Abstract
The widespread use of persistent organic polybrominated diphenyl ethers (PBDEs) as commercial flame retardants has raised concern about potential long-lived effects on human health. Epigenetic mechanisms, such as DNA methylation, are responsive to environmental influences and have long-lasting consequences. Autism spectrum disorders (ASDs) have complex neurodevelopmental origins whereby both genetic and environmental factors are implicated. Rett syndrome is an X-linked ASD caused by mutations in the epigenetic factor methyl-CpG binding protein 2 (MECP2). In this study, an Mecp2 truncation mutant mouse (Mecp2308) with social behavioral defects was used to explore the long-lasting effects of PBDE exposure in a genetically and epigenetically susceptible model. Mecp2308/+ dams were perinatally exposed daily to 2,2′,4,4′-tetrabromodiphenyl ether 47 (BDE-47) and bred to wild-type C57BL/6J males, and the offspring of each sex and genotype were examined for developmental, behavioral and epigenetic outcomes. Perinatal BDE-47 exposure negatively impacted fertility of Mecp2308/+ dams and preweaning weights of females. Global hypomethylation of adult brain DNA was observed specifically in female offspring perinatally exposed to BDE-47 and it coincided with reduced sociability in a genotype-independent manner. A reversing interaction of Mecp2 genotype on BDE-47 exposure was observed in a short-term memory test of social novelty that corresponded to increased Dnmt3a levels specifically in BDE-47-exposed Mecp2308/+ offspring. In contrast, learning and long-term memory in the Morris water maze was impaired by BDE-47 exposure in female Mecp2308/+ offspring. These results demonstrate that a genetic and environmental interaction relevant to social and cognitive behaviors shows sexual dimorphism, epigenetic dysregulation, compensatory molecular mechanisms and specific behavioral deficits.
INTRODUCTION
Autism spectrum disorder (ASD) is characterized by deficits in social interactions, impairments in language and repetitive, patterned behavior. Although ASD is highly heritable, the prevalence has risen in the last decade to an estimated 1:110 individuals (1,2). Younger ages at diagnosis, changes in diagnostic criteria and inclusion of milder cases are factors that contributed to increased autism incidence rates from 1990 through 2006, but are insufficient to explain the 600% rise in this time frame (3). A study of ASD concordance in monozygotic versus dizygotic twins found a moderate genetic heritability and a substantial environmental component (4), suggesting that the increased incidence may be related to a recent environmental trigger. Epigenetic mechanisms such as DNA methylation act at the interface of genetic and environmental factors, and methylation deficiencies have been observed in autism (5).
Polybrominated diphenyl ethers (PBDEs) are a class of persistent organic pollutants (POPs) pervasive in the environment because of their widespread use as commercial flame retardants (6). 2,2′,4,4′-Tetrabromodiphenyl ether (BDE-47) is the PBDE congener found at highest levels in human serum and breast milk, raising concerns about its potential neurotoxic effects during pregnancy and neonatal development (7). Furthermore, PBDEs are lipophilic compounds that bioaccumulate in lipid-rich environments such as the brain. In mice, neonatal exposure to a single high dose of BDE-47 resulted in reductions to synaptic plasticity and long-term potentiation in the hippocampus (8), whereas daily perinatal exposure of mice to environmentally relevant BDE-47 doses resulted in alterations to growth and motor performance in behavioral assessments (9). PBDEs are structurally similar to thyroid hormone (TH), implicating thyroid disruptions in their neurotoxic effects, although the molecular mechanisms are not well delineated. Disruption of TH signaling may mediate some aspects of PBDE neurotoxicity (6); however, BDE-47 does not interact directly with TH receptor beta (10). POPs and a wide variety of other environmental toxins are associated with reduced global levels of DNA methylation in human tissues (11,12), but neither genetic nor epigenetic interactions on neurobehavioral outcome have been investigated for BDE-47 exposures.
A genetic mutant Avy mouse has been used to successfully demonstrate proof-of-principle environmental epigenetic interactions (13,14). Few studies, however, have been designed to investigate the epigenetic interface of gene by environment interactions relevant to human neurodevelopment. Because environmental factors are likely to combine with genetic susceptibilities in the risk of neurodevelopmental disorders such as autism, we chose a ‘two hit’ model to investigate the interaction of a single gene (Mecp2, methyl-CpG binding protein 2) and a single exposure (BDE-47). Mutations in MECP2 cause Rett syndrome (RTT), a neurodevelopmental disorder where autistic features are common (15). MECP2 levels are decreased in cortical cells of ASD patients, suggesting a broad role for MECP2 in neurodevelopmental disorders (16). RTT also serves as a model disorder for understanding the role of epigenetic mechanisms in neurodevelopmental disorders, including ASD, since it involves two epigenetic mechanisms: DNA methylation and X-inactivation. MECP2 encodes methyl CpG-binding protein, an epigenetic reader of DNA methylation in the genome and a regulator of neuronal activity and maturation (17–19). MECP2 is an X-linked gene and subject to X chromosome inactivation (XCI), thus female RTT brain is a mosaic for cells expressing the mutant and wild-type alleles of MECP2 (20). Mouse models of Mecp2 deficiency show deficits in neuronal synaptic connectivity and inhibitory neurotransmission with similarities to autism (21–25). The Mecp2308/y truncation mutant mouse exhibits deficits in social behaviors and learning and memory but without the early lethality observed in Mecp2-deficient models (26–28). However, the studies to date on the Mecp2308/y mouse model have been limited to males and have not examined a potential susceptibility to environmental exposures on social or cognitive behavior.
The epigenetic and behavioral effects of perinatal exposure to BDE-47 in a mouse model of Mecp2 mutation were examined to explore potential neurodevelopmental outcomes arising from having both a genetic susceptibility and an environmental exposure. Evidence for gene by environment interactions was found for Mecp2308/+ female but not for Mecp2308/y male offspring. Perinatally exposed female mice showed significantly reduced global DNA methylation levels in the adult brain that correlated with decreased social behavior. BDE-47 exposure negatively impacted pup survival and learning of adult females, but Mecp2 mutation reversed a social novelty learning defect which corresponded to increased levels of the methyltransferase Dnmt3a. These results demonstrate complex molecular and behavioral interaction effects at the epigenetic interface of a genetic and environmental interaction relevant to human neurodevelopmental disorders.
RESULTS
Human-relevant BDE-47 exposures affect reproductive success and preweaning growth independently of pup genotype
Breeding of Mecp2308/+ dams to wild-type males resulted in litters containing four genotype groups (Mecp2+/+ or Mecp2308/+ females, Mecp2+/y or Mecp2308/y males) (26). A 10-week daily regimen of low-dose (0.03 mg/kg/day) BDE-47 was performed on Mecp2308/+ dams for 4 weeks' preconception, 3 weeks' gestation and 3 weeks during lactation (9) in two successive experiments (Exp. 1 and Exp. 2), in order to identify and characterize interactions between BDE-47 exposure and Mecp2 genotype. The BDE-47 dosing was designed to model prolonged, low-level environmental exposures. Initially, a second, higher BDE-47 dose group (0.1 mg/kg/day) was included, but this dosage group was discontinued due to poor reproductive success, with only two of eight dosed dams delivering litters that survived to postnatal day (PND) 8 when compared with 10 of 12 control dams (P = 0.02, Supplementary Material, Table S1).
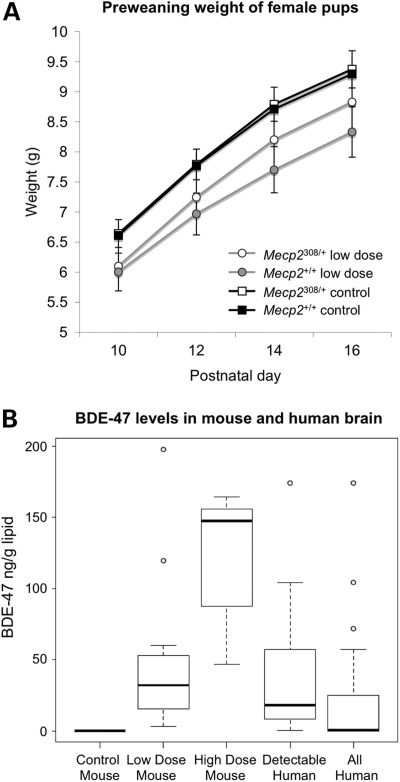
BDE-47 adversely affected pups prior to weaning. Survival (Supplementary Material, Table S1) and body weights (Fig. 1A and Supplementary Material, Fig. S1A-B) were lower in the BDE-47-treated pups in Exp. 1, where pups were separated from the dam daily from PND 8 to 18 for weighing and testing. Exp. 2 used a reduced, alternate day preweaning test schedule. Body weights were lower in female BDE-47-treated pups in Exp. 2 (Supplementary Material, Fig. S1C–D), but survival was not affected. Litter size (as determined on PND 8) was not affected by BDE-47 treatment in either experiment, but litters were larger in Exp. 2 (vehicle control, 7.1 ± 0.3; 0.03 mg/kg/day BDE-47, 7.3 ± 0.4) than in Exp. 1 (vehicle control, 5.3 ± 0.6; 0.03 mg/kg/day BDE-47, 4.6 ± 0.5), which may explain the difference in pup survival between experiments. Neither Mecp2 genotype nor BDE-47 by genotype interaction effects on survival and preweaning growth was significant in either experiment.
Figure 1.
BDE-47 dosing scheme is comparable with human exposures and significantly affects preweaning growth in females. (A) The preweaning weights of BDE-47 low-dose-exposed females were lower than those of controls (F(1,31) = 6.17, P = 0.019), with litter size as a covariate. Error bars represent SEM. Data shown are from Exp. 2, and both Exp. 1 and 2 results are shown in Supplemental Material, Figure S1. Mecp2+/+ control n = 24, Mecp2+/+ low-dose BDE-47 n = 11, Mecp2308/+ control n = 26, Mecp2308/+ low-dose BDE-47 n = 15. (B) BDE-47 levels in brains of low-dose-exposed dams are similar to detectable levels found in human brain samples. After weaning, brain tissue was collected from control (n = 6) and BDE-47-dosed dams (low dose n = 12, high dose n = 6). BDE-47 levels were quantitated using GC/MS-NCI along with human post-mortem brain samples (n = 24, mean age 26 ± SD 16.5 years). BDE-47 levels were normalized to lipid content. Human samples were also subdivided into those showing BDE-47 greater than the level of detection. No significant difference between low dose and detectable human samples (n = 13) was seen (P = 0.4), confirming the low-dose daily regiment as producing BDE-47 levels in the mouse brain which are comparable with average human brain levels.
After weaning, brain samples from BDE-47-exposed dams were analyzed for levels of BDE-47 and compared with a random sampling of 24 human postmortem brain samples with no known neurologic disorders (Fig. 1B). The average BDE-47 level in the mouse brain from the 0.03 mg/kg/day group was similar to that of human brain levels, whereas the 0.1 mg/kg/day group was similar to the highest BDE-47 level observed in the human brain.
Developmental, social and cognitive behaviors revealed a female-specific interaction for Mecp2 genotype and perinatal BDE-47 exposure
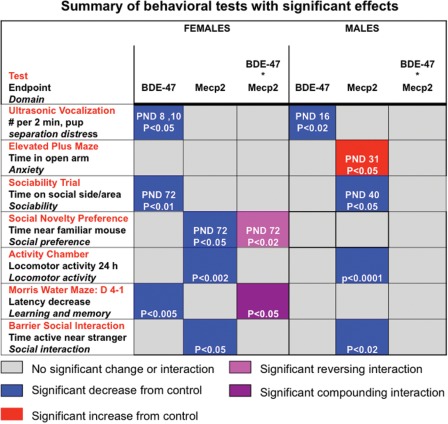
The offspring of BDE-47-exposed Mecp2308/+ dams were subjected to a battery of tests to determine the developmental and behavioral effects of BDE-47 exposure and Mecp2 mutation interaction (described in Materials and Methods). Behavioral tests that showed significant effects are summarized in Figure 2. Genotype, rather than BDE-47, had a greater influence on the behavior of males, whereas females were more sensitive to BDE-47 effects and BDE-47 interaction with Mecp2 genotype (Fig. 2). Both male and female offspring were tested in Exp. 1. In Exp. 2, postweaning behavior testing focused exclusively on females because of their increased sensitivity to BDE-47 and interaction effects.
Figure 2.
BDE-47, genotype, reversing and compounding effects are observed in behavioral tests, predominantly in females. The chart summarizes significant differences for females versus males of each genotype and treatment independently as well as the Mecp2 by BDE-47 interaction. All behavioral tests described in the Materials and Methods section were performed on all mice for each experiment. Only tests showing significant effects in both Exp. 1 and Exp. 2 are shown.
Preweaning tests of motor and sensory development and juvenile tests of motor behaviors were unaffected by BDE-47 exposure or Mecp2 genotype. BDE-47 influenced only one test in males, ultrasound vocalizations (USVs) at PND 16, and did not significantly interact with Mecp2 genotype on any of the tests. In females, perinatal exposure to BDE-47 resulted in significantly reduced USVs at PND 8 (Exp. 1) or PND 10 (Exp. 2, Supplementary Material, Fig. S2A). In the elevated plus maze, male Mecp2308/y mice spent significantly more time on the open arms, as described previously (26) (Supplementary Material, Fig. S2B). No significant effects on the elevated plus maze were observed in females.
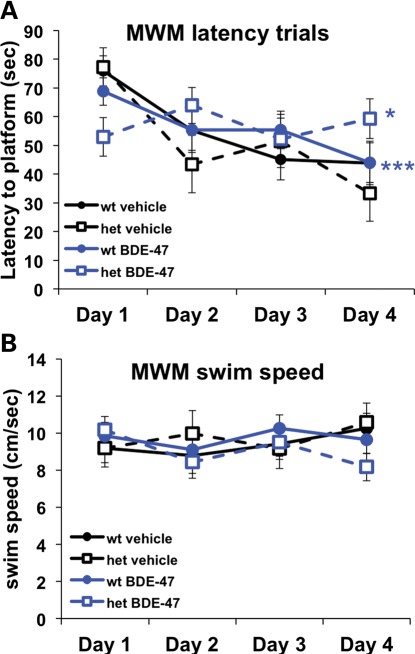
Adult locomotor activity and social barrier interaction tests showed significant defects due to Mecp2 genotype in both sexes (Fig. 2 and Supplementary Material, Fig. S3), as previously described in males (26–28). Sociability and social novelty were tested at a juvenile stage (PND 40) in Exp. 1, with an Mecp2 genotype effect observed in males that showed significantly lower sociability based on time spent in the social side. In the Morris water maze (MWM) test of learning in Exp. 1, males from all groups exhibited learning across the 4-day period, as evaluated by RMANOVA of the latency to reach the platform (F(3,42) = 13.77, P < 0.0001), with no significant genotype or PBDE effects or interactions (Fig. 2). In contrast, females showed a significant BDE-47 treatment effect of greater latency to reach the platform in both experimental replicates (Figs 2 and 5). Overall, males showed an Mecp2 genotype effect on anxiety, sociability, motor activity and barrier social interaction tests, but no interaction effects. In contrast, females showed interactions of Mecp2 genotype with BDE-47 in social and learning tests.
Figure 5.
BDE-47-induced deficits in learning and memory are compounded by genotype in females. (A) Mean latency in MWM is reduced in BDE-47-treated females. RMANOVA of the latency to reach the platform indicated significant learning across the 4-day period (F(3,33) = 7.96, P < 0.001). A BDE-47 by day interaction (F(3,33) = 3.91, P = 0.017), based on a significant lower latency in the BDE-47 group than the vehicle group observed on the first day. In addition, there was a triple interaction between day, BDE-47 and genotype (F(3,33) = 4.06, P = 0.015). The decrease in latency across days was similar in both vehicle and BDE-47 wild-type females. The decrease in latency across trials was also seen in the vehicle Mecp2308/+ group, but not in the BDE-47 Mecp2308/+ group. (B) The differences in latency shown in (A) for the BDE-47 Mecp2308/+ group were not explained by differences in swim speed. Results from Exp. 1 are shown. Group sizes for mice (litters): BDE-47 het n = 11(6), BDE-47 wt n = 12(7), vehicle het n = 6(4), vehicle wt n = 8(6).
Brain DNA hypomethylation but not XCI ratio corresponds to female-specific BDE-47 behavioral effects
To explore potential epigenetic alterations that explain the differences in behavioral analyses, a global assessment of DNA methylation levels by dot-blot analyses was performed on DNA samples taken from whole brain following the completion of behavioral tests in both experiments. Adult female mice perinatally exposed to BDE-47 showed significantly lower levels of DNA methylation in both wild-type and Mecp2308/+ mice compared with vehicle-exposed females (Fig. 3A). In contrast, adult male mice showed comparable brain DNA methylation levels across all exposure and genotype groups (Supplementary Material, Fig. S4). MeCP2 levels were examined by quantitative immunofluorescence, but no significant differences between genotype or exposure groups were found (Supplementary Material, Fig. S5). An analysis of XCI ratios using an immunofluorescence-based approach showed no significant difference in either the mean or variance of XCI ratios in BDE-47-exposed females compared with vehicle controls (Fig. 3B). Furthermore, there were no significant correlations observed between XCI ratios of individual females and performance in the USV, sociability, social novelty or MWM behavioral tests from individual Mecp2308/+ mice (Supplementary Material, Fig. S6). These combined results suggest that the female-specific and genotype-independent global DNA hypomethylation in brain tissue from mice exposed to BDE-47 could be a potential explanation for female specificity of the BDE-47 and BDE-47 by Mecp2 interaction effects observed in social and learning behaviors.
Figure 3.
BDE-47 exposure coincides with global hypomethylation but not with altered X-inactivation ratios. (A) Global DNA methylation levels were measured in wild-type and Mecp2308/+ mice. Hypomethylation was observed in BDE-47-treated females relative to corresponding vehicle control. All samples were assayed in triplicate and methylation signals were normalized to the amount of DNA loaded. Wt vehicle n = 9, wt BDE-47 n = 8, het vehicle n = 14, het BDE-47 n = 11. **P < 0.01, ***P < 0.001. All female samples were normalized to the mean of wt vehicle control samples, set to 1.0. (B) X-inactivation skewing is not observed in BDE-47-treated Mecp2308/+ mice. Cells expressing wild-type Mecp2 were quantified using immunofluorescence on a laser scanning cytometer and shown as a percent of total nuclei. Gating values were set using a hemizygous vehicle control sample (Mecp2−/y). Values were normalized to wild-type vehicle controls (100% positive for wild-type expression). Wt vehicle n = 11, wt BDE-47 n = 15, Mecp2308/+ vehicle n = 19, Mecp2308/+ BDE-47 n = 17.
BDE-47 by Mecp2 interaction reverses social novelty learning impairments, compounds learning deficits and correlates with Dnmt3a hyperexpression in the female brain
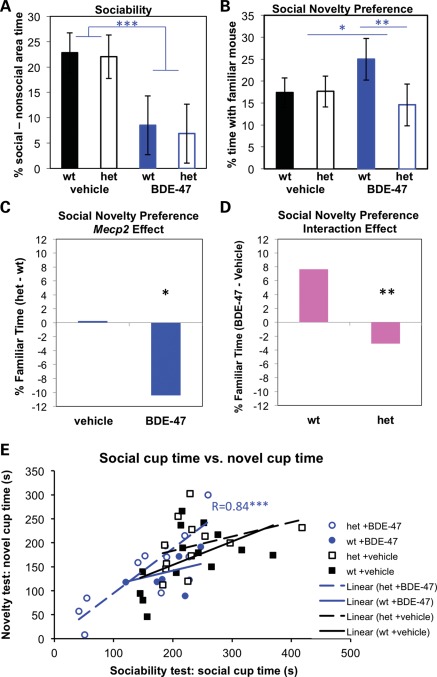
Because of the female specificity of both the DNA hypomethylation and BDE-47 effects on sociability, the sociability and social novelty tests were made more sensitive in Exp. 2 by using adult (PND 72) rather than juvenile females, as well as carrying out a more specific assessment of behavior during the socialization trial (Fig. 2, Materials and Methods). A significant reversal of BDE-47 exposure effects was observed in the social novelty test in adult females. In adult females, BDE-47 treatment led to reduced sociability without a genotype effect (Fig. 4A). In the social novelty trial, both a genotype and interaction effect was seen for time spent near the now-familiar mouse (Fig. 4B–D). BDE-47-exposed Mecp2+/+ mice spent more time near the familiar stimulus mouse compared with BDE-47-treated Mecp2308/+ mice, suggesting that the decreased social interaction in BDE-47-treated mice during the sociability trial affected recognition of the familiar mouse in the Mecp2+/+ mice but not the Mecp2308/+ mice (Fig. 4E). In contrast to the social novelty test, a significant interaction between BDE-47 treatment and Mecp2 genotype was observed in the MWM that resulted in impaired learning based on an analysis of latency to reach the platform (Fig. 5A) that was not due to differences in swim speed (Fig. 5B). That is, the effect of BDE-47 exposure on latency was compounded by the Mecp2308/+ genotype. A significant difference in the time spent in the platform-containing quadrant was also observed between BDE-treated versus vehicle-treated Mecp2308/+ mice in the MWM probe trial (Supplemental Material, Fig. S7). These combined results suggest that the reduced sociability was genotype-independent in BDE-47-exposed females, similar to the DNA hypomethylation observed in BDE-47- exposed female brain samples, but that the learned behaviors of recognition of social novelty and MWM training were both Mecp2 genotype-dependent and sensitive to BDE-47 by Mecp2 interactions.
Figure 4.
Sociability and social novelty preference are altered in female mice exposed to BDE-47. (A) Sociability is decreased in both wild-type female and Mecp2308/+ (het) mice with BDE-47 exposure. No genotype effects were seen in the sociability trial in the females, but BDE-47 exposure led to reduced sociability (time in social area minus time in non-social area, F(1,32) = 8.51, P = 0.006). (B) BDE-47 effects on social novelty preference are reversed in Mecp2308/+ het females. An Mecp2 effect and BDE-47 by Mecp2 interaction effect (F(1,31) = 6.12, P = 0.019) were seen for familiar time spent near the now-familiar mouse. BDE-47-exposed het mice spent less time near the familiar stimulus, primarily due to the lower time recorded in the BDE-47-exposed het mice compared with the control het mice. During the social novelty preference trial, no genotype or BDE-47 effects were seen for novelty preference (novel area time minus familiar area time, RMANOVA). (C) The Mecp2 effect for the social novelty preference test is individually graphed as the difference between het and wt mice for percent time, with the familiar mouse to show the significance of the decrease (F(1,31) = 5.52, P = 0.025, asterisk) specifically in BDE-47-exposed mice. (D) The BDE-47 by Mecp2 interaction effect for the social novelty test is individually graphed as the difference between BDE-47- and vehicle-exposed mice for percent time, with the familiar mouse to show the significant reversing interaction (F(1,31) = 6.12, P = 0.019). (E) Social area time in the sociability trial correlated with novel area time in the novelty trial for Mecp2308/+ females treated with BDE-47. Mecp2308/+ mice generally spent less time in the social areas (novel and familiar) than wild-type mice, but this differential was mitigated by BDE-47 treatment. Bar graphs represent least square means and error bars represent SEM. *P < 0.05, **P < 0.02, ***P < 0.01, black represents genotype effects, blue represents BDE-47 effects. Colors of bar graphs for (C) and (D) match those used in the summary table of Figure 2 (blue, decreased; pink, reversing). All data shown are from PND 72 in Exp. 2, but similar interactions effects were observed at PND 40 in Exp. 1. Litter-based statistics were performed on the following group sizes of mice (litters): BDE-47 het n = 9(6), BDE-47 wt n = 8(6), vehicle het n = 12(11), vehicle wt n = 14(12).
To explore a mechanistic basis for the complex genotype and environmental interaction effects in social and learning behaviors, transcript levels of members of the DNA methyltransferase family of genes (Dnmt1, Dnmt3a) were examined. Dnmt1 and Dnmt3a, which are both required for learning and memory in mouse brain (29), showed increased transcript levels specifically in the BDE-47-exposed Mecp2308/+ females, and this was a significant increase for Dnmt3a (Fig. 6). Neither the BDE-47 exposure nor the Mecp2 genotype alone showed a significant effect on Dnmt1 or Dnmt3a transcript levels. Dnmt3a was also significantly increased specifically in BDE-47-exposed Mecp2308/y males, similar to the females (Supplemental Material, Fig. S8). These results are consistent with the observation that BDE-47 exposure reversed the deficit in recognition of social novelty seen in female Mecp2308/+ mice.
Figure 6.
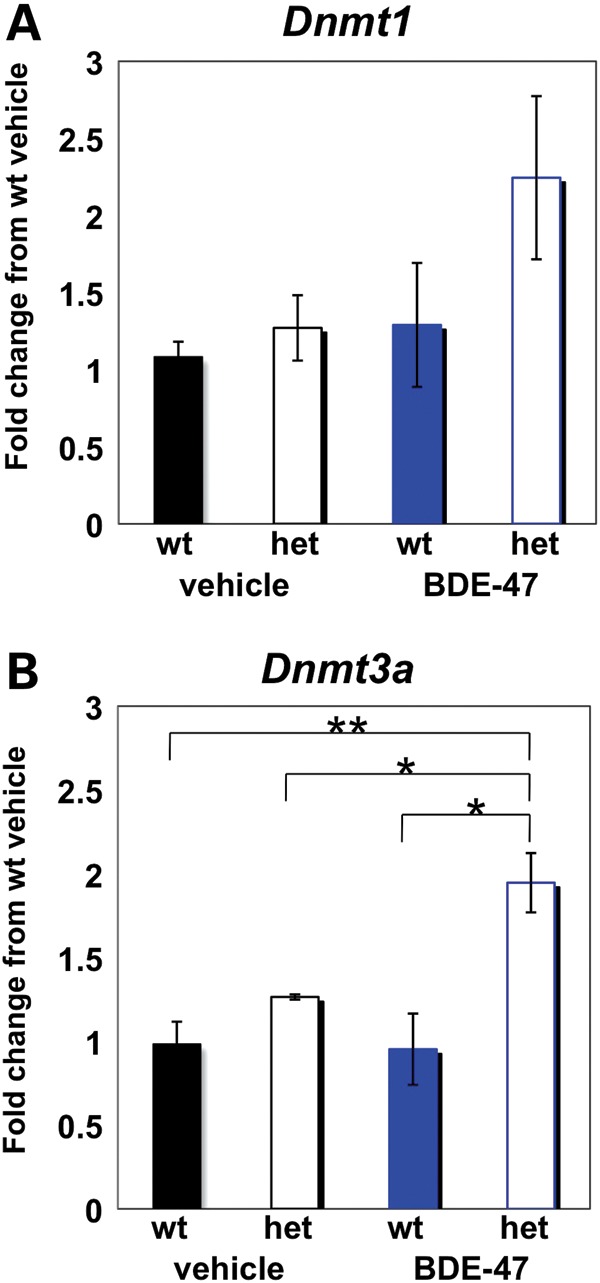
Dnmt3a expression is altered with BDE-47 exposure in Mecp2308/+ but not wild-type females. (A) qRT-PCR analysis of Dnmt1 shows no significant increase between genotype or treatment groups. (B) qRT-PCR analysis of Dnmt3a reveals a significant increase in expression in the het BDE-47 group relative to all other groups. Bar graphs show mean ± SEM, where n = 15 for wt and het vehicle and wt BDE-47 groups, analyzed as three pools of five samples each, and n = 20 for het BDE-47 group, analyzed as four pools of five samples. *P < 0.05, **P < 0.01. Data for males are shown in Supplemental Material, Figure S8.
DISCUSSION
Complex human disorders such as ASDs involve a mixture of genetic and environmental risk and protective factors. This study was designed to reduce the complexity in a controlled experimental system by examining the effects of perinatal exposure to a single environmental toxin (BDE-47) in a genetically susceptible mouse model (Mecp2308/+). From this multidimensional investigation, several novel discoveries were made that contribute to understanding the complexities of gene by environment interactions relevant to human neurodevelopment. First, perinatal BDE-47 exposure produced sexually dimorphic effects on preweaning weights, brain DNA hypomethylation and behavioral interaction effects on social novelty preference and learning. Second, brain DNA hypomethylation in females corresponded with reduced sociability following perinatal BDE-47 exposure. Third, exposure to BDE-47 coincided with a reversal of deficits in the preference for social novelty test, which coincided with increased Dnmt3a levels. In contrast, impairments in learning in the MWM were exacerbated in BDE-47-exposed female mice with an Mecp2308/+ genotype. Lastly, the analysis of post-mortem human brain samples suggested that the relative low-dose BDE-47 exposures in our study are in the range of human exposures and are consistent with adverse effects on fertility and neurodevelopmental outcomes in genetically and epigenetically susceptible humans.
Because Mecp2 is an X-linked gene subject to XCI, sex differences were expected in the behavioral analyses, as males are hemizygous and females are heterozygous and mosaic for mutant and wild-type alleles (30). A stronger genetic contribution may explain why males showed significant genotype-only effects in tests of anxiety, sociability, activity and social barrier interactions (Fig. 2) that were predicted from prior behavioral studies of Mecp2308/y mice on a 129/SvEv background (26–28). Differences in genetic background, age of testing (2 versus 5 months), prior testing experiences and MWM-reduced pool size and spatial specificity of the probe trial are likely explanations for why the deficit in learning and memory previously described in Mecp2308/y male mice (28) was not observed in our study. Female Mecp2308/+ mice have not been previously analyzed for behavior because of the confounder of variable XCI (27,28), even though RTT primarily affects females. Interestingly, similar defects in activity and social barrier interactions were demonstrated in Mecp2308/+ females that were not correlated with XCI in our study, suggesting that female mice of this model can be useful in therapy trials of RTT. The phenotype associated with the absence of Mecp2 (31–34), including abnormal clasping reflex, impaired motor ability, weight loss, respiratory abnormalities and early mortality, was not seen in our Mecp2308/+ or Mecp2308/y mice at up to 130 days of age when testing was completed.
With the exception of USVs, which were lower in BDE-47-exposed pups of both sexes, BDE-47 and BDE-47 by Mecp2 interaction effects were exclusively found in females. C57BL/6J female mice are known to show increased latency relative to males in the MWM test (35) and reduced sociability compared with males in the three-chamber test (36). In our study, female mice appeared to be more susceptible to the added ‘hit’ of an environmental exposure in these specific behavioral tests. A study in rats showed that the half-life of BDE-47 was around one-third greater in females than in males (37), providing a potential explanation for the greater impact of BDE-47 on females in our study. Similar to our study, gestational exposure to the pollutant bisphenol A resulted in social behavioral alterations specifically in female wild-type mice that corresponded to increased Dnmt3a levels in exposed animals (38). Consistent with the hypothesis that females may be more epigenetically susceptible to environmental exposures is the observation of significantly lower levels of global DNA methylation in human female peripheral blood compared with male (39). Furthermore, a recent quantitative analysis of LINE-1 methylation levels in mouse tissues has shown that male mice show higher tissue DNA methylation than female mice in two different mouse strains (40). Combined, these results suggest that because females of both species have lower levels of global DNA methylation than males, females may be more susceptible to environmental exposures that impact the saturation of DNA methylation.
Why would females have lower global DNA methylation and be thereby more influenced by BDE-47 exposure? While differences in sex hormones cannot be ruled out in our experiments, the most likely explanation is the genomic influence of X chromosome number. Females have an inactive X chromosome that is globally hypomethylated compared with the active X chromosome (41). In addition, a recent study of blood DNA samples with XO, XY, XX, XXY and XXXXY chromosome content showed that LINE-1 methylation levels at both X-linked and autosomal loci correlated with genome size, with the lowest methylation levels observed in Klinefelter's males carrying extra inactive X chromosomes (42). The effect on autosomal methylation levels may be due to a larger genomic ‘sink’ size that requires additional methyl donors to maintain high global methylation levels (43). In future studies, it would be informative to investigate global DNA methylation in individuals with copy number variation gains that are observed at a higher frequency in individuals with autism (44).
In the emerging area of environmental epigenetics, the vast majority of environmental toxins contribute to global DNA hypomethylation (12). Exposure-linked hypermethylation has been limited to a few specific gene promoters, which are a small fraction of the genome (reviewed in 12). The supply of methyl donors to DNA is dependent on the one-carbon cycle, which is biochemically linked to the glutathione (GSH) synthesis pathway, the major redox buffer in virtually all brain cells. Therefore, an enhanced GSH response to conjugate unwanted toxic chemicals in the body is predicted to reduce the supply of methyl donors, leading to global DNA hypomethylation (45). The suppression of GSH levels also promotes oxidative stress. Interestingly, deficiencies in global DNA methylation, GSH and oxidative stress pathways have been observed in autism blood samples (5,46). The human brain has particularly high levels of DNA methylation compared with other tissues genome-wide, and neuron-specific methylation domains have been observed to be enriched for genes implicated in neurodevelopment and ASDs (47). Together, these results suggest that environmental exposures may have a widespread epigenomic impact on the developing brain.
Female mice exposed to BDE-47 during the perinatal period showed reduced sociability independent of Mecp2 genotype, coincident with reduced global methylation in the brain. There appeared to be a compensatory mechanism in female mice that were both Mecp2 deficient and BDE-47 exposed for social novelty learning that may be in part explained by upregulated Dnmt3a levels seen specifically in the interaction group. Consistent with our findings, both Dnmt1 and Dnmt3a were upregulated in response to diet-induced methyl deficiency in the liver (48). In addition, Dnmt3a is increased in response to neuronal activity (49) and targets the non-proximal promoter regions of neuronal genes in postnatal neural precursor cells (50), suggesting a specific and targeted role in the brain rather than a global one. Whereas Dnmt1 protein is predominantly nuclear and levels are relatively constant in the brain, Dnmt3a is found in both cytosolic and nuclear fractions and increases with postnatal development (51). The social novelty test was dependent on short-term recognition of a mouse previously encountered 10 min prior to the test, whereas the MWM was conducted over several days and therefore required long-term memory storage of the platform position. Therefore, the reversing interaction effect observed in the social novelty test may be a reflection of a compensatory mechanism when there are ‘two hits’ to the methylation system in the brain (global hypomethylation and Mecp2 mutation) that can be observed within a specific behavioral test. These results suggest that increased Dnmt3a levels in females with two hits of global hypomethylation and Mecp2 mutation may be a compensatory mechanism of the more dynamically expressed DNA methyltransferase gene utilized in the brain. Future studies that examine the earlier developmental dynamics of brain DNA methylation and Dnmt3a levels in females versus males in this system would be needed to determine whether these epigenetic changes are independent or related events.
Brain levels of BDE-47 were at a level comparable with those measured in human post-mortem brain samples from individuals of the general population born in the last half of the 1900s. Human brain levels for individuals born in the USA within the past decade are expected to be higher, based on recent blood level detection in children (52). To our knowledge, this is the first study to quantify BDE-47 levels in the lipid-rich human brain, where these organic pollutants bioaccumulate. The dose-dependent reduction in reproductive success observed in our study is consistent with a study showing a correlation between levels of some PBDE congeners and reduced fecundity (53). Since the reproductive effect of perinatal BDE-47 exposure was not observed previously for wild-type C57Bl/6J mice (54), our results suggest that genetically susceptible mice and humans could be most impacted by PBDE exposures. Combined genetic and environmental studies are important to future investigations of both mice and humans to further understand the impact these persistent flame retardants have on fertility and brain development.
MATERIALS AND METHODS
Lipid content determination and measurement of BDE-47 levels in the brain by mass spectometry
Brain tissues were stored at −80°C until analyzed. The tissue sample was weighed and placed in a 20 ml glass vial. Three surrogate standards (BDE-75, 77 and 138) were added to each sample and mixed with hydromatrix. The hydromatrix-mixed sample was transferred to a 34 ml Dionex extraction cell and extracted with hexane (ultra-re-analyzed grade, J.T. Baker), using the Dionex Automated Solvent Extraction System (model ASE300, Dionex, Sunnyvale, CA, USA) programmed as follows: temperature 60°C, pressure 1500 psi, 5 min heat cycle, three cycles of 5 min static cycle, 150% flush volume and 90 s purge time. Extracts were collected and concentrated to ∼3 ml under ultrapure nitrogen, using the Zymark TurboVapII Concentration System (Caliper Life Sciences, Hopkinton, MA, USA). Concentrates were transferred to corresponding labeled preweighed glass vials and air-dried in a fume hood. The vial with dried extract was weighed and the weight was recorded. Lipid content of the sample was obtained by subtracting the weight of the vial from that of the vial with dried extract. For sample cleanup, the dried extracts were reconstituted in 1 ml of hexane and loaded onto preconditioned acid silica solid-phase extraction cartridges [self-packed, 1 g of acid silica (activated silica: sulfuric acid = 2:1) topped with 200 mg of activated silica, separated from acid silica by a frit in between]. The cartridges were eluted with 10 ml of hexane. Eluants were collected and concentrated to 0.5 ml. Concentrated eluants were further cleaned on preconditioned florisil sep-pak cartridges (500 mg, Waters, Milford, MA, USA). The florisil cartridges were eluted with 10 ml of hexane and concentrated to 0.1 ml under ultrapure nitrogen. An internal standard (BDE-118) was added for chromatographic analysis. GC/MS-NCI (gas chromatography coupled to mass spectrometry using negative chemical ion mode) analysis for BDE-47 was carried out with an Agilent 6890 GC /5973 MS with a capillary column (RTX-5, 10 × 0.18 mm, i.d., 0.20 μm film thickness; Restek, Bellefonte, PA, USA). The injection temperature was set at 300°C and helium gas flow rate was 0.6 ml/min. The initial oven temperature was 120°C and was increased to 240°C at 20oC/min, and then increased to 320°C at 10oC/min and held at 320°C for 1 min. Selected ions were monitored in SIM mode at ions 79 and 81. One reagent blank, two sample blanks and three QC spike samples prepared in rendered chicken fat were included in each batch run. BDE-47 concentrations were determined from a standard curve.
Analysis of global DNA methyation levels by dot-blot analyses
Genomic DNA was purified from whole brain, using the Trizol reagent. Fifty nanograms of DNA was denatured at 95°C in 25 μl of 0.4 m NaOH, 10 mm EDTA for 10 min. After denaturation, 25 μl of cold 2 m ammonium acetate, pH 7, was added. Samples were blotted in triplicate on a nitrocellulose membrane, using a BioRad DotBlot apparatus. Spots were washed twice using 2× SSC. After blotting, DNA was UV-crosslinked to the membrane and then washed in 2× SSC. The membrane was blocked using Licor Odyssey Blocking Buffer for 30 min. After blocking, membranes were incubated with anti-5-methylcytosine (Eurogentec) antibodies in LiCor Odyssey Blocking Buffer supplemented with 0.1% Tween-20 overnight at 4°C. Membranes were washed with 1× PBS 0.1% Tween-20 and then incubated with LiCor IRDye-680-conjugated secondary antibody in Blocking Buffer 0.1% Tween-20. After washing, blots were imaged using the LiCor Odyssey Infrared Imaging System. Blots were then washed in 2× SSC and equilibrated in PerfectHyb Plus (Sigma) hybridization buffer prewarmed to 42°C for 30 min. Blots were incubated overnight at 42°C with 1 μg of biotin-labeled genomic DNA and then washed for 20 min in prewarmed 0.5× saline sodium citrate 0.1% SDS. Blocking, secondary antibody incubation and imaging were repeated as described above but with the addition of LiCor IRDye-800-conjugated streptavidin. Integrated intensity of 5-methylcytosine signal was normalized to integrated intensity of corresponding DNA hybridization signal, which served as a loading control. Wild-type and heterozygous samples were analyzed independently. Normalization factors were obtained by converting control samples to 1. Calculation of significance was performed using Wilcoxon test in R. Biotin labeling was carried out using nick translation: 5 μg of genomic DNA in 1× NT buffer, 1 nmol dATP, 1 nmol dCTP, 1 nmol dGTP, 1 nmol biotin-16-dUTP, 20 U of DNA Pol 1 (NEB) and 3 μl of DNase (1/1000 dil.) in 100 μl total volume. Nick translation was performed at 15°C for 1.5 h after which the reaction was stopped with 20 μl of 0.2 m EDTA.
Quantitative reverse transcriptase real-time PCR
Total RNA was isolated using the Trizol reagent (Invitrogen). RNA was stored at −80°C until use. cDNA was synthesized according to the manufacturer's protocol, using the QuantiTect Reverse Transcription Kit (Qiagen). cDNA from groups were pooled, with each pool containing five samples. Quantitative reverse transcriptase real-time PCR (qRT-PCR) was performed as described in reference (55), with results normalized by Ct of GAPDH. Fold change is calculated relative to wild-type vehicle control. Primers are listed in Supplementary Material, Table S2.
XCI analysis by laser scanning cytometry
Percent wild-type Mecp2-expressing cells were calculated as in Braunschweig et al. (56) with minor alterations. A tissue microarray was constructed using 600 μm cores punched in triplicate from paraffin-embedded cortical tissue. Slides were immunostained with anti-MeCP2 (Cell Signaling no. 3456). Primary antibody fluorescent signals were quantified using an iCys Laser Scanning Cytometer and represented as a percentage of total cells. Hemizygous males were quantified as a negative control with not more than 5% of cells positive.
Transgenic mouse model breeding and husbandry
Mecp2308/y males and wild-type C57/Bl6 females (Jackson Laboratories) were bred to produce female offspring that were Mecp2308/+. Dams were housed in groups (maximum three per cage) and placed individually in separate cages at 5 weeks of age. Beginning at 46 days of age, heterozygous Mecp2308/+ dams were housed individually and dosed orally with either vehicle control (corn oil) or 0.03 mg BDE-47/kg/day for a 28-day period. At the end of this period, they were mated with C57Bl/6J males (obtained from JAX West, West Sacramento, CA, USA). Dosing was continued throughout mating, pregnancy and lactation. This mating scheme resulted in offspring with four possible genotypes per treatment group: Mecp2+/+, Mecp2308/+, Mecp2+/y, Mecp2308/y. Pregnant dams were left undisturbed except for dosing from gestation day 16 until PND 8 when pups were weighed and counted and began neurodevelopmental testing. Litters were weaned at 21 days of age by removing the dam to a separate cage. At 28 days of age, the litters were separated by sex for the remainder of the experiment. Pups were individually identified by foot tattoo from 8 to 21 days of age, and additionally by ear notches from weaning.
Mouse genotyping
Tail-snips were obtained at weaning (PND 21). Gentra Puregene Tissue Kit (Qiagen) was used for DNA extraction. Purified DNA was digested using restriction enzyme NotI (or HindIII) prior to PCR. Primers Mecp2 Fwd and Mecp2 Rev1 were used to detect the wild-type allele, and Mecp2 Fwd and Mecp2 Rev2 were used to detect the mutant allele. Primers are listed in Supplementary Material, Table S2.
PBDE dosing
The BDE-47 was purchased from AccuStandard, New Haven, CT, USA, supplied as a dry powder (Cat. No. BDE-047N, Lot. No. 100901MT). A stock solution of 20 mg/ml BDE-47 was made by dissolving 5 mg of BDE-47 in 250 μm of DMSO. Aliquots of this stock solution were added to Mazola corn oil (100%, cholesterol free) to yield solutions containing 0.03, 0.1 or 1.0 mg/ml stored in amber Pyrex bottles. At the time of dosing, the dam was weighed to the nearest 0.1 g. The PBDE solution or corn oil vehicle was placed by pipette on an individual corn flake (Kellogs®) as previously described for BDE-47 and PCBs (54,57). The dose of 0.03 mg of BDE-47/kg/day dose was based on previous studies (54) and the observation that a dose of 0.1 mg/kg/day had adverse effects on fertility and pup survival in the Mecp2308/+ dams. The corn flake was placed with forceps in the front corner of the mouse's home cage, where it was retrieved and eaten with species typical gnawing pattern as the mouse held it in its forepaws. Observers verified that the cornflake was completely eaten within 10 min. To avoid disturbance of newborn litters, dams were not weighed from gestation day 16 to PND 8 (dose was based on the weight measured on gestation day 16) and no dosing was given on the day of parturition and the following 2 days. From PND 15–21, pups were removed from the home cage prior to dosing. Dosing was conducted between 1100 and 1200 hours. In Exp. 1, all surviving pups from each litter were tested. In Exp. 2, some tests were repeated using a litter-based design to confirm and extend the findings. The age at testing and some aspects of the test procedure were altered for Exp. 2. Only female data are reported from Exp. 2.
Behavioral tests
Each test was conducted at the same time of day within a 3.5 h range for all mice during the light cycle. When testing multiple mice in the same apparatus, all surfaces were cleaned with Nolvasan between mice. Mice were tested in order balanced for genotype and toxicant groups. The same mice received all tests in the test battery. Testing was conducted blind for the treatment group.
Developmental test battery
Maturation of reflexes and simple behaviors was evaluated as described previously (54,58,59). These include: righting, cliff aversion, grasp, vibrissa placing, ear opening, ear twitch, screen grasp, pull and climb, dowel foot placement, eye opening, palpebral reflex, visual placement, auditory startle and popcorn (jumping) behavior. A Plexiglass test board (25.3 × 34.1 cm2) was placed on a heating pad and maintained at 36°C (surface of board). The condition of the nest was rated before the pups were removed from the cage. Pups were placed randomly in individual holding cups on a heating pad and tested. Endpoints were scored from 0 to 3, with 3 being a fully mature response. Weights were recorded following testing. For Exp. 1, pups were tested daily from PND 8 to 18. For Exp. 2, they were tested on PND 10, 12, 14 and 16.
Weanling test battery
Litters were observed daily for a 3 min period on the testing board (without heat support) for attainment of age-appropriate postures and movement. A grid (6.6 cm2) was placed under the testing board for locomotor activity scoring. In each session, the number of rearing, pivoting, sitting and grooming movements and grid lines crossed was recorded. Gait characterization categories and abnormalities were also tallied. Additionally, measures of grasp and visual placement, which mature late in lactation, were continued from the developmental test battery (58). This test was conducted on PND 19–21 in Exp. 1 only.
Ultrasonic vocalizations
Mouse pups were removed from the nest and placed within 15 s in a 7.5 cm diameter holding cup in a sound-attenuated chamber. Over the next 2 min, USVs were recorded using a duration cutoff of 10 m and a frequency range of 30–50 khz with commercial software (Ultravox, Noldus). An amplitude filter was used to eliminate extraneous peripheral noise. All pups in the litter were tested successively in a random order. This test was conducted prior to the developmental test battery on postnatal days when both tests were scheduled. Pups were tested on PND 8, 12 and 16 in Exp. 1 and PND 10, 12, 14 and 16 in Exp. 2.
Rotarod
For Exp. 1, two training trials on the rotorod (Rota-rod Treadmill for Mice 7600, Ugo Basile Biological Research Apparatus, Italy) at a speed of 16 r.p.m. for 120 s were conducted 2 h apart. During training, the mouse was replaced on the rotating rod if it fell, so as to provide equal experience for all mice. The next day, a single test trial was conduced at 24 r.p.m. and time to fall was recorded to a maximum of 120 s (58,60). The number of passive rotations was also recorded. For Exp. 2, a more challenging accelerating protocol was used in which the speed of the rotarod (rotarod: San Diego Instruments) increased from 4 to 40 r.p.m. over a 5 min period. Three trials per day for 3 consecutive days were conducted. Mice were tested on PND 29–30 in Exp. 1 and PND 66–68 in Exp. 2.
Prepulse inhibition
This uses the auditory startle reflex to assess sensory processing (60,61). This 20 min test was conducted without prior acclimation. The mouse was restrained in a Plexiglass tube (dimensions) in a sound-attenuated chamber while programmed series of auditory stimuli was delivered over a speaker in the chamber. The stimuli were: (i) 120 dB startle alone, (ii) 120 dB startle with 74 dB prepulse, (iii) 120 dB startle with 82 dB prepulse, (iv) 120 dB startle with 90 dB prepulse and (v) no stimulus (background white noise only, 70 dB). There were 50 trials, 10 for each stimulus. Each trial consisted of a 50 ms null period and a 65 ms reciroding period. The prepulses were 20 ms long and were presented 100 ms before the 40 m 120 dB mixed frequency startle stimulus. This test was conducted only in Exp. 1.
Elevated plus maze
This test of anxiety was conducted as previously described (60,61). The elevated plus-maze apparatus consisted of two open (30 × 5 × 0.25 cm3) and two closed (30 × 5 × 6 cm3) arms emanating from a central platform (5 × 5 cm2) to form a plus shape. The apparatus was made of white plastic and elevated to a height of 60 cm above the floor by a central support. Each mouse was placed onto the central platform without acclimation and allowed 2 min of free exploration in the apparatus. Mouse behavior was recorded under dim red light by a video camera (Sony Digital Handycam 460×) and analyzed using the SMART tracking system (San Diego Instrument, San Diego, CA, USA). Distance traveled, number of entries and duration of time spent in the open versus closed arms were recorded. This test was conducted only in Exp. 1.
Sociability and social novelty preference
As described by Moy et al. (62), mice were first habituated to a 24 × 15.5 in.2 chamber for 5 min. This was followed by a sociability trial (10 min) for which the chamber was divided into three sections (right side, left side, center). In one of the side sections, an object mouse was present in a small (10.2 cm) cage and the other side had an empty cage. In the final social novelty trial (10 min), a novel mouse was placed in the previously empty container. The trials were videotaped and the videos processed with the Topscan software to determine the amount of time spent in each section of the chamber and in the area around the container. The amount of time spent sniffing in the area around the container was also determined by the software. This test was conducted on PND 40 in Exp. 1 and on PND 72 in Exp. 2. In Exp. 2, a sniffing endpoint was added based on the orientation of the test mouse's nose toward the stimulus mouse while the test mouse was in the social area as identified by the video-tracking software.
Motor challenge tests
A battery of tests was administered in a single session with no prior adaptation in the following order: grip strength, wire suspension, mesh pole descent and beam traversal (58). Each test consisted of three trials averaged for analysis. Grip strength was measured by strain gauge (Columbus Instruments). The fore and hind grips were measured separately. Wire suspension: the animal was suspended by its front paws on a taut horizontal wire (2 mm diameter) raised 50 cm from a flat surface between two wooden poles. Latency was recorded as the time to fall or reach a side support (maximum of 2 min). A wire agility rating score of 0–5 (58) was recorded. Mesh pole descent: the animal was placed head down at the top of a wooden pole covered with a wire mesh (3 cm diameter × 82 cm in length). Latency was recorded as the time to reach the bottom of the pole. Beam traversal: mice were tested for the ability to maintain balance while crossing a 10 mm round beam elevated 80 cm above a flat surface. The animal was placed a body length away from one end of the beam, which was fitted with a barrier. The latency for the animal to either reach the platform (all feet on) at the other end of the beam or to fall off the beam was recorded. The number of foot faults (paw not placed firmly on the beam surface) was also recorded.
Morris water maze
The mice received four 90 s trials per day for 4 days in a 90 cm diameter tank to find a 6 × 6 cm2 platform 1 cm below the water surface in 21°C water made opaque with non-toxic paint powder, using room cues (54). After reaching the platform or after 90 s elapsed, the mouse remained on the platform for 30 s and then was removed to a warming cage for 10–15 min inter-trial interval. Training was preceded by a visible trial test (90 s) on the first day and followed by a probe trial (90 s) on the fifth day. Video tracking using the SMART system (PanLabs, US distributor) recorded latency and distance to reach the platform, and time spent in each platform quadrant. Additionally, floating time was estimated by the tester. Mice were tested starting on PND 50–54 in Exp. 1 and PND 79–83 in Exp. 2.
Neurological scoring
In this test, based on reference (31), mice were observed and rated for mobility, gait, posture in response to suspension by the tail, tremor, breathing and general condition. This test was conducted only in Exp. 1.
Activity chamber
Activity of individual mice was monitored over a 24 h period beginning at 1600 hours, using a 40.6 × 40.6 × 14.6 cm3 cage with beam break apparatus, with beams at 2.5 cm intervals in the horizontal plane and 8.3 cm in the vertical plane. Data were summarized in 3 min period for measures of distance traveled, rearing (vertical activity), non-locomotor activity and time spent in peripheral and central areas. This test was conducted at the same age in both experiments.
Barrier social interaction
Mice were housed overnight (12 h) in a single compartment (13.5 × 15.0 cm2) of a two-compartment chamber in a separate test room. At the end of this period, a novel mouse was introduced into the cage behind a perforated transparent barrier. A video tracking system (SMART) was used to measure the location and the speed of movement (rest, <2 cm/s; slow, 2–5 cm/s; fast, >5 cm/s) of the subject mouse during a 60 min period. Movement was detected as change in the location of the center of the mouse's body. This test was modified from reference (28). Mice were tested on PND 130 in Exp. 1 and PND 112 in Exp. 2. The chamber was divided into three areas (next to, midway and far from the barrier) for Exp. 1, and into two areas (next to, far from the barrier) for Exp. 2. Only female mice were tested in Exp. 2.
Statistical analyses
All pups surviving to the appropriate age were tested. Exp. 1 was conducted in four successive cohorts of mice. Prior to analysis, endpoints were examined for associations with the test cohort. If association with the endpoint was found (P ≤ 0.10), the cohort was included as a covariate. Most endpoints were analyzed with ANOVA separately by sex, using genotype and drug treatment as independent variables and including the interaction. For tests conducted prior to weaning, the litter was the unit of analysis. If more than one pup per litter had the same sex and genotype, a mean value was obtained for use in the analysis. The pup was the unit of analysis for tests conducted after weaning. RMANOVA or paired t-tests were used for sequential measures in the same mice. Nonparametric analyses were conducted with χ2 or Mann–Whitney tests. In Exp. 2, litter-based statistics were also used for preweaning measures and postweaning tests. Significance for BDE-47 brain levels and for global methylation signal were calculated using Wilcoxon test. Real-time PCR significance was calculated using Student's t-test.
SUPPLEMENTARY MATERIAL
FUNDING
This work was supported by NIH R01ES015171 (J.M.L.), 2R01HD041462 (J.M.L.), ARRA stimulus funds 3R01ES015171-04S1 (J.K.S.), T32002321 (R.W.) and the NIEHS/EPA Center for Children's Environmental Health PO1 ES11269, the US Environmental Protection Agency (US EPA) through the Science to Achieve Results (STAR) program award numbers R833292 and R829388.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank the Murine Behavioral Assessment Laboratory within the UC Davis Mouse Biology Program for behavioral testing, Dr Michael Gonzales and Sarrita Adams for critical comments on the manuscript, and Dr Irva Hertz-Picciotto for helpful discussions.
Conflict of Interest statement. The authors declare no conflict of interest.
REFERENCES
- 1.Rice C.E., Baio J., Van Naarden Braun K., Doernberg N., Meaney F.J., Kirby R.S. A public shealth collaboration for the surveillance of autism spectrum disorders. Paediatr. Perinat. Epidemiol. 2007;21:179–190. doi: 10.1111/j.1365-3016.2007.00801.x. [DOI] [PubMed] [Google Scholar]
- 2.Kim Y.S., Leventhal B.L., Koh Y.J., Fombonne E., Laska E., Lim E.C., Cheon K.A., Kim S.J., Kim Y.K., Lee H., et al. Prevalence of autism spectrum disorders in a total population sample. Am. J. Psychiatry. 2011;168:904–912. doi: 10.1176/appi.ajp.2011.10101532. [DOI] [PubMed] [Google Scholar]
- 3.Hertz-Picciotto I., Delwiche L. The rise in autism and the role of age at diagnosis. Epidemiology. 2009;20:84–90. doi: 10.1097/EDE.0b013e3181902d15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hallmayer J., Cleveland S., Torres A., Phillips J., Cohen B., Torigoe T., Miller J., Fedele A., Collins J., Smith K., et al. Genetic heritability and shared environmental factors among twin pairs with autism. Arch. Gen. Psychiatry. 2011;68:1095–1102. doi: 10.1001/archgenpsychiatry.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.James S.J., Cutler P., Melnyk S., Jernigan S., Janak L., Gaylor D.W., Neubrander J.A. Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism. Am. J. Clin. Nutr. 2004;80:1611–1617. doi: 10.1093/ajcn/80.6.1611. [DOI] [PubMed] [Google Scholar]
- 6.Messer A. Mini-review: polybrominated diphenyl ether (PBDE) flame retardants as potential autism risk factors. Physiol. Behav. 2010;100:245–249. doi: 10.1016/j.physbeh.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 7.Costa L.G., Giordano G. Developmental neurotoxicity of polybrominated diphenyl ether (PBDE) flame retardants. Neurotoxicology. 2007;28:1047–1067. doi: 10.1016/j.neuro.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dingemans M.M., Ramakers G.M., Gardoni F., van Kleef R.G., Bergman A., Di Luca M., van den Berg M., Westerink R.H., Vijverberg H.P. Neonatal exposure to brominated flame retardant BDE-47 reduces long-term potentiation and postsynaptic protein levels in mouse hippocampus. Environ. Health Perspect. 2007;115:865–870. doi: 10.1289/ehp.9860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hertz-Picciotto I., Park H.Y., Dostal M., Kocan A., Trnovec T., Sram R. Prenatal exposures to persistent and non-persistent organic compounds and effects on immune system development. Basic Clin. Pharmacol. Toxicol. 2008;102:146–154. doi: 10.1111/j.1742-7843.2007.00190.x. [DOI] [PubMed] [Google Scholar]
- 10.Suvorov A., Bissonnette C., Takser L., Langlois M.F. Does 2,2′,4,4′-tetrabromodiphenyl ether interact directly with thyroid receptor? J. Appl. Toxicol. 2011;31:179–184. doi: 10.1002/jat.1580. [DOI] [PubMed] [Google Scholar]
- 11.Rusiecki J.A., Baccarelli A., Bollati V., Tarantini L., Moore L.E., Bonefeld-Jorgensen E.C. Global DNA hypomethylation is associated with high serum-persistent organic pollutants in Greenlandic Inuit. Environ. Health Perspect. 2008;116:1547–1552. doi: 10.1289/ehp.11338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baccarelli A., Bollati V. Epigenetics and environmental chemicals. Curr. Opin. Pediatr. 2009;21:243–251. doi: 10.1097/mop.0b013e32832925cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dolinoy D.C., Weidman J.R., Waterland R.A., Jirtle R.L. Maternal genistein alters coat color and protects Avy mouse offspring from obesity by modifying the fetal epigenome. Environ. Health Perspect. 2006;114:567–572. doi: 10.1289/ehp.8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dolinoy D.C., Huang D., Jirtle R.L. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc. Natl Acad. Sci. USA. 2007;104:13056–13061. doi: 10.1073/pnas.0703739104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amir R.E., Van den Veyver I.B., Wan M., Tran C.Q., Francke U., Zoghbi H.Y. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat. Genet. 1999;23:185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- 16.Nagarajan R.P., Hogart A.R., Gwye Y., Martin M.R., Lasalle J.M. Reduced MeCP2 expression is frequent in autism frontal cortex and correlates with aberrant MECP2 promoter methylation. Epigenetics. 2006;1:172–182. doi: 10.4161/epi.1.4.3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skene P.J., Illingworth R.S., Webb S., Kerr A.R., James K.D., Turner D.J., Andrews R., Bird A.P. Neuronal MeCP2 is expressed at near histone-octamer levels and globally alters the chromatin state. Mol. Cell. 2010;37:457–468. doi: 10.1016/j.molcel.2010.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Z.W., Zak J.D., Liu H. MeCP2 is required for normal development of GABAergic circuits in the thalamus. J. Neurophysiol. 2010;103:2470–2481. doi: 10.1152/jn.00601.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martinowich K., Hattori D., Wu H., Fouse S., He F., Hu Y., Fan G., Sun Y.E. DNA methylation-related chromatin remodeling in activity-dependent BDNF gene regulation. Science. 2003;302:890–893. doi: 10.1126/science.1090842. [DOI] [PubMed] [Google Scholar]
- 20.Shahbazian M.D., Sun Y., Zoghbi H.Y. Balanced X chromosome inactivation patterns in the Rett syndrome brain. Am. J. Med. Genet. 2002;111:164–168. doi: 10.1002/ajmg.10557. [DOI] [PubMed] [Google Scholar]
- 21.Ramocki M.B., Zoghbi H.Y. Failure of neuronal homeostasis results in common neuropsychiatric phenotypes. Nature. 2008;455:912–918. doi: 10.1038/nature07457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Samaco R.C., Fryer J.D., Ren J., Fyffe S., Chao H.T., Sun Y., Greer J.J., Zoghbi H.Y., Neul J.L. A partial loss of function allele of methyl-CpG-binding protein 2 predicts a human neurodevelopmental syndrome. Hum. Mol. Genet. 2008;17:1718–1727. doi: 10.1093/hmg/ddn062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samaco R.C., Mandel-Brehm C., Chao H.T., Ward C.S., Fyffe-Maricich S.L., Ren J., Hyland K., Thaller C., Maricich S.M., Humphreys P., et al. Loss of MeCP2 in aminergic neurons causes cell-autonomous defects in neurotransmitter synthesis and specific behavioral abnormalities. Proc. Natl Acad. Sci. USA. 2009;106:21966–21971. doi: 10.1073/pnas.0912257106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dani V.S., Nelson S.B. Intact long-term potentiation but reduced connectivity between neocortical layer 5 pyramidal neurons in a mouse model of Rett syndrome. J. Neurosci. 2009;29:11263–11270. doi: 10.1523/JNEUROSCI.1019-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chao H.T., Chen H., Samaco R.C., Xue M., Chahrour M., Yoo J., Neul J.L., Gong S., Lu H.C., Heintz N., et al. Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature. 2010;468:263–269. doi: 10.1038/nature09582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shahbazian M., Young J., Yuva-Paylor L., Spencer C., Antalffy B., Noebels J., Armstrong D., Paylor R., Zoghbi H. Mice with truncated MeCP2 recapitulate many Rett syndrome features and display hyperacetylation of histone H3. Neuron. 2002;35:243–254. doi: 10.1016/s0896-6273(02)00768-7. [DOI] [PubMed] [Google Scholar]
- 27.Moretti P., Bouwknecht J.A., Teague R., Paylor R., Zoghbi H.Y. Abnormalities of social interactions and home-cage behavior in a mouse model of Rett syndrome. Hum. Mol. Genet. 2005;14:205–220. doi: 10.1093/hmg/ddi016. [DOI] [PubMed] [Google Scholar]
- 28.Moretti P., Levenson J.M., Battaglia F., Atkinson R., Teague R., Antalffy B., Armstrong D., Arancio O., Sweatt J.D., Zoghbi H.Y. Learning and memory and synaptic plasticity are impaired in a mouse model of Rett syndrome. J. Neurosci. 2006;26:319–327. doi: 10.1523/JNEUROSCI.2623-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feng J., Zhou Y., Campbell S.L., Le T., Li E., Sweatt J.D., Silva A.J., Fan G. Dnmt1 and Dnmt3a maintain DNA methylation and regulate synaptic function in adult forebrain neurons. Nat. Neurosci. 2010;13:423–430. doi: 10.1038/nn.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gonzales M.L., LaSalle J.M. The role of MeCP2 in brain development and neurodevelopmental disorders. Curr. Psychiatry Rep. 2010;12:127–134. doi: 10.1007/s11920-010-0097-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guy J., Gan J., Selfridge J., Cobb S., Bird A. Reversal of neurological defects in a mouse model of Rett syndrome. Science. 2007;315:1143–1147. doi: 10.1126/science.1138389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guy J., Hendrich B., Holmes M., Martin J.E., Bird A. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat. Genet. 2001;27:322–326. doi: 10.1038/85899. [DOI] [PubMed] [Google Scholar]
- 33.Chen R.Z., Akbarian S., Tudor M., Jaenisch R. Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nat. Genet. 2001;27:327–331. doi: 10.1038/85906. [DOI] [PubMed] [Google Scholar]
- 34.Tropea D., Giacometti E., Wilson N.R., Beard C., McCurry C., Fu D.D., Flannery R., Jaenisch R., Sur M. Partial reversal of Rett Syndrome-like symptoms in MeCP2 mutant mice. Proc. Natl Acad. Sci. USA. 2009;106:2029–2034. doi: 10.1073/pnas.0812394106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berger-Sweeney J., Arnold A., Gabeau D., Mills J. Sex differences in learning and memory in mice: effects of sequence of testing and cholinergic blockade. Behav. Neurosci. 1995;109:859–873. doi: 10.1037//0735-7044.109.5.859. [DOI] [PubMed] [Google Scholar]
- 36.Defensor E.B., Pearson B.L., Pobbe R.L., Bolivar V.J., Blanchard D.C., Blanchard R.J. A novel social proximity test suggests patterns of social avoidance and gaze aversion-like behavior in BTBR T+ tf/J mice. Behav. Brain Res. 2011;217:302–308. doi: 10.1016/j.bbr.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.von Meyerinck L., Hufnagel B., Schmoldt A., Benthe H.F. Induction of rat liver microsomal cytochrome P-450 by the pentabromo diphenyl ether Bromkal 70 and half-lives of its components in the adipose tissue. Toxicology. 1990;61:259–274. doi: 10.1016/0300-483x(90)90176-h. [DOI] [PubMed] [Google Scholar]
- 38.Wolstenholme J.T., Taylor J.A., Shetty S.R., Edwards M., Connelly J.J., Rissman E.F. Gestational exposure to low dose bisphenol A alters social behavior in juvenile mice. PLoS One. 2011;6:e25448. doi: 10.1371/journal.pone.0025448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang F.F., Cardarelli R., Carroll J., Fulda K.G., Kaur M., Gonzalez K., Vishwanatha J.K., Santella R.M., Morabia A. Significant differences in global genomic DNA methylation by gender and race/ethnicity in peripheral blood. Epigenetics. 2011;6:623–629. doi: 10.4161/epi.6.5.15335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Newman M., Blyth B., Hussey D., Jardine D., Sykes P., Ormsby R. Sensitive quantitative analysis of murine LINE1 DNA methylation using high resolution melt analysis. Epigenetics. 2012;7:92–105. doi: 10.4161/epi.7.1.18815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hellman A., Chess A. Gene body-specific methylation on the active X chromosome. Science. 2007;315:1141–1143. doi: 10.1126/science.1136352. [DOI] [PubMed] [Google Scholar]
- 42.Singer H., Walier M., Nusgen N., Meesters C., Schreiner F., Woelfle J., Fimmers R., Wienker T., Kalscheuer V.M., Becker T., et al. Methylation of L1Hs promoters is lower on the inactive X, has a tendency of being higher on autosomes in smaller genomes and shows inter-individual variability at some loci. Hum. Mol. Genet. 2012;21:219–235. doi: 10.1093/hmg/ddr456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.LaSalle J.M. A genomic point-of-view on environmental factors influencing the human brain methylome. Epigenetics. 2011;6:862–869. doi: 10.4161/epi.6.7.16353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pinto D., Pagnamenta A.T., Klei L., Anney R., Merico D., Regan R., Conroy J., Magalhaes T.R., Correia C., Abrahams B.S., et al. Functional impact of global rare copy number variation in autism spectrum disorders. Nature. 2011;466:368–372. doi: 10.1038/nature09146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee D.H., Jacobs D.R., Jr., Porta M. Hypothesis: a unifying mechanism for nutrition and chemicals as lifelong modulators of DNA hypomethylation. Environ. Health Perspect. 2009;117:1799–1802. doi: 10.1289/ehp.0900741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.James S.J., Melnyk S., Jernigan S., Hubanks A., Rose S., Gaylor D.W. Abnormal transmethylation/transsulfuration metabolism and DNA hypomethylation among parents of children with autism. J. Autism Dev. Disord. 2008;38:1976. doi: 10.1007/s10803-008-0614-2. [DOI] [PubMed] [Google Scholar]
- 47.Schroeder D.I., Lott P., Korf I., Lasalle J.M. Large-scale methylation domains mark a functional subset of neuronally expressed genes. Genome Res. 2011;10:1583–1591. doi: 10.1101/gr.119131.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ghoshal K., Li X., Datta J., Bai S., Pogribny I., Pogribny M., Huang Y., Young D., Jacob S.T. A folate- and methyl-deficient diet alters the expression of DNA methyltransferases and methyl CpG binding proteins involved in epigenetic gene silencing in livers of F344 rats. J. Nutr. 2006;136:1522–1527. doi: 10.1093/jn/136.6.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.LaPlant Q., Vialou V., Covington H.E., III, Dumitriu D., Feng J., Warren B.L., Maze I., Dietz D.M., Watts E.L., Iniguez S.D., et al. Dnmt3a regulates emotional behavior and spine plasticity in the nucleus accumbens. Nat. Neurosci. 2010;13:1137–1143. doi: 10.1038/nn.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu H., Coskun V., Tao J., Xie W., Ge W., Yoshikawa K., Li E., Zhang Y., Sun Y.E. Dnmt3a-dependent nonpromoter DNA methylation facilitates transcription of neurogenic genes. Science. 2010;329:444–448. doi: 10.1126/science.1190485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chestnut B.A., Chang Q., Price A., Lesuisse C., Wong M., Martin L.J. Epigenetic regulation of motor neuron cell death through DNA methylation. J. Neurosci. 2011;31:16619–16636. doi: 10.1523/JNEUROSCI.1639-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hertz-Picciotto I., Bergman A., Fangstrom B., Rose M., Krakowiak P., Pessah I., Hansen R., Bennett D.H. Polybrominated diphenyl ethers in relation to autism and developmental delay: a case-control study. Environ. Health. 2011;10:1. doi: 10.1186/1476-069X-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harley K.G., Marks A.R., Chevrier J., Bradman A., Sjodin A., Eskenazi B. PBDE concentrations in women's serum and fecundability. Environ. Health Perspect. 2010;118:699–704. doi: 10.1289/ehp.0901450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ta T.A., Koenig C.M., Golub M.S., Pessah I.N., Qi L., Aronov P.A., Berman R.F. Bioaccumulation and behavioral effects of 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47) in perinatally exposed mice. Neurotoxicol. Teratol. 2011;33:393–404. doi: 10.1016/j.ntt.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Feng J., Zhou Y., Campbell S.L., Le T., Li E., Sweatt J.D., Silva A.J., Fan G. Dnmt1 and Dnmt3a maintain DNA methylation and regulate synaptic function in adult forebrain neurons. Nat. Neurosci. 2010;13:423–430. doi: 10.1038/nn.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Braunschweig D., Simcox T., Samaco R.C., LaSalle J.M. X-Chromosome inactivation ratios affect wild-type MeCP2 expression within mosaic Rett syndrome and Mecp2−/+ mouse brain. Hum. Mol. Genet. 2004;13:1275–1286. doi: 10.1093/hmg/ddh142. [DOI] [PubMed] [Google Scholar]
- 57.Kenet T., Froemke R.C., Schreiner C.E., Pessah I.N., Merzenich M.M. Perinatal exposure to a noncoplanar polychlorinated biphenyl alters tonotopy, receptive fields, and plasticity in rat primary auditory cortex. Proc. Natl Acad. Sci. USA. 2007;104:7646–7651. doi: 10.1073/pnas.0701944104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Golub M.S., Germann S.L., Lloyd K.C. Behavioral characteristics of a nervous system-specific erbB4 knock-out mouse. Behav. Brain Res. 2004;153:159–170. doi: 10.1016/j.bbr.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 59.Wahlsten D. A developmental time scale for postnatal changes in brain and behavior of B6D2F2 mice. Brain Res. 1974;72:251–264. doi: 10.1016/0006-8993(74)90863-4. [DOI] [PubMed] [Google Scholar]
- 60.Jaubert P.J., Golub M.S., Lo Y.Y., Germann S.L., Dehoff M.H., Worley P.F., Kang S.H., Schwarz M.K., Seeburg P.H., Berman R.F. Complex, multimodal behavioral profile of the Homer1 knockout mouse. Genes Brain Behav. 2007;6:141–154. doi: 10.1111/j.1601-183X.2006.00240.x. [DOI] [PubMed] [Google Scholar]
- 61.Berman R.F., Pessah I.N., Mouton P.R., Mav D., Harry J. Low-level neonatal thimerosal exposure: further evaluation of altered neurotoxic potential in SJL mice. Toxicol. Sci. 2008;101:294–309. doi: 10.1093/toxsci/kfm265. [DOI] [PubMed] [Google Scholar]
- 62.Moy S.S., Nadler J.J., Perez A., Barbaro R.P., Johns J.M., Magnuson T.R., Piven J., Crawley J.N. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3:287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.