Abstract
The innate immune system differentially regulates the expression of host defense peptides to combat infection during wound healing. We enhanced the expression of a host defense peptide, human β defensin-3 (hBD-3), in keratinocytes to generate a three-dimensional biologic dressing to improve healing of infected wounds. The NIKS human keratinocyte cell line was stably transfected ex vivo with a construct containing an epidermis-specific promoter driving hBD-3(NIKShBD-3) using non-viral methods. Levels of hBD-3 mRNA and protein in three-dimensional skin tissue produced from NIKShBD-3 were determined using quantitative PCR and ELISA, respectively. Tissue architecture was characterized by hematoxylin and eosin staining, and by indirect immunofluorence using proliferation and keratinocyte differentiation markers. Antimicrobial activity was assessed using an in vitro bacterial growth assay and in vivo using a murine burn infection model. Three-dimensional full thickness skin tissues containing epidermal NIKShBD-3 or control NIKS possessed histologic features of interfollicular epidermis and exhibited normal tissue growth and differentiation. NIKShBD-3 tissue contained approximately 5-fold more hBD-3 protein than tissue containing unmodified control NIKS. In vitro studies showed that NIKShBD-3 tissue produced a significant reduction in the growth of Staphylococcusaureus (S. aureus) mprF compared to control tissue. In an in vivo infected murine burn model, NIKShBD-3 tissue resulted in a 90% reduction in bacterial growth. These results demonstrate that sustained delivery of hBD-3 by a bioengineered skin tissue results in a therapeutically relevant reduction in growth of a S. aureus strain in an animal model of infected third degree burn wounds.
Keywords: skin substitute, host defense peptide, wound infection
INTRODUCTION
Healthy intact epidermis possesses intrinsic physical and chemical barriers against infection. Terminal differentiation of keratinocytes provides a physical barrier designed to prevent the entrance of microorganisms from the environment into the body. This physical barrier is augmented by the innate immune system as well as tissue-specific expression of host defense peptides (HDPs) which act as a chemical barrier against microbial invasion.(1) Wound environments which disrupt both the physical and chemical barriers of the skin such as second and third degree burns as well as chronic, non-healing skin ulcers are often depleted of HDPs. Because the wounds provide an ideal breeding ground for microbes,(2) wound healing is typically delayed until the microbial burden is below an infective level, usually less than 105organisms/gram of tissue.(3) Treatment of infected cutaneous wounds has long relied on topical antibiotics administered alone or in combination. However, reports have documented the cytotoxic effects of topical antimicrobial therapies on keratinocytes in monolayer culture, inhuman skin substitutes and in wounds, providing direct evidence for the resultant decrease in the rate of wound healing.(4, 5) Moreover, since the beginning of the antibiotic era in the 1940s, the use and misuse of conventional antimicrobial therapies has resulted in the continual emergence of multi-drug resistant strains of bacteria further complicating the clearance of infection in cutaneous wounds.(6) Therefore, a new and innovative strategy is needed to combat infected cutaneous wounds.
HDPs exhibit antimicrobial activity against many common pathogens and are produced in part by keratinocytes of the epidermal compartment. Two main classes of HDPs have been identified in humans: cathelicidins and defensins. The β-defensins are a subclass of defensins which possess strong antimicrobial activity against gram-negative and gram-positive bacteria, yeast and viruses and are expressed at relatively high levels in epithelial tissues such as the skin, trachea, and urinary epithelium.(7–9) Chemically synthesized HDPs have been applied topically to infected wounds in a porcine animal model as a simple approach to the delivery of HDPs.(10,11) However, the therapeutic promise of topical application of synthetic HDPs in clinical practice has been hindered by the cost of peptide synthesis and peptide degradation in the wound environment.(12)
We describe in this report a novel cell-based approach for the sustained delivery of rapid, direct acting, anti-infective agents such as HDPs. NIKS keratinocytes are a genetically uniform source of non-tumorigenic, pathogen-free, epidermal progenitor cells that have completed PhaseI/IIa clinical testing.(13–15) These features make the NIKS keratinocytes ideal for ex vivo genetic engineering approaches using non-viral means to generate skin substitute tissues with enhanced anti-infective and wound healing properties.(16) The studies presented here are focused on enhanced expression of hBD-3. This HDP was selected based on its broad spectrum antimicrobial activity that is retained at physiological salt concentrations.(17) We used non-viral genetic modification of the NIKS keratinocyte progenitor cell line to generate human keratinocytes which constitutively express hBD-3 in a differentiation-specific manner. The genetically modified NIKS were used to generate an anti-infective skin substitute tissue. The hBD-3-expressing skin tissue exhibited sustained production of hBD-3 and reduced the growth of a methicillin-resistant strain of S. aureus in vitro and in an animal model of third degree burns. These findings show that sustained delivery of host defense peptides can reduce growth of antibiotic resistant S. aureus.
MATERIALS AND METHOD
Monolayer Cell Culture
Unmodified NIKS keratinocytes (13) were obtained from existing cell stocks. NIKShBD-3keratinocytes generated using the unmodified NIKS cell stocks were cultivated in standard keratinocyte culture medium composed of a mixture of Ham's F-12 medium:Dulbecco's modified Eagle's medium, (3:1, final calcium concentration 0.66 mM) supplemented with 2.5% Fetal Clone II (Hyclone, Logan, Utah), 0.4 µg/ml hydrocortisone, 8.4 ng/ml cholera toxin, 5 µg/mlinsulin, 24 µg/ml adenine, and 5–10 ng/ml epidermal growth factor. The keratinocytes were sub-cultured at weekly intervals onto mitomycin C–treated Swiss 3T3 fibroblasts (mito-C 3T3) as previously described.(13) Keratinocyte monolayer cultures used in experimental conditions were observed daily for confluence and then harvested for use in experiments the following day. These cultures are defined as “post-confluent” when they are one day after confluence.
Transfection and clonal selection
A 3.7 kb genomic DNA fragment containing the involucrin promoter was cloned into the pUb-Bsd vector (Invitrogen, Carlsbad, CA). A DNA fragment encoding hBD-3 was isolated by PCR and sequenced to verify the identity and integrity of the PCR products. This DNA fragment was identical to previously reported sequences for hBD-3.(9, 17) Subsequently, the hBD-3 gene coding region was cloned downstream of the involucrin promoter and a DNA fragment containing the rabbit beta-globin intron and poly(A) signal was inserted downstream of the hBD-3 gene coding region. To allow selection of stably-transfected NIKS keratinocytes, a gene encoding a protein that confers resistance to blasticidin was also contained in this vector backbone. The structures of the involucrin-β-defensin-Ub-Bsd constructs were confirmed by restriction enzyme mapping and DNA sequencing. NIKS cells were electroporated with the linearized hBD-3 construct and subsequently cultured on a mito-C 3T3 feeder layer in blasticidin-containing media for three weeks. Multiple independent clones expressing the β-defensin-3 transgene were isolated, expanded in monolayer culture for further characterization, and cryopreserved. After the initial selection, transgene expression was confirmed by RT-PCR and all clones were subsequently grown in antibiotic-free keratinocyte growth medium for the duration of the studies.
RNA isolation and quantitative PCR Analysis
Screening of NIKShBD-3 clones after blasticidin selection was performed by RT-PCR. Total cellular RNA was isolated from cells grown in monolayer culture using Trizol Reagent as per manufacturer’s instructions (Invitrogen, Carlsbad, CA). Following DNase I treatment of 1 µg total RNA using the DNAfree kit (Ambion, Austin, TX), reverse transcription was performed using oligo-dT primers and M-MLV reverse transcriptase (Invitrogen) according to manufacturer’s instructions. PCR was performed using the MasterTaq Kit (Eppendorf, Hamburg, Germany) and the following primer sets: hBD-3 5’- CAACCAGCACGTTGCCCAGG -3’(forward, within human hBD-3 gene) and 5’- CGCCCCGGGCCACCATGAGGATCCATTATC-3’(reverse, within rabbit β-globin region); GAPDH 5’-CCAGCCGAGCCACATCGCTC-3’(forward) and 5’-ATGAGCCCCAGCCTTCTCCAT-3’ (reverse). PCR samples were run on a1.5% agarose gel and images taken with Kodak ImageStation 2000R (Eastman Kodak Co., Rochester, New York).
Analysis of the relative levels of hBD-3 mRNA from post-confluent monolayer cells or tissue (grown for 15 days in organotypic culture) was measured using quantitative PCR (qPCR). qPCR was performed using Taqman assay probes and primers for hBD-3 (Assay ID: Hs00218678_m1)and human PPIA endogenous control (cyclophilin A; assay ID: Hs99999904_m1) (Applied Biosystems, Foster City, CA). Reactions were run on a PTC-thermocycler with a Chromo 4 fluorescence detector (MJ Research now Bio-Rad, Hercules, CA). qPCR for monolayer keratinocytes was performed on post-confluent cell monolayer replicates (n=2–4 monolayer cultures/clone). Tissue qPCR consisted of independent triplicate experiments performed with 15day old NIKS tissue (3 tissues) and NIKShBD-3 tissues (3 tissues). Primary keratinocyte results consisted of three experiments performed with single post-confluent monolayer cultures in each cell strain. Opticon Monitor Software and Genex Macro (Bio-Rad, Hercules, CA) were used for data analysis. Calculated values represent the fold expression level of NIKShBD-3 mRNA over NIKS (averaged and arbitrarily set at 1) within each experiment.
Organotypic Cell Culture
Composite human skin substitute tissues were prepared using unmodified NIKS or NIKShBD-3keratinocyte clones in an organotypic culturing system (Millipore, Billerica, MA or Corning, Corning, NY). Keratinocytes were seeded onto the surface of a cellularized dermal matrix consisting of normal human fibroblasts and type 1 collagen at a density of 1.2–1.6 ×105 cells/cm2and maintained at the air/medium interface throughout the stratification process. Tissue cultures were fed two days after seeding the dermises and every other day thereafter with the StrataLife media series (Stratatech Corp., Madison, WI). Full stratification of the skin substitute tissue is typically present after 14 days in organotypic culture.
Histology and Immunostaining
Tissues grown in organotypic culture for 15 days were fixed for two hours in 1%paraformaldehyde. Each tissue sample was then divided evenly for further cryopreservation and paraffin embedding. Paraffin embedded sections were stained with hematoxylin and eosin(H&E) by the Department of Pathology and Laboratory Medicine, University of Wisconsin (Madison, WI). For indirect immunofluorescence (IIF) of Ki67, K14 and hBD-3, cryostat sections of 5 µm thickness were fixed in acetone at −20°C. After air-drying, samples were rehydrated in phosphate buffered saline (PBS) and blocked with 3% normal goat serum (Sigma-Aldrich, St. Louis, MO) for 30 minutes at room temperature. Samples were then incubated for 1hour at 37°C with rabbit polyclonal antibodies against hBD-3 (5 µg/ml; Novus Biologicals, Littleton, CO), and mouse monoclonal antibodies against Ki67 (10 µg/ml; Oncogene, Boston, MA) or K14 (1:20 dilution; Novacastro, Newcastle, UK). Samples were then incubated for 30 minutes at room temperature with Alexa 488 goat anti-rabbit or anti-mouse IgG secondary antibodies (2 µg/ml; Molecular Probes, Eugene, OR). Finally, samples were stained with 5 µg/mlHoescht 33258 nuclear stain (Sigma-Aldrich). For IIF of filaggrin, paraffin embedded sections were deparaffinized with xylenes, rehydrated through an ethanol series, boiled in 10 mM citrate buffer for antigen exposure, washed in PBS and blocked with 5% normal goat serum for 45 minutes at room temperature. Samples were then incubated for 30 minutes at room temperature with mouse monoclonal antibodies against filaggrin (4 µg/ml; NeoMarkers, Inc., Fremont, CA), followed by a 40 minute incubation at room temperature with Alexa-488 goat anti-mouse IgG secondary antibodies (2 µg/ml; Molecular Probes). Finally, samples were stained with 5 µg/mlHoescht 33258 nuclear stain. Samples were examined using an Olympus IX-70 inverted fluorescent microscope equipped with filters for visualization of both Hoechst (360 nm, blue)and Alexa Fluor 488 (490 nm, green) fluorescence. Digital images were captured on an Optronics DEI-750 CE camera (Goleta, CA) using ImagePro Plus software (Media Cybernetics, Silver Spring, MD). Dual color images were created by overlaying single color images of the same field.
Western Blot Analysis and ELISA
Cell lysates were collected from tissues using CytoBuster lysis buffer (EMD Biosciences, Darmstadt, Germany) containing protease inhibitor cocktail 3 (EMD Biosciences) from at least three independent experiments. Cell lysis supernatant protein concentrations were determined by BCA assay (Pierce, Rockford, IL). Western blots were prepared from cell lysate supernatant samples containing 15 µg total protein or chemically synthesized hBD-3 peptide (Protein Synthesis Core Facility, University of Wisconsin, Madison, WI) separated on a 4–12% Bis-Tris NuPAGE gel (Invitrogen) and transferred to a 0.2 micron Immobilon PSQ PVDF membrane(Millipore). Blots were probed with affinity-purified polyclonal rabbit anti-human hBD-3 antibody (1µg/ml) (Novus Biologicals, Littleton, CO), followed by goat anti-rabbit IgG horseradish peroxidase (0.2 µg/ml; Promega, Madison, WI). Blots were developed using ECLA dvance Chemiluminescence detection reagent per manufacturer’s instructions (Amersham, Piscataway, NJ). Blots were imaged using Kodak Image Station IS2000R. Concentrations ofhBD-3 protein present in whole cell lysate were quantified with a commercially available hBD-3 ELISA kit (Peprotech, Rocky Hill, NJ) using a GeniosPlus plate reader (TECAN, Austria)according to the manufacturer’s instructions.
In vitro antimicrobial functional activity assay
After 14 days of growth in organotypic culture, high calcium StrataLife medium was conditioned with individual tissues generated with NIKS or NIKShBD-3 keratinocytes for 24 hours. The conditioned media was removed from the tissue and placed in a 96 well nonbinding surface plate(Corning, Acton, MA) with a solution of 106/ml S. aureus mprF (generously provided by A. Peschel, Microbial Genetics, University of Tubingen, Tubingen, Germany) in 1% Trypticase Soy Broth/ 10 mM sodium phosphate (TSB mix) in a 1:1 ratio for total volume of 150 µl. The plate was set to shake linearly for 5 hours at 37°C and optical density (OD) measurements were taken every 15 minutes with a 620nm filter using a GeniosPlus plate reader (TECAN, Austria). A blank solution containing StrataLife medium and TSB mix was subtracted from readings. Area under the curve measurements were calculated and graphed using Prism 4 graphing software(GraphPad Software Inc, San Diego, CA).
In vivo antimicrobial functional activity model
The burn wounding and sepsis protocol as previously described by Stevenson, Gamelli and Shankar (18) was utilized with modifications.(16) Mice were anesthetized, shaved, and given a12% total body surface area (TBSA) third degree scald burn using 90°C water bath immersion for 10 seconds. Mice were resuscitated with an intraperitoneal injection of 2 mL 0.9% NaCl. The eschar was allowed to cool and inoculated with 7×103 colony forming units (CFU) S. aureus mprF in Trypticase Soy Broth (TSB). 24 hours later the mice were anesthetized and the burn eschar was excised down to the fascia. NIKS and NIKShBD-3 tissues, measuring 2.8 × 2.9 cm in size, were placed dermal side down onto the excised wound bed and covered with a layer of non-adherent N-terface dressing (Winfield Laboratories, Richardson, TX). The graft was secured onto the wound site with Tegaderm transparent dressing (3M, St. Paul, MN) and VetBond (3M, St. Paul, MN). Negative control mice were treated identically with the exception of graft placement and served as the growth control to which the percentages of bacterial growth were normalized. After 72 hours, the animals were sacrificed, grafts were removed from the wound bed, and four 8 mm biopsy punches were taken from the underlying muscle bed. The skin substitute tissue and muscle tissue were weighed together and added to bacterial culturing medium. The samples were homogenized, diluted and spiral plated (Microbiology International, Frederick, MD). Colony forming units per gram (CFU/g) tissue was enumerated the following day using a plate counter (ProtoCOL, Microbiology International, Frederick, MD). All animal studies were conducted at Loyola University Medical Center (Maywood, IL) in collaboration with Stratatech Corporation. The Institutional Animal Care and Use Committee of Loyola University Medical Center approved the experimental protocol used in the animal studies.
RESULTS
Endogenous and hBD-3 transgene expression in monolayer and organotypic NIKS cultures
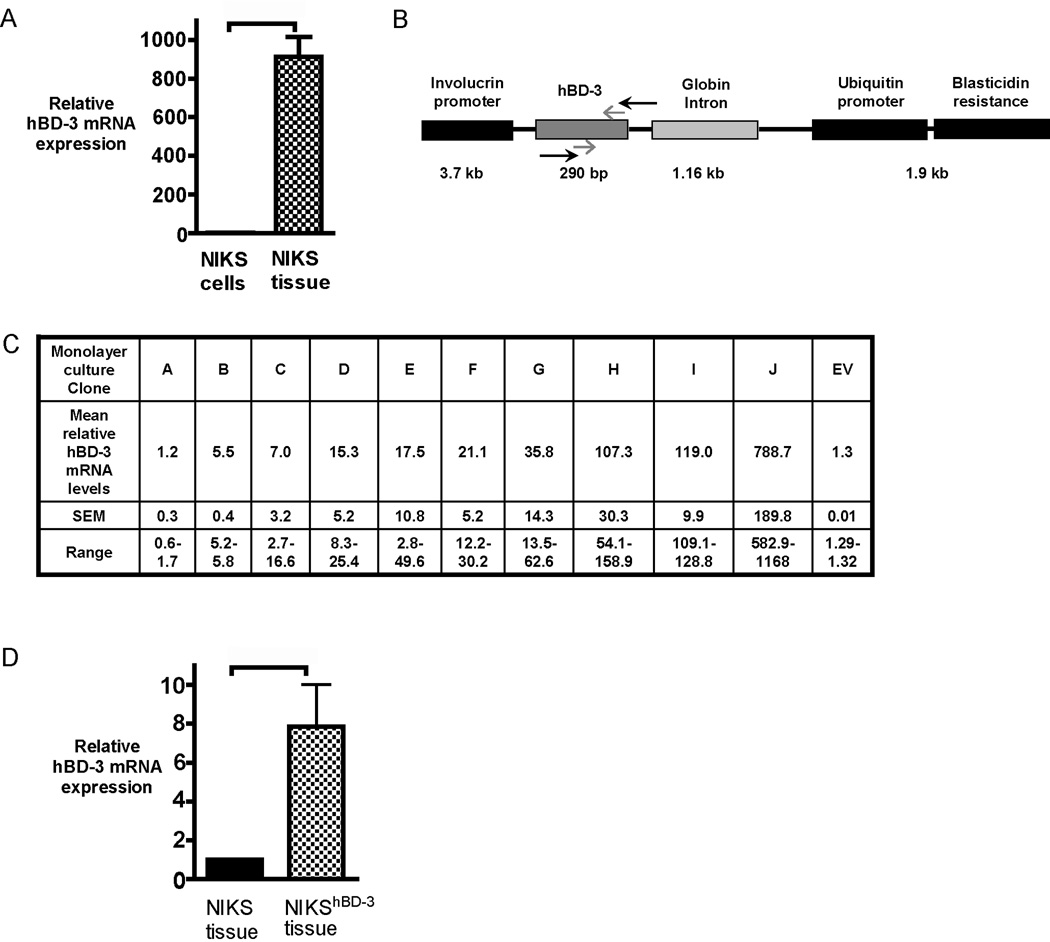
Expression of keratinocyte-specific genes are differentially regulated when cells are cultured in monolayer versus organotypic conditions. In comparison to monolayer culture, keratinocytes cultured using organotypic conditions undergo a dramatic increase in the expression of differentiation-specific genes.(19) hBD-3 gene expression has also been shown to increase when primary keratinocytes are grown as tissue using organotypic culturing methods.(20) Therefore, mRNA levels were compared using qPCR in post-confluent monolayer cultures of unmodified control NIKS keratinocytes and tissue generated from the organotypic culture of unmodified NIKS. Endogenous hBD-3 mRNA expression was substantially increased (900-fold) when the keratinocytes were grown in an organotypic culturing format (Figure 1 A). These results are in agreement with previous findings by others showing an increase in hBD-3 mRNA expression upon keratinocyte differentiation.(21)
Figure 1. Generation of a stable NIKS keratinocyte cell line exogenously expressing hBD-3.
(A) hBD-3 mRNA expression measured by qPCR of skin substitute tissue generated with NIKS keratinocytes (NIKS tissue) compared with post-confluent monolayer cultures of NIKS keratinocytes (NIKS cells). hBD-3 mRNA expression in NIKS tissue is expressed as a fold change relative to expression in NIKS monolayer arbitrarily set at 1. Bars represent mean + SEM (n=3). p value = 0.0031 calculated with the Wilcoxon signed rank test. (B) Schematic representation of the construct used for genetic engineering of NIKS keratinocytes. Black arrows represent primers used in RT-PCR to detect transgene expression for the initial clone screen to identify clones containing the construct. Grey arrows show approximate positions of qPCR amplified endogenous and transgene hBD-3. Sizes of gene products listed are not to scale. (C) Total hBD-3 mRNA expression (endogenous and exogenous) of NIKShBD-3 clones, designated A–J, in post-confluent monolayer cultures. Values are expressed as a fold change relative to hBD-3 mRNA expression in unmodified NIKS arbitrarily set at 1. Fold change is represented as the mean and standard error of the mean (SEM) of experimental. Empty Vector clone containing Inv-Ub-bsd construct without the hBD-3 insert is designated as EV. (D) hBD-3 mRNA expression measured by qPCR of NIKS tissue and NIKShBD-3 (Clone J) tissue. Values are expressed as a fold change relative to hBD-3 mRNA expression in untransfected NIKS tissue arbitrarily set at 1. Bars represent mean + SEM (n=3). p=0.0206 calculated with the Wilcoxon signed rank test, using logarithmic transformation of fold change for quantitative PCR data.
To augment the level of hBD-3 in NIKS keratinocytes monolayer cultures were genetically modified with naked DNA. To accomplish this NIKS keratinocytes were stably transfected with a linearized vector containing the full-length hBD-3 transgene by electroporation (Figure 1 B), and multiple independent transgene-expressing clones were obtained. Placement of the involucrin promoter upstream of the hBD-3 transgene allowed directed expression to the suprabasal epidermal layers in a tissue- and differentiation-specific manner. This strategy in concert with organotypic culture enabled high-level constitutive transgene expression of exogenous hBD-3 to be targeted to epidermal cells which normally produce endogenous hBD-3.(22)
Exogenous hBD-3 expression was qualitatively evaluated in monolayer culture by RT-PCR with transgene-specific primers (Figure 1 B). Clones which contained the transgene and exhibited normal keratinocyte growth characteristics in serial passage were expanded in monolayer culture and cryopreserved for further characterization. Transgene expression of hBD-3 was quantified by qPCR. qPCR analysis of post-confluent monolayer cultures of NIKShBD-3clones revealed a wide range of hBD-3 transgene expression, ranging from 1.2- to 789-fold expression compared to unmodified NIKS (Figure 1C). Clone J the highest expressing NIKShBD-3 clone, was selected for further investigation. Consistent with its high level of expression in monolayer culture (Figure 1 C), tissue produced using clone J expressed 7.9-fold more hBD-3mRNA as compared to tissue from unmodified NIKS (Figure 1 D). These results demonstrate the successful generation of a stable keratinocyte cell line with increased hBD-3 expression both in monolayer keratinocytes and in organotypic culture.
NIKShBD-3 produces normal skin substitute tissue
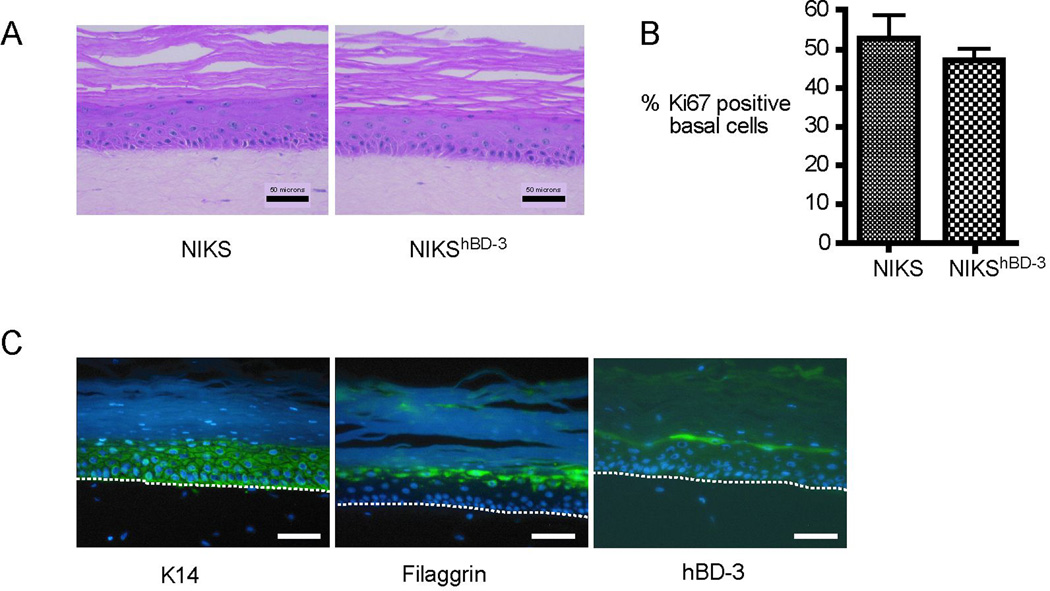
Histological assessment by H&E staining showed that NIKShBD-3 cells produce architecturally normal interfollicular skin tissue indistinguishable from that produced with unmodified NIKS cells or primary keratinocytes (Figure 2 A and data not shown).(13) The characteristic tissue architecture of skin results from the terminal differentiation program of keratinocytes as they commit to leave the proliferative basal layer. No evidence of alterations in growth and differentiation as a result of the increased hBD-3 transgene expression was observed in any tissue samples examined. NIKShBD-3 keratinocytes generate tissue with normal basal, spinous, granular and cornified layers appropriately located in the tissue.
Figure 2. Tissues created with NIKShBD-3 keratinocytes exhibit normal architecture, proliferation of basal keratinocytes, differentiation marker expression, and hBD-3 localization.
(A) NIKS and NIKShBD-3 tissues were fixed, sectioned, and stained with H&E to visualize tissue architecture. (B) Ki67 positive nuclei were counted in the basal layer of NIKS and NIKShBD-3 tissues. Proliferation is expressed as the percent of Ki67 positive cells/Hoechst positive cells per field at 100X magnification. At least 100 basal keratinocytes were scored per condition. Bars represent mean + SEM (n=3). Values are not significantly different; p=0.5 calculated with the Mann-Whitney U test. (C) IIF for K14, filaggrin, and hBD-3 was performed on NIKShBD-3 tissues. Dashed line indicates location of basement membrane of tissue. Scale bars represent 50 µm.
Proliferation of the keratinocytes in the basal layer of NIKS and NIKShBD-3 tissues was assessed by IIF for Ki67, a marker of growing, dividing cells. The staining revealed similar percentages of Ki67 positive cells indicating cell viability was comparable in both tissues (Figure 2 B). To determine whether exogenous hBD-3 expression altered the differentiation pattern of the tissue, IIF for the differentiation markers keratin 14 (K14) and filaggrin was performed on NIKShBD-3 tissues and revealed similar staining patterns as previously seen with unmodifiedNIKS keratinocytes (23) (Figure 2 C). Measurement of electrical impedance under continuous occlusion has been shown to correlate with transepidermal water loss and allows quantitation of barrier function.(24) NIKS and NIKShBD-3 tissues were assessed for barrier function using a dermal phase meter (DPM) measuring skin surface electrical impedance (SEI). (14) Measurements obtained for NIKShBD-3 tissues were comparable to those measurements obtained from the clinically tested NIKS tissues (15) (Supplemental Figure 1). Taken together, these results indicate that exogenous expression of the hBD-3 transgene in NIKS keratinocytes does not alter the expression or localization of early or late stage differentiation markers and does not affect cellular viability in organotypic culture.
Localization of hBD-3 to the differentiated layers of the epidermis has been demonstrated in intact human skin and skin substitute tissue generated by organotypic culture.(20, 22) IIF was performed to determine the localization of hBD-3 protein expression in NIKShBD-3 tissues. hBD-3 was present throughout the epidermis with a higher concentration in the upper granular layer immediately adjacent to the stratum corneum in NIKShBD-3 tissue (Figure 2 C), consistent withhBD-3’s localization in adult human skin and neonatal foreskin. These findings suggest thathBD-3 protein expression driven by the involucrin promoter is appropriately localized in NIKShBD-3 tissue. Taken together, these findings show that NIKShBD-3 tissues are architecturally and histologically comparable to interfollicular human skin.
Elevated hBD-3 protein in NIKShBD-3 tissue results in antimicrobial activity in vitro
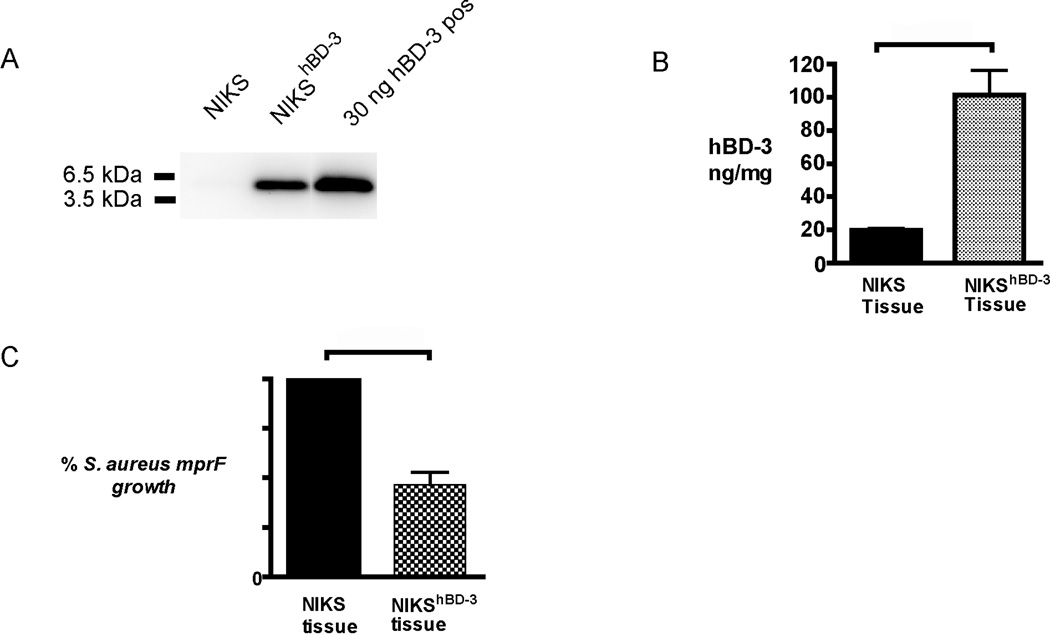
hBD-3 is a highly basic peptide with a molecular mass ~5 kDa that readily forms dimers in solution.(7) Detection of hBD-3 protein produced by keratinocytes in epidermal tissue or conditioned medium is difficult, often requiring the use of an affinity column, acid extraction, and/or concentration of the sample.(25) In our studies, hBD-3 protein was readily detected in lysate from NIKShBD-3 tissue by immunoblot analysis without use of protein enrichment techniques (Figure 3 A). An hBD-3 ELISA enabled quantification of hBD-3 protein levels in cell lysates from NIKS and NIKShBD-3 tissues and determined that NIKShBD-3 tissue contains approximately 5-fold more hBD-3 protein in cell lysate than control NIKS tissue (Figure 3 B).
Figure 3. hBD-3 protein expression and antimicrobial activity is elevated in NIKShBD-3 tissues in vitro.
(A) Lysates of NIKS and NIKShBD-3 tissues were analyzed by immunoblot. Samples containing 15 µg of total protein were loaded per lane. 30ng chemically synthesized hBD-3 was included as a positive control. Shown is a representative blot of hBD-3. (B) hBD-3 protein levels in cell lysate detected by ELISA. Protein levels are expressed as a concentration of hBD-3 present in the total protein of cell lysate Protein expression is represented as the mean and standard error of the mean (n=3) p=0.05 calculated with the Mann-Whitney U test. (C) Conditioned medium from NIKShBD-3 tissues were tested in a kinetic bacterial assay with S. aureus mprF. Values are calculated using area under the [growth] curve (AUC) from OD measurements of bacterial growth over 5 hours and are relative to conditioned medium from tissues made with unmodified NIKS set at 100%. Percent bacterial growth represents mean and SEM of AUC (n=3). p = 0.003 calculated with the Wilcoxon signed rank test.
The antimicrobial activity of medium conditioned for 24 hours by NIKS and NIKShBD-3 tissues was tested against S. aureus mprF in an in vitro kinetic bacterial assay using OD measurements to monitor bacterial growth. As the bacteria replicate, the solution becomes more turbid resulting in an increased OD. This technique has been used to estimate bacterial growth as a high-throughput substitute for agar plating methods that enumerate colony forming units.(26) S. aureus mprF exhibits an increased susceptibility to cationic peptides resulting from the deletion of the mprF virulence gene.(27) This choice of S. aureus strain allows for a sensitive discrimination in antimicrobial activity.(28) A 50% decrease in bacterial growth of S. aureus mprF was observed in the medium basally conditioned with NIKShBD-3 tissue in comparison to conditioned medium collected from control NIKS tissue (Figure 3 C). These in vitro findings demonstrate that NIKShBD-3 tissue produces and secretes sufficient hBD-3 to reduce the growth of S. aureus mprF relative to tissue made from unmodified NIKS cells.
NIKShBD-3 tissues exhibit increased antimicrobial activity in an in vivo murine burn infection model
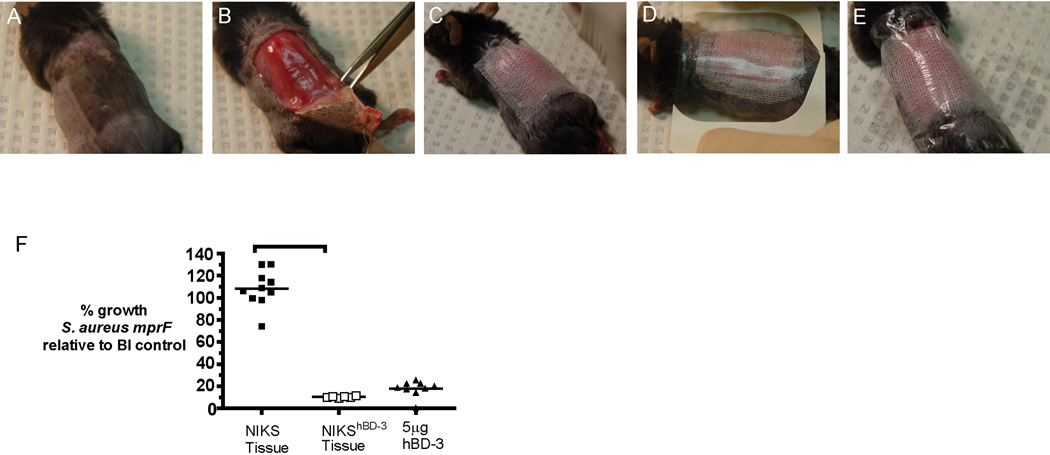
To assess the antimicrobial activity of NIKShBD-3 in vivo, we utilized a murine burn model in which C57BL/6J male mice were subjected to a 12% TBSA third degree scald burn, followed by inoculation with S. aureus mprF. NIKS and NIKShBD-3 tissues were transplanted on the excised wound 24 hours after burn and infection (Figure 4 A–E). Seventy-two hours after transplantation, the tissue and underlying muscle were harvested, weighed and homogenized in bacterial growth medium for quantitative culture. Mice treated for 3 days with NIKShBD-3 tissue exhibited a 1-log (90%) decrease in bacterial growth when compared to NIKS tissue (Figure 4 F). Treatment with NIKShBD-3 tissue resulted in a similar reduction of S. aureus mprF growth as compared to a single 5 µg dose of synthetic hBD-3 given at the same time as tissue transplantation. These findings suggest that increased tissue-specific hBD-3 expression results in enhanced antimicrobial activity of NIKShBD-3 tissue in vivo.
Figure 4. Application of NIKShBD-3 tissue for 72 hours on murine burn wounds infected with S. aureus mprF resulted in a 90% reduction in bacterial growth.
(A) Mouse receives burn and inoculation with S. aureus mprF 24 hours prior to transplantation of tissue to allow infection and invasion into subeschar muscle bed. (B) Eschar is excised immediately prior to graft placement. (C) NIKShBD-3 tissue is placed onto wound bed with a layer of N-terface non-adherent dressing. (D–E) Skin graft is secured with Tegaderm and VetBond for 72 hours. Mice were grafted with NIKS (n=10) or NIKShBD-3 (n=6) tissues. At the same time as tissue transplantation, 5 µg synthetic hBD-3 in 50 µl sterile water was topically applied onto the excised wound bed without skin substitute tissue as a positive control (n=9). (F) Quantitative cultures of the transplanted tissue and subeschar muscle were assessed 72 hours post-graft placement. Bacterial growth values are calculated as a percentage of the no graft control (BI=burn and infection only, n=8) growth set at 100%. p<0.01 calculated using one-way ANOVA corrected with Dunnett’s comparison.
DISCUSSION
Infection is a significant problem complicating the definitive closure of both acute and chronic wounds.(29) Therapeutic strategies using HDPs are an attractive alternative to traditional antibiotics due to the rapid, potent and broad spectrum nature of their antimicrobial activity coupled the inability of most pathogens to develop effective resistance to these ancient antibacterial peptides.(30) However, challenges in the production and continuous delivery of the HDPs, such as cost of peptide synthesis, peptide degradation by wound proteases, and systemic toxicities have limited development of these small bioactive peptides for therapeutic use.(31) Genetic manipulation is emerging as a therapeutic strategy for the treatment of skin disorders.(32) However, recent concerns have been raised regarding the safety, consistency, and continuous delivery of viral-based gene transfer.(33) For this reason, viral gene delivery of HDPs may be of limited therapeutic benefit and have substantial regulatory hurdles.(34–36) The NIKShBD-3 cells described here were derived from the NIKS, which are non-tumorigenic and adventitious agent-free progenitor keratinocytes amenable to genetic engineering. A Phase I/IIa study was designed primarily to evaluate the safety of the unmodified NIKS skin tissue. Completed in 2009, this clinical trial has provided critical preliminary safety data on NIKS tissue, with no evidence of safety concerns and no indication of an acute immune response to the NIKS cells or NIKS tissue. (14, 15) The tissue is currently being tested in a Phase Ib clinical trial to assess its safety and efficacy as an alternative to autografting for deep, partial thickness burns. Notably, in this clinical trial the NIKS skin tissue is allowed to remain on the wound to assess long-term endpoints. Although initial clinical testing of the tissue generated from unmodified NIKS cells is not predictive of the therapeutic benefit of bioengineered NIKS tissue, it supports the clinical feasibility. In the study described here, we demonstrate non-viral transfection of the NIKS cell line as an effective means to genetically engineer the stable tissue-specific expression of an hBD-3 transgene in keratinocytes. Furthermore, we show that skin substitute tissue expressing hBD-3 exhibits enhanced antimicrobial activity against S. aureus mprF in vitro and in vivo.
The external epithelial surfaces of the human body are covered with approximately 1012 commensal microorganisms which have co-evolved to survive in existence with their human host.(37) Constitutive expression of HDPs prevent the conversion of commensal bacteria to pathogenic bacteria and a recent report suggests that constitutive hBD-3 expression plays a direct role in maintaining the homeostatic balance between host and microorganism.(38) In a wound environment where the normal host defense mechanisms may be attenuated, it is desirable to develop a constant delivery system of hBD-3, such as the NIKShBD-3 tissue, to provide protection against microbial infection until intact healed skin is in place.
NIKS keratinocytes differentiate to form a multi-layered skin substitute tissue with normal epidermal architecture containing well-defined basal, spinous, granular and cornified layers.(13) Our results indicated that exogenous hBD-3 gene expression in NIKShBD-3 tissue did not alter the tissue architecture, barrier function, differentiation or proliferation of the tissue. The time- and concentration-dependent cytotoxicity to mammalian cells observed with naturally occurring HDPs such as protegrin-1 (39) and histone H1.2 (10) have led investigators to develop peptide engineering strategies to alter the structure of endogenous cationic peptides in an effort to decrease the negative effects on wound healing while preserving the antimicrobial activity. An example of this type of engineered peptide is proline-novispirin G10.(11) Studies with this peptide suggest that concentrations required for in vivo antimicrobial activity are cytotoxic to mammalian cells. Our strategy using a tissue-specific involucrin promoter to direct expression of the hBD-3 transgene to the differentiated layers of the epidermis results in continuous expression and secretion of the peptide similar to endogenous expression. This should mitigate the difficulties with cytotoxicity seen with synthetic peptides which require repeated applications at high concentrations for efficacy. Creation of a genetically engineered skin tissue with enhanced antimicrobial activity is desirable not only as a clinically useful antimicrobial therapeutic capable of the continuous delivery of hBD-3, but also having the potential to function as a temporary skin replacement with barrier function. Given the chemotactic properties of HDPs, it is also likely that the enhanced hBD-3 will recruit inflammatory cells to the wound area further facilitating bacterial clearance (40) Future studies will be designed to investigate the additional potential chemotactic properties of the NIKShBD-3 cells.
The complexities inherent in a wound environment are difficult to replicate in vitro and thus, analysis of in vitro antimicrobial activity is not sufficient to assess the functional immunomodulatory activities of HDPs. For example, peptide activation in response to signaling in an in vivo wound environment, including bacterial or cytokine stimulation, proteolytic processing in the wound bed, or the release of proteases from bacteria is not adequately addressed by in vitro assays.(41) Similar to our previous studies with genetically modified tissue (16), the increase in the antimicrobial activity of the NIKShBD-3 tissue in vivo (90% inhibition – 1-log kill) compared to in vitro (50% inhibition – see Figure 3 D) suggests that contact of the skin substitute tissue with the wound tissue, bacteria within the wound, or sustained delivery of hBD-3 over a longer time period resulted in enhanced antimicrobial activity of NIKShBD-3 tissue. We found that in vivo animal studies provided a more therapeutically relevant assessment of the functional bioactivity of human skin tissues genetically engineered to express hBD-3. The use of S. aureus mprF is an important first step in the assessment of antimicrobial activity of hBD-3-expressing human skin tissue. Future investigations will focus on wound pathogens such as methicillin-resistant S. aureus, Acinetobacter baumannii, and Pseudomonas aeruginosa as well as polymicrobial infections.
The wound environment is complex and dynamic throughout the phases of healing. Polymicrobial infections present in both acute and chronic wounds often require a combination of antimicrobial agents to control the bioburden.(42) hBD-3 is an attractive peptide for therapeutic use because of its broad spectrum of antimicrobial activity, lack of hemolytic activity at concentrations necessary for antimicrobial activity, and ability to recruit cellular mediators into the wound environment.(9) Reports of synergism between various HDPs and conventional antibiotics suggest the benefit of a combination therapeutic approach that would potentially exhibit lower cytotoxicity and a greater spectrum of antimicrobial activity than a single high dose antibiotic formulation.(40) Chronic wounds present unique challenges specifically related to the underlying pathology that led to the creation of the wound.(42) hBD-3 has already proven efficacious in an infected, diabetic wound animal model.(43) Furthermore, these challenges related to the wound etiology could be addressed by creating skin substitute tissue composed of a variety of genetically engineered NIKS keratinocytes expressing antimicrobial peptides and growth factors for custom-made tissues tailored to meet the needs of acute and chronic wounds.(44)
Microbial infections significantly delay healing and can result in increased morbidity and mortality.(45) Alternative therapies against microbial infection must be considered in light of the increasing prevalence of multi-drug resistant organisms that exist today. NIKS keratinocytes are amenable to non-viral, ex vivo genetic manipulation and incorporation into a skin substitute tissue, and have the potential for therapeutic use on wounds. The results of this study lay the groundwork for further testing of skin substitute tissues with elevated expression of host defense peptides in a clinical trial setting.
Supplementary Material
ACKNOWLEDGMENTS
We would like to sincerely thank Satoshi Kinoshita for the processing of histological samples, Dr. Cathy Rasmussen and Dr. Mary Lokuta for editorial assistance and Dr. Andreas Peschel for generously providing the S. aureus mprF strain. This work was supported by the National Institutes of Health NIAMS Grant R44 AR050349 awarded to LAH, Grant R01 HL074284 awarded to LAH and NIBIB Ruth L Kirschstein National Research Service Award Grant F31 EB005437 awarded to AG.
Abbreviations
- AUC
Area Under the Curve
- CFU
Colony Forming Units
- CM
Conditioned Medium
- DNA
Deoxyribonucleic Acid
- DPM
Dermal Phase Meter
- ELISA
Enzyme-linked Immunosorbent Assay
- hBD-3
Human Beta Defensin 3
- HDP
Host Defense Peptide
- H&E
Hematoxylin and Eosin
- IIF
Indirect Immunofluorescence
- K14
Keratin 14
- mRNA
messenger Ribonucleic Acid
- OD
Optical Density
- NIKS
Near-normal Immortalized Keratinocytes that produce Skin
- RT PCR
Reverse Transcriptase Polymerase Chain Reaction
- qPCR
Quantitative Polymerase Chain Reaction
- S. aureus
Staphylococcus aureus
- SEI
Surface electrical impedance
- TBSA
Total burn surface area
Footnotes
Please note that a conflict of interest exists for Stratatech affiliated authors.
REFERENCES
- 1.Niyonsaba F, Ogawa H. Protective roles of the skin against infection: Implication of naturally occurring human antimicrobial agents beta-defensins, cathelicidin LL-37 and lysozyme. J Dermatol Sci. 2005;40(3):157–168. doi: 10.1016/j.jdermsci.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 2.Edwards R, Harding KG. Bacteria and wound healing. Curr Opin Infect Dis. 2004;17(2):91–96. doi: 10.1097/00001432-200404000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Bowler PG. The 10(5) bacterial growth guideline: reassessing its clinical relevance in wound healing. Ostomy Wound Manage. 2003;49(1):44–53. [PubMed] [Google Scholar]
- 4.Bennett LL, Rosenblum RS, Perlov C, Davidson JM, Barton RM, Nanney LB. An in vivo comparison of topical agents on wound repair. Plast Reconstr Surg. 2001;108(3):675–687. doi: 10.1097/00006534-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 5.Gibson AL, Schurr MJ, Schlosser SJ, Comer AR, Allen-Hoffmann BL. Comparison of therapeutic antibiotic treatments on tissue-engineered human skin substitutes. Tissue Eng Part A. 2008;14(5):629–638. doi: 10.1089/tea.2007.0126. [DOI] [PubMed] [Google Scholar]
- 6.Lee SY, Kuti JL, Nicolau DP. Antimicrobial management of complicated skin and skin structure infections in the era of emerging resistance. Surg Infect (Larchmt) 2005;6(3):283–295. doi: 10.1089/sur.2005.6.283. [DOI] [PubMed] [Google Scholar]
- 7.Harder J, Bartels J, Christophers E, Schroder JM. Isolation and characterization of human beta -defensin-3, a novel human inducible peptide antibiotic. J Biol Chem. 2001;276(8):5707–5713. doi: 10.1074/jbc.M008557200. [DOI] [PubMed] [Google Scholar]
- 8.Pazgier M, Hoover DM, Yang D, Lu W, Lubkowski J. Human beta-defensins. Cell Mol Life Sci. 2006;63(11):1294–1313. doi: 10.1007/s00018-005-5540-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu Z, Hoover DM, Yang D, Boulegue C, Santamaria F, Oppenheim JJ, Lubkowski J, Lu W. Engineering disulfide bridges to dissect antimicrobial and chemotactic activities of human beta-defensin 3. Proc Natl Acad Sci U S A. 2003;100(15):8880–8885. doi: 10.1073/pnas.1533186100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacobsen F, Baraniskin A, Mertens J, Mittler D, Mohammadi-Tabrisi A, Schubert S, Soltau M, Lehnhardt M, Behnke B, Gatermann S, Steinau HU, Steinstraesser L. Activity of histone H1.2 in infected burn wounds. J Antimicrob Chemother. 2005;55(5):735–741. doi: 10.1093/jac/dki067. [DOI] [PubMed] [Google Scholar]
- 11.Jacobsen F, Mohammadi-Tabrisi A, Hirsch T, Mittler D, Mygind PH, Sonksen CP, Raventos D, Kristensen HH, Gatermann S, Lehnhardt M, Daigeler A, Steinau HU, Steinstraesser L. Antimicrobial activity of the recombinant designer host defence peptide P-novispirin G10 in infected full-thickness wounds of porcine skin. J Antimicrob Chemother. 2007;59(3):493–498. doi: 10.1093/jac/dkl513. [DOI] [PubMed] [Google Scholar]
- 12.Zhang L, Falla TJ. Host defense peptides for use as potential therapeutics. Curr Opin Investig Drugs. 2009;10(2):164–171. [PubMed] [Google Scholar]
- 13.Allen-Hoffmann BL, Schlosser SJ, Ivarie CA, Sattler CA, Meisner LF, O'Connor SL. Normal growth and differentiation in a spontaneously immortalized near-diploid human keratinocyte cell line, NIKS. J Invest Dermatol. 2000;114(3):444–455. doi: 10.1046/j.1523-1747.2000.00869.x. [DOI] [PubMed] [Google Scholar]
- 14.Centanni JM, Straseski JA, Wicks A, Hank JA, Rasmussen CA, Lokuta MA, Schurr MJ, Foster KN, Faucher LD, Caruso DM, Comer AR, Allen-Hoffmann BL. StrataGraft skin substitute is well-tolerated and is not acutely immunogenic in patients with traumaticwounds: results from a prospective, randomized, controlled dose escalation trial. Ann Surg. 2011;254(4):672–683. doi: 10.1097/SLA.0b013e318210f3bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schurr MJ, Foster KN, Centanni JM, Comer AR, Wicks A, Gibson AL, Thomas-Virnig CL, Schlosser SJ, Faucher LD, Lokuta MA, Allen-Hoffmann BL. Phase I/II clinical evaluation of StrataGraft: a consistent, pathogen-free human skin substitute. J Trauma. 2009;66(3):866–873. doi: 10.1097/TA.0b013e31819849d6. discussion 73-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas-Virnig CL, Centanni JM, Johnston CE, He LK, Schlosser SJ, Van Winkle KF, Chen R, Gibson AL, Szilagyi A, Li L, Shankar R, Allen-Hoffmann BL. Inhibition of multidrug-resistant Acinetobacter baumannii by nonviral expression of hCAP-18 in a bioengineered human skin tissue. Mol Ther. 2009;17(3):562–569. doi: 10.1038/mt.2008.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoover DM, Wu Z, Tucker K, Lu W, Lubkowski J. Antimicrobial characterization of human beta-defensin 3 derivatives. Antimicrob Agents Chemother. 2003;47(9):2804–2809. doi: 10.1128/AAC.47.9.2804-2809.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stevenson JM, Gamelli RL, Shankar R. A mouse model of burn wounding and sepsis. Methods Mol Med. 2003;78:95–105. doi: 10.1385/1-59259-332-1:95. [DOI] [PubMed] [Google Scholar]
- 19.Eckert RL, Crish JF, Robinson NA. The epidermal keratinocyte as a model for the study of gene regulation and cell differentiation. Physiol Rev. 1997;77(2):397–424. doi: 10.1152/physrev.1997.77.2.397. [DOI] [PubMed] [Google Scholar]
- 20.Supp DM, Karpinski AC, Boyce ST. Expression of human beta-defensins HBD-1, HBD-2, and HBD-3 in cultured keratinocytes and skin substitutes. Burns. 2004;30(7):643–648. doi: 10.1016/j.burns.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 21.Pernet I, Reymermier C, Guezennec A, Branka JE, Guesnet J, Perrier E, Dezutter-Dambuyant C, Schmitt D, Viac J. Calcium triggers beta-defensin (hBD-2 and hBD-3) and chemokine macrophage inflammatory protein-3 alpha (MIP-3alpha/CCL20) expression in monolayers of activated human keratinocytes. Exp Dermatol. 2003;12(6):755–760. doi: 10.1111/j.0906-6705.2003.00086.x. [DOI] [PubMed] [Google Scholar]
- 22.Sawamura D, Goto M, Shibaki A, Akiyama M, McMillan JR, Abiko Y, Shimizu H. Beta defensin-3 engineered epidermis shows highly protective effect for bacterial infection. Gene Ther. 2005;12(10):857–861. doi: 10.1038/sj.gt.3302472. [DOI] [PubMed] [Google Scholar]
- 23.Straseski JA, Gibson AL, Thomas-Virnig CL, Allen-Hoffmann BA. Oxygen deprivation inhibits basal keratinocyte proliferation in a model of human skin and induces regio-specific changes in the distribution of epidermal adherens junction proteins, aquaporin-3 and glycogen. Wound Repair and Regeneration. 2009;17(4):606–616. doi: 10.1111/j.1524-475X.2009.00515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wickett RR, Nath V, Tanaka R, Hoath SB. Use of continuous electrical capacitance and transepidermal water loss measurements for assessing barrier function in neonatal rat skin. Skin Pharmacol. 1995;8(4):179–185. doi: 10.1159/000211344. [DOI] [PubMed] [Google Scholar]
- 25.Sorensen OE, Thapa DR, Roupe KM, Valore EV, Sjobring U, Roberts AA, Schmidtchen A, Ganz T. Injury-induced innate immune response in human skin mediated by transactivation of the epidermal growth factor receptor. J Clin Invest. 2006;116(7):1878–1885. doi: 10.1172/JCI28422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lehtinen J, Jarvinen S, Virta M, Lilius EM. Real-time monitoring of antimicrobial activity with the multiparameter microplate assay. J Microbiol Methods. 2006;66(3):381–389. doi: 10.1016/j.mimet.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Peschel A, Jack RW, Otto M, Collins LV, Staubitz P, Nicholson G, Kalbacher H, Nieuwenhuizen WF, Jung G, Tarkowski A, van Kessel KP, van Strijp JA. Staphylococcus aureus resistance to human defensins and evasion of neutrophil killing via the novel virulence factor MprF is based on modification of membrane lipids with l-lysine. J Exp Med. 2001;193(9):1067–1076. doi: 10.1084/jem.193.9.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Braff MH, Zaiou M, Fierer J, Nizet V, Gallo RL. Keratinocyte production of cathelicidin provides direct activity against bacterial skin pathogens. Infect Immun. 2005;73(10):6771–6781. doi: 10.1128/IAI.73.10.6771-6781.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Broughton G, 2nd, Janis JE, Attinger CE. Wound healing: an overview. Plast Reconstr Surg. 2006;117(7 Suppl):1e-S–32e-S. doi: 10.1097/01.prs.0000222562.60260.f9. [DOI] [PubMed] [Google Scholar]
- 30.Hancock RE, Sahl HG. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotechnol. 2006;24(12):1551–1557. doi: 10.1038/nbt1267. [DOI] [PubMed] [Google Scholar]
- 31.Zhang L, Falla TJ. Antimicrobial peptides: therapeutic potential. Expert Opin Pharmacother. 2006;7(6):653–663. doi: 10.1517/14656566.7.6.653. [DOI] [PubMed] [Google Scholar]
- 32.Hengge UR. Gene therapy progress and prospects: the skin--easily accessible, but still far away. Gene Ther. 2006;13(22):1555–1563. doi: 10.1038/sj.gt.3302855. [DOI] [PubMed] [Google Scholar]
- 33.Scharschmidt T, Lo B. Clinical trial design issues raised during recombinant DNA advisory committee review of gene transfer protocols. Hum Gene Ther. 2006;17(4):448–454. doi: 10.1089/hum.2006.17.448. [DOI] [PubMed] [Google Scholar]
- 34.Carretero M, Del Rio M, Garcia M, Escamez MJ, Mirones I, Rivas L, Balague C, Jorcano JL, Larcher F. A cutaneous gene therapy approach to treat infection through keratinocyte-targeted overexpression of antimicrobial peptides. Faseb J. 2004;18(15):1931–1933. doi: 10.1096/fj.04-1515fje. [DOI] [PubMed] [Google Scholar]
- 35.Jacobsen F, Mittler D, Hirsch T, Gerhards A, Lehnhardt M, Voss B, Steinau HU, Steinstraesser L. Transient cutaneous adenoviral gene therapy with human host defense peptide hCAP-18/LL-37 is effective for the treatment of burn wound infections. Gene Ther. 2005;12(20):1494–1502. doi: 10.1038/sj.gt.3302568. [DOI] [PubMed] [Google Scholar]
- 36.Lee PH, Ohtake T, Zaiou M, Murakami M, Rudisill JA, Lin KH, Gallo RL. Expression of an additional cathelicidin antimicrobial peptide protects against bacterial skin infection. Proc Natl Acad Sci U S A. 2005;102(10):3750–3755. doi: 10.1073/pnas.0500268102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tlaskalova-Hogenova H, Stepankova R, Hudcovic T, Tuckova L, Cukrowska B, Lodinova-Zadnikova R, Kozáková H, Rossmann P, Bártová J, Sokol D, Funda DP, Borovská D, Reháková Z, Sinkora J, Hofman J, Drastich P, Kokesová A. Commensal bacteria (normal microflora), mucosal immunity and chronic inflammatory and autoimmune diseases. Immunol Lett. 2004;93(2–3):97–108. doi: 10.1016/j.imlet.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 38.Kisich KO, Howell MD, Boguniewicz M, Heizer HR, Watson NU, Leung DY. The Constitutive Capacity of Human Keratinocytes to Kill Staphylococcus. J Invest Dermatol. 2007;26:26. doi: 10.1038/sj.jid.5700861. [DOI] [PubMed] [Google Scholar]
- 39.Steinstraesser L, Klein RD, Aminlari A, Fan MH, Khilanani V, Remick DG, Su GL, Wang SC. Protegrin-1 enhances bacterial killing in thermally injured skin. Crit Care Med. 2001;29(7):1431–1437. doi: 10.1097/00003246-200107000-00022. [DOI] [PubMed] [Google Scholar]
- 40.Chen X, Niyonsaba F, Ushio H, Okuda D, Nagaoka I, Ikeda S, Okumura K, Ogawa H. Synergistic effect of antibacterial agents human beta-defensins, cathelicidin LL-37 and lysozyme against Staphylococcus aureus and Escherichia coli. J Dermatol Sci. 2005;40(2):123–132. doi: 10.1016/j.jdermsci.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 41.Yamasaki K, Schauber J, Coda A, Lin H, Dorschner RA, Schechter NM, Bonnart C, Descargues P, Hovnanian A, Gallo RL. Kallikrein-mediated proteolysis regulates the antimicrobial effects of cathelicidins in skin. Faseb J. 2006;20(12):2068–2080. doi: 10.1096/fj.06-6075com. [DOI] [PubMed] [Google Scholar]
- 42.Bowler PG. Wound pathophysiology, infection and therapeutic options. Ann Med. 2002;34(6):419–427. doi: 10.1080/078538902321012360. [DOI] [PubMed] [Google Scholar]
- 43.Hirsch T, Spielmann M, Zuhaili B, Fossum M, Metzig M, Koehler T, Steinau HU, Yao F, Onderdonk AB, Steinstraesser L, Eriksson E. Human beta-defensin-3 promotes wound healing in infected diabetic wounds. J Gene Med. 2009;11(3):220–228. doi: 10.1002/jgm.1287. [DOI] [PubMed] [Google Scholar]
- 44.Rasmussen CA, Gibson AL, Schlosser SJ, Schurr MJ, Allen-Hoffmann BL. Chimeric composite skin substitutes for delivery of autologous keratinocytes to promote tissue regeneration. Ann Surg. 2010;251(2):368–376. doi: 10.1097/SLA.0b013e3181c1ab5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McGuckin M, Goldman R, Bolton L, Salcido R. The clinical relevance of microbiology in acute and chronic wounds. Adv Skin Wound Care. 2003;16(1):12–23. doi: 10.1097/00129334-200301000-00011. quiz 4–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.