Abstract
We recently reported increased mitochondrial fission and decreased fusion, increased amyloid beta (Aβ) interaction with the mitochondrial fission protein Drp1, increased mitochondrial fragmentation, impaired axonal transport of mitochondria and synaptic degeneration in neurons affected by AD. In the present study, we extended our previous investigations to determine whether phosphorylated tau interacts with Drp1 and to elucidate mitochondrial damage in the progression of AD. We also investigated GTPase activity, which is critical for mitochondrial fragmentation, in postmortem brain tissues from patients with AD and brain tissues from APP, APP/PS1 and 3XTg.AD mice. Using co-immunoprecipitation and immunofluorescence analyses, for the first time, we demonstrated the physical interaction between phosphorylated tau and Drp1. Mitochondrial fission-linked GTPase activity was significantly elevated in the postmortem frontal cortex tissues from AD patients and cortical tissues from APP, APP/PS1 and 3XTg.AD mice. On the basis of these findings, we conclude that Drp1 interacts with Aβ and phosphorylated tau, likely leading to excessive mitochondrial fragmentation, and mitochondrial and synaptic deficiencies, ultimately possibly leading to neuronal damage and cognitive decline. Treatment designed to reduce the expression of Drp1, Aβ and/or phosphorylated tau may decrease the interaction between Drp1 and phosphorylated tau and the interaction between Drp1 and Aβ, conferring protection to neurons from toxic insults of excessive Drp1, Aβ and/or phosphorylated tau.
INTRODUCTION
Alzheimer's disease (AD) is an age-related, progressive, neurodegenerative disorder characterized by memory loss and multiple cognitive impairments (1). Worldwide, 36 million people older than 65 years are living with dementia, with numbers in this age group expecting to double to 66 million by 2030 and increase to 115 million by 2050 (2). With the lifespan of humans increasing, AD—already a significant health concern—will likely become even a greater concern. In addition to the personal and family hardships that AD creates, the numbers of current and expected patients with AD will translate into extremely high health-care costs. According to 2010 estimates, worldwide dementia is currently costing $604 billion annually. Histopathological investigations of AD brains have revealed changes in the brain, characterized as synaptic loss, mitochondrial abnormalities and inflammatory responses, in addition to extracellular amyloid beta (Aβ) deposits and intracellular neurofibrillary tangles (NFTs) in learning and memory regions of the brain (3–6).
The intraneuronal accumulation of Aβ is a key factor that triggers multiple cellular changes in the pathogenesis of AD. Intraneuronal Aβ precedes Aβ production and deposition, and NFT formation in the brains of AD patients and mice that were modeled for AD (7). In AD brains, intraneuronal levels of Aβ are controlled by the production, clearance and degradation of Aβ. Another factor involved in AD pathogenesis is the hyperphosphorylation of tau, a microtubule-associated protein, in brains of patients with AD. The hyperphosphorylation of tau has been found to be triggered by intraneuronal Aβ (8). However, the precise link between Aβ and tau hyperphosphorylation, and NFT formation is not well understood.
Tau hyperphosphorylation and NFT formations are late-stage events in AD progression (9–11). Recent research revealed that several factors might be involved in tau hyperphosphorylation, including Aβ-mediated caspase activation, Aβ-mediated oxidative stress, chronic oxidative stress, reduced insulin-like growth factor 1-mediated oxidative stress and mutations in the tau gene (7). NFTs are composed of hyperphosphorylated forms of tau, which is normally, abundantly present in the central nervous system and is predominantly expressed in neuronal axons (12). Normal tau performs several cellular functions, including stabilization of microtubules, promotion of neurite outgrowth, membrane interactions, facilitation of enzyme anchoring and facilitation of the transport of organelles from axons to nerve terminals (13).
In AD, tau is hyperphosphorylated, accumulates in neurons and forms paired, helical filaments. Owing to hyperphosphorylation, tau loses its capability to bind with microtubules, which ultimately leads to neurodegeneration (14). Further, over-expressed normal tau and/or hyperphosphorylated tau has also been found to impair axonal transport of mitochondria and the abnormal distribution of mitochondria in AD neurons (15,16).
Increasing evidence suggests that abnormal mitochondrial dynamics, such as increased fission and decreased fusion, are early and key factors that have been found in neurodegenerative diseases, such as Alzheimer's, Huntington's, Parkinson's and amyotrophic lateral sclerosis (17–30). In addition, these abnormal mitochondrial dynamics have been associated with abnormal changes in the structure of mitochondria. These abnormal structural changes in mitochondria have been found to be due to an imbalance in highly conserved, GTPase genes that are essential for mitochondrial fission and mitochondrial fusion. GTPase genes—dynamin-related protein 1 (Drp1), fission 1 (Fis1), mitofusins 1 and 2 (Mfn1, Mfn2) and optic atrophy 1 (Opa1)—regulate, maintain and remodel mammalian mitochondria (31–33).
Using a large number of well-characterized postmortem brain specimens from AD patients and age-matched control subjects, we recently studied mitochondrial structural genes in order to determine mitochondrial dynamics in AD-affected neurons (22). We found increased expression of Drp1 and Fis1, decreased expression of Mfn1, Mfn2, Opa1 and Tomm40, and increased expression of the matrix gene CypD. Our findings suggest that abnormal mitochondrial dynamics increase as AD progresses. Further, using these same brain specimens from AD patients, we investigated whether mitochondrial proteins interact with Aβ in AD progression. Immunoprecipitation (IP) of Drp1 and western blot analysis of the Aβ antibodies 6E10 and A11 revealed that Drp1 interacts with Aβ monomers and oligomers. Immunofluorescence analysis using Drp1 antibody and 6E10 and A11 revealed the colocalization of Drp1 and Aβ and increased abnormal interactions between Drp1 and Aβ as AD progressed. However, it is unclear whether phosphorylated tau, as a major pathological hallmark of AD, interacts with Drp1 and is involved in mitochondrial dysfunction.
In the current study, we studied postmortem brains from AD patients and brain tissues from mice that were modeled for AD, APP transgenic mice (34), APP/PS1 mice (35) and 3XTg.AD mice (36), in order to determine the interaction between Drp1, phosphorylated tau and GTPase activity, which is critical for mitochondrial fragmentation in AD.
RESULTS
Normal and phosphorylated tau in postmortem brain specimens from AD patients and 3XTg.AD mice
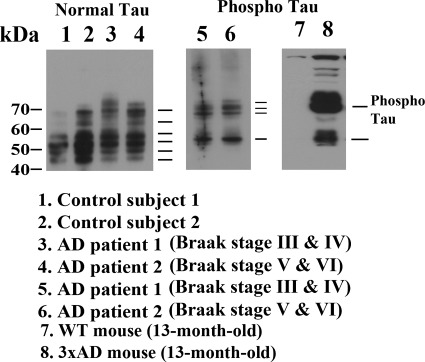
We studied postmortem cortical tissues from definite and severe AD patients and control subjects, 13-month-old 3XTg.AD mice and wild-type (WT) mice, using normal tau and phosphorylated tau-specific antibodies. As shown in Figure 1, six bands of tau were found in AD patients and control subjects (lanes 1–4). Tau was phosphorylated in the definite (Braak stages III and IV) and severe (Braak stages V and VI) AD patients (lanes 5 and 6) and in the 13-month-old 3XTg.AD mice (lane 8), but not in the WT mice (lane 7). These results confirm earlier findings that mutant tau is phosphorylated in 3XTg.AD mice, whereas normal tau is heavily phosphorylated in AD patients (even in the absence of tau mutation).
Figure 1.
A representative western blot analysis of normal and phosphorylated tau proteins in brain tissues from early (Braak stages I and II, n = 5), definite (Braak stages III and IV, n = 5) and severe (Braak stages V and VI, n = 5) AD patients, control subjects (Braak stage 0, n = 5) and 3XTg.AD mice (n = 5) and WT mice (n = 5). Normal tau is phosphorylated in AD patients and 3XTg.AD mice.
Interaction of hyperphosphorylated tau with Drp1 in AD patients
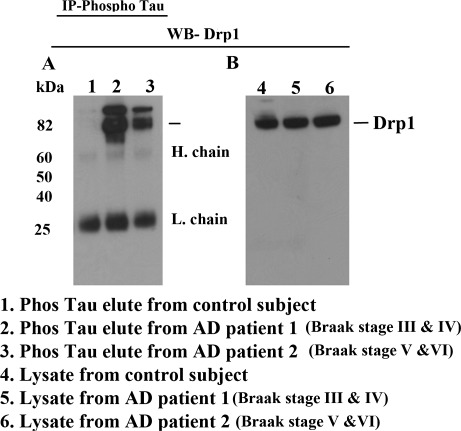
To determine whether hyperphosphorylated tau interacts with Drp1, we conducted co-IP analysis, using cortical protein lysates from postmortem brain tissues of AD definite and severe AD patients and of age-matched control subjects. We also conducted co-IP using the Drp1 antibody and western blot with the phosphorylated tau antibody. As shown in Figure 2A, we found several bands of phosphorylated tau in Drp1-IP elutes from AD patients (lanes 2 and 3) and further band intensity increased with disease progression in AD patients. Our western blot analysis of cortical protein lysates revealed several bands of phosphorylated tau in definite and severe AD patients (lanes 5 and 6) but not in the control subjects (lane 4) (Fig. 2B), indicating that the tau antibody (PHF-S396) is specific for the phosphorylated tau protein in AD patients.
Figure 2.
Co-IP analysis of Drp1 and phosphorylated tau in AD patients. (A) Results from IP with a Drp1 antibody and from western blot analysis with a phosphorylated tau antibody. Phosphorylated tau was found in Drp1-IP elutes from AD patients. (B) Western blot analysis of protein lysates from AD patients and control subjects, using the Drp1 antibody.
To verify our co-IP results and our western blot results, we performed IP with phosphorylated tau and western blot with the Drp1 antibody. As shown in Figure 3A, phosphorylated tau-IP elutes from AD patients (lanes 2 and 3) revealed a 82 kDa Drp1 protein, further confirming the interaction between Drp1 and phosphorylated tau in AD patients. Our western blot analysis of lysates from AD patients (lanes 5 and 6) and control subjects (lane 4) using the Drp1-specific antibody found a 82 kDa band, indicating that the 82 kDa band is specific for Drp1 (Fig. 3B).
Figure 3.
Co-IP analysis of phosphorylated tau and Drp1 in AD patients. (A) IP results with a phosphorylated tau antibody and results from western blot analysis with the Drp1 antibody. Drp1 was found in phosphorylated tau-IP elutes in the cortical tissues from AD patients. (B) Western blots of protein lysates from AD patients and control subjects, using the phosphorylated tau antibody.
Interaction of hyperphosphorylated tau with Drp1 in APP/PS1 and 3XTg.AD mice
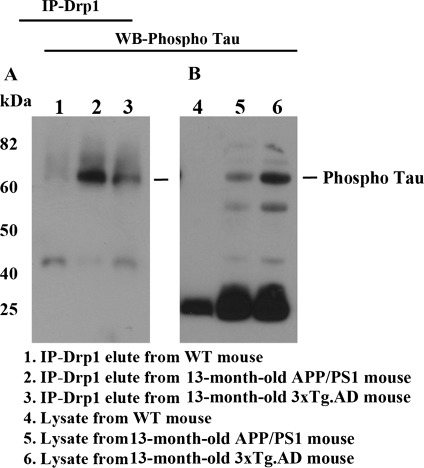
We also conducted co-IP analysis, using cortical protein lysates from brain tissues of APP/PS1 mice and 3XTg.AD mice and of age-matched WT mice, to confirm findings of Drp1 interaction with phosphorylated tau in AD patients. As shown in Figure 4A, we found phosphorylated tau in Drp1 IP-elutes from the APP/PS1 and 3XTg.AD mice (lanes 2 and 3). Our western blot analysis of cortical protein lysates revealed several bands of phosphorylated tau in the APP/PS1 and 3XTg.AD mice (lanes 5 and 6) but not in the WT mice (Fig. 4B), further confirming Drp1 interaction with phosphorylated tau in AD patients.
Figure 4.
Co-IP analysis of Drp1 and phosphorylated tau in APP/PS1 and 3XTg.AD mice. (A) IP results with the Drp1 antibody and results from western blot analysis with the phosphorylated tau antibody. Phosphorylated tau was found in Drp1-IP elutes in the cortical tissues from AD patients. (B) Western blot analysis of protein lysates from in APP/PS1 and 3XTg.AD mice, using the Drp1 antibody.
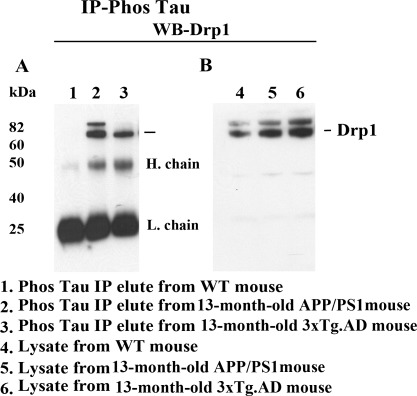
Using cortical proteins from APP/PS1, 3XTg.AD and WT, we also verified co-IP results with the Drp1 antibody and western blot with the phosphorylated tau antibody, using IP with the phosphorylated tau antibody and western blot analysis with the Drp1 antibody. As shown in Figure 5A, we found the 82 kDa Drp1 protein in the IP-tau elutes and in the cortical tissues, in APP/PS1 mice (lane 2) and 3XTg.AD mice (lane 3) but not in the WT mice (lane 1), further confirming the interaction between Drp1 and phosphorylated tau. Western blot findings of protein lysates in APP/PS1 mice (lane 5), 3XTg.AD mice (lane 6) and WT mice (lane 4) showed a band of the 82 kDa protein, indicating increased Drp1 in the APP/PS1 and 3XTg.AD mice, compared with the level of Drp1 in the WT mice (Fig. 5B).
Figure 5.
Co-IP analysis of phosphorylated tau and Drp1 in APP/PS1 and 3XTg.AD mice. (A) IP results with the phosphorylated tau antibody and results from western blot analysis with the Drp1 antibody. Drp1 was found in phosphorylated tau-IP elutes, in the cortical tissues from AD patients. (B) Results from western blot analysis of protein lysates from APP/PS1 and 3XTg.AD mice, using phosphorylated tau antibody.
Double-labeling immunofluorescence analysis of Drp1 and phosphorylated tau in AD brains
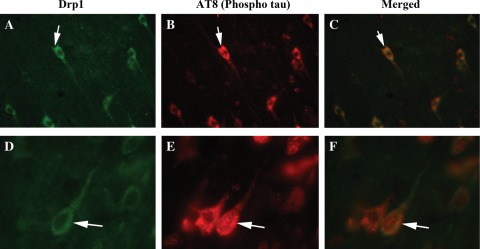
To determine whether Drp1 localizes and interacts with phosphorylated tau, we conducted double-labeling analysis of Drp1 and phosphorylated tau, using specimens from frontal cortex sections of AD patients. As shown in Figure 6, the immunoreactivity of Drp1 was colocalized with phosphorylated tau, indicating that Drp1 interacts with phosphorylated tau. These observations matched our IP findings of Drp1 and phosphorylated tau (Figs 2 and 3). Further, not all of the Drp1 immunoreactive neurons were positive with the phosphorylated tau antibody, indicating that Drp1 may selectively interact with phosphorylated tau-positive neurons in AD patients.
Figure 6.
Double-labeling immunofluorescence analysis of phosphorylated tau and Drp1 in AD patients. The localization of (A) Drp1 and (B) phosphorylated tau, and (C, merged) the colocalization of Drp1 and phosphorylated tau at 40× the original magnification. (D) Images of Drp1, (E) phosphorylated tau and (F) merged at 100× the original magnification. Arrows indicate localization of phosphorylated tau, Drp1 and colocalization of both.
Double-labeling immunofluorescence analysis of Drp1 and phosphorylated tau in 3XTg.AD mice
To determine whether Drp1 localizes and interacts with phosphorylated tau, we conducted double-labeling analysis of Drp1 and phosphorylated tau in cortex and hippocampal sections from 3XTg.AD mice. As shown in Figure 7, the immunoreactivity of Drp1 was colocalized with phosphorylated tau immunoreactivity, indicating that Drp1 interacts with phosphorylated tau. These results agreed with our IP findings of Drp1 and phosphorylated tau (Figs 4 and 5). Similar to sections from AD brain, we found not all Drp1 immunoreactive neurons were positive with the phosphorylated tau antibody, indicating that Drp1 selectively interacts with phosphorylated tau-positive neurons in the 3XTg.AD mice.
Figure 7.
Double-labeling immunofluorescence analysis of phosphorylated tau and Drp1 in 3XTg.AD mice. The localization of (A) Drp1 and (B) phosphorylated tau, and (C, merged) the colocalization of Drp1 and phosphorylated tau in the cortex and hippocampus of AD patients; images taken at 40× the original magnification; images of Drp1 and phosphorylated tau were merged at 100× the original magnification. Arrows indicate localization of phosphorylated tau, Drp1 and colocalization of both.
GTPase activity in AD patients, and APP, APP/PS1 and 3XTg.AD mice
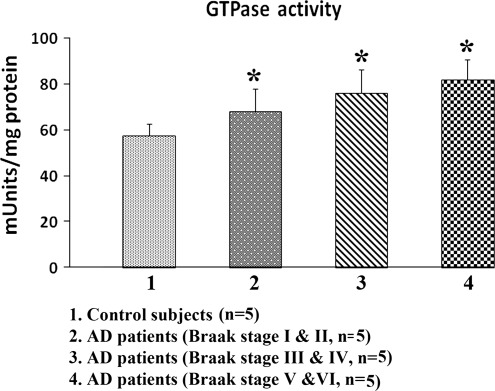
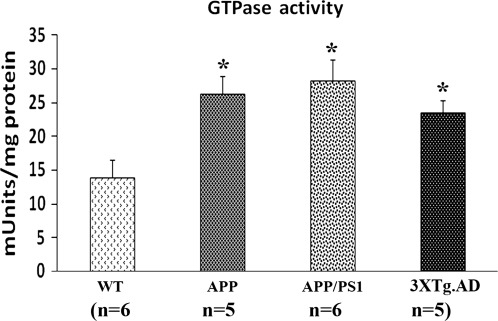
In an earlier study, we found that Drp1 interacted with Aβ in brain specimens from patients with AD brains and from APP transgenic mice (22). In the current study, we found that Drp1 interacted with hyperphosphorylated tau brain specimens from patients with AD brains and from APP/PS1 and 3XTg.AD mice. We measured GTPase enzymatic activity in all brain specimens to study, and we studied GTPase enzymatic activity in cortex tissues from AD patients and control subjects, and from 13-month-old APP, APP/PS1 and 3XTg.AD mice. As shown in Figure 8, we found significantly increased GTPase enzymatic activity in the cortex tissues from early (Braak stages I and II), definite (Braak stages III and IV) and severe (Braak stages V and VI) AD patients, relative to the control subjects (Braak stage 0) (P < 0.05). We also found significantly increased levels of GTPase enzymatic activity in the cerebral cortex tissues from the 13-month-old APP/PS1 and 3XTg.AD mice, relative to the age-matched WT mice (cortex, P < 0.05) (Fig. 9), indicating that increased interaction of hyperphosphorylated and Aβ with Drp1 is associated with enhanced GTPase enzymatic activity, leading to excessive fragmentation of mitochondria in AD neurons.
Figure 8.
GTPase enzymatic activity in cortical tissues of postmortem brains from AD patients—early AD, Braak stages I and II (n = 5), definite AD, Braak stages III and IV (n = 5), severe AD, Braak stage (n = 5) and control subjects (n = 5). GTPase enzymatic activity was significantly decreased in AD patients relative to control subjects.
Figure 9.
GTPase enzymatic activity in cortical tissues from cortical tissues from the APP (n = 6), APP/PS1 mice (n = 6), 3XTg.AD mice (n = 5) and WT mice (n = 6). GTPase enzymatic activity was significantly increased in the APP, APP/PS1 mice and 3XTg.AD mice relative to WT, non-transgenic mice.
DISCUSSION
In neurons affected by AD in patients, we recently reported abnormal mitochondrial dynamics (increased fission and decreased fusion), Aβ interaction with mitochondrial fission protein Drp1, increased mitochondrial fragmentation and impaired axonal transport and synaptic degeneration. These abnormalities increased with disease progression (21–25). In the current study, using postmortem AD brains and brain tissues from APP, APP/PS1 and 3XTg.AD mice, we extended our previous investigations to determine whether phosphorylated tau interacts with mitochondrial protein Drp1, possibly exacerbating disease progression in AD. We also investigated GTPase activity in brain specimens from patients with AD and from APP, APP/PS1 and 3XTg.AD mice. We found that phosphorylated tau interacted with Drp1 in postmortem cortical tissues from definite and severe AD patients, and this interaction occurred mainly in a late stage of disease progression. We also found GTPase activity elevated in the brain tissue from AD patients, and APP, APP/PS1 and 3XTg.AD mice. On the basis of these findings, it appears that the interaction between Drp1 and hyperphosphorylated tau exacerbates mitochondrial and synaptic deficiencies, ultimately leading to neuronal damage and cognitive decline in AD.
Tau phosphorylation in AD
In the current study, we investigated tau proteins in normal and phosphorylation states in brain specimens from AD patients and control subjects, and from 3XTg.AD and WT mice. In the adult human brain, normal tau has six isoforms (10). The normal tau antibody that we used had been identified six bands in the brains from AD patients and control subjects. However, the phosphorylated tau antibody that we used identified higher bands of phosphorylated tau, mainly in the brain specimens from AD patients (Fig. 1). In brain tissues from 13-month-old 3XTg.AD mice, we found tau to be a much more robust, hyperphosphorylated tau than in patients with AD. This hyperphosphorylated tau might be due to over-expression of mutant tau in the 3XTg.AD mice. Further, the pattern of tau phosphorylation in these mice was also different from that in human AD brains.
Interaction of Drp1 with phosphorylated tau in AD
In brain specimens from patients with AD, we recently found that Aβ interacted with Drp1, increased mitochondrial fragmentation, impaired axonal transport and impaired synaptic degeneration and that these characteristics increased with disease progression (21–25). However, it was unclear whether phosphorylated tau interacts with Drp1 and whether this interaction promotes mitochondrial fragmentation and neuronal damage in AD. In the present study, using co-IP and immunofluorescence analyses, we found such interaction and we also found mitochondrial fragmentation and neuronal damage. We observed a robust abnormal interaction in brain specimens from patients with definite and severe AD, and we found little or no such interaction in control subjects. Similarly, we observed abnormal interaction between phosphorylated tau and Drp1 in brain specimens from 13-month-old 3XTg.AD and APP/PS1 mice, and little or no interaction in 3-month-old 3XTg.AD and APP/PS1 mice (data not shown). These findings clearly suggest that Drp1 interacts with phosphorylated tau.
Tau phosphorylation and NFT formation are late-stage events in the disease progression in patients with AD and in 3XTg.AD mice (36,37). It appears that an accumulation of phosphorylated tau is an important factor in Drp1-phosphorylated tau interaction and in formation of the Drp1-phosphorylated tau complex. The interaction between Drp1 and phosphorylated tau may exacerbate mitochondrial fragmentation and synaptic and neuronal damage in neurons affected by AD, and may exacerbate the interaction between Aβ and Drp1, resulting in excessive mitochondrial fragmentation (21–25).
As described in previous studies, the production of Aβ and the subsequent accumulation of intraneuronal tau led to abnormal mitochondrial dynamics (increased fission and decreased fusion) and mitochondrial dysfunction in AD neurons (17–25). Further, that Aβ-induced abnormal mitochondrial dynamics is an early event in AD has been supported by several transmission electron microscopy studies, which revealed excessive mitochondrial fragmentation in primary neurons from over-expressed Aβ mutation in mice (23), in mouse neuroblastoma cells treated with an exogenous Aβ peptide (21) and in human neuroblastoma cells that overexpress Aβ (swe) mutation (17,18). Further, a recent global gene expression study of APP transgenic mice revealed increased expression of mitochondrially encoded genes in 2-, 5- and 18-month-old AβPP transgenic mice. This elevated mitochondrial gene expression may well be a compensatory response to mitochondrial dysfunction caused by mutant APP and soluble Aβ (38).
The current finding, that phosphorylated tau interacts with Drp1, further supports phosphorylated tau involvement in excessive mitochondrial fragmentation, which in turn may ultimately lead to mitochondrial dysfunction and neuronal damage in AD. Thus, increasing evidence suggests that mitochondrial dysfunction is linked to abnormal tau in AD (39–44). Further, increasing evidence supports the involvement of abnormal tau or hyperphosphorylated tau in impaired axonal transport and in the abnormal localization of mitochondria in AD neurons (15,16). Using neuronal and non-neuronal cell cultures, several research groups have found that tau impairs axonal transport of organelles and impairs APP trafficking and that the depletion of organelles, including mitochondria, causes oxidative stress and reduces ATP in synapses (45–47). It is possible that the interaction between Drp1 and phosphorylated tau may be involved in causing defective axonal transport of mitochondria, mitochondrial dysfunction and oxidative stress in mitochondria.
There are a couple of explanations that may account for the interaction of Drp1 and phosphorylated tau leading to excessive mitochondrial fragmentation, mitochondrial dysfunction and neuronal damage in AD. Two possibilities are that: (i) this interaction is dependent on Aβ-induced oxidative stress, which has been found to be present in neurons with mitochondrial fragmentation and dysfunction, and neuronal damage and (ii) this interaction may be associated with oxidative stress-induced phosphorylated tau. Additional research is needed to investigate these possibilities.
A possible therapeutic strategy for patients with AD: Drp1 or reducing phosphorylated tau and/or Aβ–
Recent knockout Drp1 mice studies revealed that Drp1 homozygote knockout mice (Drp1−/−) have developmental and synaptic abnormalities, particularly in the forebrain, and they die, on average, shortly after embryonic days 11.5–12.5 (48,49). However, heterozygote Drp1+/− knockout mice appear to be normal in terms of lifespan, fertility and viability. Further, Drp1+/− heterozygote knockout mice do not show significant changes in synaptic, dendritic or mitochondrial proteins. In contrast, elongated mitochondria were observed in the brains of Drp1+/− mice, indicating the presence of mitochondrial fusion. Further, significantly reduced hydrogen peroxide and lipid peroxidation levels were observed in the Drp1+/− mice, suggesting reduced oxidative stress in the Drp1+/− mice (50). These findings, taken together, suggest that partial reduction in Drp1 may have beneficial effects and a therapeutic value for patients with AD and patients with other neurodegenerative diseases, such as Huntington's since excessive mitochondrial fragmentation and increased mitochondrial dysfunction and oxidative stress have been reported in their diseased brain tissues (17–28).
From a therapeutic point of view, it may be critical to reduce the production of Aβ and phosphorylated tau to slow or stop AD progression. The reduced production of Aβ and phosphorylated tau may lead to a reduction in the interaction between Drp1 and phosphorylated tau, which may in turn protect neurons from toxic insults resulting from Drp1 and Aβ interactions, and from Drp1 and phosphorylated tau interactions.
MATERIALS AND METHODS
Twenty postmortem brain specimens from AD patients and age-matched control subjects were obtained from the Harvard Tissue Resource Center, as described previously (22). Fifteen specimens were from patients diagnosed with AD at different stages of disease progression, according to Braak criteria (9) and five were from age-matched control subjects. On the basis of quantitative pathological features, including senile plaques, NFTs and neuronal density, the AD brain specimens were classified as coming from AD patients at Braak stages 1 and II (early AD) (n = 5), III and IV (definite AD) (n = 5), V and VI (severe AD) (n = 5) and control subjects (n = 5). The specimens were from the frontal cortex and were both quick-frozen and formalin-fixed (BA9).
APP, APP×PS1 and 3XTg.AD transgenic mice
Using APP (Tg2576 line) (34), APP×PS1 (35) and 3XTg.AD mice (36) and age-matched WT littermates (controls), we studied Drp1 and its relationship to Tau in patients at different stages of AD progression. The mice were housed at the Oregon National Primate Research Center at Oregon Health and Science University (OHSU). The OHSU Institutional Animal Care and Use Committee approved all procedures for animal care, according to guidelines set forth by the National Institutes of Health.
Drp1 and tau antibodies
To characterize phosphorylated tau and Drp1, we used an anti-Drp1 antibody, and normal and anti-normal tau and anti-phosphorylated tau antibodies. The Drp1 antibody was raised against 560–736 amino acid residues of a human Drp1 and this antibody was purchased from Santa Cruz Biotechnology, Inc (Santa Cruz, CA, USA). This antibody reacts with cells from mice, rats and humans. The normal tau that we used was raised against 159–163 amino acid residues of human tau. The tau antibody recognizes normal tau from humans and bovine. We also used phosphorylated tau antibody that raised against a phosphorylated serine, 202 and threonine 205 amino acid residues of human tau and this antibody referred as AT8 in the literature. The phosphorylated tau antibody recognizes a phosphatase-sensitive epitope on PHF-tau. Both tau antibodies were purchased from Pierce Biotechnology, Inc. (Rockford, IL, USA). We also used phosphorylated tau antibody (PHF-Tau, S396) for IP analysis and this antibody was raised a phosphorylated serine, 396 amino acid of human tau, and this antibody was purchased from Abcam (Cambridge, MA, USA).
Western blot analysis of tau proteins
To determine tau phosphorylation in AD patients relative to control subjects, we performed western blot analysis of protein lysates from cortical tissues of AD patients and control subjects, and from 3XTg.AD and non-transgenic control mice, as described in Manczak et al. (51). Briefly, protein lysates were prepared, using a radioimmunoprecipitation buffer (25 mm Tris–HCl, pH 7.6, 150 mm NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate and protease inhibitors 0.5 g/ml pepstatin, 0.5 g/ml leupeptin and 1 mm phenylmethanesulfonylfluoride). Fifty micrograms of protein lysates were resolved on a 4–12% Nu-PAGE gel (Invitrogen, Temicula, CA, USA). The resolved proteins were transferred to polyvinylidene fluoride (PVDF) membranes (Novax, Inc., San Diego, CA, USA) and then were incubated for 1 h at room temperature with a blocking buffer [5% dry milk dissolved in a mixture of Tris-buffered saline and Tween 20 (TBST) buffer]. The PVDF membranes were incubated overnight with the following primary antibodies: anti-Drp1 (rabbit polyclonal 1:500 dilution; Santa Cruz Biotechnology), anti-normal tau antibody (1:200, mouse monoclonal; Pierce Biotechnology, Inc., Rockford, IL, USA) and anti-phosphorylated tau antibody (1:100, mouse monoclonal; Pierce Biotechnology, Inc.). The membranes were washed with a TBST buffer three times at 10 min intervals and then incubated for 2 h with appropriate secondary antibodies, followed by three additional washes at 10 min intervals. Details of the secondary bodies are given in Table 1. Proteins were detected with chemiluminescent reagents (Pierce Biotechnology, Inc.).
Table 1.
Summary of antibody dilutions and conditions used in western blot analysis of Drp1 and tau proteins
| Marker | Primary antibody: species and dilution | Purchased from: company, city, state | Secondary antibody: species and dilution | Purchased from: company, city, state |
|---|---|---|---|---|
| Drp1 | Rabbit Polyclonal 1:500 | Santa Cruz Biotechnology, Inc., Santa Cruz, CA | Donkey anti-rabbit HRP 1:10 000 | GE Healthcare Amersham, Piscataway, NJ |
| PHF-Tau-AT8 (S202/T-205) | Mouse Monoclonal 1:100 | Pierce Biotechnology, Inc., Rockford, IL | Sheep anti-mouse HRP 1:8,000 | GE Healthcare Amersham, Piscataway, NJ |
| Tau HT7 clone | Mouse Monoclonal 1:200 | Pierce Biotechnology, Inc., Rockford, IL | Sheep anti-mouse HRP 1:8,000 | GE Healthcare Amersham, Piscataway, NJ |
| B-actin | Mouse Monoclonal 1:500 | Sigma-Aldrich, St Luis, MO | Sheep anti-mouse HRP 1:10 000 | GE Healthcare Amersham, Piscataway, NJ |
Co-IP of Drp1 and phosphorylated tau
To determine the interaction between Drp1 and phosphorylated tau, we performed co-IP using cortical protein lysates from a large number of brains from AD patients, from control subjects and from 13-month-old 3XAD mice, as described in Manczak et al. (22). We cross-checked the results by performing co-IP experiments using both anti-Drp1 and anti-phosphorylated tau antibodies and conducted western blot analysis using (i) Drp1-IP elutes and phosphorylated tau antibody and (ii) phosphorylated tau-IP elutes and Drp1 antibody. Briefly, we used a Dynabeads Kit for IP (Invitrogen). Fifty microliters of Dynabeads containing protein G was incubated with 10 µg of anti-Drp1 and anti-phosphorylated tau antibodies for 1 h at room temperature, with rotation. Details of secondary antibodies are given in Table 2. The Dynabeads were then washed three times with a washing buffer and incubated with rotation overnight with 400 µg of lysate protein at 4°C. The incubated Dynabead–antigen/antibody complexes were washed again three times with a washing buffer, and an immunoprecipitant was eluted from the Dynabeads, using the NuPAGE LDS sample buffer. The IP elute was loaded onto a gel, followed by western blot analysis of Drp1 and phosphorylated tau antibodies.
Table 2.
Summary of antibody dilutions and conditions used in co-IP of Drp1 and phosphorylated proteins
| Marker | Primary antibody: species and dilution | Purchased from: company, city, state | Secondary antibody: species and dilution | Purchased from: company, city, state |
|---|---|---|---|---|
| PHF-Tau (S396) | Mouse Monoclonal 10 µg/400 µg protein | Abcam, Cambridge, MA | Sheep anti-mouse HRP 1:8,000 | GE Healthcare Amersham, Piscataway, NJ |
| Drp1 | Rabbit Polyclonal 10 µg/400 µg protein | Santa Cruz Biotechnology, Inc., Santa Cruz, CA | Donkey anti-rabbit HRP 1:10 000 | GE Healthcare Amersham, Piscataway, NJ |
Immunohistochemistry/immunofluorescence analysis
Using immunofluorescence techniques, we localized Drp1, phosphorylated tau in frontal cortex specimens (broadman area 9) taken from postmortem brains of 15 patients in early stages of AD (Braak stages I and II) and from postmortem brains of five control subjects (Braak stage 0) as described in our earlier publication (22). Briefly, the brain specimens were paraffin-embedded, and sections were cut into widths of 15μm. We deparaffinized the sections by washing them with xylene for 10 min and then washed them for 5 min each in a serial dilution of alcohol (95, 70 and 50%). The sections were washed once for 10 min with double-distilled H2O and then for six more times at 5 min each, with phosphate-buffered saline (PBS) (pH 7.4). To reduce the autofluorescence of brain specimens, we treated the deparaffinized sections with sodium borohydrate twice each for 30 min in a freshly prepared 0.1% sodium borohydrate solution dissolved in PBS (pH 8.0). We then washed the sections three times for 5 min each, with PBS (pH 7.4). To block the endogenous peroxidase, sections were treated for 15 min with 3% H2O2 and then with 0.5% Triton dissolved in PBS (pH 7.4). The sections were blocked for 1 h with a solution (0.5% Triton in PBS + 10% goat serum + 1% bovine serum albumin). The sections were incubated overnight at room temperature with the following antibodies: anti-Drp1 (rabbit polyclonal 1:100 dilution, Santa Cruz Biotechnology) and anti-phosphorylated tau antibody (1:100, mouse monoclonal; Pierce Biotechnology, Inc.). On the day after the primary antibody incubation, sections were washed once with 0.1% Triton in PBS and then incubated with appropriate biotinylated secondary antibodies for 1 h at room temperature. Details of secondary antibodies are given in Table 3. They were washed with PBS three times for 10 min each and then incubated for 1 h with a horseradish peroxidase (HRP)-conjugated streptavidin solution (Invitrogen). The sections were each washed three more times with PBS (pH 7.4) for 10 min each and then treated with Tyramide Alexa 594 (red) or Alexa 488 (green) (Molecular Probes, Eugene, OR) for 10 min at room temperature. They were cover-slipped with Prolong Gold and photographed with a confocal microscope.
Table 3.
Summary of antibody dilutions and conditions used in immunohistochemistry and immunofluorescence analyses
| Marker | Primary antibody: species and dilution | Purchased from: company, city, state | Secondary antibody: species and dilution | Purchased from: company, city, state |
|---|---|---|---|---|
| Drp1 | Rabbit Polyclonal 1:200 | Santa Cruz Biotechnology, Inc., Santa Cruz, CA | Goat anti-rabbit Biotin 1:300, ABC, TSA-Alexa 488 | KPL, Gaithersburg, MD |
| Vector Laboratories, Inc., Burlingame, CA | ||||
| Molecular Probe, Eugene, OR | ||||
| PHF-Tau | Mouse Monoclonal 1:100 | Pierce Biotechnology, Inc., Rockford, IL | Goat anti-mouse Biotin 1:300, ABC, TSA-Alexa 594 | KPL, Gaithersburg, MD |
| AT-8 | Vector Laboratories, Inc., Burlingame, CA | |||
| S202/T205) | Molecular Probe, Eugene, OR |
Double-labeling immunofluorescence analysis of Drp1 and phosphorylated tau
To determine the interaction between Drp1 and phosphorylated tau, we conducted double-labeling immunofluorescence analysis, using an anti-Drp1 antibody and an anti-phosphorylated tau antibody. As described earlier, postmortem brain sections from patients with AD and control subjects were deparaffinized and treated with sodium borohydrate to reduce autofluorescence. For the first labeling, the sections were incubated overnight with the anti-Drp1 antibody (1:200, rabbit polyclonal; Santa Cruz Biotechnology) at room temperature. On the day after this incubation, the sections were washed with 0.5% Triton in PBS and then incubated with a secondary biotinylated anti-rabbit antibody, at a 1:400 dilution (Vector Laboratories, Burlingame, CA, USA) or with a secondary biotinylated anti-mouse antibody (1:400) for 1 h at room temperature. They were incubated for 1 h in an HRP-conjugated streptavidin solution (Molecular Probes). The sections were then washed three times with PBS, each for 10 min at pH 7.4. They were then treated with Tyramide Alexa488 for 10 min at room temperature. For the second labeling, the sections were incubated overnight with an anti-phosphorylated tau antibody (1:100, mouse monoclonal; Pierce Biotechnology, Inc.) at room temperature. Details of the secondary antibodies are given in Table 3. Next, they were incubated with a donkey, anti-mouse secondary antibody that was labeled with Alexa 594 for 1 h at room temperature. The sections were cover-slipped with Prolong Gold and photographed with a confocal microscope.
GTPase enzymatic activity
GTPase enzymatic activity was measured in frozen cortical tissues from AD patients and control subjects, and APP, PP/PS1 and 3XTg.AD mice and WT mice as described in Manczak et al. (50). In a GTPase assay, GTPase hydrolyzes guanosine triphosphate (GTP) to guanosine diphosphate and to inorganic phosphorous (Pi). Therefore, briefly, in these methods, GTPase activity was measured, based on the amount of inorganic phosphorous that the GTP produced. By adding the ColorLock Gold (orange) substrate to the inorganic phosphorous that was generated from GTP, GTP activity was assessed, based on the inorganic complex solution (green). Calorimetric measurements (green) were read in the wavelength range of 650 nm. GTPase activity was compared in AD patients and in control subjects. Also compared was GTPase enzymatic activity in APP, APP/PS1 mice, 3XTg.AD mice and control mice.
FUNDING
This research was supported by NIH grants AG028072, AG026051, RR000163 (P.H.R.), P30-NS061800 (PI, Aicher) and a grant from the Alzheimer Association (IIRG-09-92429, P.H.R.).
ACKNOWLEDGEMENTS
We thank Dr Anda Cornea (Imaging Core of the OHSU Oregon National Primate Research Center) for her assistance with the confocal microscopy. We also thank Drs Karen Ashe, David Borchelt, Frank LaFerla and Salvatore Oddo for their generous gifts of the APP, APP/PS1 and 3XTg.AD mice.
Conflict of Interest statement. None declared.
References
- 1.Mattson M.P. Pathways towards and away from Alzheimer's disease. Nature. 2004;430:631–639. doi: 10.1038/nature02621. doi:10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Alzheimer Report. 2009.
- 3.Terry R.D., Masliah E., Salmon D.P., Butters N., DeTeresa R., Hill R., Hansen L.A., Katzman R. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. doi:10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 4.Selkoe D.J. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. doi:10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 5.Reddy P.H., Manczak M., Mao P., Calkins M.J., Reddy A.P., Shirendeb U. Amyloid-beta and mitochondria in aging and Alzheimer's disease: implications for synaptic damage and cognitive decline. J. Alzheimers Dis. 2010;20:S499–S512. doi: 10.3233/JAD-2010-100504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Querfurth H.W., LaFerla F.M. Alzheimer's disease. N. Engl. J. Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. doi:10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 7.Reddy P.H. Abnormal tau, mitochondrial dysfunction, impaired axonal transport of mitochondria, and synaptic deprivation in Alzheimer's disease. Brain Res. 2011;1415:136–148. doi: 10.1016/j.brainres.2011.07.052. doi:10.1016/j.brainres.2011.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LaFerla F.M. Pathways linking Abeta and tau pathologies. Biochem. Soc. Trans. 2010;38:993–995. doi: 10.1042/BST0380993. doi:10.1042/BST0380993. [DOI] [PubMed] [Google Scholar]
- 9.Braak H., Braak E. Demonstration of amyloid deposits and neurofibrillary changes in whole brain sections. Brain Pathol. 1991;1:213–216. doi: 10.1111/j.1750-3639.1991.tb00661.x. doi:10.1111/j.1750-3639.1991.tb00661.x. [DOI] [PubMed] [Google Scholar]
- 10.Lee V.M., Goedert M., Trojanowski J.Q. Neurodegenerative tauopathies. Annu. Rev. Neurosci. 2001;24:1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. doi:10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- 11.Selkoe D.J. Alzheimer's disease: genes, proteins, and therapy. Physiol. Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 12.Brandt R., Hundelt M., Shahani N. Tau alteration and neuronal degeneration in tauopathies: mechanisms and models. Biochim. Biophys. Acta. 2005;1739:331–354. doi: 10.1016/j.bbadis.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 13.Ittner L.M., Götz J. Amyloid-β and tau—a toxic pas de deux in Alzheimer's disease. Nat. Rev. Neurosci. 2011;12:65–72. doi: 10.1038/nrn2967. doi:10.1038/nrn2967. [DOI] [PubMed] [Google Scholar]
- 14.Garcia M.L., Cleveland D.W. Going new places using an old MAP: tau, microtubules and human neurodegenerative disease. Curr. Opin. Cell Biol. 2001;13:41–48. doi: 10.1016/s0955-0674(00)00172-1. doi:10.1016/S0955-0674(00)00172-1. [DOI] [PubMed] [Google Scholar]
- 15.Vossel K.A., Zhang K., Brodbeck J., Daub A.C., Sharma P., Finkbeiner S., Cui B., Mucke L. Tau reduction prevents Abeta-induced defects in axonal transport. Science. 2010;330:198. doi: 10.1126/science.1194653. doi:10.1126/science.1194653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kopeikina K.J., Carlson G.A., Pitstick R., Ludvigson A.E., Peters A., Luebke J.I., Koffie R.M., Frosch M.P., Hyman B.T., Spires-Jones T.L. Tau accumulation causes mitochondrial distribution deficits in neurons in a mouse model of tauopathy and in human Alzheimer's disease brain. Am. J. Pathol. 2011;179:2071–2082. doi: 10.1016/j.ajpath.2011.07.004. doi:10.1016/j.ajpath.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X., Su B., Siedlak S.L., Moreira P.I., Fujioka H., Wang Y., Casadesus G., Zhu X. Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc. Natl Acad. Sci. USA. 2008;105:19318–19323. doi: 10.1073/pnas.0804871105. doi:10.1073/pnas.0804871105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X., Su B., Lee H.G., Li X., Perry G., Smith M.A., Zhu X. Impaired balance of mitochondrial fission and fusion in Alzheimer's disease. J. Neurosci. 2009;29:9090–9103. doi: 10.1523/JNEUROSCI.1357-09.2009. doi:10.1523/JNEUROSCI.1357-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X., Perry G., Smith M.A., Zhu X. Amyloid-beta-derived diffusible ligands cause impaired axonal transport of mitochondria in neurons. Neurodegener. Dis. 2010;7:56–59. doi: 10.1159/000283484. doi:10.1159/000283484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X., Su B., Liu W., He X., Gao Y., Castellani R.J., Perry G., Smith M.A., Zhu X. DLP1-dependent mitochondrial fragmentation mediates 1-methyl-4-phenylpyridinium toxicity in neurons: implications for Parkinson's disease. Aging Cell. 2011;10:807–823. doi: 10.1111/j.1474-9726.2011.00721.x. doi:10.1111/j.1474-9726.2011.00721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manczak M., Mao P., Calkins M.J., Cornea A., Reddy A.P., Murphy M.P., Szeto H.H., Park B., Reddy P.H. Mitochondria-targeted antioxidants protect against amyloid-beta toxicity in Alzheimer's disease neurons. J. Alzheimers Dis. 2010;20(suppl. 2):S609–S631. doi: 10.3233/JAD-2010-100564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manczak M., Calkins M.J., Reddy P.H. Impaired mitochondrial dynamics and abnormal interaction of amyloid beta with mitochondrial protein Drp1 in neurons from patients with Alzheimer's disease: implications for neuronal damage. Hum. Mol. Genet. 2011;20:2495–2509. doi: 10.1093/hmg/ddr139. doi:10.1093/hmg/ddr139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calkins M.J., Manczak M., Mao P., Shirendeb U., Reddy P.H. Impaired mitochondrial biogenesis, defective axonal transport of mitochondria, abnormal mitochondrial dynamics and synaptic degeneration in a mouse model of Alzheimer's disease. Hum. Mol. Genet. 2011;20:4515–4529. doi: 10.1093/hmg/ddr381. doi:10.1093/hmg/ddr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calkins M.J., Reddy P.H. Amyloid beta impairs mitochondrial anterograde transport and degenerates synapses in Alzheimer's disease neurons. Biochim. Biophys. Acta. 2011;1812:507–513. doi: 10.1016/j.bbadis.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calkins M.J., Reddy P.H. Assessment of newly synthesized mitochondrial DNA using BrdU labeling in primary neurons from Alzheimer's disease mice: implications for impaired mitochondrial biogenesis and synaptic damage. Biochim. Biophys. Acta. 2011;1812:1182–1189. doi: 10.1016/j.bbadis.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shirendeb U.P., Calkins M.J., Manczak M., Anekonda V., Dufour B., McBride J.L., Mao P., Reddy P.H. Mutant huntingtin's interaction with mitochondrial protein Drp1 impairs mitochondrial biogenesis and causes defective axonal transport and synaptic degeneration in Huntington's disease. Hum. Mol. Genet. 2012;21:406–420. doi: 10.1093/hmg/ddr475. doi:10.1093/hmg/ddr475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shirendeb U., Reddy A.P., Manczak M., Calkins M.J., Mao P., Tagle D.A., Reddy P.H. Abnormal mitochondrial dynamics, mitochondrial loss and mutant huntingtin oligomers in Huntington's disease: implications for selective neuronal damage. Hum. Mol. Genet. 2011;20:1438–1455. doi: 10.1093/hmg/ddr024. doi:10.1093/hmg/ddr024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song W., Chen J., Petrilli A., Liot G., Klinglmayr E., Zhou Y., Poquiz P., Tjong J., Pouladi M.A., Hayden M.R., et al. Mutant huntingtin binds the mitochondrial fission GTPase dynamin-related protein-1 and increases its enzymatic activity. Nat. Med. 2011;17:377–382. doi: 10.1038/nm.2313. doi:10.1038/nm.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Magrané J., Sahawneh M.A., Przedborski S., Estévez A.G., Manfredi G. Mitochondrial dynamics and bioenergetic dysfunction is associated with synaptic alterations in mutant SOD1 motor neurons. J. Neurosci. 2012;32:229–242. doi: 10.1523/JNEUROSCI.1233-11.2012. doi:10.1523/JNEUROSCI.1233-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mórotz G.M., De Vos K.J., Vagnoni A., Ackerley S., Shaw C.E., Miller C.C. Amyotrophic lateral sclerosis-associated mutant VAPBP56S perturbs calcium homeostasis to disrupt axonal transport of mitochondria. Hum. Mol. Genet. 2012 doi: 10.1093/hmg/dds011. 2012 Jan 17. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kageyama Y., Zhang Z., Sesaki H. Mitochondrial division: molecular machinery and physiological functions. Curr. Opin. Cell Biol. 2011;23:427–434. doi: 10.1016/j.ceb.2011.04.009. doi:10.1016/j.ceb.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palmer C.S., Osellame L.D., Stojanovski D., Ryan M.T. The regulation of mitochondrial morphology: intricate mechanisms and dynamic machinery. Cell. Signal. 2011;23:1534–1545. doi: 10.1016/j.cellsig.2011.05.021. doi:10.1016/j.cellsig.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 33.Reddy P.H., Reddy T.P., Manczak M., Calkins M.J., Shirendeb U., Mao P. Dynamin-related protein 1 and mitochondrial fragmentation in neurodegenerative diseases. Brain Res. Rev. 2011;67:103–118. doi: 10.1016/j.brainresrev.2010.11.004. doi:10.1016/j.brainresrev.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsiao K., Chapman P., Nilsen S., Eckman C., Harigaya Y., Younkin S., Yang F., Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. doi:10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 35.Borchelt D.R., Thinakaran G., Eckman C.B., Lee M.K., Davenport F., Ratovitsky T., Prada C.M., Kim G., Seekins S., Yager D., et al. Familial Alzheimer's disease-linked presenilin 1 variants elevate Abeta1–42/1–40 ratio in vitro and in vivo. Neuron. 1996;17:1005–1013. doi: 10.1016/s0896-6273(00)80230-5. doi:10.1016/S0896-6273(00)80230-5. [DOI] [PubMed] [Google Scholar]
- 36.Oddo S., Caccamo A., Shepherd J.D., Murphy M.P., Golde T.E., Kayed R., Metherate R., Mattson M.P., Akbari Y., LaFerla F.M. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. doi:10.1016/S0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 37.LaFerla F.M., Oddo S. Alzheimer's disease: Abeta, tau and synaptic dysfunction. Trends Mol. Med. 2005;11:170–176. doi: 10.1016/j.molmed.2005.02.009. doi:10.1016/j.molmed.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 38.Reddy P.H., McWeeney S., Park B.S., Manczak M., Gutala R.V., Partovi D., Jung Y., Yau V., Searles R., Mori M., Quinn J. Gene expression profiles of transcripts in amyloid precursor protein transgenic mice: up-regulation of mitochondrial metabolism and apoptotic genes is an early cellular change in Alzheimer's disease. Hum. Mol. Genet. 2004;13:1225–1240. doi: 10.1093/hmg/ddh140. doi:10.1093/hmg/ddh140. [DOI] [PubMed] [Google Scholar]
- 39.Drago D., Cavaliere A., Mascetra N., Ciavardelli D., di Ilio C., Zatta P., Sensi S.L. Aluminum modulates effects of beta amyloid(1–42) on neuronal calcium homeostasis and mitochondria functioning and is altered in a triple transgenic mouse model of Alzheimer's disease. Rejuvenation Res. 2008;11:861–871. doi: 10.1089/rej.2008.0761. doi:10.1089/rej.2008.0761. [DOI] [PubMed] [Google Scholar]
- 40.Sensi S.L., Rapposelli I.G., Frazzini V., Mascetra N. Altered oxidant-mediated intraneuronal zinc mobilization in a triple transgenic mouse model of Alzheimer's disease. Exp. Gerontol. 2008;43:488–492. doi: 10.1016/j.exger.2007.10.018. doi:10.1016/j.exger.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 41.Yao J., Irwin R., Zhao L., Nilsen J., Hamilton R.T., Brinton R.D. Mitochondrial bioenergetic deficit precedes Alzheimer's pathology in female mouse model of Alzheimer's disease. Proc. Natl Acad. Sci. USA. 2009;106:14670–14675. doi: 10.1073/pnas.0903563106. doi:10.1073/pnas.0903563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Resende R., Moreira P.I., Proença T., Deshpande A., Busciglio J., Pereira C., Oliveira C.R. Brain oxidative stress in a triple-transgenic mouse model of Alzheimer disease. Free Radic. Biol. Med. 2008;44:2051–2057. doi: 10.1016/j.freeradbiomed.2008.03.012. doi:10.1016/j.freeradbiomed.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 43.Atlante A., Amadoro G., Bobba A., de Bari L., Corsetti V., Pappalardo G., Marra E., Calissano P., Passarella S. A peptide containing residues 26–44 of tau protein impairs mitochondrial oxidative phosphorylation acting at the level of the adenine nucleotide translocator. Biochim. Biophys. Acta. 2008;17771:1289–1300. doi: 10.1016/j.bbabio.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 44.Quintanilla R.A., Matthews-Roberson T.A., Dolan P.J., Johnson G.V. Caspase-cleaved tau expression induces mitochondrial dysfunction in immortalized cortical neurons: implications for the pathogenesis of Alzheimer disease. J. Biol. Chem. 2009;284:18754–18766. doi: 10.1074/jbc.M808908200. doi:10.1074/jbc.M808908200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mandelkow E.M., Stamer K., Vogel R., Thies E., Mandelkow E. Clogging of axons by tau, inhibition of axonal traffic and starvation of synapses. Neurobiol. Aging. 2003;24:1079–1085. doi: 10.1016/j.neurobiolaging.2003.04.007. doi:10.1016/j.neurobiolaging.2003.04.007. [DOI] [PubMed] [Google Scholar]
- 46.Stamer K., Vogel R., Thies E., Mandelkow E., Mandelkow E.M. Tau blocks traffic of organelles, neurofilaments, and APP vesicles in neurons and enhances oxidative stress. J. Cell Biol. 2002;156:1051–1063. doi: 10.1083/jcb.200108057. doi:10.1083/jcb.200108057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dubey M., Chaudhury P., Kabiru H., Shea T.B. Tau inhibits anterograde axonal transport and perturbs stability in growing axonal neurites in part by displacing kinesin cargo: neurofilaments attenuate tau-mediated neurite instability. Cell Motil. Cytoskeleton. 2008;65:89–99. doi: 10.1002/cm.20243. doi:10.1002/cm.20243. [DOI] [PubMed] [Google Scholar]
- 48.Ishihara N., Nomura M., Jofuku A., Kato H., Suzuki S.O., Masuda K., Otera H., Nakanishi Y., Nonaka I., Goto Y., et al. Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nat. Cell Biol. 2009;11:958–966. doi: 10.1038/ncb1907. doi:10.1038/ncb1907. [DOI] [PubMed] [Google Scholar]
- 49.Wakabayashi J., Zhang Z., Wakabayashi N., Tamura Y., Fukaya M., Kensler T.W., Iijima M., Sesaki H.H. The dynamin-related GTPase Drp1 is required for embryonic and brain development in mice. J. Cell. Biol. 2009;186:805–816. doi: 10.1083/jcb.200903065. doi:10.1083/jcb.200903065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Manczak M., Sesaki H., Reddy P.H. Heterozygote Drp1 knockout mice do not have synaptic and mitochondrial deficiencies. Biochim. Biophys. Acta. 2012 doi: 10.1016/j.bbadis.2012.02.017. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Manczak M., Anekonda T.S., Henson E., Park B.S., Quinn J., Reddy P.H. Mitochondria are a direct site of A beta accumulation in Alzheimer's disease neurons: implications for free radical generation and oxidative damage in disease progression. Hum. Mol. Genet. 2006;15:1437–1449. doi: 10.1093/hmg/ddl066. doi:10.1093/hmg/ddl066. [DOI] [PubMed] [Google Scholar]