.S.M., N.J.F., and C.K.T. approved the final version of the manuscript.
REFERENCES
- 1. Alexandre MD, Jeansonne BG, Renegar RH, Tatum R, Chen YH. The first extracellular domain of claudin-7 affects paracellular Cl− permeability. Biochem Biophys Res Commun 357: 87–91, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Angelow S, Ahlstrom R, Yu ASL. Biology of claudins. Am J Physiol Renal Physiol 295: F867–F876, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bagherie-Lachidan M, Wright SI, Kelly SP. Claudin-3 tight junction proteins in Tetraodon nigroviridis: cloning, tissue-specific expression, and a role in hydromineral balance. Am J Physiol Regul Integr Comp Physiol 294: R1638–R1647, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Bagherie-Lachidan M, Wright SI, Kelly SP. Claudin-8 and -27 tight junction proteins in puffer fish Tetraodon nigroviridis acclimated to freshwater and seawater. J Comp Physiol B 179: 419–431, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Chasiotis H, Kelly SP. Occludin immunolocalization and protein expression in goldfish. J Exp Biol 211: 1524–1534, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Chiba H, Osanai M, Murata M, Kojima T, Sawada N. Transmembrane proteins of tight junctions. BBA Biomembranes 1778: 588–600, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Colegio OR, Van Itallie CM, McCrea HJ, Rahner C, Anderson JM. Claudins create charge-selective channels in the paracellular pathway between epithelial cells. Am J Physiol Cell Physiol 283: C142–C147, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Coyne CB, Gambling TM, Boucher RC, Carson JL, Johnson LG. Role of claudin interactions in airway tight junctional permeability. Am J Physiol Lung Cell Mol Physiol 285: L1166–L1178, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Evans DH, Piermarini PM, Choe KP. The multifunctional fish gill: dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol Rev 85: 97–177, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Flores-Benitez D, Ruiz-Cabrera A, Flores-Maldonado C, Shoshani L, Cereijido M, Contreras RG. Control of tight junctional sealing: role of epidermal growth factor. Am J Physiol Renal Physiol 292: F828–F836, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Furuse M, Sasaki H, Tsukita S. Manner of interaction of heterogeneous claudin species within and between tight junction strands. J Cell Biol 147: 891–903, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hiroi J, McCormick SD, Ohtani-Kaneko R, Kaneko T. Functional classification of mitochondrion-rich cells in euryhaline Mozambique tilapia (Oreochromis mossambicus) embryos, by means of triple immunofluorescence staining for Na+/K+-ATPase, Na+/K+/2Cl− cotransporter and CFTR anion channel. J Exp Biol 208: 2023–2036, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Hiroi J, Miyazaki H, Katoh F, Ohtani-Kaneko R, Kaneko T. Chloride turnover and ion-transporting activities of yolk-sac preparations (yolk balls) separated from Mozambique tilapia embryos and incubated in freshwater and seawater. J Exp Biol 208: 3851–3858, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Hou JH, Gomes AS, Paul DL, Goodenough DA. Study of claudin function by RNA interference. J Biol Chem 281: 36117–36123, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Hwang P, Lee T, Lin L. Ion regulation in fish gills: recent progress in the cellular and molecular mechanisms. Am J Physiol Regul Integr Comp Physiol 301: R28–R47, 2011 [DOI] [PubMed] [Google Scholar]
- 17. Karnaky KJ. Structure and function of the chloride cell of Fundulus heteroclitus and other teleosts. Am Zool 26: 209–224, 1986 [Google Scholar]
- 18. Kato A, Nakamura K, Kudo H, Tran YH, Yamamoto Y, Doi H, Hirose S. Characterization of the column and autocellular junctions that define the vasculature of gill lamellae. J Histochem Cytochem 55: 941–953, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Kimizuka H, Koketsu K. Ion transport through cell membrane. J Theor Biol 6: 290–305, 1964 [DOI] [PubMed] [Google Scholar]
- 21. Li WY, Huey CL, Yu ASL. Expression of claudin-7 and -8 along the mouse nephron. Am J Physiol Renal Physiol 286: F1063–F1071, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Loh YH, Christoffels A, Brenner S, Hunziker W, Venkatesh B. Extensive expansion of the claudin gene family in the teleost fish, Fugu rubripes. Genome Res 14: 1248–1257, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Madsen SS, Jensen LN, Tipsmark CK, Kiilerich P, Borski RJ. Differential regulation of cystic fibrosis transmembrane conductance regulator and Na+,K+-ATPase in gills of striped bass, Morone saxatilis: effect of salinity and hormones. J Endocrinol 192: 249–260, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Madsen SS, Kiilerich P, Tipsmark CK. Multiplicity of expression of Na+,K+-ATPase α-subunit isoforms in the gill of Atlantic salmon (Salmo salar): cellular localisation and absolute quantification in response to salinity change. J Exp Biol 212: 78–88, 2009 [DOI] [PubMed] [Google Scholar]
- 25. Marshall WS, Lynch EM, Cozzi RR. Redistribution of immunofluorescence of CFTR anion channel and NKCC cotransporter in chloride cells during adaptation of the killifish Fundulus heteroclitus to sea water. J Exp Biol 205: 1265–1273, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Marshall WS, Ossum CG, Hoffmann EK. Hypotonic shock mediation by p38 MAPK, JNK, PKC, FAK, OSR1 and SPAK in osmosensing chloride secreting cells of killifish opercular epithelium. J Exp Biol 208: 1063–1077, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Nubel T, Preobraschenski J, Tuncay H, Weiss T, Kuhn S, Ladwein M, Langbein L, Zoller M. Claudin-7 regulates EpCAM-mediated functions in tumor progression. Mol Cancer Res 7: 285–299, 2009 [DOI] [PubMed] [Google Scholar]
- 28. Potts WTW, Eddy FB. Gill potentials and sodium fluxes in the flounder Platichthys flesus. J Comp Physiol 87: 29–48, 1973 [Google Scholar]
- 29. Rahner C, Mitic LL, Anderson JM. Heterogeneity in expression and subcellular localization of claudins 2, 3, 4, and 5 in the rat liver, pancreas, and gut. Gastroenterology 120: 411–422, 2001 [DOI] [PubMed] [Google Scholar]
- 30. Sardet C, Pisam M, Maetz J. Surface epithelium of teleostean fish gills—cellular and junctional adaptations of chloride cell in relation to salt adaptation. J Cell Biol 80: 96–117, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sandbichler AM, Egg M, Schwerte T, Pelster B. Claudin 28b and F-actin are involved in rainbow trout gill pavement cell tight junction remodelling under osmotic stress. J Exp Biol 214: 1473–1487, 2011 [DOI] [PubMed] [Google Scholar]
- 32. Shen L, Weber CR, Turner JR. The tight junction protein complex undergoes rapid and continuous molecular remodeling at steady state. J Cell Biol 181: 683–695, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sjo A, Magnusson KE, Peterson KH. Protein kinase C activation has distinct effects on the localization, phosphorylation and detergent solubility of the claudin protein family in tight and leaky epithelial cells. J Membr Biol 236: 181–189, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tipsmark CK, Madsen SS, Seidelin M, Christensen AS, Cutler CP, Cramb G. Dynamics of Na+,K+,2Cl− cotransporter and Na+,K+-ATPase expression in the branchial epithelium of brown trout (Salmo trutta) and Atlantic salmon (Salmo salar). J Exp Zool 293: 106–118, 2002 [DOI] [PubMed] [Google Scholar]
- 35. Tipsmark CK, Baltzegar DA, Ozden O, Grubb BJ, Borski RJ. Salinity regulates claudin mRNA and protein expression in the teleost gill. Am J Physiol Regul Integr Comp Physiol 294: R1004–R1014, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Tipsmark CK, Jorgensen C, Brande-Lavridsen N, Engelund M, Olesen JH, Madsen SS. Effects of cortisol, growth hormone and prolactin on gill claudin expression in Atlantic salmon. Gen Comp Endocrinol 163: 270–277, 2009 [DOI] [PubMed] [Google Scholar]
- 37. Tipsmark CK, Kiilerich P, Nilsen TO, Ebbesson LOE, Stefansson SO, Madsen SS. Branchial expression patterns of claudin isoforms in Atlantic salmon during seawater acclimation and smoltification. Am J Physiol Regul Integr Comp Physiol 294: R1563–R1574, 2008 [DOI] [PubMed] [Google Scholar]
- 40. Ussing HH, Zerahn K. Active transport of sodium as the source of electric current in the short-circuited isolated frog skin. Acta Physiol Scand 23: 110–127, 1951 [DOI] [PubMed] [Google Scholar]
- 41. Van Itallie C, Rahner C, Anderson JM. Regulated expression of claudin-4 decreases paracellular conductance through a selective decrease in sodium permeability. J Clin Invest 107: 1319–1327, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Van Itallie CM, Anderson JM. Claudins and epithelial paracellular transport. Annu Rev Physiol 68: 403–429, 2006 [DOI] [PubMed] [Google Scholar]
- 43. Van Itallie CM, Mitic LL, Anderson JM. Claudin-2 forms homodimers and is a component of a high molecular weight protein complex. J Biol Chem 286: 3442–3450, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43a. Volkov AG, Paula S, Deamer DW. Two mechanisms of permeation of small neutral molecules and hydrated ions across phospholipid bilayers. Bioelectrochem Bioenerg 42: 153–160, 1997 [Google Scholar]
- 44. Wood CM, Kelly SP, Zhou B, Fletcher M, O'Donnell M, Eletti B, Pärt P. Cultured gill epithelia as models for the freshwater fish gill. Biochim Biophys Acta 1566: 72–83, 2002 [DOI] [PubMed] [Google Scholar]
- 45. Yu ASL, Enck AH, Lencer WI, Schneeberger EE. Claudin-8 expression in Madin-Darby canine kidney cells augments the paracellular barrier to cation permeation. J Biol Chem 278: 17350–17359, 2003 [DOI] [PubMed] [Google Scholar]
- 46. Yu ASL, Cheng MH, Angelow S, Gunzel D, Kanzawa SA, Schneeberger EE, Fromm M, Coalson RD. Molecular basis for cation selectivity in claudin-2-based paracellular pores: identification of an electrostatic interaction site. J Gen Physiol 133: 111–127, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]