Abstract
Proper healing of cutaneous wounds progresses through a series of overlapping phases. Non-healing wounds are defective in one or more of these processes and represent a major clinical problem. A critical issue in developing treatments for chronic wounds is the paucity of animal models to study the mechanisms underlying the defects in healing. Here we show that deletion of Tumor Necrosis Factor Superfamily Member 14 (TNFSF14/LIGHT) leads to impaired wounds in mice that have the characteristics of non-chronic and chronic ulcers. These wounds show: (1) Excessive production of cytokines, in particular three chemokines (KC/CXCL8, MCP-1/CCL2, IP-10/CXCL10), that may be key to the abnormal initiation and resolution of inflammation; (2) defective basement membranes, explaining blood vessel leakage and disruption of dermal/epidermal interactions; (3) granulation tissue that contains high levels of Coll III whereas Coll I is virtually absent and does not form fibrils. We also see major differences between non-chronic and chronic wounds, with the latter populated by bacterial films and producing eotaxin, a chemokine that attracts leukocytes that combat multicellular organisms (which biofilms can be considered to be). This new mouse model captures many defects observed in impaired and chronic human wounds, and provides a vehicle to address their underlying cell and molecular mechanisms.
Keywords: Inflammation, repair, regeneration, cytokines, chemokines
INTRODUCTION
Cutaneous wound healing is a complex event consisting of sequential, overlapping phases that include hemostasis, inflammation, re-epithelialization, granulation tissue formation, and remodeling (1). When the wounds do not progress normally through these stages, the course of healing derails, and problematic healing ensues. Chronic wounds, such as diabetic foot ulcers, pressure ulcers and venous ulcers, have a significant impact on human health. A recent report estimates that $20M/yr is spent world wide in drugs and devices to treat chronic wounds and that the rate of growth is ~10%/yr. The US carries 40% of this market with a growth of 15%/yr (2).
Little is known about what causes impaired resolution of inflammation in chronic ulcers and whether initiation of inflammation is also impaired. Several studies have shown differences in pro-inflammatory growth factors and cytokines such as EGF, PDGF, VEGF, TNFα, TGFβ, bFGF, KGF, GM-CSF, IL1β and chemokines such as CXCL8/IL-8. However, there is disagreement regarding the extent to which these cytokines contribute to development of chronic ulcers (3–9).
Disruption of dermal-epidermal interactions and the presence of vascular fibrin “cuffs” are seen in some chronic wounds, although the underlying mechanisms remain unclear (10–13). Another aspect of chronic wounds that is defective is the interstitial extracellular matrix in the granulation tissue. Abnormal deposition of collagen in chronic wounds has been more extensively studied, and has been attributed to alterations in the profile of proteases. For example, several proteases were elevated in patients with chronic ulcers versus when compared with acute ulcers (11, 14, 15). In particular, MMPs and their inhibitors, the TIMPs, and the balance between the two appear to be altered (16, 17).
Chronic wounds are frequently colonized by bacterial species known to impair the wound healing process (18, 19). Several bacterial wound contaminants can form biofilms, complex microenvironments consisting of bacteria and associated extracellular polymeric substances (EPS), which are largely resistant to antimicrobial treatment (20). Staphylococcus aureus and epidermidis are common ulcer contaminants that can form biofilms in chronic wounds. Inhibition of these Staphylococcal biofilms promotes wound re-epithelialization. In the case of S. aureus, in vitro studies have suggested that the biofilm-induced re-epithelialization defects result from the ability of biofilms to inhibit keratinocyte proliferation and migration and increase their apoptosis (19, 21).
One of the factors delaying progress in understanding chronic wounds is lack of good animal models to investigate underlying mechanisms. In a previous study, we showed that inhibition of the angiogenic factor VEGF or its receptor in vivo results in increased presence of inflammatory cells in the wound tissue (22) (35). Moreover, we also found that VEGF induces macrophage apoptosis in vitro, which is mediated by the tumor necrosis factor SF14 also known as LIGHT, a factor that is stimulated by VEGF. Macrophage apoptosis is important for the resolution of inflammation during wound healing hence, VEGF-induced LIGHT expression may promote macrophage apoptosis in healing wounds, thereby participating in the resolution of inflammation during the wound healing process. Because LIGHT promotes macrophage apoptosis, we hypothesized that LIGHT plays a central role in the resolution of inflammation during wound healing. If true, LIGHT-deficient wounds should exhibit impaired healing with excessive inflammation. Here, we show that deletion of the LIGHT gene leads to cutaneous wounds that mimic healing and non-healing chronic ulcers in humans. We further show that the mouse wounds have excess inflammation, the inflammatory cells remain in the wound longer, and that the wounds of these mice show many of the characteristics of chronic ulcers. This novel finding led us to conclude that LIGHT−/− mice can provide a useful animal model to study the basic processes that lead to chronic wound development and potentially development of better approaches to treating these wounds.
MATERIALS AND METHODS
Materials
C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME), and LIGHT−/− mice were a gift from Carl Ware (La Jolla Institute for Allergy and Immunology, San Diego, CA). Antibodies were obtained as follows: anti-Coll IV and anti-fibrinogen from Abcam (Cambridge, MA); anti-CD45 from BioLegend (San Diego, CA); anti-F4/80 from AbD Serotec (Raleigh, NC); anti-CD3 and anti-MPO from R&D Systems (Minneapolis, MN); anti-αSMA from Sigma (St. Louis, MO); and anti-rabbit FITC and anti-rabbit Alexa 594 from Invitrogen (Carlsbad, CA). Other materials were obtained as follows: Vectashield from Vector Laboratories (Burlingame, CA); streptavidin-FITC from Invitrogen (Carlsbad, CA); Diff-quick staining kit from Andwin Scientific (Woodland Hills, CA); Luminex multi-plex kits from Invitrogen (Carlsbad, CA) and Millipore (Billerica, MA); Trichrome stain kit from ScyTek Laboratories (Logan, UT); Picrosirius red stain kit from Polysciences, Inc. (Warrington, PA); ketamine, xylazine, buprenorphine, hydrocortisone, animax, and betamethasone from Henry Schein (Melville, NY);
Mouse wounding experiments
Experiments were approved by the Institutional Animal Care and Use Committee of the University of California, Riverside. After removal of dorsal hair, excision wounds were created on the dorsum of both control and LIGHT−/− mice using a 7 mm biopsy punch (Acuderm, Inc). Wound tissues were collected using a 10 mm diameter Acupunch at various time points following injury. Half of each sample was homogenized for the molecular assays and the remainder prepared for frozen sectioning.
Immunolabeling
Frozen tissue sections were immunolabeled with various antibodies, as indicated above, mounted with Vectashield, and viewed and recorded using either a Nikon Microphot-FXA fluorescence microscope with a Nikon DS-Fi1 digital camera and Nikon NIS-Elements software or a Leica SP2 confocal microscope; for sections labeled with multiple dyes, images were merged in Photoshop.
Diff-quick staining
Frozen tissues were stained with Diff-quick, a modified Romanowski stain in which cytoplasm is stained pink and the nuclei are stained blue. Sections were incubated with the Diff-quick solution II for 30s and then counterstained with the Diff-quick solution I for 2 minutes, rinsed with ddH2O, dehydrated, and mounted. Stained tissues were then visualized using a Nikon Microphot-FXA microscope with a Nikon DS-Fi1 digital camera and Nikon NIS-Elements software.
Trichrome staining
Wound sections were subjected to a Modified Masson’s Trichrome stain according to the manufacturer’s directions. Briefly, sections were fixed with Bouin’s Fluid, then stained with Weigert’s hematoxylin, followed by incubation with Biebrich Scarlet/Acid Fuchsin, differentiation in a Phosphomolybdic/Phosphotungstic acid solution, incubation with Aniline Blue, and incubation with acetic acid. After dehydration, sections were mounted and visualized using a Nikon Microphot-FXA microscope with a Nikon DS-Fi1 digital camera.
Picrosirius red staining
Wound sections were subjected to Picrosirius Red staining (23, 24). We used the protocol proposed by the manufacturer (Polysciences, Inc., Warrington, PA). Briefly, sections were stained with Weigert’s hematoxylin, then incubated with phosphomolybdic acid hydrate, followed by solutions of picrosirius red F3BA and HCl. After dehydration, sections were mounted, visualized using a Nikon Microphot-FXA microscope, and photographed with plane-polarized light or between crossed polarizers with a Nikon DS-Fi1 digital camera.
Chronic wound treatment
We applied a series of anti-inflammatory and/or antibiotic creams to a chronic wound in order to promote healing. Treatment was initiated seven weeks after wounding with hydrocortisone cream. This cream was discontinued eleven days later, and treatment with Animax cream [containing nystatin, neomycin sulfate, thiostrepton and triamcinolone acetonide] was begun. Twenty days after this, the Animax was discontinued, and betamethasone treatment was initiated three days later and continued until complete wound closure, which occurred fifty-eight days later. Ultimately, the wounds healed after ~ five months.
Immunoblot analysis
Wound tissue was lysed in RIPA buffer (0.5% Triton X100, 0.5% Nonidet P-40, 10 mM Tris, pH 7.5, 2.5 mM KCl, 150 mM NaCl, 30 mM b-glycerophosphate, 50 mM NaF, 1 mM Na3VO4, 0.1% SDS, and protease and phosphatase inhibitors) to yield a concentration of 10μl/mg of tissue. Equal tissue weight of Zirconium oxide beads was added to the sample and then homogenized in a bullet blender at speed 8 for 5 min. The homogenized samples were centrifuged at 13000x g for 15 min at 4°C. The supernatant was divided into aliquots and stored at −80°C for further analysis. Protein concentrations were measured using the DC protein assay kit (Bio-Rad). Equal amounts of protein for each sample were mixed with sample buffer, boiled, and analyzed using 10% acrylamide SDS-PAGE. Immunoblotting was performed with the indicated primary Abs and the appropriate HRP-conjugated secondary Abs, followed by incubation with West Dura extended duration substrate (Pierce Biotechnology). Blots were then re-probed for H2B, a housekeeping protein to evaluate equal loading. Coll IV and αSMA band intensities were quantified using Image J (NIH) and then normalized against the H2B control. Fold change to the control was plotted.
Luminex multiplex assays
Wound tissue samples were homogenized in RIPA buffer using a Bullet Blender with 0.5 mm zirconium oxide beads (Next Advance, Averill Park, NY) at 4°C for 5–10 minutes. Protein concentration of wound extracts was determined using the DC protein assay (Bio-Rad, Hercules, CA). Luminex assays were conducted according to the manufacturers’ protocols (Invitrogen, Carlsbad, CA, and Millipore, Billerica, MA) and read using a Luminex™ 100 instrument (Invitrogen, Carlsbad, CA), which quantified cytokine levels by monitoring RPE fluorescence associated with each bead set.
Second Harmonic Generation (SHG) Imaging
An inverted Zeiss LSM 510 NLO META laser scanning microscope based on the Axiovert 200M inverted microscope equipped with standard illumination systems for transmitted light and epi-fluorescence detection was used. It was also equipped with an NLO interface for a femtosecond Titanium:Sapphire laser excitation source (Chameleon-Ultra, Coherent, Incorporated, Santa Clara, California) for multi-photon excitation. The Chameleon laser provided femtosecond pulses at a repetition rate of about 80 MHz, with the center frequency tunable from 690 to 1040 nm. A long working distance objective (Zeiss, 40X water, N.A. 0.8) was used to acquire images. The two-photon signals from the sample were epi-collected and discriminated by the short pass 650 nm dichroic beamsplitter. The SHG images were collected using a META detection module with signals sampled in a 394–405 nm detection range (λex = 800 nm). Each image presented in this work is 12 bit, 512×512 pixels representing 225 μm × 225 μm field of view.
Bacterial isolation and characterization
Pieces of wound tissue from control and LIGHT−/− acute wounds at 3d after wounding, as well as from LIGHT−/− chronic wounds, were diced and then combined with sterile Luria Broth (LB) to yield a ratio of 0.2 mg wound tissue/μl of LB. The bacteria were eluted and grown on Brucella agar plates containing 5% sheep red blood cells at 37°C overnight in a 10% CO2 humidified incubator. Bacteria that grew on these plates were examined for gram-staining reactivity, catalase production, coagulation activity, and hemolytic activity. In addition, isolated strains were further analyzed by PCR using primer sets designated to identify 16S.rRNA and HSP60 genomic sequences found in Staphylococcus epidermidis. The ability of isolated strains to produce biofilms was examined using the method described by Deighton et al. (25) for quantification of mature biofilms on polysterene plates under static conditions.
Statistical Analysis
For experiments with more than two treatment groups, we used GraphPad InStat software and ANOVA; groups with significant differences were then subjected to the Tukey-Kramer Multiple Comparisons Post Test. In experiments with only two groups, statistical analysis was conducted using a Student T Test.
RESULTS
LIGHT−/− wounds have excessive inflammation
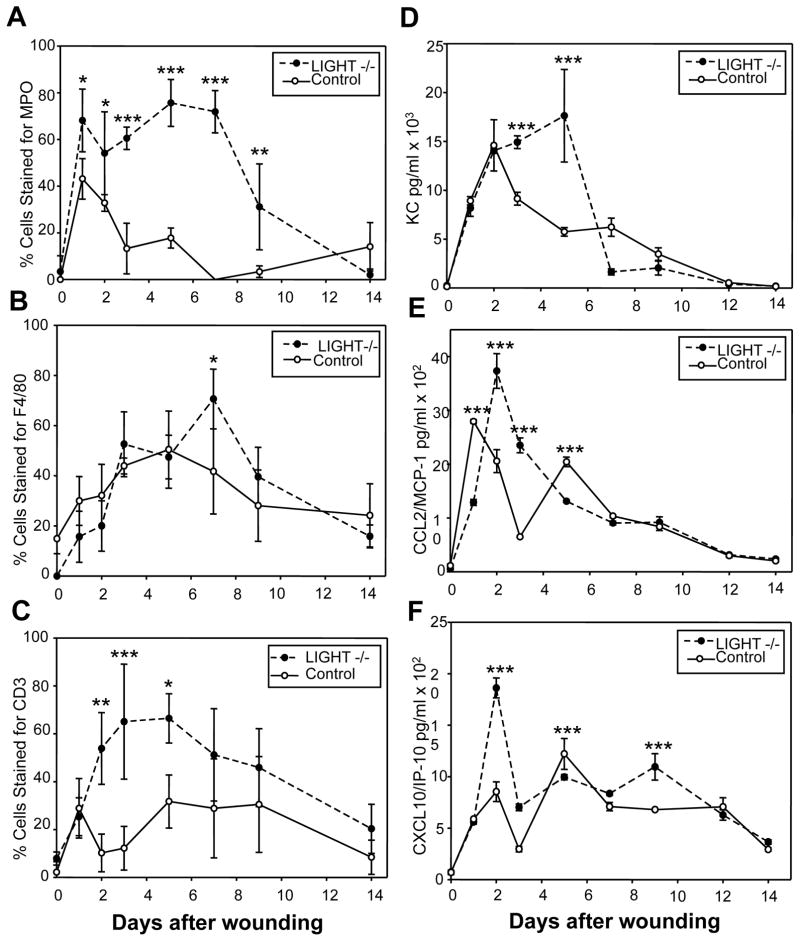
LIGHT can induce macrophage apoptosis, a process thought to contribute to resolution of inflammation (22). Thus, absence of LIGHT during wound healing could lead to increased and prolonged inflammation. Indeed, wounds of LIGHT−/− mice contain increased levels of inflammatory cells that remain in the wound much longer than in control mice (Figure 1). We immunolabeled wounded tissues to identify neutrophils (MPO), macrophages (F4/80) and T lymphocytes (CD3), and stained the nuclei with DAPI to determine % labeled cells per HPF using fluorescence microscopy (Suppl. Figure A). Neutrophils appeared a few hours after wounding in much higher numbers in LIGHT−/− wounds than in control wounds; they remained until day 9, whereas in the control wounds they declined by day 3 (Figure 1A). Macrophage numbers were higher in LIGHT−/− wounds, and their peak was reached at day 7 rather than day five in control wounds (Figure 1B). T-lymphocyte numbers were much higher in LIGHT−/− mice throughout the healing process (Figure 1C).
Figure 1. LIGHT−/− wounds exhibit a defective inflammatory response.
LIGHT−/− tissues collected at multiple time points after wounding were immunolabeled with antibodies against myeloperoxidase (MPO), a neutrophil marker (A), F4/80, a macrophage marker (B), and CD3, a T cell marker (C). (A–C) The percentage of leukocytes staining for the various antibodies, as determined by counting the total number of cells using the DAPI nuclear staining and the cells stained with the specific markers in the same high power field (HPF). Four separate fields were counted for each time point. (D–F) Homogenized tissue extracts were analyzed using Luminex multiplex assays to determine levels of multiple pro-inflammatory cytokines and chemokines. KC (D) is chemotactic for neutrophils; MCP-1/CCL2 (E) is chemotactic for monocytes; IP-10/CXCL10 (F) is chemotactic for lymphocytes. Data shown are representative of several independent experiments. *P<0.05, **P<0.01, ***P<0.001.
Pro-inflammatory cytokines and chemokines were also elevated in LIGHT−/− wounds. Three pro-inflammatory chemokines were particularly elevated: (1) KC (the mouse homologue of human IL-8/CXCL8), a neutrophil chemoattractant (26), was strongly elevated (Figure 1D), directly correlating with the prolonged presence of neutrophils in the wound tissue (compare with Figure 1A). Production of MCP-1/CCL2, a chemoattractant for monocytes (cells that become macrophages in the wound), was slightly delayed in LIGHT−/− wounds but increased to much higher levels on day 2, followed by a slow decline, much like the control (Figure 1E). However, macrophages persisted even after CCL2 began to decline (compare with Figure 1B). Levels of IP-10/CXCL10, a T-lymphocyte chemoattractant, peaks early (at day 2), and remain high through day 9 (Figure 1F), directly correlating with the pattern for T-lymphocytes (compare with Figure 1C) (for review on the role of chemokines in wound healing see 27). Thus, in LIGHT−/− mice, the inflammatory response is abnormal both in initiation and in resolution, much like in human chronic wounds (for review see 28). Furthermore, we show for the first time that, in LIGHT−/− wounds, there are abnormal levels of specific pro-inflammatory chemokines that have not been previously identified in impaired wounds.
Abnormal development of granulation tissue in LIGHT−/− mice
Microvessels are defective and immature in LIGHT−/− wounds
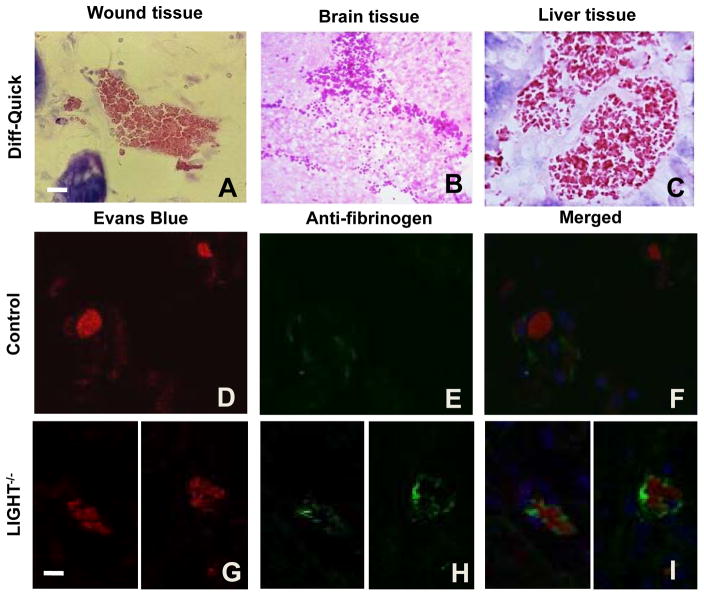
We observed that LIGHT−/− blood vessels of the wound tissue and various organs such as brain, liver, lung, heart, showed clumping of blood cells within the microvessels, suggesting intravascular coagulation (Figure 2A–C and data not shown). To determine whether this is in fact true, we chose to immunostain wound tissues with an anti-fibrinogen antibody (see Suppl. Fig 1B for antibody specificity). To visualize the red blood cells, Evans blue dye, which binds to proteins and fluoresces red, was used. We found clusters of blood cells and fibrin in the blood vessels of LIGHT−/− tissue (Figure 2G–I) but essentially none in controls (Figure 2D–F). The fact that we find this phenotype in the microvessels of other tissues in this genetically modified mouse model indicates that this is a blood vessel defect that could contribute significantly to the impaired healing.
Figure 2. Intravascular coagulation in LIGHT−/− wounds.
(A–C) Blood vessels from LIGHT−/− wound tissue, brain, and liver containing clumped blood cells. (D–I) Sections were stained with Evans blue dye, which binds to blood cells, causing them to fluoresce red, and immunolabeled with anti-fibrinogen (green). (D–F) Control wound tissue. (G–I) LIGHT−/− wound tissue. Scale bar = 20μm.
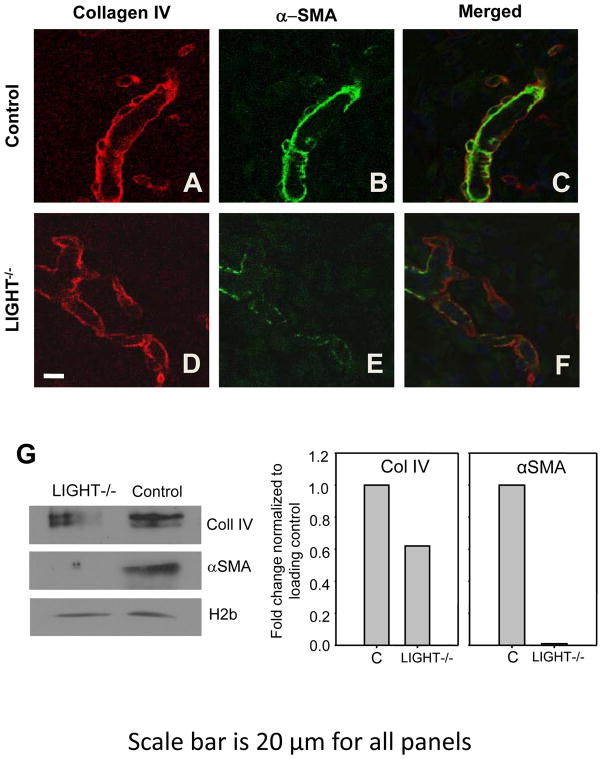
The presence of intravascular coagulation in LIGHT−/− wounds strongly suggests that the blood vessels are defective or damaged. Therefore, we examined the vasculature using the basement membrane marker Coll IV and the periendothelial cell marker α-SMA. Both these cells and the basement membrane confer stability to blood vessels. In control wounds, blood vessels were well delineated by Coll IV and by the presence of αSMA (Figure 3A–C) whereas the LIGHT−/− mice wound vessels were convoluted and misshapen, with some sprouts terminating abruptly to form “dead ends” (Figure 3D–F). These results were confirmed using immunoblot analysis for Coll IV and α-SMA (Figure 3G). The defects in the microvessel basement membrane correlate well with those found in the basement membrane of the epithelium (see next section). Taken together, these findings show that microvessels in LIGHT−/− wound tissue and other tissues are defective and/or immature compared to the wild type control animals.
Figure 3. Blood vessels in LIGHT−/− wounds are irregularly-shaped and immature.
Co-staining control (A–C) and LIGHT−/− (D–F) wound tissues with antibodies against Coll IV (red) and αSMA (green) to delineate the blood vessels. Scale bar = 20 μm. Data shown are representative of two experiments. (G) Immunoblot analysis and quantification of Coll IV (Col IV) and αSMA in the wounds of control and LIGHT−/− mice. Data shown are representative of two experiments.
Impaired epidermal maturation in LIGHT−/− wounds
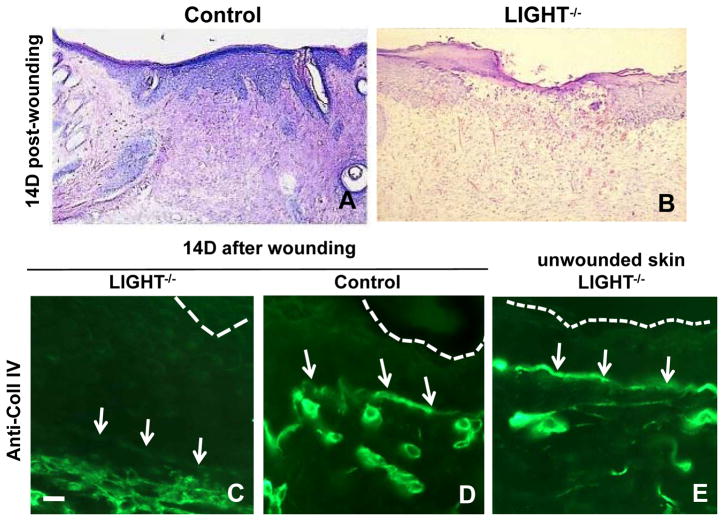
Histological examination showed that the epidermis of LIGHT−/− wounds was thicker than in control wounds and had fewer rete ridges (Figure 4A,B). In addition, the basement membrane underlying the nascent epithelium fourteen days after wounding was discontinuous in LIGHT−/− wounds, as shown by immunolabeling for Coll IV (Figure 4C,D); in non-wounded skin of LIGHT−/− mice the basement membrane was continuous (Figure 4E) but proved to be weak; indeed when removing the hair of these mice we have to be very careful to not cause blisters. We also observed that the granulation tissue underlying the dermis was disrupted even when the wound appeared closed (Figure 4B). Indeed, all of these mice showed disrupted wound healing despite the fact that some of them, upon visual inspection, seemed to close in a similar manner to that of the controls.
Figure 4. Basement membrane deposition is reduced in LIGHT−/− wounds.
(A, B) Histological appearance of wound tissues 14 days post-wounding for control and LIGHT−/− respectively. (C–D) Immunolabeled with anti-Coll IV 14 days after wounding; control shows a normal basement membrane whereas LIGHT−/− shows almost not basement membrane deposition. (E) Unwounded skin of LIGHT−/− mice have a continuous basement membrane. The top of the epithelium is delineated with dotted lines and arrows point to the Coll IV staining, or lack thereof, in the basement membrane. Scale bar = 20μm.
Collagen matrix is abnormal in LIGHT−/− wound tissue
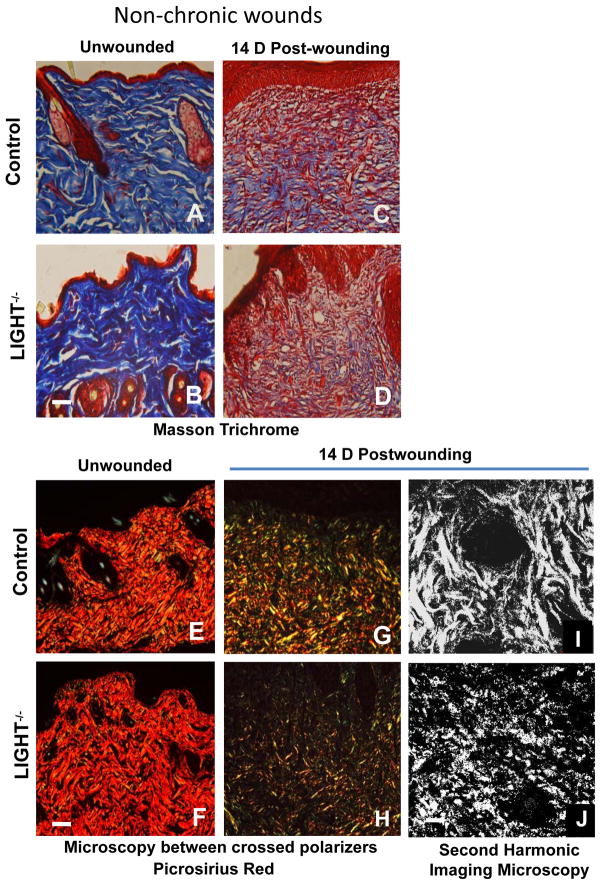
Proper extracellular matrix deposition and remodeling in the healing tissue is necessary for wound maturation and scar formation. Coll I deposition and structure appear similar in unwounded control and LIGHT−/− skin (Figure 5A,B). However, fourteen days after wounding, LIGHT−/− wound tissue contains less collagen than the control and the fibers appear thin and more loosely distributed, suggesting immature collagen deposition (Figure 5C,D). We also stained wound tissues with picrosirius red, a dye that can distinguish between collagen types when viewed between crossed polarizers; in standard 10μm thick tissue sections, thicker collagen fibers such as Coll I appear red to orange, whereas thinner collagen fibers such as Coll III appear yellow to green (23, 24). In unwounded skin of both control and LIGHT−/− mice, the majority of the dermal matrix stained red to orange, suggesting the predominance of Coll I fibers (Figure 5E,F). In the granulation tissue of control wounds, substantial amounts of Coll I (red/orange) and less Coll III (yellow/green) were present, indicative of wound maturation and remodeling (Figure 5G) similar to the trichrome staining. In contrast, the granulation tissue of LIGHT−/− wounds contained little Coll I (red/orange) and more Coll III (Figure 5H). Second-harmonic imaging microscopy (29) showed that the granulation tissue of control wounds contains thick, “well-organized” Coll I fibers, whereas LIGHT−/− wounds have little fibrillar organization of Coll I (Figure 5I,J), and in some areas very little Coll I was present (not shown). All these imaging techniques show that the collagen in LIGHT−/− wound tissue is immature and/or abnormal, suggesting that there could be a problem with matrix deposition or that the enzymes that remodel and maintain the ECM and their modulators are altered.
Figure 5. Collagen deposition is reduced and immature in LIGHT−/− non-chronic and chronic wounds.
(A–D) Control and LIGHT−/− unwounded skin and 14D wound tissue were stained using the Masson trichrome staining which stains the nuclei dark with Weigert’s hematoxylin, the cytoplasm and muscle fibers red with the Biebrich scarlet dye, and the collagen blue with aniline blue dye. Data shown are representative of two experiments. (E, F) Control and LIGHT−/− unwounded skin and (G,H) 14D post-wounding tissues were stained using picrosirius red, in which thick Coll I fibers appear red/orange in color and thinner Coll III fibers appear yellow/green in standard 10μm thick tissue sections viewed between crossed polarizers. Scale bar = 50μm. (I,J) Second-harmonic imaging microscopy (SHIM) of the structure of Coll I fibers in 14D wild-type and LIGHT−/− wounds Scale bar = 100μm. Data shown are representative of two independent experiments.
LIGHT−/− wounds can become chronic
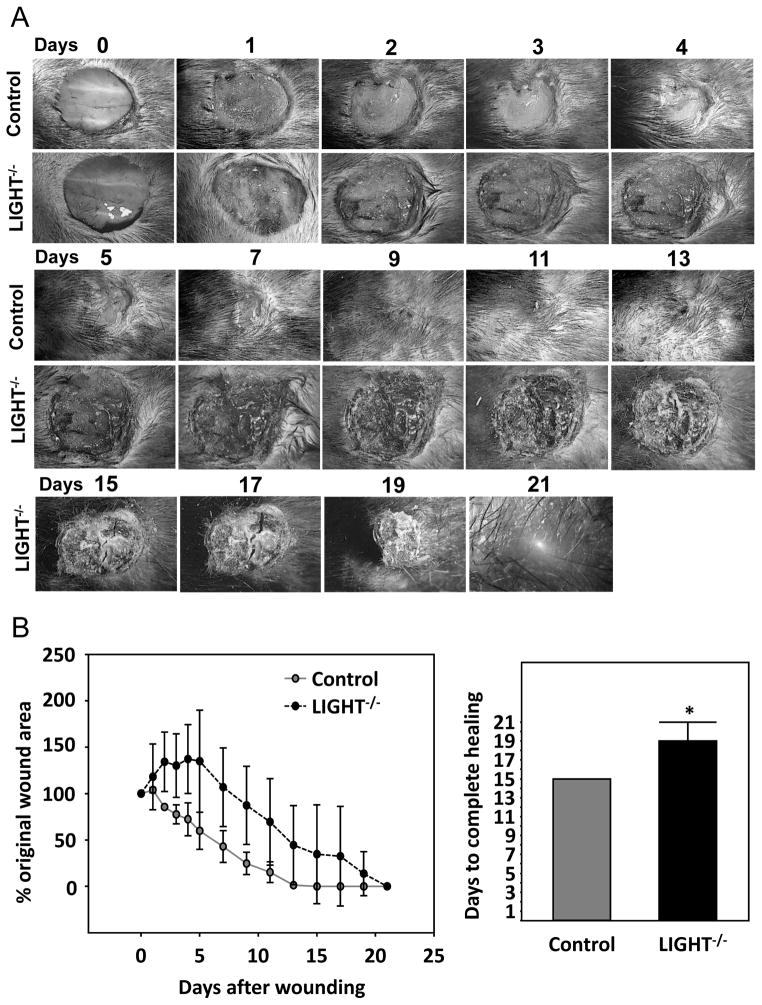
In all of the mice we studied, ~ 42% LIGHT−/− mouse wounds re-epithelialized at apparently the same rate as the control wounds. Despite the apparently normal closure rate of these wounds, they showed excessive prolonged inflammation, blood vessels were leaky and formed fibrin “cuffs”, the collagen matrix was disrupted, and the dermis underlying the epidermis showed immature/abnormal healing (Figures 1–5). Of the remaining LIGHT−/− wounds, 25% had delayed closure (Figure 6), ~17% developed chronic wounds, and ~17 % died during the healing process, potentially due to generalized microvessel defects. The LIGHT−/− mice that developed chronic wounds showed signs of systemic infection shortly after wounding, and were put on oral systemic antibiotics for a week. Despite the fact that the infection resolved quickly, the wounds continued to worsen and became chronic. The non-healing ulcers became crusty, oozed fluid, and opened and closed repeatedly (Figure 7A). Seven weeks after initial wounding, the rules of our institution required us to begin treatment of these wounds. Hydrocortisone cream was first prescribed without success, followed a few days later by Animax, an anti-bacterial and anti-inflammatory mixture of nystatin, neomycin sulfate, thiostrepton and triamcinolone acetonide, for twenty days. Because no progress was made, we discontinued Animax and initiated betamethasone treatment for fifty-eight days, at which point the wounds closed. In total, wound closure required nearly five months.
Figure 6. Delayed closure in non-chronic wounds of LIGHT−/− mice.
Of the twelve mice we wounded and examined for closure, 25% showed delayed wound closure as depicted in (A). The wounds remained large, became crusty, and did not close until much after the control. (B) % wound closure over time. *P<0.05.
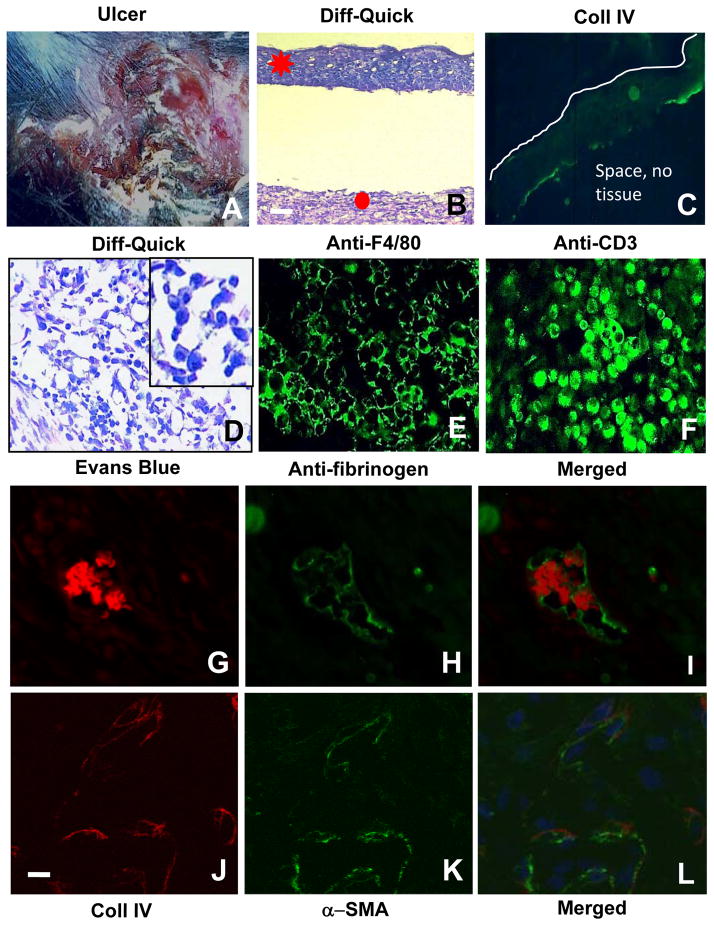
Figure 7. Defects in the LIGHT−/− mice chronic wounds are exacerbated when compared with non-chronic wounds.
(A) General appearance of the chronic wounds from the LIGHT−/− mice. (B) Highly impaired epithelial-dermal connectivity. (C) Chronic wound epidermis is immature; immunolabeling for Coll IV shows a discontinuous epithelial basement membrane (green). Below the staining there is an empty space much like in B. Line delineates the top of the epidermis (D) Histological examination showed the presence of large numbers of small round and dense cells, likely inflammatory cells; insert show enlargement of the cells). (E–F) Immunolabeling of macrophages with anti-F4/80 (E) and T-lymphocytes with anti-CD3 (F) in the chronic wounds. (G–I) Chronic LIGHT−/− wound tissue was labeled with Evans blue (red fluorescence) and anti-fibrinogen antibody (green fluorescence) to detect intravascular coagulation. (J–L) Co-staining with antibodies against Coll IV (red) and αSMA (green) in chronic LIGHT−/− wounds shows very low levels of both. Blue fluorescence is DAPI. Scale bar for B–D = 50μm for E–L = 20μm. Data shown are representative of staining of multiple sections of the chronic wounds.
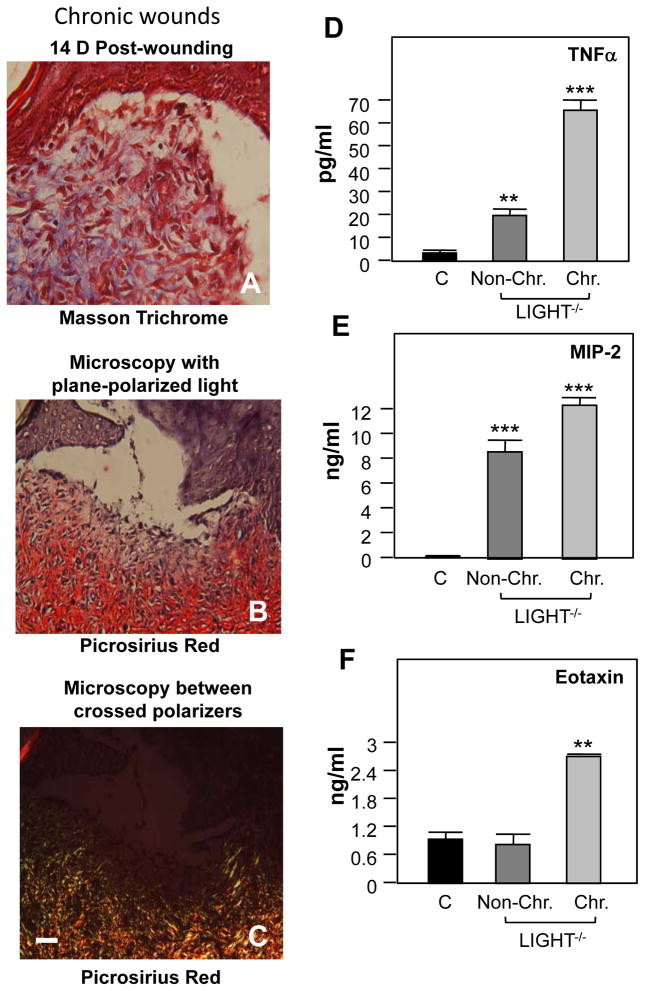
Like the non-chronic LIGHT−/− wounds, chronic wounds exhibited a decreased epidermal-dermal connectivity and a discontinuous basement membrane (Figure 7B,C). The white line in C delineates the surface of the epithelium and we also indicate that the space below the staining is empty. This is similar to what is shown in B. These wounds contained large numbers of inflammatory cells that were identified as macrophages and T-lymphocytes (Figure 7D–F). The vasculature was also impaired, much like in the non-chronic wounds. We found intravascular coagulation (Figure 7G–I), decreased αSMA-positive cells, and decreased Coll IV deposition (Figure 7J–L), indicating defects in the basement membrane and in the microvasculature supporting cells. Chronic wounds in LIGHT−/− mice also exhibit an immature extracellular matrix (Figure 8 A–C). Luminex multiplex array assays in LIGHT−/− wound tissues showed that two factors were upregulated in both non-chronic and chronic LIGHT−/− wounds -- TNFα (Figure 8D) and MIP-2 (Figure 8E) – and one factor, eotaxin, was upregulated in chronic wounds only (Figure 8F). All of the phenotypes in LIGHT−/− chronic wounds were exacerbated in comparison to the non-chronic wounds.
Figure 8. Collagen deposition and pro-inflammatory cytokine levels in LIGHT−/− chronic wound tissues.
LIGHT−/− chronic wound tissue was sectioned and stained with Masson trichrome (A). (B,C) Picrosirius red staining of LIGHT−/− chronic wound tissues under plane-polarized light (B) or between crossed polarizers (C). Scale bar = 50μm. (D–F) Homogenized chronic wound tissue was analyzed using Luminex multiplex arrays to determine levels of multiple pro-inflammatory cytokines and chemokines. Cytokine levels were compared at 14 days between control wounds and LIGHT−/− non-chronic and chronic wounds. Both LIGHT−/− wounds contain higher levels of TNFα (D) and MIP-2 (E) than control wounds, but eotaxin is upregulated only in chronic LIGHT−/− wounds (F).
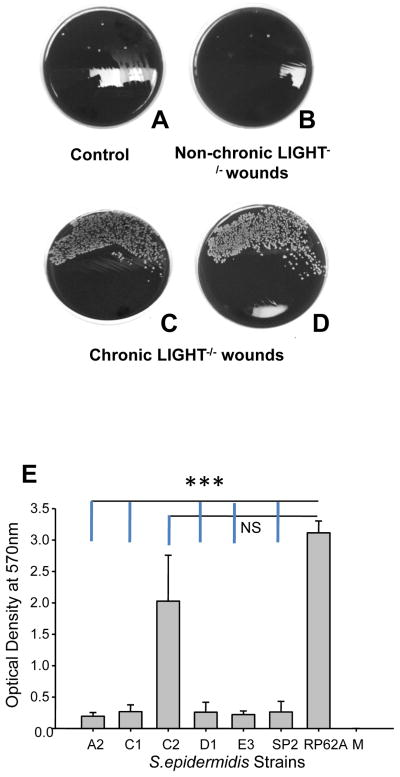
Another major difference we found between the LIGHT−/− non-chronic and chronic wounds was the presence of large amounts of bacteria in chronic wounds (Figure 9A–D). The predominant isolates found in these chronic wound specimens, which were collected prior to any treatment, were Gram-positive cocci in clusters, strongly catalase positive, coagulase negative, produced little or no beta-hemolytic activity, and were identified as coagulase-negative staphylococci, presumptively Staphylococcus epidermidis (isolates C1, C2 and D1; Figure 9E). We also isolated a few similar-appearing colonies from the non-chronic wound specimens that showed similar characteristics (strainsA2 and E3). We used S. epidermidis 16S-rRNA and HSP60 genomic primer sequences as previously described (30) to confirm that these 5 strains were S. epidermidis strains (not shown).
Figure 9. Bacterial contamination in chronic LIGHT−/− wounds.
Non-chronic and chronic wound tissues were plated on blood agar plates, and the resulting colonies identified and characterized. Control (A), non-chronic LIGHT−/− (B), chronic LIGHT−/− wound (C,D). (E) Quantification of biofilm formation on polystyrene plates was performed as described in Materials and Methods. Isolates A2, C1, C2, D1 and E3 were obtained from mouse wounds, while strains SP2 (negative control) and RP62A (positive control) were purchased from ATCC. M = media without bacteria. Data represent the mean and standard deviations based on 3 separate experiments, with 3 replicates per strain in each experiment.
In order to determine whether these isolates could form biofilms, we used a previously described protocol (25) to quantify biofilm formation on polystyrene plates (Figure 9E). In this experiment, we used 2 control strains purchased from the American Type Culture Collection – S. epidermidis RP62A (ATCC 35984), a known biofilm producer, and S. hominis SP2 (ATCC 35983), which is biofilm negative (25). Of the 5 strains isolated from the wound samples, only isolate C2 produced significant levels of biofilm. This result is significant because isolate C2 was recovered in relatively high numbers from 2 separate chronic wound samples, but was not isolated from control samples or non-chronic wound exudates. In addition, this strain was the second most abundant bacteria recovered from each of the chronic wound samples examined.
DISCUSSION
The studies presented here show that LIGHT−/− mice have impaired healing that mimics human chronic ulcers. This is the first animal model to show the various characteristics of these ulcers. Using this model system, we have uncovered new deregulated molecules that contribute to poor healing. We find that: (1) The chemokines KC/IL-8, MCP-1/CCL2 and IP-10/CXCL10 are highly elevated shortly after wounding, and that this elevation remains over times at which they should not be present in the wound, leading to excess number of neutrophils, macrophages and T-lymphocytes, respectively. (2) The blood vessels are often clogged with erythrocytes, have fibrin “cuffs” (as in venous ulcers), have a basement membrane that is deficient in Coll IV, and the periendothelial cells are either absent or produce low levels of αSMA. (3) Coll I is deficient and does not form fibrils; Coll III, which is normally present primarily during the early stages of granulation tissue development, is the predominant collagen in LIGHT−/− wounds. (4) The dermal-epidermal junction is disrupted and Coll IV is virtually absent in the basement membrane of the epithelium. (5) Some of the ulcers become chronic and contain biofilm-forming bacteria and high levels of the chemokine eotaxin that are not found in either control or LIGHT−/− non-chronic wounds. These findings contribute to the understanding of many defects found in chronic human ulcers, and strongly suggest that this model can be used to further elucidate the mechanisms underlying the pathology of these wounds.
Elevated pro-inflammatory chemokine production plays a critical role in the excessive attraction of immune cells to the wound site in LIGHT−/− mice. In the case of neutrophils, the problem is two-fold: (1) They arrive in very higher numbers at the very early stages of healing, suggesting a problem with regulation of inflammation initiation. (2) Because they produce high levels of proteases and reactive oxygen species to destroy pathogens, and are “sloppy” in their activities, they cause considerable damage to the tissue resulting in increased inflammation (31, 32). The increased duration of macrophage presence in the wound tissue likely leads to increased secretion or activation of inflammatory cytokines and proteases (33), resulting in further tissue damage. More importantly, macrophages may not undergo apoptosis at the appropriate time during wound healing if they lack the LIGHT molecule (22).
Perhaps the most critical observation in the inflammatory response of LIGHT−/− mice is that T-lymphocytes are present in large numbers early in the healing process; these cells normally arrive later and facilitate the remodeling process (34). Our data strongly suggest that the primary reason that these cells come to the wound earlier than they should is abnormally high levels of IP-10/CXCL10 at the very early stages of healing in the LIGHT−/− wounds. The persistence of lymphocytes in the wound tissue further suggests that remodeling signals are being produced at a time when the granulation tissue is normally developing rather than remodeling.
The excessive and prolonged inflammation in the LIGHT−/− wounds also has a significant effect on the deposition of the nascent extracellular matrix in the granulation/scar tissues. In particular, it affects the interstitial collagens (I and III), the Coll IV in the basement membrane and the periendothelial cells that lend support to the endothelium. This likely destabilizes the endothelium and causes the endothelial cells to lose their adhesions, allowing the extravasation of blood contents and coagulation. We also find absence of Coll IV in the basement membrane of the epithelium in LIGHT−/− wounds, indicating that this defect in matrix deposition is fundamental in impaired wounds (35, 36), in particular in blistering of the skin. Coll I, the main type of collagen that is normally present after wound closure, having replaced the Coll III deposited earlier, is virtually absent, and, when present, does not form fibrils. This condition, in conjunction with the weak dermal-epidermal interaction, contributes to the fragility of the healing wound and results in repeated opening and closing of chronic wounds. With our model, the mechanisms underlying these abnormalities can now be studied.
One major difference between the non-chronic and chronic wounds lies in the levels of eotaxin, which are significantly increased in chronic wounds. Eotaxin is a chemokine that attracts eosinophils, leukocytes that are effective in combating multicellular pathogens. These wounds also contain Staphylococcus epidermidis, a bacterium that forms biofilms in humans and has been found in chronic wounds (37, 38). Because biofilms are similar in many ways to multicellular organisms, it is possible that the production of eotaxin in these wounds is the animal’s attempt to eliminate the bacterial biofilms by recruiting eosinophils to the wound site.
The demonstration of biofilm-producing coagulase-negative staphylococci in the chronic wound samples, but not in the non-chronic wound samples, strongly suggests that these bacteria are responsible for chronicity. This conclusion is consistent with the growing awareness of the contribution of bacterial biofilms to pathophysiology of chronic wounds in humans (19, 39, 40).
Clinically, chronic wounds are treated first by debridement to remove dead tissue and bacteria, and to stimulate the “healthier” tissue surrounding the wound to initiate the process of normal healing. However, this approach leads to non-predictable outcomes because it is not yet known what causes a wound to become chronic. Our LIGHT−/− mouse model shows a number of features seen in most chronic wounds. Failure of dermal epidermal connection is common in many skin diseases and chronic wounds and so is chronic inflammation. Both of these defects are found in the LIGHT−/− mice. Chronic wounds in humans show persistent bacterial infections with formation of biofilms, often have altered matrix deposition, in particular interstitial collagens, the blood vessels are defective and in venous ulcers form fibrin “cuffs”. Our mouse model also depicts these defects including fibrin “cuffs”. The implication of our findings for chronic wounds in humans is that our model shows most of the defects observed in humans. Therefore, it can be used to understand the cellular and molecular mechanisms involved in these defects with the goal of finding potential targets for treatment of chronic wounds in humans.
In conclusion, we show that LIGHT plays multiple roles in inflammatory and healing processes. A particularly intriguing possibility is that bacterial films may be a critical (perhaps the critical) difference between non-chronic impaired wounds and chronic wounds, and that the chemokine eotaxin plays an important role in attracting eosinophils, leukocytes that are critical for elimination of multicellular organisms, which biofilms can be considered to be. On a larger scale, our studies provide new information about the types of defects that can occur when inflammation persists, and show that this mouse model captures a wide variety of defects observed in chronic wounds in humans, providing a vehicle to address their underlying cell and molecular mechanisms.
Supplementary Material
Acknowledgments
We thank V. Capasso (Massachusetts General Hospital) for helpful discussion regarding the chronic LIGHT−/− wounds, T. Mustoe (Northwestern University) for comments on the manuscript, Harry Green for assistance with polarized light microscopy, the UC Riverside Stem Cell Core Facility for assistance with Luminex multi-plex arrays, Allen Wang with help in submitting the manuscript, and Jim Sinclair for assistance with the animal work. This work was funded in part by an NIH grant to MMG and a Wound Healing Foundation 3M fellowship to MLP.
Abbreviations
- bFGF
basic Fibroblast Growth Factor
- EGF
Epidermal Growth Factor
- GM-CSF
Granulocyte Macrophage-Colony Stimulating Factor
- HSP60
Heat Shock Protein 60
- IL-1b
Interleukin 1b
- IP10/CXCL10
interferon gamma-induced protein 10
- IL-8
Interleukin-8
- KC
keratinocyte-derived cytokine
- KGF
Keratinocyte Growth Factor
- MCP-1/CCL2
Monocyte Chemoattractant Protein-1
- MIP-2
Macrophage Inflammatory Protein-2
- MMP
Matrix Metalloprotease
- PDGF
Platelet-Derived Growth Factor
- αSMA
α–Smooth Muscle Actin
- TGFβ
Transforming Growth Factor b
- TIMP
Tissue Inhibtor of Metalloprotease
- TNFα
Tumor Necrosis Factor a
- VEGF
Vascular Endothelial Growth
Footnotes
CONFLICT OF INTEREST
None of the authors have conflict of interest related to this work.
References
- 1.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341(10):738–46. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 2.Higgins M. Industry Overview-Advanced Wound Care Market. Specialty Pharmaceuticals Industry coverage. Roodman and Renshaw Equity Research. 2010 [Google Scholar]
- 3.Wallace HJ, Stacey MC. Levels of tumor necrosis factor-alpha (TNF-alpha) and soluble TNF receptors in chronic venous leg ulcers--correlations to healing status. J Invest Dermatol. 1998;110(3):292–6. doi: 10.1046/j.1523-1747.1998.00113.x. [DOI] [PubMed] [Google Scholar]
- 4.Trengove NJ, Bielefeldt-Ohmann H, Stacey MC. Mitogenic activity and cytokine levels in non-healing and healing chronic leg ulcers. Wound Repair Regen. 2000;8(1):13–25. doi: 10.1046/j.1524-475x.2000.00013.x. [DOI] [PubMed] [Google Scholar]
- 5.Murphy MA, Joyce WP, Condron C, Bouchier-Hayes D. A reduction in serum cytokine levels parallels healing of venous ulcers in patients undergoing compression therapy. Eur J Vasc Endovasc Surg. 2002;23(4):349–52. doi: 10.1053/ejvs.2002.1597. [DOI] [PubMed] [Google Scholar]
- 6.Brem H, Stojadinovic O, Diegelmann RF, Entero H, Lee B, Pastar I, Golinko M, Rosenberg H, Tomic-Canic M. Molecular markers in patients with chronic wounds to guide surgical debridement. Mol Med. 2007;13(1–2):30–9. doi: 10.2119/2006-00054.Brem. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gohel MS, Windhaber RA, Tarlton JF, Whyman MR, Poskitt KR. The relationship between cytokine concentrations and wound healing in chronic venous ulceration. J Vasc Surg. 2008;48(5):1272–7. doi: 10.1016/j.jvs.2008.06.042. [DOI] [PubMed] [Google Scholar]
- 8.Charles CA, Romanelli P, Martinez ZB, Ma F, Roberts B, Kirsner RS. Tumor necrosis factor-alfa in nonhealing venous leg ulcers. J Am Acad Dermatol. 2009;60(6):951–5. doi: 10.1016/j.jaad.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Zimmerman K, Stojadinovic O, Lebrun E, Tomic-Canic J, Brem H. Cellular and molecular mechanism of chronic wounds. In: Sen C, editor. Adv Wound Care. New Rochelle, NY: Mary Ann Liebert; 2010. pp. 165–70. [Google Scholar]
- 10.Coleridge Smith PD, Thomas P, Scurr JH, Dormandy JA. Causes of venous ulceration: a new hypothesis. Br Med J (Clin Res Ed) 1988;296(6638):1726–7. doi: 10.1136/bmj.296.6638.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palolahti M, Lauharanta J, Stephens RW, Kuusela P, Vaheri A. Proteolytic activity in leg ulcer exudate. Exp Dermatol. 1993;2(1):29–37. doi: 10.1111/j.1600-0625.1993.tb00196.x. [DOI] [PubMed] [Google Scholar]
- 12.Neumann HA, van den Broek MJ, Boersma IH, Veraart JC. Transcutaneous oxygen tension in patients with and without pericapillary fibrin cuffs in chronic venous insufficiency, porphyria cutanea tarda and non-venous leg ulcers. Vasa. 1996;25(2):127–33. [PubMed] [Google Scholar]
- 13.Joshi A, Sloan P. Role of “fibrin” cuffs in chronic nonspecific oral ulceration. Wound Repair Regen. 2004;12(1):18–23. doi: 10.1111/j.1067-1927.2004.012106.x. [DOI] [PubMed] [Google Scholar]
- 14.Lim CS, Shalhoub J, Gohel MS, Shepherd AC, Davies AH. Matrix metalloproteinases in vascular disease--a potential therapeutic target? Curr Vasc Pharmacol. 2010;8(1):75–85. doi: 10.2174/157016110790226697. [DOI] [PubMed] [Google Scholar]
- 15.Beidler SK, Douillet CD, Berndt DF, Keagy BA, Rich PB, Marston WA. Multiplexed analysis of matrix metalloproteinases in leg ulcer tissue of patients with chronic venous insufficiency before and after compression therapy. Wound Repair Regen. 2008;16(5):642–8. doi: 10.1111/j.1524-475X.2008.00415.x. [DOI] [PubMed] [Google Scholar]
- 16.Gill SE, Parks WC. Metalloproteinases and their inhibitors: regulators of wound healing. Int J Biochem Cell Biol. 2008;40(6–7):1334–47. doi: 10.1016/j.biocel.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mwaura B, Mahendran B, Hynes N, Defreitas D, Avalos G, Adegbola T, Adham M, Connolly CE, Sultan S. The impact of differential expression of extracellular matrix metalloproteinase inducer, matrix metalloproteinase-2, tissue inhibitor of matrix metalloproteinase-2 and PDGF-AA on the chronicity of venous leg ulcers. Eur J Vasc Endovasc Surg. 2006;31(3):306–10. doi: 10.1016/j.ejvs.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 18.Bowler PG, Duerden BI, Armstrong DG. Wound microbiology and associated approaches to wound management. Clin Microbiol Rev. 2001;14(2):244–69. doi: 10.1128/CMR.14.2.244-269.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gurjala AN, Schierle CF, Galiano RD, Leung KP, Mustoe TA. Animal models of biofilm-infected wound healing. In: Sen C, editor. Advances in Wound Care. New Rochelle, NY: Mary Ann Liebert; 2010. pp. 305–10. [Google Scholar]
- 20.Flemming HC, Wingender J. The biofilm matrix. Nat Rev Microbiol. 2010;8(9):623–33. doi: 10.1038/nrmicro2415. [DOI] [PubMed] [Google Scholar]
- 21.Kirker KR, Secor PR, James GA, Fleckman P, Olerud JE, Stewart PS. Loss of viability and induction of apoptosis in human keratinocytes exposed to Staphylococcus aureus biofilms in vitro. Wound Repair Regen. 2009;17(5):690–9. doi: 10.1111/j.1524-475X.2009.00523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petreaca ML, Yao M, Ware C, Martins-Green MM. Vascular endothelial growth factor promotes macrophage apoptosis through stimulation of tumor necrosis factor superfamily member 14 (TNFSF14/LIGHT) Wound Repair Regen. 2008;16(5):602–14. doi: 10.1111/j.1524-475X.2008.00411.x. [DOI] [PubMed] [Google Scholar]
- 23.Junqueira LC, Cossermelli W, Brentani R. Differential staining of collagens type I, II and III by Sirius Red and polarization microscopy. Arch Histol Jpn. 1978;41(3):267–74. doi: 10.1679/aohc1950.41.267. [DOI] [PubMed] [Google Scholar]
- 24.Rich LaWP. Collagen and picrosirius red staining: a polarized light assessment of fibrillar hue and spatial distribution. Braz J Morphol Sci. 2005;22:97–104. [Google Scholar]
- 25.Deighton MA, Capstick J, Domalewski E, van Nguyen T. Methods for studying biofilms produced by Staphylococcus epidermidis. Methods Enzymol. 2001;336:177–95. doi: 10.1016/s0076-6879(01)36589-8. [DOI] [PubMed] [Google Scholar]
- 26.Bozic CR, Kolakowski LF, Jr, Gerard NP, Garcia-Rodriguez C, von Uexkull-Guldenband C, Conklyn MJ, Breslow R, Showell HJ, Gerard C. Expression and biologic characterization of the murine chemokine KC. J Immunol. 1995;154(11):6048–57. [PubMed] [Google Scholar]
- 27.Martins-Green M. A Family of Small Cytokines Called Chemokines Is Important in Regulation of Wound Repair. In: Sen C, editor. Advances in Wound Care. New Rochelle, NY: Mary Ann Liebert; 2010. [Google Scholar]
- 28.Willenborg SKJ, Ranjan R, Krieg T, Eming SA. Chronic Wounds and Inflammation. In: Sen C, editor. Advances in Wound Care. New Rochelle, NY: Mary Ann Liebert; 2010. [Google Scholar]
- 29.Campagnola PJ, Loew LM. Second-harmonic imaging microscopy for visualizing biomolecular arrays in cells, tissues and organisms. Nat Biotechnol. 2003;21(11):1356–60. doi: 10.1038/nbt894. [DOI] [PubMed] [Google Scholar]
- 30.Vandecasteele SJ, Peetermans WE, Merckx R, Van Eldere J. Quantification of expression of Staphylococcus epidermidis housekeeping genes with Taqman quantitative PCR during in vitro growth and under different conditions. J Bacteriol. 2001;183(24):7094–101. doi: 10.1128/JB.183.24.7094-7101.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abbott RE, Corral CJ, MacIvor DM, Lin X, Ley TJ, Mustoe TA. Augmented inflammatory responses and altered wound healing in cathepsin G-deficient mice. Arch Surg. 1998;133(9):1002–6. doi: 10.1001/archsurg.133.9.1002. [DOI] [PubMed] [Google Scholar]
- 32.Dovi JV, Szpaderska AM, DiPietro LA. Neutrophil function in the healing wound: adding insult to injury? Thromb Haemost. 2004;92(2):275–80. doi: 10.1160/TH03-11-0720. [DOI] [PubMed] [Google Scholar]
- 33.Szekanecz Z, Koch AE. Macrophages and their products in rheumatoid arthritis. Curr Opin Rheumatol. 2007;19(3):289–95. doi: 10.1097/BOR.0b013e32805e87ae. [DOI] [PubMed] [Google Scholar]
- 34.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214(2):199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aumailley M, Has C, Tunggal L, Bruckner-Tuderman L. Molecular basis of inherited skin-blistering disorders, and therapeutic implications. Expert Rev Mol Med. 2006;8(24):1–21. doi: 10.1017/S1462399406000123. [DOI] [PubMed] [Google Scholar]
- 36.Coulombe PA, Kerns ML, Fuchs E. Epidermolysis bullosa simplex: a paradigm for disorders of tissue fragility. J Clin Invest. 2009;119(7):1784–93. doi: 10.1172/JCI38177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gotz F. Staphylococcus and biofilms. Mol Microbiol. 2002;43(6):1367–78. doi: 10.1046/j.1365-2958.2002.02827.x. [DOI] [PubMed] [Google Scholar]
- 38.Hill KE, Davies CE, Wilson MJ, Stephens P, Harding KG, Thomas DW. Molecular analysis of the microflora in chronic venous leg ulceration. J Med Microbiol. 2003;52(Pt 4):365–9. doi: 10.1099/jmm.0.05030-0. [DOI] [PubMed] [Google Scholar]
- 39.James GA, Swogger E, Wolcott R, Pulcini E, Secor P, Sestrich J, Costerton JW, Stewart PS. Biofilms in chronic wounds. Wound Repair Regen. 2008;16(1):37–44. doi: 10.1111/j.1524-475X.2007.00321.x. [DOI] [PubMed] [Google Scholar]
- 40.Schierle CF, De la Garza M, Mustoe TA, Galiano RD. Staphylococcal biofilms impair wound healing by delaying reepithelialization in a murine cutaneous wound model. Wound Repair Regen. 2009;17(3):354–9. doi: 10.1111/j.1524-475X.2009.00489.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.