Abstract
The study was carried out to determine the in vitro effect of Plantago major extract on calcium oxalate crystals and to compare the effects of Plantago major extract with clinically used drugs like allopurinol and potassium citrate (positive controls). Modified Schneider slide gel method was used for the in vitro study and the crystals formed were measured by Image Analyser system KS 300, 3.0 Carl Zeiss. The concentrations of Plantago major extract used were from 100ppm to 350ppm. Plantago major extract at concentrations in the range of (100ppm–350ppm) significantly inhibited the size of calcium oxate crystals (dihydrate variety) against negative control (p<0.05) and against positive controls (p<0.05). However the inhibition concentration 50 (IC50) values on the size of calcium oxalate crystal for the extract, potassium citrate and allopurinol were 300ppm, 350ppm and 450ppm respectively. Extract of Plantago major also has inhibition effect on the number of crystals but it was not significant. In conclusion extract of Plantago major was better than allopurinol and potassium citrate in inhibiting the size of the calcium oxalate crystal in-vitro.
Keywords: Plantago Major, urolithiasis, in Vitro
Introduction
Urolithiasis is a condition where there is a formation of stone in the urinary system, i.e in the kidney, ureter, urinary bladder or in the urethra (1). Generally there are five different types of stones of which calcium oxalate is the most common stone (80%), calcium phosphate stone (5%), magnesium ammonium phosphate, cistine and uric acid stone (2). There are two varieties in calcium oxalate stone, i.e monohydrate type (in the form of dump bell or oval) and dihydrate type (in the form of double pyramid) (3). The cause is multifactorial including diet, genetic and environmental (4). Many treatments have been tried for the treatment of urolithiasis in Malaysia and other parts of the World. It recurs back within five years (50%) and there is no one standard treatment that can prevent the recurrences (5). Plantago Major Linn. belonging to the family Plantaginaceae is a perennial herb found wild throughout the whole of Europe and temperate Asia (6). Every part of the plant has been used in many traditional medicines to treat cough, diarrhoea, dysentery, urinary tract calculus (6,7,8). Allopurinol (Zyloric) is a uricosuric agent that has been clinically used for the follow up patients with stone as it reduced the production of uric acid in urine and in fact uric acid may be a nidus for calcium oxalate crystals (9). Potassium citrate is a low molecular weight inhibitor for crystallization. The present study reports on the inhibiting effects of the ethanol extract of Plantago Major on calcium oxalate crystals in vitro and to compare the effects of this extract with the clinically used drugs like allopurinol and potassium citrate.
Materials and Methods
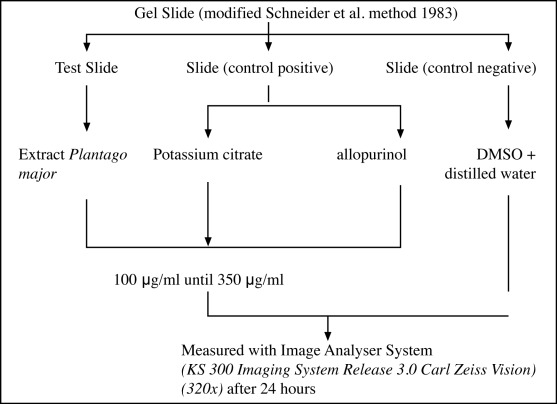
The present study was carried out in Makmal 1 of Jabatan Sains Bioperubatan, Fakulti Sains Kesihatan Bersekutu in the year 2003–2004. The whole plant of Plantago major was collected from Cameron Highland and was identified at the Malaysian Agricultural Research & Development Institute (MARDI). The sample studied was the ethanol extract of Plantago major (the whole plant) soxhlet extracted after drying at room temperature for a week (10). Each extract was diluted with dimethylsulphoxide (DMSO) to get different concentrations of the plantago major extract, allopurinol and potassium citrate. Dimethyl sulphoxide (DMSO) was used as a negative control and allopurinol standard and potassium citrate were used as positive controls. Allopurinol standard of 0.1mg/ml was obtained by crushing Allopurinol tablets (100mg) into power and dissolving 0.001g of the powder in 5ml DMSO. The solution was then diluted further with distilled water to obtain a final concentration of 0.1mg/ml. The slides were coated with 1.5ml of 1% Bactoagar and each slide was equally divided into two areas. Eight equal wells with a distance of 1.25 × 0.5 cm were made on each slide when the gel was about to solidify. Calcium oxalate crystals were prepared by introducing equal amount of 20ul of 0.2M solution of calcium chloride and ammonium oxalate in the horizontal wells and sample and controls were put in the vertical wells. Modification of Schneider slide Gel method (1983) was used for this study and number and size of the crystal were measured after 24 hours by using Image analyser KS 300, 3.0 Carl Zeiss (11). The inhibition concentrations of Plantago major extract, allopurinol and potassium citrate were calculated and inhibition concentrations 50(IC50) were determined for extract, allopurinol and potassium citrate (Fig. 1). Comparison between results of DMSO, Plantago major, allopurinol and potassium citrate were analysed using One way ANOVA (SPSS 11.0). Student ‘t’ test (unpaired) was also used to compare results between any two groups. Level of significance was set at p<0.05.
Figure 1:
Methology
Results
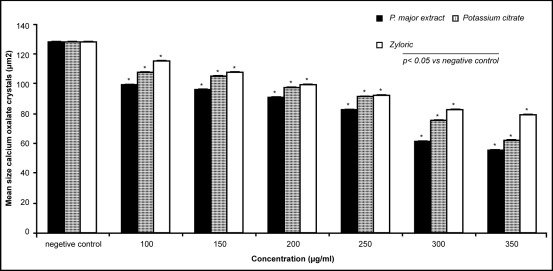
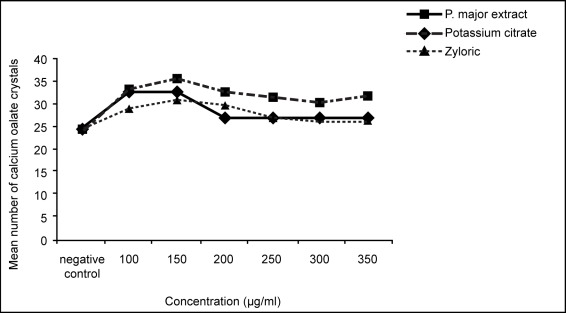
There was a white vertical line of formation of calcium oxalate crystals in each slide and the number of slides for each sample was twelve (n=12). The measurement was done for three areas on each white vertical line for the number and size of the crystals by computerised Image Analyser and the mean ± SEM was calculated. There were significant reductions in the size of calcium oxalate crystals (dihydrate variety) by extract of Plantago major compared to control (99.9 ± 0.6μm2; 96.9 ± 0.5μm2; 91.0 ± 0.5μm2; 83.1 ± 0.5μm2; 61.9 ± 0.4μm2; 56.5 ± 0.4μm2 vs 128.5 ± 0.8μm2) (*p<0.05) (n=60). Similarly the results for potassium citrate were (107.8 ± 0.4μm2; 105.0 ± 0.3μm2; 97.9 ± 0.2μm2; 91.3 ± 0.1μm2; 75.7 ± 0.2μm2; 62.4 ± 0.2μm2 vs 128.5 ± 0.8 μm2) (*p<0.05) (n=60) and for allopurinol the results were (115.2 ± 0.5μm2; 107.2 ± 0.5μm2; 99.7 ± 0.4μm2; 92.3 ± 0.4μm2; 84.2 ± 0.4μm2; 80.6 ± 0.3 μm2 vs 128.5 ± 0.8μm2). (*p<0.05) (n=60) (Fig. 2). The reduction on the number of calcium oxalate cryatals (dihydrate variety) for extract of Plantago major were (33.2 ± 2.6; 32.8 ± 3.2; 26.9 ± 1.3; 26.7 ± 2.2; 26.6 ± 3.2; 26.4 ± 1.9 vs 24.3 ± 1.5) but was not significant (p>0.05). Similarly the reduction on the number of crystals for potassium citrate were (33.4 ± 2.8; 35.7 ± 3.1; 32.8 ± 2.3; 31.4 ± 2.2; 30.5 ± 2.2; 31.8 ± 1.9 vs 24.3 + 1.5 ) and for allopurinol were (28.8 ± 0.4; 30.8 ± 0.9; 29.7 ± 0.7; 27.2 ± 0.5; 26 ± 0.9; 25.8 ± 0.7 vs 24.3 ± 1.5). (Fig. 3). IC50 values (inhibition concentration 50) for the reduction on the size of crystals for plantago major was 300mg/ml, 350 for potassium citrate and 450 for allopurinol. (Table 1).
Figure 2 :
Effects of Plantago major extract, potassium citrate and Allopurinol on the size of calcium oxalate crystals.
Figure 3 :
Effects of Plantago major extract, potassium citrate and allopurinol on the number of calcium oxalate crystals.
Table 1 :
IC50 values of Plantago major extract, potassium citrate and allopurinol on the size of calcium oxalate crystals in vitro
| IC50 values | Mean size cystal (μm2) | |
|---|---|---|
| Negative control (DMSO) | - | 128.50 ± 0.75 |
| Extract to Plantago major | 300 μg/ml | 61.68 ± 0.42 |
| Patassium citrate (positive control) | 350 μg/ml | 62.36 ± 0.15 |
| Allopurinol (positive control) | 450 μg/ml | 63.70 ± 0.39 |
Discussion
The calcium oxalate crystals that have been produced in this in vitro study were similar to the crystals in the urine of patient with stone (12). Most of the crystals measured in this study were calcium oxalate (dihydrate variety) since 90% of monohydrate variety were formed only after 48 hours (13). Plantago major extract has signifcantly reduced the size of calcium oxalate crystals (dihydrate variety) (P<0.05). The higher the concentrations of extract the more will be the size reduction (Fig. 2). The active ingredients in the Plantago major are polysaccharides, fat, caffein acid derivatives, flavonoids, glycosides, irinoid and terpenoids (14). It may be one of the active ingredients that may have reduced the size of the crystals in this study. The mechanism of action was not known as yet. It may be the active ingredient in the ethanol extract of Plantago major which may have prevented the crystal formation or may have dissolved the preformed crystals (12). The action of potassium citrate may be that citrate may have combined with calcium ion to form calcium citrate so that there will be less chance of formation of calcium oxalate crystals (15). Since the core of calcium oxalate crystal contains 10–12 % of uric acid (9) allopurinol may have prevented the formation of calcium oxalate crystals by reducing the content of uric acid in the urine by acting through the enzyme xanthine oxidase (16). On the number of calcium oxalate crystals the lower concentrations of Plantago major up till 150mg/ml has no inhibition effect on the number of calcium oxalate crystals but the higher concentrations of Plantago major from 200mg/ml upwards do have inhibition effects but were not significant (p>0.05). According to the results obtained by IC50 on the size of the crystals, extract of Plantago major was the best inhibitor on the size of calcium oxalate crystals followed by potassium citrate and allopurinol.
Conclusion
Plantago major, potassium citrate and allopurinol do have significant inhibition effects on the size of calcium oxalate crystals (*p<0.05). All these three samples studied do have inhibition effects on the number of crystals but was not significant(p>0.05). According to IC50 values on the size of crystals, Plantago major was the best inhibitor on the size of crystals followed by potassium citrate and allopurinol.
References
- 1.Chandrasoma P, Taylor CR. Concise Pathology. 3rd Edition. USA: Appleton & Lange; 1998. The ureter, urinary bladder & urethra; pp. 738–739. [Google Scholar]
- 2.Free AH, Free HM. Renal Lithiasis. In Urinalysis in Clinical Laboratory Practice, CRC Press; 1975. pp. 229–243. [Google Scholar]
- 3.Kannabiran K, Selva MR. Induction of renal nukleus oxalate binding activity in experimental hyperoxaluric rats. Nephron. 1997;(2):219. doi: 10.1159/000189535. Basel 75; [DOI] [PubMed] [Google Scholar]
- 4.Anderson CK. Urinary Calculous disease. Edinburgh: Churchill Livingstone; 1979. The anatomical aetiology of renal urolithiasis; pp. 40–68. Wickham. J.E.A. [Google Scholar]
- 5.Schenkman NS, Stroller ML. Epitomes-urology : Urolithiasis. WJM. 169(2):110–111. [PMC free article] [PubMed] [Google Scholar]
- 6.Burkhill IH. A dictionary of the Economic products of the Malay Peninsula. Vol. 2. Kuala Lumpur: Ministry of Agriculture and co-operatives, Malaysia; 1966. [Google Scholar]
- 7.Mabey R. The complete new herbal. London: Elm; 1988. Tree Books. [Google Scholar]
- 8.Muhamed Z, Mustafa AM. Traditional Malay Medicinal Plants. Kuala Lumpur: Penerbit Fajar Bakti Sdn; 1994. Bhd. [Google Scholar]
- 9.Ismail SI, Zhari I, Norakmal, Abdul RS. Urolithiasis : A review on the formation, epidemiology, treatment and the present state. Berita Farmasi. 1984;11(2):8–14. [Google Scholar]
- 10.Ibrahim J, Yong HK, Dae YS, Byung HH. Inhibition effects of Malaysian Med. Plants on the platelet-activating factor (PAF) receptor binding. Natural product sciences. 1996;2(2):86–89. [Google Scholar]
- 11.Schneider HJ, Bothor W, Berg RH, Borner RH, Tacob M. A gel model for measuring Crystallization Inhibiter Activities. Urol Int. 19838:33–38. doi: 10.1159/000280858. [DOI] [PubMed] [Google Scholar]
- 12.Zhari I, Norhayati I, Temumen A, Sirajinisa Potensi beberapa tumbuhan tempatan sebagi bahan ubat penyakit batu karang. Dlm. Azizol, A.K., Khozirah, S., Ibrahim, J, Jamaludin, Z Nik, M.N. (pnyt). Pembangunan Industri Tumbuhan Ubatan : Peranan Agensi Kerajaan, Industri dan Saintis. Pros. Konvensyen Kebangsaan Tumbuhan Ubatan, 13–15 Oktober 1995. hlm. 25. Kuala lumpur: Institute Penyelidikan Perhutanan Malaysia (FRIM).
- 13.Grases F, Millan A, Conte A. Product of calcium oxalate monohydrate, dihydrate or trihydrate. Urol. Res. 1989;18:17–20. doi: 10.1007/BF00294575. [DOI] [PubMed] [Google Scholar]
- 14.Samuelson AB. The traditional uses, chemical constituents and biological activities of Plantago major. J. Ethnopharmacol. 2000;71(1–2):1–21. doi: 10.1016/S0378-8741(00)00212-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baumann JM. Physio-chemical aspects of calcium stone formation. Urol. Res. 1990;18(1):S25–S30. doi: 10.1007/BF00301524. [DOI] [PubMed] [Google Scholar]
- 16.Abramson SB. Treatment of gout and crystal arthropathies and uses and mechanisms of action of nonsteroidal anti-inflammatory drugs. Curr Opin Rheumatol. 1992;4(3):295–300. doi: 10.1097/00002281-199206000-00002. [DOI] [PubMed] [Google Scholar]