Abstract
In obstructive sleep apnea patients, elevated activity of the lingual muscles during wakefulness protects the upper airway against occlusions. A possibly related form of respiratory neuroplasticity is present in rats exposed to acute and chronic intermittent hypoxia (CIH). Since rats exposed to CIH have increased density of noradrenergic terminals and increased α1-adrenoceptor immunoreactivity in the hypoglossal (XII) nucleus, we investigated whether these anatomic indexes of increased noradrenergic innervation translate to increased sensitivity of XII motoneurons to noradrenergic activation. Adult male Sprague-Dawley rats were subjected to CIH for 35 days, with O2 level varying between 24% and 7% with 180-s period for 10 h/day. They were then anesthetized, vagotomized, paralyzed, and artificially ventilated. The dorsal medulla was exposed, and phenylephrine (2 mM, 10 nl) and then the α1-adrenoceptor antagonist prazosin (0.2 mM, 3 × 40 nl) were microinjected into the XII nucleus while XII nerve activity (XIIa) was recorded. The area under integrated XIIa was measured before and at different times after microinjections. The excitatory effect of phenylephrine on XII motoneurons was similar in sham- and CIH-treated rats. In contrast, spontaneous XIIa was more profoundly reduced following prazosin injections in CIH- than sham-treated rats [to 21 ± 7% (SE) vs. 40 ± 8% of baseline, P < 0.05] without significant changes in central respiratory rate, arterial blood pressure, or heart rate. Thus, consistent with increased neuroanatomic measures of noradrenergic innervation of XII motoneurons following exposure to CIH, prazosin injections revealed a stronger endogenous noradrenergic excitatory drive to XII motoneurons in CIH- than sham-treated anesthetized rats.
Keywords: genioglossus, obstructive sleep apnea, norepinephrine, upper airway
long-term facilitation (LTF) is a form of respiratory neuroplasticity that manifests as a long-lasting increase of respiratory motor output following acute intermittent hypoxia or repeated stimulation of arterial chemoreceptors (for reviews see Refs. 26 and 28). In animal models, LTF following acute intermittent hypoxia can be observed in the motor output to respiratory pump muscles (17) and in upper airway motor nerves, such as the hypoglossal (XII) nerve, which innervates lingual muscles (16). Also, exposure to chronic intermittent hypoxia (CIH) enhances the magnitude of LTF (25, 34), an effect termed metaplasticity to indicate modulation of the acutely elicited LTF by prior experience (1).
Clinically significant respiratory adaptation occurs in obstructive sleep apnea (OSA) patients, who experience repetitive upper airway narrowing or collapse and hypoxic episodes that disrupt ventilation and sleep (8, 40). Specifically, OSA patients exhibit hyperactivity of upper airway muscles, including those of the tongue (22, 31, 52). This protects their upper airway against occlusions and allows for adequate ventilation during wakefulness. OSA patients also have increased ventilatory response following experimentally imposed acute hypoxia (20).
XII motoneurons innervate the genioglossus and other lingual muscles. In OSA patients, these muscles play an important role in the maintenance of upper airway patency (46) and have sleep-wake state-dependent activity that is maximal during wakefulness, reduced during non-rapid eye movement (REM) sleep, and minimal during REM sleep (10, 23, 32, 44, 58). This activity pattern is importantly driven by pontomedullary noradrenergic and serotonergic neurons; these neurons have axonal projections to the XII and other orofacial motor nuclei (3, 27, 43), and their activity is maximal during wakefulness and lowest or absent during REM sleep (4, 13, 42, 54). Indeed, data indicate that XII motoneurons are under a tonic noradrenergic excitatory drive in anesthetized and unanesthetized rats (7, 15) and that intermittent noradrenergic stimulation of XII motoneurons in vitro elicits their prolonged activation, similar to that elicited in vivo under anesthesia by acute intermittent hypoxia (36, 45).
A recent neuroanatomic study demonstrated that noradrenergic terminal density and the excitatory α1-adrenoceptor immunoreactivity are increased in the XII nucleus in rats exposed to CIH (41). This suggested that exposure to CIH may lead to increased sensitivity of XII motoneurons to noradrenergic inputs and, by implication, that CIH is one of the factors responsible for hyperactivity of upper airway muscles in OSA patients. Accordingly, the present experiments were undertaken to test the hypothesis that exposure to CIH results in increased sensitivity of XII motoneurons to exogenous and/or endogenous noradrenergic excitatory drive. We found that microinjections of phenylephrine (PE), an α1-adrenoceptor agonist, into the XII nucleus produced similar activation of XII nerve activity (XIIa) in anesthetized rats previously exposed to CIH and those exposed to sham treatment. However, microinjections of prazosin (PZ), an α1-adrenoceptor antagonist, resulted in larger decrements of spontaneous XIIa in CIH- than sham-treated rats. Thus our data suggest that exposure to CIH increases endogenous noradrenergic excitatory drive to XII motoneurons.
MATERIALS AND METHODS
Animals and administration of CIH.
Adult male Sprague-Dawley rats [308 ± 8 (SE) g body wt, n = 24] were obtained from Charles River Laboratories (Wilmington, MA). All animal procedures followed the guidelines of the National Institutes of Health (NIH Publication 80-23 with subsequent revisions) and were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania. The rats were housed, two to three per standard cage, under a 12:12-h light-dark cycle (lights on at 7 AM), with standard chow diet (5001/AIN76, Nestle Purina, St. Louis, MO) and water provided ad libitum. The cages were placed inside 28.5 × 30.0 × 51.5-cm chambers in which the O2 level was controlled by alternating flows of N2 and O2 (Oxycycler, Biospherix, Redfield, NY). Sham-treated rats were housed in adjacent chambers in which the flow of compressed room air occurred with the timing controlled by gas flows in one of the experimental chambers. The exposures lasted ∼35 (range 33–37) days. O2 levels oscillated between 24% and 7% with 180-s period for 10 h/day (7 AM–5 PM) (41). During the remaining time, all chambers were continuously ventilated with room air to prevent CO2 accumulation. Each animal was individually weighed when it was entered into the study, after the 1st day of exposure, and then every other day throughout the period of exposure to CIH or sham treatment. At 5 PM on the last day of exposure, each animal was transferred to a new cage and placed outside the chamber. The interval between the end of the last CIH exposure and the start of animal preparation for the acute experiment on the following day was ∼17 h.
Animal preparation for the acute experiment and the experimental protocol.
Rats were anesthetized with isoflurane (3%) followed by urethane (1.0 g/kg iv via a tail vein catheter) and tracheotomized, and a femoral artery and vein were catheterized for arterial blood pressure monitoring and fluid/drug injections, respectively. The medial branch of the right XII nerve was dissected and placed in a cuff-type recording electrode (modified after Ref. 12). Both cervical vagus nerves were cut to enhance XIIa and make it independent of mechanical ventilation. The animal's head was placed in a stereotaxic holder, and the dorsal surface of the caudal medulla was exposed for insertion of a microinjection pipette into the right XII nucleus. Rectal temperature was maintained at 37.0°C with a servo-controlled heating pad.
The animals were paralyzed with pancuronium bromide (1 mg/kg iv; Sigma, St. Louis, MO) and artificially ventilated with an air-O2 mixture (30–60% O2). The central respiratory drive was set by first ventilating the animals to the apneic threshold and then gradually reducing the tidal volume of the ventilator until a steady respiratory modulation of XIIa was established. The rate and volume of artificial ventilation were kept constant thereafter. Subsequently, a capnograph (Micro Capnometer, Columbus Instruments, Columbus, OH) was used to verify the stability of gas exchange by monitoring the end-expiratory CO2 level for the reminder of the experiment. In two rats, Pco2 in arterial blood (PaCO2) was measured when steady respiratory modulation of XIIa was established using the i-STAT 1 analyzer and i-STAT G3+ cartridge (Abbott Point of Care, Abbott Park, IL). In these two rats, PaCO2 levels were 52.7 and 53.1 Torr. Adequate level of anesthesia was verified based on stability of the amplitude and constant rate of inspiratory bursts recorded from the XII nerve, stable heart rate, and arterial blood pressure. If needed, supplemental doses of urethane were administered in 0.2–0.3 mg/kg increments. Adequate paralysis was maintained by continuous infusion of pancuronium bromide (0.6 mg·kg−1·h−1 iv).
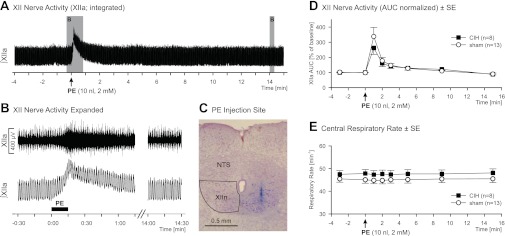
All drugs for microinjections were obtained from Sigma and diluted from frozen aliquots in 0.9% NaCl before each experiment. We used PE, an α1-adrenoceptor agonist (2 mM), and PZ, an α1-adrenoceptor antagonist (0.2 mM). A PE solution containing 2% pontamine sky blue dye (ICN Biomedicals, Aurora, OH) was used to mark the drug injection sites. The microinjections were made using glass pipettes pulled to obtain tip diameters of 20–25 μm (model 626800, A-M Systems, Carlsborg, WA). The drugs were injected by application of pressure to the fluid in the pipette, while the movement of the meniscus was monitored with a calibrated microscope with 1-nl resolution. For PE injections, the pipette was inserted into the ventral region of the caudal half of the right XII nucleus, 0.3 mm lateral to the midline, 0.15 mm rostral to the obex, and 1.15 mm below the dorsal medullary surface, because this region contains XII motoneurons that innervate the base of the tongue, including the genioglossus muscle, has the most dense noradrenergic innervation, and sends motor axons in the medial branch of the XII nerve (6, 41). After recording of baseline XIIa, the animals received up to three (1.8 on average) PE injections, 10 nl each, separated by ≥15-min intervals. PE has a relatively high Kd of ∼22 μM (33), which results in a relatively short-lasting activation of α1-adrenoceptors and allows multiple PE injections and independent observation of their effects. If more than one PE injection was made in an animal, the mean value for each measure of the effect was calculated prior to averaging across animals. All animals, 13 sham- and 11 CIH-treated rats, were used for PE injections. PE experiments in three CIH-treated rats were excluded from analysis for technical reasons. After completion of the 15-min observation period following the last PE injection and an additional interval of 37 ± 4 (SD) min, PZ was injected successively at three sites (40 nl each) targeting the XII nucleus at three rostrocaudal levels to antagonize α1-adrenoceptors in the entire XII nucleus in 10 sham- and 7 CIH-treated rats. The first injection was placed at the same coordinates used for the PE injections, followed by one injection 0.65 mm caudal and one injection 0.65 mm rostral to the first one (14, 15). The three PZ injections were completed within 8.0 ± 0.5 (SE) min. PZ has a low Kd of ∼0.002 μM (33), which results in long-lasting binding to α1-adrenoceptors. This and gradual diffusion of PZ allow one to observe summation of PZ effects following multiple injections at different rostrocaudal levels within the XII nucleus. Figure 1 shows the sequence of drug injections and observations/measurements collected before and after successive PE and PZ injections.
Fig. 1.
Experimental protocol and sequence of phenylephrine (PE) and prazosin (PZ) microinjections into the hypoglossal (XII) nucleus and data collection. Gray bars represent 1-min intervals during which mean XII nerve activity (area under the curve), central respiratory rate, blood pressure, and heart rate were measured before and after microinjections. Arrows indicate times of PE and PZ injections into the XII nucleus during the course of the experimental protocol.
At the conclusion of the experiment, the animal received an additional dose of urethane (1.0 g/kg) and was intra-arterially perfused with cold 0.9% saline followed by 10% formalin. The brain was extracted, postfixed, and cryoprotected in 30% sucrose, and 50-μm medullary sections were cut on a cryostat and collected sequentially. The sections containing the blue dye were mounted and stained with neutral red for localization of the injection sites.
Data acquisition and analysis.
The XII nerve recording electrode was connected to a differential amplifier (Neurolog 100, Digitimer, Hertfordshire, UK), and XIIa was amplified and filtered (150 Hz–3 kHz; Neurolog modules 104 and 126). Arterial blood pressure was measured using a pressure transducer (model P23Db, Statham, Hato Rey, Puerto Rico). The raw and integrated XIIa (100-ms time constant; MA-821RSP moving averager, CWE, Ardmore, PA), arterial blood pressure, end-expiratory CO2, rectal temperature, and event markers were digitized (Micro1401-3 data acquisition unit, Cambridge Electronic Design, Cambridge, UK) and stored on a computer (Spike-2 software version 7, Cambridge Electronic Design) using a sampling rate of 2 kHz for the raw XIIa and 100 Hz for all other signals. The effects of microinjections on XIIa were measured from integrated XIIa by calculation of the area under the curve (AUC) relative to the expiratory minimum prior to drug injections. XIIa was absent during expiration under the baseline conditions in both treatment groups. The instantaneous central respiratory rate and heart rate were automatically derived from integrated XIIa and arterial blood pressure, respectively.
Cardiorespiratory parameters (XIIa AUC, central respiratory rate, heart rate, and blood pressure) were calculated over a 4-min interval prior to each microinjection and also over 1-min intervals before and at different times after each microinjection. The data obtained from the 4-min interval were used to normalize XIIa AUC derived from 1-min intervals before and after each microinjection. For PE microinjections, measurements were taken at 4 and 1 min before and 1, 2, 3, 5, 9, and 15 min after injection. For PZ microinjections, measurements were taken at 4 and 1 min before and 10, 20, 30, 45, and 60 min after injection (Fig. 1). The different observation periods and different distributions of 1-min measurement periods reflect the longer duration of the effects of PZ than PE. The complete data set contained measures of XIIa and respiratory rate for all 1-min measurement periods before and at different times after PE and PZ injections from each rat. The corresponding mean arterial blood pressure and heart rate measurements were obtained from most rats, except in two sham- and three CIH-treated animals, in which they were not available because of arterial catheter blockage.
Statistical analysis.
Data analysis was performed with SigmaPlot 11.2 software (Systat Software, San Jose, CA). Normality of the distributions was tested using the Shapiro-Wilks test. Values are means ± SE, unless indicated otherwise.
In the case of normally distributed data sets, two-sided, unpaired Student's t-test was used for comparison of baseline cardiorespiratory parameters, integrated XIIa amplitudes, and latency and duration of PE effects between sham- and CIH-treated animals. In the case of a nonnormal distribution, the Mann-Whitney U-test was used, and, in addition to the mean ± SE, median and interquartile range (IQR) are indicated. Two-way repeated-measures ANOVA with Holm-Sidak post hoc analysis was used to evaluate the effects of treatments (CIH vs. sham) over time after drug injections (PE or PZ), except Kruskal-Wallis nonparametric ANOVA was applied to evaluate the differences of XIIa AUC between sham and CIH treatment following PE injections and one-way repeated-measures Friedman's ANOVA with Tukey's post hoc analysis was used to evaluate XIIa following PE injections vs. baseline, because these data sets were not normally distributed. P < 0.05 was considered significant.
RESULTS
We investigated the effect of CIH on noradrenergic activation of XII motoneurons by microinjecting PE and PZ into the XII nucleus in urethane-anesthetized, paralyzed, and artificially ventilated rats. The body weight of the rats did not differ at the beginning of CIH exposure and sham treatment [311 ± 14 g (n = 11) vs. 305 ± 10 g (n = 13), P = 0.729]. At the end of exposures, CIH-treated animals gained less weight than sham-treated animals (440 ± 13 vs. 482 ± 14 g, P = 0.027).
PE microinjections.
The effect of PE on XIIa was assessed in 13 sham- and 8 CIH-treated rats, each receiving up to three PE microinjections that targeted the ventral region of the caudal half of the XII nucleus (Fig. 2C). The control levels of XIIa prior to PE injections did not differ between sham- and CIH-treated animals. The XIIa AUC was 2.4 ± 0.5 V·min (median 1.8 V·min, IQR 1.0–3.3 V·min) in sham-treated rats and 1.8 ± 0.4 V·min (median 1.7 V·min, IQR 0.7–3.0 V·min, P = 0.69) in CIH-treated rats when measured in arbitrary units over the 4-min period prior to PE injections. During the same period, the mean amplitude of respiratory modulation measured from integrated XIIa was not different between sham- and CIH-treated rats: 0.12 ± 0.025 V (median 0.094 V, IQR 0.058–0.16 V) vs. 0.092 ± 0.020 V (median 0.082 V, IQR 0.036–0.15 V) (P = 0.49). The baseline central respiratory rate was 45.2 ± 1.5 and 47.6 ± 1.7 min−1 in sham- and CIH-treated rats, respectively (P = 0.31). Comparable magnitudes of XIIa and central respiratory rates under the baseline conditions indirectly point to similar CO2 levels in both treatment groups. The mean baseline arterial blood pressure and heart rate also were not different: 67.7 ± 4.7 and 70.4 ± 6.4 mmHg in sham- and CIH-treated rats, respectively (P = 0.72), and 426 ± 8 and 423 ± 11 min−1 in sham- and CIH-treated rats, respectively (P = 0.77).
Fig. 2.
Effects of PE injections into the XII nucleus in rats exposed to chronic intermittent hypoxia (CIH) and sham-treated rats. A: integrated XII nerve activity (∫XIIa) prior to and following PE injection in a sham-treated rat. B: expanded segments of raw and integrated XIIa corresponding to the shaded areas in A. C: PE injection site for the experiment illustrated in A. Pontamine sky blue dye was injected with PE and is seen in the right XII nucleus (XIIn) in a neutral red-stained coronal section. Nucleus of the solitary tract (NTS) is shown as an orientation landmark. D and E: mean effects of PE injections into the XII nucleus on XIIa area under the curve (AUC) and central respiratory rate in CIH- and sham-treated rats. Values (means ± SE) for each measurement period are plotted at the time corresponding to the end of that period. Excitatory effect of PE on XIIa was of similar magnitude in both groups, and injections did not affect central respiratory rate. XIIa was significantly increased compared with the baseline level up to min 3 following PE injection when both treatment groups were combined (Friedman's ANOVA, Tukey-corrected multiple comparisons, P < 0.05).
The PE-evoked changes of XIIa were expressed relative to the preinjection baseline. PE injections led to a rapid increase of XIIa in both treatment groups, with mostly the tonic component of XIIa increased and small changes in the amplitude of respiratory modulation of XIIa. This was followed by a gradual decrease of the tonic activation toward the baseline level within the subsequent 4–8 min (Fig. 2, A and B). The duration of PE effects was not different between the treatment groups, measured as the time from the onset of the effect to the point when XIIa returned to 25% of its peak increase: 175.9 ± 57.6 s (median 97.5 s, IQR 49.1–210.8 s) for sham-treated rats and 90.3 ± 21.4 s (median 64.8 s, IQR 57.2–146.1 s) for CIH-treated rats (P = 0.69). During the 1st min following PE injection, XIIa AUC reached 338 ± 60% of the preinjection level (median 246%, IQR 203–415%) in sham-treated rats and 262 ± 43% (median 247%, IQR 172–316%) in CIH-treated rats and was not different between the two groups (P = 0.61; Fig. 2D). The interval from the onset of PE injection to the peak of the PE effect was similar in both treatment groups: 13.5 ± 1.3 and 14.7 ± 1.9 s in sham- and CIH-treated rats, respectively (P = 0.57). The mean amplitude (including tonic and phasic XIIa) at the peak of the PE effect was increased to 228 ± 39% (median 175%, IQR 159–269%) in sham-treated rats and 216 ± 30% (median 237, IQR 124–280%) in CIH-treated rats (P = 0.97) relative to the baseline conditions. At the peak of the PE effect, the mean amplitude of phasic XIIa was 87 ± 6% and 101 ± 11% in sham- and CIH-treated rats, respectively (P = 0.23), relative to the baseline conditions, and the mean absolute amplitude of tonic XIIa was 0.14 ± 0.028 V (median 0.12 V, IQR 0.077–0.17 V) in sham-treated rats and 0.11 ± 0.039 V (median 0.083 V, IQR 0.026–0.16 V) in CIH-treated rats (P = 0.36). At 15 min after PE injection, XIIa was fully stabilized at a level slightly below the preinjection level (90 ± 6%, median 93%, IQR 80–102% in sham-treated rats and 88 ± 4%, median 90%, IQR 85–94% in CIH-treated rats, P = 0.59; Fig. 2D). PE injections into the XII nucleus did not have a significant effect on the central respiratory rate (Fig. 2E), arterial blood pressure, or heart rate (data not shown).
PZ microinjections.
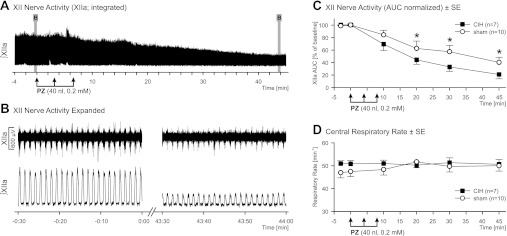
The effect of PZ on XIIa was assessed in 10 sham- and 7 CIH-treated rats, each receiving three 40-nl microinjections that targeted the ventral XII nucleus at three rostrocaudal levels.
The control measurements taken prior to PZ injections did not differ between sham- and CIH-treated animals. The XIIa AUC was 1.5 ± 0.3 V·min (median 1.2 V·min, IQR 0.7–1.9 V·min) in sham-treated rats and 1.3 ± 0.3 V·min (median 1.0 V·min, IQR 0.8–1.7 V·min, P = 0.66) in CIH-treated rats. The central respiratory rate was 47.3 ± 2.1 and 50.8 ± 1.3 min−1 in sham- and CIH-treated rats, respectively (P = 0.23). The mean baseline arterial blood pressure and heart rate also were not different: 69.6 ± 5.5 and 66.7 ± 6.7 mmHg in sham- and CIH-treated rats, respectively (P = 0.75), and 433 ± 9 and 439 ± 7 min−1 in sham- and CIH-treated animals, respectively (P = 0.63).
The PZ-evoked changes in XIIa were expressed as percentage of the preinjection level of activity. The three successive PZ injections led to a gradual decline of XIIa in both treatment groups (Fig. 3, A–C). In contrast, the expiratory level of integrated XIIa following PZ injections did not decline (Fig. 3A), which confirmed the absence of tonic XIIa during baseline conditions in either treatment group. The PZ effect was significant compared with baseline activity (4 or 1 min preinjection) from 20 min after injection for the sham-treated group and from 10 min after injection for the CIH group (P ≤ 0.002). The magnitude of XIIa decline was more pronounced in CIH- than sham-treated rats at 20 min after PZ injection and at all subsequent time points (Fig. 3C). The Holm-Sidak-corrected pair-wise multiple comparisons revealed significantly different XIIa AUC between sham- and CIH-treated animals at 20, 30, 45, and 60 min after PZ injection (P = 0.048–0.014). The stronger effect of PZ on XIIa in CIH- than sham-treated rats was also reflected in a significant treatment effect across all postinjection time points (F1,15 = 4.712, P = 0.046), and the treatment-by-time interaction was nearly statistically significant (F1,6,15 = 2.193, P = 0.052). Under the influence of PZ, XIIa AUC was reduced to 40.3 ± 8.3% and 21.0 ± 6.9% of the preinjection baseline in sham- and CIH-treated rats, respectively, when measured 45 min after PZ injections (P = 0.048; the latest time point shown in Fig. 3C) and to 32.3 ± 7.4% and 10.9 ± 5.1% in sham- and CIH-treated rats, respectively, when measured 60 min after PZ injections (P = 0.043). PZ injections did not significantly affect the central respiratory rate (Fig. 3D) or arterial blood pressure or heart rate (data not shown).
Fig. 3.
Effects of PZ injections in CIH- and sham-treated rats. A: integrated XIIa prior to and following 3 successive injections of 40 nl of PZ into the XII nucleus at 3 rostrocaudal levels in a CIH-treated rat. Transient fluctuations of XIIa prior to the 2nd and 3rd PZ injections are caused by insertions of the microinjection pipette into the XII nucleus and the resulting mechanical stimulation of XII motoneurons. B: expanded segments of raw and integrated XIIa corresponding to the shaded areas in A. C and D: mean effects of PZ injections into the XII nucleus on XIIa AUC and central respiratory rate in CIH- and sham-treated rats. Values (means ± SE) for each measurement period are plotted at the time corresponding to the end of that period. *Significantly different from CIH (P < 0.05). PZ injections did not elicit changes in central respiratory rate.
DISCUSSION
We investigated whether exposure to CIH alters sensitivity of XII motoneurons to noradrenergic stimulation and found that endogenous noradrenergic excitatory drive was stronger in XII motoneurons in CIH- than sham-treated rats when assessed by local microinjections of the α1-adrenoceptor antagonist PZ. We also found that the response to exogenously injected agonist (PE) into the XII nucleus was similar in CIH- and sham-treated rats. These results suggest that exposure to CIH enhances endogenous noradrenergic excitation of XII motoneurons. Extension of this result to the conditions in OSA patients leads us to suggest that the hyperactivity of upper airway muscles that characterizes this population may be caused, at least in part, by CIH, which represents a major component of OSA.
Noradrenergic activation of XII motoneurons following CIH.
Rats subjected to a CIH protocol similar to that used in the present study exhibit a prominent increase of noradrenergic terminal density and increased α1-adrenoceptor immunoreactivity in the XII nucleus (41). These findings suggest that CIH may also functionally alter noradrenergic effects on XII motoneurons. We found that microinjections of the α1-adrenoceptor antagonist PZ into the XII nucleus resulted in a significantly stronger suppression of XIIa in CIH- than sham-treated rats. Endogenous norepinephrine activates lingual muscle activity in wakefulness and, to a lesser extent, also in non-REM sleep, mainly via α1-adrenoceptors (7, 11, 56). As in our study, the α1-adrenoceptor antagonist PZ also reduced XIIa in urethane-anesthetized rats (15), but changes in the magnitude of endogenous noradrenergic activation of XII motoneurons resulting from the exposure to CIH have not been studied. Our present results show that the previously observed increase in anatomic measures of noradrenergic innervation of the XII nucleus (41) is associated with a stronger endogenous noradrenergic activation of XII motoneurons when tested under urethane anesthesia. The mechanisms underlying this enhancement may include an increased sensitivity of α1-adrenoceptors in CIH rats, increased level of activity in brain stem noradrenergic neurons that innervate the XII nucleus, or a combination of these effects.
To more directly address the issue of increased α1-adrenoceptor sensitivity in CIH rats, we also tested the excitatory effect of exogenous stimulation of α1-adrenoceptors in the XII nucleus with PE. Excitatory effects of PE on XII motoneurons have been previously reported in rats (2) and mice in vitro (19, 49) and in one in vivo study in rats (11). In our study, PE evoked a strong, mainly tonic, activation of XIIa that was consistent with those previous studies. However, in contrast to a clear difference between CIH- and sham-treated rats revealed by PZ injections, we did not detect a difference in the effects of PE between CIH- and sham-treated rats, despite our use of a rather large number of animals for this type of study (8 CIH- and 13 sham-treated rats). Therefore, we believe that the absence of a significant difference cannot be explained by a relatively high intersubject variability in the magnitude of the excitatory effect of PE. This result is not consistent with the possibility that α1-adrenoceptors are more sensitive, or more numerous, in CIH- than sham-treated rats. Three important variables that are unknown and could affect the outcome from PE injection experiments may explain the absence of a difference between CIH- and sham-treated rats. 1) The relative receptor occupancy could be higher in CIH- than sham-treated rats, thus effectively masking a potentially stronger effect of PE in CIH rats. Indeed, our result with PZ may be taken to suggest that noradrenergic cells are more active in CIH-treated rats, causing an increased occupancy of endogenous α1-adrenoceptors. A difference in receptor occupancy, if present, could result in different absolute baseline XIIa levels between the treatment groups as a result of different levels of respiratory drive in CIH- and sham-treated animals. This, however, could escape detection, because we set the baseline level of respiratory output in our animals relative to the apneic threshold by decreasing the level of mechanical ventilation from the apneic threshold until a steady respiratory modulation of XIIa was established, rather than by measuring CO2 levels at the apneic threshold and at baseline. On the basis of direct measurements conducted in two rats, this approach resulted in PaCO2 levels that differed by <0.5 Torr when baseline ventilation was set. Nevertheless, the absolute baseline CO2 levels were not measured in all rats, and we cannot fully exclude the possibility that they were different between the two treatment groups. Ultimately, it remains to be determined whether lingual muscle activity is elevated in spontaneously breathing and freely behaving CIH-exposed rats compared with sham-treated animals and whether the set points and/or slopes of their ventilatory CO2 curves differ. 2) Treatment-related differences in the rate of degradation and removal of exogenous PE may be another reason for similar effects of PE in both groups. There is no information on the effect of CIH on adrenergic transporters in the brain stem, but an increase in their activity following CIH could prevent detection of a stronger effect of PE in CIH-treated rats in our experimental setting. 3) The prior study suggesting increased α1-adrenoceptor immunoreactivity in XII motoneurons (41) did not differentiate between receptors bound to motoneuronal membrane and those located within the cytosol. Accordingly, the finding of increased α1-adrenoceptor immunoreactivity in CIH-treated rats could be indicative of stronger endogenous noradrenergic activation and increased turnover of α1-adrenoceptors, rather than an increased availability of membrane-bound fraction of these receptors (57, 59). Collectively, our present data with PZ and PE can be best explained by an increased endogenous noradrenergic activation of XII motoneurons in CIH-treated rats that occurs with or without altered responsiveness of α1-adrenoceptors on XII motoneurons.
While we have not detected a change in sensitivity of XII motoneurons to exogenous PE in CIH-exposed rats, serotonergic activation of XII motoneurons was reduced in CIH-exposed rats (55) and immunoreactivity for 5-HT2A receptors, the main receptor type that mediates serotonergic excitatory effects in XII motoneurons, tended to be reduced in the XII nucleus of rats subjected to CIH (41). So, in that case, the pharmacological and immunohistochemical study pointed to reduced responsiveness of XII motoneurons to 5-HT following exposure to CIH. Thus, it is plausible that CIH exerts different effects on the expression of different aminergic receptors, such as a downregulation of 5-HT2A receptors and no change or upregulation of α1-adrenoceptors. The ultimate functional consequences of these changes still depend on additional factors residing on the input side to motoneurons and within motoneurons.
In the context of our study, it is worth noting that acute intermittent hypoxia elicits a well-studied form of respiratory neuroplasticity, referred to as LTF, which leads, among other effects, to enhanced upper airway muscle activity (for reviews see Refs. 26, 28, 34). In vivo and in vitro studies show that hypoxia-induced LTF is dependent on stimulation of α1-adrenoceptors and 5-HT2 receptors (5, 18, 25, 30, 36). Furthermore, antagonism of α1-adrenoceptors by intravenous infusion of PZ blocks development of intermittent hypoxia-elicited LTF of XII motoneurons in rats in vivo (36). Thus, α1-adrenoceptors are important for the initiation and maintenance of LTF. In addition, after pretreatment with CIH, rats subjected to a few episodes of hypoxia exhibit larger LTF than naive rats (34). Recently, it has been also demonstrated in anesthetized rats that acute repeated manipulations with vagal input, with or without asphyxia, trigger a form of respiratory plasticity that also is dependent on α1-adrenoceptors (53). This expands the range of physiological and pathophysiological conditions under which the noradrenergic system facilitates occurrence of long-lasting changes in the motor output from the XII nucleus. In our study, we did not explore the magnitude of LTF elicited by CIH. Rather, we found that tonic noradrenergic activation of XII motoneurons is enhanced by CIH when measured nearly 24 h after the last exposure to CIH. Thus the effects of CIH on tonic noradrenergic activation of XII motoneurons that we observed many hours after cessation of the last exposure to CIH and the enhancement of LTF elicited by acute episodic hypoxia may represent two distinct phenomena that, nevertheless, depend on a common set of aminergic inputs to motoneurons.
In this study, we focused on the impact of CIH on noradrenergic transmission to, and noradrenergic activation of, XII motoneurons. Whether CIH-induced alterations of noradrenergic transmission similar to those observed in the XII nucleus in our study also occur in other motor nuclei and which aspects of CIH cause these alterations remain to be determined. Similar to the anatomic findings in the XII nucleus (41), noradrenergic terminal density is increased following CIH also in the spinal trigeminal sensory and trigeminal motor nuclei, albeit to a lesser extent (35). Different magnitudes of CIH-induced alteration of noradrenergic innervation may be due to different functions of motoneurons and different sources of their noradrenergic innervation. For example, XII motoneurons are strongly activated by PE and related compounds in vivo and in vitro (present study; 2, 19, 36), whereas trigeminal motoneurons are only weakly activated by PE in anesthetized rats, unless they also receive concomitant glutamatergic inputs (48). However, comparison of noradrenergic excitability of XII and trigeminal motoneurons is difficult, because noradrenergic effects were observed in XII motoneurons when they were rhythmically active (present study; 2, 19, 36), whereas trigeminal motoneurons were much less active or entirely silent (48). Stress is among the factors that may link CIH to increased noradrenergic transmission to XII motoneurons through its actions on noradrenergic cells mediated by the adrenocorticotropic hormone and glucocorticoids. For example, stress and elevated glucocorticoid levels increase expression of tyrosine hydroxylase and dopamine β-hydroxylase, two key enzymes in the synthesis of norepinephrine (9, 37, 50). However, whether and when CIH of moderate severity, such as that used in our study, is associated with stress and increased glucocorticoid levels is not clear. Gozal et al. (21) suggested that CIH causes stress only during the first few days of exposure based on only a transient occurrence of sleep fragmentation and altered sleep architecture. Direct measurements of corticosterone levels as a marker of stress during and following CIH are rare, and the results are contradictory. In one study, plasma corticosterone levels were not increased following 3 days of CIH, whereas the same duration of paradoxical sleep deprivation without CIH led to a significant increase of plasma corticosterone (39). Combination of paradoxical sleep deprivation with CIH did not result in an additional significant increase of the plasma corticosterone concentration (39). A second study reported moderately elevated plasma corticosterone levels following 35 days of exposure to a severe CIH protocol (60). There is also evidence that CIH-evoked increase of norepinephrine levels in the periphery is dependent on the hypoxia-inducible factor-1α, rather than the hypothalamic-pituitary-adrenal axis, because CIH-induced changes in plasma norepinephrine occurred in wild-type mice, but not in partially hypoxia-inducible factor-1α-deficient animals (38).
Relevance to OSA.
OSA patients commonly have structural abnormalities that result in narrowed upper airways and collapsible pharyngeal walls (8, 47). These conditions lead to repeated obstructive apneas and hypoventilation during sleep, when upper airway muscle activity decreases (reviewed in Refs. 8 and 24). However, during wakefulness, OSA patients maintain a fully patent upper airway. XII motoneurons innervate the genioglossus and other lingual muscles, and, in OSA patients, their activation helps maintain upper airway patency (40, 46). This is possible, because upper airway muscle activity is enhanced in OSA patients compared with healthy persons (22, 31, 52). Our results imply that CIH leads to enhanced endogenous noradrenergic drive to upper airway motoneurons, and this may contribute to increased upper airway muscle activity during wakefulness in OSA patients.
Despite the severe negative health impact of OSA, the availability of suitable experimental animal models with which to study the disorder is limited. Rodents exposed to intermittent hypoxia are most commonly used to study distinct mechanisms unique to OSA (8, 26, 29). Intermittent hypoxia effects in rodents importantly help understand OSA pathogenesis, but translation of the results to humans is difficult because of uncertainty regarding the quantitative relationships between different intermittent hypoxia protocols in rodents and OSA severity in humans, as well as any qualitative and/or quantitative differences in the neurochemical control of the upper airway and breathing in humans vs. rodents (for reviews see Refs. 8 and 29).
Conclusion.
Antagonism of α1-adrenoceptors in the XII nucleus revealed an increased endogenous noradrenergic excitatory drive to XII motoneurons in urethane-anesthetized rats previously exposed to CIH compared with sham-treated animals. This is consistent with data showing that rats subjected to CIH exhibit anatomic measures of an increased excitatory noradrenergic input to XII motoneurons (41). Our data suggest that CIH alone, without upper airway obstruction, can lead to upregulation of the endogenous noradrenergic excitatory drive to XII motoneurons. Accordingly, in OSA patients, CIH may contribute to the increased upper airway muscle tone during wakefulness that characterizes this patient group. Thus, CIH may activate an airway protective response.
GRANTS
The study was supported by National Heart, Lung, and Blood Institute Grant HL-047600 and a research fellowship from the Deutsche Forschungsgemeinschaft (DFG Ste1899/1-1) to G. M. Stettner.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
G.M.S., V.B.F., and L.K. are responsible for conception and design of the research; G.M.S., V.B.F., and L.K. performed the experiments; G.M.S. analyzed the data; G.M.S., V.B.F., and L.K. interpreted the results of the experiments; G.M.S. prepared the figures; G.M.S. and L.K. drafted the manuscript; G.M.S., V.B.F., and L.K. edited and revised the manuscript; G.M.S., V.B.F., and L.K. approved the final version of the manuscript.
ACKNOWLEDGMENTS
The authors thank Pari Mody for excellent technical assistance.
A preliminary report has been published in abstract form (51).
REFERENCES
- 1. Abraham WC, Tate WP. Metaplasticity: a new vista across the field of synaptic plasticity. Prog Neurobiol 52: 303– 323, 1997 [DOI] [PubMed] [Google Scholar]
- 2. Al-Zubaidy ZA, Erickson RL, Greer JJ. Serotonergic and noradrenergic effects on respiratory neural discharge in the medullary slice preparation of neonatal rats. Pflügers Arch 431: 942– 949, 1996 [DOI] [PubMed] [Google Scholar]
- 3. Aldes LD, Chapman ME, Chronister RB, Haycock JW. Sources of noradrenergic afferents to the hypoglossal nucleus in the rat. Brain Res Bull 29: 931– 942, 1992 [DOI] [PubMed] [Google Scholar]
- 4. Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci 1: 876– 886, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bocchiaro CM, Feldman JL. Synaptic activity-independent persistent plasticity in endogenously active mammalian motoneurons. Proc Natl Acad Sci USA 101: 4292– 4295, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brennick MJ, Trouard TP, Gmitro AF, Fregosi RF. MRI study of pharyngeal airway changes during stimulation of the hypoglossal nerve branches in rats. J Appl Physiol 90: 1373– 1384, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Chan E, Steenland HW, Liu H, Horner RL. Endogenous excitatory drive modulating respiratory muscle activity across sleep-wake states. Am J Respir Crit Care Med 174: 1264– 1273, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Dempsey JA, Veasey SC, Morgan BJ, O'Donnell CP. Pathophysiology of sleep apnea. Physiol Rev 90: 47– 112, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dent GW, Smith MA, Levine S. Stress-induced alterations in locus coeruleus gene expression during ontogeny. Brain Res Dev Brain Res 127: 23– 30, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Eckert DJ, Malhotra A, Lo YL, White DP, Jordan AS. The influence of obstructive sleep apnea and gender on genioglossus activity during rapid eye movement sleep. Chest 135: 957– 964, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fenik V, Davies RO, Kubin L. Adrenergic receptor subtypes mediating excitatory effects in hypoglossal motoneurons. Sleep 22 Suppl: S37, 1999 [Google Scholar]
- 12. Fenik V, Fenik P, Kubin L. A simple cuff electrode for nerve recording and stimulation in acute experiments on small animals. J Neurosci Methods 116: 147– 150, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Fenik V, Marchenko V, Janssen P, Davies RO, Kubin L. A5 cells are silenced when REM sleep-like signs are elicited by pontine carbachol. J Appl Physiol 93: 1448– 1456, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Fenik VB, Davies RO, Kubin L. Noradrenergic, serotonergic and GABAergic antagonists injected together into the XII nucleus abolish the REM sleep-like depression of hypoglossal motoneuronal activity. J Sleep Res 14: 419– 429, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Fenik VB, Davies RO, Kubin L. REM sleep-like atonia of hypoglossal (XII) motoneurons is caused by loss of noradrenergic and serotonergic inputs. Am J Respir Crit Care Med 172: 1322– 1330, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fuller DD. Episodic hypoxia induces long-term facilitation of neural drive to tongue protrudor and retractor muscles. J Appl Physiol 98: 1761– 1767, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Fuller DD, Bach KB, Baker TL, Kinkead R, Mitchell GS. Long term facilitation of phrenic motor output. Respir Physiol 121: 135– 146, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Fuller DD, Zabka AG, Baker TL, Mitchell GS. Phrenic long-term facilitation requires 5-HT receptor activation during but not following episodic hypoxia. J Appl Physiol 90: 2001– 2006, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Funk GD, Smith JC, Feldman JL. Development of thyrotropin-releasing hormone and norepinephrine potentiation of inspiratory-related hypoglossal motoneuron discharge in neonatal and juvenile mice in vitro. J Neurophysiol 72: 2538– 2541, 1994 [DOI] [PubMed] [Google Scholar]
- 20. Gerst DG, 3rd, Yokhana SS, Carney LM, Lee DS, Badr MS, Qureshi T, Anthouard MN, Mateika JH. The hypoxic ventilatory response and ventilatory long-term facilitation are altered by time of day and repeated daily exposure to intermittent hypoxia. J Appl Physiol 110: 15– 28, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gozal D, Daniel JM, Dohanich GP. Behavioral and anatomical correlates of chronic episodic hypoxia during sleep in the rat. J Neurosci 21: 2442– 2450, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Katz ES, White DP. Genioglossus activity in children with obstructive sleep apnea during wakefulness and sleep onset. Am J Respir Crit Care Med 168: 664– 670, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Katz ES, White DP. Genioglossus activity during sleep in normal control subjects and children with obstructive sleep apnea. Am J Respir Crit Care Med 170: 553– 560, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Kubin L, Davies RO. Mechanisms of airway hypotonia. In: Sleep Apnea. Pathogenesis, Diagnosis, and Treatment, edited by Pack AI. New York: Dekker, 2002, p. 99–154 [Google Scholar]
- 25. Ling L, Fuller DD, Bach KB, Kinkead R, Olson EB, Jr, Mitchell GS. Chronic intermittent hypoxia elicits serotonin-dependent plasticity in the central neural control of breathing. J Neurosci 21: 5381– 5388, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mahamed S, Mitchell GS. Is there a link between intermittent hypoxia-induced respiratory plasticity and obstructive sleep apnoea? Exp Physiol 92: 27– 37, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Manaker S, Tischler LJ. Origin of serotonergic afferents to the hypoglossal nucleus in the rat. J Comp Neurol 334: 466– 476, 1993 [DOI] [PubMed] [Google Scholar]
- 28. Mateika JH, Narwani G. Intermittent hypoxia and respiratory plasticity in humans and other animals: does exposure to intermittent hypoxia promote or mitigate sleep apnoea? Exp Physiol 94: 279– 296, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mateika JH, Sandhu KS. Experimental protocols and preparations to study respiratory long term facilitation. Respir Physiol Neurobiol 176: 1– 11, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McGuire M, Zhang Y, White DP, Ling L. Serotonin receptor subtypes required for ventilatory long-term facilitation and its enhancement after chronic intermittent hypoxia in awake rats. Am J Physiol Regul Integr Comp Physiol 286: R334– R341, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Mezzanotte WS, Tangel DJ, White DP. Waking genioglossal electromyogram in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanism). J Clin Invest 89: 1571– 1579, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mezzanotte WS, Tangel DJ, White DP. Influence of sleep onset on upper-airway muscle activity in apnea patients versus normal controls. Am J Respir Crit Care Med 153: 1880– 1887, 1996 [DOI] [PubMed] [Google Scholar]
- 33. Minneman KP. Binding properties of α1-adrenergic receptors in rat cerebral cortex: similarity to smooth muscle. J Pharmacol Exp Ther 227: 605– 612, 1983 [PubMed] [Google Scholar]
- 34. Mitchell GS, Johnson SM. Neuroplasticity in respiratory motor control. J Appl Physiol 94: 358– 374, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Mody P, Rukhadze I, Kubin L. Rats subjected to chronic-intermittent hypoxia have increased density of noradrenergic terminals in the trigeminal sensory and motor nuclei. Neurosci Lett 505: 176– 179, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Neverova NV, Saywell SA, Nashold LJ, Mitchell GS, Feldman JL. Episodic stimulation of α1-adrenoreceptors induces protein kinase C-dependent persistent changes in motoneuronal excitability. J Neurosci 27: 4435– 4442, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nunez C, Foldes A, Perez-Flores D, Garcia-Borron JC, Laorden ML, Kovacs KJ, Milanes MV. Elevated glucocorticoid levels are responsible for induction of tyrosine hydroxylase mRNA expression, phosphorylation, and enzyme activity in the nucleus of the solitary tract during morphine withdrawal. Endocrinology 150: 3118– 3127, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Peng YJ, Yuan G, Ramakrishnan D, Sharma SD, Bosch-Marce M, Kumar GK, Semenza GL, Prabhakar NR. Heterozygous HIF-1α deficiency impairs carotid body-mediated systemic responses and reactive oxygen species generation in mice exposed to intermittent hypoxia. J Physiol 577: 705– 716, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Perry JC, D'Almeida V, Antunes IB, Tufik S. Distinct behavioral and neurochemical alterations induced by intermittent hypoxia or paradoxical sleep deprivation in rats. Prog Neuropsychopharmacol Biol Psychiatry 32: 87– 94, 2008 [DOI] [PubMed] [Google Scholar]
- 40. Remmers JE, DeGroot WJ, Sauerland EK, Anch AM. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol 44: 931– 938, 1978 [DOI] [PubMed] [Google Scholar]
- 41. Rukhadze I, Fenik VB, Benincasa KE, Price A, Kubin L. Chronic intermittent hypoxia alters density of aminergic terminals and receptors in the hypoglossal motor nucleus. Am J Respir Crit Care Med 182: 1321– 1329, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rukhadze I, Fenik VB, Branconi JL, Kubin L. Fos expression in pontomedullary catecholaminergic cells following REM sleep-like episodes elicited by pontine carbachol in urethane-anesthetized rats. Neuroscience 152: 208– 222, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rukhadze I, Kubin L. Differential pontomedullary catecholaminergic projections to hypoglossal motor nucleus and viscerosensory nucleus of the solitary tract. J Chem Neuroanat 33: 23– 33, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Sauerland EK, Harper RM. The human tongue during sleep: electromyographic activity of the genioglossus muscle. Exp Neurol 51: 160– 170, 1976 [DOI] [PubMed] [Google Scholar]
- 45. Saywell SA, Babiec WE, Neverova NV, Feldman JL. Protein kinase G-dependent mechanisms modulate hypoglossal motoneuronal excitability and long-term facilitation. J Physiol 588: 4431– 4439, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schwartz AR, Eisele DW, Hari A, Testerman R, Erickson D, Smith PL. Electrical stimulation of the lingual musculature in obstructive sleep apnea. J Appl Physiol 81: 643– 652, 1996 [DOI] [PubMed] [Google Scholar]
- 47. Schwartz AR, Patil SP, Squier S, Schneider H, Kirkness JP, Smith PL. Obesity and upper airway control during sleep. J Appl Physiol 108: 430– 435, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schwarz PB, Yee N, Mir S, Peever JH. Noradrenaline triggers muscle tone by amplifying glutamate-driven excitation of somatic motoneurones in anaesthetized rats. J Physiol 586: 5787– 5802, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Selvaratnam SR, Parkis MA, Funk GD. Developmental modulation of mouse hypoglossal nerve inspiratory output in vitro by noradrenergic receptor agonists. Brain Res 805: 104– 115, 1998 [DOI] [PubMed] [Google Scholar]
- 50. Serova LI, Gueorguiev V, Cheng SY, Sabban EL. Adrenocorticotropic hormone elevates gene expression for catecholamine biosynthesis in rat superior cervical ganglia and locus coeruleus by an adrenal independent mechanism. Neuroscience 153: 1380– 1389, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Stettner GM, Fenik VB, Kubin L. Effect of chronic-intermittent hypoxia (CIH) on noradrenergic activation of hypoglossal (XII) motoneurons (Abstract). Sleep 34 Suppl: A52, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Suratt PM, McTier RF, Wilhoit SC. Upper airway muscle activation is augmented in patients with obstructive sleep apnea compared with that in normal subjects. Am Rev Respir Dis 137: 889– 894, 1988 [DOI] [PubMed] [Google Scholar]
- 53. Tadjalli A, Duffin J, Peever J. Identification of a novel form of noradrenergic-dependent respiratory motor plasticity triggered by vagal feedback. J Neurosci 30: 16886– 16895, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Trulson ME, Trulson VM. Activity of nucleus raphe pallidus neurons across the sleep-waking cycle in freely moving cats. Brain Res 237: 232– 237, 1982 [DOI] [PubMed] [Google Scholar]
- 55. Veasey SC, Zhan G, Fenik P, Pratico D. Long-term intermittent hypoxia: reduced excitatory hypoglossal nerve output. Am J Respir Crit Care Med 170: 665– 672, 2004 [DOI] [PubMed] [Google Scholar]
- 56. Volgin DV, Mackiewicz M, Kubin L. α1B Receptors are the main postsynaptic mediators of adrenergic excitation in brainstem motoneurons, a single-cell RT-PCR study. J Chem Neuroanat 22: 157– 166, 2001 [DOI] [PubMed] [Google Scholar]
- 57. Wise A, Lee TW, MacEwan DJ, Milligan G. Degradation of G11α/Gqα is accelerated by agonist occupancy of α1A/D, α1B, and α1C adrenergic receptors. J Biol Chem 270: 17196– 17203, 1995 [DOI] [PubMed] [Google Scholar]
- 58. Worsnop C, Kay A, Pierce R, Kim Y, Trinder J. Activity of respiratory pump and upper airway muscles during sleep onset. J Appl Physiol 85: 908– 920, 1998 [DOI] [PubMed] [Google Scholar]
- 59. Wu D, Katz A, Lee CH, Simon MI. Activation of phospholipase C by α1-adrenergic receptors is mediated by the α-subunits of Gq family. J Biol Chem 267: 25798– 25802, 1992 [PubMed] [Google Scholar]
- 60. Zoccal DB, Bonagamba LG, Antunes-Rodrigues J, Machado BH. Plasma corticosterone levels is elevated in rats submitted to chronic intermittent hypoxia. Auton Neurosci 134: 115– 117, 2007 [DOI] [PubMed] [Google Scholar]