Abstract
Previous reports suggest that glucagon-like peptide (GLP-1), a peptide secreted from the distal small intestine, is an endocrine satiation signal. Nevertheless, there are conflicting reports regarding the site where circulating GLP-1 acts to reduce food intake. To test the hypothesis that vagal afferents are necessary for reduction of food intake by circulating GLP-1, we measured intake of 15% sucrose during intravenous GLP-1 infusion in intact, vagotomized, and capsaicin-treated rats. We also measured sucrose intake during intravenous infusion of cholecystokinin, a peptide known to reduce food intake via abdominal vagal afferents. We found that reduction of intake by GLP-1 was not diminished by capsaicin treatment or vagotomy. In fact, reduction of sucrose intake by our highest GLP-1 dose was enhanced in vagotomized and capsaicin-treated rats. Intravenous GLP-1 induced comparable increases of hindbrain c-Fos immunoreactivity in intact, capsaicin-treated, and vagotomized rats. Plasma concentrations of active GLP-1 in capsaicin-treated rats did not differ from those of controls during the intravenous infusions. Finally, capsaicin treatment was not associated with altered GLP-1R mRNA in the brain, but nodose ganglia GLP-1R mRNA was significantly reduced in capsaicin-treated rats. Although reduction of food intake by intraperitoneal cholecystokinin was abolished in vagotomized and capsaicin-treated rats, reduction of intake by intravenous cholecystokinin was only partially attenuated. These results indicate that vagal or capsaicin-sensitive neurons are not necessary for reduction of food intake by circulating (endocrine) GLP-1, or cholecystokinin. Vagal participation in satiation by these peptides may be limited to paracrine effects exerted near the sites of their secretion.
Keywords: satiety, gut-brain peptides, gastrointestinal, rat
glucagon-like peptide-1 (glp-1) is derived from the precursor peptide, pre-pro-glucagon (PPG), by tissue-specific enzymatic cleavage (9, 23). PPG mRNA is expressed only in very restricted areas of the rat brain, specifically in small groups of neurons in the olfactory glomeruli, in the ventral caudal medulla, and in the nucleus of the solitary tract (NTS) (18, 23). Peripherally, most GLP-1 is synthesized and secreted by a population of enteroendocrine cells (L-cells), which are most prevalent in the mucosa of the distal small intestine and colon (10). Intestinal GLP-1 is secreted into intestinal extracellular space in response to nutrients in the intestinal lumen (19, 29, 32).
GLP-1 affects metabolic homeostasis via multiple mechanisms. For example, GLP-1 and GLP-1 analogs enhance insulin secretion (24) and increase insulin sensitivity in both rodents and humans (46, 49). In addition, GLP-1 inhibits gastric emptying (15), which serves to reduce the rate of presentation of nutrients, including glucose, for intestinal absorption. Finally, GLP-1 and GLP-1 analogs reduce food intake when administered systemically (27, 33) or into the forebrain or hindbrain ventricles (17, 40, 43).
The effects of GLP-1 and GLP-1 analogs are mediated through G protein-coupled receptors (GLP-1R). In the brain, GLP-1Rs are widely but discretely distributed (12, 41), including in areas involved in control of food intake, such as the hypothalamus and the dorsal vagal complex (DVC), which includes the area postrema (AP) and the NTS. Although some brain neurons that express GLP-1R mRNA reside in areas innervated by PPG-expressing neurons, some areas that exhibit GLP-1Rs are not recipients of known GLP-1 neuronal inputs (23), suggesting that these areas might be targets of GLP-1 from other sources, including blood-borne GLP-1. In situ hybridization to localize GLP-1R transcript indicates that the AP is among those mismatched areas that express GLP-1R mRNA but lack detectable input from PPG-expressing neurons (23). In the periphery, GLP-1Rs are expressed by a variety of tissues, including pancreatic β cells, cells in the lung, the gastric pits of the stomach, and the crypts of the duodenum, as well as by vagal afferent neurons (7, 26).
Several lines of investigation indicate that GLP-1Rs expressed by vagal afferents mediate GLP-1's effects on gastric motility and its inhibition of gastric emptying (15, 44). However, the role of vagal afferents in control of food intake by GLP-1 remains uncertain (1, 34). Therefore, to test the hypothesis that vagal afferents are necessary for reduction of food intake by circulating GLP-1, we measured intake of 15% sucrose during intravenous infusion of GLP-1 in intact, vagotomized, or capsaicin-treated rats. We also measured sucrose intake during intravenous infusion of sulfated CCK-8, a peptide known to reduce food intake via its action on abdominal vagal afferents following intraperitoneal injection.
MATERIALS AND METHODS
Animals
A total of 60 adult male Sprague-Dawley rats (Simonsen Laboratories, Gilroy, CA, USA) weighing an average of 313 ± 2.5 g were subjects for these experiments. All rats were housed in individual hanging cages in a vivarium under conditions of controlled lighting (12:12-h light-dark cycle, lights on at 0700), humidity (70%), and temperature (22 ± 2°C). Rats were handled daily and habituated to laboratory conditions before surgery or behavioral testing began. They had ad libitum access to pelleted chow (Teklad) and water, except during experiments and overnight fasts. All animal procedures were approved by the Washington State University Institutional Animal Care and Use Committee.
Peptides
GLP-1 (7–36) amide (Bachem, Torrance, CA) for intravenous infusion was dissolved in sterile pyrogen-free 0.15 M NaCl in concentrations ranging from 1.75 to 14 μg/ml. Sulfated CCK-8 (Peptide Institute, Osaka, Japan) also was dissolved in sterile 0.15 M NaCl. For intravenous administration, CCK-8 concentrations were 2 or 4 μg/ml, while for intraperitoneal administration, the CCK-8 solution was at a concentration of 2 μg/ml and administered in a dose of 0.1 ml/100 g body wt. Peptide solutions were prepared immediately before the start of the experiments on each testing day.
Surgeries and Capsaicin Treatment
Bilateral subdiaphragmatic vagotomy.
In five rats (n = 5), both dorsal and ventral subdiaphragmatic vagal trunks were sectioned just caudal to the diaphragmatic hiatus. Sham-vagotomized control rats (n = 8) were prepared by surgically approaching the vagi, but not sectioning them. These procedures have previously been described in detail (42, 48). Briefly, rats were anesthetized with a mixture containing ketamine (50 mg/kg), acepromazine (2 mg/kg), and xylazine (25 mg/kg) (0.1 ml mixture/100 g body wt). Rats were placed in a dorsal recumbent position, and the stomach and abdominal portion of the esophagus were exposed via a ventral midline laparotomy. The dorsal and ventral vagal nerve trunks were identified and isolated. Using fine scissors, we dissected and removed a segment ∼3 mm long from each vagal trunk, rostral to the hepatic and accessory celiac vagal branches. The abdominal incision was closed with a combination of 4–0 gut suture and skin staples. Sham surgeries were conducted in an identical manner except that, rather than resecting the vagal trunks, each nerve was merely touched with a wet cotton-tip applicator. The postsurgical recovery period before the catheterization surgeries were performed was 14 days or the time required for a rat to consistently maintain stable body weight, whichever was longer. Sham-vagotomized rats were maintained on pelleted rodent diet, while vagotomized rats were maintained on the same diet in powdered ground form.
Capsaicin treatment.
Capsaicin (Sigma, St. Louis, MO) was dissolved in a vehicle consisting of Tween 80 (10%), ethanol (10%), and 0.9% NaCl (80%) to achieve capsaicin concentrations of either 25 or 50 mg/ml. Systemic treatment with capsaicin (n = 8) was used to destroy small unmyelinated primary afferent neurons, including those in the vagi, as described previously (30, 38, 47). Briefly, a series of three capsaicin doses (25, 50, and 50 mg/kg) was injected intraperitoneally over a 36-h period. Controls (n = 8) were injected intraperitoneally with the vehicle solution under the same conditions as capsaicin, and according to an identical schedule. Rats were maintained at a surgical plane of general anesthesia using isoflurane for all capsaicin and vehicle injections, and positive pressure ventilation was administered as required using a mechanical ventilator. The rats were allowed a minimum 2-wk recovery period between capsaicin or vehicle treatment and catheterization surgeries. During this period, both capsaicin and vehicle-treated animals gained weight, and there was no difference between the body weights of capsaicin and vehicle-treated groups at the time of experimental testing. After the recovery period, efficacy of the capsaicin treatment was assessed by using a corneal chemosensory response (30, 38). This test revealed whether our capsaicin treatment eliminated corneal chemosensation, which is mediated by small unmyelinated trigeminal afferents. The test involved instillation of a drop of 1% NH4OH to the eye. All of our vehicle-treated rats responded to this test with one to five rapid wipes of the eye. However, none of the capsaicin-treated rats exhibited an eye wipe in response to the test, confirming that our capsaicin treatment successfully destroyed afferent C-fibers remote from those innervating the abdomen.
Food intake following intraperitoneal CCK-8 injection.
An intact vagus nerve is essential for reduction of food intake in response to intraperitoneal CCK-8 (37). Likewise, destruction of small unmyelinated vagal afferents by capsaicin attenuates reduction of food intake by intraperitoneal CCK-8 (30, 38). Therefore, after all intravenous infusion tests were complete, or prior to the use of rats for immunohistochemical or PCR experiments, we tested for reduction of food intake in response to intraperitoneal CCK-8 to assess success and persistence of the subdiaphragmatic vagotomy and capsaicin treatment. All vagotomized and sham-vagotomized rats were adapted to eating powdered rodent diet in spill-resistant cups for at least 7 days before tests. Capsaicin-treated and vehicle-treated rats were maintained and tested with pelleted rodent diet. Rats were deprived of food overnight for 16 h, and preweighed diet was returned following intraperitoneal injections of CCK-8 or saline. Food remaining and spillage were measured at 30, 60, 120, and 240 min.
Jugular vein catheterization.
Rats were anesthetized using a mixture of ketamine (50 mg/kg), acepromazine (2 mg/kg), and xylazine (25 mg/kg) (0.1 ml mixture/100 g body wt). Via a ventral cervical incision, a silicone rubber catheter (0.64 mm internal, 1.19 mm external diameter) (Silastic Laboratory Tubing, Dow Corning, Midland, MI) was introduced into the right jugular vein and secured into place with the tip resting about 3.5 cm caudal to the internal/external jugular bifurcation, within the right cardiac atrium. The patency of the catheter was verified by drawing blood with a syringe connected to the free end. The patent catheter was flushed and filled with the following solutions in the respective order: heparinized saline (100 U of heparin/ml saline), sterile saline, and blocking solution (50% glycerol with 5% gentamicin and 100-U/ml heparin). The free end was then routed subcutaneously to the top of the head, where it was connected to a stainless-steel elbow and secured onto the skull using stainless-steel screws and acrylic polymer. Rats were allowed to recover from the catheterization surgeries for 7–10 days. Twice weekly, flushing the catheter with sterile heparinized saline and refilling the catheter with fresh blocking solution maintained the patency of the catheters. After completion of all feeding experiments, patency of the catheters was confirmed prior to necropsy by infusing 5% pentobarbital sodium via the catheter. Rats that lost the righting reflex within 2 s of beginning pentobarbital infusion were considered to have had patent catheters during the conduct of the experiments, and thus, their data were included for subsequent statistical analyses.
Intravenous infusions and feeding tests.
Non-food-deprived rats, implanted with jugular intravenous catheters, were removed from their home, placed in individual black Plexiglas infusion cages, and adapted to receiving a 60-min continuous intravenous infusion of 0.9% NaCl and to consuming a highly palatable 15% sucrose solution from calibrated sipper tubes over a 60-min test period, beginning at 1300. At the end of the infusion/sucrose consumption period, the rats were immediately returned to their home cages, where water and rat chow were available ad libitum. The experimental procedure was identical to the adaptation procedure, except that prior to experiments, food, but not water, was removed from the rats' cages at 0900 to limit variation that might be introduced by daytime nibbling during the four daytime hours preceding the sucrose test. The rats were transferred to the infusion chambers and intravenous infusions began at 1300. Ten minutes after the infusion began, rats were presented with graduated glass tubes filled with 15% sucrose solution. Intakes of the sucrose solution were measured to the nearest 0.1 ml at 5-min intervals for the first 30 min, then at 15-min intervals until 60 min after the sucrose solution was available. All rats received a 60-min continuous intravenous infusion of GLP-1, CCK-8, or saline. All infusions started 10 min before sucrose presentation. Infusions and feeding tests were conducted at 48-h intervals. Four doses of GLP-1 (1.25, 2.5, 5, and 10 μg/rat) were tested in the ascending order, followed by two doses of CCK-8 (1.4, and 2.8 μg/rat), and each peptide infusion was separated from the next by a saline infusion. Infusions were performed using Razel syringe pumps (Razel Scientific Instruments, Stamford, CT) at the rate of 0.012 ml/min.
c-Fos immunohistochemistry and cell counts.
Ten rats were subjected to capsaicin treatment or vehicle treatment. Nine rats were subjected to vagotomy or sham vagotomy. All rats were equipped with jugular vein catheters and were habituated to 60-min intravenous infusion. Rats were fasted overnight for 16 h and were randomly assigned (2–4 per group) to receive intravenous infusion of either 0.9% NaCl or GLP-1 (10 μg/rat). Sixty minutes after the infusion ended, rats were deeply anesthetized with isoflurane and perfused with 0.9% PBS solution followed by 4% formaldehyde solution. Brains were then removed and prepared for immunohistochemical examination of c-Fos immunoreactivity. Brains were sectioned at 30 μm, and the sections were incubated in a primary antibody against c-Fos (1:10,000, Ab-5; Oncogene, San Diego, CA), followed by a biotinylated secondary antibody (1:500; Jackson ImmunoResearch Laboratories, West Grove, PA). The tissues were then incubated in horseradish peroxidase-conjugated avidin (1:1,500, Sigma), after which they were washed and processed to reveal peroxidase activity using diaminobenzidine (Sigma) intensified with nickel. Cells with nuclear c-Fos immunoreactivity were counted in the AP, NTS, and dorsal motor nucleus of the vagus (DMV). Because we could detect no difference in the levels of c-Fos immunoreactivity between vehicle-treated rats and sham-vagotomized rats following saline infusion, and their levels of c-Fos immunoreactivity were also comparable following GLP-1 infusion, these two groups of rats were combined to make a single control group. Subsequently, slides were coded and counts of c-Fos-immunoreactive nuclei were performed with the help of automated image analysis software (NIS-Elements AR 3.0) and an operator blind to the treatments. Four levels of the hindbrain were selected for counting: hindbrain level caudal to the AP, caudal edge of the AP, middle of the AP, and hindbrain level rostral to the AP. These levels correspond to plates −5.30 mm, −5.08 mm, −4.68 mm, and −4.30 mm caudal to the interaural plane, as identified from the rat brain atlas of Paxinos and Watson (28).
Assay of active GLP-1.
Vehicle- (n = 8) or capsaicin- (n = 5) treated rats implanted with jugular catheters were adapted to receiving a 60-min continuous intravenous infusion, which took place in the individual infusion chambers, as previously described. However, these rats were deprived of food, but not water, overnight for 16 h, and their hind limbs were shaved for blood sampling via saphenous venipuncture (4). On the testing day, the rats received an intravenous infusion of 10 μg GLP-1 over 60 min, and 100-μl blood samples were collected from the saphenous vein at 0, 15, 25, 40, and 70 min. Blood samples were collected into 1.5-ml tubes containing 10 μl EDTA (in 15% NaCl, Tyco Healthcare Group LP, Mansfield, MA) and 1 μl dipeptidyl peptidase IV, an enzyme that degrades active GLP-1 into its inactive form) (22) inhibitor (Millipore, Billerica, MA). Blood samples were mixed by vortex and kept on ice immediately, and centrifuged within 20 min. Plasma samples were then removed and kept at −80°C until utilized in the analysis. ELISA assay kits for active GLP-1 were obtained from Millipore (Billerica, MA) and utilized according to the manufacturer's instructions to determine active GLP-1 levels using a fluorescence microplate reader (Synergy HT, BioTek, Winooski, VT). After completion of blood sampling, patency of the catheters was confirmed by an infusion of 0.1 ml brevital sodium (methohexital sodium, 10 mg/ml) into the jugular vein catheters. Rats that lost consciousness within 2 s following the infusion were considered valid with patent catheters, and thus, their data were included in our analysis.
Real-time PCR.
Eighteen rats were subjected to systemic vehicle (n = 9) or capsaicin (n = 9) treatments, as previously described. Because of the small amount of available RNA, an additional 12 rats (6 per group) were used to collect nodose ganglia. Rats were deeply anesthetized with isoflurane and decapitated, and the brains were immediately removed and frozen on dry ice. Hindbrain and hypothalamus were dissected using the method described by Zhao et al. (50). A 7-mm coronal section of hypothalamic area was made from the optic chiasm to the mammillary bodies. A horizontal cut was made at the dorsal edge of the ventral third ventricle tangential to the ventral interthalamic adhesion. The resulting lower portion was cut parasaggitally at the hypothalamic sulci. Medial hindbrain, including AP, NTS, and DMV were collected as a contiguous tissue block. The cerebellum was gently retracted, and a coronal cut was made ∼1.0 mm caudal to the AP. A second coronal cut was made ∼1.0 mm rostral to this landmark. A horizontal cut was made through the central canal and caudal fourth ventricle, tangential to the dorsal border of the hypoglossal nucleus. The remaining dorsal portion of the section was further dissected by making bilateral parasagittal cuts tangential to the lateral edges of the right and left cuneate nuclei. The portion of the section medial to and between these two latter cuts was retained for PCR. Microtubes containing the regional tissue samples were immediately immersed in liquid nitrogen before being stored at −80°C until utilized for quantitative real-time PCR. Total RNA was isolated from the tissues using TRIzol reagent (Invitrogen, Carlsbad, CA), and RNA quantity was determined by absorbance using a UV-1800 spectrophotometer (Shimadzu Scientific Instruments, Columbia, MD). Genomic DNA contamination was removed by digestion with RNase-free DNase I at 37°C for 90 min. First-strand cDNAs were synthesized by incubating the total RNA from hindbrain, hypothalamus, or nodose ganglia with oligo(dT) primer using Superscript III (Invitrogen, Carlsbad, CA). Expressions of GLP-1R and cyclophilin A were tested in all samples. Other genes tested include neuropeptide Y (NPY) and proopiomelanocortin (POMC) in the hypothalamus, tyrosine hydroxylase (TH) in the hindbrain, and CCK type 1 receptor (CCK-1R) in the nodose ganglia. PCR primers were designed using Primer3 software. The sequences of primers used are listed in Table 1. Real-time PCR was performed in triplicate using the Platinum Taq DNA polymerase (Invitrogen) with 5 μl of diluted cDNA (1:10 to 1:40 with water) in a final reaction volume of 25 μl. The amplification was followed by 40 cycles of denaturation at 95°C for 10 s, annealing at 58°C for 10 s and extension at 72°C for 20 s. The threshold cycle (Ct) was determined using SYBR Green fluorescence with Bio-Rad CFX96 real-time system (Bio-Rad Laboratories, Hercules, CA). Gene expressions were normalized to cyclophilin A expression.
Table 1.
Primers for quantitative real-time PCR
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Cyclophilin A | AAA TGC TGG ACC AAA CAC AAA | CTC ATG CCT TCT TTC ACC TTC |
| GLP-1R | AGT CTG CAT TTG ATG TCG GTC TT | CCG GGT CAT CTG CAT CGT |
| CCK-1R | GAT CAA CCT CAA CCT TCA AAA GA | CAC ACT GAG AAG GAA TAT GAT GGA |
| NPY | AGA GAT CCA GCC CTG AGA CA | TTT CAT TTC CCA TCA CCA CA |
| POMC | CTC CTG CTT CAG ACC TCC AT | TTT CAG TCA AGG GCT GTT CA |
| TH | ACA CAG CGG AAG AGA TTG CT | GGA TGC TGT CCT CTC GGT AG |
GLP-1R, glucagon-like peptide-1R; NPY, neuropeptide Y; POMC, proopiomelanocortin; TH, tyrosine hydroxylase. Primer sequences for rat genes measured in the quantitative real-time PCR are listed.
Statistical analysis.
Effects of GLP-1 and CCK-8 on intake of 15% sucrose or rodent diet are expressed in the text as percent reduction of intake. Percent reduction of intake was calculated according to the following equation: % percentage reduction of intake = −100·(intake following saline infusion − intake following peptide infusion) / intake following saline infusion. Therefore, reductions of intake are graphed as negative numbers and increases are graphed as positive numbers. Graphic presentations of percent reductions of intake are presented as means ± SE of percent reductions calculated for individual rats. Raw cumulative intake data for each treatment group (vagotomy, sham-operated, capsaicin-treated, or vehicle-treated) were tested for GLP-1- or CCK-8-induced reduction of intake using two-way repeated-measures ANOVA, with peptide dose and time postinjection being the two factors. The ANOVA were followed by Holm-Sidak comparisons to determine the times and dosages at which individual differences occurred. Potential differences between treatment groups in percent reduction of intake were assessed at each time point using repeated-measures ANOVA, with the dose of peptide being the repeated factor, and pairwise comparisons again were tested using the Holm-Sidak method. Counts of c-Fos immunoreactive nuclei are expressed as means ± SE for each area counted. Differences in c-Fos-immunoreactive cell counts were analyzed using two-way repeated-measures ANOVA, with treatment group and brain area counted as the two factors. Plasma GLP-1 levels were normalized to fasting level and expressed as means ± SE in fold increase. Again, repeated-measures ANOVA was used to analyze these data, with time of sampling being the repeated factor. Gene expression was normalized to cyclophilin A expression control transcript, and statistical comparisons were made using Student's t-test.
RESULTS
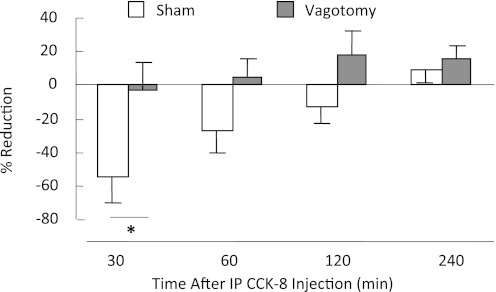
Sham-operated control rats and vagotomized rats differed significantly with regard to reduction of food intake by intraperitoneal CCK-8 [F(1, 33) = 3.882, P < 0.05]. Specifically, CCK-8 reduced powdered rodent diet intake in sham-operated rats by an average of 54.3 ± 15.5% (P = 0.011), while in vagotomized rats, intake was reduced by an insignificant 2.9 ± 16.2%, compared with saline-injected condition, confirming that abdominal vagal fibers are necessary for reduction of food intake by intraperitoneal CCK-8, and that the vagi were successfully sectioned in these rats (Fig. 1).
Fig. 1.
Percent reduction of intake of powdered rodent diet following intraperitoneal injection of CCK-8 (2 μg/kg) in vagotomized and sham-vagotomized rats. Compared with intraperitoneal saline injection, intraperitoneal CCK-8 injection significantly reduced food intake in 16-h, food-deprived, sham-operated control rats (sham, n = 8), but it did not reduce food intake in vagotomized rats (n = 5). Following saline injection, sham-operated control rats ate 5.3 ± 0.3 g in 30 min, which was significantly reduced to 2.4 ± 0.8 g following intraperitoneal CCK-8 (P < 0.005). By contrast, vagotomized rats ate 4.5 ± 0.6 g after saline injection, which was not significantly different from their intake after intraperitoneal CCK-8 (4.3 ± 1.0g; P > 0.5). *Significant difference (P < 0.01) in CCK-induced reduction of intake between vagotomized and sham-vagotomized rats.
Intravenous infusions of GLP-1 (1.25, 2.5, 5, and 10 μg/rat) reduced sucrose intake in both vagotomized rats [F(4,32) = 9.425, P < 0.001] and sham-operated rats [F(4,56) = 10.391, P < 0.001] (Fig. 2). Moreover, ANOVA detected a GLP-1 dose-related difference between vagotomized and sham-operated rats with regard to percent reduction of sucrose at the 15, 30, and 60 min time points [F(3,33) = 2.881, P = 0.05, 3.602 P = 0.024, 6.029, P < 0.002]. This difference, however, was specific only to the 10 μg/rat dose condition. Specifically, while there was no difference in reduction of intake between groups for the 1.25, 2.5, and 5 μg/rat doses, vagotomized rats reduced their sucrose intake significantly more than sham-operated controls at 15, 30, and 60 min (P = 0.039, 0.005, and < 0.001, respectively).
Fig. 2.
Percent reduction of 15% sucrose intake in response to intravenous glucagon-like peptide 1 (GLP-1) or intravenous CCK-8 in vagotomized and sham-vagotomized rats. Intravenous infusions of GLP-1, 5, or 10 μg/rat, reduced sucrose intake in both vagotomized (n = 5) and sham-vagotomized (n = 8) rats, whereas the 1.25 and 2.5 μg/rat doses were ineffective in both groups. At the highest dose (10 μg/rat), percent reduction of food intake by intravenous GLP-1 was significantly greater for vagotomized than sham-operated rats at all time points, 15, 30, and 60 min (P = 0.039, 0.005, <0.001, respectively). Reduction of intake by intravenous CCK-8 (1.4 and 2.8 μg/rat) did not differ between sham-operated and vagotomized rats at the 15- and 30-min time points. Likewise, percent reductions of intake produced by the 2.8 μg/rat CCK-8 dose did not differ between sham-operated and vagotomized rats at the 60-min time point. However, at 60 min, percent reduction of food intake by 1.4 μg/rat CCK-8 was significantly greater in sham-operated rats than in vagotomized rats (P = 0.05). *Significant difference between percent reduction of intake.
Intravenous infusions of CCK-8 reduced sucrose intake significantly in both vagotomized [F(2,16) = 10.583, P = 0.006] and sham-operated rats [F(2,24) = 5.314, P = 0.022] (Fig. 2). Sham-operated and vagotomized rats did not differ with regard to percent reduction of intake at either CCK-8 dose at the 15- and 30-min time points [F(1,10) = 1.197, 1.899, P > 0.2]. However, at the 60-min time point, ANOVA detected a difference between the two groups in percent reduction of intake [F(1,10) = 6.621, P = 0.03]. Holm-Sidak comparisons revealed no difference in percent reduction of intake between sham-operated and vagotomized rats at the 2.8 μg/rat CCK-8 dose (P = 0.5). However, at this time point, the 1.4 μg/rat dose produced greater percent reduction of intake in sham-operated rats than in vagotomized rats (P = 0.05).
Capsaicin-treated rats and vehicle-treated controls differed significantly with regard to reduction of food intake by intraperitoneal CCK-8 [F(1,21) = 3.907, P = 0.023]. Specifically, vehicle-treated rats reduced 30-min intake an average of 60.1 ± 12.4%, relative to intake after intraperitoneal saline injection. By contrast in capsaicin-treated rats, intake following intraperitoneal CCK-8 did not reduce intake in response to intraperitoneal CCK-8, confirming that the capsaicin-sensitive afferents that are necessary for reduction of food intake by intraperitoneal CCK-8 were destroyed by systemic capsaicin treatment in these rats (Fig. 3).
Fig. 3.
Percent reduction of intake of pelleted rodent diet following intraperitoneal injection of CCK-8 (2 μg/kg) in capsaicin- and vehicle-treated rats. Compared with intraperitoneal saline injection, intraperitoneal CCK-8 injection significantly reduced food intake in 16-h, food-deprived, vehicle-treated rats (n = 8, P < 0.05), but it did not reduce food intake in capsaicin-treated rats (n = 8, P > 0.4 compared with saline injection). *Significant difference (P < 0.01) in CCK-induced reduction of intake between capsaicin-treated and vehicle-treated rats.
Intravenous infusions of GLP-1 (1.25, 2.5, 5, and 10 μg/rat) reduced sucrose intake in both vehicle-treated [F(4,56) = 10.895, P < 0.001] and capsaicin-treated rats [F(4,56) = 8.734, P < 0.001] (Fig. 4). Percent reduction of intake in capsaicin-treated rats did not differ from that of vehicle-treated rats at 15 or 30 min following GLP-1 infusion [F(1, 42) = 0.272, P = 0.610 at 15 min, 0.0128, P > 0.5 at 30 min]. At the 60-min time point, there also was not a difference in reduction of intake between groups for the three lower GLP-1 doses [F(1,42) = 1.833, P = 0.197]. However, at this time point, percent reduction of sucrose intake in capsaicin-treated rats was significantly enhanced (P < 0.05) (51.2 ± 6.4%) in response to our highest GLP-1 dose (10 μg/rat) compared with vehicle-treated rats (29.0 ± 6.6%).
Fig. 4.
Percent reduction of 15% sucrose intake in response to intravenous GLP-1 or CCK-8 in vehicle-treated and capsaicin-treated rats. Intravenous infusions of GLP-1, 5, or 10 μg/rat, reduced sucrose intake in both vehicle- (n = 8) and capsaicin-treated (n = 8) rats, whereas the 1.25 and 2.5 μg/rat doses were ineffective in both groups. At the highest GLP-1 dose (10 μg/rat), percent reduction of food intake by intravenous GLP-1 was significantly greater for capsaicin-treated than vehicle-treated rats at the 60-min time point (*P < 0.05). Intravenous infusions of CCK-8 (2.8 μg/rat) produced reduction of sucrose intake in both capsaicin- and vehicle-treated rats at all three time points (P < 0.05), while 1.4 μg/rat failed to reduce intake significantly in either group. There were no statistically significant differences in intakes between the groups at any dose or time point.
Intravenous infusions of CCK-8 (1.4 and 2.8 μg/rat) reduced sucrose intake in both vehicle-treated [F(2,28) = 7.773, P = 0.005] and capsaicin-treated rats [F(2,28) = 6.998, P = 0.008], and percent reductions of intake for the two groups did not differ significantly at any time point [F(1, 14) = 3.880 at 15 min, 1.231 at 30 min, and 0.695 at 60 min, P > 0.1].
Examination of c-Fos immunoreactivity in the rat hindbrain revealed increased numbers of c-Fos-immunoreactive nuclei in the AP, medial NTS, and DMV following intravenous GLP-1 infusion (10 μg/rat), compared with intravenous saline infusion [F(1,13) = 27.347, P < 0.001]. However, neither capsaicin treatment nor vagotomy altered the number of c-Fos-immunoreactive nuclei compared with control rats infused with GLP-1 [F(2, 13) = 0.708, P > 0.5, Table 2].
Table 2.
Numbers of c-Fos-immunoreactive neuronal nuclei in dorsal vagal complex after intravenous infusions of saline or GLP-1 in capsaicin-treated, vagotomized, and control rats
| AP | NTS | DMV | |
|---|---|---|---|
| Control/saline (n = 4) | 47.5 ± 6.5 | 75.3 ± 31.2 | 13.3 ± 4.5 |
| Capsaicin/saline (n = 3) | 77.7 ± 10.2 | 144.3 ± 13.0 | 22.3 ± 3.4 |
| VagX/saline (n = 2) | 57.5 ± 12.5 | 67.5 ± 48.5 | 6.0 ± 5.0 |
| Control/GLP-1 (n = 4) | 189.8 ± 25.6# | 383.5 ± 92.7# | 61.5 ± 13.0# |
| Capsaicin/GLP-1 (n = 3) | 241.3 ± 10.9# | 449.7 ± 74.5# | 54.7 ± 4.2# |
| VagX/GLP-1 (n = 3) | 328.3 ± 78.3# | 558.7 ± 207.9# | 72.0 ± 24.6# |
Rats were equipped with jugular vein catheters and were infused intravenously with either saline or GLP-1 (10 μg/rat) (n = 2–4 per group) over a 60-min period. Hindbrains were collected 60 min after the completion of the infusion. Cells with c-Fos-immunoreactive nuclei were counted in the area postrema (AP), nucleus of the solitary tract (NTS), and dorsal motor nucleus of the vagus (DMV). Counts of c-Fos-immunoreactive cells are expressed as means ± SE. Intravenous GLP-1 infusion significantly increased c-Fos immunoreactivity in all areas for all groups (#P < 0.001) when compared to saline infusion. However, neither capsaicin treatment nor vagotomy altered the number of c-Fos-immunoreactive nuclei compared to control rats infused with GLP-1 (P > 0.5). Vgx, vagotomized.
P < 0.001 compared to saline infusion within the group.
Fasting plasma concentrations of endogenous active GLP-1 were statistically indistinguishable for capsaicin-treated rats and vehicle-treated rats. Sixty-minute intravenous infusion of GLP-1 (10 μg/rat) increased plasma levels of active GLP-1 within the first 15 min of infusion, and active GLP-1 levels remained significantly elevated (6.8–10.6-fold) for the duration of the 60-min infusion [F(4, 50) = 25.763, P < 0.001]. Nevertheless, plasma levels of active GLP-1 did not differ between capsaicin-treated rats and vehicle-treated rats at any time before, during, or after the GLP-1 infusion [F(1, 50) = 0.350, P > 0.5, Fig. 5].
Fig. 5.
Normalized concentrations of plasma active GLP-1 before, during, and after continuous intravenous GLP-1 infusion in capsaicin- and vehicle-treated rats. GLP-1 (10 μg·rat−1·h−1) was infused intravenously over 60 min in capsaicin-treated (n = 5) and vehicle-treated (n = 7) rats. Blood was sampled from the saphenous vein before and throughout the infusion for measurement of plasma-active GLP-1 by ELISA. Capsaicin-treated rats and vehicle-treated rats had comparable fasting levels of endogenous GLP-1. Intravenous GLP-1 infusion increased the plasma levels of active GLP-1 within 15 min, and significantly elevated levels were maintained throughout the 60-min infusion in both groups. GLP-1 levels began to drop after the infusion stopped, but they were still elevated above fasting levels at the end of the experiment. At all time points before, during, and after the infusion, plasma levels of active GLP-1 did not differ between capsaicin-treated rats and vehicle-treated rats (P > 0.5). *Significant increase over preinfusion GLP-1 levels for both groups (P < 0.001). The period of infusion is indicated by the heavy double-headed line beneath the x-axis.
Real-time PCR measurements indicated that, compared with vehicle treatment, capsaicin-treatment did not alter expression of GLP-1R, NPY, or POMC in the hypothalamus (Fig. 6A, P > 0.1), or the expression of GLP-1R or TH in the hindbrain (Fig. 6B, P > 0.1). Likewise, in nodose ganglia CCK-1R expression in capsaicin-treated rats and vehicle-treated rats did not differ significantly (Fig. 6C, P > 0.1); while GLP-1R expression was significantly decreased in capsaicin-treated rats (P < 0.05).
Fig. 6.
Gene transcript levels in brain and nodose ganglia of capsaicin- and vehicle-treated rats measured by quantitative real-time PCR. A: hypothalamic expression of mRNAs coding for GLP-1R, NPY, and POMC in capsaicin-treated (n = 8) and vehicle-treated rats (n = 8). B: hindbrain expression of mRNAs coding for GLP-1R and TH in capsaicin-treated (n = 9) and vehicle-treated rats (n = 9). C: expression of GLP-1R and CCK-1R mRNA in nodose ganglia from capsaicin-treated (n = 7) and vehicle-treated rats (n = 7). Expression of cyclophilin A mRNA was used as a reference transcript, and the expression of other genes was calculated as a ratio to cyclophilin A mRNA. Expressions of GLP-1R, NPY, and POMC genes in the hypothalamus did not differ between capsaicin-treated and vehicle-treated rats (P > 0.1). Expression of both GLP-1R and TH genes in the hindbrain also did not differ between capsaicin-treated and vehicle-treated rats (P > 0.1). In the nodose ganglia, CCK-1R expression was comparable in capsaicin-treated rats and vehicle-treated rats (P > 0.1), while GLP-1R expression was decreased in capsaicin-treated rats (*P < 0.05).
DISCUSSION
We found that GLP-1 infused intravenously over 60 min reduced food intake in a dose-related manner, confirming similar observations by other investigators (8, 34). Moreover, the total doses of GLP-1 administered in our experiments generally were below or comparable to effective doses reported by others. For example, Chelikani et al. (8) reported that intravenous infusions of GLP-1 at rates from 0.5 to 170 pmol·kg−1·min−1, infused over a 3-h period, reduced real feeding and sham feeding in rats. The GLP-1 peptide doses that we used were 1.25, 2.5, 5, and 10 μg/rat, which correspond to 17, 34, 68, and 137 pmol·kg−1·min−1. Hence, our GLP-1 infusate concentrations were within the concentration range used by Chelikani et al. However, because our infusions occurred over just 60 min, vs. 3 h in the Chelikani et al. study, the total amount of exogenous GLP-1 peptide that we infused was about 1/3 that administered by Chelikani et al. In another more recent publication, Ruttimann et al. (34) reported that GLP-1 (1 or 3 nmol/kg) infused intravenously over 2.5 to 5 min, just after the onset of the first night-time meal, reduced meal size. In our experiments, we found that intravenous GLP-1, at our two higher doses (5 and 10 μg/rat), reduced sucrose intake significantly 15 min after the start of infusion. At this time point, the total GLP-1 dose administered was 1 and 2 nmol/kg, which was quite comparable to doses that reduced meal size in the Ruttimann et al. (34) study. Hence, while our infusion parameters were not physiological, they fall well within GLP-1 dosage regimens reported by others to reduce food intake. Another point worth mentioning is that Ruttimann et al. (34) compared portal vein GLP-1 with infusion via the jugular vein. They found comparable reductions of food intake by either route, and, as in our experiments, reduction of food intake by intravenous GLP-1 was not attenuated in vagotomized rats (34).
In our experiments, we measured intake of 15% sucrose solution to assess the effects of intravenous GLP-1 on ingestion. In our hands, responses to this liquid food are quite comparable to responses made to solid diets. Moreover, the use of 15% sucrose enables monitoring of intake over short 5-min intervals without disturbing the animal to remove solid food or spillage. In this regard, it is reassuring that our results using sucrose intake are in quite good agreement with those reported using intake of solid diets.
GLP-1Rs are expressed by a subpopulation of vagal afferent neurons (26). Moreover, GLP-1 activates some vagal afferents in vivo (6, 25) and excites cultured vagal afferents by decreasing an outward potassium conductance (11). Nevertheless, at this time, there is little experimental evidence documenting behavioral or other physiological responses depending on vagal afferent sensitivity to GLP-1. For some physiological responses, it appears that GLP-1 acts at receptors within the brain to activate vagal efferents. For example, Holmes et al. (14) presented compelling evidence that activation of brain GLP-1 receptors inhibits gastric motility via activation of noncholinergic/nonadrenergic vagal efferent pathways (14). Part of GLP-1's inhibitory effects on food intake could be mediated via such a pathway. However, we found that complete subdiaphragmatic vagotomy did not attenuate reduction of food intake by intravenous infusions of GLP-1. Hence, our results do not support a role for either vagal afferents or efferents in reduction of food intake by circulating GLP-1.
In contrast with our findings that an intact vagus is not required for reduction of food intake induced by intravenous infused GLP-1, two groups have reported that reduction of food intake by very high doses of GLP-1 delivered intraperitoneally are attenuated in rats with vagal damage. H. Abbott et al. (1) reported that intraperitoneal injection of 100 nmol/kg GLP-1 reduced food intake of intact rats but did not do so in rats with bilateral abdominal vagotomy. Similarly, Ruttimann et al. (34) recently reported that GLP-1 (10 nmol/kg) infused via an intraperitoneal catheter reduced food intake of control rats but not of vagal deafferented rats (34). A possible explanation for the apparent discrepancy between our data and those of Abbott et al. (1) and Ruttimann et al. (34) is that reduction of food intake following intraperitoneal GLP-1 may be the result of very high concentrations of GLP-1 at vagal afferent terminals in the abdominal cavity. It is noteworthy, however, that in both the Abbott et al. and the Ruttimann et al. studies, the total amount of GLP-1 administered as a bolus, or over a short time period, was 1.2- to 12-fold higher than our highest intravenous dose, which we infused over a period of 60 min. Our 10 μg/rat GLP-1 intravenous infusion elevated active GLP-1 plasma concentrations by 6.8- to 10.6-fold during the period of infusion. Abbott et al. (1) and Ruttimann et al. (34) did not assay plasma-active GLP-1. Nevertheless, given the high intraperitoneal GLP-1 doses they administered, one might have expected that peak plasma GLP-1 concentrations would have activated the nonvagal substrates that mediated reduction of intake by our intravenous GLP-1 infusions, as well as any vagal substrates activated locally in the abdomen. Because, either bilateral vagotomy (1) or vagal deafferentation (34) completely abolished reduction of food intake induced by intraperitoneal GLP-1, we would infer that intraperitoneal GLP-1 used in their experiments was degraded in the abdomen and did not elevate plasma GLP-1 sufficiently to access nonvagal mechanisms that mediate reduction of food intake by circulating GLP-1. The site(s) of action for circulating GLP-1 is likely to be in the brain. As mentioned earlier, some brain nuclei, such as the AP, express GLP-1R mRNA but lack prominent input from known PPG-expressing neurons. Responsiveness to GLP-1 by one or more brain areas might mediate reduction of food intake in response to circulating GLP-1 in vagotomized rats.
An alternative to central mediation of GLP-1's effects on ingestive behavior is that GLP-1-sensitive nonvagal neural afferents might mediate reduction of food intake by circulating GLP-1. To date, there have been no reports of GLP-1 receptor expression by spinal afferents. Moreover, there are no reports of electrophysiological responses of spinal afferents to GLP-1. Nevertheless, a few reports suggest that capsaicin-induced destruction of small unmyelinated afferents impair some responses to GLP-1 receptor agonists. For example, Imeryuz et al. (15) reported that GLP-1-induced inhibition of gastric emptying was attenuated in capsaicin-treated rats (15). Likewise, capsaicin treatment reportedly attenuated the incretin effects of low but not high doses of GLP-1 in mice (2). Finally, Talsania et al. (39) reported that reduction of food intake by intraperitoneal exendin-4, a GLP-1 receptor agonist that is not rapidly inactivated by dipeptidyl peptidase IV, was attenuated in capsaicin-treated mice. In our hands, however, destruction of small unmyelinated primary afferent neurons with systemic capsaicin did not attenuate reduction of sucrose intake by intravenous GLP-1. While it could be argued that our capsaicin treatments were not rigorous enough to destroy the substrate activated in the Talsania et al. study, our capsaicin treatment protocol has repeatedly been shown to destroy small unmyelinated primary afferents in vagal and nonvagal peripheral nerves (16, 31). Moreover, the capsaicin-treated rats that we found responsive to intravenous GLP-1 were insensitive to intraperitoneal CCK-8, indicating that the treatment had destroyed vagal C-type fibers. In addition, our rats did not respond to chemical stimulation of the cornea, indicating that C-fibers in the spinal trigeminal nerve also were ablated. Consequently, we conclude that reduction of food intake by intravenous GLP-1 is not mediated by capsaicin-sensitive vagal or nonvagal neurons.
Our most surprising result was that, at the highest intravenous GLP-1 dose (10 μg/rat) capsaicin-treated and vagotomized rats actually reduced their sucrose intake significantly more than their respective controls, suggesting an increased responsiveness to GLP-1. Although it might be argued that GLP-1 might have reduced intake more dramatically in vagotomized rats because their body weights were lower than those of sham-operated controls (354.6 ± 9.1g vs. 392.0 ± 8.0 g, respectively), vehicle-treated rats, and capsaicin-treated rats did not differ in body weight at the time of infusions (375.6 ± 10.5 g vs. 376.6 ± 10.3 g, respectively). Yet, like vagotomized rats, capsaicin-treated rats reduced their intake significantly more in response to the high dose of GLP-1 than did vehicle-treated controls. Therefore, the increased response to GLP-1 by capsaicin-treated and vagotomized rats is unlikely to be due to dosage differences between lesioned and control rats.
GLP-1 has a very short half-life in the circulation, about half a minute in rat (31). It is possible that capsaicin treatment or vagotomy altered catabolism or clearance of infused GLP-1, such that GLP-1 plasma levels were higher during the feeding test. However, we found that active GLP-1 plasma concentrations did not differ between capsaicin-treated rats and vehicle-treated rats before, during or following intravenous GLP-1. These results indicate that increased response to GLP-1 in capsaicin-treated rats probably cannot be explained by alterations in plasma GLP-1 concentrations or its catabolism or clearance from the systemic circulation.
To assess the possibility that increased response to GLP-1 might be related to a capsaicin-induced change in expression of GLP-1Rs, GLP-1R mRNA was quantified in the nodose ganglia, hindbrain, and forebrain areas. As expected, expression of GLP-1R mRNA in the nodose ganglia was decreased in capsaicin-treated rats. This observation is consistent with previous reports of GLP-1R mRNA in nodose ganglion (26) and indicates that capsaicin-sensitive neurons represent a significant portion of the vagal afferents expressing GLP-1Rs. We did not detect any change in the expressions of GLP-1R in the hindbrain or hypothalamus. Therefore, it does not seem likely that increased response to GLP-1 in capsaicin-treated rats was associated with increased GLP-1 receptor transcription in the brain or the nodose ganglia. Nevertheless, we cannot rule out the possibility that GLP-1r binding, or GLP-1 induced intracellular signaling is enhanced following capsaicin treatment.
If GLP-1 binding or signaling were increased in brain areas associated with ingestive control, one might suspect that GLP-1-evoked activation in selected brain areas might be enhanced in capsaicin-treated or vagotomized rats. However, our 10 μg/rat GLP-1 infusion induced comparable numbers of c-Fos-immunoreactive neurons in the DVC of capsaicin-treated, vagotomized, and control rats. Nevertheless, we cannot definitively rule out the possibility that GLP-1 produces greater activation of neurons beyond the NTS and AP in capsaicin-treated and vagotomized rats, because we did not quantify c-Fos immunoreactivity in brain sites beyond the dorsal hindbrain following GLP-1 infusion.
Lee et al. (20) found a significantly lower concentration of NPY-immunopositive cells in the arcuate and paraventricular nuclei in capsaicin-treated rats. Therefore, it is plausible that increased response to high-dose GLP-1 is mediated by a shift in the balance between orexogenic and anorexogenic hypothalamic peptide expression. However, we detected no capsaicin-induced changes in NPY or POMC mRNA, measured by real-time PCR in the hypothalamus. Similarly, Yamamoto et al. (45) reported that intravenous administration of a GLP-1R agonist induced increased TH transcription in the AP, raising the possibility that capsaicin treatment might elevate TH expression in the AP and thereby enhance the response to GLP-1. However, we found no difference in TH expression between capsaicin-treated rats and their controls. Therefore, we find no evidence that increased response to high-dose GLP-1 in capsaicin-treated rats is related to changes in central NPY, POMC, or TH expression.
Consistent with many prior reports, we found that intraperitoneal CCK-8 injection caused significant reduction of 30-min food intake in intact food-deprived rats (vehicle-treated and sham-vagotomized controls), while either subdiaphragmatic vagotomy or capsaicin treatment abolished reduction of food intake by intraperitoneal CCK-8 (30, 37). In contrast to the lack of response to intraperitoneal CCK-8, intravenous CCK-8 reduced food intake similarly but not identically in both controls, vagotomized, and capsaicin-treated rats. At the 2.8 μg/h CCK-8 dose, there was no difference in reduction of intake between vagotomized and sham-operated controls at any time point. However, at the lower of our two intravenous CCK-8 doses (1.4 μg/h), there was a trend toward attenuation of CCK-8-induced reduction of food intake in vagotomized rats by 30 min, and vagotomized rats were significantly different from sham-operated rats at 60 min. Thus, at lower circulating levels of CCK-8, the vagus nerve may provide an important contribution to reduction of food intake by this peptide. However, at higher infusate concentrations, vagal contributions are difficult to detect, and another nonvagal mechanism mediates reduction of food intake.
There are a variety of nonvagal site(s) through which CCK-8 might reduce food intake. For example, Schick et al. (35) reported that infusion of CCK-8 reduced food intake in rats when it was injected into the hypothalamus, the medial pontine area, and lateral medulla, in the vicinity of the NTS. Blevins et al. (5) also reported that CCK-8, at doses that did not elevate plasma CCK-8 concentrations, reduced food intake when injected into several hypothalamic sites, as well as when it was injected into the NTS. Moreover, electrophysiological experiments suggest that CCK-8 might act directly on central vagal afferent terminals in the NTS to increase the release of glutamate (3). Hence, it is possible that reduction of food intake in response to intravenous CCK-8 in vagotomized rats might be mediated by CCK-8's action at surviving central terminals of vagal afferent neurons. In this regard, it is interesting to examine sucrose intakes during the first 5 min of ingestion, when rats are being infused intravenously with 2.8 μg/h CCK-8. Subdiaphragmatically vagotomized rats exhibited a marked and significant reduction of food intake in response to CCK-8 during the first 5 min of the ingestion test. However, in capsaicin-treated rats, intake during the first 5 min of the test was not significantly lower during CCK-8 infusion than during saline infusion. Given that subdiaphragmatic vagotomy leaves central vagal terminals intact, while capsaicin treatment destroys both central and peripheral terminals of small unmyelinated vagal afferents, it is conceivable that reduction of food intake in response to intravenous CCK-8, particularly early during intravenous infusion, is mediated, in part, by direct action of CCK-8 on central vagal terminals. Of course, since CCK-8 continues to reduce cumulative sucrose intake in capsaicin-treated rats, there must be still other mechanisms to account for CCK-8's actions.
Our data indicate that both vagotomized and capsaicin-treated rats consumed a larger volume of sucrose under saline-injected conditions compared with control rats. This increased intake is consistent with impaired short-term satiation, due to reduced gastrointestinal feedback, but alterations in gastrointestinal transit of ingested sucrose might also contribute to these differences. For example, Schwartz et al. (36) reported that gastric branch vagotomy completely blocked the reduction of gastric emptying by intragastric fat, protein, carbohydrate, and acid loads. This compromised inhibition and enhanced “dumping” of liquid diet from the stomach into the small intestine might trigger increased intake in rats with vagal lesions.
Changes in sweet taste sensitivity could also play a role in the increase in sucrose intake after capsaicin treatment or vagotomy. Martin et al. (21) reported that GLP-1 and its receptor (GLP-1R) are expressed in mammalian taste buds and that GLP-1R knockout mice exhibit a dramatic reduction in sweet taste sensitivity. Moreover, oral pretreatment with capsaicin increased consumption of sweet solutions in rats (13), and real-time RT-PCR data revealed decreased expression of sweet receptors T1R2 and T1R3, along with capsaicin receptor VR1 in the circumvallate papillae of the tongue. Although these results suggest that decreased expression of sweet receptors may be related to increased sweet diet consumption in oral capsaicin-treated rats, it is difficult to imagine how comparable changes would occur following subdiaphragmatic vagotomy, which produces effects similar to capsaicin on baseline food intake and responsiveness to GLP-1.
Perspectives and Significance
Our behavioral results indicate that abdominal vagal or capsaicin-sensitive neurons are not necessary for reduction of food intake by circulating (endocrine) GLP-1 or CCK-8. In fact, vagotomy or capsaicin-treatment actually enhanced responses to intravenous GLP-1. The fact that both vagotomy and capsaicin treatment abolished reduction of sucrose intake in response to intraperitoneal injected CCK-8, while leaving the response in intravenously infused peptides mostly intact, suggests that vagal afferents might mediate reduction of food intake in response to paracrine secretion of these peptides close to their site of release in the gastrointestinal tract. On the other hand, reduction of food intake by GLP-1 and CCK-8 in the systemic circulation involves action at a site beyond the abdominal vagal afferent endings.
GRANTS
This work was supported by National Institutes of Health Grant NS-20561, and National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-52849.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: J.Z. and R.C.R. conception and design of research; J.Z. performed experiments; J.Z. and R.C.R. analyzed data; J.Z. and R.C.R. interpreted results of experiments; J.Z. prepared figures; J.Z. drafted manuscript; J.Z. and R.C.R. edited and revised manuscript; J.Z. and R.C.R. approved final version of manuscript.
REFERENCES
- 1. Abbott CR, Monteiro M, Small CJ, Sajedi A, Smith KL, Parkinson JR, Ghatei MA, Bloom SR. The inhibitory effects of peripheral administration of peptide YY(3–36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res 1044: 127–131, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Ahren B. Sensory nerves contribute to insulin secretion by glucagon-like peptide-1 in mice. Am J Physiol Regul Integr Comp Physiol 286: R269–R272, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Baptista V, Zheng ZL, Coleman FH, Rogers RC, Travagli RA. Cholecystokinin octapeptide increases spontaneous glutamatergic synaptic transmission to neurons of the nucleus tractus solitarius centralis. J Neurophysiol 94: 2763–2771, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Beeton C, Garcia A, Chandy KG. Drawing blood from rats through the saphenous vein and by cardiac puncture. J Vis Exp: 266, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blevins JE, Stanley BG, Reidelberger RD. Brain regions where cholecystokinin suppresses feeding in rats. Brain Res 860: 1–10, 2000 [DOI] [PubMed] [Google Scholar]
- 6. Bucinskaite V, Tolessa T, Pedersen J, Rydqvist B, Zerihun L, Holst JJ, Hellstrom PM. Receptor-mediated activation of gastric vagal afferents by glucagon-like peptide-1 in the rat. Neurogastroenterol Motil 21: e978–e978, 2009 [DOI] [PubMed] [Google Scholar]
- 7. Bullock BP, Heller RS, Habener JF. Tissue distribution of messenger ribonucleic acid encoding the rat glucagon-like peptide-1 receptor. Endocrinology 137: 2968–2978, 1996 [DOI] [PubMed] [Google Scholar]
- 8. Chelikani PK, Haver AC, Reidelberger RD. Intravenous infusion of glucagon-like peptide-1 potently inhibits food intake, sham feeding, and gastric emptying in rats. Am J Physiol Regul Integr Comp Physiol 288: R1695–R1706, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Dhanvantari S, Seidah NG, Brubaker PL. Role of prohormone convertases in the tissue-specific processing of proglucagon. Mol Endocrinol 10: 342–355, 1996 [DOI] [PubMed] [Google Scholar]
- 10. Eissele R, Goke R, Willemer S, Harthus HP, Vermeer H, Arnold R, Goke B. Glucagon-like peptide-1 cells in the gastrointestinal tract and pancreas of rat, pig and man. Eur J Clin Invest 22: 283–291, 1992 [DOI] [PubMed] [Google Scholar]
- 11. Gaisano GG, Park SJ, Daly DM, Beyak MJ. Glucagon-like peptide-1 inhibits voltage-gated potassium currents in mouse nodose ganglion neurons. Neurogastroenterol Motil 22: 470–479, e111, 2010 [DOI] [PubMed] [Google Scholar]
- 12. Goke R, Larsen PJ, Mikkelsen JD, Sheikh SP. Distribution of GLP-1 binding sites in the rat brain: evidence that exendin-4 is a ligand of brain GLP-1 binding sites. Eur J Neurosci 7: 2294–2300, 1995 [DOI] [PubMed] [Google Scholar]
- 13. Gu XF, Lee JH, Yoo SB, Moon YW, Jahng JW. Intra-oral pre-treatment with capsaicin increases consumption of sweet solutions in rats. Nutr Neurosci 12: 149–154, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Holmes GM, Browning KN, Tong M, Qualls-Creekmore E, Travagli RA. Vagally mediated effects of glucagon-like peptide 1: in vitro and in vivo gastric actions. J Physiol 587: 4749–4759, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Imeryuz N, Yegen BC, Bozkurt A, Coskun T, Villanueva-Penacarrillo ML, Ulusoy NB. Glucagon-like peptide-1 inhibits gastric emptying via vagal afferent-mediated central mechanisms. Am J Physiol Gastrointest Liver Physiol 273: G920–G927, 1997 [DOI] [PubMed] [Google Scholar]
- 16. Jancso G, Kiraly E, Such G, Joo F, Nagy A. Neurotoxic effect of capsaicin in mammals. Acta Physiol Hung 69: 295–313, 1987 [PubMed] [Google Scholar]
- 17. Kinzig KP, D'Alessio DA, Seeley RJ. The diverse roles of specific GLP-1 receptors in the control of food intake and the response to visceral illness. J Neurosci 22: 10470–10476, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Larsen PJ, Tang-Christensen M, Holst JJ, Orskov C. Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience 77: 257–270, 1997 [DOI] [PubMed] [Google Scholar]
- 19. Layer P, Holst JJ, Grandt D, Goebell H. Ileal release of glucagon-like peptide-1 (GLP-1). Association with inhibition of gastric acid secretion in humans. Dig Dis Sci 40: 1074–1082, 1995 [DOI] [PubMed] [Google Scholar]
- 20. Lee IS, Nam YS, Lee CH, Chung DW, Yoon YS, Kim JS, Yi SJ, Pai T, Lee HS. Expressional changes of neuropeptide Y and cholecystokinin in the arcuate and paraventricular nuclei after capsaicin administration. J Nutr Sci Vitaminol (Tokyo) 50: 144–148, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Martin B, Dotson CD, Shin YK, Ji S, Drucker DJ, Maudsley S, Munger SD. Modulation of taste sensitivity by GLP-1 signaling in taste buds. Ann N Y Acad Sci 1170: 98–101, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mentlein R, Gallwitz B, Schmidt WE. Dipeptidyl-peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon-like peptide-1(7–36) amide, peptide histidine methionine and is responsible for their degradation in human serum. Eur J Biochem 214: 829–835, 1993 [DOI] [PubMed] [Google Scholar]
- 23. Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol 403: 261–280, 1999 [DOI] [PubMed] [Google Scholar]
- 24. Mojsov S, Weir GC, Habener JF. Insulinotropin: glucagon-like peptide I (7–37) co-encoded in the glucagon gene is a potent stimulator of insulin release in the perfused rat pancreas. J Clin Invest 79: 616–619, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nakabayashi H, Nishizawa M, Nakagawa A, Takeda R, Niijima A. Vagal hepatopancreatic reflex effect evoked by intraportal appearance of tGLP-1. Am J Physiol Endocrinol Metab 271: E808–E813, 1996 [DOI] [PubMed] [Google Scholar]
- 26. Nakagawa A, Satake H, Nakabayashi H, Nishizawa M, Furuya K, Nakano S, Kigoshi T, Nakayama K, Uchida K. Receptor gene expression of glucagon-like peptide-1, but not glucose-dependent insulinotropic polypeptide, in rat nodose ganglion cells. Auton Neurosci 110: 36–43, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Naslund E, King N, Mansten S, Adner N, Holst JJ, Gutniak M, Hellstrom PM. Prandial subcutaneous injections of glucagon-like peptide-1 cause weight loss in obese human subjects. Br J Nutr 91: 439–446, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Paxinos G, Watson C. The Rat Brain in Sterotaxic Coordinates. San Diego, CA: Academic Press, 1997 [Google Scholar]
- 29. Qualmann C, Nauck MA, Holst JJ, Orskov C, Creutzfeldt W. Glucagon-like peptide 1 (7–36 amide) secretion in response to luminal sucrose from the upper and lower gut. A study using alpha-glucosidase inhibition (acarbose). Scand J Gastroenterol 30: 892–896, 1995 [DOI] [PubMed] [Google Scholar]
- 30. Ritter RC, Ladenheim EE. Capsaicin pretreatment attenuates suppression of food intake by cholecystokinin. Am J Physiol Regul Integr Comp Physiol 248: R501–R504, 1985 [DOI] [PubMed] [Google Scholar]
- 31. Ritter S, Dinh TT. Capsaicin-induced neuronal degeneration: silver impregnation of cell bodies, axons, and terminals in the central nervous system of the adult rat. J Comp Neurol 271: 79–90, 1988 [DOI] [PubMed] [Google Scholar]
- 32. Roberge JN, Brubaker PL. Regulation of intestinal proglucagon-derived peptide secretion by glucose-dependent insulinotropic peptide in a novel enteroendocrine loop. Endocrinology 133: 233–240, 1993 [DOI] [PubMed] [Google Scholar]
- 33. Rodriquez de Fonseca F, Navarro M, Alvarez E, Roncero I, Chowen JA, Maestre O, Gomez R, Munoz RM, Eng J, Blazquez E. Peripheral versus central effects of glucagon-like peptide-1 receptor agonists on satiety and body weight loss in Zucker obese rats. Metabolism 49: 709–717, 2000 [DOI] [PubMed] [Google Scholar]
- 34. Ruttimann EB, Arnold M, Hillebrand JJ, Geary N, Langhans W. Intrameal hepatic portal and intraperitoneal infusions of glucagon-like peptide-1 reduce spontaneous meal size in the rat via different mechanisms. Endocrinology 150: 1174–1181, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schick RR, Harty GJ, Yaksh TL, Go VL. Sites in the brain at which cholecystokinin octapeptide (CCK-8) acts to suppress feeding in rats: a mapping study. Neuropharmacology 29: 109–118, 1990 [DOI] [PubMed] [Google Scholar]
- 36. Schwartz GJ, Berkow G, McHugh PR, Moran TH. Gastric branch vagotomy blocks nutrient and cholecystokinin-induced suppression of gastric emptying. Am J Physiol Regul Integr Comp Physiol 264: R630–R637, 1993 [DOI] [PubMed] [Google Scholar]
- 37. Smith GP, Jerome C, Cushin BJ, Eterno R, Simansky KJ. Abdominal vagotomy blocks the satiety effect of cholecystokinin in the rat. Science 213: 1036–1037, 1981 [DOI] [PubMed] [Google Scholar]
- 38. South EH, Ritter RC. Capsaicin application to central or peripheral vagal fibers attenuates CCK satiety. Peptides 9: 601–612, 1988 [DOI] [PubMed] [Google Scholar]
- 39. Talsania T, Anini Y, Siu S, Drucker DJ, Brubaker PL. Peripheral exendin-4 and peptide YY(3–36) synergistically reduce food intake through different mechanisms in mice. Endocrinology 146: 3748–3756, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Tang-Christensen M, Larsen PJ, Goke R, Fink-Jensen A, Jessop DS, Moller M, Sheikh SP. Central administration of GLP-1-(7–36) amide inhibits food and water intake in rats. Am J Physiol Regul Integr Comp Physiol 271: R848–R856, 1996 [DOI] [PubMed] [Google Scholar]
- 41. Uttenthal LO, Toledano A, Blazquez E. Autoradiographic localization of receptors for glucagon-like peptide-1 (7–36) amide in rat brain. Neuropeptides 21: 143–146, 1992 [DOI] [PubMed] [Google Scholar]
- 42. van de Wall EH, Duffy P, Ritter RC. CCK enhances response to gastric distension by acting on capsaicin-insensitive vagal afferents. Am J Physiol Regul Integr Comp Physiol 289: R695–R703, 2005 [DOI] [PubMed] [Google Scholar]
- 43. Wang T, Edwards GL, Baile CA. Glucagon-like peptide-1 (7–36) amide administered into the third cerebroventricle inhibits water intake in rats. Proc Soc Exp Biol Med 219: 85–91, 1998 [DOI] [PubMed] [Google Scholar]
- 44. Wettergren A, Schjoldager B, Mortensen PE, Myhre J, Christiansen J, Holst JJ. Truncated GLP-1 (proglucagon 78–107-amide) inhibits gastric and pancreatic functions in man. Dig Dis Sci 38: 665–673, 1993 [DOI] [PubMed] [Google Scholar]
- 45. Yamamoto H, Kishi T, Lee CE, Choi BJ, Fang H, Hollenberg AN, Drucker DJ, Elmquist JK. Glucagon-like peptide-1-responsive catecholamine neurons in the area postrema link peripheral glucagon-like peptide-1 with central autonomic control sites. J Neurosci 23: 2939–2946, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Young AA, Gedulin BR, Bhavsar S, Bodkin N, Jodka C, Hansen B, Denaro M. Glucose-lowering and insulin-sensitizing actions of exendin-4: studies in obese diabetic (ob/ob, db/db) mice, diabetic fatty Zucker rats, and diabetic rhesus monkeys (Macaca mulatta). Diabetes 48: 1026–1034, 1999 [DOI] [PubMed] [Google Scholar]
- 47. Yox DP, Ritter RC. Capsaicin attenuates suppression of sham feeding induced by intestinal nutrients. Am J Physiol Regul Integr Comp Physiol 255: R569–R574, 1988 [DOI] [PubMed] [Google Scholar]
- 48. Yox DP, Stokesberry H, Ritter RC. Vagotomy attenuates suppression of sham feeding induced by intestinal nutrients. Am J Physiol Regul Integr Comp Physiol 260: R503–R508, 1991 [DOI] [PubMed] [Google Scholar]
- 49. Zander M, Madsbad S, Madsen JL, Holst JJ. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study. Lancet 359: 824–830, 2002 [DOI] [PubMed] [Google Scholar]
- 50. Zhao H, Sprunger LK, Simasko SM. Expression of transient receptor potential channels and two-pore potassium channels in subtypes of vagal afferent neurons in rat. Am J Physiol Gastrointest Liver Physiol 298: G212–G221, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]