Abstract
Chlorpyrifos is one of the commonly used organophosphorus insecticides that are implicated in serious environmental and human health problems. To evaluate plant potential for uptake of chlorpyrifos, several plant species of poplar (Populus sp.) and willow (Salix sp.) were investigated. Chlorpyrifos was taken up from nutrient solution by all seven plant species. Significant amounts of chlorpyrifos accumulated in plant tissues, and roots accumulated higher concentrations of chlorpyrifos than did shoots. Chlorpyrifos did not persist in the plant tissues, suggesting further metabolism of chlorpyrifos in plant tissue. To our knowledge, this work represents the first report for phytoremediation of chlorpyrifos using poplar and willow plants.
Keywords: phytoremediation, organophosphorus (OP) insecticide, chlorpyrifos (CPS), Populus, Sali
INTRODUCTION
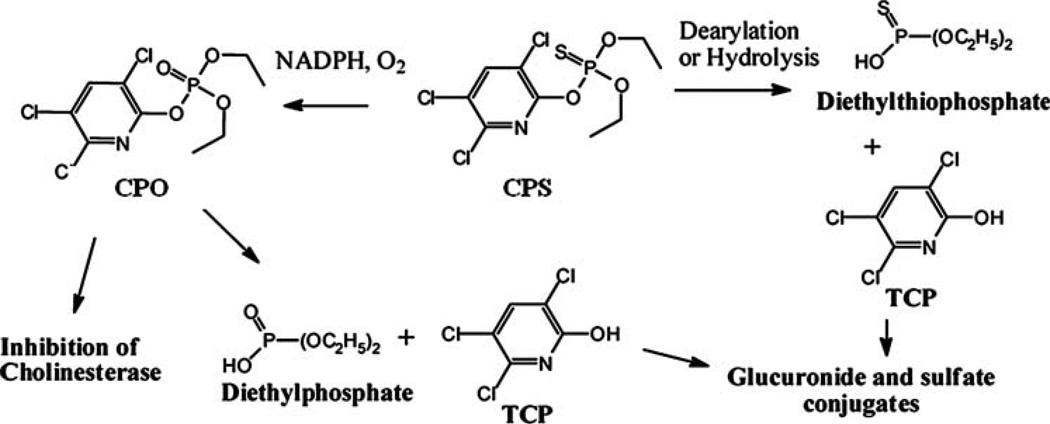
Chlorpyrifos (CPS), [O,O-diethylO-(3,5,6-trichloro-2-pyridinyl)-phosphorothioate], is one of the most widely used organophosphorus (OP) insecticides worldwide. It has been used to control foliage- and soil-borne insect pests on a variety of food and feed crops since its first use in 1965. Conversion of the parent organophosphorothionate to its highly toxic oxon generates a potent inhibitor of butyrylcholinesterase and acetylcholinesterase, key enzymes necessary for the proper functioning of the nervous system, including the brain (ATSDR 1997; USEPA 2000, 2002). CPS itself is not toxic, but it creates a toxic form when it is transformed by the environment or in vivo by cytochrome P450 to chlorpyrifos-oxon (CPO) (Figure 1), which is about 3000 times as potent as CPS itself (Williamson et al. 2006).
Figure 1.
Biotransformation of CPS in experimental animals (adapted from Eaton et al. 2008).
CPS adversely affects other organisms besides the pests it is designed to kill. CPS/CPO exposures kill beneficial arthropods including bees, ladybird beetles, and parasitic wasps, and other animals including fish and other aquatic organisms, birds, cats, pigs, monkeys, and humans, because of their broad-spectrum effects on non-target organisms (Cox 1995). According to the US-EPA (U.S. Environmental Protection Agency), exposure of the U.S. population to CPS and its metabolites is widespread and has been related to a variety of nerve disorders in humans. Symptoms of acute poisoning include headache, nausea, dizziness, confusion, and in some extreme cases even respiratory paralysis and death. Human birth defects and male infertility have also been associated with exposure to CPS and its products. To mitigate risks from the exposures, the use of CPS has been restricted for nearly all residential uses and even for some agricultural purposes in the United States and in some European countries (USEPA 2002). However, it continues to be used in developing countries like India, to control crop damage from insects in agriculture (Eaton et al. 2008). Not only animals, but plants are damaged by exposure to CPS. Delayed seedling emergence (Sinclair et al. 1992), fruit deformities (Beck et al. 1991), and abnormal cell division (Amer and Farah 1983) have all resulted from CPS exposure.
These environmental and human health problems caused by CPS may be mitigated by using phytoremediation, which is the use of plants for the cleanup of environmental contaminants (Cunningham and Berti 1993; Salt et al. 1998; Pilon-Smits 2005). Phytore-mediation has been shown to be useful in the removal of CPS (Moore et al. 2002). However, CPS remediation studies have been limited to plant-associated microorganisms (Singh et al. 2004; Yang et al. 2005; Yu et al. 2006). It remains to be seen whether plants are able to take up and transform OP pesticides without the participation of associated microbes. Removal of CPS by plants is based on the ability of plants to take up CPS into tissues and to degrade it. Although the fate of CPS has been documented in the environment, little is known about the fate of this contaminant and its metabolites within a plant system (Smith et al. 1967a, 1967b; Rouchaud et al. 1991). In addition, most plant species that have been tested so far for CPS uptake are herbaceous plants. Compared to herbaceous plants, trees such as poplar and willow offer some distinct advantages for treatment of contaminated sites. These species are perennial, long-lived (80–100 years), hardy, fast growing, and easily propagated, providing high biomass and an extensive root system. Recent findings which suggest that they can take up and degrade organic contaminants also support this plant family as the one of choice for the phytoremediation studies (Burken and Schnoor 1997; Newman et al. 1997; Pilon-Smits et al. 1998; Robinson et al. 2000). It is expected that these types of trees will be significant in phytoremediation research in the future. For these reasons, trees of the Salicaceae family, the genus Populus—which includes poplars and cottonwoods – and the Salix –which includes willows—were chosen for this study.
In this study we report plants with phytoremediation capabilities for removing CPS from hydroponic solutions. To this end, the ability of Populus and Salix spp. to take up CPS, the levels of CPS accumulated in plant tissues, as well as plant metabolism of CPS are examined in detail.
MATERIAL AND METHODS
Chemicals
Chlorpyrifos Pestanal® (CPS, 99.2% purity) and 3,5,6-trichloro-2-pyridinol (TCP, 99.5% purity) were purchased from Sigma-Aldrich (Milwaukee, WI, USA) and Chem Service Inc. (West Chester, PA, USA), respectively. Both CPS and TCP solutions were prepared by dissolving the appropriate volumes in methanol (99.9% purity) for 10 mg/mL stock solutions which were diluted serially in methyl tert-butyl ether (MTBE, ≥ 99.8%, Sigma Aldrich) for standard solutions.
Plant Materials
Seven clones of Populus and Salix spp. were selected for the study of CPS uptake from hydroponic solution (See Table S1 in Supporting Information). In vitro-grown, 50-to 60-day-old plantlets maintained on MS medium (Murashige and Skoog 1962) (Caisson Labs) served as explants sources, and they were washed with sterile water to aseptically remove traces of agar before being transferred for the CPS treatment. Among the seven clones, the hybrid poplar clone INRA 717-1B4 was chosen for most of the experiments because of its fast and easy propagation through tissue culture and its successful utilization in previous phytoremediation experiments (Pilon-Smits et al. 1998; Doty et al. 2007). All of the plant materials were incubated at 24°C with a 14-h photoperiod in a growth chamber (Percival).
CPS Toxicity in Plants
To evaluate the toxicity of CPS, poplar clones INRA 717-1B4, in triplicate, were exposed to various concentrations of CPS (See Fig. S1 in Supporting Information). Plant cuttings were placed in sterile 40-mL clear Volatile Organics Analysis (VOA) vials containing 10 mL of MS broth (MS medium without agar). The liquid concentrations of CPS were 0, 25, 50, 75, 100, 150, 200, 250, 300, and 350 µg/mL (0, 0.07, 0.14, 0.21, 0.29, 0.43, 0.57, 0.71, 0.86, and 1.00 mM), respectively. Health of the plants was monitored visually.
CPS Removal by Plants from Hydroponic Solution
To evaluate plant potential for uptake of CPS, three plants of each species were place in sterile 40-mL clear VOA vials containing MS broth and capped with septum valve caps (Mininert). For controls, there were additional six vials containing MS broth without plants. CPS was added to the solution to a final concentration of 25 µg/mL through the mini-nert valves using a glass gastight syringe (Hamilton, Reno, NV). All vials were incubated under a 14-h photoperiod for 7 days, but three unplanted vials were covered with aluminum foil to prevent light penetration in order to determine if there is a significant difference in the abiotic CPS degradation between under light and dark treatment. The 4 mL of liquid samples were removed from each vial at intervals of 0, 3, and 7 days.
The Fate of CPS in Plant Tissue
In order to determine whether plants metabolize CPS, poplar clones ‘INRA 717-1B4’ were treated with CPS, and the amount of CPS remaining in plants, in quintuplicate, was monitored over a time course (5 weeks). A total of 25 plants were placed in sterile 125-mL clear glass screw-cap jars containing MS broth. CPS was added to the solution to a final concentration of 20 µg/mL. At the end of 7 days, one set (5 plants) of plant samples were analyzed for the CPS extraction, and the rest of the plants were moved to medium devoid of CPS. CPS was extracted from the other set of plant samples every week. In order to localize the CPS within different plant tissues, stem, leaf, and root tissues were extracted separately.
Extraction of CPS and TCP from Samples
To extract CPS from the hydroponic solution, 4 mL of the solution were sampled and added deep into a 15-mL amber vial containing 2 mL 10% NaCl and 5 mL MTBE. The vials were inverted repeatedly for one minute, and 1 mL of the MTBE layer was removed and run on gas chromatography (GC). For the analysis of TCP, an additional 1 mL of the solution was filtered using a 0.22 µm porosity nylon syringe filter (Restek, Bellefonte, PA) and analyzed by HPLC (high performance liquid chromatography).
Plants were subjected to extraction of the chlorinated compounds as described by Shang et al. (2001). All plant samples were washed out with sterile water to remove CPS remaining on the surfaces of tissues prior to grinding in liquid nitrogen, and transferred to chilled 25-mL glass centrifuge tubes (Corex). Two mL of 1 N H2SO4/10% NaCl solution was added and the tubes were shaken vigorously for 1 min. After 5 mL of MTBE was added, the aqueous extract was clarified by centrifugation at 8,000 rpm for 10 min. The 3.5 mL of MTBE layer was transferred to 15-mL amber vials containing 2 g Na2SO4 and incubated at room temperature for 1 h. 1 mL of the MTBE layer was removed and run on GC. An additional 1mL of the MTBE was concentrated using a nitrogen evaporator (Organomation, Berlin, MA). The residue was redissolved in methanol and analyzed by HPLC. The methods for GC and HPLC analysis are described in Supporting Information, Text S1.
RESULTS
CPS Phytotoxicity
Cuttings of poplar clone INRA 717-1B4 were exposed to a range of concentrations (25–350 µg/mL) of CPS. After seven days of exposure, cuttings exhibited significant blackening of themesophyllic (non-vein) leaf tissue, and mortality at CPS concentrations of 150 µg/mL and higher (See Fig. S1 in Supporting Information). After another seven days, the plant cuttings at 100 µg/mL also showed mortality and the cuttings at 75 µg/mL were dead after another four weeks. The highest non-lethal dose of CPS was 50 µg/mL for six-week exposure.
CPS Uptake by Plants
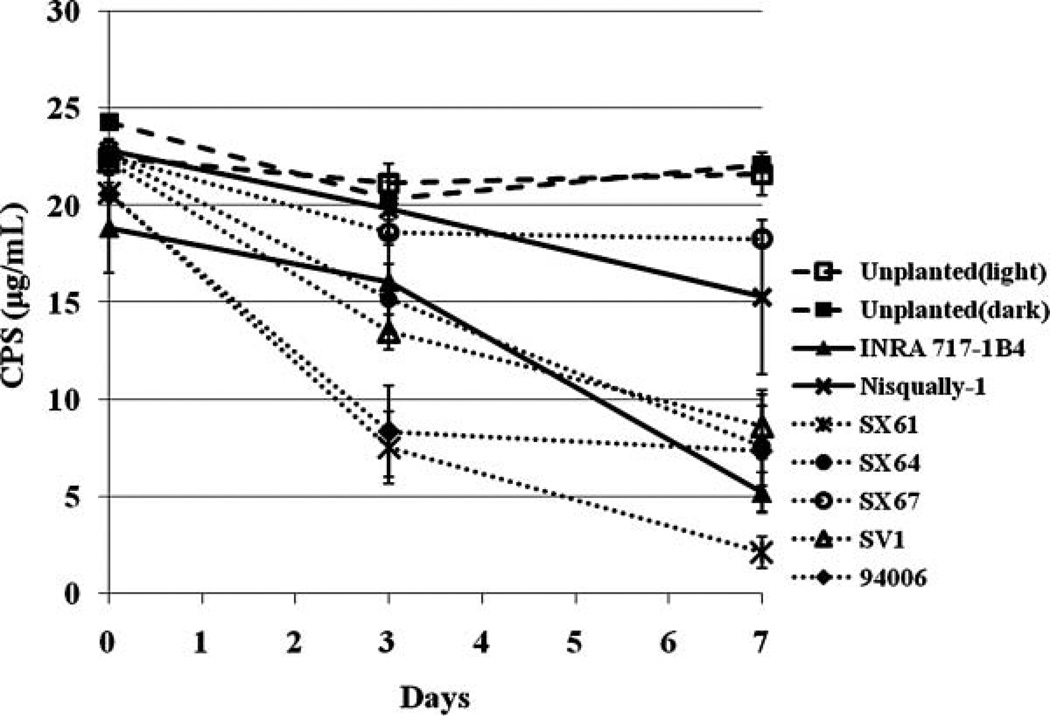
To study whether CPS was taken up by plants from a nutrient solution, the concentration of CPS in the liquid growth medium was monitored. During the 1-week growth, there were no adverse effects of CPS exposure on plant growth and appearance. The results indicated that CPS was taken up from the nutrient solution by the plants (Figure 2 and Table 1), and the uptake of CPS varies among different plants. While a trace amount of CPS was lost from the unplanted vials, removal of CPS from the planted vials was 2–10-fold greater. Of the initial dose, 46.0 ± 2.9% and 34.4 ± 0.0% was removed by SX61 and 94006 clones, respectively. The best performing line, SX64, removed CPS with the highest uptake rate of 21.3 ± 2.1 µg of CPS · day−1 · g−1 plant wet weight. There was a significant difference between three clones (INRA 717-1B4, Nisqually-1, and SX61) and SX64 at the 5% significance level (p = 0.011). There was no significant difference between light and dark controls. All statistical analyses were performed by using one-way Analysis of Variance (ANOVA) with Tukey’s HSD (honestly significant difference) post hoc analysis. From the analysis of the nutrient solution, it was apparent that the total quantity of CPS in the nutrient solution decreased with time.
Figure 2.
The decrease of CPS in hydroponic solution. The concentrations of CPS in the hydroponic solution were monitored for seven days. CPS was taken up from the nutrient solution as the plants were being grown. The data are shown as the mean ± SEM from three samples. The solid lines correspond to two poplar clones, dashed lines unplanted vials, and dotted lines five willow clones.
Table 1.
Removal of CPS from hydroponic solution by plants for 7 days
| Plant line | Removal* (%) | Removal rate |
|---|---|---|
| Unplanted (under light) | 8.4 ± 3.4 | **5.08 ± 2.07 |
| Unplanted (under dark) | 5.6 ± 0.0 | **3.38 ± 0.00 |
| INRA 717-1B4 | 25.7 ± 2.2 | ***11.44 ± 1.53a |
| Nisqually-1 | 16.1 ± 7.1 | ***10.65 ± 1.85a |
| SX61 | 46.0 ± 2.9 | ***11.58 ± 1.96a |
| SX64 | 33.4 ± 5.7 | ***21.27 ± 2.09b |
| SX67 | 11.8 ± 1.7 | ***15.17 ± 2.16ab |
| SV1 | 32.8 ± 3.2 | ***15.45 ± 1.40ab |
| 94006 | 34.4 ± 0.0 | ***18.86 ± 2.26ab |
Data is not normalized to plant mass.
Micrograms of CPS per day ± SEM (standard error of the mean).
Micrograms of CPS per day per gram of fresh weight ± SEM.
The data are shown as the mean ± SEM from three samples.
Different letters denote significant differences at P < 0.05.
Accumulation of CPS in Plant Tissue
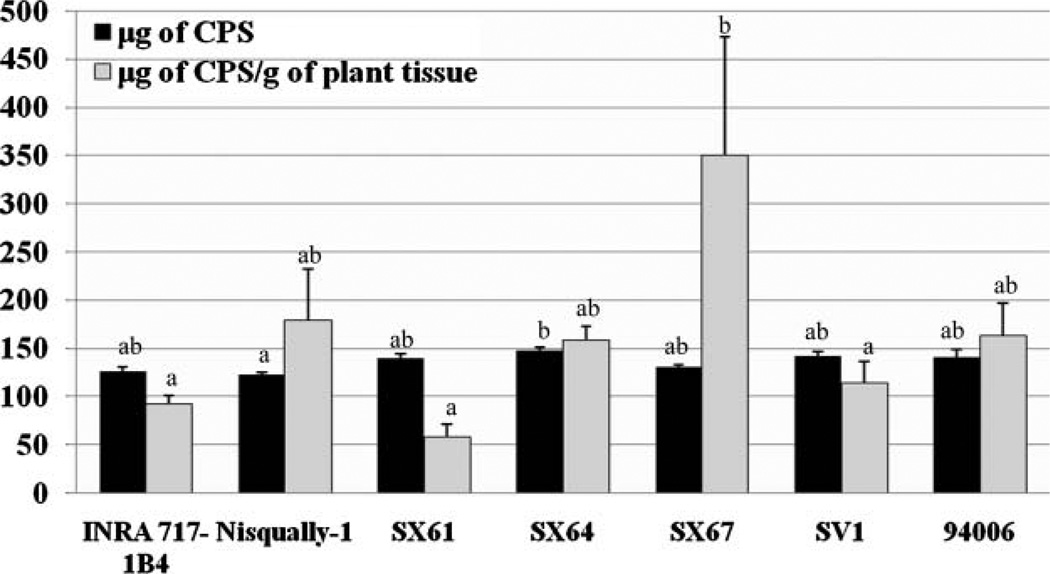
After 1 week of exposure to CPS, all plant samples were analyzed for the extraction of CPS. Considerable amounts of CPS accumulated in all plants (Figure 3), and the bioaccumulation of CPS varies among different plants. Higher (17%) CPS accumulation was measured in SX64, which removed CPS with the highest uptake rate, than in Nisqually-1 (P < 0.05). The clone that accumulated the most CPS in 1 g of tissue was SX67, and higher (68–83%) CPS accumulation occurred in SX67 compared to INRA 717-1B4, SX61, and SV1. The mean% of CPS accumulation in plants was about 80 to 99% of the total removal.
Figure 3.
The amounts of CPS accumulated in plant tissue. After 1 week of exposure to CPS, all of the plant explants were ground and treated for the CPS extraction from plant tissue. The results show that considerable amounts of CPS were accumulated in plant tissue. Dark-colored column: microgram of CPS per plant; light-colored column: microgram of CPS normalized to plant weight. The data are shown as the mean ± SEM from three samples. Different letters denote significant differences at P < 0.05. P values for the microgram of CPS and microgram of CPS · g−1 are 0.03 and 0.01, respectively.
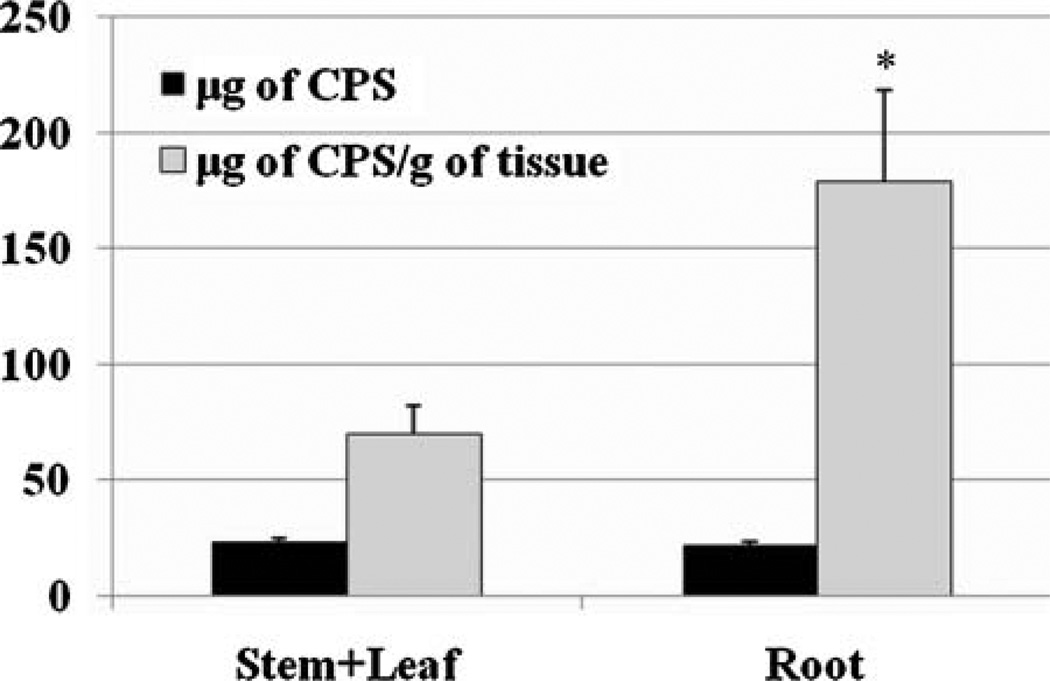
To determine whether plants accumulate CPS in shoot or in root, the distribution of CPS among individual tissues was investigated for the poplar clone INRA 717-1B4. After 7 days of CPS exposure, the plants were harvested, and shoots and roots were analyzed separately. CPS was nearly equally divided between shoots and roots (See Table S2 in Supporting Information). However, levels of CPS normalized to gram of tissue showed that roots have 155.1% higher CPS contents than shoots and the difference was significant (P = 0.029) (Figure 4).We propose, therefore, that some amounts of CPS taken up by roots were translocated to upper plant biomass in poplar and that a much higher concentration of CPS was shown in roots than in shoots.
Figure 4.
The distribution of CPS among plant tissues. After 1 week of exposure to CPS, the amount of CPS accumulated in plant tissue was investigated from shoots and roots separately from a poplar clone INRA 717-1B4. Roots accumulated much higher concentrations of CPS than did shoots. Dark-colored column: microgram of CPS per plant tissue; light-colored column: microgram of CPS normalized to plant weight. The data are shown as the mean ± SEM from five plant samples. Significant difference (P < 0.05) is indicated by asterisk (*).
The Fate of CPS in Plant Tissue
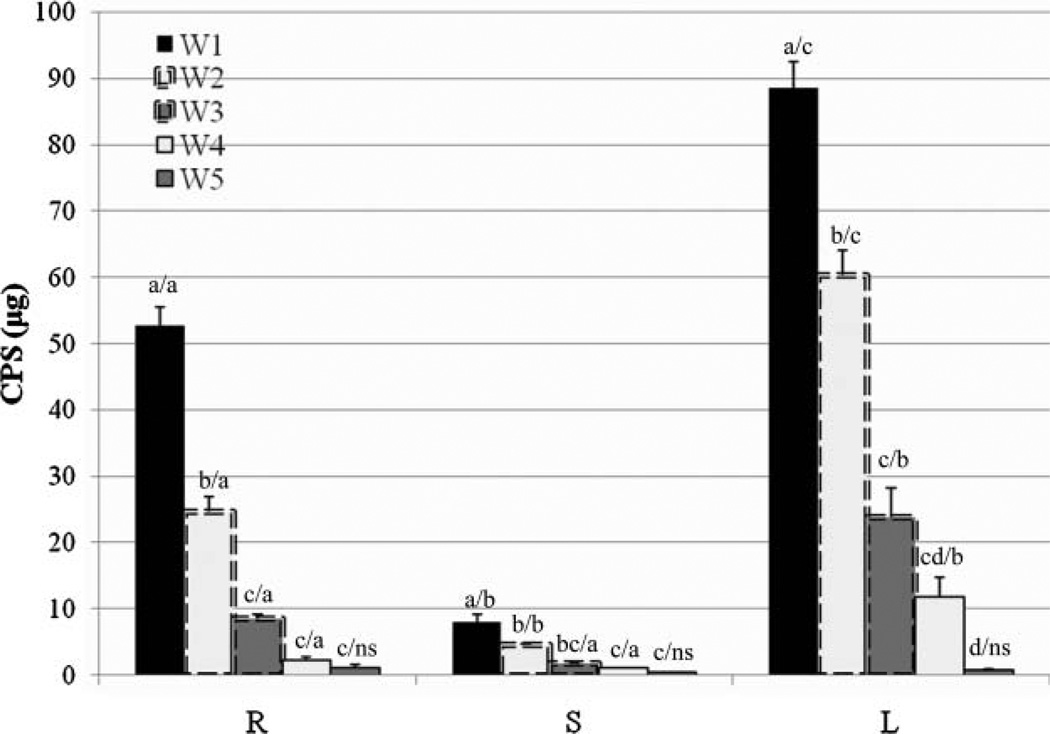
During the 1-week growth, no visual symptoms of CPS toxicity were observed for the plants growing in mediawith 25 µg/mL CPS. During the following 4-week growth, after the plants were transferred to media devoid of CPS, most plants showed enhanced plant growth with auxiliary shoot proliferation (data not shown). Figure 5 shows that the amount of CPS decreased dramatically within the hydroponic poplar plants. The total amounts of CPS (µg) decreased at a rate of 40.1% over the first week, 61.7% over the second week, 55.2% over the third week, and 84.3% over the fourth week. Overall, the results indicated that 97.7% (R), 94.8% (S), and 99.1% (L) of CPS accumulated in plants were metabolized within the plant tissue. Comparing the five time periods for a given tissue, the difference in all three plant tissues was statistically significant (P < 0.000). Comparing the three different plant tissues for a given time period, the difference between each time point decreased with time, and there was no significant difference in week 5 (W5) when compared CPS remaining in root, stem, and leaf (P = 0.276). Importantly, there was no CPS released back to the nutrient solution from plants (data not shown).
Figure 5.
The amounts of CPS remaining in hydroponic poplar plants 1 (W1), 2 (W2), 3 (W3), 4 (W4), and 5 weeks (W5) after CPS dose. Plants INRA 717-1B4 were treated with CPS for 7 days, then moved to media devoid of CPS for 4 more weeks. CPS was extracted from root (R), stem (S), and leaf (L), separately at each time point. As the results show, CPS did not persist in the plants, suggesting further metabolism of CPS in plant tissue. The data are shown as the mean ± SEM from five plant samples. Different letters denote significant differences at P < 0.05 (first letter: five time periods for a given plant tissue; second letter: three parts of plant for a given time period); ns denotes non-significant difference at the 0.05 level. P values for the microgram of CPS are 0.000 (R, S, L, W1, and W2), 0.001 (W3 and W4), and 0.276 (W5).
DISCUSSION
Since CPS is an insecticide, it was not expected to be toxic to plants. Surprisingly, CPS has been shown to adversely affect a variety of plants including alfalfa (Medicago sativa), clover (Melilotus alba and Trifolium pratense) (Smith et al. 1978), Pinus halepensis (Olofinboba and Kozlowski 1982), and Arabidopsis thaliana (Aben et al. 1992). However, information is limited on CPS toxicity to plants compared to a number of animal toxicity studies. It is well known that CPS and CPO are directly toxic to the nervous system in insects and other animals primarily by inhibition of cholinesterase (ChE). Interestingly, ChE has also been found in plants. ChE has been isolated from mung bean roots (Riov and Jaffe 1973), and the ChE activity assay in 118 plant species confirmed widespread distribution of ChE in the plant kingdom (Gupta and Gupta 1997). The ChE from plant tissues is not identical with any animal ChE but shows similarity in several important properties to ChE. In addition, CPS exposure has also been shown to inhibit enzymes other than ChE in the laboratory animals (Cox 1995). ChE may participate in several neurophysiological-like processes such as modulating hormone levels in plants, or binding to certain plant proteins. In addition, plants may be affected by CPS indirectly. Sardar and Kole (2005) conducted a laboratory experiment to evaluate the effect of CPS on the availability of the major plant nutrients (N, P, and K) in soil. Their results show that there was a significant decrease in the available N and P content in soil treated with CPS in comparison to the control set. Decrease in availability of these nutrients might be due to the inhibitory effect of CPS or its metabolites indirectly on microorganisms involved in mobilizing these elements, such as di-nitrogen fixing bacteria and phosphate solubilizing microorganisms or directly on the soil nitrification and the phosphatase enzyme in soil. We hypothesize, therefore, that CPS and its metabolites adversely affect plants directly by inhibition of ChE and other enzymes in plant tissues or soil, and also affects plants indirectly by limiting the uptake of nutrients through inhibiting microbial growth.
The uptake of CPS by herbaceous plants has been investigated previously. In general, it has been shown that negligible levels of CPS enter the plant via the roots, indicating its non-systemic nature. However, most studies have focused on CPS uptake by plants in soil (Smith et al. 1967a; Bauriedel et al. 1976; Bauriedel and Miller 1986a, 1986b), and the results were similar (i.e., negligible uptake) to the results obtained in nutrient solution (Smith and Watson 1964). While previous study have shown its uptake by plants to be insignificant, our results show that significant amounts of CPS were taken up from the nutrient solution by woody plants, poplar and willow. The fact that only living plants were capable of taking up CPS into its tissue, while CPS was released back from tissues to the media when plants were dead also supports the idea of CPS uptake by plants (data not shown). Further, not only rooted poplar cuttings but unrooted poplar cuttings removed CPS from nutrient solution to similar degrees (µg/g) (data not shown). Since pesticides’ bioavailability and translocation in plant was found to be octanol-water partition coefficient (Kow)-dependent (Bromilow and Chamberlain 1995), CPS, which is a very lipophilic compound with a higher Kow (4.7 ~ 5.3), is expected to quickly cross biomembranes and then sorb to the roots (Burken and Schnoor 1996; Trapp 2000, 2004). Once introduced inside the plant, CPS was distributed throughout the plant in this work as well as in other studies (Smith et al. 1967b). Further, in poplar plants dosed with CPS, accumulation of CPS was more pronounced in roots than in shoots on a per gram basis (Figure 4) and this finding corresponds to that of Azmat et al. (2009). It has been observed that roots were important in accumulating compounds due to their direct exposure of toxic chemicals as underground parts, and transporting the compounds to aboveground organs (shoots) (Azmat et al. 2009).
The dramatic decline of CPS accumulated in plants (Figure 5) indicates further metabolism of CPS in plant tissue. It also demonstrates that the poplar clone ‘INRA 717-1B4’ is a very efficient plant material to use for phytoremediation due to the ability to biodegrade CPS. This characteristic is an advantage since the harvested plants would not be considered hazardous waste as is the case for phytoremediation of non-degradable pollutants such as heavy metals. Judging by the increased growth of the plants following removal from CPS (data not shown), it can be assumed that the degradative products were not phytotoxic. It is important to know what happens to CPS when it enters the plant. Since pesticides taken up from soil or water are usually metabolized into less toxic or non-toxic products by several metabolic processes in plants (Peuke and Rennenberg 2005; Laurent et al. 2006), we first hypothesized that 3,5,6,-trichloro-2-pyridinol (TCP), a primary metabolite of CPS (Racke 1993) (Figure 1), might be detected in both hydroponic solution and plant tissue as CPS is taken up by plants and its concentration decreases in tissue with time. However, no TCP was found as CPS levels declined in hydroponic poplar plants. CPS may have degraded to TCP within the plant, and then further rapidly degraded either partially or completely in plant tissues to form unidentified metabolites. Interestingly, TCP is further mineralized in animals to carbon dioxide and water (Eaton et al. 2008), and plants fix this pesticide-derived carbon as natural, cellular materials (e.g., starch and cellulose) through anabolic activity (Racke 1993). Metabolism also includes liberation of chloride and the formation of trivial amounts of several unidentified water soluble decomposition products (Smith et al. 1967b). Thus, further metabolism and mineralization of TCP may have occurred in plant tissue via unknown mechanisms. Another possible pathway for CPS degradation in poplar could result in unextractable compounds. Bauriedel and Miller (1986a, 1986b) conducted a series of studies with sugar beets and corn in CPS-treated soil and found that there were only traces of CPS and TCP (1–3%) whereas the major residues (62–79%) were unextractable products, indicating that themetabolites were in a conjugated form. Conjugated compounds are then usually sequestered into the vacuole or become part of cell wall material (Dietz and Schnoor 2001).
CONCLUSIONS
This report demonstrates that poplar and willow trees, unlike the herbaceous plants in previous studies, have a strong ability to take up CPS and to translocate it within the plants and thus, assist in removing it from the hydroponic solution. CPS did not persist in the plants, suggesting further metabolism of CPS in plant tissue. Further study is necessary to determine the potential uptake mechanism and CPS metabolism pathway of plants not only in CPS-treated hydroponic solution but also in soil and soil-applied residues, since a number of soil physicochemical factors such as moisture, redox conditions, pH, temperature, organic matter, and nutrients, affect microbial activity, chemical diffusion in soils, and further the uptake and translocation of pesticide by plants in soil. To our knowledge, the poplar and willow species presented in this study were the first woody plants to be tested for potential phytoremediation applications for CPS. The results of this study demonstrate that phytoremediation of CPS may be possible through the use of poplar and willow trees. Since poplar and willow are able to efficiently take up CPS, there is the possibility of enhancing the CPS degradative potential by genetic manipulation of metabolism in planta. The research of enhancing phytoremediation of CPS using transgenic plants expressing enzymes which are known to be involved in OP insecticide degradation pathways is in progress. The results presented here indicate that phytoremediation of CPS and other OP insecticides will be a fertile area for future development.
Supplementary Material
ACKNOWLEDGMENTS
The authors are grateful to Jud Isebrands and Prof. Steve Strazuss for providing us plant cuttings and to Prof. Clement E. Furlong for his inspiring guidance and suggestions in the completion of this manuscript. We are also thankful to Peter F. Andeer for providing us useful advice and technical support for HPLC analysis. This work was sponsored by the University of Washington Superfund Research Program (SRP), Grant # NIEHS P42ES04696.
Footnotes
Publisher's Disclaimer: Full terms and conditions of use: http://www.tandfonline.com/page/terms-and-conditions
This article may be used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan, sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden.
The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
REFERENCES
- Aben WJM, Houx NWH, Leistra M. Toxicity of pentachlorophenol and chlorpyrifos in soil and in solution to a nematode and a plant species. Rep. No. 59. Wageningen (The Netherlands): U.S. Dep. of Commerce, Agric. Res. Dept. Winand Staring Center for Integrated Land, Soil and Water Res. (U.S. NTIS PB93-221216); 1992. p. 39. [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological profile for chlorpyrifos. Atlanta (GA): U.S. Department of Health and Human Services, Public Health Service; 1997. [PubMed] [Google Scholar]
- Amer SM, Farah OR. Cytological effects of pesticides XII. Effects of the phosphorothioate insecticide Dursban on the mitosis of Vicia faba. Cytologia. 1983;48:27–33. [Google Scholar]
- Azmat R, Haider S, Riaz M. An inverse relation between Pb2+ and Ca2+ ions accumulation in Phaseolus mungo and Lens culinaris under Pb stress. Pak J Bot. 2009;41:2289–2295. [Google Scholar]
- Bauriedel WR, McKellar RL, Miller JH. A rotational crop study using 14C-labeled chlorpyrifos. Indianapolis (IN): Dow Elanco; 1976. (unpublished report). [Google Scholar]
- Bauriedel WR, Miller JH. The metabolic fate of 14C-chlorpyrifos applied to field corn at planting (soil application) and in mid-season (foliar application) Indianapolis (IN): Dow Elanco; 1986a. (unpublished report). [Google Scholar]
- Bauriedel WR, Miller JH. The metabolic fate of 14C-chlorpyrifos applied to sugar beets at planting (soil application) and in mid-season (foliar application) Indianapolis (IN): Dow Elanco; 1986b. (unpublished report). [Google Scholar]
- Beck NG, Arpaia ML, Eckard KJ, Reints JS, Lord EM. The effect of chlorpyrifos on flower and fruit development in grapefruit, Citrus paradisi Macfayden. Sci Horticul. 1991;47:35–50. [Google Scholar]
- Bromilow RH, Chamberlain K. Principles governing uptake and transport of chemicals. In: Trapp S, McFarlane JC, editors. Plant contamination: modeling and simulation of organic chemical processes. Boca Raton (FL): Lewis Publishers; 1995. pp. 37–68. [Google Scholar]
- Burken JG, Schnoor JL. Phytoremediation: Plant uptake of atrazine and role of root exudates. J Environ Eng. 1996;122:958–963. [Google Scholar]
- Burken JG, Schnoor JL. Uptake and metabolism of atrazine by poplar trees. Environ Sci Technol. 1997;31:1399–1406. [Google Scholar]
- Cox C. Chlorpyrifos, part3: ecological effects. J Pest Reform. 1995;15:13–19. [Google Scholar]
- Cunningham SD, Berti WR. Remediation of contaminated soils with green plants: an overview. In Vitro Cell Dev Biol. 1993;29:207–212. [Google Scholar]
- Dietz AC, Schnoor JL. Advances in phytoremediation. Environ Health Perspect. 2001;109:163–168. doi: 10.1289/ehp.01109s1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty SL, James CA, Moore AL, Vajzovic A, Singleton GL, Ma C, Khan Z, Xin G, Kang JW, Park JY, Meilan R, Strauss SH, Wilkerson J, Farin F, Strand SE. Enhanced phytoremediation of volatile environmental pollutants with transgenic trees. PNAS. 2007;104:16816–16821. doi: 10.1073/pnas.0703276104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton DL, Daroff RB, Autrup H, Bridges J, Buffler P, Costa LG, Coyle J, McKhann G, Mobley WC, Nadel L, Neubert D, Schulte-Hermann R, Spencer PS. Review of the toxicology of chlorpyrifos with an emphasis on human exposure and neurodevelopment. Crit Rev Toxicol. 2008;38:1–125. doi: 10.1080/10408440802272158. [DOI] [PubMed] [Google Scholar]
- Gupta A, Gupta R. A survey of plants for presence of cholinesterase activity. Phytochemistry. 1997;46:827–831. [Google Scholar]
- Laurent F, Debrauwer L, Pascal-Lorber S. Metabolism of [14C]-2,4-dichlorophenol in edible plants. Pest Manage Sci. 2006;62:558–564. doi: 10.1002/ps.1213. [DOI] [PubMed] [Google Scholar]
- Moore MT, Schulz R, Cooper CM, Smith S, Jr, Rodgers JH., Jr Mitigation of chlorpyrifos runoff using constructed wetlands. Chemosphere. 2002;46:827–835. doi: 10.1016/s0045-6535(01)00189-8. [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant. 1962;15:473–497. [Google Scholar]
- Newman LA, Strand SE, Choe N, Duffy J, Ekuan G, Ruszaj M, Shurtleff BB, Wilmoth J, Heilman P, Gordon MP. Uptake and biotransformation of trichloroethylene by hybrid poplars. Environ Sci Technol. 1997;31:1062–1067. doi: 10.1289/ehp.98106s41001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofinboba MO, Kozlowski TT. Effects of three systemic insecticides on seed germination and growth of Pinus halepensis seedlings. Plant Soil. 1982;64:255–258. [Google Scholar]
- Peuke AD, Rennenberg H. Phytoremediation. EMBO Rep. 2005;6:497–501. doi: 10.1038/sj.embor.7400445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilon-Smits E. Phytoremediation. Annu Rev Plant Biol. 2005;56:15–39. doi: 10.1146/annurev.arplant.56.032604.144214. [DOI] [PubMed] [Google Scholar]
- Pilon-Smits EAH, de Souza MP, Lytle CM, Shang C, Lugo T, Terry N. Selenium volatilization and assimilation by hybrid poplar (Populus tremula x alba) J Exp Bot. 1998;49:1889–1892. [Google Scholar]
- Racke KD. Environmental fate of chlorpyrifos. Rev Environ Contam Toxicol. 1993;131:1–151. doi: 10.1007/978-1-4612-4362-5_1. [DOI] [PubMed] [Google Scholar]
- Riov J, Jaffe MJ. Cholinesterases from plant tissues. I. Purification and characterization of a cholinesterase from mung bean roots. Plant Physiol. 1973;51:520–528. doi: 10.1104/pp.51.3.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson BH, Mills TM, Petit D, Fung LE, Green SR, Clothier BE. Natural and induced cadmium-accumulation in poplar and willow: implications for phytoremediation. Plant Soil. 2000;227:301–306. [Google Scholar]
- Rogers MR, Clark GJ, DiVincenzo JP. Analysis of chlorpyrifos by gas chromatography: methodology concerns. EJEAFChe. 2006;5:1509–1514. [Google Scholar]
- Rouchaud J, Gustin F, van de Steene F, Pelerents C, Vanparys L, Gillet J, Benoit F, Ceustermans N. Transport of the insecticides chlorpyrifos, chlorfenvinphos, carbofuran, carbosulfan, and furathiocarb from soil into the foliage of cauliflower and Brussels sprouts plants grown in the field. Toxicol Environ Chem. 1991;30:79–94. [Google Scholar]
- Salt DE, Smith RD, Raskin I. Phytoremediation. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:643–668. doi: 10.1146/annurev.arplant.49.1.643. [DOI] [PubMed] [Google Scholar]
- Sardar D, Kole RK. Metabolism of chlorpyrifos in relation to its effect on the availability of some plant nutrients in soil. Chemosphere. 2005;61:1273–1280. doi: 10.1016/j.chemosphere.2005.03.078. [DOI] [PubMed] [Google Scholar]
- Shang QT, Doty SL, Wilson AM, Howald WN, Gordon MP. Trichloroethylene oxidative metabolism in plants: the trichloroethanol pathway. Phytochemistry. 2001;58:1055–1065. doi: 10.1016/s0031-9422(01)00369-7. [DOI] [PubMed] [Google Scholar]
- Sinclair PJ, Neeson RJ, Williams PA. Phytotoxicity of some organophosphate insecticides to onions and carrots during germination and emergence. Plant Prot Q. 1992;7:23–25. [Google Scholar]
- Singh BK, Walker A, Morgan JAW, Wright DJ. Biodegradation of chlorpyrifos by Enterobacter strain B-14 and its use in bioremediation of contaminated soils. Appl Environ Microb. 2004;70:4855–4863. doi: 10.1128/AEM.70.8.4855-4863.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CR, Funke BR, Schultz JT. Effects of insecticides on acetylene reduction by alfalfa, red clover and sweet clover. Soil Biol Biochem. 1978;10:463–466. [Google Scholar]
- Smith GN, Watson BS. Uptake and translocation of ethel-36Cl in plants. Indianapolis (IN): Dow Elanco; 1964. (unpublished report). [Google Scholar]
- Smith GN, Watson BS, Fischer FS. Investigations of dursban insecticide: uptake and translocation of [36Cl] O,O-diethyl O-3,5,6-trichloro-2-pyridyl phosphorothioate and [14C] O,O-diethyl O-3,5,6-trichloro-2-pyridyl phosphorothioate by beans and corn. J Agric Food Chem. 1967a;15:127–131. [Google Scholar]
- Smith GN, Watson BS, Fischer FS. Investigations of dursban insecticide: metabolism of O,Odiethyl O-3,5,6-trichloro-2-pyridyl phosphorothioate and 3,5,6-trichloro-2-pyridinol in plants. J Agric Food Chem. 1967b;15:870–877. [Google Scholar]
- Trapp S. Modeling uptake into roots and subsequent translocation of neutral and ionisable organic compounds. Pest Manage Sci. 2000;56:767–778. [Google Scholar]
- Trapp S. Plant uptake and transport models for neutral and ionic chemicals. Environ Sci Poll Res. 2004;11:33–39. doi: 10.1065/espr2003.08.169. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency (USEPA) Human health risk assessment: Chlorpyrifos. Office of Pesticide Programs, Health Effects Division 7509C; 2000. 8 June 2000. [Google Scholar]
- U.S. Environmental Protection Agency (USEPA) Interim reregistration eligibility decision for chlorpyrifos. Prevention, Pesticides and Toxic Substances (7508C), EPA 738-R-01-007; 2002. February 2002. [Google Scholar]
- Williamson LN, Terry AV, Jr, Bartlett MG. Determination of chlorpyrifos and its metabolites in rat brain tissue using coupled-column liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom. 2006;20:2689–2695. doi: 10.1002/rcm.2647. [DOI] [PubMed] [Google Scholar]
- Yang L, Zhao YH, Zhang BX, Yang CH, Zhang X. Isolation and characterization of a chlorpyrifos and 3, 5, 6-trichloro-2-pyridinol degrading bacterium. FEMS Microbiol Lett. 2005;251:67–73. doi: 10.1016/j.femsle.2005.07.031. [DOI] [PubMed] [Google Scholar]
- Yu YL, Fang H, Wang X, Wu XM, Shan M, Yu JQ. Characterization of a fungal strain capable of degrading chlorpyrifos and its use in detoxification of the insecticide on vegetables. Biodegradation. 2006;17:487–494. doi: 10.1007/s10532-005-9020-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.