Abstract
Background: Dietary variety is a factor that influences consumption but has received little attention in obesity treatment.
Objective: This study examined the effect of limiting the variety of different non-nutrient-dense, energy-dense foods (NND-EDFs) (ie, chips, ice cream, cookies) on dietary intake and weight loss during an 18-mo lifestyle intervention.
Design: Two hundred two adults aged 51.3 ± 9.5 y with a BMI (in kg/m2) of 34.9 ± 4.3 (57.8% women, 92.2% white) were randomly assigned to 1 of 2 interventions: Lifestyle (1200–1500 kcal/d, ≤30% of energy as fat; n = 101) or Lifestyle + limited variety (LV) (limit variety of NND-EDFs, ie, 2 choices; n = 101). Both interventions involved 48 group sessions. Dietary intake, NND-EDF hedonics, NND-EDF variety in the home, and weight were assessed at 0, 6, 12, and 18 mo.
Results: Intent-to-treat analyses showed that the Lifestyle+LV group consumed less variety (P < 0.01) and energy daily (P < 0.05) from NND-EDFs than did the Lifestyle group at 6, 12, and 18 mo. The Lifestyle+LV group consumed less total energy daily (P < 0.05) at 6 mo than did the Lifestyle group. The Lifestyle+LV group reported less (P < 0.05) NND-EDF variety in the home at 6 and 18 mo than did the Lifestyle group. The hedonics of one chosen NND-EDF decreased more (P < 0.05) in the Lifestyle+LV group. Despite these effects, no difference in percentage weight loss occurred at 18 mo (Lifestyle+LV: −9.9 ± 7.6%; Lifestyle: −9.6 ± 9.2%).
Conclusions: Limitations in dietary variety decreased intakes in the targeted area but did not affect weight loss. Limiting variety in more areas may be needed to improve weight loss and weight-loss maintenance. This trial was registered at clinicaltrials.gov as NCT01096719.
INTRODUCTION
Approximately 2 of every 3 adults in the United States are overweight or obese (1), which negatively affects the health of the population (2–5). The Dietary Guidelines for Americans, 2010 (6) highlight the importance of consuming fewer calories while making healthy food choices to reduce morbidity and mortality. One dietary factor that is known to influence consumption and dietary quality, but has received little attention in the treatment of obesity, is dietary variety (7, 8).
Experimental research in animals and humans has found that meals and diets containing less variety result in reduced intakes (7). Within observational research when the relation between dietary variety and weight management has been examined, results indicate that consuming less variety in the energy-dense food groups is associated with greater weight loss and long-term weight-loss maintenance (9, 10). The research in variety suggests that a prescription that focuses on reducing variety in energy-dense food groups may assist with decreasing overall energy and fat intake, thus improving weight loss and weight-loss maintenance.
One proposed mechanism for the effect of reduced variety on intake is monotony, in which food hedonics (ie, liking) decrease across time with repeated consumption of a food (8). As hedonics decrease, consumption is expected to decrease. With limited variety (LV) in a food group, there should be a decrease in hedonics for the few foods consistently consumed; therefore, intake from these few foods should decrease, causing an overall decrease in consumption from that food group. However, not all research has found a reduced intake of a food when monotony occurs (11).
Within the context of a lifestyle intervention, a prescription to limit variety within a food group may also alter the amount of variety in the targeted food group available in the home environment. If the number of different foods within the targeted food group is reduced in the home environment, intake of that food group may decrease, because a food environmental cue (variety) that prompts consumption has been removed (12).
Thus, this investigation examined the influence of a novel dietary prescription that limited the number of different non-nutrient-dense, energy-dense foods (NND-EDFs) (ie, chips, ice cream, and cookies) consumed during an 18-mo weight-loss intervention. Two hundred two overweight and obese participants were randomly assigned to a standard behavioral lifestyle intervention (Lifestyle) or to a standard behavioral lifestyle intervention that also limited the number of different NND-EDFs consumed to 2 (Lifestyle+LV). NND-EDF variety, dietary intake, NND-EDF hedonics, home availability of NND-EDF variety, and weight were assessed at 0, 6, 12, and 18 mo. Compared with the Lifestyle group, it was hypothesized that the Lifestyle+LV group would consume less energy from NND-EDFs, consume less total energy and percentage energy from fat, show greater reductions in NND-EDF hedonics and home availability of NND-EDF variety, and have a greater percentage of weight loss.
SUBJECTS AND METHODS
Participants
Between July 2006 and August 2008, potential participants were recruited through newspaper advertisements, flyers, and posters and phone-screened for eligibility in Providence, RI, and Knoxville, TN. The eligibility criteria included an age of 21 to 65 y and a BMI (in kg/m2) between 27 and 45. Individuals were excluded if they could not walk 2 blocks; reported a heart condition, chest pain during periods of activity or rest, or loss of consciousness on the Physical Activity Readiness Questionnaire (13); were taking weight-loss medications or participating in a weight-loss program; had undergone bariatric surgery; were pregnant, lactating, or <6 mo postpartum or planned to become pregnant during the time frame of the investigation; were allergic to foods used in hedonic measures; and were consuming <5 different types of NND-EDFs. The protocol was approved by the Institutional Review Board at The Miriam Hospital in Providence, RI, and at the University of Tennessee, Knoxville, TN.
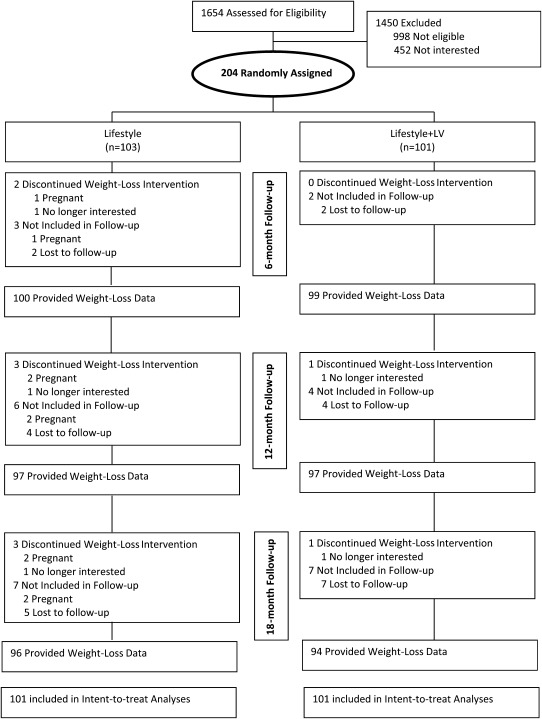
A total of 1654 participants (813 in Rhode Island and 841 in Tennessee) were phone-screened for eligibility, and those participants who were initially eligible were invited to an in-person orientation during which informed consent was obtained by the research staff. Three hundred sixty potential participants attended the orientation, and 204 participants (95 in Providence, RI, and 109 in Knoxville, TN) provided signed consent, completed baseline assessments, and were randomly assigned to 1 of the 2 conditions. Of the 204 participants, 2 participants were withdrawn from the study because they became pregnant during the 18-mo intervention; thus, the total number of randomly assigned participants included in all analyses was 202. See Figure 1 for participant flow.
FIGURE 1.
Participant flow.
Study design
Participants were assigned by using permuted-block randomization within strata (sex) to 1 of the 2 conditions in a 1:1 allocation ratio. Randomization and randomization assignment were conducted by the study statistician (JLF). Participants were informed at the start of the first group session of their randomization assignment by the group interventionist; thus, both participant and interventionist were aware of randomization assignment. Whereas participants were aware that there were 2 groups within the trial, participants were unaware of the study hypothesis and which measures assessed proposed mediators. The primary dependent variables were dietary intake and percentage weight loss. Additional measures included change in NND-EDF hedonics and home availability of NND-EDF variety. Measures were taken at 0, 6, 12, and 18 mo, with participants receiving $25 (6 and 12 mo) and $50 (18 mo) in compensation for completing follow-up assessments.
Sample size
Our sample size calculations presumed 2-sided hypothesis testing at the 18-mo follow-up, with type 1 error rate (α) equal to 0.05. One hundred participants randomly assigned to each condition were required to reject with 80% power the null hypothesis of no treatment difference compared with the alternative that the treatment difference is ≥0.4 (effect size) (14).
Intervention
Lifestyle interventions contain 3 components: a cognitive behavioral intervention, a diet prescription, and a physical activity prescription. The 3 components are designed to assist participants with a developing a healthy lifestyle to achieve weight loss of ≥10% (12, 15, 16).
Treatment structure
The 18-mo intervention consisted of 48 group meetings lasting 60 min each. These meetings occurred weekly from month 1 to month 6 and then twice a month from month 7 to month 18. The meetings were led by an experienced research interventionist (either master or doctoral level) with expertise in nutrition, exercise physiology, and behavior modification and were delivered in a research setting. Separate group meetings occurred for the 2 conditions. Sessions covered lessons on behavioral and cognitive skills (self-monitoring, stimulus control, problem-solving, preplanning, goal setting, cognitive restructuring, social support development, and relapse prevention) to help with changing dietary and physical activity behaviors and were modeled after lessons used in the Diabetes Prevention Program (17). Each session began with a group discussion on progress on intervention goals, which was followed by a lesson and the assignment of homework that would assist participants in meeting intervention goals.
Energy and fat goals
Participants were instructed to consume a standard energy- and fat-restricted diet. Daily calorie goals were based on study entry weight: with 1200 kcal/d prescribed for an entry weight ≤90.9 kg (200 lb) and 1500 kcal/d prescribed for an entry weight of >90.9 kg (200 lb). Fat intake was restricted to 30% of energy from fat. A sample meal plan, based on recommendations of MyPyramid (18), was provided to help participants consume a balanced diet while meeting energy and fat goals. Participants used a diary to record their daily energy and fat gram intake from food items and beverages. Diaries were turned in weekly for months 1–6, and twice a month from months 7–18, so that written feedback on food choices, dietary goals, and other problematic eating behaviors could be provided to participants. Participants were also educated on how to adjust caloric intake for weight maintenance, to prevent weight regain, in sessions focused on relapse prevention (eg, increase caloric intake by 100 kcal/d for 1 wk, keeping other aspects of the diet and physical activity the same, until weight maintenance occurred).
Physical activity goals
Participants were instructed to gradually increase moderate-intensity physical activity to ≥40 min/d 5 times/wk. Participants were encouraged to walk briskly and accumulate time spent being physically active by engaging in multiple short bouts (eg, ≥10 min in length). Participants were also given a pedometer and a goal of 10,000 steps per day (19). Daily steps and minutes of physical activity were recorded in the same diary in which consumption was recorded.
Limiting variety prescription
The LV prescription was designed to reduce the number of different NND-EDFs consumed to only 2 self-selected, chosen NND-EDFs. NND-EDFs were described to participants as foods that were energy-dense, of low nutrient value, and consumed as any component of a meal or snack and were grouped into the specific food groups from MyPyramid (18). NND-EDFs included baked goods, granola/snack bars, high-fat crackers, and flavored popcorn (bread, cereal, rice, and pasta); flavored dairy drinks, frozen dairy-based desserts, frozen yogurt, ice cream, ice milk, and pudding (milk, yogurt, and cheese); and candy, chips, salty snacks, chocolate, frozen desserts, gelatin desserts, and sherbet (fats, oils, and sweets). Modified NND-EDFs (eg, reduced-fat cookies), except for calorie-free modified foods (eg, sugar-free gelatin), were included in this category because they compete with healthier, more nutrient-dense food choices and still can be a significant source of calories. Moreover, they are sensory-similar to their nonmodified counterparts. Participants were informed that reducing variety in the NND-EDF group helped reduce intake from this food group, which assisted them in meeting daily energy and fat gram goals.
During the baseline assessments and before randomization, participants listed all NND-EDFs, including all flavors of the NND-EDFs (ie, chocolate chip cookie rather than just cookie), that they had consumed within the previous 28 d as part of any meal or snack. Participants also reported their liking of each NND-EDF, using a Likert-type scale, and their frequency of consumption of each NND-EDF over the previous 28 d. Each NND-EDF reported consumed received a score, which was the product of the liking × the frequency score. The 6 NND-EDFs with the highest product scores were presented to the participants, and from this list participants were asked to choose 2 that they would like to include in their diet during the intervention. Participants were encouraged to pick NND-EDFs that they felt they could consume while also working toward consuming a low-energy, low-fat diet (ie, they were advised to not choose an NND-EDF in which it would be challenging to consume only one serving at a time). The flavor of NND-EDFs was also defined. For example, rather than just selecting ice cream, participants chose the flavor of ice cream, such as strawberry ice cream. Seventy-nine percent of participants selected a sweet and savory NND-EDF, 19% selected 2 sweet NND-EDFs, and 2% selected 2 savory NND-EDFs for their 2 chosen snack foods.
In the initial treatment session, after being made aware of randomization assignment, participants in Lifestyle+LV were reminded of the 2 NND-EDFs that they selected. Participants in this condition were instructed to eat no other NND-EDFs, except the 2 selected, throughout the intervention as any part of a meal or snack. Participants were not given instructions regarding any specific amount of the chosen NND-EDFs to consume or the frequency of consumption of these foods. Participants recorded NND-EDF consumption in the daily diary, indicating whether the NND-EDF was a “chosen” NND-EDF or “other” NND-EDF. Participants were informed that the goal was to limit NND-EDF consumption to only the 2 chosen NND-EDFs.
Measures
Dependent measures were collected in a research setting by trained research staff blinded to the randomization assignment at 0, 6, 12, and 18 mo, unless otherwise indicated.
Demographic characteristics and anthropometric measures
Basic demographic information (eg, sex, age, and education level) was obtained by self-report at baseline only. Height was measured to the nearest millimeter at entry into the trial by using a stadiometer, and weight was measured with a calibrated digital scale at 0, 6, 12, and 18 mo while the participants were wearing light street clothes and recorded to the nearest 0.05 kg. BMI was calculated as weight (in kg)/height (in m)2. Percentage weight loss was calculated as follows: amount of weight loss from baseline/baseline weight × 100.
Dietary intake
The number of different NND-EDFs consumed, energy intake from NND-EDFs, overall energy and percentage energy from the macronutrients, and grams of saturated and trans fat, grams of added sugars, and mg of cholesterol per 1000 kcal were assessed by 3 random (2 weekdays and 1 weekend day) 24-h dietary recalls collected by phone over a 1-wk period at 0, 6, 12, and 18 mo.
Trained interviewers blinded to the intervention and intervention assignment, from the Cincinnati Center for Nutritional Research and Analysis, conducted the 24-h dietary recalls. Each 24-h recall was completed by using the Nutrition Data System Software for Research developed by the Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN. Participants were given 2-dimensional food shapes to help with estimating portion sizes. Nutrient data were computed from this information, and the nutrient food grouping system in Nutrition Data System Software for Research was used to calculate information specific to NND-EDFs. All dietary variables, except for the variety of NND-EDFs, were calculated as means over the 3 d of recall. The variety of NND-EDFs was calculated as the number of different NND-EDFs consumed over the 3 d, regardless of the quantity consumed. NND-EDFs in the same category but of a different flavor (ie, chocolate chip cookies and peanut butter cookies) were counted as 2 NND-EDFs.
Because the variety of food choices in the diet has been found to greatly increase from day 1 to day 3, but to show very little increase after 10 d (20), NND-EDF variety consumed was also calculated over a 28-d period. Participants were asked to recall events in the 28 d occurring before the assessment appointment that may affect eating (ie, vacations, celebrations, visitors, etc), similar to what is done for the Eating Disorders Examination (21). A list of NND-EDFs was provided to participants, and they indicated whether the food had been eaten as part of a meal or snack during the previous 28 d, regardless of the quantity consumed. For each food consumed, participants were also asked to note the flavor consumed. Total variety was the sum of different foods consumed. This measure was also completed at 0, 6, 12, and 18 mo.
Change in hedonic ratings of NND-EDFs
To assess for long-term monotony, participants were asked to taste and rate the pleasantness of 2 NND-EDFs (3-g samples each) (22) by using 100-mm visual analog scales, anchored on the left with “very unpleasant” and on the right with “very pleasant.” Measures were taken at the same time of day at 0, 3, 6, 12, and 18 mo during the intervention meetings. Participants were instructed to not eat 2 h before the intervention session in which the measure occurred. When participants came to the session, they were given a small preload of ∼140 kcal (cereal bar) that did not contain flavors of any participant's chosen NND-EDF to consume to ensure that participants were not overly hungry during the taste test. After consuming the preload, the participants were given the samples to taste. For participants in the Lifestyle+LV group, the NND-EDFs provided to them were their chosen 2 NND-EDFs. Participants in the Lifestyle group were matched (yoked) to a participant in the Lifestyle+LV condition who had chosen sensory-similar NND-EDFs at the baseline assessments, so that they also received NND-EDFs in the taste test that they highly liked and preferred (ie, one of the Lifestyle+LV participant's chosen NND-EDFs = chocolate ice cream; one of the yoked Lifestyle participant's chosen NND-EDFs = chocolate pudding; both participants received chocolate ice cream for the measure). The same NND-EDFs were presented to the same participants at each taste test. Change in ratings from baseline to each measure were calculated for each of the 2 foods separately, with negative values indicating a decrease in pleasantness and positive numbers indicating an increase in pleasantness (23). Using the previously chosen NND-EDF example, changes in ratings from baseline to each measure for chocolate ice cream were calculated and compared between the Lifestyle+LV participant and their yoked Lifestyle counterpart.
Home availability of NND-EDF variety
The participants completed a checklist of the NND-EDFs available in their homes (ie, stored in their refrigerator and cabinets) at 0, 6, 12, and 18 mo. This self-report questionnaire has acceptable test-retest and interrater reliability (24). The checklist included only NND-EDF categories. The participants were asked to list the specific flavors of the NND-EDFs that they had in their homes that fell within the larger category (ie, list all flavors of cookies). The total number of different NND-EDFs listed was summed.
Physical activity
Self-reported physical activity was assessed by using the Paffenbarger Activity Questionnaire (25). This questionnaire yields estimates of the total energy expended in all self-reported physical activity per week based on flights of stairs climbed per day, city blocks walked per day (assesses for both structured walking and lifestyle walking), and hours of structured activity acquired within a typical week. The Paffenbarger Activity Questionnaire has been shown to be significantly correlated with an objective measure of physical activity (26).
Compliance and retention
Compliance was calculated as the mean number of sessions and number of diaries completed by each condition. Follow-up rates at the 6-, 12-, and 18-mo assessments were calculated to determine retention rates.
Serious adverse effects
At each assessment, participants were asked to report any injury, allergy, or health condition potentially related to the intervention
Statistical analysis
Differences in baseline characteristics between the conditions were examined by using chi-square and independent t tests for nominal and interval/ratio data, respectively. Differences were examined by intervention site, and the data collected from both sites were collapsed because no differences occurred. For the analyses of dietary intake and NND-EDF home availability, condition differences at 0, 6, 12, and 18 mo were examined by using a mixed-factor ANOVA. Percentage weight loss was also examined by using a mixed-factor ANOVA, with condition (Lifestyle+LV and Lifestyle) as the between-subjects factor and time (6, 12, and 18 mo) as the within-subjects factor. These analyses were conducted by using an intent-to-treat analysis, with missing data filled by using a multiple imputation strategy (27). If it is believed that missingness or dropout depends on program adherence and weight loss (ie, if those who are gaining weight more rapidly are more likely to drop out), then analyses must proceed using assumptions that cannot be empirically verified (28); therefore, the model was fit under several assumptions. Participants were assumed to have returned, on average, to their baseline value. Instead of simply filling in the baseline value, a multiple imputation strategy to account for uncertainty in the filling-in process was used (27). Specifically, for each participant with a missing value, 5 random variables from a normal distribution that has a mean equal to the baseline variable and variance equal to the estimated variance for the variables of other participants at the time when the value is missing was created. This process led to 5 complete data sets, each of which was analyzed by using the ANOVA model, and treatment effects were computed by averaging the appropriate regression coefficient across models. When appropriate, Greenhouse-Geisser probability levels were used to adjust for sphericity. Significant outcomes of analyses were followed up with pairwise comparisons by using Bonferroni corrections.
The change in hedonic ratings was analyzed by using doubly-repeated ANOVAs, with the within-subject factors of yoking (Lifestyle+LV matched to Lifestyle) and time (0, 3, 6, 12, and 18 mo). The analyses were conducted for each NND-EDF separately to be able to ascertain whether potential differences in the frequency of consumption of each NND-EDF in Lifestyle+LV, an important component for establishing the occurrence of monotony, were related to potential differences in change in hedonic ratings in each NND-EDF between Lifestyle+LV and their yoked counterparts in Lifestyle. Greenhouse-Geisser probability levels were used to adjust for sphericity. Compliance rates for attending the sessions and turning in diaries were analyzed with independent t tests, and retention rates at the follow-up assessment were analyzed with a chi-square analysis. Analyses were conducted by using SPSS 19.0 (SPSS, Inc). The α-level was set at 0.05.
RESULTS
No differences in baseline characteristics or anthropometric measures were found between the 2 conditions (Table 1). Participants were aged 51.3 ± 9.5 y, had a BMI of 34.9 ± 4.3, and were 57.8% female; 92.2% of the participants were white, 98.0% were non-Hispanic, 74.0% were married, and 87.8% had some college education.
TABLE 1.
Baseline characteristics of participants by intervention1
| Lifestyle+LV(n = 101) | Lifestyle(n = 101) | |
| Age (y) | 51.7 ± 8.92 | 51.9 ± 9.0 |
| BMI (kg/m2) | 34.5 ± 4.1 | 35.3 ± 4.5 |
| Female (%) | 58.4 | 57.3 |
| White (%) | 91.1 | 93.2 |
| Non-Hispanic (%) | 97.0 | 99.0 |
| Married (%) | 74.3 | 73.8 |
| Some college education (%) | 88.1 | 87.4 |
No significant differences were found between the groups by using independent t tests and chi-square tests. LV, limited variety.
Mean ± SD (all such values).
NND-EDF intake
No differences between conditions were found at 0 mo in NND-EDF variety (as assessed by 24-h dietary recall or 28-d period) or energy consumed from NND-EDFs. Analyses of the number of different NND-EDFs consumed, assessed by 24-h recall and over a 28-d period, found a significant (P < 0.01) interaction; both measures of NND-EDF variety showed significantly (P < 0.01) fewer different NND-EDFs consumed at 6, 12, and 18 mo in the Lifestyle+LV group than in the Lifestyle group (Figure 2, A and B). Effect sizes (d), a metric-free effect size (29), for differences in the number of NND-EDFs consumed ranged from 0.56 to 0.83, with measures from the 24-h recall having the smaller effect size. Energy intake from the NND-EDFs also showed a significant (P < 0.05) interaction; the Lifestyle+LV group consumed significantly (P < 0.05) less energy from NND-EDFs at 6, 12, and 18 mo than did the Lifestyle group (Figure 3). Effect sizes (d) for energy intake from the NND-EDFs ranged from 0.75 to 0.65, and the largest effect occurred at 6 mo.
FIGURE 2.
Mean (±SD) number of different non-nutrient-dense, energy-dense foods consumed, assessed by 24-h dietary recall (A) and during the previous 28 d (B) at 0, 6, 12, and 18 mo by intervention. In a mixed-factor ANOVA, significant (P < 0.01) interactions occurred with both methods of assessment; the Lifestyle+LV group (n = 101) consumed significantly fewer different foods than did the Lifestyle group (n = 101) at each time point (***P < 0.001 and **P < 0.01). LV, limited variety.
FIGURE 3.
Mean (±SD) daily energy consumption from non-nutrient-dense, energy-dense foods at 0, 6, 12, and 18 mo by intervention. In a mixed-factor ANOVA, a significant (P < 0.05) interaction occurred; the Lifestyle+LV group (n = 101) consumed significantly less energy than did the Lifestyle group (n = 101) at each time point (**P < 0.01 and *P < 0.05). LV, limited variety.
Overall dietary intake
Overall dietary intake for both conditions at 0, 6, 12, and 18 mo is shown in Table 2. No differences in intake between the conditions at 0 mo were found for any overall dietary intake measures. For energy intake, a significant (P < 0.05) interaction occurred; the Lifestyle+LV group consumed significantly (P < 0.05) less energy at 6 mo than did the Lifestyle group. However, no significant differences in total energy intake were found between the conditions at 12 and 18 mo. Additionally, there was a significant (P < 0.001) main effect of time, in which energy intake at each time point was significantly (P < 0.05) different from one another; energy intake at 6, 12, and 18 mo was significantly (P < 0.001) lower than that at 0 mo.
TABLE 2.
Overall dietary intake at 0, 6, 12, and 18 mo by intervention1
| Lifestyle+LV(n = 101) | Lifestyle(n = 101) | |
| Energy (kcal)2 | ||
| 0 mo | 2082 ± 645a | 1934 ± 579a |
| 6 mo | 1351 ± 424b | 1395 ± 416c |
| 12 mo | 1462 ± 426d | 1477 ± 450d |
| 18 mo | 1529 ± 537e | 1547 ± 499e |
| Fat (% of energy)3 | ||
| 0 mo | 35.2 ± 6.2a | 35.2 ± 6.7a |
| 6 mo | 25.1 ± 6.3b | 25.3 ± 6.3b |
| 12 mo | 27.0 ± 6.5c | 27.5 ± 6.3c |
| 18 mo | 28.9 ± 7.3d | 28.8 ± 6.9d |
| Carbohydrate (% of energy)3 | ||
| 0 mo | 47.6 ± 7.2a | 48.0 ± 8.0a |
| 6 mo | 54.9 ± 7.9b | 56.2 ± 8.6b |
| 12 mo | 53.7 ± 7.8c | 54.4 ± 8.0c |
| 18 mo | 52.9 ± 8.4c | 53.0 ± 7.4c |
| Protein (% of energy)3 | ||
| 0 mo | 17.1 ± 4.1a | 17.6 ± 4.6a |
| 6 mo | 20.2 ± 5.3b | 19.8 ± 4.8b |
| 12 mo | 20.6 ± 4.4b | 19.3 ± 4.2b |
| 18 mo | 19.8 ± 5.4b | 19.5 ± 4.3b |
| Saturated fat (g/1000 kcal)3 | ||
| 0 mo | 13.2 ± 3.4a | 12.6 ± 2.9a |
| 6 mo | 8.9 ± 2.8b | 9.0 ± 2.9b |
| 12 mo | 9.7 ± 3.0c | 9.7 ± 3.0c |
| 18 mo | 10.1 ± 3.4c | 10.3 ± 3.0c |
| trans Fat (g/1000 kcal)3 | ||
| 0 mo | 2.4 ± 1.1a | 2.5 ± 1.3a |
| 6 mo | 1.4 ± 0.9b | 1.3 ± 0.8b |
| 12 mo | 1.3 ± 0.8b | 1.4 ± 0.8b |
| 18 mo | 1.4 ± 0.8b | 1.3 ± 0.9b |
| Added sugars (g/1000 kcal)3 | ||
| 0 mo | 30.2 ± 12.7a | 29.0 ± 15.3a |
| 6 mo | 26.2 ± 12.4b | 27.9 ± 11.6b |
| 12 mo | 24.6 ± 11.7b | 27.5 ± 11.4b |
| 18 mo | 27.6 ± 15.8b | 27.4 ± 11.7b |
| Cholesterol (mg/1000 kcal)3 | ||
| 0 mo | 148.8 ± 56.1a | 137.4 ± 64.5a |
| 6 mo | 125.2 ± 59.7b | 121.8 ± 63.7b |
| 12 mo | 140.1 ± 61.7a | 120.9 ± 65.3a |
| 18 mo | 131.9 ± 56.1b | 126.4 ± 55.0b |
All 8 values for each variable were included in the mixed-factor ANOVA. Means in a row with different superscript letters are significantly different, P < 0.05. LV, limited variety.
Significant condition × time interaction, P < 0.05.
Significant main effect of time, P < 0.01.
For all other dietary variables, significant (P < 0.01) main effects of time were found; however, no differences occurred between conditions (Table 2). All dietary variables, except for percentage energy from carbohydrate and protein, decreased over time. The percentage of energy from carbohydrate and protein increased over time.
Change in hedonic ratings of NND-EDFs and home availability of NND-EDF variety
For analyses of changes in hedonic ratings of each of the 2 chosen NND-EDFs, only one chosen NND-EDF had a significant (P < 0.05) main effect of yoking for changes in hedonics; those in the Lifestyle+LV group had a greater reduction in hedonics than did their yoked counterpart in the Lifestyle group (−7.4 ± 13.4 and −1.4 ± 12.3 mm, respectively), with an effect size of d = 0.47. No interaction or main effect of yoking or time occurred for changes in hedonics of the other chosen NND-EDF. A paired t test was conducted in participants from the Lifestyle+LV group to examine whether there were differences in the percentage of assessments in which either chosen NND-EDF was reported consumed in the 28-d period before the assessment. No significant difference occurred between the 2 chosen NND-EDFs: the chosen NND-EDF showing a reduction in hedonics when compared with the yoked Lifestyle counterpart was reported consumed at 65.3 ± 36.5% of the assessments, and the chosen NND-EDF not showing a reduction in hedonics when compared with the yoked counterpart was reported consumed at 61.7 ± 36.6% of the assessments. Additionally, when the relation between change in hedonics of each chosen NND-EDF and energy intake from NND-EDFs was examined in the Lifestyle+LV group at 6, 12, and 18 mo, no significant relation was observed.
No differences in the number of different NND-EDFs available in the home were found at baseline. For home NND-EDF availability, a significant (P < 0.05) interaction occurred, such that the number of different NND-EDFs available in the home at 6 and 18 mo was lower in the Lifestyle+LV group than in the Lifestyle group (6 mo: 8.5 ± 7.2 foods compared with 10.8 ± 7.6 foods, P < 0.05; 18 mo: 7.6 ± 6.9 foods compared with 10.1 ± 6.8 foods, P < 0.05), and no difference occurred between the conditions at 12 mo (Lifestyle+LV: 8.9 ± 6.4 foods; Lifestyle: 10.1 ± 6.9 foods). Associations between home availability of different NND-EDFs and energy intake from NND-EDFs were also examined at 6, 12, and 18 mo. At 18 mo, a significant relation between these variables occurred (r = 0.33, P < 0.01) in the Lifestyle+LV group but not in the Lifestyle group.
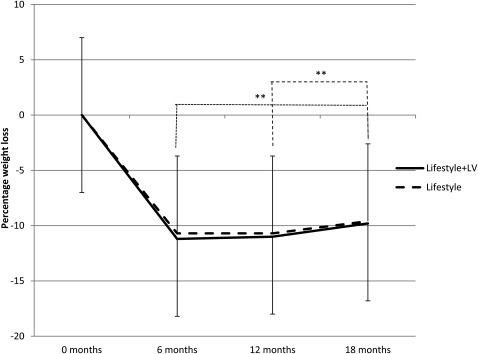
Percentage weight loss
Percentage weight loss is shown in Figure 4. A significant (P < 0.001) main effect of time was found for percentage weight loss, which was significantly lower (P < 0.01) at 6 and 12 mo than at 18 mo. The mean percentages of weight loss across the conditions were −10.9 ± 5.8%, −10.8 ± 8.0%, and −9.7 ± 8.4%, at 6, 12, and 18 mo, respectively. Associations between energy intake from NND-EDFs and percentage weight loss were also examined at 6, 12, and 18 mo. No significant relations occurred between these variables in either condition or with the conditions combined.
FIGURE 4.
Mean (±SD) percentage weight loss at 6, 12, and 18 mo by intervention. In a mixed-factor ANOVA, a significant main effect of time occurred; percentage weight loss was significantly (**P < 0.01) greater at 6 and 12 mo than at 18 mo (Lifestyle+LV: n = 101; Lifestyle: n = 101). LV, limited variety.
Physical activity
A significant (P < 0.001) main effect of time was found for self-reported energy expenditure from physical activity, which was significantly greater (P < 0.001) at 6, 12, and 18 mo than at 0 mo and significantly (P < 0.01) greater at 6 mo than at 12 mo. No significant difference between conditions occurred. Mean self-reported energy expenditures from physical activity were 1051 ± 1348, 2057 ± 2013, 1651 ± 1360, and 1921 ± 1820 kcal/wk at 0, 6, 12, and 18 mo, respectively.
Compliance and retention
No significant differences in the number of sessions attended (31.6 ± 13.1) or in the number of diaries turned in (31.5 ± 21.7) were found between the conditions or in retention rates for follow-up assessments: 97.5%, 95.1%, and 93.1% at 6, 12, and 18 mo, respectively. No serious adverse effects were reported in the trial.
DISCUSSION
This investigation found that a dietary prescription to limit variety in NND-EDFs was successful at reducing energy intake from NND-EDFs and that this reduction in energy intake from NND-EDFs was maintained across the 18 mo of the intervention. These outcomes are consistent with previous research that investigated the variety and consumption (7, 8). This reduction in energy intake from NND-EDFs appeared to be successful in assisting with the reduction in overall energy intake at 6 mo. However, limiting NND-EDF variety did not affect energy intake at 12 or 18 mo, because there was no difference in overall energy intake between the 2 interventions at these time points. Moreover, the percentage weight loss between the interventions was not different. Finally, because no differences in the reductions in percentage energy from fat, saturated fat, trans fat, added sugars, or cholesterol occurred between the 2 interventions, the prescription to limit variety in NND-EDFs did not appear to influence the overall quality of the diet.
The greater reduction in energy intake from NND-EDFs but not in overall energy intake at 12 and 18 mo indicates that Lifestyle+LV “compensated” for the additional energy decrease in NND-EDFs by increasing energy intake in other areas of the diet as compared with Lifestyle. Because additional analyses did not find significant differences in the consumption of other food groups between the conditions, it appears that this compensation was achieved through small global differences in energy intake in other food groups. Most importantly, the LV prescription did not cause a shift to increase intake in food groups that are nutrient-dense and low in energy density (ie, fruit and vegetables)—foods that are commonly proposed to be substitutes for NND-EDFs (30, 31). This suggests that for a dietary variety prescription to have an effect on diet quality and weight loss, greater areas of the diet need to be targeted to address the potential for compensation. For example, targeting a reduction in the variety of other energy-dense food categories (ie, high-fat meats and dairy products) may assist with a reduction in overall energy intake. Limiting other areas of food-related variety, such as within-meal variety (the number of different foods consumed within an eating bout) and across-meal variety (the number of different foods used as the main item of a meal over a specific time, such as a week), may also assist with a reduction in intake (7, 8). Furthermore, to shift intake to more nutrient-dense, low-energy-dense foods, a prescription to increase variety in these foods may be required.
As with most dietary prescriptions focused on reducing intake, the challenge is developing a prescription that can be implemented successfully and adhered to over a long period (32). It is important to note that, whereas the Lifestyle+LV intervention reduced NND-EDF variety more than did the Lifestyle intervention, the Lifestyle+LV group reported consuming >2 different NND-EDFs (2.2 ± 1.5 different foods from the 24-h dietary recalls and 8.4 ± 4.5 different foods from the 28-d recall period) and >100 kcal/d from these foods at 6 mo. This may also suggest that, whereas the LV prescription did reduce the number of different NND-EDFs consumed, it may not have reduced NND-EDF variety and consequential energy intake from NND-EDFs enough to affect overall energy intake and weight loss. Measures of the number of different NND-EDFs available in the home indicated that there were >2 different NND-EDFs available at home at each time point, which may have hampered the overall adherence to the LV prescription.
Although this study did not show that LV influenced weight loss, LV did reduce energy intake from the target food that was limited in the diet. This investigation examined 2 potential mechanisms for this effect. The first was monotony, and, whereas this study found a reduction in hedonics over the 18-mo intervention, this reduction was found in only one of the chosen NND-EDFs and did not appear to be related to frequency of consumption of the chosen NND-EDFs. However, because frequency of consumption was assessed only in the 28 d before each assessment, to better understand the differential reduction in hedonics of the chosen NND-EDFs, measures regarding frequency of consumption of the 2 chosen NND-EDFs may need to capture a longer time frame of intake. Nonetheless, no relation was found between changes in the hedonics of either chosen NND-EDF and energy intake from NND-EDFs, which suggests that monotony was not the mechanism by which limiting NND-EDF variety affected a reduction in energy intake from NND-EDFs. Perhaps a decrease in another process separate from hedonics, which is also known to influence consumption and decrease with repeated exposure to a food, the motivational value (wanting) of a food, may have influenced the reduction in consumption of energy from NND-EDFs (33–35).
LV may also affect consumption through stimulus control. The Lifestyle+LV group did report having fewer different NND-EDFs available at home at 6 and 18 mo than did the Lifestyle group when less energy from NND-EDFs was consumed by Lifestyle+LV. Moreover, a significant positive association between these different NND-EDFs available at home and energy intake from NND-EDFs was found in the Lifestyle+LV group at 18 mo. Thus, some evidence is available that less variety in NND-EDFs may have reduced the intake of these foods. However, because a significant relation was not found between NND-EDF variety availability and NND-EDF energy intake in the Lifestyle+LV group at 6 and 12 mo, other variables not measured, such as total quantity of NND-EDFs available in the home, may factor into this relation. To assist with a better understanding of whether stimulus control may be a mechanism by which a reduction in variety decreases consumption, the quantity, along with variety, of the limited foods available within the home environment should be assessed.
Limitations of the study include a sample that was fairly homogeneous with regard to race and ethnicity, self-reported dietary intake, and an inability to determine the dose of exposure to the chosen NND-EDFs across the 18 mo of the intervention. Strengths include a sample that was fairly balanced in terms of number of participating males and females, a high retention rate, and multiple measures of implementation of the LV prescription, with both measures indicating a difference between the 2 interventions in this variable.
In conclusion, this investigation, which was the first to examine the influence of a dietary prescription limiting variety on weight loss and diet quality, indicates that a prescription to limit variety in the diet can be implemented and that limiting variety can reduce energy intake of the component of the diet that has been targeted for limiting variety. The finding that overall energy intake was not reduced enough to produce a greater percentage of weight loss than what occurs with a hypocaloric, low-fat diet suggests that a prescription to limit variety may need to target more areas in the diet, not just one energy-dense food category, to potentially improve weight loss and weight-loss maintenance.
Acknowledgments
The authors’ responsibilities were as follows—HAR: conceived the project, oversaw the study, and wrote the first draft of the manuscript; HAR and RRW: developed the overall research plan; HAR, EAS, and JH: conducted the research; and HAR and JLF: performed the statistical analyses. All authors had primary responsibility for the final content. None of the authors had a personal or financial conflict of interest.
REFERENCES
- 1.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA 2006;295:1549–55 [DOI] [PubMed] [Google Scholar]
- 2.Wannamethee SG, Shaper AG, Walker M. Overweight and obesity and weight change in middle aged men: impact on cardiovascular disease and diabetes. J Epidemiol Community Health 2005;59:134–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harris MI, Flegal KM, Cowie CC, Eberhardt MS, Goldstein DE, Little RR, Hsiao-Mei W, Byrd-Holt DD. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey, 1988–1994. Diabetes Care 1998;21:518–24 [DOI] [PubMed] [Google Scholar]
- 4.National Heart, Lung, and Blood Institute Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: the evidence report. Obes Res 1998;6:51S–209S [PubMed] [Google Scholar]
- 5.Anderson JW, Konz EC. Obesity and disease management: effect of weight loss on comorbid conditions. Obes Res 2001;9:326S–34S [DOI] [PubMed] [Google Scholar]
- 6.US Department of Health and Human Services Dietary Guidelines for Americans, 2010. 7th ed. Washington, DC: U.S. Government Printing Office, 2010 [Google Scholar]
- 7.Raynor HA, Epstein LH. Dietary variety, energy regulation, and obesity. Psychol Bull 2001;127:325–41 [DOI] [PubMed] [Google Scholar]
- 8.Remick AK, Polivy J, Pliner P. Internal and external moderators of the effect of variety on food intake. Psychol Bull 2009;135:434–51 [DOI] [PubMed] [Google Scholar]
- 9.Raynor HA, Jeffery RW, Phelan S, Hill JO, Wing RR. Amount of food group variety consumed in the diet and long-term weight loss maintenance. Obes Res 2005;13:883–90 [DOI] [PubMed] [Google Scholar]
- 10.Raynor HA, Jeffery RW, Tate DF, Wing RR. Relationship between changes in food group variety, dietary intake, and weight during obesity treatment. Int J Obes Relat Metab Disord 2004;28:813–20 [DOI] [PubMed] [Google Scholar]
- 11.Raynor HA, Wing RR. Effect of limiting snack food variety across days on hedonics and consumption. Appetite 2006;46:168–76 [DOI] [PubMed] [Google Scholar]
- 12.Foster GD, Makris AP, Bailer BA. Behavioral treatment of obesity. Am J Clin Nutr 2005;82:230S–5S [DOI] [PubMed] [Google Scholar]
- 13.Thomas S, Reading J, Shephard R. Revision of the Physical Activity Readiness Questionnaire (PAR-Q). Can J Sport Sci 1992;17:338–45 [PubMed] [Google Scholar]
- 14.Raynor HA, Niemeier HN, Wing RR. Effect of limiting snack food variety on long-term sensory-specific satiety and monotony during obesity treatment. Eat Behav 2006;7:1–14 [DOI] [PubMed] [Google Scholar]
- 15.Wadden TA, Butryn ML, Byrne KJ. Efficacy of lifestyle modifications for long-term weight control. Obes Res 2004;12:151S–62S [DOI] [PubMed] [Google Scholar]
- 16.Wadden TA, Crerand CE, Brock J. Behavioral treatment of obesity. Psychiatr Clin North Am 2005;28:151–70 [DOI] [PubMed] [Google Scholar]
- 17.The Diabetes Prevention Program Research Group The Diabetes Prevention Program: description of the lifestyle intervention. Diabetes Care 2002;25:2165–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.US Department of Agriculture MyPyramid. Center for Nutriton Policy and Promotion Number 16. Washington, DC, USDA, 2005 [Google Scholar]
- 19.Wilde BE, Sidman CL, Corbin CB. A 10,000-step count as a physical activity target for sedentary women. Res Q Exerc Sport 2001;72:411–4 [DOI] [PubMed] [Google Scholar]
- 20.Drewnowski A, Henderson SA, Driscoll A, Rolls BJ. The dietary variety score: assessing diet quality in healthy young and older adults. J Am Diet Assoc 1997;97:266–71 [DOI] [PubMed] [Google Scholar]
- 21.Cooper Z, Fairburn CG. The Eating Disorder Examination: a semi-structured interview for the assessment of the specific psychopathology of eating disorders. Int J Eat Disord 1987;6:1–8 [Google Scholar]
- 22.Rolls BJ, Rowe EA, Rolls ET. How sensory properties of foods affect human feeding behavior. Physiol Behav 1982;29:409–17 [DOI] [PubMed] [Google Scholar]
- 23.Rolls BJ. The role of sensory-specific satiety in food intake and food selection In: Capaldi ED, Powley TL. eds. Taste, experience, and feeding. Washington, DC: American Psychological Association, 1990:197–209 [Google Scholar]
- 24.Raynor HA, Polley BA, Wing RR, Jeffery RW. Is dietary fat intake related to liking or household availability of high- and low-fat foods? Obes Res 2004;12:816–23 [DOI] [PubMed] [Google Scholar]
- 25.Paffenbarger RS, Jr, Hyde RT, Wing AL, Lee IM, Jung DL, Kamert JB. The association of changes in physical-activity level and other lifestyle characteristics with mortality among men. N Engl J Med 1993;328:538–45 [DOI] [PubMed] [Google Scholar]
- 26.Rauh MJD, Hovell MF, Hosfstetter CR, Sallis JF, Gleghorn A. Reliability and validity of a self-reported physical activity questionnaire in Latinos. Int J Epidemiol 1992;21:966–71 [DOI] [PubMed] [Google Scholar]
- 27.Rubin DB. Multiple imputation for nonresponse in surveys. New York, NY: Wiley & Sons, 1987 [Google Scholar]
- 28.Little R. Statistical analysis with missing data. New York, NY: Wiley & Sons, 1987 [Google Scholar]
- 29.Kline RB. Beyond signficance testing: reforming data analysis methods in behavioral research. Washington, DC: American Psychological Association, 2004 [Google Scholar]
- 30.Epstein LH, Gordy CC, Raynor HA, Beddome M, Kilanowski CK, Paluch R. Increasing fruit and vegetable intake and decreasing fat and sugar intake in families at risk for childhood obesity. Obes Res 2001;9:171–8 [DOI] [PubMed] [Google Scholar]
- 31.Epstein LH, Paluch RA, Beecher MD, Roemmich JN. Increasing healthy foods vs. reducing high energy-dense food to treat pediatric obesity. Obesity (Silver Spring) 2008;16:318–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lapointe A, Weisnagel SJ, Provencher V, Begin C, Dufour-Bouchard AA, Trudeau C, Lemieux S. Using restrictive messages to limit high-fat foods or non-restrictive messages to increase fruit and vegetable intake: what works better for postmenopausal women. Eur J Clin Nutr 2010;64:194–202 [DOI] [PubMed] [Google Scholar]
- 33.Epstein LH, Carr KA, Cavanaugh MD, Paluch RA, Bouton ME. Long-term habituation to food in obese and nonobese women. Am J Clin Nutr 2011;94:371–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Epstein LH, Temple JL, Roemmich JN, Bouton ME. Habituation as a determinant of food intake. Psychol Rev 2009;116:384–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Epstein LH, Truesdale R, Wojcik A, Paluch RA, Raynor HA. Effects of deprivation on hedonics and reinforcing value of food. Physiol Behav 2003;78:221–7 [DOI] [PubMed] [Google Scholar]