Abstract
A dose range study to assess the teratogenic potential of aqueous extract of Labisia pumila var. alata (Kacip Fatimah) was conducted in rodents. The extract at doses of 0 (control), 2, 20, 200, 400, 1000 mg/kg/day were respectively administered by gavaging to 6 groups of pregnant Sprague Dawley rats from day 6 through day 16 of pregnancy and sacrificed on day 21. No significant agent-related effects including changes in maternal body weight (MBW) nor weight gain were observed. The corrected maternal body weights (CMBW) were slightly higher in animals receiving low dose extracts (2 mg/kg/day) as compared to all groups of animals. However, body weight differences were not statistically significant. Gravid uterine weight, number of corpora lutea, number of implantation sites, percentage of foetal resorptions, number of life foetuses, foetal weight and foetal sex ratio showed no significant differences among all group animals. None of the foetuses from all dams showed evidence of external congenital malformations. These findings may suggest that aqueous extracts of Labisia pumila var. alata up to 1000 mg/kg/day statistically do not show any significant teratogenic effects in rats but do affect the maternal body weight and this is dose dependent.
Keywords: Labisia pumila var. alata, aqueous extract, teratogenicity
Introduction
Labisia pumila or popularly known in Malaysia as Kacip Fatimah (KF) is a member of the small genus of slightly woody plants of the family Myrsinaceae (1). There are at least four known varieties of Labisia pumila found in Malaysia. Though, only three of them are popularly used by the Malays and are recognized as Labisia pumila var. pumila (LPP), Labisia pumila var. alata (LPA) and Labisia pumila var. lanceolata (LPL) (2).
This plant has been utilized by many generations of Malay women for the purpose of inducing and in the facilitation of labour. Water decoctions of the root or whole plant were and are still given to pregnant woman one to two months prior to childbirth (1, 2). It is also used as a postpartum medication (2) in the form of mixed preparation to help in the contraction of uterus. Further, KF is reported to delay fertility and to help regain body strength of mothers (3).
KF is very popular amongst the local womenfolk and it is frequently consumed by women of reproductive age. As a result of this popularity, to date several commercial products in the form of capsules (4) and canned drinks containing this herb have emerged in the Malaysian market (5). These products are claimed to contain the grinded or extracted parts of the plant (4, 5). However, there is no standardized preparation or any scientific data authenticating or providing evaluation of the quality, safety and efficacy of this herb (4). Furthermore, little is known about the chemical constituents of this plant. No international publication regarding to this plant available as well (6).
Since KF is consumed by women during reproductive period, it is reasonable that the present study focus on accessing the possible teratogenic and reproductive toxicity of this herb. A teratogenic agent could be defined as any substance which increases the chances of offspring being born with a gross structural or functionally abnormality (7, 8). The purposes of conducting this study are to ensure and establish the safety profiles of KF by evaluating the effects of the aqueous extract of Labisia pumila var. alata (LPE) on embryonic development (teratogenic study) in rats through Segment II Study.
The study was started by looking at possible dose related teratogenic properties of the herb (Segment II– Dose range study). If it is proven that the extract has potential teratogenic properties, then a definitive teratogenic study will be conducted (9, 10). The results obtained are expected to provide useful information on the safety profile of this herb and thereby help to alleviate concerns over its use.
Materials and Methodology
Research and ethical committee approval
Proposal of the Evaluation of the Teratogenicity (Segment II) was submitted to research and ethics committee for evaluation and was approved by Universiti Sains Malaysia’s Animal Ethics Committee (USM/PPSF/050(1) Jld.1) and Universiti Sains Malaysia Health Campus’s Animals Research and Ethics Committee (No 013).
Plant materials
The standardized aqueous extract of Labisia pumila var. alata (LPE) was provided by Institute for Medical Research (IMR), Malaysia. The raw material was previously identified and authenticated by ethno botanist of the Forest Research Institute of Malaysia (FRIM). Subsequently, the plant was sent to Chemical Engineering Pilot Plant, Universiti Teknologi Malaysia for further processing. The dosage preparation was carried out at the Pharmacology Laboratory, Universiti Sains Malaysia Health Campus using the lyophilized powdered sample obtained from the IMR.
Dosage preparation
The Kacip Fatimah (KF) or Labisia pumila dried extract (LPE) was reconstituted with distilled water at 5 different dosages. Doses selected in this experiment were based on reported pharmacological dose (dose gives maximum favorable effect without adverse effect) which is 16.7 mg/kg/day (11).
The prepared extract were kept in small aliquots 0.2 or 0.6ml at (−40°C) for experiments at the following concentrations: Group 1, Control group, LPE 0 mg/kg/day; Group 2, LPE 2 mg/kg/day; Group 3, LPE 20 mg/kg/day; Group 4, LPE 200 mg/kg/day; Group 5, LPE 400 mg/kg/day and Group 6, LPE 1000 mg/kg/day.
Animals
Female, virgin, Sprague Dawley rats weighing between 180 – 200 g and proven fertile adult males of body weights (b.w) between 200 – 250 g for mating purposes were used in this experiment. These animals were procured by Animal House, Universiti Sains Malaysia Health Campus. All rats were healthy and maintained according to the USM ethical guidelines under standard laboratory conditions. They were acclimatized to the laboratory environment for 2 weeks prior to use. Animals were housed at 20±2°C with 12 h light /dark cycle (lights on from 0700 to 1900). The animals had free access to commercially obtained pelleted rat chow and water ad libitum.
Mating procedures
Daily vaginal smear was performed in all females. Pregnancy was then induced by separately caging each female with a confirmed fertile male in the evening of proestrus. A sperm positive vaginal smear in the next morning was considered as day 0 (D0) of pregnancy (pc) (9, 10, 12).
Treatment
Forty-eight (48) pregnant rats were randomly divided into 6 groups (8 animals per group). Group 1 (Control group) received vehicle treatment (distilled water (DW)) 0.2ml daily, whereas Group 2, 3, 4, 5 and 6 received herbal extract at a dose of 2, 20, 200, 400 (0.2ml) and 1000 mg/kg/day (0.6ml) respectively. All treatment were administered during D6 through D16pc (period of organogenesis) which is defined as the critical period for the structural development span of the embryonic stage for rats (13, 14, 15).
Behavioral observations
During the treatment and post-treatment periods, the animals were observed closely for any signs of toxicity. Daily body weight was recorded for all animals.
Assessment on dams and foetuses
On D21 of pregnancy, all dams were sacrificed by CO2 asphyxiation and were immediately laparotomised. Examinations of maternal visceral organs such as uterus, ovaries, adrenal glands, liver, spleen, kidney and gut were then performed macroscopically. The gravid uterus and ovaries were subsequently removed, cleared of adhering tissue and weighed. The isolated uteri were cut open and position of implantations, early resorption (no embryonic tissue visible at termination) or late resorption (placental and embryonic tissue visible at termination) and dead or live foetuses were counted. Uteri of non-pregnant dams were stained in 0.5% ammonium sulfide to confirm the absence of implantation sites (9).
The umbilical cord of each foetus was cut and foetuses were removed, blotted dry, weighed and checked for their sex. Male and female foetuses were differentiated by examination of the location of the genital papilla (which is further away from the base of the tail in males) (16). Head, eyes, palate, nares, limbs, neck, spine, chest, abdomen, orifices, tails and genitals were examined under dissecting stereomicroscope (Motic Digital Stereomicroscope, DM-143 PAL System).
Outcome measures
Several parameters were recorded in this study which includes fates of females such as survivability and death. Clinical signs, maternal visceral changes, daily maternal body weight (MBW) (D0-21) and their weight gain (D6-16, D6-21 and D16-21) were monitored and recorded.
Gravid uterine weight, corrected maternal body weight (maternal body weight D21 – gravid uterus weight) (CMBW), percentage change in body weight compared to expected body weight (corrected maternal body weight of control animal-corrected maternal body weight of treated animal / corrected maternal body weight of control animal × 100), number of corpora lutea per litter, number of implantation sites per litter, percentage of pre implantation loss (no. corpora lutea – no. implantation / no. corpora lutea × 100), number of resorption sites (early and late resorption) and percentage of post implantation death (no. implantation – no. live foetuses / no. implantation × 100) were noted.
The live foetuses were counted and weighed, sex distributed (male and female) and organs checked for external abnormality. Any malformation was reported as per treatment group and per litter.
Statistical evaluation
Firstly, all the parameters were checked for normality using Normality Test as well as Levene’s Test to check for homogeneity of variance and to determine if the groups have unequal variances at the 5% level of significance (17). Subsequently, to the Normality and Levene’s Test (if the data showed normal distribution and homogeny), parametric test was performed. Nonparametric test was used for skewed and non homogenous data. The 0.05 level of probability was used as the criterion for significance.
Data on maternal body weight (MBW) (D0-21 pc) was analyzed by General Linear Model Repeated Measures. Maternal weight gain, corrected maternal body weight (CMBW), maternal organ weights (ovaries and uterus), number of corpora lutea per litter, number of implantation sites per litter, number of life foetuses and foetal body weight were analyzed by One-way analysis of variance (ANOVA), followed by Scheffe Test if differences were found. Additionally, Kruskal Wallis test (nonparametric) was used to assess the overall effects of percentage of pre-implantation loss and percentage of post-implantation death. The parametric data were evaluated and expressed as Mean (Standard Error of Mean) (SEM) or ratio while the non-parametric data were expressed as Median (Interquartile Range) (IQR).
Results
Fates of females and other clinical signs
Treatment with Labisia pumila aqueous extract (LPE) did not adversely affect the progress of pregnancy and clinical condition of the rats (Figure 1). All dams did not exhibit abnormal findings as they grew normally and healthily. No mortality and morbidity were recorded throughout this study. There were also no abnormal findings at autopsy.
Figure 1:
Picture showing the pregnant dam of control group animal at sacrificed. Treatment was given from D6 through D16 of pregnancy. Dam was sacrificed on D21 of pregnancy. There were no abnormal gross findings at autopsy in all animals throughout study.
Maternal body weight (MBW) (D0-21), weight gain (D6-16, D6-21 & D16-21), gravid uterine weight and corrected maternal body weight (CMBW)
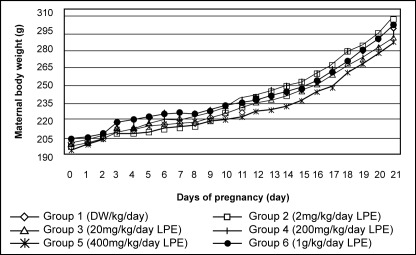
There were no significant differences (P>0.05, GLM Repeated Measures) in maternal body weights (MBW) on D0-21 pc (Figure 2) whilst gravid uterine weight and maternal weight gain from D6-16, D6-21 & D16-21 (one-way ANOVA) (Table 1) between treated and control group animals. The corrected maternal body weights (CMBW) were slightly reduced in groups treated with 400 and 1000 mg/kg/day LPE as compared to control. While animals that received 2 mg LPE/kg/day (Group 2) showed the highest corrected maternal body weight among all group of animals. The animals that received extract of 400 mg/kg/day gained less weight. The differences in the corrected maternal body weight were however, not statistically significance (Figure 3).
Figure 2:
Graph showing the maternal body weights of treated and control group animals. Treatment was given from D6 through D16 of pregnancy. There were no significant differences in the maternal body weights of treated and control group animals from day 0 through day 21 of pregnancy.
Table 1:
Reproductive and foetal parameters in rats treated with aqueous extract of Labisia pumila var. alata (LPE) from day 6 through day 16 of pregnancy.
| Parameters |
LPE (mg/kg/day) |
|||||
|---|---|---|---|---|---|---|
| 0 (Control) | 2 | 20 | 200 | 400 | 1000 | |
| No. of pregnant rats | 8 | 8 | 8 | 8 | 8 | 8 |
| Percentage of pregnancy (%) | 100 | 100 | 100 | 100 | 100 | 100 |
| Change in body weight compared to expected body weight (%) | 0 | +5.19 | +1.87 | +3.98 | +3.97 | −0.55 |
| Gravid uterine weight (g)a | 63.22 (1.68) | 57.71 (7.18) | 50.31 (9.29) | 51.57 (6.55) | 59.48 (5.40) | 65.03 (2.93) |
| No. of corpora lutea/litera | 10.13 (0.67) | 11.13 (0.52) | 10.25 (0.80) | 10.88 (0.72) | 10.38 (0.65) | 11.75 (0.31) |
| No. of pre-implantation sites/litera | 10.00 (0.60) | 9.50 (1.15) | 8.13 (1.47) | 8.88 (1.11) | 9.38 (0.82) | 11.13 (0.40) |
| % of pre-implantation loss/literb | 0 | 8.55 (34.55) | 3.57 (57.50) | 15.55 (33.22) | 3.57 (24.31) | 0 (15.72) |
| % of post implantation death (resorption)/literb | 0 | 0 | 0 | 0 (24.98) | 0 | 0 (12.50) |
| Liters with early resorption | 0 | 1 | 1 | 2 | 1 | 2 |
| Liters with late resorption | 0 | 0 | 0 | 0 | 0 | 0 |
| No. of life foetuses/litera | 10.00 (0.60) | 8.88 (1.90) | 8.00 (1.55) | 8.38 (1.35) | 9.25 (0.82) | 10.63 (0.46) |
| Pups male: female ratio | 1.50:1 | 1.03:1 | 1:1 | 1:1.16 | 1:1.47 | 1:1.02 |
Mean (SEM)
Median (IQR)
Significantly different from the control, P <0.05
Figure 3:
Histogram showing the means of corrected maternal body weights of treated and control group animals. Treatment was given from D6 through D16 of pregnancy. There were no significant differences in the means of corrected maternal body weights among all group animals.
Maternal visceral changes and organ weights
No gross maternal visceral changes that were considered unusual were observed both in treated and the control group animals.
There was no significant difference (P>0.05, one-way ANOVA) in maternal reproductive organ weights (ovaries, adrenal glands and uterus) among all group animals (data not shown).
Number of corpora lutea and number of implantation sites per litter
There were no significant differences (P>0.05) in number of corpora lutea and number of implantation sites per litter between treated and control group animals (Table 1).
Post-implantation death and foetal resorption
There was no statistical differences (P>0.05; Kruskal Wallis test) in percentage of post implantation death between treated and control group animals.
Few early foetal resorptions and postimplantation death were noted in the treated group of animals. Control group animal given distilled water did not show any foetal resorption. However, there were no significant differences (P>0.05) when compared to the control group for both the early resorptions and post-implantation death (Table 1). No late resorption was noted in any of the animals.
Number of life foetuses per litter
All foetuses in every group were alive at autopsy (D21). This was confirmed by observation of breathing or when they could be induced to respond. There was a slightly reduction in the number of life foetuses in animals of Group 2, 3, 4 and 5 as compared to the control group. However, animals in Group 6 that received high dose (1000 mg/kg/day LPE) showed the highest number of life foetuses among all groups of animals (Table 1). It was however, not significantly different (P>0.05) when compared to all groups of animals.
Sex distribution and foetal weight (male and female)
Based on one-way ANOVA, the number of male and female foetuses were similar (P>0.05) within and among all group animals. The overall male: female foetus sex ratio was equal (1:1.01).
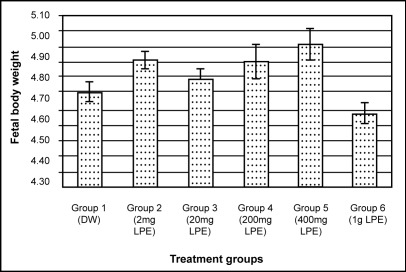
Likewise, no significant difference (P>0.05, one-way ANOVA) was observed in foetal weight for all group animals (Figure 4). Foetal body weight of animals which received herbal extracts was commonly higher than control group except for those in Group 6 (1000 mg/kg/day LPE) but no significant difference was seen when compared to control group.
Figure 4:
Histogram showing the means of fetal body weights of treated and control group animals. Treatment was given from D6 through D16 of pregnancy. There were no significant differences in the means of fetal body weights among all group animals.
External malformations
Based on the observations and examinations under dissecting stereomicroscope, there were no external malformations noted on the head, eyes, palate, nares, limbs, neck, spine, chest, abdomen, orifices, tails and genitals of all pups of treated and control group animals (Figure 5). Overall, a total of 361 pups of treated group animals and 80 pups of control group did not show evidence of external congenital malformations.
Figure 5:
Picture showing the fetuses of control group dam. Treatment was given from D6 through D16 of pregnancy. Caesarean section was performed on D21 of pregnancy. Examination under dissecting stereo microscope did not indicate any external malformation in any of the fetuses of all groups of animals.
Discussion
The literature provides limited number of controlled studies with Kacip Fatimah. The highly popular nature of Kacip Fatimah amongst Malay women warrants a detailed scientific study. In this regard, the present study was carried out using LPE at doses of 2 to 1000 mg/kg/day. This dose range is selected to reflect the doses used in the practice of the Malay women. This study is the first to evaluate the teratogenicity of this herb.
Results obtained showed that the exposure of rats to the LPE of up to 1000 mg/kg daily (as suggested by most guidelines for toxicity studies) during period of organogenesis did not show any significant deleterious effect on rats. Slightly less maternal weight gain was noted in animals that received extract (400 mg/kg) during period of organogenesis (D6-16 of pregnancy). However, no significant difference was found when compared to the control group. This decrease was inconsistent and may be attributed to some possible toxicological effect but may not be toxicologically significance. Additionally, there were no statistical differences (P> 0.05) in maternal body weights (MBW) of treated and control group animals from D0 through D21 of pregnancy. These findings suggest that the herb do not have any significant deleterious effect on the progression of pregnancy in rats.
The corrected maternal body weight (CMBW) was slightly higher in animals receiving low dose (2 mg/kg) compared to the control and those receiving higher doses of the herbal extract (20, 200, 400 and 1000 mg/kg) in the present study. However, they were not statistically different when compared to all groups of animals. The percentage change in body weight also showed a similar trend to finding of the corrected maternal body weight. These suggest that the herb may affect maternal body weight and that it was dose dependent.
Percentages of early foetal resorption and post implantation death showed no significant findings (P>0.05). This result is encouraging as it may imply that LPE is unlikely to be fetotoxic in rats. Incidence of early or late resorptions in the control animals were absence in the present study and this could be considered normal. The small number of animals used in this study could well be the reason for the reproductive effects (early foetal resorption and post implantation death) not showing up in the control females. Foetal resorptions on the other hand can still occur in untreated as well as treated rodents (18).
Gravid uterine weights were lower in treated group of animals compared to control group except for those receiving the highest dose (1000 mg/kg/day). However, the differences were not statistically significant. In addition, uterine weight can be influenced by litter size, viable foetuses and foetal gender where females tend to be smaller than males (16, 18).
Foetal body weight, on the other hand was higher in the treated group animals (Group 2, 3, 4 and 5) except for Group 6 but show no statistically significant difference when compared to control group. The slightly higher foetal body weight could be due to compensatory effect of slightly lower number of life foetuses in the specific treated groups (Group 2, 3, 4 and 5) (refer to Table 1). Foetal body weights are influenced by intrauterine growth rates, litter size and gestation length. Individual foetuses in large litters tend to be smaller than foetuses in smaller litters. Thus, reduction in their weights that can be attributed to the large litter size should not be considered as an adverse effect unless the increased litter size is treatment related (18). Examination under dissecting stereomicroscope did not indicate any gross malformation in any of the foetuses. The male and female foetal ratio was equal in control and treated group animals.
The above findings may show some evidence that Labisia pumila aqueous extract lacks any observable fetotoxic effect when given at doses of up to 1000 mg/kg/day to rats during the period of organogenesis. Further, no external malformation in the pups observed in this study substantiates the above conclusion.
Conclusion
In conclusion, the present data demonstrate that Kacip Fatimah or Labisia pumila aqueous extract might affect the body weight of dams and the effect was dose dependent. The differences in maternal weight gained in the treated group of animals did not adversely affect foetal growth and well being as indicated by a reasonably good foetal body weight. Early foetal resorption (early post implantation death) was not significantly increased in treated group animals. No late foetal resorption was noted in any of the rats. None of foetuses in treated group animals showed evidence of external congenital malformations. Findings of the current study suggest that aqueous extract of Labisia pumila did not cause significant fetotoxic effect in rats. No observable adverse effect level (NOAEL) for LPE in this study was determined at 1000 mg/kg/day. However, this study alone is insufficient to make an overall conclusion of teratogenicity. Thus, to clarify this observation, a more definitive study and a thorough investigation using a much larger cohort of animals are required to confirm the safety profile of this herb on the female. In addition, human studies utilizing cell-lines should be designed to measure the developmental toxicity and not just teratogenicity prior to comparative assessment between species.
Acknowledgments
The authors acknowledge IRPA Top Down Grant (no: 304 PPSP 6112213) of Malaysia for its financial support, Animal House and Department of Pharmacology, USM Health Campus for laboratory infrastructural facilities. Special thanks to Dr Wan Nazaimoon Wan Mohamud from Institute Medical Research for providing us standardized extract of Kacip Fatimah.
References
- 1.Burkill IH. A dictionary of the economic products of the Malay Peninsula. London: Crown Agent; 1935. [Google Scholar]
- 2.Stone BC. Notes on the Genus Labisia Lindl. (Myrsinaceae) Malayan Nature Journal. 1988;42:43–51. [Google Scholar]
- 3.Zakaria M, Mohd MA. Traditional Malay medicinal plants. Kuala Lumpur: Penerbit Fajar Bakti Sdn. Bhd; 1994. [Google Scholar]
- 4.Houghton PJ, Jamal JA, Milligan S. Studies on Labisia pumila herb and its commercial products. Journal Pharm. Pharmacol. 1999;51(Supplement):236. [Google Scholar]
- 5.Nazaimoon WMW. Estrogenic and androgenic activities of Kacip Fatimah (Labisia pumila var. alata and var. pumila) Project summary. 2002 [Google Scholar]
- 6.Ismail N, Manaf MA, Ismail Z. Trends in Traditional Medicine Research Proceedings of the International Conference on the Use of Traditional Medicine & Other Natural Products in Health Care. Penang; Malaysia: 1993. Pharmacognosical characterisation of Labisia Pothoina; p. 574. [Google Scholar]
- 7.Smithells RW. The demonstration of teratogenic effects of drugs in humans. In: Hawkins DF, editor. Drugs and pregnancy: Human teratogenesis and related problems. 2 ed. Edinburgh: Churchill Livingstone; 1987. [Google Scholar]
- 8.Neubert D, Jodicke B, Welsch F. Reproduction and Development. In: Marquardt H, Schafer SG, McClellan R, Welsch F, editors. Toxicology. San Diego: Academic Press; 1999. pp. 493–4. [Google Scholar]
- 9.Manson JM, Kang YJ. Test methods for assessing female reproductive and developmental toxicology. In: Hayes AW, editor. Principles and methods of toxicology. 3 ed. New York: Raven Press Ltd; 1994. pp. 1026–33. [Google Scholar]
- 10.Detection of Toxicity to Reproduction for Medicinal Products. ICH Harmonised Tripartite Guideline. Jun 24, 1993. p. 5. Endorsed by the ICH Steering Committee at Step 4 of the ICH Process; 24 June 1993.
- 11.Nuradilah M, Sani MNH, Anom HS, Balaspramaniam A, Aliah T, Nazaimoon WMW. Effects of Kacip Fatimah water extract on hormonal profile of normal female rats. The 4th International Traditional / Complementary Medicine Conference and Exhibition (INTRACOM 2002); 2002; Sunway Pyramid Convention Centre. [Google Scholar]
- 12.Paumgartten FRJ, De-Carvalho RR, Souza CAM, Madi K, Chahoud I. Study of the effects of B-myrcene on rat fertility and general reproductive performance. Brazillian Journal of Medical and Biological Research. 1997;31:955–965. doi: 10.1590/s0100-879x1998000700012. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization (WHO) Environmental Health Criteria. Principles for evaluating health risks to reproduction associated with exposure to chemicals. WHO (World Health Organization); 2001. pp. 1–187. [Google Scholar]
- 14.Manson JM, Zenick H, Costlow RD. Teratology test methods for laboratory animals. In: Hayes AW, editor. Principles of toxicology. New York: Raven Press; 1982. pp. 141–184. [Google Scholar]
- 15.Organization for Economic Co-operation and Development (OECD) Guideline for the testing of chemicals. Proposal for updating guideline 414. Prenatal development toxicity study. Organization for Economic Co-operation and Development (OECD); 2001. pp. 1–13. [Google Scholar]
- 16.Sharp PE, Regina MCL. Important biological features. In: Suckow MA, editor. The laboratory rat A volume in the laboratory animal pocket reference series. Boca Raton: CRC Press LLC; 1998. p. 7. [Google Scholar]
- 17.Green SB, Salkind NJ, Akey TM. Using SPSS for windows: Analyzing and understanding data. 2 ed. Prentice-Hall; 2000. [Google Scholar]
- 18.U.S Environmental Protectian Agency . Guidelines for Reproductive Toxicity Risk Assesment. Office of Research and Development, ed: U.S Environmental Protectian Agency; 1996. pp. 1–163. [Google Scholar]