Abstract
Background: Currently, there is a lack of clarity in the literature as to whether there is a definitive difference between the effects of vitamins D2 and D3 in the raising of serum 25-hydroxyvitamin D [25(OH)D].
Objective: The objective of this article was to report a systematic review and meta-analysis of randomized controlled trials (RCTs) that have directly compared the effects of vitamin D2 and vitamin D3 on serum 25(OH)D concentrations in humans.
Design: The ISI Web of Knowledge (January 1966 to July 2011) database was searched electronically for all relevant studies in adults that directly compared vitamin D3 with vitamin D2. The Cochrane Clinical Trials Registry, International Standard Randomized Controlled Trials Number register, and clinicaltrials.gov were also searched for any unpublished trials.
Results: A meta-analysis of RCTs indicated that supplementation with vitamin D3 had a significant and positive effect in the raising of serum 25(OH)D concentrations compared with the effect of vitamin D2 (P = 0.001). When the frequency of dosage administration was compared, there was a significant response for vitamin D3 when given as a bolus dose (P = 0.0002) compared with administration of vitamin D2, but the effect was lost with daily supplementation.
Conclusions: This meta-analysis indicates that vitamin D3 is more efficacious at raising serum 25(OH)D concentrations than is vitamin D2, and thus vitamin D3 could potentially become the preferred choice for supplementation. However, additional research is required to examine the metabolic pathways involved in oral and intramuscular administration of vitamin D and the effects across age, sex, and ethnicity, which this review was unable to verify.
See corresponding editorial on page 1299.
INTRODUCTION
Compared with the other known vitamins essential to health, vitamin D is unique in its role because of the diverse sources available. Ergocalciferol (vitamin D2) is sourced from the UV irradiation of ergosterol, which is a steroid found in some plants but largely in fungi (1). Cholecalciferol (vitamin D3) is synthesized via the UV irradiation of 7-dehydrocholesterol to previtamin D3 in the skin of animals at UVB wavelengths of 290–320 nm, with a further thermal isomerization step to form vitamin D3 (1, 2). Therefore, humans have a combination of vitamins D2 and D3 available to them as part of a typical lifestyle from ambient UV exposure (vitamin D3), habitual dietary intakes of vitamin D3–rich foods (egg yolks and oily fish), fortified foods (margarine and breakfast cereals, which generally have vitamin D2 fortification), and vitamin supplements (both vitamins D2 and D3 are available) (2).
Vitamins D2 and D3 function as prohormones (which, therefore, have no biological effect), with the only differentiation between the two being the structure of their side chains, and thus, theoretically are used by the body in an identical manner (2). The conversion of vitamins D2 and D3 into active compounds (irrespective of source) requires a 2-step enzymatic hydroxylation process to occur (2). At the first site, the liver, vitamins D2 and D3 are converted to 25-hydroxyvitamin D [25(OH)D]4 via the action of 25-hydroxylases (ie, microsomal cytochrome P450 2R1 and mitochondrial cytochrome P450 27A1), which are known to be from the cytochrome P450 group (2, 3). The kidney is the second site of activity where 1α-hydroxylase (cytochrome P450 27B1) converts 25(OH)D to 1,25-dihydroxyvitamin D2 or D3 (calcitriol) (1, 2). However the rate of conversion is under homeostatic control because of its dependence on circulating parathyroid hormone concentrations (1). Once calcitriol is available, the known systemic effects center on the maintenance of serum calcium and phosphate concentrations via the control of intestinal absorption of calcium, renal resorption of phosphate, and the release of calcium from the skeleton (1).
Although vitamins D2 and D3 have previously been described as undertaking identical hydroxylation processes, which, thus, result in the same active metabolite (calcitriol), data have arisen to show that, in comparison, there may be a difference in their respective efficacies in the raising of serum 25(OH)D (4–7), which is the established marker of vitamin D status (1). Data suggested that these proposed differences between the 2 calciferols are due to their differing affinities for the vitamin D receptor (VDR), which appears to be linked to an additional step of 24-hydroxylation that inactivates calcitriol (8). In addition, it is thought that vitamin D3 is potentially the preferred substrate for hepatic 25-hydroxylase (9), which in combination with the possible difference in the 24-hydroxylation rate, only reinforces the importance of determining whether these metabolic anomalies impact on health.
Given the essential role vitamin D plays in maintaining bone health and the gathering proof of widespread deficiency within the UK population (10), it is essential that the most efficacious source of vitamin D is elucidated. Therefore, the aim of this systematic review and meta-analysis was to determine whether there is a difference in the efficacy of vitamin D2 compared with vitamin D3 in the raising of the serum 25(OH)D status within the context of randomized controlled trials (RCTs).
METHODS
Identification of studies and quality assessment
A systematic search of the current literature was completed via the use of the ISI Web of Knowledge (including MEDLINE and Web of Science databases, http://wok.mimas.ac.uk/) and the Cochrane Central Register of Controlled Trials (http://onlinelibrary.wiley.com/o/cochrane/cochrane_clcentral_articles_fs.html), and the International Standard Randomized Controlled Trials Number Register (www.isrctn.org) and clinicaltrials.gov databases were also searched for any unpublished trials; the search covered from January 1950 to November 2011. Search terms used were as follows: “vitamin D2 and D3” or “ergocalciferol and cholecalciferol” and “supplementation” and “25 hydroxyvitamin D.”
Two authors (HL and LT) screened article titles and abstracts to identify studies that may be suitable for analysis. Full articles were independently assessed (by HL, SL-N, and LT) for final inclusion by using a scoring system for assessing quality (11). All authors agreed on the final decision of the studies to be included.
Study eligibility criteria
Any randomized intervention trials that involved human adults (men and women) that directly compared the effects of vitamin D2 and vitamin D3 supplementation and used serum 25(OH)D concentrations as a primary outcome were initially included for consideration.
In addition to healthy participants, studies that had participants with acute or chronic medical conditions associated with vitamin D insufficiency or poor musculoskeletal health were also included for the initial review of articles. No studies were included that involved children, adolescents, or pregnant or breastfeeding women. Variations between the extracted studies for supplement dosage, frequency of supplementation, use of either intramuscular or oral delivery methods, and lack of a control or placebo group were acceptable and were not cause for exclusion.
Data collection
All relevant information was extracted from eligible studies as follows: first author, publication year, country of origin, study design, supplement dosage, frequency of supplementation, method of delivery (intramuscular compared with oral), serum 25(OH)D concentrations, and demographic characteristics of the study population. Any other information pertinent to the review such as potential confounders to the RCTs (ie, the season during which the RCT took place and risk of ambient UV exposure), the lack of a control group, the analysis technique chosen to assess serum 25(OH)D, and the dropout rate were also noted when reported.
For all studies, the mean, SD (or equivalent), and n for absolute change of serum 25(OH)D from baseline in addition to baseline and postintervention measurements of serum 25(OH)D were recorded. Depending on the author, data for serum 25(OH)D concentrations were extracted as both nanograms per milliliter and nanomoles per liter, with results converted to the SI unit of nanomoles per liter for this review. If results were obtained as SEMs, then they were converted to SDs by using the formula
Six articles had data missing that were required for review. Five authors were contacted; current contact details were not available for the sixth author (12). Of the authors contacted, 2 authors did not respond to the request for additional data (13, 14); thus, these 2 studies were not included in the meta-analysis but were still reviewed on a qualitative basis. Three authors responded and were able to provide the necessary information (15–17) and were subsequently included in the meta-analysis. No articles were excluded from the entire review because of missing data. Data quality was assured by comparing all studies to the Consolidated Standards of Reporting Trials statement checklist (18).
Data analysis
All studies were reviewed against a qualitative analysis score, and studies with complete data were quantitatively analyzed with RevMan software (version 5.1.4) (19) with the outcome represented via forest plots (see figures). Results for the meta-analysis could be obtained only when ≥2 studies were included that had complete data for the specific outcome that was being assessed. Two initial meta-analyses were completed by looking at whether there was an effect on the efficacy of vitamins D2 and D3 in the raising of serum 25(OH)D concentrations that was dependent on the frequency of dosage [ie, daily administration and single or infrequent bolus (either intramuscular or oral)]. The aim of the main meta-analysis (irrespective of dosage frequency) was to determine whether the change in serum 25(OH)D after intervention was the same between vitamins D2 and D3 (which indicated equal efficacy) or whether the change from baseline was greater with vitamin D3.
Assessment of heterogeneity, sensitivity, and risk of bias
To account for the heterogeneity between included studies, a random-effects model was used, in addition to the use of the weighted mean difference. The I2 statistic was reported to indicate the heterogeneity across the studies used for each meta-analysis. A result >75% was deemed likely to indicate a high level of heterogeneity between studies, and ≤25% indicated a low level of heterogeneity (20). Because of the practical difficulties of obtaining individual participant data and the small number of studies available for review, a meta-regression was not possible to further explore the cause of any possible heterogeneity. There were insufficient studies available to complete an analysis of publication bias; however, a search of clinical trials registries was completed to assess the number of studies that declared a vitamin D intervention compared with studies subsequently reported in the literature in an attempt to assess the potential for publication bias. All included studies were qualitatively assessed for allocation, attrition, and reporting bias.
RESULTS
Search results
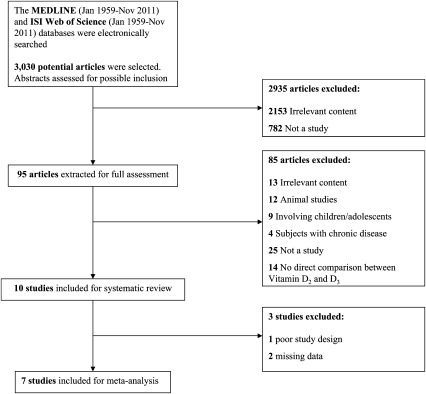
As per the Quality of Reporting of Meta-analyses flow diagram (Figure 1), 7 of 10 possible studies were included in the meta-analysis, with one study that did not meet the criteria for inclusion in the meta-analysis because of a lack of random assignment of participants; however, the study was still suitable for qualitative review. Two studies had missing data or the authors could not be contacted. A total of 3020 studies did not meet the criteria for both quantitative and qualitative review because of a number of reasons, such as an irrelevance to the subject, a lack of random assignment of participants, a lack of direct comparison between ergocalciferol and cholecalciferol, or studies that were conducted in animals, cell models, or children.
FIGURE 1.
QUOROM statement flow diagram (29) indicating numbers of articles reviewed and later excluded or included for the systematic review and meta-analysis. QUOROM, Quality of Reporting of Meta-analyses.
Study characteristics
In the collective of 10 studies, there were 1016 participants aged 18–97 y. The participant ratio of men to women was ∼1:3, with one study (16) that did not declare the sex of participants (n = 95). The studies were conducted in the United States, Canada, United Kingdom, Australia, Denmark, and Italy; all studies were single-center studies. Seven studies were conducted in healthy, free-living participants (4, 6, 7, 12, 13, 15, 17); one study focused on elderly women who were residents of a nursing home and were deficient in vitamin D (5), whereas another study instead focused on hospital inpatients who had been diagnosed with a hip fracture and were vitamin D deficient (16). The final study recruited participants who were under the care of a rheumatology outpatient clinic (diagnosis unspecified) and were vitamin D insufficient (14).
All intervention studies, except one study (14), declared that the studies had a randomized, parallel design. Only 4 studies used a control or placebo group to compare against intervention groups (4, 6, 7, 13), and of those, only 3 studies implemented double blinding of the intervention (4, 6, 7). Of the 6 studies that chose not to compare intervention groups against a control group (5, 12, 14–17), 3 studies were double blind to the intervention (12, 15, 16). The remaining 3 studies used either a single-blinded (presumed that the participant was blinded to the intervention) (5, 17) or an unblinded strategy (14).
The interventions used for all studies were vitamins D2 and D3 of varying dosages and treatment time periods. One study chose to give a single bolus orally of 50,000 IU (13); one study chose to administer one single large bolus of vitamin D2 or D3 (300,000 IU) and to also compare the route of administration (ie, intramuscular and oral) (5). Six studies preferred daily oral supplementation strategies by using dosages between 1000 and 4000 IU (4, 6, 7, 12, 15, 16). One study chose a weekly intervention of 50,000 IU (17), and another study chose a daily compared with monthly intervention by using 1600 and 50,000 IU, respectively (15). The final study compared a single intramuscular injection of 300,000 IU of vitamin D2 compared with a single oral dose of 300,000 IU of vitamin D3 (14). Treatment follow-up times varied from 28 d to 24 wk for the bolus-intervention studies, whereas the daily and weekly study designs had intervention periods that ranged from 14 d to 12 mo.
Compliance was reported to be >90% in 6 studies (5–7, 14, 15, 17) and invariably was verified via a pill count or stated by the research team if doses were administered by or in the presence of a clinician. For studies that involved bolus dosages, compliance was assumed at 100% because of the study design because it was presumed from the details of the study design that a clinician was present to administer the dose or, at minimum, observed the participant when the dose was consumed. One study reported that 53% of participants completed the study at a compliance rate ≥80% (16); 3 studies did not report the monitoring of compliance to the intervention (4, 12, 13).
All studies, except 3 studies (5, 14, 16), stated that the vitamin D content of intervention products was verified to ensure consistency. In addition to the vitamin D supplementation, 3 studies also supplemented with between 350 and 600 mg Ca/d (7, 12, 16). The additional supplementation was either due to the calcium already being present as part of the intervention product or as a deliberate concomitant use of calcium as part of the study design. One study conducted a 2-mo run-in diet before commencement of the intervention period that contained 1000–1500 mg Ca/d, but it was not clear whether this dietary program continued for the duration of the vitamin D–supplementation period.
Methodologic quality
As a general overview, all studies included in the meta-analysis showed a moderately clear methodology and subsequent reporting of results (studies are described in Table 1). However, across all studies, there were some notable omissions with respect to the statistical analysis strategy, reasoning behind the study design, and an overall general lack of attention to detail that would make the dissemination of results much more fruitful for all interested parties. The recruitment criterion for each study was briefly described, and all studies stated that a random assignment of participants had occurred (when it was part of the study design); however, few studies detailed the actual randomization strategy (ie, block randomization or computer-generated code) (7, 12, 16). As previously described, all studies, except one study, were of a randomized, parallel design. Four of 10 studies used a control group to compare against intervention groups, and 6 studies of 10 were double blinded. Thus, only 3 of 10 possible studies included in the meta-analysis were randomized, controlled, double-blind, parallel intervention studies, which is the gold-standard design in nutrition research. Therefore, because of the lack of specific information surrounding the randomization and allocation of intervention to participants in all studies, there was an unknown degree of selection, performance, and detection bias. Concern was also raised regarding stratification during the randomization process for sex. Three studies had very high proportions of female participants, which appeared to be unevenly distributed between intervention groups (6, 7, 14). One study also had very mismatched participant numbers when the 2 intervention groups of ergocalciferol (n = 50) and cholecalciferol (n = 19) were compared, which (in combination with a lack of control group and an unblinded study design) resulted in a tremendous weakening of its methodologic quality and, thus, resulted in the study not being included in the meta-analysis.
TABLE 1.
Study characteristics and outcomes of those relevant intervention studies included in meta-analysis and systematic review1
| Study and country | Intervention, dose, and frequency | n | Sex and age | Follow-up | Results |
| Armas et al, 2004 (13); United States |
1) No supplement (seasonal effect acting as control) 2) 1 tablet of 50,000 IU (1.25 mg) of vitamin D2 3) 10 tablets of 5000 IU (125 μg) of vitamin D3 |
30 | All M20–61 y | 28 d | 28-d AUC was significantly greater for vitamin D3 supplementation group than vitamin D2 supplementation groups (P < 0.002). |
| Biancuzzo et al, 2010 (7); United States |
1) Placebo capsule + placebo OJ 2) Placebo capsule + 1000 IU vitamin D3 OJ 3) Placebo capsule + 1000 IU vitamin D2 OJ 4) 1000 IU vitamin D3 capsule + placebo OJ 5) 1000 IU vitamin D2 capsule + placebo OJ |
86 | 59 F27 M18–79 y | 11 wk | No significant difference was shown in AUC for 25(OH)D when vitamins D2 and vitamin D3 were compared, irrespective of the intervention vehicle (capsule or juice). |
| Binkley et al, 2011 (15); United States | 1) 1600 IU of vitamin D2/d | 64 | 43 F | 12 mo | Vitamin D3 was shown to be significantly more effective than vitamin D2 at raising serum 25(OH)D concentrations for the daily dosage (P = 0.05) and for daily and monthly dosage groups combined (P = 0.01). NS for monthly dosage group. |
| 2) 1600 IU of vitamin D3/d | 21 M | ||||
| 3) 50,000 IU of vitamin D2/mo | ≥65 y | ||||
| 4) 50,000 IU of vitamin D3/mo | |||||
| Glendenning et al, 2009 (16); Australia | 1) Ergocalciferol 1000 IU/d | 70 | Sex | 3 mo | Vitamin D3 supplementation was associated with a 31% greater increase in concentrations of serum 25(OH)D than was vitamin D2 supplementation (P = 0.01). |
| 2) Cholecalciferol 1000 IU/d | Unknown | ||||
| 82–84 y | |||||
| Heaney et al, 2011 (17); United States |
1) 1 capsule of 50,000 IU vitamin D2 2) 5 capsules of 10,000 IU vitamin D3 |
33 | 30 F3 M49.5 ± 9.8 y | 12 wk | 12-wk induced AUC was significantly greater for the vitamin D3 supplementation group than for the vitamin D2 group (P < 0.001). Vitamin D3 was calculated as 87% more potent at raising 25(OH)D. |
| Holick et al, 2008 (6); United States | 1) Placebo | 68 | 47 F | 11 wk | At the end of the intervention, there was no significant difference in 25(OH)D concentrations between vitamin D2 and D3 groups. |
| 2) 1000 IU vitamin D2 capsule3) 1000 IU vitamin D3 capsule4) 500 IU D2 + 500 IU D3 capsule | 21 M18–84 y | ||||
| Leventis and Kiely 2009 (14); United Kingdom | Study 1: single intramuscular injection of 300,000 IU vitamin D2 | 69 | Study 1: 43 F and 7 M | 24 wk | Greater increases in serum 25(OH)D were achieved with vitamin D3 intervention |
| Study 2: single 100-mL oral dose of 300,000 IU vitamin D3 | Study 2: 15 F and 4 M | ||||
| 23–82 y | |||||
| Romagnoli et al, 2008 (5); Italy |
1) Single oral dose of 300,000 IU vitamin D3 2) Single intramuscular dose of 300,000 IU vitamin D3 3) Single oral dose of 300,000 IU vitamin D2 4) Single intramuscular dose of 300,000 IU vitamin D2 |
32 | All F66–97 y | 60 d | Vitamin D3 significantly more potent at raising serum 25(OH)D concentrations than was vitamin D2 for both oral and intramuscular administration. |
| Tjellesen et al, 1986 (12); Denmark |
1) 4000 IU vitamin D3 2) 4000 IU vitamin D2 |
19 | All F22–49 y | 8 wk | Vitamin D3 was linked to a greater increase in serum 25(OH)D compared with that in the vitamin D2 intervention group. No direct statistical comparison results were available. |
| Trang et al, 1998 (4); Canada |
1) Untreated 2) 4000 IU vitamin D2 3) 4000 IU vitamin D3 |
89 | 48 F23 M18 Unknown38 ± 9 y | 14 d | Greater increase in serum 25(OH)D concentrations with vitamin D3 group than with vitamin D2 group (P = 0.03). |
OJ, orange juice; 25(OH)D, 25-hydroxyvitamin D.
Attrition bias was difficult to qualify because of a lack of detail given by all studies. Two studies made specific reference to dropouts and sought to give some clarification about the reasons for dropout or noncompliance (15, 16), whereas 3 studies recorded dropout rates but gave no satisfactory detail as to the reasons for dropout (6, 7, 17). The remaining studies either gave no mention of dropouts (4, 12–14) or alternatively stated there was full compliance to the study (5).
With respect to reporting bias, 8 of 10 studies reported all outcomes as set out by their stated study aims and objectives (4–7, 12, 13, 16, 17). However, in some cases, additional data that detailed absolute changes in serum 25(OH)D concentrations, alongside data already reported, would have proved helpful to understand the outcomes and implications of these studies to a greater degree. Two studies did not fully report on all outcomes that were measured (14, 15), which, for both studies, centered on markers of bone health that were not detailed, despite being stated as measured in the study design and methodology.
A concern for all studies was the lack of consensus in the analysis of serum 25(OH)D concentrations. Methods used for analysis were reverse-phase HPLC, HPLC (UV detection), and liquid chromatography–mass-spectrometry, of which CVs were reported of ∼9–12% for both intraassay and interassay (6, 7, 16). Radioimmunoassay was also used, with CVs of ∼8% intraassay and a 10% interassay reported (5). ELISA and chemiluminescence were implemented but lacked details on sensitivity apart from one study that reported an intraassay CV of 2.6% for the chemiluminescence assay (17). The competency of individuals who conducted the assays that measured serum markers was invariably given little detail, which only added to concerns for an additional potential bias when it was not known in full whether all studies had serum analysis carried out by individuals or contracted laboratories that followed rigorous procedures. Furthermore, in one study (14), because of the large passage of time (2 y) between the completion of the 2 intervention groups, the serum analysis was completed by 2 different laboratories that used 2 different techniques (chemiluminescence and ELISA), which was a major contributory factor in the decision to remove this study from the final meta-analysis.
The approach to statistical analysis in all studies generally appeared sound, although in some areas, there was a lack of specific details. For only one study, there was an estimated sample size required to detect a treatment effect (16). Omissions of power calculations caused great concern because of the relatively small sizes of intervention groups for all the studies included in this review.
Meta-analysis
Eight intervention studies showed that, no matter the dosage, the frequency or administration (oral compared with intramuscular) of cholecalciferol supplementation produced a significantly greater absolute increase from baseline of serum 25(OH)D compared with ergocalciferol (4, 5, 12, 13, 14–17). Two studies (6, 7) showed that ergocalciferol and cholecalciferol were equally efficacious.
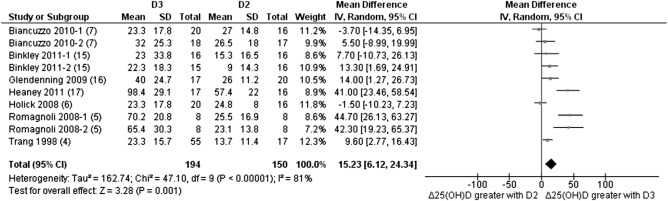
Ten studies were available for the meta-analysis; however, only 7 studies had sufficient and available data to be included in the primary analysis by using the random effects model. Overall, the analysis showed that the greater absolute change in serum 25(OH)D from baseline favored the cholecalciferol intervention, with a weighted mean difference of 15.23 (95% CI: 6.12, 24.34; Z = 3.28; P = 0.001; Figure 2). The heterogeneity between studies was shown to be high (I2 = 81%), although this unfortunately could not be investigated further by using meta-regression because of the small number of studies available.
FIGURE 2.
Random-effects meta-analysis comparing the effects of daily and bolus supplementation of D3 with that of D2 on net changes in serum 25(OH)D concentrations. The forest plot indicates that the absolute change in 25(OH)D from baseline favored the D3 intervention. In the figure, “Δ25(OH)D” denotes the change in serum 25(OH)D concentrations from baseline (net change), squares denote mean differences [with 95% CIs (lines)], and “Total” denotes the cumulative n from all included studies. With the use of a random-effects model, overall, there was a significantly greater effect in the raising of serum 25(OH)D concentrations over time for D3 supplementation than for D2 supplementation (mean difference: 15.23; 95% CI: 6.12, 24.34; P = 0.001). D2, vitamin D2; D3, vitamin D3; IV, inverse variance; 25(OH)D, 25-hydroxyvitamin D.
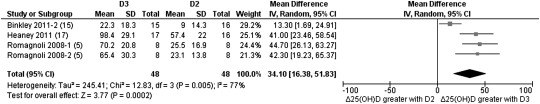
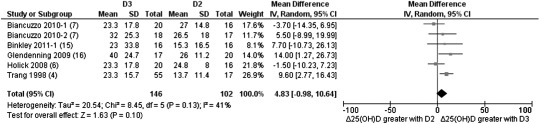
Separate analyses were performed to determine whether the frequency of dosage had any effect on the favoring of either ergocalciferol or cholecalciferol supplementation. Four studies used either a single or multiple (weekly or monthly) bolus dosage of 50,000–300,000 IU with anywhere from 4 wk to 1 y of follow-up (5, 13, 15, 17). Three of these studies had data available that showed that bolus doses favored cholecalciferol with a weighted mean difference of 34.10 (95% CI: 16.38, 51.83; Z = 3.77; P = 0.0002; Figure 3). When we focused on studies that chose daily supplementation (4, 6, 7, 12, 15, 16), the differentiation between the 2 forms of vitamin D was not as clear cut. Although a clear preference was shown for cholecalciferol supplementation (1000–4000 IU/d), the result was nonsignificant with a weight mean difference of 4.83 (95% CI: −0.98, 10.64; Z = 1.63; P = 0.10; Figure 4).
FIGURE 3.
Random-effects meta-analysis comparing the effects of bolus supplementation of D3 with that of D2 on net changes in serum 25(OH)D concentrations. The forest plot indicates that the absolute change in 25(OH)D from baseline favored the D3 intervention when administered as a bolus dose. In the figure, “Δ25(OH)D” denotes the change in serum 25(OH)D concentrations from baseline (net change), squares denote mean differences [with 95% CIs (lines)], and “Total” denotes the cumulative n from all included studies. With the use of a random-effects model, overall, there was a significantly greater effect in the raising of serum 25(OH)D concentrations over time for D3 supplementation as a bolus dosage (single and multiple bolus) than for D2 supplementation (mean difference: 34.10; 95% CI: 16.38, 51.83; P = 0.0002). D2, vitamin D2; D3, vitamin D3; IV, inverse variance; 25(OH)D, 25-hydroxyvitamin D.
FIGURE 4.
Random-effects meta-analysis comparing the effects of daily supplementation of D3 with that of D2 on net changes in serum 25(OH)D concentrations. The forest plot indicates that the absolute change in 25(OH)D from baseline favored the D3 intervention as a daily supplement. “Δ25(OH)D” denotes the change in serum 25(OH)D concentrations from baseline (net change), squares denote mean differences [with 95% CIs (lines)], and “Total” denotes the cumulative n from all included studies. With the use of a random-effects model, overall, there was no significant difference between D2 and D3 interventions in the raising of serum 25(OH)D concentrations when taken as a daily supplement (mean difference: 4.83; 95% CI: −0.98, 10.64; P = 0.10). D2, vitamin D2; D3, vitamin D3; IV, inverse variance; 25(OH)D, 25-hydroxyvitamin D.
The heterogeneity for additional analyses was mixed; the bolus analysis reported a high level of heterogeneity between studies (I2 = 77%), but the daily supplementation analysis showed more moderate levels of heterogeneity (I2 = 41%).
DISCUSSION
To our knowledge, our reported work is the first-ever systematic review and meta-analysis of the comparative effectiveness of ergocalciferol compared with cholecalciferol in the raising of serum 25(OH)D concentrations. We consider this study to be a key investigation because of the widespread perception that ergocalciferol and cholecalciferol are equally efficacious in their respective abilities to raise serum 25(OH)D concentrations (21). As shown in our meta-analysis results, after we reviewed all known studies, there was a clear favoring of cholecalciferol supplementation in the raising of serum 25(OH)D concentrations compared with that of ergocalciferol supplementation (Figure 2). We have also shown that, regardless of whether supplementation with vitamin D was in small daily doses or in larger and more infrequent bolus dosages, the favoring toward cholecalciferol was still evident. The reasons why cholecalciferol is much more proficient than ergocalciferol at raising serum 25(OH)D concentrations must be examined. Evidence has been accumulating that specifically looks at the metabolism of vitamin D, especially that focuses on the hydroxylation steps at the liver and kidney that were required to convert the inert ergocalciferol and cholecalciferol to the active calcitriol (1,25-dihydroxyvitamin D). As described in the review of Houghton and Vieth (8) of the evidence in 2006, it is clear that there are stark differences in the metabolic fates of ergocalciferol and cholecalciferol that should not be ignored. By centering on the differences in side chains between the 2 forms of vitamin D [ergocalciferol has an additional methyl group on carbon 24 (22)], there have been reports that this difference directly affects the rate of ergocalciferol conversion to serum 25(OH)D (9, 23, 24) and also its affinity for vitamin D binding protein and VDR (8), which are all critical steps involved in the activation of vitamin D. Once the 2-step 25-hydroxylation process has been completed and 1,25-dihydroxyvitamin D has been formed, an additional step occurs that involves 24-hydroxylation at the kidney to form 1,24,25(OH)3D [1,24,25-trihydroxyvitamin D; 25(OH)D can also be converted to 24,25-dihydroxyvitamin D at this point] (8). As reported by Horst et al (25), it is this 24-hydroxylation step that truly demarcates the impact of ergocalciferol compared with that of cholecalciferol. This differentiation between ergocalciferol and cholecalciferol is due to the fact that once 1,24,25(OH)3D2 has been formed, ergocalciferol has been deactivated and, therefore, is irretrievable (25). In contrast, cholecalciferol [now 1,24,25(OH)3D3] retains its capacity to bind to the VDR (8) and still requires an additional side-chain oxidation to become deactivated (25). Thus, this additional step gives a vast advantage and potential for cholecalciferol to remain biologically active and, thus, maintain vitamin D status, which only strengthen the hypothesis that cholecalciferol is the preferred substrate compared with ergocalciferol.
This complication in the hydroxylation and deactivation processes of ergocalciferol and cholecalciferol that could impact efficacy is explored to some degree in a small number of the clinical trials included in this review. Armas et al (13) and Heaney et al (17) showed that, over a time course, cholecalciferol induced a quicker response in the production of serum 25(OH)D that was sustained for longer and at higher concentrations than did ergocalciferol. In Heaney et al (17), weekly doses of 50,000 IU (for 12 wk) induced AUC values for cholecalciferol that were significantly higher than those for ergocalciferol; Heaney et al (17) also noted that, once the doses of vitamin D were stopped at week 12, there was evidence of far greater rates of degradation of serum 25(OH)D2 (ergocalciferol) than serum 25(OH)D3 (cholecalciferol) over a 6-wk time period. Armas et al (13) chose a single bolus of 50,000 IU that again showed a significantly greater AUC for cholecalciferol than for ergocalciferol, with serum 25(OH)D2 concentrations that fell rapidly back to baseline after only 14 d, whereas serum 25(OH)D3 concentrations peaked at the same time point and had not returned to baseline at the end of the 28-d intervention.
When the evidence from the studies that focused on vitamin D metabolism at the cellular level is compared with the evidence from clinical trials, it is clear that, overall, there was consistency in the results that shows cholecalciferol appears to have advantageous biological qualities that allows it to sustain its systemic influence for far longer and at far greater concentrations than does ergocalciferol. However, not all the clinical trials agree with this outcome, and 2 clinical trials showed no difference in the efficacy of ergocalciferol compared with cholecalciferol (6, 7), which indicated the possibility that the not all possible influences on vitamin D metabolism have been accounted for in the research completed to date.
The main limitation of this review was the small number of studies available within the literature, which, in turn, limited, to some degree, the ability to extrapolate the outcomes of this review toward realistic public health recommendations when referring to ergocalciferol and cholecalciferol supplementation. In addition, as described previously, the small number of studies also limited, to some degree, the confidence in our knowledge of the metabolism of vitamin D and whether all influences have been examined and quantified.
The intervention strategy across all studies was diverse with respect to the chosen dosage of vitamin D, the frequency of supplementation, and the method of administration used (oral compared with intramuscular), which inevitably contributed to the high levels of heterogeneity (Figure 2). In addition, there were recurring issues across all studies in terms of the depth and detail of reporting. Many omissions were shown in the reporting of the randomization strategy, power calculations, and subject compliance. In scientific reporting, this lack of detail is unacceptable, especially given the crucial role of such studies in establishing key data within the vitamin D field. A lack of serious bias was only assured because of the clear detail that referred to the blinding of subjects and investigators and the overall attention to detail when methodologies were reported.
It was also noted that all studies gave ergocalciferol and cholecalciferol supplementation dosages far above the amount currently recommended [Recommended Dietary Allowance of 600 IU for males and females aged 1–70 y (26)]. Although this strategy can be a useful methodology to force a physiologic change (and, thus, to possibly piece together a mechanism of action), these studies offered little information for lower doses, which are more realistic in terms of what individuals are likely to be able to consume within their daily diet and gain from sunlight exposure and the concentrations of ergocalciferol and cholecalciferol available in commercial supplements. Thus, in combination with the small and underpowered study populations (n = 19–89) and lack of data in lower doses, there is a clear need for additional research to ascertain whether the results obtained within the studies included in this review translate across the entire dose-response curve.
In conclusion, our results suggest a favoring toward cholecalciferol rather than ergocalciferol supplementation with respect to the more effective improvement of vitamin D status. When the frequency of dosage administration was compared, there was a significant response for vitamin D3 when given as a bolus dose (P = 0.0002) compared with administration of vitamin D2, but the effect was lost with daily supplementation. Far larger, more robust trials are now required that not only monitor serum 25(OH)D concentrations but also explore much further the potential mechanism behind this apparent discrepancy in effectiveness between the 2 forms of vitamin D available. This mechanistic approach should encompass the obvious need for a description of genetic polymorphisms linked to vitamin D metabolism and, also, the critical enzymes involved in the hydroxylation process. These studies would provide an ideal opportunity to identify the levels of enzymatic activity (microsomal cytochrome P450 2R1, mitochondrial cytochrome P450 27A1, and cytochrome P450 27B1) and the spectrum of vitamin D metabolites that are produced as part of the hydroxylation process that would be able to account for the deactivation process of ergocalciferol and cholecalciferol that appears to be the central cause for the discrepancy between efficacies at this point in time.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—LT and SL-N: design of review; LT, HL, and SL-N: data collection; LT and SL-N: writing of the manuscript; and SL-N, EH, KH, GC, SP, CPS, GB, JB, and RV: review of the manuscript. None of the authors had a conflict of interest.
Footnotes
Abbreviations used: RCT, randomized controlled trial; VDR, vitamin D receptor; 1,24,25(OH)3D, 1,24,25-trihydroxyvitamin D; 25(OH)D, 25-hydroxyvitamin D.
REFERENCES
- 1.Calman K. ed. Nutrition and bone health with particular reference to calcium and vitamin D: Report of the Subgroup on Bone Health (Working Group on the Nutritional Status of the Population) of the Committee on Medical Aspects of Food and Nutrition Policy London, United Kingdom: The Stationary Office, 1998 [Google Scholar]
- 2.Ross CA, Taylor CL, Yaktime AL, Del Valle HB. eds. Dietary Reference Intakes for calcium and vitamin. Committee to Review Dietary Reference Intakes for Vitamin D and Calcium; Institute of Medicine Washington, DC: National Academies Press, 2010 [Google Scholar]
- 3.Jones G. Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr 2008;88:582S–6S [DOI] [PubMed] [Google Scholar]
- 4.Trang HM, Cole DE, Rubin LA, Pierratos A, Siu S, Vieth R. Evidence that vitamin D3 increases serum 25-hydroxyvitamin D more efficiently than does vitamin D2. Am J Clin Nutr 1998;68:854–8 [DOI] [PubMed] [Google Scholar]
- 5.Romagnoli E, Mascia ML, Cipriani C, Fassino V, Mazzei F, D'Erasmo E, Carnevale V, Scillitani A, Minisola S. Short and long-term variations in serum calciotropic hormones after a single very large dose of ergocalciferol (vitamin D2) or cholecalciferol (vitamin D3) in the elderly. J Clin Endocrinol Metab 2008;93:3015–20 [DOI] [PubMed] [Google Scholar]
- 6.Holick MF, Biancuzzo RM, Chen TC, Klein EK, Young A, Bibuld D, Reitz R, Salameh W, Ameri A, Tannenbaum AD. Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvitamin D. J Clin Endocrinol Metab 2008;93:677–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biancuzzo RM, Young A, Bibuld D, Cai MH, Winter MR, Klein EK, Ameri A, Reitz R, Salameh W, Chen TC, et al. Fortification of orange juice with vitamin D(2) or vitamin D(3) is as effective as an oral supplement in maintaining vitamin D status in adults. Am J Clin Nutr 2010;91:1621–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Houghton LA, Vieth R. The case against ergocalciferol (vitamin D-2) as a vitamin supplement. Am J Clin Nutr 2006;84:694–7 [DOI] [PubMed] [Google Scholar]
- 9.Holmberg I, Berlin T, Ewerth S, Bjorkhem I. 25-Hydroxylase activity in subcellular fractions from human liver. Evidence for different rates of mitochondrial hydroxylation of vitamin D2 and D3. Scand J Clin Lab Invest 1986;46:785–90 [DOI] [PubMed] [Google Scholar]
- 10.Hyppönen E, Power C. Hypovitaminosis D in British adults at age 45 y: nationwide cohort study of dietary and lifestyle predictors. Am J Clin Nutr 2007;85:860–8 [DOI] [PubMed] [Google Scholar]
- 11.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: Is blinding necessary?. Control Clin Trials 1996;17:1–12 [DOI] [PubMed] [Google Scholar]
- 12.Tjellesen L, Hummer L, Christiansen C, Rodbro P. Serum concentration of vitamin-d metabolites during treatment with vitamin-D2 and vitamin-D3 in normal premenopausal women. Bone Miner 1986;1:407–13 [PubMed] [Google Scholar]
- 13.Armas LA, Hollis BW, Heaney RP. Vitamin D(2) is much less effective than vitamin D(3) in humans. J Clin Endocrinol Metab 2004;89:5387–91 [DOI] [PubMed] [Google Scholar]
- 14.Leventis P, Kiely PD. The tolerability and biochemical effects of high-dose bolus vitamin D2 and D3 supplementation in patients with vitamin D insufficiency. Scand J Rheumatol 2009;38:149–53 [DOI] [PubMed] [Google Scholar]
- 15.Binkley N, Gemar D, Engelke J, Gangnon R, Ramamurthy R, Krueger D, Drezner MK. Evaluation of ergocalciferol or cholecalciferol dosing, 1,600 IU daily or 50,000 IU monthly in older adults. J Clin Endocrinol Metab 2011;96:981–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glendenning P, Chew GT, Seymour HM, Gillett MJ, Goldswain PR, Inderjeeth CA, Vasikaran SD, Taranto M, Musk AA, Fraser WD. Serum 25-hydroxyvitamin D levels in vitamin D-insufficient hip fracture patients after supplementation with ergocalciferol and cholecalciferol. Bone 2009;45:870–5 [DOI] [PubMed] [Google Scholar]
- 17.Heaney RP, Recker RR, Grote J, Horst RL, Armas LAG. Vitamin D(3) is more potent than vitamin D(2) in humans. J Clin Endocrinol Metab 2011;96:E447–52 [DOI] [PubMed] [Google Scholar]
- 18.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. J Clin Epidemiol 2010;63:834–40 [DOI] [PubMed] [Google Scholar]
- 19.Review Manager (RevMan) Copenhagen, Denmark: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011 [Google Scholar]
- 20.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holick MF. Resurrection of vitamin D deficiency and rickets. J Clin Invest 2006;116:2062–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hollis BW. Comparison of equilibrium and disequilibrium assay conditions for ergocalciferol, cholecalciferol and their major metabolites. J Steroid Biochem 1984;21:81–6 [DOI] [PubMed] [Google Scholar]
- 23.Cheng JB, Motola DL, Mangelsdorf DJ, Russell DW. De-orphanization of cytochrome P450 2R1: a microsomal vitamin D 25-hydroxylase. J Biol Chem 2003;278:38084–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng JB, Levine MA, Bell NH, Mangelsdorf DJ, Russell DW. Genetic evidence that the human CYP2R1 enzyme is a key vitamin D 25-hydroxylase. Proc Natl Acad Sci USA 2004;101:7711–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horst RL, Reinhardt TA, Ramberg CF, Koszewski NJ, Napoli JL. 24-Hydroxylation of 1,25dihydroxyergocalciferol—an unambiguous deactivation process. J Biol Chem 1986;261:9250–6 [PubMed] [Google Scholar]
- 26.IOM Dietary Reference Intakes for calcium and vitamin D. Washington, DC: The National Academies Press, 2011 [PubMed] [Google Scholar]
- 27.Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta-analyses. Lancet 1999;354:1896–900 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.