Abstract
Background: Nutrient stimulation of muscle protein anabolism is blunted with aging and may contribute to the development and progression of sarcopenia in older adults. This is likely due to insulin resistance of protein metabolism and/or endothelial dysfunction with a reduction in nutritive flow, both of which can be improved by aerobic exercise.
Objective: Our objective was to determine whether increasing physical activity can enhance the muscle protein anabolic effect of essential amino acid (EAA) + sucrose intake in older subjects by improving nutritive flow and/or insulin signaling.
Design: Using a randomized crossover design, we measured in older subjects [n = 6, 70 ± 3 y of age, BMI (in kg/m2) of 25 ± 1] the acute effects of increasing physical activity with aerobic exercise, as compared with normal sedentary lifestyle, on the response of blood flow, microvascular perfusion, insulin signaling, and muscle protein kinetics to EAA+sucrose intake.
Results: No differences between treatment groups were found in the basal state. The change from the basal state in blood flow, muscle perfusion, phenylalanine delivery, net balance, and muscle protein synthesis during the consumption of EAA+sucrose was significantly higher after the exercise than after the control treatment (P < 0.05). Insulin signaling increased during EAA+sucrose ingestion in both groups (P < 0.05).
Conclusions: Our data indicate that a prior bout of aerobic exercise increases the anabolic effect of nutrient intake in older adults. This effect appears to be mediated by an exercise-induced improvement in nutrient-stimulated vasodilation and nutrient delivery to muscle rather than to improved insulin signaling. This trial was registered at clinicaltrials.gov as NCT00690534.
INTRODUCTION
The age-related loss of skeletal muscle mass and function, or sarcopenia, is a major contributor to physical frailty in older adults (1–5). The rate of muscle loss or gain is a function of the net balance between muscle protein synthesis and breakdown. Whereas healthy, active, older adults have a postabsorptive net protein balance comparable with that of healthy younger individuals (6), they are less responsive to the combined anabolic effect of elevated amino acid and insulin concentrations, mainly because of blunted protein synthesis (7, 8).
A reduced response to amino acids at low doses may contribute to the blunted protein anabolism observed in older adults after mixed nutrient intake (9). However, this phenomenon seems to be dependent on the leucine content of the amino acid mixture (10) and is not detectable when amino acids or intact protein are administered after exercise or at the standard doses of a main meal (11–15).
Growing evidence indicates that the inability to mount an anabolic response to a meal may be primarily attributable to a reduced responsiveness of skeletal muscle to the vasodilatory and protein anabolic effects of insulin (16–18). Insulin has been shown to elicit skeletal muscle anabolism in young healthy adults (19–23) but not in healthy nondiabetic older adults (7, 16, 18). This age disparity of the muscle protein anabolic response to hyperinsulinemia is a true insulin resistance, because it can be overcome by elevating insulin to supraphysiologic concentrations (24). In recent studies, we have found that insulin's anabolic effects on skeletal muscle proteins are mediated by its ability to stimulate endothelial-dependent increases in blood flow, muscle perfusion, and amino acid delivery to the muscle, which are impaired in older adults (16, 18, 22). We have also shown that aerobic exercise before an insulin challenge can normalize vasodilation and restore muscle protein anabolism in response to isolated hyperinsulinemia in healthy older adults (25). However, whereas it is quite useful to define mechanisms, isolated hyperinsulinemia does not occur physiologically because insulin is normally secreted in conjunction with macronutrient intake. There is currently no direct evidence that increased aerobic activity may also enhance the muscle protein anabolic effects of essential amino acids (EAA)4 and carbohydrate intake in older adults.
The purpose of the current study was to determine whether an acute and moderate increase in physical activity with aerobic exercise improves the response of muscle protein anabolism to subsequent intake of EAA+sucrose in older adults. We hypothesized that a moderately intense bout of aerobic exercise performed the night before ingestion of EAA+sucrose would enhance muscle vasodilation and/or insulin signaling and, consequently, improve muscle protein synthesis and net protein balance in sedentary healthy older adults.
SUBJECTS AND METHODS
Subjects
Six healthy independent but sedentary subjects were recruited through the Claude D. Pepper Older Americans Independence Center Volunteer Registry. A clinical history, physical examination, and laboratory tests—including a standard 75-g oral-glucose-tolerance test—were performed on each volunteer to determine eligibility. Volunteers with a stable body weight for ≥3 mo and screening results within the normal range were included in the study (Table 1). After approval by the Institutional Review Board of the University of Texas Medical Branch (Galveston, TX) and the US Food and Drug Administration (IND 73870), all subjects read and signed a written informed consent form before enrollment.
TABLE 1.
Subject characteristics1
| Subjects (n = 3 M, 3 F) | |
| Age (y) | 70 ± 3 |
| Weight (kg) | 71 ± 5 |
| Height (m) | 1.70 ± 0.03 |
| BMI (kg/m2) | 25 ± 1 |
| Leg volume (mL) | 9489 ± 801 |
All values are means ± SEs.
Study design
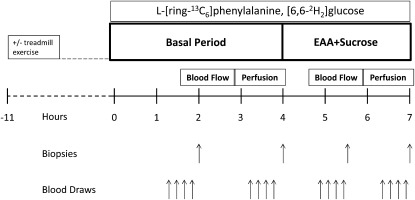
We used a randomized crossover design. Each subject participated in 2 EAA+sucrose ingestion experiments, separated by a 4–6-wk washout period during which subjects maintained their usual diet and activity level. The 2 experiments were identical, with the exception of the level of physical activity performed on the night before ingestion of EAA+sucrose: on one occasion subjects completed a bout of aerobic exercise (60–70% of heart rate reserve for 45 min on a treadmill), and on the other occasion they did not exercise (Figure 1).
FIGURE 1.
Study design. EAA, essential amino acids.
Before both experiments, subjects were instructed to refrain from strenuous physical activity before admission to our Institute for Translational Sciences–Clinical Research Center (ITS-CRC). To monitor the subjects’ prestudy activity and ensure that our exercise intervention resulted in increased physical activity over the day preceding the exercise infusion experiment, we used StepWatch step activity monitors (Orthocare Innovations LLC), which subjects wore for the 3 d preceding admission to the ITS-CRC. The average number of daily steps over the 3 d preceding the control treatment were as follows: day 1: 3613 ± 547; day 2: 4984 ± 602; and day 3: 4217 ± 430. The average number of steps preceding the aerobic exercise treatment were as follows: day 1: 3999 ± 222; day 2: 4383 ± 543, and day 3: 6239 ± 277. Steps for days 1 and 2 were not significantly different between treatments, whereas steps on day 3 were significantly higher (P < 0.05) in the exercise treatment than in the control period. To control for macronutrient intake, subjects consumed a standardized research dinner (10 kcal/kg body weight; 60% carbohydrate, 20% fat, and 20% protein) and received a standard snack at ∼2300. After the snack, subjects were allowed only water ad libitum until consuming the experimental meal on the following day.
On the next morning at ∼0600, a polyethylene catheter was inserted into an antecubital vein for the infusion of stable-isotope tracers (Cambridge Isotopes Laboratories Inc). A retrograde catheter was placed in the contralateral hand and warmed to ∼60°C to obtain arterialized blood for the measurement of systemic insulin, glucose, and indocyanine green (ICG) concentrations. With the use of local anesthesia and aseptic technique, 3-French (21 gauge) retrograde catheters were placed into the common femoral artery and vein of one leg for blood sampling. The arterial catheter was also used for the infusion of ICG. After background samples were obtained, a primed continuous infusion of l-[ring-13C6]phenylalanine (priming dose: 2 μmol/kg, infusion rate: 0.05 μmol · kg−1 · min−1) and d-[6,6-2H2]glucose (priming dose: 19 μmol/kg, infusion rate: 0.05 μmol · kg−1 · min−1) were started to measure phenylalanine and glucose kinetics.
After a 4-h basal period, subjects began consuming a solution of EAA+sucrose containing 20 g crystalline EAA (Sigma Aldrich; 20% histidine, 10% isoleucine, 18% leucine, 16% lysine, 3% methionine, 16% phenylalanine, 14% threonine, and 12% valine) and 35 g carbohydrate (sucrose), which were dissolved in 540 mL flavored water. To maintain the isotopic steady state in the arterial blood, based on our previous experience in older adults given phenylalanine and glucose tracers at the same rate (18), we enriched the meal with 9% l-[ring-13C6]phenylalanine and 2% d-[6,6-2H2]glucose. The enriched drink was given in small boluses (30 mL) every 10 min for 3 h to minimize fluctuations in blood phenylalanine and glucose concentrations, thus maintaining a metabolic steady state. Throughout the infusion experiment, frequent Doppler ultrasound (HDI-5000 ultrasound system; Philips ATL Ultrasound) measurements of the superficial femoral artery were taken to determine leg blood flow. Leg blood flow was also measured between 2.5 and 3 h (basal state) and between 5.5 and 6 h (EAA+sucrose) via infusion of ICG (0.5 mg/mL) into the common femoral artery and subsequent blood sampling from the femoral vein and hand vein. After ICG infusion, Perflutren Lipid Microspheres (Definity; Lantheus Medical Imaging) were infused into the antecubital vein to measure blood perfusion into the vastus lateralis muscle by contrast-enhanced ultrasound imaging. After perfusion was measured between 3.5 and 4 h (basal state) and 6.5 and 7 h (EAA+sucrose), 4 blood samples were taken from the femoral artery and vein to measure the concentrations of amino acids, glucose, insulin, and free phenylalanine and glucose enrichments. Muscle biopsy samples were taken from the lateral aspect of the vastus lateralis at 2, 4, 5.5, and 7 h of tracer infusion with the use of aseptic technique, local anesthesia, and a 5-mm Bergström biopsy needle. The first 2 biopsy samples (basal period) were taken from the same incision at different angles to ensure that the sampling sites were ≥5 cm apart. Biopsy samples 3 and 4 (EAA+sucrose period) were collected from a new incision ∼10 cm proximal to the first incision. On collection, biopsied muscle tissue was rinsed in ice-cold saline, blotted, and frozen in liquid nitrogen. Samples were then stored at −80°C until analyzed. After the last biopsy sample was collected, the tracer infusion was stopped, the catheters were removed, and the subjects were fed and discharged from the ITS-CRC after 2 h of observation.
Analytic methods
Plasma glucose concentrations were measured with the use of an automated glucose analyzer (Yellow Springs Instrument Co). Insulin concentrations were measured by enzyme-linked immunosorbent assay (Linco) by using a microplate reader (Bio-Rad). Serum ICG concentrations in the femoral vein and hand vein were measured spectrophotometrically (Beckman Coulter) at λ = 805, which allowed for the calculation of blood flow as previously described (26, 27).
Muscle microvascular perfusion was measured in the basal period and during EAA+sucrose consumption by using contrast-enhanced ultrasound as previously described (17, 18, 28–31). Ultrasound imaging of the vastus lateralis muscle was performed in a transaxial plane ∼15–20 cm above the patella over the midportion of the vastus lateralis muscle by using a P4-2 phased array transducer. A suspension of perflutren lipid microspheres (Definity; Lantheus Medical Imaging) was infused intravenously (3.5 mL/min for 8 min) by using a mechanical index of 1.3 and a compression of 80%. Once the systemic microsphere concentrations reached steady state (∼2 min), background images were obtained at a frame rate of 1/s. Intermittent imaging was then performed by using an internal timer at pulsing intervals ranging from 1 to 25 s, allowing for progressively greater replenishment of the ultrasound beam elevation between destructive pulses. Depth, focus, and gain were optimized at the beginning of each experiment and held constant throughout. Data were recorded on a Magneto-Optical Disk (Fujitsu) and digitized for analysis by using an offline system. A minimum of 3 images were acquired at each pulsing interval. The background subtracted video intensity at each pulsing interval was measured from a region of interest within the vastus lateralis muscle. Pulsing intervals compared with video intensity data were curve fitted to the function:
where y is the video intensity at pulsing interval t, A is the plateau video intensity (an index of microvascular blood volume), and β is the rate of microvascular refilling (an indicator of microvascular flow velocity) (28).
Muscle tissue samples were used to measure phosphorylated and total protein expression of protein kinase B (Akt), mammalian target of rapamycin (mTOR), and 4E-binding protein 1 (4E-BP1). The primary phospho-antibodies (Cell Signaling) used were phospho-Akt (serine 473, 1:1000), phospho-mTOR (serine 2448, 1:1000 in 5% nonfat dairy milk), and phospho–4E-BP1 (threonine 37/46, 1:1000). A dilution of 1:1000 was used for total expression of each protein. Fifty micrograms of total protein homogenate was loaded in duplicate into each lane, and the samples were separated on a 7.5% or 15% polyacrylamide gel (150 V, 1 h) (Criterion; Bio-Rad), depending on the size of the target protein. Specific details of our immunoblotting technique have been reported elsewhere (32). All samples were normalized to a rodent internal loading control, and final data were reported as phosphorylated protein relative to total protein.
Free phenylalanine enrichment and 13C6 concentrations in blood and tissue fluid were measured by gas chromatography–mass spectrometry (GC-MS; 6890 Plus GC, 5973N MSD, 7683 autosampler, Agilent Technologies) after addition of an internal standard ([l-15N]phenylalanine), extraction, purification, and tert-butyldimethylsilyl derivatization (33). [2H2]Glucose enrichment was measured by GC-MS after extraction by ion-exchange chromatography and penta-acetate derivatization (33). Muscle tissue samples were mechanically homogenized, and intracellular free amino acids and muscle proteins were extracted as described previously (34). The incorporation of labeled phenylalanine into the mixed muscle proteins was measured by GC-MS, after protein hydrolysis and amino acid extraction, by using the external standard curve approach (35).
Calculations
The kinetics of intracellular free phenylalanine in response to the intake of EAA+sucrose preceded by either an acute bout of aerobic exercise or rest were calculated by using 2-pool, 3-pool, and precursor-product models. With the 2-pool model, phenylalanine enrichments and concentrations in the femoral artery and vein were measured to estimate muscle protein synthesis, breakdown, and net balance without any consideration of the intracellular recycling of amino acids from breakdown to synthesis (33). The 3-pool model also included intracellular amino acid kinetics.
The 2-pool and 3-pool model parameters were calculated as follows:
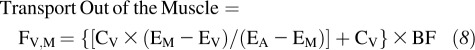 |
where CA and CV are the blood amino acid concentrations in the femoral artery and vein, respectively; EA, EV, and EM are the amino acid enrichments, expressed as tracer/tracee ratios, in the femoral artery, vein, and muscle, respectively; and BF is leg blood flow as calculated from the steady state ICG concentration values in the common femoral and wrist veins (26, 27). Data were expressed per 100 mL leg volume.
With the precursor-product model, we calculated the fractional synthetic rate (FSR) of mixed muscle proteins as follows:
where ΔEp is the increment in protein-bound phenylalanine enrichment between 2 biopsy samples, t is the time between the 2 biopsy samples, and EM(1) and EM(2) are the phenylalanine enrichments in the free intracellular pool in the 2 biopsy samples. Data are expressed as percentages per hour (33).
Leg glucose utilization was calculated as the net glucose uptake across the leg:
where GA and GV are blood glucose arterial and venous concentrations, respectively. Data are reported per 100 mL leg volume.
Basal endogenous glucose production was calculated, by using the single-pool model, as the ratio between the tracer infusion rate and the arterial glucose enrichment (EA) (33):
This equation was also used during nutrient intake because the nutritive solution was enriched with labeled glucose to match the basal arterial glucose enrichment (see above).
Statistical analyses
Statistical analyses were performed by using the statistical software SAS version 9.2 (SAS Institute Inc). The primary endpoints were measures of blood flow, muscle perfusion, muscle protein synthesis, and net muscle phenylalanine balance. Secondary endpoints were all remaining measures of muscle amino acid turnover and glucose kinetics. Paired t tests were used to make 3 comparisons: 1) a comparison of basal values between treatments (control and exercise conditions) to determine whether basal values were significantly different between the 2 treatment conditions, 2) a comparison of basal with EAA+sucrose values within a treatment (control or exercise condition) to determine whether there were significant within-treatment effects, and 3) a comparison of delta scores (EAA+sucrose values – basal values) between treatments (control and exercise conditions) to determine whether the magnitude of the effect was significantly different between treatments. Paired t tests were additionally used to make comparisons of step counts on the 3 d preceding each treatment. For data that did not pass the normality test, the values were transformed by using the natural ln or the reciprocal of the value. Blood flow, phenylalanine delivery to the leg, phenylalanine output from the leg, and phenylalanine rate of disappearance were transformed by using ln. Phenylalanine transport into the muscle was transformed by using the reciprocal of the value. The medians and interquartile ranges for these variables are reported elsewhere (see Supplemental Table 1 under “Supplemental data” in the online issue). Statistical analyses were performed on the transformed data, but all data are presented as means ± SEMs. Differences were considered significant at P < 0.05.
RESULTS
Blood flow and microvascular perfusion
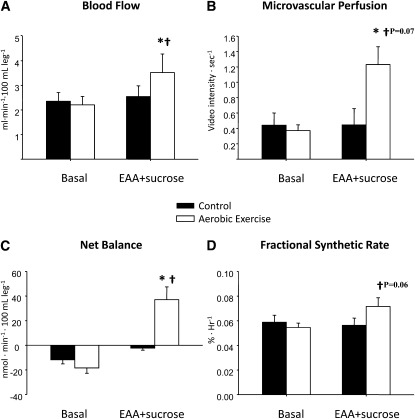
No basal differences for blood flow or microvascular perfusion were found between the treatment conditions (control and exercise). During the ingestion of EAA+sucrose, the change in blood flow from the basal state was significantly greater after the exercise than after the control treatment (P < 0.05). This difference was due to a significant increase (P < 0.05) in blood flow from the basal state with EAA+sucrose in the exercise condition, which was not observed in the control condition (P = NS) (Figure 2A; P < 0.05). Microvascular perfusion increased significantly within the exercise treatment during EAA+sucrose ingestion, and there was a trend for the change in perfusion to be higher after the exercise than after the control treatment (Figure 2B; P = 0.07). Representative contrast ultrasound images are shown for one subject during each of the 4 conditions (control:basal; control:EAA+sucrose; exercise:basal; and exercise:EAA+sucrose) (Figure 3).
FIGURE 2.
Blood flow, microvascular perfusion, phenylalanine net balance across the leg, and mixed-muscle-protein fractional synthetic rate in the basal period and during the consumption of EAA+sucrose in healthy older adults after a control or aerobic exercise treatment. n = 6 for both treatments by using a randomized crossover design. Paired t tests were used to compare basal with EAA+sucrose within treatments (control and exercise) and delta scores between treatments. *Significantly different from basal, P < 0.05. †Exercise:EAA+sucrose delta score > control:EAA+sucrose delta score (P < 0.05 unless otherwise noted). EAA, essential amino acids.
FIGURE 3.
Representative contrast ultrasound images of Definity microspheres infused into the vastus lateralis of one subject during each of the 4 conditions: control:basal, control:EAA+sucrose, exercise:basal, and exercise:EAA+sucrose. Each image was captured from the 10-s pulsing interval. EAA, essential amino acids.
Insulin and glucose kinetics
Data for insulin and glucose kinetics are presented in Table 2. No treatment differences for either insulin or glucose were found in the basal period. Systemic and femoral vein concentrations of insulin increased significantly during nutrient consumption under both treatment conditions (P < 0.05). Arterial and venous glucose increased during nutrient consumption compared with basal concentrations during both treatments (P < 0.05). Leg glucose uptake increased (P < 0.05) and endogenous glucose production decreased (P < 0.05) with nutrient consumption under both treatment conditions, with no differences in delta scores between treatments.
TABLE 2.
Insulin and glucose concentrations and kinetics in healthy older subjects in the basal state and during EAA+sucrose ingestion and the change (delta score) from the basal state during EAA+sucrose ingestion after a prior bout of aerobic exercise (n = 6) or a control period (n = 6)1
| Control |
Aerobic exercise |
|||||
| Basal | EAA+sucrose | Delta score | Basal | EAA+sucrose | Delta score | |
| Insulin | ||||||
| Systemic concentration (pmol/L) | 28 ± 4 | 62 ± 11* | 34 ± 8 | 31 ± 9 | 67 ± 13* | 37 ± 9 |
| Femoral vein concentration (pmol/L) | 24 ± 4 | 58 ± 10* | 34 ± 8 | 23 ± 6 | 57 ± 7* | 35 ± 7 |
| Glucose | ||||||
| Arterial concentration (mmol/L) | 5.1 ± 0.1 | 5.6 ± 0.2* | 0.4 ± 0.1 | 5.1 ± 0.1 | 5.5 ± 0.1* | 0.4 ± 0.1 |
| Venous concentration (mmol/L) | 5.1 ± 0.2 | 5.4 ± 0.2* | 0.4 ± 0.2 | 5.0 ± 0.2 | 5.4 ± 0.2* | 0.4 ± 0.1 |
| Leg uptake (μmol · min−1 · 100 mL leg volume−1) | 0.10 ± 0.03 | 0.36 ± 0.18* | 0.26 ± 0.13 | 0.23 ± 0.05 | 0.47 ± 0.06* | 0.22 ± 0.09 |
| Endogenous production (μmol · kg−1 · min−1) | 9.3 ± 0.4 | 5.9 ± 0.2* | −3.4 ± 0.3 | 9.4 ± 0.3 | 5.9 ± 0.1* | −3.5 ± 0.3 |
All values are means ± SEs. A randomized crossover design was used. *Significantly different from basal, P < 0.05. EAA, essential amino acids.
Phenylalanine concentrations and enrichments
The concentrations of phenylalanine in the artery, vein, and muscle (Table 3) were not different between treatments in the basal period and increased significantly during consumption of EAA+sucrose under both treatment conditions. No differences were found in the delta scores for these concentrations between treatments. Consumption of EAA+sucrose resulted in a slight, yet significant (P < 0.05), increase in phenylalanine enrichment in femoral vein and muscle under both treatment conditions, and no differences in the delta scores between treatments were observed. A slight yet significant decrease in phenylalanine enrichment in the artery was found during consumption of EAA+sucrose under both treatment conditions (P < 0.05).
TABLE 3.
Leg free phenylalanine concentrations, enrichments, and kinetics in healthy older subjects in the basal period and during EAA+sucrose ingestion and the change from the basal state (delta score) during EAA+sucrose ingestion after a prior bout of aerobic exercise (n = 6) or a control period (n = 6)1
| Control |
Aerobic exercise |
|||||
| Basal | EAA+sucrose | Delta score | Basal | EAA+sucrose | Delta score | |
| Phenylalanine concentration (μmol/L) | ||||||
| Femoral artery | 63 ± 5 | 187 ± 11* | 124 ± 12 | 64 ± 3 | 216 ± 15* | 151 ± 15 |
| Femoral vein | 68 ± 5 | 187 ± 10* | 119 ± 11 | 73 ± 4 | 203 ± 15* | 130 ± 16 |
| Muscle | 63 ± 6 | 136 ± 16* | 73 ± 13 | 60 ± 6 | 154 ± 22* | 94 ± 19 |
| Phenylalanine enrichment (tracer/tracee, %) | ||||||
| Femoral artery | 9.7 ± 0.4 | 9.2 ± 0.3* | −0.46 ± 0.25 | 9.9 ± 0.6 | 9.1 ± 0.3* | −0.74 ± 0.36 |
| Femoral vein | 7.9 ± 0.4 | 8.5 ± 0.3* | 0.63 ± 0.24 | 7.6 ± 0.6 | 8.4 ± 0.3* | 0.80 ± 0.32 |
| Muscle | 6.1 ± 0.5 | 7.5 ± 0.3* | 1.3 ± 0.30 | 5.5 ± 0.4 | 7.0 ± 0.5* | 1.53 ± 0.39 |
| Phenylalanine kinetics (nmol · min−1 · 100 mL leg volume−1) | ||||||
| Delivery to the leg | 143 ± 22 | 473 ± 88* | 330 ± 76 | 138 ± 14 | 726 ± 117* | 588 ± 111† |
| Output from the leg | 154 ± 23 | 475 ± 89* | 320 ± 74 | 156 ± 16 | 689 ± 118* | 532 ± 113 |
| Leg rate of appearance | 36 ± 8 | 37 ± 8 | 1.0 ± 2.5 | 42 ± 6 | 57 ± 9* | 15 ± 6 |
| Leg rate of disappearance | 24 ± 7 | 35 ± 7 | 10.6 ± 5 | 24 ± 3 | 94 ± 8* | 71 ± 8† |
| Transport into the muscle | 72 ± 17 | 215 ± 54* | 143 ± 52 | 64 ± 8 | 262 ± 26* | 198 ± 30 |
| Transport out of the muscle | 83 ± 17 | 217 ± 55* | 134 ± 51 | 83 ± 11 | 225 ± 22* | 142 ± 28 |
| Release from proteolysis | 42 ± 10 | 42 ± 9 | 0.15 ± 3.20 | 51 ± 6 | 77 ± 13 | 26 ± 11 |
| Utilization for protein synthesis | 30 ± 8 | 40 ± 8 | 10 ± 6 | 32 ± 3 | 114 ± 10* | 82 ± 12† |
| Intracellular availability | 114 ± 25 | 257 ± 56* | 143 ± 51 | 114 ± 13 | 338 ± 23* | 224 ± 47 |
| Efficiency of synthesis | 24 ± 3 | 19 ± 4 | −5 ± 4 | 28 ± 3 | 34 ± 3 | 5.4 ± 5.7 |
| Recycling (%) | 14 ± 1 | 12 ± 3 | −0.02 ± 0.03 | 18 ± 4 | 24 ± 2 | 0.06 ± 0.06 |
All values are means ± SEs. *Significantly different from basal, P < 0.05. †Exercise:EAA+sucrose delta score > control:EAA+sucrose delta score (P < 0.05). EAA, essential amino acids.
Phenylalanine kinetics
No differences in phenylalanine kinetics in the basal state were found between treatment conditions (Table 3). Phenylalanine delivery to the leg, output from the leg, transport into the muscle, transport out of the muscle, and intracellular availability increased significantly from basal values during EAA+sucrose consumption under both treatment conditions (P < 0.05). However, the delta score for phenylalanine delivery to the leg was greater after exercise than after the control treatment (P < 0.05). Phenylalanine rate of appearance, the 2-pool model measure of muscle protein breakdown, increased significantly during consumption of EAA+sucrose after the aerobic exercise treatment (36% from basal value; P < 0.05), but the change from the basal state was not significantly different from that observed in the control condition. Phenylalanine rate of disappearance and utilization for protein synthesis, the 2- and 3-pool model measures of muscle protein synthesis, increased significantly with EAA+sucrose consumption after the exercise treatment (301% and 254% from basal values, respectively; P < 0.05), whereas they did not change in the control treatment. The delta score for both measures of synthesis was significantly higher after exercise than after the control treatment (P < 0.05). Accordingly, phenylalanine net balance across the leg increased significantly after the exercise treatment (P < 0.05), and the value shifted from negative in the basal state (−18 ± 4 nmol · min−1 · 100 mL leg volume−1 = net muscle protein breakdown) to positive during EAA+sucrose consumption (37 ± 10 nmol · min−1 · 100 mL leg volume−1 = net muscle protein accretion). In contrast, net balance remained negative (−2 ± 2 nmol · min−1 · 100 mL leg volume−1) during EAA+sucrose consumption after the control treatment (Figure 2C; P < 0.05). The change in net balance was significantly greater after aerobic exercise than after the control treatment. The changes in efficiency of protein synthesis and intracellular recycling of protein were not different between treatments because of an outlier. However, when the raw values for these 2 variables were compared by using a paired t test between treatments, they were significantly higher during EAA+sucrose consumption after the exercise treatment than after the control treatment.
Muscle-protein fractional synthetic rate
The fractional synthetic rate of mixed skeletal muscle protein was not different in the basal state between treatment conditions (control: 0.059 ± 0.005%/h; exercise: 0.054 ± 0.008%/h), but there was a trend for the delta score to be higher after the exercise than after the control treatment (Figure 2D; P = 0.06).
Cell signaling
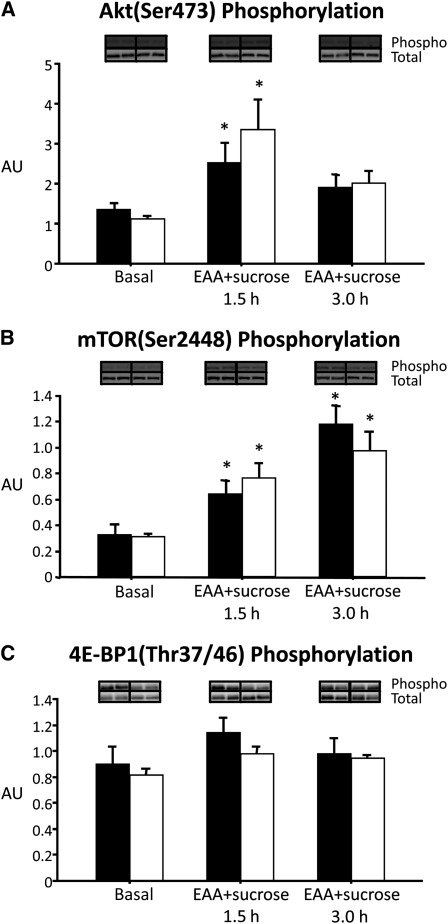
Phosphorylation of skeletal muscle Akt, mTOR, and 4E-BP1 did not differ in the basal state between treatment conditions (Figure 4). Akt phosphorylation increased with EAA+sucrose consumption 1.5 h after nutrient intake began under both treatment conditions (P < 0.05), and no difference in delta scores was found between treatments at 1.5 or 3.0 h of EAA+sucrose consumption. mTOR phosphorylation increased from the basal state at 1.5 and 3.0 h of nutrient consumption intake under both treatment conditions (P < 0.05), and no difference in delta scores was found between treatment conditions. No significant differences in 4E-BP1 were observed.
FIGURE 4.
Skeletal muscle Akt, mTOR, and 4E-BP1 phosphorylation (relative to total protein expression) in the basal state and at 1.5 and 3.0 h of EAA+sucrose consumption in healthy older adults after a control (▪) or aerobic exercise (□) treatment. n = 6 for each treatment by using a randomized crossover design. Representative basal images are included for comparison. Paired t tests were used to compare the basal state with EAA+sucrose at 1.5 and 3.0 h within treatments (control and exercise) and delta scores between treatments.*Significantly different from basal. P < 0.05. Akt, protein kinase B; AU, arbitrary units; EAA, essential amino acids; mTor, mammalian target of rapamycin; 4E-BP1, 4E-binding protein 1.
DISCUSSION
The novel finding of this study was that an acute increase in physical activity with a moderate bout of aerobic exercise enhanced the subsequent anabolic response to intake of amino acids and carbohydrate in healthy older adults, which suggests that aerobic exercise may be an important tool for the prevention and treatment of sarcopenia. This positive effect of acute aerobic exercise on skeletal muscle protein anabolism appears to be linked to an increase in microvascular perfusion and amino acid delivery, rather than to an improvement in skeletal muscle insulin signaling.
Exercising the night before nutrient intake significantly elevated total leg blood flow, microvascular perfusion, amino acid delivery to the leg, muscle protein synthesis, and net balance in these older adults to a greater extent than did the control treatment. The resulting increase in delivery of amino acids likely contributed to the enhanced anabolic response to nutrients. Conversely, when the same older subjects consumed the same nutrients after a sedentary period, blood flow and microvascular perfusion did not increase, and amino acid delivery to the leg increased less than after aerobic exercise, and no significant changes in either muscle protein synthesis or net balance was found.
Hyperaminoacidemia with hyperinsulinemia increases muscle protein synthesis (7, 8) and stimulates blood flow (8) and microvascular perfusion (30) in young subjects, whereas older subjects have been reported to be resistant to the anabolic effects of these anabolic stimuli (7, 8), which is consistent with our current findings in the control, resting condition. Of note, this was the first study to show that nutrient consumption fails to stimulate muscle microvascular flow and perfusion in older adults. These data support the notion that there is a link between endothelial function and muscle protein anabolism that is not only limited to isolated anabolic stimuli (eg, isolated hyperinsulinemia) as previously reported (16–18, 22, 24), but is also critical in complex physiologic conditions such as the response to mixed nutrients (ie, EAA+sucrose) in older adults. The nutrient-induced vasodilation was likely due to a direct effect of endogenous hyperinsulinemia on endothelial-dependent nitric oxide synthesis—a process that is impaired in sedentary older adults (8, 16, 36, 37) but can be restored by aerobic physical activity (25, 38).
Whereas insulin and glucose kinetics were equivalent in response to nutrient ingestion, regardless of treatment (control or aerobic exercise), significant treatment differences in phenylalanine kinetics were observed. Phenylalanine delivery to the leg was elevated to a greater extent when consumption of nutrients was preceded by aerobic exercise than when preceded by a sedentary behavior. Thus, it was not surprising to find that phenylalanine utilization for protein synthesis was also markedly elevated during EAA+sucrose intake after aerobic exercise only.
Interestingly, consumption of nutrients after aerobic exercise resulted in a modest, yet statistically significant, increase in phenylalanine rate of appearance. This response was somewhat unexpected, because elevated concentrations of insulin have been reported by some to blunt proteolysis (39, 40). However, in older subjects it has been suggested that insulin's ability to decrease proteolysis is attenuated (41). Moreover, the insulin response achieved in this study was blunted as a result of the continuous administration of nutrients over 3 h. Another possibility is that the increase in protein breakdown could have been due to a kinetic artifact induced by increased blood flow (washout effect). However, if the effects of exercise on proteolysis were solely due to a washout effect, we should have observed an equal increase in protein synthesis and proteolysis during nutrient intake, and no changes in net balance (which is the difference between synthesis and breakdown) would have been observed. This was not the case, because net balance increased and became positive with nutrient intake after exercise. To further determine the relative contribution of blood flow, we adjusted the 2- and 3-pool kinetic parameters by blood flow, in other words, we calculated the simple chemical balances between the various model compartments. The effect of exercise on amino acid release was still present, although smaller and no longer significant, whereas the measures of amino acid uptake remained significantly higher with exercise. Thus, whereas increased blood flow might have somewhat magnified the changes in proteolysis, it is likely that the increased protein breakdown rate was simply reflective of increased intracellular protein turnover during nutrient intake after exercise.
Specifically, whereas the delta scores of amino acid recycling from breakdown to synthesis and protein synthesis efficiency were not significantly different, when we compared the mean values during nutrient ingestion we found that the exercise treatment resulted in higher amino acid recycling from muscle protein breakdown to synthesis and higher protein synthesis efficiency than did the control treatment. Increased tissue exposure (muscle perfusion) to nutrients (amino acids) and hormones (insulin) probably increased amino acid turnover and were the driving forces behind the elevated protein synthesis, protein synthesis efficiency and net muscle protein anabolism observed during EAA+sucrose consumption after aerobic exercise in our group of older adults.
Because aerobic exercise can improve insulin signaling, we measured Akt/PKB and components of the mTOR signaling pathway. Akt/PKB phosphorylation increased 1.5 h after nutrient consumption was started under both treatment conditions. There was a trend for the change in Akt/PKB phosphorylation from the basal state (P = 0.12; 1.5 h during nutrient consumption) to be higher during EAA+sucrose consumption after the exercise than after the sedentary control condition. However, mTOR signaling increased to the same extent during nutrient consumption, regardless of treatment. It is possible that while nutrient intake activated the translational machinery to comparable levels under both treatment conditions, substrate (amino acids) availability and/or differences in the amount of muscle tissue exposed to the dietary stimuli were the rate-limiting steps for increased synthesis. In other words, the increased amino acid delivery and perfusion observed during nutrient consumption after the exercise treatment allowed for a significantly greater increase in muscle protein synthesis. However, others have also reported a disassociation between the effects of amino acid intake and mTOR signaling in skeletal muscle (42). We cannot exclude that under certain conditions, such as those of this and the other study (42), cell signaling events were missed because of the timing of the muscle biopsy sample collection.
Limitations of the current study include the following. First, we cannot draw final conclusions regarding the potential long-term benefits of aerobic exercise or increased physical activity level on muscle protein turnover after ingestion of a regular meal because the nutritional stimulus did not contain lipids and was given acutely, in a continuous manner. Second, we collected only 2 biopsy samples during the consumption of EAA+sucrose. Thus, it is possible that we missed treatment-related differences in mTOR signaling that may have occurred during nutrient consumption. Third, whereas we had enough power to detect changes in the primary outcomes (blood flow, protein synthesis, and net balance), the limited sample size did not allow for extensive extrapolation of the results of this study to the general population. Larger clinical trials are warranted. Fourth, the study was not powered to detect interactions for secondary variables, such as amino acid recycling and protein synthesis efficiency. Thus, these data should be interpreted as preliminary estimates of treatment differences for future experiments. Finally, the study was unblinded, because the treatment was exercise. However, because of the objective, the biological nature of the primary outcome measures and the randomized crossover design, we believe that the results of this study are unbiased.
In conclusion, the results of this study indicate that in healthy older adults a moderate acute increase in physical activity with aerobic exercise enhances the skeletal muscle protein synthetic and anabolic responses to subsequent amino acid and carbohydrate intake via improved nutrient delivery. It is important to underscore that, whereas the exercise bout was moderately intense (70% heart rate reserve), the overall level of physical activity remained well below the recommended 10,000 steps/d (43). These very encouraging acute data suggest that aerobic exercise and/or moderately increasing physical activity status may help prevent and treat muscle loss with aging.
Supplementary Material
Acknowledgments
We thank Shelley Medina and Ming Zheng for their superb technical assistance and the nurses and personnel of the University of Texas Medical Branch Institute for Translational Sciences Clinical Research Center for their assistance in the clinical conduct of this study.
The authors’ responsibilities were as follows—EV: designed the research; KLT, SD, ELG, CSF, MJD, and EV: conducted the research; KLT, KJ, BBR, and EV: analyzed the data; KLT, BBR, and EV: wrote the manuscript; and KTL and EV: had primary responsibility for the final content. The authors reported no conflicts of interest.
Footnotes
Abbreviations used: Akt, protein kinase B; EAA, essential amino acids; GC-MS, gas chromatography–mass spectrometry; ICG, indocyanine green; ITS-CRC, Institute for Translational Sciences–Clinical Research Center; mTOR, mammalian target of rapamycin; 4E-BP1, 4E-binding protein 1.
REFERENCES
- 1.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fried LP, Hadley EC, Walston JD, Newman AB, Guralnik JM, Studenski S, Harris TB, Ershler WB, Ferrucci L. From bedside to bench: research agenda for frailty. Sci Aging Knowledge Environ 2005;2005:pe24 [DOI] [PubMed] [Google Scholar]
- 3.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc 2002;50:889–96 [DOI] [PubMed] [Google Scholar]
- 4.Janssen I, Baumgartner RN, Ross R, Rosenberg IH, Roubenoff R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol 2004;159:413–21 [DOI] [PubMed] [Google Scholar]
- 5.Sayer AA, Syddall HE, Martin HJ, Dennison EM, Anderson FH, Cooper C. Falls, sarcopenia, and growth in early life: findings from the Hertfordshire cohort study. Am J Epidemiol 2006;164:665–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Volpi E, Sheffield-Moore M, Rasmussen BB, Wolfe RR. Basal muscle amino acid kinetics and protein synthesis in healthy young and older men. JAMA 2001;286:1206–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guillet C, Prod'homme M, Balage M, Gachon P, Giraudet C, Morin L, Grizard J, Boirie Y. Impaired anabolic response of muscle protein synthesis is associated with S6K1 dysregulation in elderly humans. FASEB J 2004;18:1586–7 [DOI] [PubMed] [Google Scholar]
- 8.Volpi E, Mittendorfer B, Rasmussen BB, Wolfe RR. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J Clin Endocrinol Metab 2000;85:4481–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am J Clin Nutr 2005;82:1065–73 [DOI] [PubMed] [Google Scholar]
- 10.Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab 2006;291:E381–7 [DOI] [PubMed] [Google Scholar]
- 11.Paddon-Jones D, Sheffield-Moore M, Zhang XJ, Volpi E, Wolf SE, Aarsland A, Ferrando AA, Wolfe RR. Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am J Physiol Endocrinol Metab 2004;286:E321–8 [DOI] [PubMed] [Google Scholar]
- 12.Rasmussen BB, Wolfe RR, Volpi E. Oral and intravenously administered amino acids produce similar effects on muscle protein synthesis in the elderly. J Nutr Health Aging 2002;6:358–62 [PMC free article] [PubMed] [Google Scholar]
- 13.Symons TB, Schutzler SE, Cocke TL, Chinkes DL, Wolfe RR, Paddon-Jones D. Aging does not impair the anabolic response to a protein-rich meal. Am J Clin Nutr 2007;86:451–6 [DOI] [PubMed] [Google Scholar]
- 14.Symons TB, Sheffield-Moore M, Wolfe RR, Paddon-Jones D. A moderate serving of high-quality protein maximally stimulates skeletal muscle protein synthesis in young and elderly subjects. J Am Diet Assoc 2009;109:1582–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Volpi E, Mittendorfer B, Wolf SE, Wolfe RR. Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first-pass splanchnic extraction. Am J Physiol 1999;277:E513–20 [DOI] [PubMed] [Google Scholar]
- 16.Rasmussen BB, Fujita S, Wolfe RR, Mittendorfer B, Roy M, Rowe VL, Volpi E. Insulin resistance of muscle protein metabolism in aging. FASEB J 2006;20:768–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Timmerman KL, Lee JL, Dreyer HC, Dhanani S, Glynn EL, Fry CS, Drummond MJ, Sheffield-Moore M, Rasmussen BB, Volpi E. Insulin stimulates human skeletal muscle protein synthesis via an indirect mechanism involving endothelial-dependent vasodilation and mammalian target of rapamycin complex 1 signaling. J Clin Endocrinol Metab 2010;95:3848–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Timmerman KL, Lee JL, Fujita S, Dhanani S, Dreyer HC, Fry CS, Drummond MJ, Sheffield-Moore M, Rasmussen BB, Volpi E. Pharmacological vasodilation improves insulin-stimulated muscle protein anabolism but not glucose utilization in older adults. Diabetes 2010;59:2764–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bennet WM, Connacher AA, Scrimgeour CM, Jung RT, Rennie MJ. Euglycemic hyperinsulinemia augments amino acid uptake by human leg tissues during hyperaminoacidemia. Am J Physiol 1990;259:E185–94 [DOI] [PubMed] [Google Scholar]
- 20.Bennet WM, Rennie MJ. Protein anabolic actions of insulin in the human body. Diabet Med 1991;8:199–207 [DOI] [PubMed] [Google Scholar]
- 21.Biolo G, Declan Fleming RY, Wolfe RR. Physiologic hyperinsulinemia stimulates protein synthesis and enhances transport of selected amino acids in human skeletal muscle. J Clin Invest 1995;95:811–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujita S, Rasmussen BB, Cadenas JG, Grady JJ, Volpi E. Effect of insulin on human skeletal muscle protein synthesis is modulated by insulin-induced changes in muscle blood flow and amino acid availability. Am J Physiol Endocrinol Metab 2006;291:E745–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hillier TA, Fryburg DA, Jahn LA, Barrett EJ. Extreme hyperinsulinemia unmasks insulin's effect to stimulate protein synthesis in the human forearm. Am J Physiol 1998;274:E1067–74 [DOI] [PubMed] [Google Scholar]
- 24.Fujita S, Glynn EL, Timmerman KL, Rasmussen BB, Volpi E. Supraphysiological hyperinsulinaemia is necessary to stimulate skeletal muscle protein anabolism in older adults: evidence of a true age-related insulin resistance of muscle protein metabolism. Diabetologia 2009;52:1889–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fujita S, Rasmussen BB, Cadenas JG, Drummond MJ, Glynn EL, Sattler FR, Volpi E. Aerobic exercise overcomes the age-related insulin resistance of muscle protein metabolism by improving endothelial function and Akt/mammalian target of rapamycin signaling. Diabetes 2007;56:1615–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jorfeldt L, Wahren J. Leg blood flow during exercise in man. Clin Sci 1971;41:459–73 [DOI] [PubMed] [Google Scholar]
- 27.Jorfeldt L, Juhlin-Dannfelt A. The influence of ethanol on splanchnic and skeletal muscle metabolism in man. Metabolism 1978;27:97–106 [DOI] [PubMed] [Google Scholar]
- 28.Clerk LH, Vincent MA, Jahn LA, Liu Z, Lindner JR, Barrett EJ. Obesity blunts insulin-mediated microvascular recruitment in human forearm muscle. Diabetes 2006;55:1436–42 [DOI] [PubMed] [Google Scholar]
- 29.Dawson D, Vincent MA, Barrett EJ, Kaul S, Clark A, Leong-Poi H, Lindner JR. Vascular recruitment in skeletal muscle during exercise and hyperinsulinemia assessed by contrast ultrasound. Am J Physiol Endocrinol Metab 2002;282:E714–20 [DOI] [PubMed] [Google Scholar]
- 30.Vincent MA, Clerk LH, Lindner JR, Price WJ, Jahn LA, Leong-Poi H, Barrett EJ. Mixed meal and light exercise each recruit muscle capillaries in healthy humans. Am J Physiol Endocrinol Metab 2006;290:E1191–7 [DOI] [PubMed] [Google Scholar]
- 31.Wei K, Jayaweera AR, Firoozan S, Linka A, Skyba DM, Kaul S. Quantification of myocardial blood flow with ultrasound-induced destruction of microbubbles administered as a constant venous infusion. Circulation 1998;97:473–83 [DOI] [PubMed] [Google Scholar]
- 32.Dreyer HC, Fujita S, Cadenas JG, Chinkes DL, Volpi E, Rasmussen BB. Resistance exercise increases AMPK activity and reduces 4E-BP1 phosphorylation and protein synthesis in human skeletal muscle. J Physiol 2006;576:613–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolfe RR, Chinkes DL. Isotope tracers in metabolic research. Principles and practice of kinetic analysis. New York, NY: Wiley-Liss, 2005 [Google Scholar]
- 34.Wolfe RR. Radioactive and stable isotope tracers in biomedicine. Principles and practice of kinetic analysis. New York, NY: Wiley-Liss, 1992 [Google Scholar]
- 35.Calder AG, Anderson SE, Grant I, McNurlan MA, Garlick PJ. The determination of low d5-phenylalanine enrichment (0.002-0.09 atom percent excess), after conversion to phenylethylamine, in relation to protein turnover studies by gas chromatography/electron ionization mass spectrometry. Rapid Commun Mass Spectrom 1992;6:421–4 [DOI] [PubMed] [Google Scholar]
- 36.Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, Salvetti A. Age-related reduction of NO availability and oxidative stress in humans. Hypertension 2001;38:274–9 [DOI] [PubMed] [Google Scholar]
- 37.Galetta F, Franzoni F, Plantinga Y, Ghiadoni L, Rossi M, Prattichizzo F, Carpi A, Taddei S, Santoro G. Ambulatory blood pressure monitoring and endothelium-dependent vasodilation in the elderly athletes. Biomed Pharmacother 2006;60:443–7 [DOI] [PubMed] [Google Scholar]
- 38.DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation 2000;102:1351–7 [DOI] [PubMed] [Google Scholar]
- 39.Chow LS, Albright RC, Bigelow ML, Toffolo G, Cobelli C, Nair KS. Mechanism of insulin's anabolic effect on muscle: measurements of muscle protein synthesis and breakdown using aminoacyl-tRNA and other surrogate measures. Am J Physiol Endocrinol Metab 2006;291:E729–36 [DOI] [PubMed] [Google Scholar]
- 40.Möller-Loswick AC, Zachrisson H, Hyltander A, Korner U, Matthews DE, Lundholm K. Insulin selectively attenuates breakdown of nonmyofibrillar proteins in peripheral tissues of normal men. Am J Physiol 1994;266:E645–52 [DOI] [PubMed] [Google Scholar]
- 41.Wilkes EA, Selby AL, Atherton PJ, Patel R, Rankin D, Smith K, Rennie MJ. Blunting of insulin inhibition of proteolysis in legs of older subjects may contribute to age-related sarcopenia. Am J Clin Nutr 2009;90:1343–50 [DOI] [PubMed] [Google Scholar]
- 42.Greenhaff PL, Karagounis LG, Peirce N, Simpson EJ, Hazell M, Layfield R, Wackerhage H, Smith K, Atherton P, Selby A, et al. Disassociation between the effects of amino acids and insulin on signaling, ubiquitin ligases, and protein turnover in human muscle. Am J Physiol Endocrinol Metab 2008;295:E595–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tudor-Locke C, Bassett DR., Jr How many steps/day are enough? Preliminary pedometer indices for public health. Sports Med 2004;34:1–8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.