Abstract
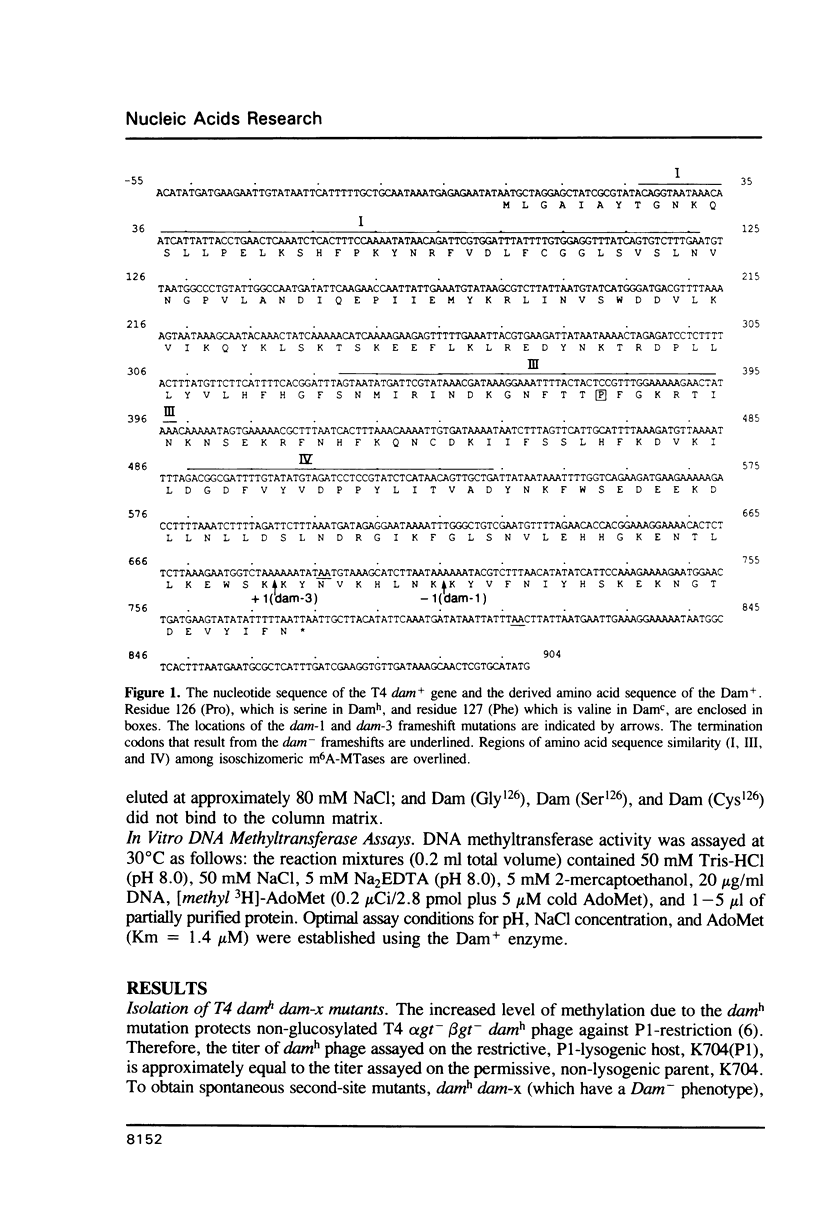
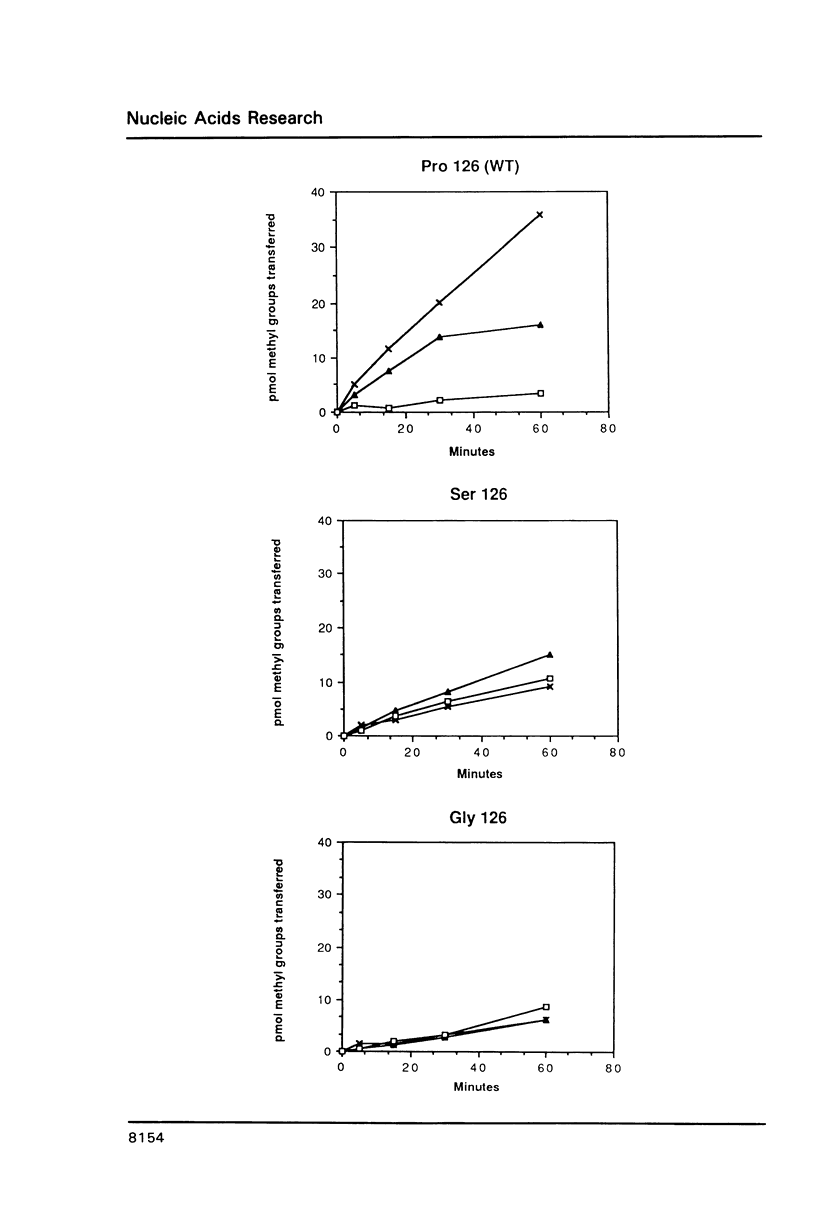
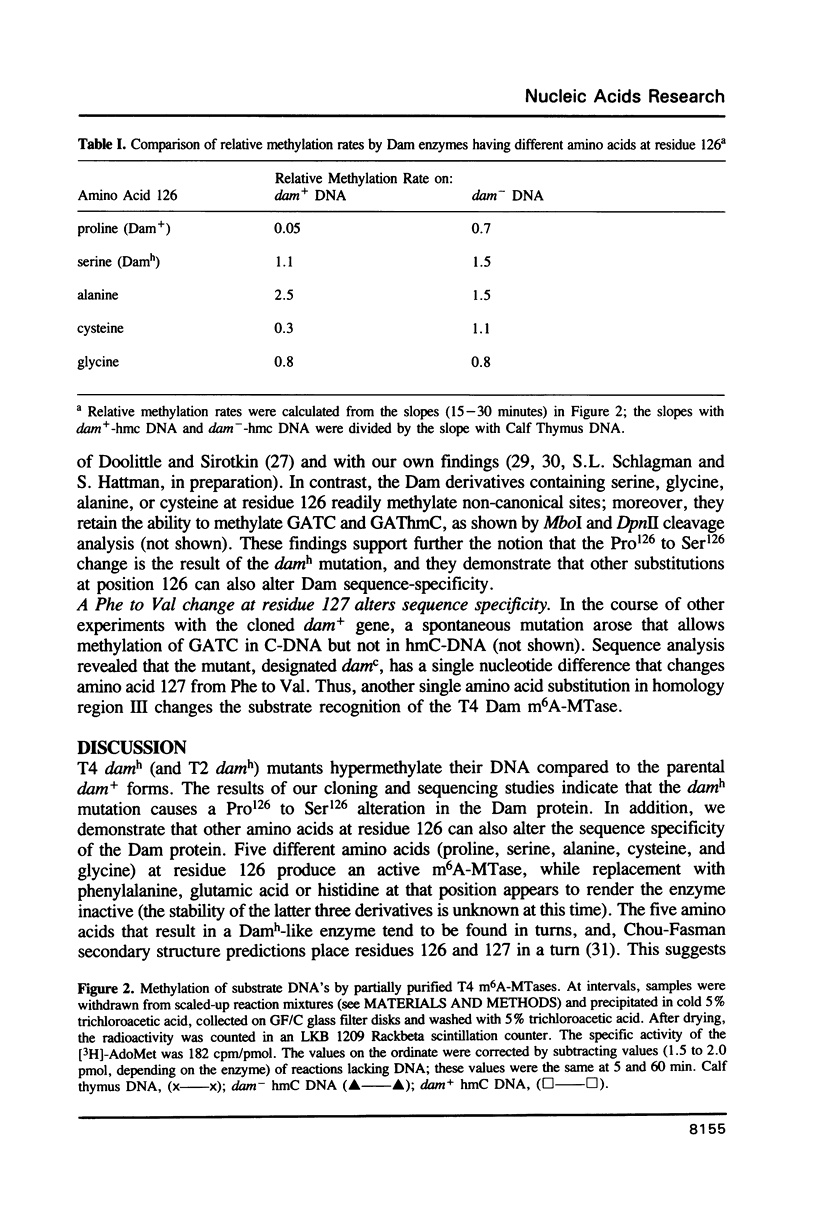
Bacteriophage T4 codes for a DNA-[N6-adenine] methyltransferase (Dam) which recognizes primarily the sequence GATC in both cytosine- and hydroxymethylcytosine-containing DNA. Hypermethylating mutants, damh, exhibit a relaxation in sequence specificity, that is, they are readily able to methylate non-canonical sites. We have determined that the damh mutation produces a single amino acid change (Pro126 to Ser126) in a region of homology (III) shared by three DNA-adenine methyltransferases; viz, T4 Dam, Escherichia coli Dam, and the DpnII modification enzyme of Streptococcus pneumoniae. We also describe another mutant, damc, which methylates GATC in cytosine-containing DNA, but not in hydroxymethylcytosine-containing DNA. This mutation also alters a single amino acid (Phe127 to Val127). These results implicate homology region III as a domain involved in DNA sequence recognition. The effect of several different amino acids at residue 126 was examined by creating a polypeptide chain terminating codon at that position and comparing the methylation capability of partially purified enzymes produced in the presence of various suppressors. No enzyme activity is detected when phenylalanine, glutamic acid, or histidine is inserted at position 126. However, insertion of alanine, cysteine, or glycine at residue 126 produces enzymatic activity similar to Damh.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brooks J. E., Hattman S. In vitro methylation of bacteriophage lambda DNA by wild type (dam+) and mutant (damh) forms of the phage T2 DNA adenine methylase. J Mol Biol. 1978 Dec 15;126(3):381–394. doi: 10.1016/0022-2836(78)90047-5. [DOI] [PubMed] [Google Scholar]
- Bächi B., Reiser J., Pirrotta V. Methylation and cleavage sequences of the EcoP1 restriction-modification enzyme. J Mol Biol. 1979 Feb 25;128(2):143–163. doi: 10.1016/0022-2836(79)90123-2. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cupples C. G., Miller J. H. Effects of amino acid substitutions at the active site in Escherichia coli beta-galactosidase. Genetics. 1988 Nov;120(3):637–644. doi: 10.1093/genetics/120.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle M. M., Sirotkin K. Bacteriophage T2 and T4, dam+ and damh and Eco dam+ methylation: preference at different sites. Biochim Biophys Acta. 1988 Feb 28;949(2):240–246. doi: 10.1016/0167-4781(88)90088-7. [DOI] [PubMed] [Google Scholar]
- Gefter M., Hausmann R., Gold M., Hurwitz J. The enzymatic methylation of ribonucleic acid and deoxyribonucleic acid. X. Bacteriophage T3-induced S-adenosylmethionine cleavage. J Biol Chem. 1966 May 10;241(9):1995–2006. [PubMed] [Google Scholar]
- Guschlbauer W. The DNA and S-adenosylmethionine-binding regions of EcoDam and related methyltransferases. Gene. 1988 Dec 25;74(1):211–214. doi: 10.1016/0378-1119(88)90289-2. [DOI] [PubMed] [Google Scholar]
- Hattman S., Brooks J. E., Masurekar M. Sequence specificity of the P1 modification methylase (M.Eco P1) and the DNA methylase (M.Eco dam) controlled by the Escherichia coli dam gene. J Mol Biol. 1978 Dec 15;126(3):367–380. doi: 10.1016/0022-2836(78)90046-3. [DOI] [PubMed] [Google Scholar]
- Hattman S. DNA methylation of T-even bacteriophages and of their nonglucosylated mutants: its role in P1-directed restriction. Virology. 1970 Oct;42(2):359–367. doi: 10.1016/0042-6822(70)90279-5. [DOI] [PubMed] [Google Scholar]
- Hattman S., Wilkinson J., Swinton D., Schlagman S., Macdonald P. M., Mosig G. Common evolutionary origin of the phage T4 dam and host Escherichia coli dam DNA-adenine methyltransferase genes. J Bacteriol. 1985 Nov;164(2):932–937. doi: 10.1128/jb.164.2.932-937.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattman S., van Ormondt H., de Waard A. Sequence specificity of the wild-type dam+) and mutant (damh) forms of bacteriophage T2 DNA adenine methylase. J Mol Biol. 1978 Mar 5;119(3):361–376. doi: 10.1016/0022-2836(78)90219-x. [DOI] [PubMed] [Google Scholar]
- Herman G. E., Modrich P. Escherichia coli K-12 clones that overproduce dam methylase are hypermutable. J Bacteriol. 1981 Jan;145(1):644–646. doi: 10.1128/jb.145.1.644-646.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Levinson A., Silver D., Seed B. Minimal size plasmids containing an M13 origin for production of single-strand transducing particles. J Mol Appl Genet. 1984;2(6):507–517. [PubMed] [Google Scholar]
- Macdonald P. M., Mosig G. Regulation of a new bacteriophage T4 gene, 69, that spans an origin of DNA replication. EMBO J. 1984 Dec 1;3(12):2863–2871. doi: 10.1002/j.1460-2075.1984.tb02221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinus M. G., Morris N. R. Isolation of deoxyribonucleic acid methylase mutants of Escherichia coli K-12. J Bacteriol. 1973 Jun;114(3):1143–1150. doi: 10.1128/jb.114.3.1143-1150.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner Z., Hattman S. Molecular cloning, sequencing, and mapping of the bacteriophage T2 dam gene. J Bacteriol. 1988 Nov;170(11):5177–5184. doi: 10.1128/jb.170.11.5177-5184.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers R. M., Lerman L. S., Maniatis T. A general method for saturation mutagenesis of cloned DNA fragments. Science. 1985 Jul 19;229(4710):242–247. doi: 10.1126/science.2990046. [DOI] [PubMed] [Google Scholar]
- Normanly J., Masson J. M., Kleina L. G., Abelson J., Miller J. H. Construction of two Escherichia coli amber suppressor genes: tRNAPheCUA and tRNACysCUA. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6548–6552. doi: 10.1073/pnas.83.17.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revel H. R., Hattman S. M. Mutants of T2gt with altered DNA methylase activity: relation to restriction by prophage P1. Virology. 1971 Aug;45(2):484–495. doi: 10.1016/0042-6822(71)90348-5. [DOI] [PubMed] [Google Scholar]
- Revel H. R., Luria S. E. DNA-glucosylation in T-even phage: genetic determination and role in phagehost interaction. Annu Rev Genet. 1970;4(0):177–192. doi: 10.1146/annurev.ge.04.120170.001141. [DOI] [PubMed] [Google Scholar]
- Revel H. R. Restriction of nonglucosylated T-even bacteriophage: properties of permissive mutants of Escherichia coli B and K12. Virology. 1967 Apr;31(4):688–701. doi: 10.1016/0042-6822(67)90197-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlagman S. L., Hattman S., Marinus M. G. Direct role of the Escherichia coli Dam DNA methyltransferase in methylation-directed mismatch repair. J Bacteriol. 1986 Mar;165(3):896–900. doi: 10.1128/jb.165.3.896-900.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlagman S. L., Hattman S. Molecular cloning of a functional dam+ gene coding for phage T4 DNA adenine methylase. Gene. 1983 May-Jun;22(2-3):139–156. doi: 10.1016/0378-1119(83)90098-7. [DOI] [PubMed] [Google Scholar]
- Zengel J. M., Lindahl L. High-efficiency, temperature-sensitive suppression of amber mutations in Escherichia coli. J Bacteriol. 1981 Jan;145(1):459–465. doi: 10.1128/jb.145.1.459-465.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ormondt H., Gorter J., Havelaar K. J., de Waard A. Specificity of deoxyribonucleic acid transmethylase induced by bacteriophage T2. I. Nucleotide sequences isolated from tmicrococcus luteus DNA methylated in vitro. Nucleic Acids Res. 1975 Aug;2(8):1391–1400. doi: 10.1093/nar/2.8.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]



