Abstract
Background: Isoflavones, having chemical structures similar to estrogens, are believed to stimulate nitric oxide production and thus lower blood pressure. The efficacy of soy isoflavone supplementation to stimulate nitric oxide production and lower blood pressure in menopausal women with high normal blood pressure remains unknown.
Objective: The objective was to test the effect of soy isoflavone supplementation on nitric oxide production and blood pressure in menopausal women with high normal blood pressure.
Design: A randomized, double-blind, parallel, placebo-controlled 6-wk trial was conducted to assess the effects of daily supplementation with 80 mg soy hypocotyl isoflavones (in aglycone units) on nitric oxide metabolism and blood pressure in 24 menopausal women with 12 women per group. Changes in nitric oxide metabolism were assessed via a primed, constant-infusion protocol with [15N]arginine and [13C]- and [2H]citrulline. Changes in blood pressure and associated vascular hemodynamics were assessed via office and 24-h ambulatory blood pressure monitoring, forearm blood flow, and indexes of arterial compliance.
Results: When compared with placebo and after control for pretreatment values, soy isoflavone supplementation had no effect on arginine flux, citrulline flux, nitric oxide synthesis, blood pressure, forearm blood flow, or estimates of arterial stiffness.
Conclusion: Daily supplementation with 80 mg soy hypocotyl isoflavones over a 6-wk period had no effect on nitric oxide metabolism or blood pressure and associated vascular hemodynamics in menopausal women with high normal blood pressure.
INTRODUCTION
Cardiovascular disease is the leading cause of death among menopausal women in the United States (1). Although the cardiovascular mortality rate among women aged ≤45 y is only half of that observed in men, it increases 4-fold in women aged 45–55 y and exceeds that reported in men of comparable age in menopausal women (2, 3). Hormone replacement therapy increases the risk of cardiovascular disease in menopausal women (4), even though estrogen replacement therapy has been shown to improve endothelium-dependent vasodilation (5). The beneficial effect of estrogen therapy on vasodilation is nullified by a nitric oxide (NO)5 synthase inhibitor (5), which suggests that NO plays an important role in the vasodilatory capacity of menopausal women. NO has been identified as an endothelium-derived relaxing factor that regulates vascular smooth muscle relaxation (6). Furthermore, estrogen therapy has been shown to increase plasma nitrate concentrations in menopausal women (7), which indicates that NO production might have been stimulated. Despite the many possible health benefits of estrogen replacement therapy (8–10), it is not an acceptable treatment in many women because of its association with an increased risk of coronary artery disease (4) and cancer, particularly breast cancer (11, 12).
Genistein and daidzein, the 2 most abundant isoflavones found in soybeans, have chemical structures similar to estradiol and possess anticancer properties (13). Improved arterial compliance has been reported in menopausal women taking a daily dose of soy isoflavones (14). In another study, infusion of genistein produced a dose-dependent increase in forearm blood flow (FBF) similar in potency to 17β-estradiol in men and in premenopausal women (15). Because the responses to both genistein and estradiol were inhibited to the same degree by NO synthase inhibitor, the genistein-induced forearm vasodilation is anticipated to be via the l-arginine/NO-dependent pathway.
In the current study, we sought to determine whether over a 6-wk interval, daily supplementation with 80 mg soy hypocotyl aglycone isoflavones could stimulate NO synthesis and improvement in hemodynamic measurements in menopausal women with high normal blood pressure. In vivo NO synthesis was measured by using an innovative primed, constant-infusion stable-isotope method. Hemodynamic measures were assessed via FBF, estimates of arterial stiffness, and 24-h ambulatory blood pressure monitoring.
SUBJECTS AND METHODS
Study design
The randomized, double-blind, parallel, placebo-controlled study was conducted at Baylor College of Medicine with initial recruitment starting in July of 2003. The study subjects provided written informed consent, and the study protocol was approved by the Institutional Review Board for Human Studies at Baylor College of Medicine.
Menopausal women (40–60 y of age) with a BMI (in kg/m2) <30 and a high normal blood pressure [systolic (SBP)/diastolic: 130–139 mm Hg/85–89 mm Hg], who had not had menses for ≥12 mo and had a serum follicle-stimulating hormone concentration >30 IU/mL, were eligible to participate in the study.
Exclusion criteria included an abnormal blood chemistry profile, BMI >30, a history of cancer, and active liver, kidney, gallbladder, or heart disease. Women were excluded from the study if they were being treated with antihypertensive medications or drugs/herbals known to alter blood pressure, bisphosphonates, calcitonin, fluoride, corticosteroids, tamoxifen, raloxifene, letrozole, or hormone therapy. Women also were excluded from the study if they were taking supplements such as black cohosh, blue cohosh, dong quai, caltrate 600+soy, Estroven (Estroven), ginseng (GNC), Healthy Women (Johnson & Johnson), Opti-Soy (Revive Personal Product), PhytoFem (Bio Nutri Food), ProBalance (Nestles), Promensil (Novogen), Remifemin (Enzymatic Therapy Inc), Rimostil (Novogen), or Trinovin (Novogen).
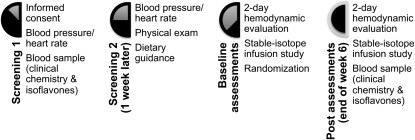
As shown in Figure 1, the study consisted of 2 periods: a screening phase and a double-blind study period of 6 wk. At screening, informed consent was obtained, overnight fasting blood samples were obtained, and blood pressure measurements were made by using a calibrated mercury sphygmomanometer. Blood pressure and heart rate measurements were made 3 times in a lying position, 3 times in a sitting position, and 3 times in a standing position on the nondominant arm with a 2-min rest period between measurements. Eligible subjects returned 1 wk later for repeated assessment of blood pressure and a physical examination and received guidance from a registered dietitian on foods high in nitrates to avoid before the infusion study. At baseline, a 2-d hemodynamic evaluation was conducted on each subject at the Cardiovascular Clinical Pharmacology Laboratory at Baylor College of Medicine, which was followed by the stable-isotope-infusion study at the General Clinical Research Center at the Methodist Hospital. After the baseline studies, the women were randomly assigned to receive either 80 mg soy isoflavones/d or a placebo for 6 wk. Treatment allocation codes were maintained by a research pharmacist who was also responsible for generating the randomization scheme before the beginning of the study and for providing study drugs to the subjects. All the investigators, research staff, and subjects were blinded to the treatment codes. The hemodynamic evaluation and the stable-isotope-infusion study were repeated at the end of 6 wk of the soy isoflavone supplementation phase or the placebo phase.
FIGURE 1.
Schematic diagram of the study design. At screening 1, informed consent was obtained from all potential study subjects followed by blood pressure and heart rate measurements. A fasting blood sample was collected for clinical blood chemistry, thyroid function, and follicle-stimulating hormone and isoflavone concentrations. At screening 2, study subjects who met the study inclusion criteria returned 1 wk later for a physical examination, dietary guidance to avoid foods high in nitrates, and blood pressure and heart rate measurements. The 2-d hemodynamic evaluation and stable-isotope infusion study were done on each subject at baseline and at the end of the 6-wk treatment period. The subjects were randomly assigned after the baseline assessments. All subjects continued to consume the placebo or isoflavone tablets until all posttreatment assessments had been done. Another fasting blood sample was collected at the end of the 6-wk treatment period to assess compliance.
Stable-isotope protocol
A modified primed, constant-infusion protocol with stable-isotope tracers was used to quantify in vivo NO production in the menopausal women. On the morning of the study, a plastic catheter was inserted into a vein of one of the arms of each woman. A fasting baseline blood sample (∼20 mL) was collected for measurements of arginine, citrulline, nitrite, nitrate, and isoflavones. Subsequently, the subject received intravenously a priming dose containing 8 μmol guanidino-[15N2]arginine/kg, 1 μmol l-citrulline (5-13C; 4,4,5,5-D4)/kg, and 0.16 μmol ureido-[15N1]citrulline/kg followed by a continuous intravenous infusion at a rate of 8 μmol guanidino-[15N2]arginine · kg−1 · h−1 and 1 μmol l-citrulline (5-13C; 4,4,5,5-D4) · kg−1 · h−1 for a total of 6 h. The inclusion of l-citrulline (5-13C; 4,4,5,5-D4) in the protocol allowed us to estimate citrulline flux (16). The priming dose of [ureido-15N1]citrulline allowed us to estimate citrulline enrichment because its low synthesis rate (0.5–1% of arginine production) (17) would have made it difficult to measure. The independently measured citrulline flux via l-citrulline (5-13C; 4,4,5,5-D4) together with the citrulline enrichment provided a direct measure of NO production. The stable-isotope tracers were manufactured by Cambridge Isotope Laboratories. All stable-isotope tracers were tested and certified to be sterile and pyrogen-free by the manufacturer. All intravenous solutions to support the primed, constant-infusion protocol were prepared by the Methodist Hospital's investigational pharmacy and approved for intravenous administration. Hourly blood samples (4 mL each) were collected during the 6-h infusion period and 1 h after completion of the infusion.
Liquid chromatographyndashmass spectrometry
Because of poor ionization efficiency and chromatography, plasma amino acids were converted to the N,N-dimethylaminonaphthalene sulfonamide (DANS) derivative by treatment of 100 μL plasma buffered to pH 9.0 with 50 μL of 0.1 mol sodium tetraborate/L, which was then allowed to react with 100 μL of 20 mmol 5-(dimethylamino)-1-napthalenesulfonyl chloride/L for 20 min. Proteins were precipitated with 1.0 mL chilled acetonitrile and removed by centrifugation. The supernatant fluid was evaporated, and the DANS amino acids were dissolved in 200 μL HPLC mobile phase consisting of acetonitrile, HPLC grade water, and formic acid at a ratio of 5:95:0.5. After being vortex-mixed, the mixture was centrifuged, and 150 μL supernatant fluid was transferred to an autosampler vial and 10 μL of the supernatant fluid was injected onto a Synergi 4 μ MAX-RP 80 Å, 150 × 2.00 mm HPLC column (Phenomenex). The DANS amino acids were eluted with an acetonitrile:water:formic acid gradient (5:95:0.5–95:5:0.5). Isotopic enrichment was measured with a Thermo Scientific Quantum Discovery triple quadrupole instrument by using electrospray ionization, collision-induced dissociation, and selected reaction monitoring. The retention times for arginine and citrulline were determined to be 5.4 and 6.2 min, respectively. Specific selected reaction monitoring conditions for the collision-induced dissociation of each amino acid (and isotopologues) were as follows: arginine m/z 408 (410, 413) →170 at 34 eV, citrulline m/z 409 (410) →392 at 14 eV, and citrulline m/z 414 →397 at 14 eV. Precision of the enrichment measurements in the baseline blood samples was ± 0.01–0.03% for an individual isotopomer. Absolute accuracy of the natural abundance baseline samples is typically ±0.1%. Enrichment data were expressed as mole percentage excess (MPE). Plateau enrichment in the primary amino acid compartment during the infusion study was typically 13–18 MPE (arginine) and 6–10 MPE (citrulline). Enrichment in the secondary amino acid was 0.2–0.6 MPE (citrulline derived from arginine) and 0.4–1.3 MPE (arginine derived from citrulline).
Flux calculations
Enrichments are expressed in units of MPE. Plasma arginine and citrulline fluxes were calculated from plasma arginine or citrulline enrichment during steady state infusion as follows (18):
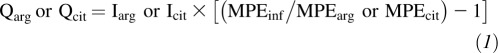 |
where Qarg and Qcit are the arginine and citrulline fluxes (μmol · kg−1 · h−1), Iarg and Icit are the infusion rates of labeled arginine and citrulline (μmol · kg−1 · h−1), MPEinf is the enrichment of the infused arginine or citrulline in MPE, and MPEarg and MPEcit are the plasma arginine and citrulline enrichments under steady state conditions. The NO production rate (QNO; μmol · kg−1 · h−1) was calculated as follows:
Hemodynamic measurements
At the Cardiovascular Clinical Pharmacology Laboratory, blood pressure measurements were repeated for each study participant in the supine, seated, and standing positions 3 times with a calibrated mercury sphygmomanometer. Each woman was then fitted with an arm cuff attached to a Space Laboratories model 90207 arterial blood pressure monitor, and blood pressure was measured every 20 min during the day and every 30 min at night for 24 h. For use in this study, ≥80% of the readings had to be valid and there had to be at least one valid reading every hour. At the laboratory, arterial compliance was measured with a cardiovascular profiling instrument (Hypertension Diagnostics Inc). Basal and postischemic (5-min brachial artery occlusion) FBF and brachial artery blood pressure were measured with a strain-gauge plethysmograph (DE Hokanson) and a Colin Pilot hemodynamic monitoring device that provided beat-to-beat blood pressure measurements. Three FBF measurements were made at 45-s intervals before and immediately after release of the brachial artery occlusion. Forearm vascular resistance before and after occlusion was calculated by dividing the average SBP by each FBF value. The highest FBF after release of the brachial artery occlusion was considered to represent peak reactive hyperemia (PRH). Flow-mediated dilation (FMD) was defined as the difference between PRH and the average of the 3 FBF measurements made before brachial artery occlusion.
Soy isoflavone and placebo tablets
The soy hypocotyl isoflavones were manufactured and provided by Frutarom Netherlands BV, whereas the placebo and isoflavone-containing tablets were manufactured and packaged by Pharma Consulting & Industries BV. Analysis of the isoflavone aglycone and glucoside content was carried out by Nutrilab BV by using an HPLC method based on Song et al (19). The average content of each isoflavone tablet contained 40.51 mg aglycone equivalent of total isoflavones (daidzein: 22.01 mg; glycitein: 13.54 mg; genistein: 4.96 mg) with most (>95%) in the form of glycosides reflecting the natural composition of the soy germ. The placebo tablets contained <1.0 mg aglycone equivalent per tablet. The placebo and isoflavone tablets each contained the same filler materials and common processing aids, yielding tablets that were identical in appearance. Women assigned to the treatment group ingested 2 isoflavone tablets each day, one in the morning and one in the evening. Women assigned to the placebo group ingested 2 placebo tablets each day, again one in the morning and one in the evening. Both groups of women ingested a tablet in the morning before entering the General Clinical Research Center for posttreatment assessments and continued to ingest the tablets until all posttreatment assessments had been completed.
Compliance measure
Compliance was confirmed on the basis of blood isoflavone measurements. An HPLC-mass spectrometry procedure for the rapid, sensitive, and specific measurement of daidzein, genistein, glycitein, and equol in human plasma was used (20). Synthetic radiolabeled genistein conjugates were used for evaluation of optimum conditions for solid-phase extraction. Biochanin A was added to plasma as a recovery marker for isoflavones and phenolphthalein glucuronide, and 4-methylumbelliferone sulfate were added to ensure completeness of hydrolysis with β-glucuronidase/sulfatase. Isoflavones in plasma extracts were separated by using an isocratic HPLC method and analyzed by negative-ion multiple reaction ion monitoring–mass spectrometry by using a heated nebulizer-atmospheric pressure chemical ionization interface. Within-assay and between-assay CVs for measurement of daidzein and genistein in 5 aliquots of the same plasma sample were 8.51% and 7.76% and 5.98% and 6.12%, respectively.
Study outcomes
The primary outcome was the change in arginine flux, citrulline flux, and NO production rate from baseline over 6 wk. Secondary outcomes were the change in FBF, in estimates of arterial compliance, and in average daytime, nighttime, and full 24-h ambulatory blood pressure measurements from baseline to 6 wk of active therapy or placebo.
Statistical analysis
Potential confounding variables were assessed by comparing treatment groups with respect to baseline demographic and clinical characteristics. Characteristics found to be different among groups to a clinically important degree were included as covariates in the analyses. A generalized linear model was used to assess each outcome with respect to the effects of treatment group, while accounting for confounders and the corresponding baseline value of the outcome variable. Analyses were performed using SPSS software (Windows version 16). The manufacturers of the supplements were not involved in the study design or data analysis. The academic authors had full and unrestricted rights to analyze, interpret, and publish the results.
RESULTS
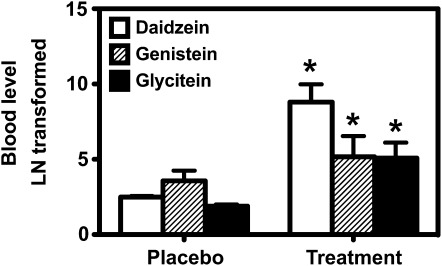
Baseline characteristics of the study subjects are listed in Table 1. Age, body weight, height, BMI, and blood pressure were similar between the treatment groups. Compliance with the study protocol was confirmed by measurements of blood isoflavone concentrations at the beginning and again at the end of the 6-wk study period. As shown in Figure 2, the natural logarithmic transformation of the skewed blood concentrations of daidzein, genistein, and glycitein were significantly elevated (P < 0.01) among the menopausal women receiving the soy isoflavone supplementation when compared with placebo. Only 3 women among the 12 women in the treatment group were able to produce equol.
TABLE 1.
Baseline descriptive characteristics of the study subjects
| Placebo (n = 12) | Soy isoflavone treatment (n = 12) | P1 | |
| Age (y) | 55.5 ± 4.32 | 55.8 ± 4.4 | 0.85 |
| Weight (kg) | 68.9 ± 9.8 | 67.6 ± 11.8 | 0.78 |
| Height (cm) | 164.4 ± 5.5 | 163.7 ± 8.4 | 0.83 |
| BMI (kg/m2) | 25.4 ± 2.7 | 25.3 ± 4.2 | 0.92 |
| Blood pressure (mm Hg)3 | |||
| Systolic | 140.7 ± 7.4 | 140.1 ± 8.3 | 0.86 |
| Diastolic | 82.2 ± 8.7 | 82.8 ± 6.2 | 0.85 |
| Ethnicity (n) | 0.30 | ||
| White | 8 | 5 | |
| African American | 4 | 4 | |
| Hispanic | 0 | 2 | |
| Asian | 0 | 1 |
Derived by using an independent-samples t test for the comparison between the placebo and treatment groups. A chi-square test was used to compare the ethnic distribution between the 2 groups.
Mean ± SD (all such values).
Seated clinic blood pressure; average of 3 measurements for each subject.
FIGURE 2.
Natural logarithmic transformation of blood isoflavone concentrations in the placebo group and the soy isoflavone–supplemented group at the end of the 6-wk study period. The asterisks above the columns denote significant differences (P < 0.01) between the 2 groups. The error bar above each column represents SD.
The clinical chemistry profiles of the study participants are summarized in Table 2. Both groups of women were considered healthy based on their laboratory chemistry profiles. No difference was detected in the lipid profiles between the 2 groups although both groups were found to have elevated serum concentrations of total cholesterol and LDL cholesterol. Their menopausal status was confirmed by elevated blood follicle-stimulating hormone concentrations.
TABLE 2.
Clinical chemistry profiles of the study participants1
| Variables (normal range) | Placebo (n = 12) | Soy isoflavone treatment (n = 12) | P2 |
| Serum chemistry profile | |||
| Sodium (135–145 mmol/L) | 142.2 ± 1.83 | 143.2 ± 1.5 | 0.15 |
| Potassium (3.3–5.0 mmol/L) | 4.1 ± 0.3 | 4.0 ± 0.5 | 0.65 |
| Chloride (95–110 mmol/L) | 105.1 ± 2.7 | 106.5 ± 1.3 | 0.12 |
| Calcium (8.6–10.5 mg/dL) | 9.6 ± 0.5 | 9.7 ± 0.5 | 0.57 |
| Carbon dioxide (24–32 mmol/L) | 27.9 ± 2.2 | 27.3 ± 2.2 | 0.53 |
| Glucose (70–110 mg/dL) | 98.0 ± 15.0 | 95.8 ± 13.5 | 0.71 |
| Uric acid (3.5–8.5 mg/dL) | 4.0 ± 0.9 | 4.3 ± 1.3 | 0.54 |
| BUN (8–22 mg/dL) | 14.1 ± 3.2 | 13.6 ± 2.8 | 0.69 |
| Creatinine (0.5–1.3 mg/dL) | 0.9 ± 0.2 | 0.9 ± 0.1 | 0.62 |
| Total bilirubin (0.3–1.3 mg/dL) | 0.6 ± 0.2 | 0.5 ± 0.2 | 0.29 |
| ALT (5–54 U/L) | 23.3 ± 7.7 | 21.4 ± 10.9 | 0.64 |
| AST (15–43 U/L) | 23.0 ± 6.4 | 19.8 ± 3.4 | 0.13 |
| Alkaline phosphatase (35–115 U/L) | 75.4 ± 17.4 | 81.8 ± 15.0 | 0.34 |
| Albumin (3.4–4.8 g/dL) | 4.8 ± 0.2 | 4.8 ± 0.2 | 0.78 |
| Total protein (6.3–8.3 g/dL) | 7.8 ± 0.5 | 7.7 ± 0.5 | 0.76 |
| Lipid profile (fasting) | |||
| Total cholesterol (0–200 mg/dL) | 218.9 ± 32.3 | 221.5 ± 36.3 | 0.86 |
| Triglyceride (35–160 mg/dL) | 114.2 ± 34.8 | 113.6 ± 50.1 | 0.97 |
| HDL cholesterol (>35 mg/dL) | 64.0 ± 18.5 | 64.3 ± 16.1 | 0.97 |
| LDL cholesterol (<130 mg/dL) | 132.1 ± 27.7 | 135.3 ± 28.1 | 0.79 |
| Endocrine profile | |||
| TSH (0.35–5.5 μIU/mL) | 2.0 ± 1.0 | 1.3 ± 0.7 | 0.07 |
| FT4 (0.80–1.80 ng/dL) | 1.1 ± 0.2 | 2.8 ± 3.1 | 0.06 |
| Hormone profile | |||
| FSH (23–117 mIU/mL)4 | 72.3 ± 29.1 | 61.9 ± 25.7 | 0.36 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; FSH, follicle-stimulating hormone; FT4, free thyroxine; TSH, thyroid-stimulating hormone.
Derived by using an independent-samples t test for the comparison between the placebo and treatment groups.
Mean ± SD (all such values).
Range of values for postmenopausal females.
The changes in arginine flux, citrulline flux, NO synthesis rate, and fractional conversion rate among the 2 groups after the 6-wk study period are shown in Table 3. The results indicate that arginine flux, citrulline flux, NO synthesis rate, and fractional conversion rate were similar between the 2 groups of menopausal women at baseline. Soy isoflavone supplementation at 80 mg/d for 6 wk had no effect on arginine flux, citrulline flux, NO synthesis rate, or fractional conversion rate when compared with placebo.
TABLE 3.
Baseline and posttreatment data for arginine and citrulline fluxes and nitric oxide synthesis
| Variable and timeline | Placebo (n = 12) | Soy isoflavone treatment (n = 12) | P1 | P2 |
| Arginine flux (μmol · kg−1 · h−1) | ||||
| Baseline | 50.1 ± 8.43 | 51.0 ± 8.1 | 0.78 | |
| 6 wk | 52.9 ± 12.4 | 50.4 ± 5.7 | 0.33 | |
| Citrulline flux (μmol · kg−1 · h−1) | ||||
| Baseline | 9.2 ± 2.4 | 8.5 ± 2.5 | 0.52 | |
| 6 wk | 8.7 ± 1.7 | 8.5 ± 2.3 | 0.82 | |
| Nitric oxide synthesis (μmol · kg−1 · h−1) | ||||
| Baseline | 0.15 ± 0.05 | 0.15 ± 0.03 | 0.96 | |
| 6 wk | 0.15 ± 0.05 | 0.16 ± 0.04 | 0.36 | |
| Fractional conversion rate | ||||
| Baseline | 0.017 ± 0.005 | 0.019 ± 0.005 | 0.37 | |
| 6 wk | 0.012 ± 0.005 | 0.019 ± 0.007 | 0.99 |
Derived by using an independent-samples t test for the comparison between the placebo and treatment groups.
Derived by using a generalized linear model for the comparison between the placebo and treatment groups with adjustment for baseline measures.
Mean ± SD (all such values).
The baseline and posttreatment hemodynamic measures of the study participants are summarized in Table 4. No significant differences were observed in FBF, forearm vascular resistance, 24-h ambulatory blood pressure monitoring measures, large or small artery elasticity indexes, preocclusion, and PRH after adjustment for baseline values between the 2 groups. Subjects were classified as dippers (nighttime SBP values were >10% below their average daytime SBP) or nondippers because nondippers have a greater 24-h blood pressure burden than do dippers and have been reported to have reduced flow mediated vasodilation (21–23). Our results showed no significant difference in the number of dippers and nondippers between the 2 groups either at baseline (P = 0.11) or after 6 wk of treatment (P = 0.21).
TABLE 4.
Baseline and posttreatment hemodynamic measures of study participants1
| Variable and timeline | Placebo (n = 12) | Soy isoflavone treatment (n = 12) | P2 | P3 |
| Arterial compliance | ||||
| SBP (mm Hg) | ||||
| Baseline | 140.7 ± 7.44 | 140.1 ± 8.3 | 0.86 | |
| 6 wk | 135.9 ± 9.8 | 137.3 ± 9.7 | 0.15 | |
| DBP (mm Hg) | ||||
| Baseline | 82.2 ± 8.7 | 82.8 ± 6.2 | 0.85 | |
| 6 wk | 79.5 ± 7.1 | 79.9 ± 6.0 | 0.57 | |
| Large-artery elasticity index (mL/mm Hg · 100) | ||||
| Baseline | 15.2 ± 8.3 | 12.3 ± 4.2 | 0.28 | |
| 6 wk | 13.8 ± 3.7 | 13.8 ± 3.8 | 0.34 | |
| Small-artery elasticity index (mL/mm Hg · 100) | ||||
| Baseline | 3.1 ± 1.6 | 3.0 ± 1.5 | 0.90 | |
| 6 wk | 3.5 ± 2.2 | 4.0 ± 2.5 | 0.61 | |
| FBF and vascular resistance | ||||
| Baseline FBF (mL · min−1 · 100 g−1 tissue) | ||||
| Baseline | 2.06 ± 0.62 | 1.78 ± 0.46 | 0.22 | |
| 6 wk | 1.92 ± 0.55 | 1.55 ± 0.70 | 0.35 | |
| PRH (mL · min−1 · 100 g−1 tissue)5 | ||||
| Baseline | 23.1 ± 7.4 | 17.7 ± 3.8 | 0.03 | |
| 6 wk | 22.3 ± 8.0 | 17.0 ± 8.1 | 0.08 | |
| Vascular resistance (pressure/flow)6 | ||||
| Baseline | 71.1 ± 36.3 | 77.7 ± 26.8 | 0.62 | |
| 6 wk | 74.7 ± 37.5 | 86.3 ± 30.2 | 0.52 | |
| 24-h Ambulatory blood pressure monitoring | ||||
| 24-h Mean SBP (mm Hg) | ||||
| Baseline | 132.0 ± 7.0 | 132.0 ± 6.5 | 0.99 | |
| 6 wk | 130.9 ± 8.2 | 129.7 ± 8.4 | 0.61 | |
| 24-h Mean DBP (mm Hg) | ||||
| Baseline | 78.5 ± 7.9 | 78.7 ± 7.6 | 0.96 | |
| 6 wk | 77.8 ± 6.3 | 77.4 ± 6.3 | 0.62 | |
| 24-h Mean HR (beats/min) | ||||
| Baseline | 78.2 ± 5.3 | 75.3 ± 5.1 | 0.19 | |
| 6 wk | 81.1 ± 9.8 | 76.2 ± 5.5 | 0.43 | |
| Daytime mean SBP (mm Hg) | ||||
| Baseline | 135.6 ± 5.7 | 137.5 ± 7.9 | 0.52 | |
| 6 wk | 134.6 ± 9.9 | 134.2 ± 6.8 | 0.59 | |
| Daytime mean DBP (mm Hg) | ||||
| Baseline | 81.0 ± 8.2 | 82.6 ± 8.2 | 0.62 | |
| 6 wk | 80.2 ± 7.0 | 81.1 ± 6.7 | 0.84 | |
| Daytime mean HR (beats/min) | ||||
| Baseline | 81.6 ± 6.6 | 77.9 ± 5.9 | 0.16 | |
| 6 wk | 84.7 ± 9.2 | 77.7 ± 5.8 | 0.12 | |
| Nighttime mean SBP (mm Hg) | ||||
| Baseline | 124.4 ± 10.4 | 121.0 ± 11.0 | 0.45 | |
| 6 wk | 121.8 ± 7.6 | 118.9 ± 11.2 | 0.74 | |
| Nighttime mean DBP (mm Hg) | ||||
| Baseline | 72.9 ± 7.9 | 70.0 ± 9.14 | 0.41 | |
| 6 wk | 70.6 ± 5.4 | 68.4 ± 7.0 | 0.68 | |
| Nighttime mean HR (beats/min) | ||||
| Baseline | 70.5 ± 8.1 | 69.9 ± 6.1 | 0.83 | |
| 6 wk | 71.7 ± 10.6 | 71.5 ± 7.4 | 0.94 | |
| Dipper SBP (fraction)7 | ||||
| Baseline | 0.25 ± 0.45 | 0.58 ± 0.52 | 0.11 | |
| 6 wk | 0.36 ± 0.51 | 0.67 ± 0.49 | 0.21 |
DBP, diastolic blood pressure; FBF, forearm blood flow; HR, heart rate; PRH, peak reactive hyperemia; SBP, systolic blood pressure.
Derived by using an independent-samples t test for the comparison between the placebo and treatment groups.
Derived by using a generalized linear model for the comparison between the placebo and treatment groups with adjustment for baseline measures.
Mean ± SD (all such values).
Represents the highest FBF after release of the brachial artery occlusion.
Change in forearm vascular resistance before ischemia to the maximum reduction in forearm vascular resistance during PRH (flow-mediated vasodilation).
Proportion of subjects whose average nighttime SBP dropped >10% compared with their average daytime SBP.
A positive relation (P ≤ 0.04) was observed between pretreatment and posttreatment FMD, but no statistically significant differences were found between the isoflavone and placebo groups (P = 0.08). No statistically significant relation (P ≥ 0.37) was found between pre- and posttreatment FMD and the change in NO synthesis rate for either treatment group.
DISCUSSION
Our study showed that soy isoflavone supplementation at 80 mg/d had no effect on blood pressure measurements obtained either in the office or by 24-h ambulatory blood pressure monitoring. Six other clinical trials on menopausal women also reported similar findings at isoflavone dosages ranging from 60 to 80 mg/d (24–29). Only 3 clinical trials reported a significant decrease in blood pressure (29–31) with isoflavone supplementation. One of these 3 clinical trials showed beneficial effects on blood pressure only among equol producers (31). Among nonequol producers, the clinical trial showed a significant increase in SBP measurement (31), in contradiction to the anticipated outcome.
Individuals who do not lower their nighttime blood pressure by >10% below their daytime average have been identified as nondippers. In clinical trials, this group, when compared with dippers, has been reported to have a higher incidence of left ventricular hypertrophy (21) and a higher incidence of obstructive sleep apnea (22), and they are at greater risk of coronary artery disease, stroke, and cardiovascular death (23). In this study, dipper status did not correlate with a change in FMD in either the placebo or isoflavone-treated group. The number of subjects who were classified as dippers after isoflavone supplementation increased, but not significantly so, perhaps because of the small number of subjects in each group.
Arterial compliance was not affected by soy isoflavone supplementation over a 6-wk period in our study. Although improvement in arterial compliance was reported in one clinical trial (26), this and one other study reported no effect (28). The lack of effect of soy isoflavone supplementation on FBF reported in our study is similar to that reported in another clinical trial, which looked at the hemodynamic effect of soy isoflavone supplementation in menopausal women (28).
The effect of isoflavones on FMD is contradictory. Whereas 6 clinical trials (27–29, 32–34) showed significant improvement in FMD with isoflavone therapy, 7 other clinical trials (24–26, 28, 31, 35, 36) documented no improvement in FMD with isoflavone therapy. A meta-analysis of these randomized placebo-controlled clinical trials showed that oral isoflavone supplementation improved FMD, measured as changes in brachial artery diameter, only in women with low baseline FMD levels (37). In this study, FMD was measured by forearm venous occlusion plethysmography. Using this method, the greatest FMD response to treatment in our subjects occurred in those with high pretreatment FMD, regardless of treatment assignment. Although differences in methods could account for the differences in our results and those reported in the meta-analysis, it is unlikely because a strong correlation between FMD measurements obtained by the 2 modalities has been reported (38).
Because NO has been identified to be the endothelium-derived relaxing factor that regulates vascular smooth muscle relaxation (6), a significant increase in plasma NO concentration might be a good indicator that isoflavone supplementation might have stimulated NO synthesis. Four clinical trials (27–29, 32) reported significant elevations in plasma NO concentrations after isoflavone supplementation, whereas 2 other trials (33, 38) reported no such effect. As shown in Table 3, using an innovative stable-isotope-infusion method we found no effect of soy isoflavone supplementation on arginine flux, citrulline flux, in vivo NO synthesis, or the fractional conversion rate.
We were unable to determine whether equol would have changed the outcome because of the small sample size. Because soy hypocotyl material has a higher amount of daidzein than genistein, it is possible that equol production could have altered the outcome of the study. However, the small sample size did not allow assessment of equol's role because equol was detected only in 3 of the 12 women receiving the soy hypocotyl material. However, the blood isoflavone measurements, as shown in Figure 2, clearly showed that our study participants were in compliance with the study protocol.
Our study had potential limitations. In an infusion study, genistein was found to stimulate NO-dependent dilation of human forearm vasculature (15). In an in vitro study, genistein was found to acutely stimulate NO synthesis in vascular endothelial cells (39). Therefore, the use of a soy supplement with a higher abundance of genistein might have produced different results. However, the result reported by Walker et al (15) might not be applicable to menopausal women because the study was conducted in men and premenopausal women. The infusion study (15) and in vitro study (39) are not applicable to the general population seeking potential sustainable health benefits from dietary supplements such as soy isoflavones, including menopausal women. According to the Office of Dietary Supplements at the NIH and the Dietary Supplement Health and Education Act, any botanicals to be classified as dietary supplements must be taken by mouth as a pill, capsule, tablet, or liquid. Furthermore, isoflavones taken by mouth are first hydrolyzed in the gut to remove the sugar moiety. Once they are in the intestinal cells, they undergo glucuronidation and sulfonation and emerge into the mesenteric blood mostly conjugated. Therefore, the use of nonconjugated genistein in the infusion study or in the in vitro study are not natural or physiologic. The studies reported by Walker et al (15) and Liu et al (39) and the test meal study reported by Hall et al (29) looked at the acute responses of FBF, nitrogen oxides, and FMD to isoflavone treatment, whereas our study was designed to look at the sustainable and possible long-term benefits of daily soy isoflavone supplementation over a 6-wk period on the hemodynamic measures and in vivo NO production. Therefore, our results are not comparable because the acute responses reported by these authors (15, 29, 39) do not guarantee any sustainable benefits.
In an acute human dietary study (29), improvement in FMD was found to coincide with peak serum isoflavone concentrations. Therefore, standardizing the hemodynamic and NO production measurements with peak serum isoflavone concentration might have altered our findings. However, it is not possible to time the stable-isotope constant-infusion study with peak serum isoflavone concentrations, because the infusion protocol took 7 h to complete. This also cannot be done with 24-h ambulatory blood pressure monitoring.
Including a sufficient number of equol producers in the study might have changed the outcomes of our study. Unfortunately, our limited resources would not allow us to pretest a large number of study subjects to identify the equol producers. Because equol is not a natural botanical, a much larger clinical trial might be more appropriate to determine whether equol has any beneficial effects against cardiovascular diseases in menopausal women. In conclusion, our results clearly showed that soy isoflavone supplementation at 80 mg/d over a 6-wk period had no beneficial effect on blood pressure, FBF, FMD, or NO synthesis.
Acknowledgments
The author's responsibilities were as follows—EOS: performed the statistical analyses; AAT: performed the physical examination and the hemodynamic assessment; SB: performed the blood isoflavone measurements; and DLH: developed and performed the LCMS measurements. All authors were involved in the study design, the execution of the clinical trial, and the preparation of the manuscript. None of the authors had any conflicts of interest to declare.
Footnotes
Abbreviations used: DANS, N,N-dimethylaminonaphthalene sulfonamide; FBF, forearm blood flow; FMD, flow-mediated dilation; MPE, mole percentage excess; NO, nitric oxide; PRH, peak reactive hyperemia; SBP, systolic blood pressure.
REFERENCES
- 1.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Camethon MR, Dai S, de Simone G, Ford ES, et al. Heart disease and stroke statistics–2011 update: a report from the American Heart Association. Circulation 2011;123:e18–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002;360:1903–13 [DOI] [PubMed] [Google Scholar]
- 3.Kannel WB, Hjortland MC, McNamara PM, Gordon T. Menopause and risk of cardiovascular disease: the Framingham Study. Ann Intern Med 1976;85:447–52 [DOI] [PubMed] [Google Scholar]
- 4.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johson KC, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA 2002;288:321–33 [DOI] [PubMed] [Google Scholar]
- 5.Guetta V, Quyyumi AA, Prasad A, Panza JA, Waclawiw M, Cannon RO., III The role of nitric oxide in coronary vascular effects of estrogen in postmenopausal women. Circulation 1997;96:2795–801 [DOI] [PubMed] [Google Scholar]
- 6.Palmer RMJ, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 1987;327:524–6 [DOI] [PubMed] [Google Scholar]
- 7.Best PJ, Berger PB, Miller VM, Lerman A. The effect of estrogen replacement therapy on plasma nitric oxide and endothelin-I levels in postmenopausal women. Ann Intern Med 1998;128:285–8 [DOI] [PubMed] [Google Scholar]
- 8.Lindsay R, Hart DM, Aitken JM, MacDonald EB, Anderson JB, Clarke AC. Long-term prevention of postmenopausal osteoporosis by oestrogen. Evidence for an increased bone mass after delayed onset of oestrogen treatment. Lancet 1976;1:1038–41 [DOI] [PubMed] [Google Scholar]
- 9.Cauley JA, Seeley DG, Ensrud K, Ettinger B, Black D, Cummings SR. Estrogen replacement therapy and fractures in older women. Ann Intern Med 1995;122:9–16 [DOI] [PubMed] [Google Scholar]
- 10.Walsh BW, Spiegelman D, Morrissey M, Sacks FM. Relationship between serum estradiol levels and the increases in high-density lipoprotein levels in postmenopausal women treated with oral estradiol. J Clin Endocrinol Metab 1999;84:985–9 [DOI] [PubMed] [Google Scholar]
- 11.Colditz GA, Hankinson SE, Hunter DJ, Willett WC, Manson JE, Stampfer MJ, Hennekens CH, Rosner B, Speizer FE. The use of estrogen and progestins and the risk of breast cancer in postmenopausal women. N Engl J Med 1995;332:1589–93 [DOI] [PubMed] [Google Scholar]
- 12.Steinberg KK, Thacker SB, Smith SJ, Stroup DF, Zack MM, Flanders WD, Berkelman RL. A meta-analysis of the effect of estrogen replacement therapy on the risk of breast cancer. JAMA 1991;265:1985–90 [PubMed] [Google Scholar]
- 13.Knight DC, Eden JA. A review of the clinical effects of phytoestrogens. Obstet Gynecol 1996;87:897–904 [PubMed] [Google Scholar]
- 14.Nestel PJ, Yamashita T, Sasahara T, Pomeroy S, Dart A, Komesaroff P, Owen A, Abbey M. Soy isoflavones improve systemic arterial compliance but not plasma lipids in menopausal and perimenopausal women. Arterioscler Thromb Vasc Biol 1997;17:3392–8 [DOI] [PubMed] [Google Scholar]
- 15.Walker HA, Dean TS, Sanders TAB, Jackson G, Ritter JM, Chowienczyk PJ. The phytoestrogen genistein produces acute nitric oxide-dependent dilation of human forearm vasculature with similar potency to 17β-estradiol. Circulation 2001;103:258–62 [DOI] [PubMed] [Google Scholar]
- 16.Castillo L, Beaumier L, Ajami AM, Young VR. Whole body nitric oxide synthesis in healthy men determined from [15N]arginine-to-[15N]citrulline labeling. Proc Natl Acad Sci USA 1996;93:11460–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luiking YC, Deutz NE. Isotopic investigation of nitric oxide metabolism in disease. Curr Opin Clin Nutr Metab Care 2003;6:103–8 [DOI] [PubMed] [Google Scholar]
- 18.Clarke JT, Bier DM. The conversion of phenylalanine to tyrosine in man. Direct measurement by continuous intravenous tracer infusions of L-[ring-2H5]phenylalanine and L-[1-13C] tyrosine in the postabsorptive state. Metabolism 1982;31:999–1005 [DOI] [PubMed] [Google Scholar]
- 19.Song T, Barua K, Buseman G, Murphy PA. Soy isoflavone analysis: quality control and a new internal standard. Am J Clin Nutr 1998;68(suppl):1474S–9S [DOI] [PubMed] [Google Scholar]
- 20.Coward L, Kirk M, Albin N, Barnes S. Analysis of plasma isoflavones by reversed-phase HPLC-multiple reaction ion monitoring-mass spectrometry. Clin Chim Acta 1996;247:121–42 [DOI] [PubMed] [Google Scholar]
- 21.Cuspidi C, Meani S, Salerno M, Valerio C, Fusi V, Severgnini B, Lonati L, Magrini F, Zanchetti A. Cardiovascular target organ damage in essential hypertensives with or without reproducible nocturnal fall in blood pressure. J Hypertens 2004;22:273–80 [DOI] [PubMed] [Google Scholar]
- 22.Baguet JP, Hammer L, Levy P, Pierre H, Rossini E, Mouret S, Ormezzano O, Mallion JM, Pepin JL. Night-time and diastolic hypertension are common and underestimated conditions in newly diagnosed apnoeic patients. J Hypertens 2005;23:521–7 [DOI] [PubMed] [Google Scholar]
- 23.Fagard RH, Celis H, Thijs L, Staessen JA, Clement DL, De Buyzere ML, De Bacquer DA. Daytime and nighttime blood pressure as predictors of death and cause-specific cardiovascular events in hypertension. Hypertension 2008;51:55–61 [DOI] [PubMed] [Google Scholar]
- 24.Simons LA, von Konigsmark M, Simons J, Celermajer DS. Phytoestrogens do not influence lipoprotein levels or endothelial function in healthy, postmenopausal women. Am J Cardiol. 2000;85:1297–301. [DOI] [PubMed]
- 25.Hale G, Paul-Labrador M, Dwyer JH, Merz CN. Isoflavone supplementation and endothelial function in menopausal women. Clin Endocrinol (Oxf) 2002;56:693–701 [DOI] [PubMed] [Google Scholar]
- 26.Teede HJ, McGrath BP, DeSilva L, Cehun M, Fassoulakis A, Nestel PJ. Isoflavones reduce arterial stiffness: a placebo-controlled study in men and postmenopausal women. Arterioscler Thromb Vasc Biol 2003;23:1066–71 [DOI] [PubMed] [Google Scholar]
- 27.Colacurci N, Chiàntera A, Fornaro F, de Novellis V, Manzella D, Arciello A, Chiàntera V, Improta L, Paolisso G. Effects of soy isoflavones on endothelial function in healthy postmenopausal women. Menopause 2005;12:299–307 [DOI] [PubMed] [Google Scholar]
- 28.Hallund J, Bugel S, Tholstrup T, Ferrari M, Talbot D, Hall WL, Reimann M, Williams CM, Winberg N. Soya isoflavone-enriched cereal bars affect markers of endothelial function in postmenopausal women. Br J Nutr 2006;95:1120–6 [DOI] [PubMed] [Google Scholar]
- 29.Hall WL, Formanuik NL, Harnpanich D, Cheung M, Talbot D, Chowienczyk PJ, Sanders TA. A meal enriched with soy isoflavones increases nitric oxide-mediated vasodilation in healthy postmenopausal women. J Nutr 2008;138:1288–92 [DOI] [PubMed] [Google Scholar]
- 30.Teede HJ, Dalais FS, Kotsopoulos D, Liang Y, Davis S, McGrath BP. Dietary soy has both beneficial and potentially adverse cardiovascular effects: a placebo-controlled study in men and postmenopausal women. J Clin Endocrinol Metab 2001;86:3053–60. [DOI] [PubMed]
- 31.Kreijkamp-Kaspers S, Kok L, Bots ML, Grobbee DE, Lampe JW, van der Schouw YT. Randomized controlled trial of the effects of soy protein containing isoflavones on vascular function in postmenopausal women. Am J Clin Nutr 2005;81:189–95 [DOI] [PubMed] [Google Scholar]
- 32.Squadrito F, Altavilla D, Crisafulli A, Saitta A, Cucinotta D, Morabito N, D'Anna R, Corrado F, Ruggeri P, Frisina N, et al. Effect of genistein on endothelial function in postmenopausal women: a randomized, double-blind, controlled study. Am J Med 2003;114:470–6 [DOI] [PubMed] [Google Scholar]
- 33.Lissin LW, Oka R, Lakshmi S, Cooke JP. Isoflavones improve vascular reactivity in post-menopausal women with hypercholesterolemia. Vasc Med 2004;9:26–30 [DOI] [PubMed] [Google Scholar]
- 34.Cuevas AM, Irribarra VL, Castillo OA, Yanez MD, Germain AM. Isolated soy protein improves endothelial function in postmenopausal hypercholesterolemic women. Eur J Clin Nutr 2003;57:889–94 [DOI] [PubMed] [Google Scholar]
- 35.Evans M, Njike VY, Hoxley M, Pearson M, Katz DL. Effect of soy isoflavone protein and soy lecithin on endothelial function in healthy postmenopausal women. Menopause 2007;14:141–9 [DOI] [PubMed] [Google Scholar]
- 36.Katz DL, Evans MA, Njike VY, Hoxley ML, Nawaz H, Comerford BP, Sarrel PM. Raloxifene, soy phytoestrogens and endothelial function in postmenopausal women. Climacteric 2007;10:500–7 [DOI] [PubMed] [Google Scholar]
- 37.Li SH, Liu XX, Bai YY, Wang XJ, Sun K, Chen JZ, Hui RT. Effect of oral isoflavone supplementation on vascular endothelial function in postmenopausal women: a meta-analysis of randomized placebo-controlled trials. Am J Clin Nutr 2010;91:480–6 [DOI] [PubMed] [Google Scholar]
- 38.Nikander E, Metsa-Heikkila M, Tiitinen A, Ylikorkala O. Evidence of a lack of effect of a phytoestrogen regimen on the levels of C-reactive protein, E-selectin, and nitrate in postmenopausal women. J Clin Endocrinol Metab 2003;88:5180–5 [DOI] [PubMed] [Google Scholar]
- 39.Liu D, Homan LL, Dillon JS. Genistein acutely stimulates nitric oxide synthesis in vascular endothelial cells by a cyclic adenosine 5′-monophosphate-dependent mechanism. Endocrinology 2004;145:5532–9 [DOI] [PubMed] [Google Scholar]