Abstract
Background: The replacement of caloric beverages such as sugar-sweetened beverages (SSBs) and fruit juices with noncaloric beverages such as plain water has been recommended for diabetes prevention.
Objective: We evaluated the relation of plain-water intake and the substitution of plain water for SSBs and fruit juices with incident type 2 diabetes (T2D) in US women.
Design: We prospectively followed 82,902 women in the Nurses’ Health Study II who were free of diabetes, cardiovascular disease, or cancer at baseline. Diet, including various beverages, was assessed by using validated food-frequency questionnaires and updated every 4 y. Incident T2D was confirmed by using a validated supplementary questionnaire. We used a 4-y lagged analysis to minimize reverse causation (ie, increased water consumption that was due to early stage of diabetes).
Results: During 1,115,427 person-years of follow-up, we documented 2718 incident T2D cases. Plain-water intake was not associated with T2D risk in the multivariable-adjusted model that included age, BMI, diet, and lifestyle factors; RRs (95% CIs) across categories (<1, 1, 2–3, 4–5, and ≥6 cups/d) were 1.00, 0.93 (0.82, 1.05), 0.93 (0.83, 1.05), 1.09 (0.96, 1.24), and 1.06 (0.91, 1.23), respectively (P-trend = 0.15). We estimated that the replacement of 1 serving SSBs and fruit juices/d by 1 cup plain water/d was associated with 7% (3%, 11%) and 8% (2%, 13%) lower risk of T2D, respectively.
Conclusions: Plain-water intake, per se, was not significantly associated with risk of T2D. However, substitution of plain water for SSBs or fruit juices was estimated to be associated with modestly lower risk of T2D.
INTRODUCTION
Extensive studies have been conducted to investigate the relation between intakes of certain beverages and risk of type 2 diabetes (T2D)5. There is growing evidence that coffee intake is associated with lower T2D risk (1), whereas caloric beverages such as sugar-sweetened beverages (SSBs) and fruit juices are associated with elevated risk (2, 3). Beverage intakes have changed dramatically in the past several decades, and SSBs and fruit juices have become major sources of fluids and also contributed to >10% of daily calories in the American diet (4). Therefore, the replacement of SSBs and fruit juices with noncaloric or low-caloric beverages such as plain water has been recommended to lower T2D risk. Some studies have suggested that the replacement of SSBs and fruit juices with plain water may be associated with a lower energy intake (5) and may promote weight loss (6). However, few studies have examined the association between plain-water intake and risk of T2D (7), and no study, to our knowledge, has examined whether the replacement of SSBs and fruit juices with plain water is associated with lower risk of T2D.
Therefore, we prospectively examined the association of plain-water intake with T2D risk in a cohort of young and middle-aged women and estimated potential benefits of the replacement of SSBs or fruit juices with plain water on T2D risk. In addition, we also examined the relation between total beverage intake and risk of T2D in this cohort.
SUBJECTS AND METHODS
Study population
The Nurses’ Health Study (NHS) II was established in 1989 and comprised 116,671 female registered nurses, aged 25–42 y, who responded to a baseline questionnaire about their lifestyles and medical histories. The cohort has been updated with biennial questionnaires to collect information on lifestyle habits and the occurrence of chronic diseases. The follow-up rate of participants in this cohort has been >90% for every 2-y period (8). We used 1991 as the baseline year because the dietary information was first collected in 1991 by using a food-frequency questionnaire (FFQ), and a total of 97,650 women returned their baseline FFQs. Women with >10 blank items on the FFQ (n = 619), unusual total energy intakes (ie, daily energy intake <500 or >3500 kcal/d; n = 2137), or missing values for any of the beverages (n = 2003) were excluded from the analysis. Women who reported a history of cardiovascular disease (myocardial infarction or stroke; n = 708), or cancer (except nonmelanoma skin cancer; n = 1232) before 1991 were excluded. Because we used a 4-y lagged analysis, self-reported diabetes cases (including type 1 diabetes, T2D, and gestational diabetes; n = 5503) before 1995 were also excluded. In addition, participants with missing baseline information of age (n = 194) or body weight (n = 2352) were excluded because body weight was shown to be a major confounding factor for the association. These exclusions left a total of 82,902 participants for this analysis. The NHS II was approved by the institutional review boards of Brigham and Women's Hospital and Harvard School of Public Health.
Assessment of water and other beverage intakes
In 1991, a 133-item FFQ was administered to NHS II participants to collect information on their usual intakes of foods and beverages over the previous year. Similar FFQs were administered in 1995, 1999, and 2003. All FFQs asked participants how often, on average, they consumed each food by using standard portion sizes (one standard serving, cup, glass, can, or bottle). There were 9 possible responses that ranged from never or <1 time/mo to ≥6 times/d. Questionnaire items on beverage intakes included one item on plain water (bottled or tap), 4 items on SSBs (Coke, Pepsi, or other cola with sugar; caffeine-free Coke, Pepsi, or other cola with sugar; other carbonated beverages with sugar; and Hawaiian Punch, lemonade, or other noncarbonated fruit drinks), 3 items on artificially sweetened beverages (ASBs), including low-calorie cola with caffeine, low-calorie caffeine-free cola, and other low-calorie beverages), 4 items on fruit juices (apple, orange, grapefruit, and other juices), 2 items on coffee (decaffeinated coffee and coffee with caffeine), 2 items on milk (skim or low-fat milk; whole milk), and 1 item on tea. We summed the intakes of all single beverage items to create a total beverage intake. The reproducibility and validity of the FFQ have been demonstrated in detail previously in NHS I, which was a similar cohort in middle-aged and elderly nurses (9). The deattenuated correlation coefficients between the FFQ and multiple dietary records were 0.36 for ASBs, 0.56 for fruit punch, 0.62 for whole milk, 0.78 for coffee, 0.81 for low-fat milk, 0.84 for orange juice, 0.84 for Coke and Pepsi colas, and 0.93 for tea (9). The correlation coefficient for plain water was not available in this validation study; however, a validation study in 127 male health professionals, which is a parallel cohort of the NHS II with similar FFQs, showed a correlation coefficient of 0.53 for plain-water intake (10).
Assessment of covariates
In the biennial follow-up questionnaires, we updated information on lifestyle and other risk factors for chronic diseases, including body weight, cigarette smoking, physical activity, medication use, menopausal status, postmenopausal hormone use, oral contraceptive use, and history of chronic diseases, including hypertension and hypercholesterolemia. Information about family history of diabetes and race was also collected.
Assessment of diabetes
Women who reported a new diagnosis of diabetes on any of the biennial questionnaires were sent supplementary questionnaires that asked about the diagnosis and treatment of their diabetes. A case of T2D was considered confirmed if at least one of the following items was reported on the supplementary questionnaire according to the National Diabetes Data Group criteria (11): 1) one or more classic symptoms (excessive thirst, polyuria, weight loss, or hunger) plus fasting plasma glucose concentrations ≥7.8 mmol/L or random plasma glucose concentrations ≥11.1 mmol/L; 2) ≥2 elevated plasma glucose concentrations on different occasions (fasting concentrations ≥7.8 mmol/L, random plasma glucose concentrations ≥11.1 mmol/L, and/or concentrations ≥11.1 mmol/L after ≥2 h shown by oral-glucose-tolerance testing) in the absence of symptoms; or 3) treatment with hypoglycemic medication (insulin or oral hypoglycemic agent). The diagnostic criteria changed in June 1998, and a fasting plasma glucose concentration of 7.0 mmol/L was considered the threshold for a diagnosis of diabetes instead of a concentration of 7.8 mmol/L according to the American Diabetes Association criteria (12).
The validity of the supplementary questionnaire has been documented previously in the NHS I; of 62 cases that were confirmed by the supplementary questionnaire, 61 cases (98%) were reconfirmed by medical records (13). Moreover, we conducted another substudy to assess the specificity of self-reported diabetes status. In a random sample of participants (n = 200) who reported no diabetes, only one participant (0.5%) had an elevated fasting plasma glucose or plasma fructosamine concentration in the diabetic range, and her concentrations were barely above the diagnostic cutoffs (14). Only cases confirmed by the supplemental questionnaires were included in the current analysis.
Statistical analysis
We calculated the person-years of each individual from the date of return of the baseline questionnaire to the date of diagnosis of T2D, death, or the end of the follow-up (30 June 2009), whichever came first. We used time-dependent Cox proportional hazards models to estimate the RR for T2D associated with plain-water intake. In the multivariable analysis, we simultaneously controlled for various potential confounding factors, including age, race (white or nonwhite), family history of diabetes (yes or no), BMI (in kg/m2) (<23.0, 23.0–24.9, 25.0–29.9, 30.0–34.9, or ≥35.0), smoking status [never, past, or current (1–14, 15–24, or ≥25 cigarettes/d)], alcohol intake (0, 0.1–4.9, 5.0–14.9, or ≥15.0 g/d), physical activity [<3.0, 3.0–8.9, 9.0–17.9, 18.0–26.9, or ≥27.0 metabolic equivalent task hours (MET-h)/wk], postmenopausal status, and menopausal hormone use [premenopausal or postmenopausal (never, past, or current hormone use)], oral contraceptive use (yes or no), and overall dietary quality represented by the Alternative Healthy Eating Index (AHEI; in quintiles) (15). The AHEI targeted food choices and nutrients that have been associated with reduced chronic disease risk (15). These components included vegetables, fruit, nuts, and soy protein, the ratio of white to red meat, cereal fiber, trans fat, the ratio of polyunsaturated to saturated fatty acids, and multivitamin use. Most of the nondietary variables were updated every 2 y (except for race and family history of diabetes), dietary data were updated every 4 y, and time-varying covariates were included in the models.
Because increased water consumption is an early symptom of T2D, we used a 4-y lagged analysis in which plain-water and beverage intakes were used to predict diabetes outcomes that occurred 4 y later to minimize reverse causation. To better represent the long-term diet and to minimize within-person variation, we created cumulative averages of food and beverage intakes from baseline to censoring events (16). We stopped updating dietary variables when participants reported a diagnosis of stroke, myocardial infarction, angina, or cancer because these conditions might lead to changes in diet and beverage intakes (16). Missing values during the follow-up were replaced by using the carry-forward method. We estimated the association of substituting a serving of one beverage for another by including both as continuous variables in the same multivariable model. Differences in their β coefficients were used to estimate the RR for the substitution association, and their variances and covariance matrix were used to derive the 95% CI (16). We conducted the following several sensitivity analyses to test the robustness of our results: 1) we used only baseline water intake in the 1991 FFQ as the exposure, 2) we repeated the analysis by using simply updated water intake with the 4-y lag as the exposure, and 3) we used the cumulative average of water intake without the 4-y lag as the exposure.
Stratified analyses were performed by using BMI (<30 or ≥30), physical activity level (<9 or ≥9 MET-h/wk), and history of hypertension or hypercholesterolemia (yes or no). The likelihood-ratio test of the cross-product terms was used to test for interactions. The P value for the linear trend was estimated by treating water intake as a continuous variable in the models. The proportional hazards assumption was tested by including an interaction term between water intake and the time to event in the model. No significant violations of the assumption were shown (all P > 0.10). Data were analyzed with the Statistical Analysis Systems software package (version 9.2; SAS Institute Inc), and a 2-tailed P < 0.05 was considered statistically significant.
RESULTS
A total of 82,902 women were included in the analysis. The mean (±SD) age of subjects was 36.0 ± 4.7 y in 1991 (range: 26–45 y). The median intake for plain water was 2.5 cups/d (IQR: 1.0–4.0 cups/d), and the median intake for total fluid was 8.2 servings/d (IQR range: 6.1–10.1 servings/d). The distribution of baseline characteristics according to categories of plain-water intake are shown in Table 1. Water intake was strongly associated with increased BMI (P-trend < 0.001). In contrast, a greater plain-water intake was associated with a healthier lifestyle [ie, a higher level of physical activity, a lower prevalence of smoking, and a higher diet quality measured by the AHEI score (all P-trend < 0.001)]. The intake of plain water was positively associated with intakes of whole grain, fish, fruit, and vegetables but negatively associated with red-meat consumption (all P-trend < 0.001). In addition, the intake of plain water was not related to intakes of tea, coffee, and fruit juices but was weakly correlated with milk intake (Spearman's correlation coefficient = 0.11) and SSB intake (Spearman correlation coefficient = −0.15; see Table 1 under “Supplemental data” in the online issue). Intake of water and other beverages remained relatively constant during the follow-up, whereas intakes of coffee substantially declined and of SSBs slightly increased (see Table 2 under “Supplemental data” in the online issue).
TABLE 1.
Characteristics of participants according to plain-water consumption1
| Characteristics | Plain-water consumption2 |
||||
| <1 cup/d | 1 cup/d | 2–3 cups/d | 4–5 cups/d | ≥6 cups/d | |
| Participants (n) | 17,524 | 11,820 | 25,707 | 15,422 | 12,429 |
| Age (y) | 36.1 ± 4.7 | 36.3 ± 4.6 | 36.1 ± 4.6 | 36.1 ± 4.7 | 36.1 ± 4.7 |
| BMI (kg/m2) | 24.0 ± 5.1 | 23.9 ± 4.8 | 24.3 ± 5.1 | 24.8 ± 5.2 | 25.4 ± 5.5 |
| Physical activity (MET-h/wk)3 | 17.3 ± 24.4 | 18.2 ± 25.2 | 20.2 ± 26.0 | 23.1 ± 28.4 | 28.0 ± 32.3 |
| Family history of diabetes (%) | 33.2 | 33.2 | 32.9 | 33.9 | 34.9 |
| Postmenopausal women (%) | 3.0 | 3.0 | 3.1 | 3.2 | 3.5 |
| Postmenopausal hormone use (%) | 4.4 | 4.1 | 4.3 | 4.5 | 5.0 |
| Oral contraceptive use (%) | 12.0 | 10.6 | 11.1 | 10.4 | 10.0 |
| History of hypertension (%) | 5.3 | 4.7 | 5.8 | 6.3 | 7.3 |
| History of hypercholesterolemia (%) | 14.4 | 13.3 | 13.8 | 14.0 | 15.3 |
| Current smoker (%) | 16.6 | 13.4 | 11.5 | 9.2 | 9.1 |
| Race [white (%)] | 96.1 | 97.5 | 97.1 | 96.1 | 96.0 |
| Alcohol consumption (g/d) | 2.9 ± 6.0 | 3.3 ± 6.2 | 3.3 ± 6.0 | 3.2 ± 6.2 | 3.0 ± 6.0 |
| Total calorie intake (kcal/d) | 1677 ± 544 | 1769 ± 538 | 1812 ± 536 | 1843 ± 547 | 1829 ± 554 |
| Alternative Healthy Eating Index score | 29.4 ± 8.4 | 30.8 ± 8.4 | 32.9 ± 8.8 | 35.1 ± 9.2 | 37.8 ± 9.9 |
| Whole-grain intake (servings/d) | 1.02 ± 0.97 | 1.20 ± 1.02 | 1.40 ± 1.12 | 1.56 ± 1.20 | 1.71 ± 1.35 |
| Regular-grain intake (servings/d) | 1.45 ± 0.95 | 1.57 ± 1.00 | 1.57 ± 0.98 | 1.57 ± 0.99 | 1.54 ± 1.00 |
| Red- or processed-meat intake (servings/d) | 0.97 ± 0.66 | 0.99 ± 0.66 | 0.97 ± 0.64 | 0.92 ± 0.64 | 0.82 ± 0.65 |
| Fish intake (servings/d) | 0.25 ± 0.25 | 0.25 ± 0.23 | 0.28 ± 0.25 | 0.32 ± 0.29 | 0.36 ± 0.36 |
| Vegetable intake (servings/d) | 2.64 ± 1.69 | 2.87 ± 1.73 | 3.15 ± 1.80 | 3.52 ± 1.99 | 4.09 ± 2.56 |
| Fruit intake (servings/d) | 0.92 ± 0.81 | 1.01 ± 0.81 | 1.17 ± 0.87 | 1.37 ± 0.97 | 1.61 ± 1.17 |
| Tea (cups/d) | 0.68 ± 1.17 | 0.77 ± 1.14 | 0.72 ± 1.09 | 0.66 ± 1.08 | 0.61 ± 1.12 |
| Coffee (cups/d) | 1.52 ± 1.74 | 1.59 ± 1.67 | 1.56 ± 1.64 | 1.52 ± 1.65 | 1.52 ± 1.72 |
| Milk (cups/d) | 0.90 ± 0.97 | 1.00 ± 0.96 | 1.12 ± 1.02 | 1.19 ± 1.07 | 1.11 ± 1.11 |
| Fruit juice (cups/d) | 0.60 ± 0.78 | 0.69 ± 0.79 | 0.74 ± 0.81 | 0.76 ± 0.83 | 0.69 ± 0.83 |
| Sugar-sweetened beverage (servings/d) | 0.62 ± 1.04 | 0.60 ± 0.95 | 0.48 ± 0.80 | 0.38 ± 0.69 | 0.25 ± 0.60 |
| Artificially sweetened beverage (servings/d) | 1.23 ± 1.68 | 1.13 ± 1.47 | 0.98 ± 1.30 | 0.86 ± 1.22 | 0.87 ± 1.31 |
| Total beverages (servings/d) | 5.88 ± 2.55 | 6.79 ± 2.39 | 8.09 ± 2.42 | 9.87 ± 2.55 | 11.15 ± 2.77 |
Mean ± SD (all such values) or percentage.
One cup = 240 mL.
MET-h, metabolic equivalent task hours.
We documented 2718 incident T2D cases during 1,115,427 person-years of follow-up. RRs of T2D according to plain-water intake by using the 4-y lagged analysis are shown in Table 2. In age- and BMI-adjusted models, plain-water intake was not significantly associated with risk of developing T2D across categories. Compared with the reference group (<1 cup/d), RRs (95% CIs) of T2D were 0.91 (0.81, 1.03) for 1 cup/d, 0.88 (0.78, 0.98) for 2–3 cups/d, 1.00 (0.88, 1.14) for 4–5 cups/d, and 0.95 (0.83, 1.10) for ≥6 cups/d (P-trend = 0.92). The results were not materially altered after additional adjustment for other lifestyle and risk factors including diet quality, and the corresponding RRs (95% CIs) were 0.93 (0.82, 1.05), 0.93 (0.83, 1.05), 1.09 (0.96, 1.24), and 1.06 (0.91, 1.23), respectively (P-trend = 0.15).
TABLE 2.
Type 2 diabetes according to plain-water intake in 4-y lagged analysis
| Plain-water intake | Cases/person-years | Age- and BMI-adjusted model1 | Multivariable model12 |
| <1 cup/d | 503/215,788 | 1.00 | 1.00 |
| 1 cup/d | 551/245,514 | 0.91 (0.81, 1.03) | 0.93 (0.82, 1.05) |
| 2–3 cups/d | 868/374,218 | 0.88 (0.78, 0.98) | 0.93 (0.83, 1.05) |
| 4–5 cups/d | 485/173,941 | 1.00 (0.88, 1.14) | 1.09 (0.96, 1.24) |
| ≥6 cups/d | 311/105,967 | 0.95 (0.83, 1.10) | 1.06 (0.91, 1.23) |
| P-linear trend3 | 0.92 | 0.15 |
All values are RRs; 95% CIs in parentheses.
Multivariable model was adjusted for age, race, family history of diabetes, BMI (in kg/m2) categories (<23.0, 23.0–24.9, 25.0–29.9, 30.0–34.9, or ≥35.0), smoking status [never, past, or current (1–14, 15–24, or ≥25 cigarettes/d)], alcohol intake (never or <5.0, 5.0–15.0, or ≥15.0 g/d), menopausal status and hormone use, oral contraceptive use, physical activity level (<3.0, 3.0–8.9, 9.0–17.9, 18.0–26.9, or ≥27.0 metabolic equivalent task hours per week), and Alternative Healthy Eating Index (quintiles).
Calculated by treating plain-water intake as a continuous variable.
In a sensitivity analysis that used only baseline water intakes, compared with the reference group (<1 cup/d), RRs (95% CIs) of T2D were 0.84 (0.73, 0.96) for 1 cup/d, 0.89 (0.80, 1.00) for 2–3 cups/d, 1.05 (0.93, 1.18) for 4–5 cups/d, and 0.99 (0.87, 1.12) for ≥6 cups/d (P-trend = 0.13; see Table 3 under “Supplemental data” in the online issue). An alternative analysis that used simply updated water intake yielded similar results. In another sensitivity analysis without the 4-y lag between exposure and T2D incidence, a modest positive association was observed for the highest category of plain-water intake (≥6 cups/d) compared with the reference category of <1 cup/d (RR: 1.18; 95% CI: 1.02, 1.36; P-trend = 0.01), but RRs for other categories were not significant compared with the reference group (see Table 3 under “Supplemental data” in the online issue).
In a priori–determined stratified analyses (Table 3), there was a trend toward increased risk in more active women (>9 MET-h/wk; P-trend = 0.10 in this subgroup) and women with hypercholesterolemia (P-trend = 0.07 in this subgroup); however, the P values for interactions were not significant (P-interaction > 0.10) for both tests.
TABLE 3.
Type 2 diabetes according to plain-water intake in the 4-y lagged analysis: stratified analyses1
| Plain-water consumption | ||||||
| <1 cup/d | 1 cup/d | 2–3 cups/d | 4–5 cups/d | ≥6 cups/d | P-linear trend | |
| Stratified by BMI | ||||||
| <30 kg/m2 (661 cases) | 1.00 | 1.03 (0.82, 1.30) | 0.95 (0.76, 1.19) | 0.95 (0.72, 1.24) | 1.09 (0.80, 1.49) | 0.66 |
| ≥30 kg/m2 (2057 cases) | 1.00 | 0.92 (0.79, 1.06) | 0.93 (0.81, 1.06) | 1.12 (0.97, 1.30) | 1.01 (0.85, 1.19) | 0.21 |
| Stratified by physical activity level | ||||||
| <9 MET-h2/wk (1411 cases) | 1.00 | 0.83 (0.71, 0.98) | 0.90 (0.78, 1.05) | 1.11 (0.93, 1.32) | 1.04 (0.84, 1.29) | 0.27 |
| ≥9 MET-h/wk (1106 cases) | 1.00 | 1.11 (0.89, 1.38) | 1.11 (0.91, 1.35) | 1.21 (0.97, 1.50) | 1.23 (0.97, 1.55) | 0.10 |
| Stratified by hypertension | ||||||
| Yes (1080 cases) | 1.00 | 0.85 (0.70, 1.04) | 0.82 (0.69, 0.99) | 0.99 (0.81, 1.22) | 1.01 (0.80, 1.27) | 0.72 |
| No (1638 cases) | 1.00 | 0.99 (0.84, 1.16) | 0.98 (0.85, 1.13) | 1.14 (0.97, 1.35) | 1.03 (0.85, 1.25) | 0.33 |
| Stratified by high cholesterol | ||||||
| Yes (1247 cases) | 1.00 | 1.03 (0.86, 1.24) | 1.06 (0.89, 1.25) | 1.10 (0.90, 1.34) | 1.21 (0.97, 1.51) | 0.07 |
| No (1471 cases) | 1.00 | 0.86 (0.73, 1.01) | 0.84 (0.72, 0.98) | 1.08 (0.91, 1.29) | 0.96 (0.78, 1.17) | 0.78 |
All values are RRs; 95% CIs in parentheses. Models were adjusted for age, race, family history of diabetes, BMI (in kg/m2) categories (<23.0, 23.0–24.9, 25.0–29.9, 30.0–34.9, or ≥35.0, but a continuous variable in the stratified analysis by BMI), smoking status (never, past, or current (1–14, 15–24, or ≥25 cigarettes/d)], alcohol intake (never or <5.0, 5.0–15.0, or ≥15.0 g/d), menopausal status and hormone use, oral contraceptive use, physical activity level (<3.0, 3.0–8.9, 9.0–17.9, 18.0–26.9, or ≥27.0 metabolic equivalent task hours per week, but a continuous variable in the stratified analysis by physical activity level), and the Alternative Healthy Eating Index (quintiles). None of the interaction tests were significant (all P-interaction > 0.10). P-linear trend was calculated by treating plain-water intake as a continuous variable.
Thirty-five women were excluded from the analysis because of missing values for physical activity level. MET-h, metabolic equivalent task hours.
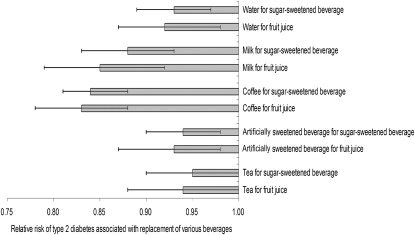
The total beverage intake was not significantly associated with risk of T2D (see Table 4 under “Supplemental data” in the online issue). Among individual beverages, 1 serving coffee/d was associated with 10% (RR: 0.90; 95% CI: 0.87, 0.92) lower risk of T2D, 1 serving SSBs/d was related with 9% (RR: 1.09; 95% CI: 1.05, 1.14) increased risk, and 1 serving fruit juices/d was associated with 11% (RR: 1.11; 95% CI: 1.05, 1.17) elevated risk, and risks for other beverages (ASBs, tea, and milk) were very minor (see Table 4 under “Supplemental data” in the online issue). We estimated that the substitution of 1 cup plain water/d for 1 serving SSBs or fruit juices/d was associated with 7% (95% CI: 3%, 11%) and 8% (95% CI: 2%, 13%) lower risk of T2D, respectively (Figure 1). We also estimated that the substitution of coffee or milk for SSBs or fruit juices was associated with a 12–17% significant reduction in risk, and the replacement of ASBs or tea for SSBs or fruit juices was related to 5–7% lower risk.
FIGURE 1.
RRs (95% CIs) of type 2 diabetes associated with replacement of various beverages. Results were adjusted for age, race, family history of diabetes, BMI (in kg/m2) categories (<23.0, 23.0–24.9, 25.0–29.9, 30.0–34.9, or ≥35.0), smoking status [never, past, or current (1–14, 15–24, or ≥25 cigarettes/d)], alcohol intake (never or <5.0, 5.0–15.0, or ≥15.0 g/d), menopausal status and hormone use, oral contraceptive use, physical activity level (<3.0, 3.0–8.9, 9.0–17.9, 18.0–26.9, or ≥27.0 metabolic equivalent tasks hours per week), and the Alternative Healthy Eating Index (quintiles). The 2 beverages for substitution were entered into the model as continuous variables. Error bars represents 95% CIs of substitution estimates.
DISCUSSION
In this large prospective cohort of US young and middle-aged women, we showed no overall association between the intake of plain water and risk of T2D. However, the substitution of plain water for SSBs or fruit juices was associated with a moderately lower risk.
The median intake of total fluid for US adults (>18 y old) in 2005–2006 as assessed by the NHANES was 3.5 L/d (∼14.6 cups, assuming 240 mL/cup) for men and 2.9 L/d (∼12.1 cups) for women, and these values remained relatively stable over time (17). In the NHANES, water intake was assessed by using 24-h dietary recalls, and the total fluid intake included plain water, beverages, and moisture in foods (17). In our current analysis, water and beverage intakes were collected by using a validated FFQ, but moisture contents in foods were not available; therefore, the total fluid only included intakes of plain water and other beverages. The median intake for total fluid was 8.2 servings/d for women aged 26–45 y, which was comparable to the NHANES data given that moisture in foods represented ∼20% of the total fluid intake in NHANES participants (17). However, the reported intake of plain water was lower in our cohort (median: 0.6 L or 2.5 cups) than that reported in female adults in the NHANES (average intake: 1.1 L) (17). The main reason for this difference is that we used predefined categories for water intake in our questionnaires with a highest category of ≥6 cups/d. Consistent with the NHANES data, women who drank more plain water had higher BMI values but were also more physically active.
The intake of plain water or total fluids was not significantly associated with risk of T2D in our cohort. A recent study in a French cohort suggested that a low plain-water intake may increase risk of new-onset hyperglycemia during a 9-y follow-up period; compared with participants who drank <0.5 L plain water/d (∼2 cups plain water/d), the odds ratio was 0.68 (95% CI: 0.52, 0.89) for individuals who drank 0.5–1.0 L/d (∼2–4 cups/d)and 0.79 (95% CI: 0.59, 1.05) for individuals who drank >1.0 L/d (∼4 cups/d; P-trend = 0.02) (7). However, the association with hyperglycemia was not significant in women (P-trend = 0.54), and the association was not significant for new-onset diabetes (P-trend = 0.36). This study did not compare the association of plain water with that of other beverages such as SSBs or fruit juices.
Total-fluid and plain-water intakes are influenced by many factors, including age, body size, physical activity level, comorbidities, environmental temperature, and food intakes (18, 19). In our cohort, water intake was strongly associated with BMI at baseline, which reflected a greater hydration requirement for a larger body size. Increased water consumption in obese individuals may also be due to symptoms that result from prediabetes or undiagnosed diabetes or because of medication use for other conditions such as hypertension or high cholesterol. The reverse causation may lead to artificially elevated risk of diabetes for increased consumption of water. Therefore, BMI was an important confounder for this analysis. Furthermore, because thirst is an early symptom of diabetes, a high water intake may be due to prediabetes or an early stage of diabetes. In our sensitivity analysis without the 4-y lag, there was a slightly increased risk in the highest category of plain-water intake, which may have resulted from the reverse causation. To alleviate this problem, we conducted a lagged analysis, whereby plain-water intake was used to predict diabetes outcomes that occurred 4 y later. In the 4-y lagged analysis, we showed little association between higher plain water consumption and T2D. The baseline-only analysis yielded similar results.
We estimated that the substitution of plain water for SSBs or fruit juices was associated with a moderately lower risk of T2D. However, the substitution associations may have been underestimated because SSBs and fruit juices were associated with a greater increased risk of T2D in the analysis without the 4-y lag (3, 8). A growing body of evidence has shown that intakes of SSBs or fruit juices is associated with elevated risk of T2D (2, 3), and they contribute more than 10% of daily calories in adults and adolescents (4, 20). Therefore, the replacement of SSBs and fruit juices with plain water could substantially reduce T2D risk in the general population. In a secondary data analysis of the 12-mo A-to-Z weight loss intervention in 173 premenopausal overweight women, the replacement of SSBs with an increased plain-water intake was associated with lower energy intakes (21) and significant losses of body weight and fat over time (6). Daniels and Popkin (5) summarized data from short-term consumption trials in adults and concluded that the drinking of SSBs before a single meal was associated with a 7.8% higher total energy intake compared with the drinking of plain water, and the drinking of juices was also associated with a 14.4% higher total energy intake. Therefore, the beneficial effects of replacing SSBs and fruit juices with plain water may be due to reduced energy intakes. A recent randomized clinical trial in 318 overweight and obese individuals showed that the replacement of SSBs with plain water led to significant reduction in fasting glucose and an improvement in hydration at 6 mo (22). In addition, participants in the noncaloric-beverage (plain water or diet drinks) intervention groups significantly decreased the energy from beverages and were more likely to achieve a 5% weight loss than were individuals in the attention control arm (22). Certainly, more studies are needed to investigate the beneficial effects of the replacement of SSBs or fruit juices with plain water on glucose homeostasis and diabetes risk.
In our analyses, the substitution estimation of coffee or milk for SSBs or fruit juices conferred a greater reduction in risk of T2D. This result was consistent with our previous findings that have shown a significant inverse association between coffee (23) or dairy products including milk (24) and risks of T2D. Several components in coffee (eg, chlorogenic acid, quinic acid, trigonelline, and lignans) and milk (eg, calcium, magnesium, lactose, and dairy protein) may provide additional benefits for the reduction of T2D risk (1, 23, 24). Furthermore, ASBs and tea were only marginally associated with increased T2D risk, and the replacement of SSBs or fruit juices by ASBs or tea was estimated to have a modest reduction of T2D risk in our analysis, which was consistent with our recent findings in men (25).
The strengths of the current study included a large sample size, high follow-up rate, and repeated assessments of diet and lifestyle variables. The current study has several potential limitations. First, our study populations primarily consisted of nurses with European ancestry. Although the homogeneity of socioeconomic status helped reduce confounding, the observed associations may not be generalizable to other populations. However, because the positive association between SSBs and T2D has also been observed in other ethnic groups, we believe that our results are broadly applicable to the general population. Second, because diet was assessed by using FFQs, some measurement errors of water and beverage assessments were inevitable. However, the FFQs used in these studies were validated against multiple diet records, and reasonable correlation coefficients for beverage intakes were observed (range: 0.36–0.94). Moreover, we calculated cumulative averages for dietary variables to minimize the random measurement error caused by within-person variation and to best reflect a long-term diet habit. Third, the underascertainment and misclassification of diabetes outcome are possible because the incident cases were self-reported. However, self-reported diabetes were confirmed by using supplemental questionnaires, and our validity study indicated that self-reported diabetes was highly reliable in this group of health professionals who had ready access to the health care system, and we used a 4-y lagged analysis to minimize the influence of undiagnosed diabetes. Last, because of the observational nature of our study, unmeasured and residual confounding was still possible, although we carefully controlled for known diabetes risk factors in our analyses.
In conclusion, these data indicate that plain-water intake was not significantly associated with risk of T2D, but the substitution of plain water for SSBs or fruit juices was estimated to confer moderately lower risk of T2D. These findings can be used to help guide healthy beverage choices to reduce risk of diabetes.
Supplementary Material
Acknowledgments
We are indebted to the participants in the NHS II for their continuing outstanding support and to our colleagues who are working in these studies for their valuable help.
The authors’ responsibilities were as follows—AP: designed and conducted the analysis, interpreted the data, and wrote the manuscript; VSM and MBS: assisted in interpreting the data and edited the manuscript; JEM and WCW: obtained funding and edited the manuscript; and FBH: obtained funding, designed and conducted the analysis, interpreted the data, and edited the manuscript. None of the authors had a conflict of interest.
Footnotes
Abbreviations used: AHEI, Alternative Healthy Eating Index; ASB, artificially sweetened beverage; FFQ, food-frequency questionnaire; MET-h, metabolic equivalent task hours; NHS, Nurses’ Health Study; SSB, sugar-sweetened beverage; T2D, type 2 diabetes
REFERENCES
- 1.Huxley R, Lee CM, Barzi F, Timmermeister L, Czernichow S, Perkovic V, Grobbee DE, Batty D, Woodward M. Coffee, decaffeinated coffee, and tea consumption in relation to incident type 2 diabetes mellitus: a systematic review with meta-analysis. Arch Intern Med 2009;169:2053–63 [DOI] [PubMed] [Google Scholar]
- 2.Malik VS, Popkin BM, Bray GA, Despres JP, Willett WC, Hu FB. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care 2010;33:2477–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bazzano LA, Li TY, Joshipura KJ, Hu FB. Intake of fruit, vegetables, and fruit juices and risk of diabetes in women. Diabetes Care 2008;31:1311–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bleich SN, Wang YC, Wang Y, Gortmaker SL. Increasing consumption of sugar-sweetened beverages among US adults: 1988-1994 to 1999-2004. Am J Clin Nutr 2009;89:372–81 [DOI] [PubMed] [Google Scholar]
- 5.Daniels MC, Popkin BM. Impact of water intake on energy intake and weight status: a systematic review. Nutr Rev 2010;68:505–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stookey JD, Constant F, Popkin BM, Gardner CD. Drinking water is associated with weight loss in overweight dieting women independent of diet and activity. Obesity (Silver Spring) 2008;16:2481–8 [DOI] [PubMed] [Google Scholar]
- 7.Roussel R, Fezeu L, Bouby N, Balkau B, Lantieri O, Alhenc-Gelas F, Marre M, Bankir L. for the D.E.S.I.R. Study Group. Low water intake and risk for new-onset hyperglycemia. Diabetes Care 2011;34:2551–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schulze MB, Manson JE, Ludwig DS, Colditz GA, Stampfer MJ, Willett WC, Hu FB. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA 2004;292:927–34 [DOI] [PubMed] [Google Scholar]
- 9.Salvini S, Hunter DJ, Sampson L, Stampfer MJ, Colditz GA, Rosner B, Willett WC. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol 1989;18:858–67 [DOI] [PubMed] [Google Scholar]
- 10.Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, Litin LB, Willett WC. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc 1993;93:790–6 [DOI] [PubMed] [Google Scholar]
- 11.National Diabetes Data Group Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979;28:1039–57 [DOI] [PubMed] [Google Scholar]
- 12.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Diabetes Care 1997;20:1183–97 [DOI] [PubMed] [Google Scholar]
- 13.Manson JE, Rimm EB, Stampfer MJ, Colditz GA, Willett WC, Krolewski AS, Rosner B, Hennekens CH, Speizer FE. Physical activity and incidence of non-insulin-dependent diabetes mellitus in women. Lancet 1991;338:774–8 [DOI] [PubMed] [Google Scholar]
- 14.Field AE, Coakley EH, Must A, Spadano JL, Laird N, Dietz WH, Rimm E, Colditz GA. Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Arch Intern Med 2001;161:1581–6 [DOI] [PubMed] [Google Scholar]
- 15.McCullough ML, Feskanich D, Stampfer MJ, Giovannucci EL, Rimm EB, Hu FB, Spiegelman D, Hunter DJ, Colditz GA, Willett WC. Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance. Am J Clin Nutr 2002;76:1261–71 [DOI] [PubMed] [Google Scholar]
- 16.Hu FB, Stampfer MJ, Rimm E, Ascherio A, Rosner BA, Spiegelman D, Willett WC. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol 1999;149:531–40 [DOI] [PubMed] [Google Scholar]
- 17.Kant AK, Graubard BI, Atchison EA. Intakes of plain water, moisture in foods and beverages, and total water in the adult US population–nutritional, meal pattern, and body weight correlates: National Health and Nutrition Examination Surveys 1999-2006. Am J Clin Nutr 2009;90:655–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jéquier E, Constant F. Water as an essential nutrient: the physiological basis of hydration. Eur J Clin Nutr 2010;64:115–23 [DOI] [PubMed] [Google Scholar]
- 19.Popkin BM, D'Anci KE, Rosenberg IH. Water, hydration, and health. Nutr Rev 2010;68:439–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang YC, Bleich SN, Gortmaker SL. Increasing caloric contribution from sugar-sweetened beverages and 100% fruit juices among US children and adolescents, 1988-2004. Pediatrics 2008;121:e1604–14 [DOI] [PubMed] [Google Scholar]
- 21.Stookey JD, Constant F, Gardner CD, Popkin BM. Replacing sweetened caloric beverages with drinking water is associated with lower energy intake. Obesity (Silver Spring) 2007;15:3013–22 [DOI] [PubMed] [Google Scholar]
- 22.Tate DF, Turner-McGrievy G, Lyons E, Stevens J, Erickson K, Polzien K, Diamond M, Wang X, Popkin BM. Replacing caloric beverages with water or diet beverages for weight loss in adults: main results of the Choose Healthy Options Consciously Everyday (CHOICE) randomized clinical trial. Am J Clin Nutr 2012;95(3):555–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Dam RM, Willett WC, Manson JE, Hu FB. Coffee, caffeine, and risk of type 2 diabetes. Diabetes Care 2006;29:398–403 [DOI] [PubMed] [Google Scholar]
- 24.Malik VS, Sun Q, van Dam RM, Rimm EB, Willett WC, Rosner B, Hu FB. Adolescent dairy product consumption and risk of type 2 diabetes in middle-aged women. Am J Clin Nutr 2011;94:854–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Koning L, Malik VS, Rimm EB, Willett WC, Hu FB. Sugar-sweetened and artificially sweetened beverage consumption and risk of type 2 diabetes in men. Am J Clin Nutr 2011;93:1321–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.