Abstract
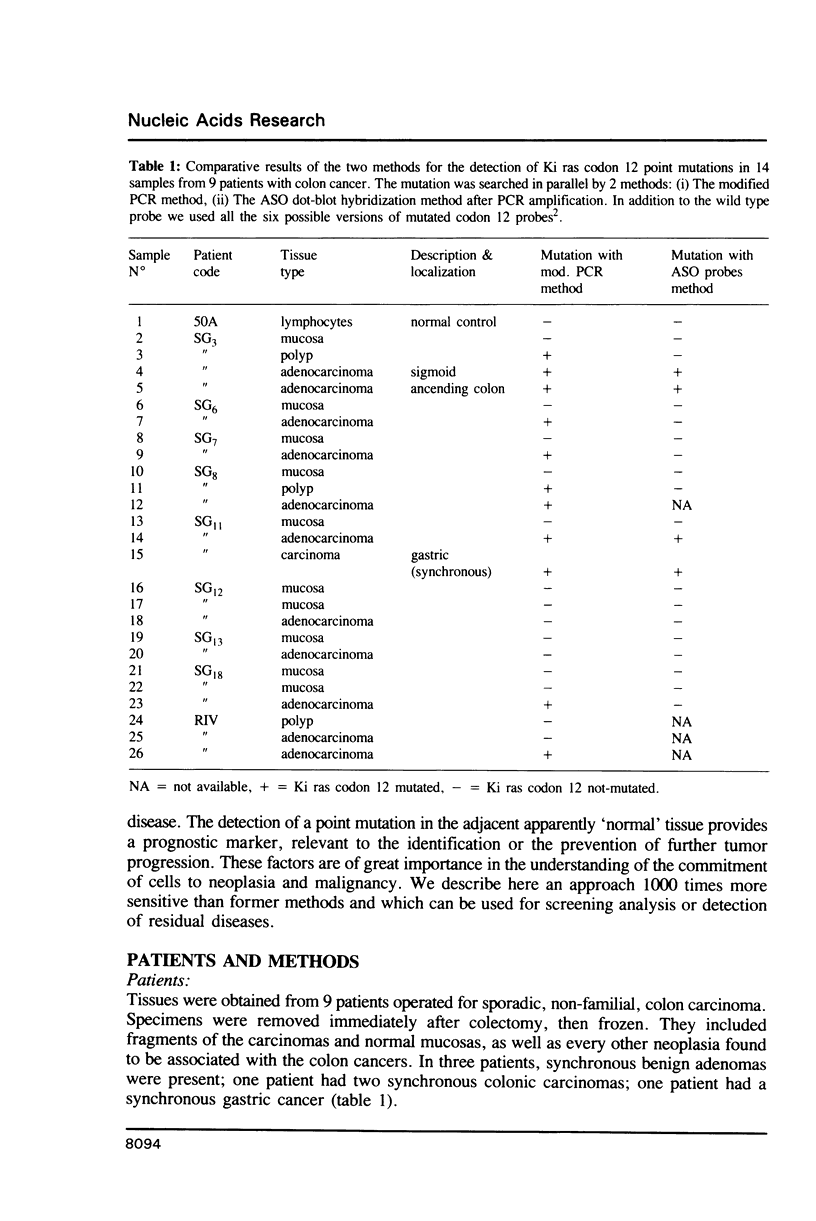
The detection of point mutations correlated with diseases, in enzymatically amplified DNA sequences (Polymerase Chain Reaction), is currently performed by digestion of PCR products when an existing restriction site disappears at least in one allele of the amplified mutated sequence or by allele specific radiolabeled probes in all other cases. These methods are the most sensitive but they cannot detect a mutation if it is present in less than 5% of the studied cells. We describe here a method based on the introduction of an artificial restriction site, using a modified primer during the PCR, which creates a RFLP indicative of the studied mutation. This RFLP is detected by a radiolabeled oligonucleotide probe which is not related to the mutation. Our approach multiplies the sensitivity by a factor of 1000 and it is practical for use in screening purposes and the detection, after treatment, of the residual disease in human malignancies. Using this method we detected 20% more mutations at codon 12 in the Ki ras oncogene in DNA from colorectal cancers that were undetectable with all the previous methods.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bos J. L., Fearon E. R., Hamilton S. R., Verlaan-de Vries M., van Boom J. H., van der Eb A. J., Vogelstein B. Prevalence of ras gene mutations in human colorectal cancers. 1987 May 28-Jun 3Nature. 327(6120):293–297. doi: 10.1038/327293a0. [DOI] [PubMed] [Google Scholar]
- Bos J. L., Fearon E. R., Hamilton S. R., Verlaan-de Vries M., van Boom J. H., van der Eb A. J., Vogelstein B. Prevalence of ras gene mutations in human colorectal cancers. 1987 May 28-Jun 3Nature. 327(6120):293–297. doi: 10.1038/327293a0. [DOI] [PubMed] [Google Scholar]
- Cannon-Albright L. A., Skolnick M. H., Bishop D. T., Lee R. G., Burt R. W. Common inheritance of susceptibility to colonic adenomatous polyps and associated colorectal cancers. N Engl J Med. 1988 Sep 1;319(9):533–537. doi: 10.1056/NEJM198809013190902. [DOI] [PubMed] [Google Scholar]
- Cannon-Albright L. A., Skolnick M. H., Bishop D. T., Lee R. G., Burt R. W. Common inheritance of susceptibility to colonic adenomatous polyps and associated colorectal cancers. N Engl J Med. 1988 Sep 1;319(9):533–537. doi: 10.1056/NEJM198809013190902. [DOI] [PubMed] [Google Scholar]
- Ehlen T., Dubeau L. Detection of ras point mutations by polymerase chain reaction using mutation-specific, inosine-containing oligonucleotide primers. Biochem Biophys Res Commun. 1989 Apr 28;160(2):441–447. doi: 10.1016/0006-291x(89)92452-2. [DOI] [PubMed] [Google Scholar]
- Forrester K., Almoguera C., Han K., Grizzle W. E., Perucho M. Detection of high incidence of K-ras oncogenes during human colon tumorigenesis. 1987 May 28-Jun 3Nature. 327(6120):298–303. doi: 10.1038/327298a0. [DOI] [PubMed] [Google Scholar]
- Haliassos A., Chomel J. C., Tesson L., Baudis M., Kruh J., Kaplan J. C., Kitzis A. Modification of enzymatically amplified DNA for the detection of point mutations. Nucleic Acids Res. 1989 May 11;17(9):3606–3606. doi: 10.1093/nar/17.9.3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haliassos A., Chomel J. C., Tesson L., Baudis M., Kruh J., Kaplan J. C., Kitzis A. Modification of enzymatically amplified DNA for the detection of point mutations. Nucleic Acids Res. 1989 May 11;17(9):3606–3606. doi: 10.1093/nar/17.9.3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanpierre M. A rapid method for the purification of DNA from blood. Nucleic Acids Res. 1987 Nov 25;15(22):9611–9611. doi: 10.1093/nar/15.22.9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krontiris T. G., Cooper G. M. Transforming activity of human tumor DNAs. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1181–1184. doi: 10.1073/pnas.78.2.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R., Barbacid M. Oncogene detection at the single cell level. Oncogene. 1988 Dec;3(6):647–651. [PubMed] [Google Scholar]
- Kumar R., Dunn L. L. Designed diagnostic restriction fragment length polymorphisms for the detection of point mutations in ras oncogenes. Oncogene Res. 1989;4(3):235–241. [PubMed] [Google Scholar]
- Lench N., Stanier P., Williamson R. Simple non-invasive method to obtain DNA for gene analysis. Lancet. 1988 Jun 18;1(8599):1356–1358. doi: 10.1016/s0140-6736(88)92178-2. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Verlaan-de Vries M., Bogaard M. E., van den Elst H., van Boom J. H., van der Eb A. J., Bos J. L. A dot-blot screening procedure for mutated ras oncogenes using synthetic oligodeoxynucleotides. Gene. 1986;50(1-3):313–320. doi: 10.1016/0378-1119(86)90335-5. [DOI] [PubMed] [Google Scholar]






