Abstract
The R6/2 mouse is the most frequently used model for experimental and preclinical drug trials in Huntington's disease (HD). When the R6/2 mouse was first developed, it carried exon 1 of the huntingtin gene with ∼150 cytosine-adenine-guanine (CAG) repeats. The model presented with a rapid and aggressive phenotype that shared many features with the human condition and was particularly similar to juvenile HD. However, instability in the CAG repeat length due to different breeding practices has led to both decreases and increases in average CAG repeat lengths among colonies. Given the inverse relationship in human HD between CAG repeat length and age at onset and to a degree, the direct relationship with severity of disease, we have investigated the effect of altered CAG repeat length. Four lines, carrying ∼110, ∼160, ∼210, and ∼310 CAG repeats, were examined using a battery of tests designed to assess the basic R6/2 phenotype. These included electrophysiological properties of striatal medium-sized spiny neurons, motor activity, inclusion formation, and protein expression. The results showed an unpredicted, inverted “U-shaped” relationship between CAG repeat length and phenotype; increasing the CAG repeat length from 110 to 160 exacerbated the R6/2 phenotype, whereas further increases to 210 and 310 CAG repeats greatly ameliorated the phenotype. These findings demonstrate that the expected relationship between CAG repeat length and disease severity observed in humans is lost in the R6/2 mouse model and highlight the importance of CAG repeat-length determination in preclinical drug trials that use this model.
Keywords: electrophysiology, synaptic currents, NMDA receptors, aggregates, behavior
the r6/2 mouse was the first transgenic mouse carrying the human gene responsible for Huntington's disease (HD), and because of the rapidity and reproducibility of phenotypic progression, this mouse remains the most widely studied model of the disorder for both in vitro and in vivo experiments and for preclinical drug screening (Bates and Hockly 2003). When this mouse was first produced, the transgene included exon 1 of the human HD gene with ∼150 cytosine-adenine-guanine (CAG) repeats (Mangiarini et al. 1996). Since then, differences in breeding practices have led to alterations in average CAG repeat length among colonies. Given that R6/2 transgenic female mice are sterile (Mangiarini et al. 1996), colonies are commonly maintained through the male line, and similar to anticipation in HD families (Kremer et al. 1995), increases in average CAG repeats have been associated with paternal transmission in the mice (Mangiarini et al. 1997). To overcome the very short breeding window of the R6/2 mice, wild-type (WT) female mice with transplanted ovaries from R6/2 females also have been used to generate colonies. In these colonies, a decrease in average CAG repeat length has occurred.
Given the strong inverse relationship in HD patients between CAG repeat length and age of disease onset and to a certain extent, the direct relationship with severity of the disease (Harper and Jones 2002), we examined the impact of mean CAG repeat length on the R6/2 phenotype. A battery of tests—designed to assess motor function, electrophysiology of medium-sized spiny neurons (MSNs), previously shown to be altered in HD mouse models (Carter et al. 1999; Cepeda et al. 2003, 2007; Cummings et al. 2009; Davies et al. 1997; Scherzinger et al. 1997; Starling et al. 2005), inclusion formation, and protein expression—was used to examine in R6/2 mice the effects of CAG repeat lengths ranging from 97 to 345. A surprising, inverted “U-shaped” function of severity of phenotype became apparent, whereby the expected relationship was observed with repeats up to ∼160, but mice carrying over ∼210 repeats appeared to be protected and displayed a protracted period of phenotype development. Two recent studies reported ameliorative effects of highly expanded CAG repeats on the R6/2 phenotype (Dragatsis et al. 2009; Morton et al. 2009). However, no study thus far has examined electrophysiological changes and if amelioration of behavioral and pathological alterations is associated with improvement of electrophysiological indices.
MATERIALS AND METHODS
Animals
All procedures were performed in accordance with the U.S. Public Health Service Guide for Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the University of California, Los Angeles (UCLA). Mice were obtained from four separate R6/2 breeding colonies at UCLA. R6/2 colonies were maintained by crosses between WT C57BL6xCBA-F1 mice, supplied by The Jackson Laboratory (Bar Harbor, ME), and either transgenic R6/2 males or R6/2 ovary-transplanted WT females, carrying one of four different, average CAG repeats. Transgenic breeding animals for the first line (CAG110) were R6/2 ovary-transplanted WT mice obtained from The Jackson Laboratory (formerly line 2810, now renamed line 6494). This colony had a CAG repeat length of 111 ± 5.5 (mean ± SD) across all transgenic mice (n = 310) in this line during the experimental period. Transgenic breeding mice for the second colony (CAG160) were again R6/2 ovary-transplanted WTs supplied by The Jackson Laboratory (new line 2810), and this colony had a CAG repeat length of 161.5 ± 5.4 across all transgenic mice in the colony during the experimental period (n = 94). Transgenic founding animals for the third line (CAG210) were a gift from Gillian Bates (Department of Medical and Molecular Genetics, King's College London, UK), and male transgenic animals from our colony were used for breeding. This line had 214 ± 8.3 CAG repeats across all transgenic animals in this colony during the experimental period (n = 252). The fourth colony (CAG310) was founded initially from mice supplied by The Jackson Laboratory (line 4601), and subsequently, males were used for breeding. This line had a CAG repeat length of 315 ± 17.9 across all transgenic animals in the colony during the experimental period (n = 256). The four lines of mice will be referred to by approximate CAG repeat lengths: CAG110, CAG160, CAG210, and CAG310, respectively.
Genotyping was performed using PCR of DNA obtained from tail samples, once at weaning and again following use for experimentation to confirm the genotype. CAG repeat lengths were also obtained for each transgenic animal, and these were determined by Laragen (Culver City, CA). Unless stated otherwise, data from both males and females were combined.
All of the four lines of mice were tested on each parameter for up to 80–90 days of age. At that age, CAG110 and CAG160 mice had lost weight, and much of the behavioral testing could not be performed. Therefore, we did not systematically test all mice after this age. For some of the analyses, we examined CAG210 and CAG310 mice up to 120 days.
Electrophysiology in Brain Slices
Mice were anesthetized with halothane and then decapitated and the brain rapidly removed to ice-cold dissection artificial cerebrospinal fluid (ACSF) containing (in mM): 130 NaCl, 3 KCl, 26 NaHCO3, 1.25 NaHPO4, 10 glucose, 5 MgCl2, 1 CaCl2 (pH 7.2; aerated with 95% O2/5% CO2; 290–300 mOsm/l). The brain was trimmed using a razor blade and glued to the stage of a vibrating microtome (Model VT1000 S, Leica Microsystems, Buffalo Grove, IL), and 350 μm coronal slices, which included dorsolateral striatum, were cut.
Following preparation, slices were stored in oxygenated ACSF (same composition as dissection ACSF, except MgCl2 and CaCl2 were 2 mM) for at least 1 h prior to experimentation. A single slice was then transferred to a submerged recording chamber fixed to an upright microscope (BX50WI, Olympus, Center Valley, PA). Whole-cell, patch-clamp recordings were obtained from MSNs visualized in the dorsolateral striatum using infrared illumination with differential interference contrast optics (i.e., IR-DIC microscopy) and identified by somatic size and basic membrane properties. The patch pipette (4–6 MΩ) was filled with cesium methanesulfonate solution containing (in mM): 125 cesium methanesulfonate, 4 NaCl, 3 KCl, 1 MgCl2, 9 EGTA, 8 HEPES, 5 MgATP, 1 TrisGTP, 10 disodium phosphocreatine, 0.1 leupeptin (pH 7.2; 270–280 mOsm/l). Upon obtaining the whole-cell configuration and stabilization of the cell, basic membrane properties were recorded in voltage-clamp mode at a membrane holding potential (Vhold) of −70 mV. Only data from cells with access resistance values <30 MΩ, and which did not vary >20% during the course of the experiment, were included. Membrane capacitances and input resistances were measured by applying a 10-mV depolarizing step voltage command and using the membrane test function integrated in pClamp 8.2 (Axon Instruments, Molecular Devices, Sunnyvale, CA).
Spontaneous excitatory postsynaptic currents (EPSCs) were recorded in gap-free mode. Membrane current was filtered at 1 kHz and digitized at 100 μs. The GABAA receptor antagonist bicuculline methobromide (BIC; 20 μM) was applied to block inhibitory currents mediated by GABAA receptors. To assess spontaneous inhibitory postsynaptic currents (IPSCs) before BIC was applied, membranes were stepped to +10 mV to minimize glutamate receptor-mediated currents and maximize GABAA receptor-mediated currents. To optimize collection of data, ionotropic glutamate receptors were not blocked. Whereas some of the currents recorded in this condition may have been mediated by glutamate receptors, we have found their contribution to be negligible. In addition, BIC blocked all spontaneous activity. Spontaneous synaptic currents were analyzed offline using the Mini Analysis Program (Synaptosoft, Fort Lee, NJ). Event counts were done by experimenters blind to genotype. Threshold for event detection (5 pA for EPSCs; 10 pA for IPSCs) was set above root-mean-square noise (1–2 pA at Vhold = −70 mV; 2–3 pA at Vhold = +10 mV).
Electrophysiology in Isolated Cells
Some slices were also used to record from acutely isolated MSNs. With the aid of a dissecting microscope, the dorsal striatum was dissected and incubated for at least 1 h at room temperature in a NaHCO3-buffered Earle's balanced salts solution (Sigma-Aldrich, St. Louis, MO), supplemented with (in mM): 1 pyruvic acid, 0.005 glutathione, 0.1 NG-nitro-l-arginine, and 1 kynurenic acid (pH 7.4; aerated with 95% O2/5% CO2; 300–310 mOsm/l). After incubation, a slice was placed in an oxygenated cell-stir chamber (Wheaton Industries, Millville, NJ), enzymatically treated for 20 min with papain (0.5 mg/ml; Calbiochem, La Jolla, CA) at 35°C in a HEPES-buffered HBSS (Sigma-Aldrich), and supplemented as described above. After enzymatic digestion, the tissue was rinsed with low Ca2+ HEPES-buffered Na+ isethionate solution containing (in mM): 140 Na+ isethionate, 2 KCl, 2 MgCl2, 0.1 CaCl2, 23 glucose, and 15 HEPES (pH 7.4; 300–310 mOsm/l). Striatal slices were then mechanically dissociated with a series of graded, fire-polished Pasteur pipettes. The cell suspension was plated into a 35-mm Nunclon Petri dish containing a HEPES-buffered salt solution (in mM: 140 NaCl, 23 glucose, 15 HEPES, 2 KCl, 2 MgCl2, 1 CaCl2, pH 7.4; 300–310 mOsm/l) and mounted on the stage of an upright, fixed-stage microscope (Axio Scope, Zeiss, Thornwood, NY).
The whole-cell patch-clamp technique in voltage-clamp mode was used for recordings. Signals were detected with an Axoclamp 2B amplifier (Axon Instruments, Molecular Devices). Glass pipettes (2.5–3.5 MΩ) were filled with an internal solution consisting of (in mM) 175 N-methyl-d-glucamine, 40 HEPES, 2 MgCl2, 10 EGTA, 12 phosphocreatine, 2 Na2ATP, 0.2 Na2GTP, and 0.1 leupeptin (pH 7.25; 265–270 mOsm/l). The Mg2+-free external solution consisted of the following (in mM): 135 NaCl, 20 CsCl, 3 BaCl2, 2 CaCl2, 10 glucose, 10 HEPES, 0.02 glycine, 0.0003 TTX (pH 7.4; 300–310 mOsm/l). After obtaining a GΩ seal and rupturing the membrane to create the whole-cell configuration, access resistances were <20 MΩ. Series resistance was then compensated (70–90%) and monitored throughout the experiment.
Drugs were applied through an array of capillaries positioned 500–600 μm from the cell using a pressure-driven fast perfusion system. Solution changes were performed by changing the position of the array with a direct current drive system controlled by a SF-77B perfusion system (Warner Instruments, Hamden, CT). N-methyl-d-aspartate (NMDA) was applied for 3 s every 30 s. Responsiveness of cells to 100 μM NMDA in the absence or presence of 50 μM Mg2+ was examined at a Vhold of −70 mV. The NMDA receptor-selective antagonist 2-amino-5-phosphonovalerate (AP5) was applied to confirm the specificity of NMDA-induced currents (data not shown). Rundown of the NMDA current was negligible during the time frame of experiments. Values for peak currents and current densities were calculated for all neurons in the absence and presence of Mg2+ (50 μM). Current densities were normalized to cell size by dividing the peak current by the cell capacitance.
Body Weights and Hind-Limb Clasping
Body weights were assessed in all WT and R6/2 mice. Each mouse was weighed from weaning until euthanasia. For these data, mice were separated by gender. Weights were then pooled by age into 10-day intervals. In parallel, the presence of hind-limb clasping, which has been reported in these and other HD mice, was assessed. A mouse was considered to display hindlimb clasping if the hindlimbs were pulled into the body and clasped by the forelimbs. Mice were considered positive for clasping if this position was assumed in more than three of 10 30-s trials (15-s intertrial interval). The proportion of mice clasping was then calculated/10-day age interval.
Rotarod
Motor coordination and balance were assessed using an accelerating rotarod (Ugo Basile, Varese, Italy). The same groups of mice were tested at 30, 40, 60, and 80 days of age. Experimenters were blind to genotype for the rotarod and pole-test paradigms. The latency for each mouse to fall from the accelerating rotarod (four to 40 revolutions/min over 10 min) was recorded for two trials at each age and the average latency to fall calculated. Occasionally, a mouse held on to the apparatus. If three complete turns were made while the mouse held on, it was considered to have stopped running, and that time was recorded.
Pole-Test
The same mice tested in the rotarod paradigm were also tested using a vertical pole-test (1 cm in diameter; 60 cm high). The training phase consisted of two “head-down” trials (no rest periods), in which mice were placed at the top of the pole, facing downward, and allowed to descend the pole. Two consecutive training phases, separated by 5-min intervals, were performed on the same day as testing. The testing phase consisted of five “head-up” trials, in which mice were placed at the top of the pole, facing upward, and the time taken to turn, time taken to descend, and total time taken to complete the task were recorded. Trials were separated by 5-min periods. In the CAG310 cohort, there were a number of WT and transgenic mice that performed poorly on this test. Data from mice with latencies >2 SD from the mean were excluded as outliers (Grubbs' test).
Seizure Susceptibility
To assess the susceptibility of R6/2 mice to seizures, the GABAA receptor antagonist picrotoxin (PTX) was injected intraperitoneally (4 mg/kg), and latency to the first tonic-clonic seizure was recorded. Previous results obtained from R6/2 mice in our laboratory (Cummings et al. 2009) have shown that inconsistent data are obtained on this test using female mice, and therefore, only males at 40 days of age were used. They were tested in pairs (R6/2 and appropriate WT littermate) from each of the four lines. Mice were placed in a transparent plexiglass chamber (15” × 8” × 7”; separated into two equal compartments) and allowed to habituate for >10 min. PTX (prepared fresh daily in buffered physiological saline; pH 7.4) was injected by an experimenter blind to genotype. Experiments were videotaped for rescoring of the latency-to-key stages of seizures using the Racine scale (Racine 1972). Upon onset of a tonic-clonic seizure, mice were euthanized with halothane followed by decapitation.
Immunohistochemistry
The EM48 antibody, which recognizes the first 256 amino acids of the N-terminus of huntingtin (htt), was used to visualize mutant htt within the striatum of each of the lines of R6/2 mice. Briefly, animals were anesthetized and transcardially perfused with 4% paraformaldehyde in 0.1 M PBS. Brains were removed, post fixed for 16 h in 4% paraformaldehyde, transferred to 30% sucrose in 0.1 M PBS at 4°C for 24 h, mounted with Tissue-Tek OCT, and frozen with powdered dry ice, and 20 μm coronal sections were cut using a cryostat (HM500 OM, Microm International, Thermo Fisher Scientific, Waldorf, Germany). Coronal sections were then histochemically treated as free-floating sections to examine the expression of human htt protein. Sections were washed in PBS and then in a 1% H2O2/0.5% Triton X-100 in PBS solution for 30 min to block endogenous peroxidase activity and to permeabilize membranes. Nonspecific binding then was blocked using 10% normal goat serum for 30 min. Sections were incubated in the primary antibody [1:100 EM48 (Chemicon International, Temecula, CA); diluted in PBS containing 2% normal goat serum and 0.2% Triton X-100] overnight at room temperature. Control sections were incubated in mouse IgG (1:100 in the same diluents as the primary) and processed in parallel. Sections then were washed for 30 min in PBS and incubated in the secondary antibody [biotinylated goat anti-mouse IgG (1:400; Chemicon International)] for 2 h. The avidin-biotin complex (ABC) method was used per the manufacturer's directions to detect the secondary antibody (Elite ABC Kit, Vector Laboratories, Burlingame, CA). Immunoreactivity was visualized by incubation in 0.03% 3,3′-diaminobenzidine tetrachloride (Sigma-Aldrich) and 0.0006% H2O2 in 0.1 M Tris buffer (pH 7.6). The sections then were rinsed in Tris buffer, mounted, dehydrated in a series of ethanols (three washes in 95%, followed by three washes in 100% ethanol), cleared through xylene, and coverslipped. A single slice/area of interest/animal/group was examined using Stereo Investigator 5.00 software (MicroBrightField, Williston, VT). In each section, the striatum was outlined using the software at 5× magnification. A counting frame with a volume of 1,800 μm3 was examined in each section, and the number of diffusely stained nuclei, number of nuclei containing microaggregates (identified as small, usually ovoid bodies within nuclei), number of neuronal intranuclear inclusions (NIIs; identified as large, usually spherical bodies within nuclei), and number of neuropil aggregates (extranuclear bodies) were counted at a 100× magnification [1.4 numerical aperture (NA) lens; 1.4 NA oil condenser; DM LB, Leica Microsystems] with a real-time digital camera (DVC 1310C). Density (per μm3) of each protein conformation was calculated in each of the three regions of the striatum (anterior, medial, and posterior). Significant differences were not observed among these regions, an average density was calculated from the three slices for each animal, and mean ± SE calculated from a total of five animals from each line.
Immunoblotting
Individual striata from R6/2 mice from each line and age-matched WT littermates at 21 days (S830) and 40 days [mouse EM48 (mEM48)] were dissected and frozen at −80°C. Whole-cell homogenates were prepared from striatal tissue, and samples (50 μg total protein) were resolved by SDS-PAGE (5% stacking gel/12% resolving gel) and Western immunoblotting, as described previously (Watson et al. 2009). Polyvinylidene fluoride membranes were probed with a primary mouse MAb, mEM48 (1:400 dilution; Millipore, Billerica, MA), which preferentially recognizes human htt aggregates (Zhou et al. 2003) or a sheep polyclonal antibody, S830 (1:1,500 dilution; gift from Gillian Bates, Department of Medical and Molecular Genetics, King's College London), selective for both aggregated and soluble exon 1 human htt protein (Benn et al. 2005). A β-actin mouse MAb (1:10,000 dilution; Sigma-Aldrich) was used as a loading control. Membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (anti-mouse for monoclonals, anti-goat for S830 antibody; 1:10,000 dilution), and bound antibodies were visualized by ECL-Plus fluorescence (GE Healthcare Life Sciences, Piscataway, NJ). Fluorescent images were acquired using the Typhoon 9410 imaging system (GE Healthcare Life Sciences) in the UCLA Biological Chemistry Imaging Facility. Quantitative analyses were performed with ImageQuant 5.2 software by Molecular Dynamics (Sunnyvale, CA). For quantification, density values (total pixel volume) for htt bands were normalized to density values for β-actin controls obtained in the same lane of the identical blot.
Statistical Analyses
Unless stated otherwise, values in figures and text are presented as mean ± SE. Differences among group means were assessed with appropriately designed ANOVA (one- or two-way with repeated measures), followed by Fisher's least significant difference post hoc test, only if a significant P value (P ≤ 0.05) occurred. Proportions were assessed with Z-tests. All statistical analyses were performed using SigmaPlot software (v2.03).
RESULTS
Membrane Properties and Spontaneous Postsynaptic Currents of MSNs in Slices
Previously, we and others (Ariano et al. 2005; Cepeda et al. 2003; Klapstein et al. 2001; Rossi et al. 2006) reported a lower membrane capacitance and higher input resistance in R6/2 MSNs, as well as a progressive reduction in frequency of spontaneous EPSCs. In contrast, higher frequency (HF) of spontaneous IPSCs occurred in a population of R6/2 MSNs (Cepeda et al. 2004). We assessed each of these parameters in the four groups of R6/2 mice and their respective WTs. There were no differences in any of the electrophysiological measures among the WTs, and data were pooled into one WT group for each measure. We found a CAG repeat dependency for each of the phenotypes at 40 and 80 days of age. As the greatest dysfunctions were consistently observed in MSNs of the CAG160 line, we also assessed each of the parameters at 21 days in this line. As no significant differences were detected, MSNs from mice with other CAG repeat lengths were not examined at 21 days.
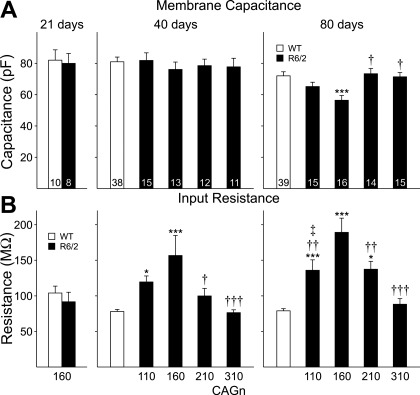
Basic membrane properties were measured with the membrane potential voltage clamped at −70 mV (Fig. 1). Cell capacitance (Fig. 1A) was similar to that of WTs in all R6/2 MSNs at 21 and 40 days but was reduced with respect to WTs at 80 days in MSNs of CAG160 (P < 0.001) but not CAG210 or CAG310 mice (Fig. 1A). There was a trend that failed to reach significance (P = 0.06) for capacitance to be reduced in CAG110 mice compared with WTs. The decrease in capacitance in the CAG160 group was also significantly different from values in CAG210 (P < 0.02) and CAG310 (P < 0.001) groups.
Fig. 1.
Cell membrane capacitance (A) and input resistance (B) for medium-sized spiny neurons (MSNs) recorded in acute slices prepared from R6/2s and pooled wild-types (WTs) at 21, 40, and 80 days of age. *P < 0.05, ***P < 0.001 with respect to WTs; †P < 0.05, ††P < 0.01, †††P < 0.001 with respect to cytosine-adenine-guanine (CAG)160; ‡P < 0.05 with respect to CAG310. In Figs. 1–5, numbers within bars indicate the number of cells.
Input resistance (Fig. 1B) was similar to that of WTs at 21 days but increased at 40 and 80 days of age. At 40 days, the largest increase occurred in MSNs from CAG160 mice (P < 0.001 vs. WTs; P < 0.02 vs. CAG210; P < 0.001 vs. CAG310). The increase in input resistance in CAG110 was significantly greater than that of WTs (P < 0.01). MSNs from CAG210 mice showed a slight increase, but this failed to reach statistical significance. At 80 days, CAG160 MSNs again displayed the highest input resistances (P < 0.001 vs. WTs; P < 0.05 vs. CAG110; P < 0.05 vs. CAG210; P < 0.001 vs. CAG310), whereas CAG110 (P < 0.001 vs. WT; P = 0.04 vs. CAG310) and CAG210 (P < 0.001 vs. WT; P = 0.036 vs. CAG310) MSNs showed intermediate increases. MSNs of the CAG310 line displayed no significant changes in input resistance at any age tested. No difference was identified for membrane time constant among MSNs of R6/2 mice with each repeat length and their WT controls (data not shown).
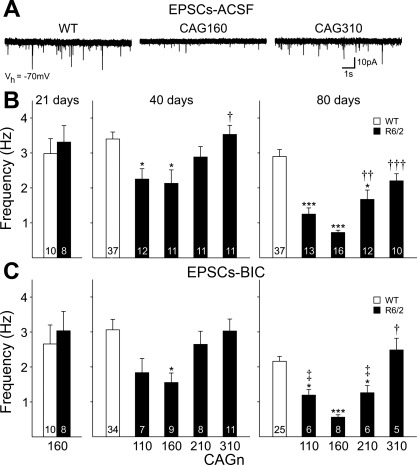
Spontaneous EPSCs were recorded with the membrane potential voltage clamped at −70 mV (Fig. 2). At 21 days, EPSC frequency of MSNs from CAG160 mice was similar to that of MSNs from WTs. By 40 days, significant reductions in frequencies were observed in MSNs of CAG160 (P < 0.05 vs. WTs; P < 0.05 vs. CAG310) and CAG110 (P < 0.05 vs. WTs) but not CAG210 or CAG310 mice. At 80 days, MSNs of CAG160 mice showed the greatest reduction in spontaneous EPSC frequency (P < 0.001 vs. WTs; P < 0.001 vs. CAG310), whereas MSNs of CAG110 and CAG210 mice showed an intermediate reduction in frequency (P < 0.001 CAG110 vs. WTs; P < 0.05 CAG210 vs. WTs). Frequencies of spontaneous EPSCs in MSNs of both CAG210 (P < 0.01) and CAG310 (P < 0.001) were also significantly different from CAG160 at this age.
Fig. 2.
A: typical traces recorded in gap-free mode at −70 mV membrane holding potential [Vhold (Vh)] in standard artificial cerebrospinal fluid (ACSF). EPSC, excitatory postsynaptic current. Traces were obtained from WT, CAG160, and CAG310 R6/2 mice at 80 days. B: bar graphs show mean (±SE) spontaneous EPSC frequency recorded in standard ACSF for each group at the ages indicated. C: bar graphs show mean (±SE) spontaneous EPSC frequency recorded in standard ACSF with 20 μM bicuculline methobromide (BIC) for each group at the ages indicated. At 21 days, in both B and C, data were obtained only from the CAG160 line and their WTs. *P < 0.05, ***P < 0.001 with respect to WT; †P < 0.05, ††P < 0.01, †††P < 0.001 with respect to CAG160; ‡P < 0.05 with respect to CAG310.
Previously, we showed that when cell membranes were voltage clamped at −70 mV, ∼10–20% of spontaneous currents were due to activation of GABAA receptors (Cepeda et al. 2003). To isolate spontaneous EPSCs, after recording spontaneous IPSCs (see below), membrane potential was returned to −70 mV, and the GABAA receptor antagonist BIC (20 μM) was applied (Fig. 2C). The addition of BIC reduced PSC frequencies in all groups of MSNs. The differences in spontaneous EPSC frequency between R6/2 MSNs and their respective WTs in standard ACSF generally were maintained when BIC was present. At 40 days, the largest decrease still occurred in MSNs from the CAG160 line (P < 0.05 vs. WTs). At 80 days, MSNs from CAG160 mice still had the lowest frequency (LF; P < 0.001 vs. WTs; P < 0.001 vs. CAG310), followed by CAG110 (P < 0.05 vs. WT; P < 0.01 vs. CAG310) and CAG210 (P < 0.05 vs. WT; P < 0.05 vs. CAG310) lines.
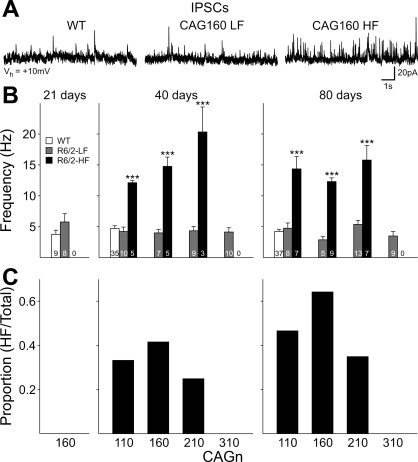
Spontaneous IPSCs were recorded at a membrane Vhold of +10 mV (Fig. 3). Although the ionotropic glutamatergic receptor antagonists 6-cyano-7-nitroquinoxaline-2,3-dione and AP5 were not used, the contributions of glutamate receptor-mediated currents are minimized at this Vhold. At 21 days, spontaneous IPSC frequency tended to be slightly higher in CAG160 MSNs compared with WT, but this failed to reach statistical significance (P = 0.17). We previously described two populations of cells in R6/2s, ≥40 days of age (Cepeda et al. 2004). The first population displayed a LF of spontaneous IPSCs and was statistically identical to WTs, and the second displayed HF spontaneous IPSCs. HF IPSCs were never observed in WT mice. At both 40 and 80 days, different subgroups of MSNs from CAG110, 160, and 210 lines displayed LF or HF IPSC properties. At both ages, LF cells from each line displayed similar frequencies of spontaneous IPSCs as WTs. HF MSNs from each line displayed significantly higher frequencies than WTs or LF MSNs at each age (40 and 80 days: P < 0.001 CAG110, CAG160, CAG210 vs. WT). At 40 days, only the mean frequency for IPSCs obtained from MSNs of the CAG210 line was also significantly higher than IPSCs from the CAG110 line (P = 0.003). The proportion of cells identified as HF differed with CAG repeat length (Fig. 3C). At both 40 and 80 days, HF IPSC cells were most common in the CAG160 group and occurred less frequently in CAG110 and CAG210 MSNs. HF IPSCs were never observed in MSNs from CAG310 mice.
Fig. 3.
A: typical traces recorded in gap-free mode at +10 mV membrane Vhold in standard ACSF for MSNs with low-frequency (LF) and high-frequency (HF) inhibitory postsynaptic currents (IPSCs) from CAG160 R6/2s at 80 days and an age-matched WT. B: bar graphs show mean (±SE) spontaneous IPSC frequency for LF and HF cells for each CAG repeat line at the ages indicated. At 21 days, data were obtained only from the CAG160 line and their WTs. ***P < 0.001 with respect to WT. C: proportion of HF cells in each CAG repeat line.
Mg2+ Sensitivity of NMDA-Induced Currents in Acutely Isolated MSNs
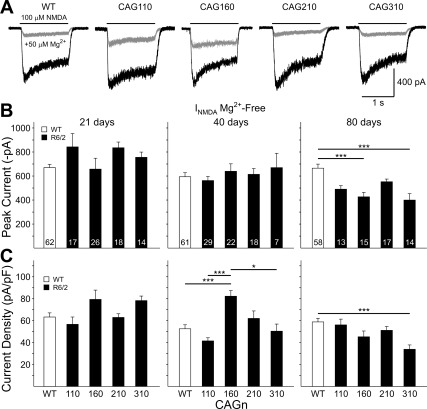
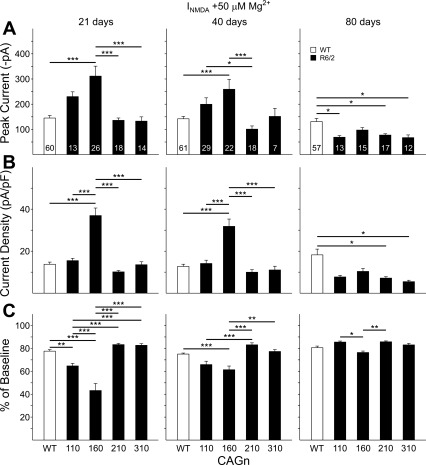
We also assessed NMDA-induced currents in acutely isolated MSNs. Previously, we showed that MSNs from R6/2 mice in the CAG160 group are less sensitive to blockade by Mg2+ at ages ranging between 15 and 40 days (Starling et al. 2005). We did not re-examine these data points for CAG160 mice in the present sample but show the data from MSNs at 21 and 40 days from our previously published study in Figs. 4 and 5 as a comparison with the data obtained from the other CAG repeat groups. We did obtain new data for CAG160 MSNs at 80 days. We recorded currents in response to NMDA (100 μM) application in Mg2+-free ACSF at a membrane Vhold of −70 mV (Fig. 4, A and B). Again, there were no significant differences in the electrophysiological measures among the cells of the WT groups, and data were pooled into one group for each measure. Similar to our previous data on CAG160 MSNs, at 21 days, there were no significant differences in average peak currents or peak current densities among the groups. Also, in concurrence with the previous data, no difference in peak current was observed at 40 days. However, peak current density for CAG160 MSNs was increased significantly at 40 days compared with WTs (P < 0.001), CAG110 cells (P < 0.001), and CAG310 cells (P < 0.05). At 80 days, NMDA peak currents were reduced in all groups with respect to WTs. The differences between WTs and CAG160 and CAG310 groups were statistically significant (P < 0.001). When peak currents were normalized by current density only, the difference between WTs and CAG310 cells remained significant (P < 0.001).
Fig. 4.
A: examples of typical current traces of acutely isolated MSNs in response to the application of 100 μM N-methyl-d-aspartate (INMDA) in the absence (black traces) and presence (gray traces) of 50 μM Mg2+ for a WT, CAG110, CAG160, CAG210, and CAG310 R6/2 at 21 days of age. B: mean (±SE) peak current amplitude in Mg2+-free ACSF for pooled WTs and each CAG repeat group at 21, 40, and 80 days. C: mean (±SE) peak current density (peak current/capacitance) for the same groups shown in B. Lines over bars indicate statistically significant group comparisons: *P < 0.05, ***P < 0.001. Data from 21- and 40-day CAG160 lines were published previously (Starling et al. 2005).
Fig. 5.
A: mean (±SE) peak current amplitude in ACSF containing 50 μM Mg2+ for pooled WTs and each CAG repeat group at 21, 40, and 80 days. Example traces are shown in Fig. 4. B: mean (±SE) peak current density (peak current/capacitance) for the same groups shown in A. C: mean (±SE) percent reduction in peak current produced by the addition of 50 μM Mg2+ in the ACSF for the same groups shown in A and B. Lines over bars indicate statistically significant group comparisons: *P < 0.05, **P < 0.01, ***P < 0.001. Data from 21- and 40-day CAG160 line were published previously (Starling et al. 2005).
We then applied 50 μM Mg2+, a concentration that was previously found to produce a partial blockade of NMDA-induced currents (Starling et al. 2005). In the presence of Mg2+, peak NMDA-induced currents were significantly larger in 21-day CAG160 MSNs compared with WTs (P < 0.001) and CAG210 and CAG310 cells (P < 0.001; Fig. 5A). A similar effect occurred for current densities with the addition of significant differences between CAG160 and CAG210 MSNs (P < 0.001; Fig. 5B). When Mg2+ sensitivity was calculated (percent change from Mg2+-free ACSF to the 50-μM Mg2+ condition), CAG 110 and CAG160 MSNs displayed significantly less reduction than WT cells (P < 0.01 for CAG110; P < 0.001 for CAG160) and were also significantly different from CAG210 and CAG310 cells (P < 0.001; Fig. 5C). In general, similar effects occurred in the 40-day groups, and CAG160 cells displayed the greatest changes, followed by CAG110 cells. At 80 days, in the presence of Mg2+, decreases in current and current density occurred in all CAG repeat groups compared with WTs (P < 0.05 for CAG110, 210, and 310 compared with WTs for current; P < 0.05 for the same CAG groups vs. WTs for current density). At this age, the CAG160 cells displayed significantly less reduction in current in the presence of Mg2+ but only compared with CAG110 (P < 0.05) and CAG210 MSNs (P < 0.01).
Body Weight
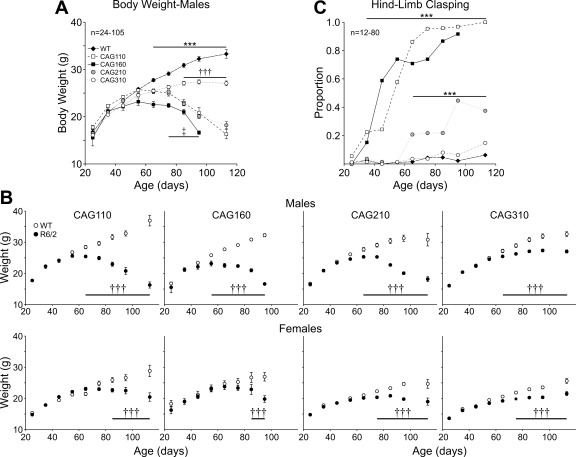
The progressive loss in body weight of R6/2 mice has been documented consistently (Carter et al. 1999; Dunnett et al. 1998; Hickey et al. 2005; Mangiarini et al. 1996). Because adult male and female mice differ in weight, and males show a more pronounced weight loss than females, mice were separated by gender for body-weight analysis. No statistically significant differences were found among the weights of male WT mice of each R6/2 line, and data were pooled (Fig. 6A). Males of all four lines of R6/2 showed typical weight gains similar to WTs, up to ∼50 days (Fig. 6A). After 60 days, CAG110, 160, and 210 R6/2 lines significantly lost weight (P < 0.05–P < 0.001). CAG310 mice continued to gain weight but at a slower rate than WTs (P < 0.05–P < 0.001). There was also a significant difference in weight between CAG310 mice and those of the other three CAG repeat lines in mice over 80 days (P < 0.05–P < 0.001). Whereas female mice generally weighed less than their male counterparts, similar trends were observed in the four lines of R6/2 mice (Fig. 6B).
Fig. 6.
A: body weight of male mice divided into 10-day age bins. WTs are pooled from all R6/2 lines. Line with *** indicates age range at which WTs are significantly heavier than mice from all 4 CAG repeat lines (P < 0.05–P < 0.001). Line with ††† indicates age range at which CAG110, CAG160, and CAG210 lines have significantly lost more weight than CAG310 mice (P < 0.05–P < 0.001). Line with ‡ indicates age range at which CAG160 mice have significantly lost more weight than CAG 110 and CAG210 mice. B: body weights of male (top) and female (bottom) R6/2 and WT mice of each respective CAG repeat line plotted against age (binned in 10-day age groups). Significant difference is indicated between R6/2 and respective WT: †††P < 0.001. C: proportion of mice displaying hind-limb clasping in each of the 4 CAG repeat lines and pooled WTs. Data have been divided into 10-day age bins. Line with *** indicates age range for statistically significant differences from WTs for both the CAG110 and CAG160 lines (top line) and CAG210 line (bottom line).
Hind-Limb Clasping
The development of hind-limb clasping has been described for the R6/2 phenotype (Hickey et al. 2005; Mangiarini et al. 1996). Clasping rarely occurred in WT mice, and no differences in clasping between genders were observed. Therefore, data were pooled for WTs and across gender. Hind-limb clasping was first seen in a significant (P < 0.01) proportion of R6/2 mice with 110 and 160 CAG repeats at ∼30 days, and after 70 days, >75% of mice in these two lines displayed clasping (Fig. 6C). Clasping was not observed in the CAG210 line until over 60 days, and no more than 45% of mice clasped, even at the oldest ages tested. CAG310 mice did not display a significant degree of clasping at any age tested.
Rotarod
Deficits in rotarod performance have been reported previously in the R6/2 mouse (Carter et al. 1999; Hickey et al. 2005). Similar performance was seen across both genders and among WTs of each R6/2 line, and data were pooled. At 30 days of age, no significant differences were observed between the R6/2 mouse lines and WT mice, although trends for impaired performance in the CAG110 and CAG160 lines were apparent (Fig. 7A). By 40 days, the CAG110 and the CAG160 lines showed significant impairment (P < 0.001 and P < 0.04, respectively). At 60 and 80 days, all four R6/2 lines showed impairments compared with WTs. The most affected were the CAG160 mice (P < 0.001), followed by CAG110 mice (P < 0.001). CAG210 mice (P < 0.01) and CAG310 mice (P < 0.05) were least affected. Furthermore, at 80 days, a significant difference was observed between CAG310 and both the CAG110 and CAG160 mice, respectively (P < 0.05).
Fig. 7.
A: latency to fall from an accelerating rotarod. B: latency to complete the pole-test task (time taken to turn, plus time taken to descend pole). In this (and see Figs. 8 and 9), numbers within the bars indicate the number of animals tested. Significance from WT (asterisks), significance from CAG110 line (daggers), significance from CAG160 line (double-dagger); */†/‡P < 0.05, **/††P < 0.01, ***/†††P < 0.001. Data from WTs have been pooled. Note the reduction in sample sizes in progressive age groups resulting from euthanasia due to advanced phenotypic progression or use for electrophysiology.
Pole-Test
To our knowledge, there are no published data for performance of R6/2 mice on the vertical pole-test, although deficits on this task have been identified in the CAG140 HD knock-in model (Hickey et al. 2008). At 30 days, there were no significant differences between WTs and any of the R6/2 lines (Fig. 7B). By 40 days, CAG110 mice were impaired with respect to WTs. This difference was maintained at 60 and 80 days (P < 0.001). At 80 days, CAG160 mice also were impaired (P < 0.05) compared with WTs. Neither CAG210 nor CAG310 mice were impaired on this test compared with WTs at the 80-day test period.
Seizure Susceptibility
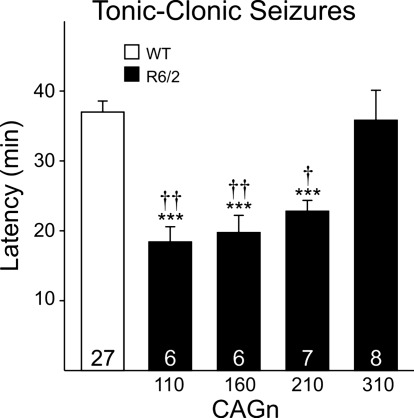
R6/2 mice are more susceptible to seizures (Cummings et al. 2009; Mangiarini et al. 1996), similar to individuals with juvenile HD (Gambardella et al. 2001; Seneca et al. 2004; Wojaczynska-Stanek et al. 2006). We assessed the effect of CAG repeat length on latency to tonic-clonic seizures induced by an intraperitoneal injection of PTX in male R6/2 mice (Fig. 8). Again, there were no differences in latency to seize among the WTs, and data were pooled into one WT group. CAG110, CAG160, and CAG210 mice developed seizures at the shortest latencies (P < 0.001 vs. WTs for all CAG groups). CAG210 mice took slightly longer to seize than the CAG110 or 160 mice, but the differences were not statistically significant. In contrast, CAG310 mice showed no difference in latency to seize compared with their respective WTs. CAG110, CAG160, and CAG210 mice displayed tonic-clonic seizures significantly faster than CAG310 mice (P = 0.002, P = 0.005, and P = 0.026, respectively).
Fig. 8.
Latency to tonic-clonic seizure in 40-day male mice from each CAG repeat line and for a pooled WT group. Statistically significant differences from WTs (asterisks), significance from CAG310 mice (daggers); ***P < 0.001, †P < 0.05, ††P < 0.01.
htt Inclusion Formation
We used the EM48 antibody, which recognizes the first 256 amino acids of the N-terminus of htt (Gutekunst et al. 1999) to visualize mutant htt within the striatum of each of the lines (Fig. 9A). We used an unbiased, stereological determination of the density of diffuse htt staining, nuclear microaggregates, NIIs, and neuropil aggregates in different regions of the striatum (anterior, medial, and posterior). No differences were observed among these regions, and we pooled density values across the striatum for each animal.
Fig. 9.
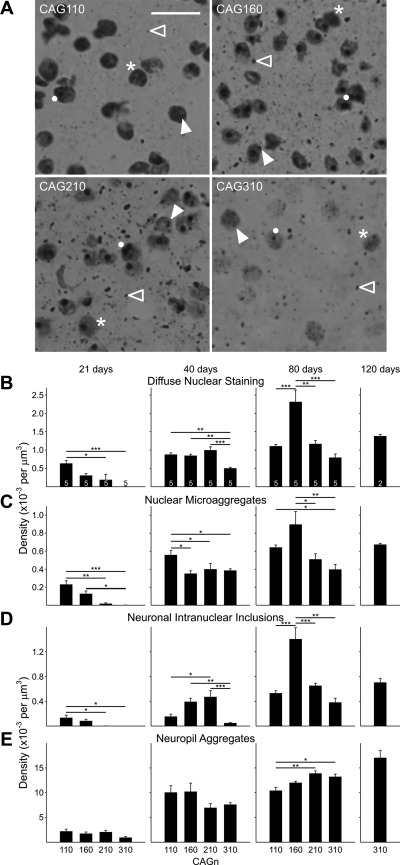
A: photomicrographs of EM48-stained striatal sections from 80-day-old mice. Each image shows an example of a diffusely stained nucleus (asterisk), a nuclear microaggregate (closed arrowhead), a neuronal intranuclear inclusion (NII; solid dot), and a neuropil aggregate (open arrowhead). Note that an individual cell could be counted multiple times. For example, a cell may contain 2 NIIs, may also contain nuclear microaggregates, or also have diffuse nuclear staining. Original scale bar (in CAG110 image) is 10 μm and refers to all images. B–E: mean (±SE) density plotted at each age and for each protein conformation for the 4 different CAG repeat lengths. Sample size (number of animals) is indicated in B as numbers inside (or above) the bar. Lines over bars indicate statistically significant group comparisons: *P < 0.05, **P < 0.01, ***P < 0.001.
Diffuse nuclear staining.
Diffuse nuclear staining of htt (Fig. 9B) was detected at 21 days in the three lines carrying up to 210 CAG repeats. The density of nuclei displaying diffuse staining was reduced with increasing CAG repeats, and significant differences were observed between CAG110 and both CAG210 (P < 0.05) and CAG310 mice (P < 0.001), respectively. The density of nuclei with diffuse htt staining increased at 40 days in each of the four lines. Similar densities were observed in CAG110, CAG160, and CAG210 mice, whereas CAG310 mice had a significantly lower density of nuclei with diffuse staining than each of the other lines (P < 0.01 vs. CAG110 and 160; P < 0.001 vs. CAG210). There was a further increase in the densities of diffusely stained nuclei in each of the lines at 80 days. At this age, CAG160 mice displayed a significantly higher density of diffusely stained nuclei than each of the other lines (P < 0.001 vs. CAG110 and 310; P < 0.01 vs. CAG210). CAG110 and CAG210 mice possessed a similar density of nuclei with diffuse staining. At 120 days, only tissue from CAG310 mice could be examined. A similar density as those seen at 80 days for the CAG110 and CAG210 mice was observed.
Nuclear microaggregates.
At 21 days, striatal tissue from CAG110, CAG160, and CAG210 mice displayed a low density of nuclear microaggregates (Fig. 9C), which were not observed in CAG310 mice at this age. The differences between the densities of microaggregates in CAG110 and both CAG210 (P < 0.01) and CAG310 mice (P < 0.001) were statistically significant. Also, the difference between CAG160 and CAG310 mice was significant (P < 0.05). The difference between CAG160 and CAG210 mice was near significance (P = 0.065). At 40 days, microaggregates were most prevalent in CAG110 mice, and the differences were significant with respect to each of the other lines of mice (P < 0.05). No significant differences were observed among the CAG160, CAG210, or CAG310 lines. At 80 days of age, the densities of nuclear microaggregates were significantly higher in the CAG160 mice compared with CAG210 (P < 0.05) and CAG310 mice (P < 0.01); the difference between CAG110 and CAG160 mice did not reach significance (P = 0.30), but the difference between CAG110 and CAG310 was significant (P < 0.05). The density of nuclear microaggregates increased further in the CAG310 line at 120 compared with 80 days.
NIIs.
At 21 days, low densities of NIIs (Fig. 9D) occurred in CAG110 and CAG160 mice but not in CAG210 or CAG310 mice. The densities of NIIs in CAG110 mice were significantly greater than in CAG210 and CAG310 mice (P < 0.05). By 40 days, NIIs were detected in the striatum of all four lines of mice. NII density was highest in the striatum of CAG210 and CAG160 mice, both of which contained a higher density of NIIs than CAG310 mice (P < 0.001 vs. CAG210; P < 0.01 vs. CAG160). The lowest density of NIIs occurred in the striatum of CAG110 and CAG310 mice. CAG110 was only significantly lower than the density of CAG210 mice (P < 0.05). By 80 days, the density of inclusions was greater than at 40 days in each line. Moreover, the density of NIIs was significantly greater in the CAG160 line than in the other lines (P < 0.001 for CAG110 and 210; P < 0.01 for CAG 310). CAG110 and CAG210 mice had similar NII densities. The CAG310 mice had the lowest density of NIIs. In CAG310 mice at 120 days, NII density was similar to that seen in CAG110 and CAG210 mice at 80 days.
Neuropil aggregates.
Neuropil aggregates were the most prevalently identified formation of htt at all ages. At 21 days, a low density of neuropil aggregates (Fig. 9E) was evident in all lines. No significant differences among lines occurred. At 40 days, neuropil aggregates increased in density, and similar densities were observed in all lines. The tendency for CAG210 and CAG310 mice to have a lower density of neuropil aggregates than either CAG110 or CAG160 mice was not statistically significant. At 80 days, the densities of neuropil aggregates increased compared with 40 days. The increase was greater in CAG210 (P < 0.01) and CAG310 mice (P < 0.05) compared with CAG110 mice. A further increase was observed in the density of neuropil aggregates in the CAG310 mice at 120 days.
htt Protein Expression
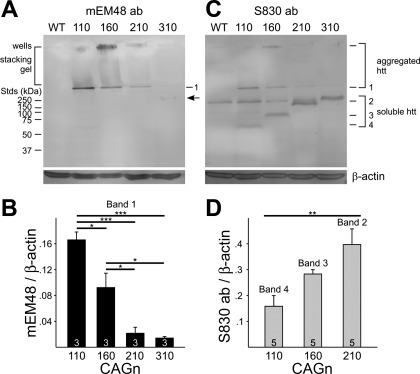
The Western blot analysis assessing protein expression levels in 40-day-old mice detected three immunoreactive htt protein bands in striatal extracts from the different R6/2 mouse lines with the mEM48 antibody selective for aggregates (Fig. 10A). A top band, at the level of the loading wells, was detected variably in the wells of the different CAG repeat lines, but a smaller middle band of ∼300 kDa was detected in the resolving gel just below the stacking gel and was seen in striata from all mice. Finally, a faint bottom band was seen in the CAG310 striata, corresponding to ∼175 kDa. No htt bands were evident for striata from WTs. Remarkably, the intensity of the ∼300-kDa band (band 1) was highly dependent on CAG repeat length and significantly decreased in intensity with higher CAG repeat lengths (Fig. 10B). As the mEM48 antibody has a high affinity for aggregated protein, the top two bands (wells and band 1) probably represent different conformations of aggregated protein. The faint band at ∼175 kDa may correspond to soluble htt protein, including long PolyQ repeats that form an epitope large enough to be detected by mEM48.
Fig. 10.
A: Western blots of striatal extracts from 40-day-old mice using the mouse EM48 (mEM48) antibody (ab). Note the presence of 3 polypeptide bands: in the loading wells, at ∼300 kDa (band 1), and at ∼175 kDa [arrow; evident in the CAG310 mouse line; standards (Stds) are on the left of the blot]. Striata from 3 mice at each of the 4 different CAG repeat R6/2 lines, plus 2 WT counterparts, were analyzed. B: intensities of the ∼300-kDa aggregated band (band 1) for each CAG repeat line were normalized to β-actin, and means (±SE) are plotted. Significance is indicated by *P < 0.05, ***P < 0.001. C: Western blots of striatal extracts from 21-day-old mice using the S830 antibody. Likely aggregated huntingtin (htt) is detected again in the wells and at ∼300 kDa, 1,046 corresponding to band 1, previously detected with mEM48 antibody in A. New bands 2, 3, and 4, corresponding in size to ∼120, 90, and 60 kDa, respectively, soluble forms of htt, are uniquely expressed in the CAG210, CAG160, and CAG110 R6/2 mouse lines, respectively. An additional, soluble htt band, similar in size to the ∼175 kDa detected with the mEM48 antibody (A), was also evident in the CAG310 line but was not quantified due to an obscuring nearby common band found in the WT fractions. Striata from 5 mice of each of the 4 different CAG repeat R6/2 mice were analyzed. D: intensities of the bands 2, 3, and 4 uniquely detected in each of the CAG210, 160, 110 repeat lines, respectively, were normalized to β-actin and means (±SE) plotted. Significance is indicated by **P = 0.007.
To assess the presence of soluble forms of htt with variable CAG repeat lengths, we used the S830 antibody to detect both aggregates and soluble exon 1 htt in striatal extracts (Benn et al. 2005) from a new set of R6/2 mice at 21 days—a time when very few aggregates were present. An identical band of ∼300 kDa comigrated with the aggregated form (band 1) seen with the mEM48 antibody (Fig. 10C). This was most evident in the CAG110 repeat lane (n = 5), but due to its low amount in the other CAG repeat lanes relative to background, it could not be quantified accurately. However, quantifiable forms of soluble htt (bands 2, 3, 4: ∼120, 90, 60 kDa, respectively, in size) were clearly detected with the S830 antibody. Although the R6/2 exon 1 with ∼150–160 CAG repeats should encode an ∼23-kDa protein, band 3 (CAG160 htt) runs aberrantly on gels at ∼90 kDa, as reported previously (Mangiarini et al. 1996). The sizes of bands 2 and 4 are close to their predicted sizes (118 kDa and 62 kDa, respectively) when calculated, based on their number of CAG repeats relative to band 3. Interestingly, the intensities of soluble bands increased as the CAG repeat lengthened (Fig. 10D; P = 0.007). An additional, soluble 310 CAG htt band, similar in size to the ∼175-kDa band, previously detected with the mEM48 antibody, was also evident. It appeared that the CAG310 band's intensity increased relative to soluble bands 2–4, but it could not be accurately quantified due to the presence of a slightly smaller, common band found in the WT fractions. This common band may represent cross-immunoreactivity with endogenous mouse htt.
DISCUSSION
We examined the progression of the HD phenotype in the R6/2 mouse model using a battery of electrophysiological, behavioral, and morphological indices to assess the impact of different CAG repeat lengths. Indices measured included MSN membrane capacitance and input resistance, spontaneous EPSCs and IPSCs, Mg2+ sensitivity of NMDA currents, body weight, clasping, rotarod, pole-test, seizure susceptibility, inclusion formation, and protein expression. Although some variations occurred in the findings, the general conclusion is that the phenotype is most severe in mice with ∼160 CAG repeats, whereas mice with 110 and 210 CAG repeats are less affected, and mice with 310 CAG repeats are minimally affected within the age range examined (summarized in Table 1). At some point during development of the phenotype, all but five of the 20 indices that we measured displayed an inverted U-shaped function. When this did not happen, it was either because the test was not sensitive enough (e.g., pole-test), because the test may have been more nonspecific (hindlimb clasping), or because the role of the measure in determining the degree of severity of the phenotype remains unclear (e.g., neuropil aggregates). Those indices that did show an inverted U-shaped function displayed a decrease in severity of effect as the number of CAG repeats increased. In humans carrying the mutated gene for HD, an inverse relationship between CAG repeat length and age at onset and to some degree, a direct relationship with severity of disease are evident, and therefore, the reduced effect in mice with over 210 CAG repeats was unpredicted.
Table 1.
Summary of measures displaying inverted U-shaped function
| Age (Days) | ||||
|---|---|---|---|---|
| 20/30 | 40 | 60 | 80 | |
| Capacitance | N | √ | ||
| Input resistance | √ | √ | ||
| EPSC frequency | √ | √ | ||
| EPSC-BIC frequency | √ | √ | ||
| IPSC frequency | N | N | ||
| Proportion of cells with high frequency IPSCs | √ | √ | ||
| NMDA peak current (Mg2+-free) | N | N | N | |
| NMDA current density (Mg2+-free) | N | √ | N | |
| NMDA peak current (50 μM Mg2+) | √ | √ | N | |
| NMDA current density (50 μM Mg2+) | √ | √ | N | |
| NMDA 50 μM Mg2+ % baseline | √ | √ | √ | |
| Body weight | N | N | N | √ |
| Hind-limb clasping | N | N | N | N |
| rotarod | N | √ | √ | √ |
| Pole test | N | N | N | N |
| Seizure susceptibility | √ | |||
| Diffuse nuclear staining | N | N | √ | |
| Nuclear microaggregates | N | N | √ | |
| Neuronal intranuclear inclusions | N | √ | √ | |
| Neuropil aggregates | N | N | N | |
√ indicates that an inverted U-shaped function was displayed. N indicates that an inverted U-shaped function was not displayed. Blank indicates the measure was not, or could not be, assessed. EPSC, excitatory postsynaptic current; BIC, bicuculline methobromide; IPSC, inhibitory postsynaptic current; NMDA, N-methyl-d-aspartate.
The present findings clearly demonstrate for the first time that the synaptic phenotype of MSNs of the R6/2 mouse is dependent on CAG repeat length. The reduced frequency of excitatory inputs coupled with an increase in inhibitory drive onto MSNs, which has been reported previously (Cepeda et al. 2003, 2004), became more pronounced when CAG repeat length was increased from 110 to 160, but further increases to 210 and 310 repeats made the changes in both excitatory and inhibitory inputs to MSNs less evident. Similar effects occurred for NMDA receptor-mediated currents and especially their sensitivity to the Mg2+ block. At 40 days of age, NMDA currents were altered most in MSNs from CAG160 mice. The earliest and greatest alterations in NMDA current sensitivity to Mg2+ occurred in MSNs from CAG160 mice. The reduction in Mg2+ sensitivity would mean that MSNs could become susceptible to activation of NMDA receptors at less-depolarized membrane potentials. This is consistent with the data of Graham and colleagues (2009), which demonstrated increased sensitivity to quinolinic acid in presymptomatic animals but decreased in symptomatic animals.
Interestingly, as the R6/2 phenotype progresses, NMDA currents tend to decrease. This occurred in all of the CAG repeat groups in the older mice. This effect occurs in other models (Graham et al. 2009; Joshi et al. 2009) and may be due to decreased excitatory receptors, as a recent study using electron microscopy demonstrates a significant reduction in excitatory synapses onto striatal neurons in 12-mo-old yeast artificial chromosome (YAC)128 mice (Singaraja et al. 2011). Data obtained from a mouse model that conditionally expresses mutant htt in striatum, cortex, or whole brain showed that altered NMDA currents require cell–cell interactions, whereas altered Mg2+ sensitivity of NMDA currents is a cell-autonomous process (Gu et al. 2007). Such differential effects may have been obtained here depending on the extent of impaired cell–cell interactions and cell-autonomous deficits imparted by altering CAG repeats.
Our data show a decrease in membrane capacitance, which correlates directly with a loss of neuronal surface area and thus may be indicative of retraction of neuronal processes and hence, synapse loss. The reduced membrane surface area and therefore, fewer transmembrane ion channels may also explain the increase in membrane input resistance. Importantly, on these parameters, the degree of change was consistent, and 160 CAG repeats had the most toxic effect.
We also show that body weight, clasping behavior, rotarod, and pole-test performance are more impaired in R6/2 mice with 110 and 160 CAG repeats. On some of these indices, the CAG110 mice were more impaired or impaired earlier than the CAG160 mice, and on some others, the degree of impairment between CAG110 and CAG160 mice reversed. In particular, the results of the pole-test varied, probably due to a low sensitivity in this test and also a smaller sample size in the CAG160 mice. Similarly, the CAG110 and 160 seized earlier than the other two groups.
We examined how htt aggregation is altered by the differing CAG repeats. Whereas the neurotoxic or neuroprotective effect of mutant htt aggregation is still widely debated, one possibility for the protective effect could be differential formation of aggregates. Once again, diffuse nuclear inclusions, microaggregates, NIIs, and neuropil aggregates were either present earlier in mice with 110 or 160 CAG repeats or displayed a greater density. Combined immunoblotting data showed that as the htt repeat length increases from 110 to 310 CAG repeats in the R6/2 striatum, a specific, aggregated form of ∼300 kDa decreases, whereas the respective soluble forms conversely increase in amount. These observations suggest that there may be a dynamic continuum of disassembly of aggregated mutant htt into soluble forms as the CAG repeat is lengthened. Alternatively, increases in repeat length may prevent aggregate formation. Interestingly, although fewer nuclear aggregates were seen in the highest CAG repeat mice, more neuropil aggregates were identified. It is therefore tempting to speculate that aggregates that form within the nucleus may be a toxic species, whereas aggregates in the neuropil are neuroprotective.
Similar ameliorative effects of highly expanded CAG repeats on the R6/2 phenotype have been reported recently. Dragatsis and colleagues (2009) compared the R6/2x mouse, carrying over 335 CAG repeats, with the CAG150 R6/2 and found a prolonged lifespan and fewer NIIs but more prevalent neuropil aggregates in both striatum and cortex. Importantly, these alterations corresponded to a reduction to one-third in both messenger RNA and transgenic protein expression. Thus they suggest that the protection or delayed phenotype is due to less htt expression. A second study (Morton et al. 2009) examined survival, body weight, neuromuscular abnormalities, and inclusion formation within the hippocampus of R6/2 mice with repeat lengths ranging between 170 and 450. Again, longer repeat lengths resulted in an amelioration of the phenotype. Furthermore, NIIs were of a different morphology in mice with the highest repeats. Morton and coworkers (2009) suggest that the htt produced by the highly expanded CAG repeats is expressed, as double-mutant mice die earlier than CAG repeat-length-matched mice carrying single copies of the transgene. A recent report suggests further that the prolonged onset and course of progression in R6/2 mice with expanded CAG repeats may result from differential upregulation of genes related to protein regulation and clearance (Tang et al. 2011).
The present report significantly adds to the previous studies by showing that R6/2 mice with CAG repeats between 110 and 160 displayed the expected relationship between CAG repeat length and phenotype, and the protective effects of highly expanded CAG repeats were observed at >210, a repeat length considerably shorter than that shown by others. Furthermore, we demonstrate that the electrophysiological phenotypes of striatal MSNs, which are likely to underlie some of the motor effects observed in these mice, possess an inverted U-shaped relationship with CAG repeat length.
The correlation between CAG repeat length and phenotype severity for CAG repeats up to 160 is similar to that observed in other mice, including another strain of the R6 line, the R6/1. This mouse originally carried ∼116 CAG repeats (Mangiarini et al. 1996), but it was reported recently that a single breeding male underwent a spontaneous deletion within the CAG tract, resulting in a line of R6/1 mice carrying ∼89 CAG repeats (Vatsavayai et al. 2007). These mice had an ameliorated phenotype, showing less weight loss, a lower incidence of clasping, and fewer NIIs compared with CAG116 R6/1 mice (Vatsavayai et al. 2007). Similarly, a full-length model, the YAC-HD mouse carrying 18, 46, 72, and 128 CAG repeats, also shows the expected inverse relationship between CAG repeat length and phenotypic severity, as determined by assessment of motor activity, immunohistochemistry, and electrophysiology (Hodgson et al. 1999; Milnerwood and Raymond 2007; Slow et al. 2003). Finally, the expected inverse relationship is also observed in knock-in mouse models carrying between 94 and 140 CAG repeats (Hickey et al. 2008; Menalled et al. 2002, 2003) or between 150 (Heng et al. 2007) and 200 CAG repeats (Heng et al. 2010).
The question therefore arises as to whether such full-length models would also show a similar protective effect to that seen in the truncated transgene model (the R6/2) if long repeats were to be expressed. Moreover, would a similar protective effect be seen in humans with highly expanded CAG repeats within the huntingtin gene? Individuals with the juvenile variant of HD typically have an age of onset of under 20 years and can have >70 CAG repeats (Alonso et al. 1997; Sanchez et al. 1996; Telenius et al. 1993). Whereas a strong relationship is found between age at onset and CAG repeat lengths up to ∼60, the addition of extra CAG repeats above this seems to have little effect (Andresen et al. 2007). Indeed, children with highly expanded CAG repeats show very little variation in age of onset, and those carrying repeats between 100 and >250 CAG repeats present with symptoms at a similar age (Nahhas et al. 2005; Nance et al. 1999; Papapetropoulos et al. 2005; Seneca et al. 2004; Wojaczynska-Stanek et al. 2006). Thus although it would appear that humans do not show a full protective effect with repeats in a similar range to those studied here in the R6/2, there is by no means a linear relationship between disease state and long CAG repeats. It is possible that a similar mechanism underlies the attenuation seen in R6/2 and humans, and in the presence of full-length htt, a much longer CAG repeat may be required before a protective effect is achieved. In addition, more studies are necessary to examine how normal htt affects disease onset in transgenic mice. In humans, there is evidence for an interaction between expanded and unexpanded CAG repeat lengths so that an increase in repeat size of the normal allele mitigates and delays manifestations of HD in individuals with the highest expanded repeat size (Djoussé et al. 2003). It is tempting to speculate that normal htt can somehow neutralize the deleterious effects of the mutant htt protein, possibly by preferential binding to mutant soluble forms with longer CAG repeat lengths (210–310), effectively sequestering toxic htt.
The present findings have important implications for experimental and preclinical drug trials that use the R6/2 mouse model. To compare data over time and among laboratories, the determination of CAG repeat length is of great importance. Despite the unexpected dependency of the phenotype to CAG repeat length observed with long repeats, the R6/2 mouse still appears to be a very useful model of HD. Importantly, when strain background and CAG repeat length were controlled for, a knock-in model carrying 150 CAG repeats showed similar pathology and motor changes at 2 yr to those seen in the R6/2 at 12 wk (Woodman et al. 2007); both the YAC128 and a knock-in mouse carrying 140 CAG repeats showed similar alterations in cortical spontaneous synaptic currents, as the R6/2 (Cummings et al. 2010) and the CAG140 and R6/2 mice both show a loss of correlated neuronal activity in the striatum (Miller et al. 2008) and cortex (Walker et al. 2008). It would appear therefore that the R6/2 mouse remains a suitable model in which to study HD and can provide further insight into the effects of highly expanded CAG repeats, which may underlie the similar age of disease onset of juvenile HD patients carrying CAG repeats between 100 and over 250. Despite the fact that R6/2 mice show many phenotypes similar to other mouse models of HD, it should be emphasized that confirmation of any finding in a transgenic mouse model of a disease should be sought in multiple independent models, as there could be unforeseen alterations in phenotype, as highlighted here, or for example, by the random nature of the integration site of the transgene, leading to silencing of another gene.
In summary, we show a unique and unpredicted relationship between CAG repeat length and the R6/2 phenotype, starting at just over 200 CAG repeats and based on evidence obtained from multiple indices, including electrophysiology, motor behavior, and aggregate and protein expression. In contrast to the inverse relationship seen in HD patients, an inverted U-shaped function exists, in which increasing CAG repeats from 110 to 160 gives rise to the expected development of a more severe phenotype, whereas further increases beyond 200, up to 310 CAG repeats, reduce the severity of the phenotype.
GRANTS
Funding was provided by CHDI Foundation and National Institute of Neurological Disorders and Stroke Grant NS41574.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: D.M.C., C.C., and M.S.L. conception and design of research; D.M.C., Y.A., M.A.H., P.R.J., S.C.H., C.Z., T.K.A., V.M.A., C.C., and J.B.W. performed experiments; D.M.C., Y.A., M.A.H., P.R.J., S.C.H., C.Z., T.K.A., V.M.A., C.C., and J.B.W. analyzed data; D.M.C., C.C., and M.S.L. interpreted results of experiments; D.M.C., C.C., and M.S.L. prepared figures; D.M.C. and C.C. drafted manuscript; D.M.C., V.M.A., C.C., J.B.W., and M.S.L. edited and revised manuscript; M.S.L. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Gillian Bates and Kirupa Sathasivam for providing CAG210 R6/2 founder mice. We thank Donna Crandall for help with illustrations and Alex Halim for assistance with data analyses. Present address of D. M. Cummings: Dept. of Neuroscience, Physiology & Pharmacology, University College London, Gower St., London WC1E 6BT, UK.
REFERENCES
- Alonso ME, Yescas P, Cisneros B, Martinez C, Silva G, Ochoa A, Montanez C. Analysis of the (CAG)n repeat causing Huntington's disease in a Mexican population. Clin Genet 51: 225–230, 1997 [DOI] [PubMed] [Google Scholar]
- Andresen JM, Gayan J, Djousse L, Roberts S, Brocklebank D, Cherny SS, Cardon LR, Gusella JF, MacDonald ME, Myers RH, Housman DE, Wexler NS. The relationship between CAG repeat length and age of onset differs for Huntington's disease patients with juvenile onset or adult onset. Ann Hum Genet 71: 295–301, 2007 [DOI] [PubMed] [Google Scholar]
- Ariano MA, Cepeda C, Calvert CR, Flores-Hernandez J, Hernandez-Echeagaray E, Klapstein GJ, Chandler SH, Aronin N, DiFiglia M, Levine MS. Striatal potassium channel dysfunction in Huntington's disease transgenic mice. J Neurophysiol 93: 2565–2574, 2005 [DOI] [PubMed] [Google Scholar]
- Bates GP, Hockly E. Experimental therapeutics in Huntington's disease: are models useful for therapeutic trials? Curr Opin Neurol 16: 465–470, 2003 [DOI] [PubMed] [Google Scholar]
- Benn CL, Landles C, Li H, Strand AD, Woodman B, Sathasivam K, Li SH, Ghazi-Noori S, Hockly E, Faruque SM, Cha JH, Sharpe PT, Olson JM, Li XJ, Bates GP. Contribution of nuclear and extranuclear PolyQ to neurological phenotypes in mouse models of Huntington's disease. Hum Mol Genet 14: 3065–3078, 2005 [DOI] [PubMed] [Google Scholar]
- Carter RJ, Lione LA, Humby T, Mangiarini L, Mahal A, Bates GP, Dunnett SB, Morton AJ. Characterization of progressive motor deficits in mice transgenic for the human Huntington's disease mutation. J Neurosci 19: 3248–3257, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda C, Hurst RS, Calvert CR, Hernandez-Echeagaray E, Nguyen OK, Jocoy E, Christian LJ, Ariano MA, Levine MS. Transient and progressive electrophysiological alterations in the corticostriatal pathway in a mouse model of Huntington's disease. J Neurosci 23: 961–969, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda C, Starling AJ, Wu N, Nguyen OK, Uzgil B, Soda T, André VM, Ariano MA, Levine MS. Increased GABAergic function in mouse models of Huntington's disease: reversal by BDNF. J Neurosci Res 78: 855–867, 2004 [DOI] [PubMed] [Google Scholar]
- Cepeda C, Wu N, André VM, Cummings DM, Levine MS. The corticostriatal pathway in Huntington's disease. Prog Neurobiol 81: 253–271, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings DM, André VM, Uzgil BO, Gee SM, Fisher YE, Cepeda C, Levine MS. Alterations in cortical excitation and inhibition in genetic mouse models of Huntington's disease. J Neurosci 29: 10371–10386, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings DM, Cepeda C, Levine MS. Alterations in striatal synaptic transmission are consistent across genetic mouse models of Huntington's disease. ASN Neuro 2: e00036, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies SW, Turmaine M, Cozens BA, DiFiglia M, Sharp AH, Ross CA, Scherzinger E, Wanker EE, Mangiarini L, Bates GP. Formation of neuronal intranuclear inclusions underlies the neurological dysfunction in mice transgenic for the HD mutation. Cell 90: 537–548, 1997 [DOI] [PubMed] [Google Scholar]
- Djoussé L, Knowlton B, Hayden M, Almqvist EW, Brinkman R, Ross C, Margolis R, Rosenblatt A, Durr A, Dode C, Morrison PJ, Novelletto A, Frontali M, Trent RJ, McCusker E, Gómez-Tortosa E, Mayo D, Jones R, Zanko A, Nance M, Abramson R, Suchowersky O, Paulsen J, Harrison M, Yang Q, Cupples LA, Gusella JF, MacDonald ME, Myers RH. Interaction of normal and expanded CAG repeat sizes influences age at onset of Huntington disease. Am J Med Genet 119A: , 2003279–282 [DOI] [PubMed] [Google Scholar]
- Dragatsis I, Goldowitz D, Del Mar N, Deng YP, Meade CA, Liu L, Sun Z, Dietrich P, Yue J, Reiner A. CAG repeat lengths > or =335 attenuate the phenotype in the R6/2 Huntington's disease transgenic mouse. Neurobiol Dis 33: 315–330, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunnett SB, Carter RJ, Watts C, Torres EM, Mahal A, Mangiarini L, Bates G, Morton AJ. Striatal transplantation in a transgenic mouse model of Huntington's disease. Exp Neurol 154: 31–40, 1998 [DOI] [PubMed] [Google Scholar]
- Gambardella A, Muglia M, Labate A, Magariello A, Gabriele AL, Mazzei R, Pirritano D, Conforti FL, Patitucci A, Valentino P, Zappia M, Quattrone A. Juvenile Huntington's disease presenting as progressive myoclonic epilepsy. Neurology 57: 708–711, 2001 [DOI] [PubMed] [Google Scholar]
- Graham RK, Pouladi MA, Joshi P, Lu G, Deng Y, Wu NP, Figueroa BE, Metzler M, André VM, Slow EJ, Raymond L, Friedlander R, Levine MS, Leavitt BR, Hayden MR. Differential susceptibility to excitotoxic stress in YAC128 mouse models of Huntington disease between initiation and progression of disease. J Neurosci 29: 2193–2204, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, André VM, Cepeda C, Li SH, Li XJ, Levine MS, Yang XW. Pathological cell-cell interactions are necessary for striatal pathogenesis in a conditional mouse model of Huntington's disease. Mol Neurodegener 2: 8, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutekunst CA, Li SH, Yi H, Mulroy JS, Kuemmerle S, Jones R, Rye D, Ferrante RJ, Hersch SM, Li XJ. Nuclear and neuropil aggregates in Huntington's disease: relationship to neuropathology. J Neurosci 19: 2522–2534, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper PS, Jones L. Huntington's disease: genetic and molecular studies. In: Huntington's Disease, edited by Bates GP, Harper PS, Jones L. Oxford, UK, Oxford University Press, 2002, p. 113–158 [Google Scholar]
- Heng MY, Duong DK, Albin RL, Tallaksen-Greene SJ, Hunter JM, Lesort MJ, Osmand A, Paulson HL, Detloff PJ. Early autophagic response in a novel knock-in model of Huntington disease. Hum Mol Genet 19: 3702–3720, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng MY, Tallaksen-Greene SJ, Detloff PJ, Albin RL. Longitudinal evaluation of the Hdh(CAG)150 knock-in murine model of Huntington's disease. J Neurosci 27: 8989–8998, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey MA, Gallant K, Gross GG, Levine MS, Chesselet MF. Early behavioral deficits in R6/2 mice suitable for use in preclinical drug testing. Neurobiol Dis 20: 1–11, 2005 [DOI] [PubMed] [Google Scholar]
- Hickey MA, Kosmalska A, Enayati J, Cohen R, Zeitlin S, Levine MS, Chesselet MF. Extensive early motor and non-motor behavioral deficits are followed by striatal neuronal loss in knock-in Huntington's disease mice. Neuroscience 157: 280–295, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson JG, Agopyan N, Gutekunst CA, Leavitt BR, LePiane F, Singaraja R, Smith DJ, Bissada N, McCutcheon K, Nasir J, Jamot L, Li XJ, Stevens ME, Rosemond E, Roder JC, Phillips AG, Rubin EM, Hersch SM, Hayden MR. A YAC mouse model for Huntington's disease with full-length mutant huntingtin, cytoplasmic toxicity, and selective striatal neurodegeneration. Neuron 23: 181–192, 1999 [DOI] [PubMed] [Google Scholar]
- Joshi PR, Wu NP, André VM, Cummings DM, Cepeda C, Joyce JA, Carroll JB, Leavitt BR, Hayden MR, Levine MS, Bamford NS. Age-dependent alterations of corticostriatal activity in the YAC128 mouse model of Huntington disease. J Neurosci 29: 2414–2427, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klapstein GJ, Fisher RS, Zanjani H, Cepeda C, Jokel ES, Chesselet MF, Levine MS. Electrophysiological and morphological changes in striatal spiny neurons in R6/2 Huntington's disease transgenic mice. J Neurophysiol 86: 2667–2677, 2001 [DOI] [PubMed] [Google Scholar]
- Kremer B, Almqvist E, Theilmann J, Spence N, Telenius H, Goldberg YP, Hayden MR. Sex-dependent mechanisms for expansions and contractions of the CAG repeat on affected Huntington disease chromosomes. Am J Hum Genet 57: 343–350, 1995 [PMC free article] [PubMed] [Google Scholar]
- Mangiarini L, Sathasivam K, Mahal A, Mott R, Seller M, Bates GP. Instability of highly expanded CAG repeats in mice transgenic for the Huntington's disease mutation. Nat Genet 15: 197–200, 1997 [DOI] [PubMed] [Google Scholar]
- Mangiarini L, Sathasivam K, Seller M, Cozens B, Harper A, Hetherington C, Lawton M, Trottier Y, Lehrach H, Davies SW, Bates GP. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell 87: 493–506, 1996 [DOI] [PubMed] [Google Scholar]
- Menalled LB, Sison JD, Dragatsis I, Zeitlin S, Chesselet MF. Time course of early motor and neuropathological anomalies in a knock-in mouse model of Huntington's disease with 140 CAG repeats. J Comp Neurol 465: 11–26, 2003 [DOI] [PubMed] [Google Scholar]
- Menalled LB, Sison JD, Wu Y, Olivieri M, Li XJ, Li H, Zeitlin S, Chesselet MF. Early motor dysfunction and striosomal distribution of huntingtin microaggregates in Huntington's disease knock-in mice. J Neurosci 22: 8266–8276, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BR, Walker AG, Shah AS, Barton SJ, Rebec GV. Dysregulated information processing by medium spiny neurons in striatum of freely behaving mouse models of Huntington's disease. J Neurophysiol 100: 2205–2216, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milnerwood AJ, Raymond LA. Corticostriatal synaptic function in mouse models of Huntington's disease: early effects of huntingtin repeat length and protein load. J Physiol 585: 817–831, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton AJ, Glynn D, Leavens W, Zheng Z, Faull RL, Skepper JN, Wight JM. Paradoxical delay in the onset of disease caused by super-long CAG repeat expansions in R6/2 mice. Neurobiol Dis 33: 331–341, 2009 [DOI] [PubMed] [Google Scholar]
- Nahhas FA, Garbern J, Krajewski KM, Roa BB, Feldman GL. Juvenile onset Huntington disease resulting from a very large maternal expansion. Am J Med Genet A 137A: , 2005328–331 [DOI] [PubMed] [Google Scholar]
- Nance MA, Mathias-Hagen V, Breningstall G, Wick MJ, McGlennen RC. Analysis of a very large trinucleotide repeat in a patient with juvenile Huntington's disease. Neurology 52, 392–394, 1999 [DOI] [PubMed] [Google Scholar]
- Papapetropoulos S, Lopez-Alberola R, Baumbach L, Russell A, Gonzalez MA, Bowen BC, Singer C. Case of maternally transmitted juvenile Huntington's disease with a very large trinucleotide repeat. Mov Disord 20: 1380–1383, 2005 [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol 32: 281–294, 1972 [DOI] [PubMed] [Google Scholar]
- Rossi S, Prosperetti C, Picconi B, De Chiara V, Mataluni G, Bernardi G, Calabresi P, Centonze D. Deficits of glutamate transmission in the striatum of toxic and genetic models of Huntington's disease. Neurosci Lett 410: 6–10, 2006 [DOI] [PubMed] [Google Scholar]
- Sanchez A, Castellvi-Bel S, Mila M, Genis D, Calopa M, Jimenez D, Estivill X. Huntington's disease: confirmation of diagnosis and presymptomatic testing in Spanish families by genetic analysis. J Neurol Neurosurg Psychiatry 61: 625–627, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherzinger E, Lurz R, Turmaine M, Mangiarini L, Hollenbach B, Hasenbank R, Bates GP, Davies Lehrach H SW, Wanker EE. Huntingtin-encoded polyglutamine expansions form amyloid-like protein aggregates in vitro and in vivo. Cell 90: 549–558, 1997 [DOI] [PubMed] [Google Scholar]
- Seneca S, Fagnart D, Keymolen K, Lissens W, Hasaerts D, Debulpaep S, Desprechins B, Liebaers I, De Meirleir L. Early onset Huntington disease: a neuronal degeneration syndrome. Eur J Pediatr 163: 717–721, 2004 [DOI] [PubMed] [Google Scholar]
- Singaraja RR, Huang K, Sanders SS, Milnerwood A, Hines R, Lerch J, Franciosi S, Drisdel R, Vaid K, Young FB, Doty C, Wan J, Bissada N, Henkelman RM, Green WN, Davis NG, Raymond LA, Hayden MR. Altered palmitoylation and neuropathological deficits in mice lacking HIP14. Hum Mol Genet 20: 3899–3909, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slow EJ, van Raamsdonk J, Rogers D, Coleman SH, Graham RK, Deng Y, Oh R, Bissada N, Hossain SM, Yang YZ, Li XJ, Simpson EM, Gutekunst CA, Leavitt BR, Hayden MR. Selective striatal neuronal loss in a YAC128 mouse model of Huntington disease. Hum Mol Genet 12: 1555–1567, 2003 [DOI] [PubMed] [Google Scholar]
- Starling AJ, André VM, Cepeda C, de Lima M, Chandler SH, Levine MS. Alterations in N-methyl-d-aspartate receptor sensitivity and magnesium blockade occur early in development in the R6/2 mouse model of Huntington's disease. J Neurosci Res 82: 377–386, 2005 [DOI] [PubMed] [Google Scholar]
- Tang B, Seredenina T, Coppola G, Kuhn A, Geschwind DH, Luthi-Carter R, Thomas EA. Gene expression profiling of R6/2 transgenic mice with different CAG repeat lengths reveals genes associated with disease onset and progression in Huntington's disease. Neurobiol Dis 42: 459–467, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telenius H, Kremer HP, Theilmann J, Andrew SE, Almqvist E, Anvret M, Greenberg C, Greenberg J, Lucotte G, Squitieri F, Starr E, Goldberg YP, Hayden MR. Molecular analysis of juvenile Huntington disease: the major influence on (CAG)n repeat length is the sex of the affected parent. Hum Mol Genet 2: 1535–1540, 1993 [DOI] [PubMed] [Google Scholar]
- Vatsavayai SC, Dallérac GM, Milnerwood AJ, Cummings DM, Rezaie P, Murphy KPSJ, Hirst MC. Progressive CAG expansion in the brain of a novel R6/1–89Q mouse model of Huntington's disease with delayed phenotypic onset. Brain Res Bull 72: 98–102, 2007 [DOI] [PubMed] [Google Scholar]
- Walker AG, Miller BR, Fritsch JN, Barton SJ, Rebec GV. Altered information processing in the prefrontal cortex of Huntington's disease mouse models. J Neurosci 28: 8973–8982, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson JB, Hatami A, David H, Masliah E, Roberts K, Evans CE, Levine MS. Alterations in corticostriatal synaptic plasticity in mice over-expressing human α-synuclein. Neuroscience 159: 501–513, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojaczynska-Stanek K, Adamek D, Marszal E, Hoffman-Zacharska D. Huntington disease in a 9-year-old boy: clinical course and neuropathologic examination. J Child Neurol 21: 1068–1073, 2006 [DOI] [PubMed] [Google Scholar]
- Woodman B, Butler R, Landles C, Lupton MK, Tse J, Hockly E, Moffitt H, Sathasivam K, Bates GP. The Hdh(Q150/Q150) knock-in mouse model of HD and the R6/2 exon 1 model develop comparable and widespread molecular phenotypes. Brain Res Bull 72: 83–97, 2007 [DOI] [PubMed] [Google Scholar]
- Zhou H, Cao F, Wang Z, Yu ZX, Nguyen HP, Evans J, Li SH, Li XJ. Huntingtin forms toxic NH2-terminal fragment complexes that are promoted by the age-dependent decrease in proteasome activity. J Cell Biol 163: 109–118, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]