Abstract
The lake sturgeon, Acipenser fulvescens, belongs to one of the few extant nonteleost ray-finned fishes and diverged from the main vertebrate lineage about 250 million years ago. The aim of this study was to use this species to explore the peripheral neural coding strategies for sound direction and compare these results to modern bony fishes (teleosts). Extracellular recordings were made from afferent neurons innervating the saccule and lagena of the inner ear while the fish was stimulated using a shaker system. Afferents were highly directional and strongly phase locked to the stimulus. Directional response profiles resembled cosine functions, and directional preferences occurred at a wide range of stimulus intensities (spanning at least 60 dB re 1 nm displacement). Seventy-six percent of afferents were directionally selective for stimuli in the vertical plane near 90° (up down) and did not respond to horizontal stimulation. Sixty-two percent of afferents responsive to horizontal stimulation had their best axis in azimuths near 0° (front back). These findings suggest that in the lake sturgeon, in contrast to teleosts, the saccule and lagena may convey more limited information about the direction of a sound source, raising the possibility that this species uses a different mechanism for localizing sound. For azimuth, a mechanism could involve the utricle or perhaps the computation of arrival time differences. For elevation, behavioral strategies such as directing the head to maximize input to the area of best sensitivity may be used. Alternatively, the lake sturgeon may have a more limited ability for sound source localization compared with teleosts.
Keywords: sound source localization, auditory code, particle motion
for humans and most other vertebrates (possibly including fish), the ability to localize sound sources is critical for survival and facilitates the identification of target sounds in a complex acoustic environment (Popper and Fay 2005). The lake sturgeon, Acipenser fulvescens (Rafinesque 1817), belongs to the ray-finned fishes (Actinopterygians). The majority of Actinopterygians are teleosts (more than 26,000 species; Nelson 2006). In contrast, there are only 48 extant nonteleost Actinopterygian species (Liem et al. 2001), of which 25 species are sturgeons (family: Acipenseridae) and 2 are paddlefishes (family: Polyodontidae). Both families belong to the order Acipenseriformes, a group that originated in the early Triassic (about 250 million years ago; Grande and Bemis 1996). Because of the early occurrence in evolution and phylogenetic position closer to the common ancestor of bony fish (Osteichthyes), studies using Acipenseriformes are useful for comparison with teleosts because they can provide insight into which character traits are evolutionary ancestral (may represent the ancestral condition) and which character traits are modern (Rupp and Northcutt 1998).
Lake sturgeon occur in the freshwater of North America and Canada and usually live on the bottom of a riverbed or lake. These fish may migrate up to 200 km to find a suitable habitat for spawning in rivers (Auer 1996). They tend to prefer certain spawning sites (Bemis and Kynard 1997). Hearing may be useful in providing cues about the location and characteristics of sound sources (Fay and Popper 2000), which may assist sturgeon in their migration between rivers and lakes for spawning as well as feeding. In addition, vocalizations by male sturgeons have been observed during spawning season in the wild (Bruch and Binkowski 2002). However, no data were obtained on the spectral content of the sounds.
The current hypothesis of sound source localization in fish suggests that, unlike in many tetrapods, the direction of a sound source is initially computed and encoded in the auditory periphery (e.g., Christensen-Daalsgard et al. 2011; Fay and Edds-Walton 1997a; Zeddies et al. 2010). This hypothesis is based on the idea that fish ears are mainly adapted to detect the particle motion component of sound (instead of pressure) by functioning as inertial accelerometers (de Vries 1950; Popper 1976; reviewed in Fay 2005; Rogers and Zeddies 2008; Sand and Bleckmann 2008). Particle motion is a vector quantity and carries information about the magnitude and direction of a sound source (Rogers and Cox 1988). Animals such as fish (Edds-Walton et al. 1999; Fay 1984), which have evolved ears suited to detect particle motion, could use this information to locate a sound source. Each otolith organ in fish has a sensory epithelium consisting of hair cells and a calcareous otolith (Popper and Fay 1999). The axis of acoustic particle motion is encoded by hair cells that are physiologically polarized and have diverse directional best axes (Flock 1964; Hudspeth and Corey 1977).
Recordings from afferents innervating otolith organs in teleost fish have demonstrated direction-dependent responses to linear acceleration. Stimulation along the best axis of hair cells leads to maximal responses from the afferents innervating them. The response falls off in a graded manner as the direction of stimulation deviates from the best axis. The graded response profile is well fit by cosine tuning curves as predicted by the “vector detector” model (Schuijf and Buwalda 1975). Generally, past studies in teleosts have shown that best response directions of primary afferents generally matched the hair cell orientation of the epithelium that the neurons innervated, as well as the orientation of the epithelium in space (Fay 1984; Fay and Edds-Walton 1997a; Lu et al. 1998; Lu and Popper 2001).
In the current study, we investigated the response of primary eighth nerve afferents innervating the saccule and lagena in the lake sturgeon to directional stimulation using a shaker system (e.g., Fay 1984). The data presented represent the first detailed electrophysiological study investigating coding strategies for sound direction in a nonteleost Actinopterygian bony fish, thereby seeking to add to the understanding of the mechanisms for sound source localization in fish and of the origin of the mechanisms for directional hearing in vertebrates.
MATERIAL AND METHODS
Experimental animals.
Juvenile lake sturgeon (between 1 and 2 yr old) with body lengths ranging from 25 to 37 cm (total length) and body masses from 45 to 115 g were used in this study. Fish were obtained from the Wild Rose fish hatchery of the Wisconsin Department of Natural Resources and housed at the University of Maryland in 45-gallon tanks at 17–18°C kept on a 12:12-h light-dark cycle. Animals were fed with frozen krill (Jehm). We used the convention aa, bb, cc, etc., to identify specific animals and numbers to identify units (e.g., oo9 stands for unit number 9 recorded from animal oo). The University of Maryland Institutional Animal Care and Use Committee approved all experimental procedures.
Surgery and anesthesia.
For a detailed description of the methods, see Meyer et al. (2010). Fish were lightly anesthetized in a bath containing 0.05% buffered 3-aminobenzoic acid methane-sulfonate salt (Sigma, St. Louis, MO) dissolved in tank water and after that were immobilized with an intramuscular injection of gallamine triethiodide in goldfish Ringer solution (43 μg/g body mass). They were then transferred to a surgical dish. Aerated freshwater was continuously pumped through the fish's mouth for artificial respiration at a flow rate of 100–200 ml/min. For analgesia, the local anesthetic lidocaine (2.5%; Astra Chemicals, Wedel, Germany) was applied to the skin at the dorsal surface of the head. Next, the brain surface was exposed above the eighth nerve at the level of the cerebellum and the medulla.
On completion of the surgery, the fish was transferred to a cylindrical stimulus dish (diameter, 23 cm; height, 6 cm) and the mouth was fitted over, and then clamped to, an aluminum respirator tube delivering aerated tank water of 17–18°C via a Neslab RTE-111 chiller pump (Cole-Parmer, Vernon Hills, IL), which was rigidly attached to the dish.
Stimulation system.
The stimulus system was developed to measure directional responses in fish to whole body translatory movement simulating the effects of particle motion of underwater sound in a natural environment (Fay 1984; Fay and Edds-Walton 1997a, 1997b; Weeg et al. 2002). The system consisted of five mechanical shakers. For linear acceleration of the fish in the horizontal plane, one pair of shakers (Bruel and Kjaer no. 4810; Naerum, Denmark) was mounted front to back and the other in a side-to-side position of the fish. A fifth shaker (Bruel and Kjaer no. 4809) was attached via a rod to the bottom center of the dish and oscillated the fish vertically. To minimize relative motion between the fish head and the dish, the animal was tightly attached to the dish via a head holder specifically designed for lake sturgeon. The entire shaker system was positioned on a Micro-G pneumatic vibration isolation table.
The dish movement was calibrated for each experiment after the fish was positioned in the stimulus dish with the use of three orthogonally oriented accelerometers (model 002A10, Flexcel; PCB Piezotronics). With the use of a calibration program (Fay 1984), the accelerometer output was used to make adjustments to the phases and amplitudes required for the shakers to create sinusoidal, linear oscillations of the dish along axes in the horizontal and midsagittal planes.
During recordings, a search stimulus was presented at 100 Hz. This search stimulus provided the fish with a constantly changing directional stimulation to maximize the chances of detecting a cell with any directional preference. Once a unit was identified, the directional stimuli were presented in six azimuthal axes in the horizontal plane at 90°, 60°, 30°, 0°, −30°, and −60° and six elevational axes in the midsagittal plane at 0°, 30°, 60°, 90°, 120°, and 150°. The directional stimuli were set at 100 Hz, which was at or near the most excitatory frequency (best frequency, BF) for most afferents of the sturgeon. Test frequencies were 50, 100, 141, 185, 244, 303, 409, and 714 Hz.
If a unit showed directional selectivity, the directional stimulus protocol was repeated at different signal intensities to determine the affect of level on directional response profiles. Signals to the shaker system were digitally synthesized sinusoids, 500 ms long, with 20-ms rise and fall times repeated 8 times. The signals were independently read out of the 3 channels of a 16-bit digital-to-analog converter (TDT DA; Tucker-Davis Technologies) at a 10-kHz sampling rate. Each signal was low-pass filtered at 2 kHz, controlled in amplitude by TDT PA4 programmable attenuators, and amplified by a Techron power amplifier (model 5507). The amplified signals were further attenuated by 32 dB using a resistor network to improve signal-to-noise ratio at the shaker inputs.
Recording and data acquisition.
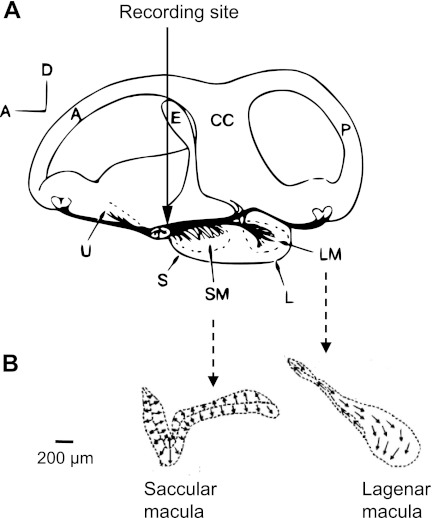
Extracellular recordings were made from the portion of the eighth nerve innervating the saccule and lagena of the right ear (posterior ramus, Fig. 1A) using 2 M NaCl-filled glass pipettes with resistances between 20 and 50 MΩ (micropipette puller P97; Sutter Instruments, Novato, CA). It was not possible to access individual nerve branches that just innervated the saccule or the lagena without potentially harming those epithelia (Fig. 1A; see also Meyer et al. 2010 for a more detailed explanation of the innervation). The part of the posterior ramus we recorded from (see arrow, Fig. 1A) innervated the saccule, lagena, posterior crista, and macula neglecta. Several extracellular labeling experiments in the lake sturgeon indicated at least one phase-locking afferent projecting to the saccule and one projecting to the lagena in two different animals. The number of labeled fibers, however, was not sufficient for statistical analysis.
Fig. 1.
A: diagram of a medial view of the right ear showing the spatial orientation of the saccule and lagena (in 1 pouch) and innervations in the Baltic sturgeon (Acipenser sturio) (Retzius 1881). A, anterior semicircular canal; CC, crus commune; E, endolymphatic duct; L, lagena; LM, lagenar macula; P, posterior semicircular canal; S, saccule; SM, saccular macula; U, utricle. Arrow marks the recording location. B: outline of the saccule and lagena in the lake sturgeon (Acipenser fulvescens) showing the orientation of the hair cells. Arrows point in the direction of the kinocilium. [Adapted from Lovell et al. 2005 with permission.]
To gain access to the nerve, the brain was carefully retracted toward the left with the use of a plastic retractor, providing a large enough area to record from (at least 1.5 mm long and 0.8 mm wide) and access many different fibers within the nerve during each experiment. Special care was taken to record from as many locations of the nerve as possible during each experiment.
The microelectrode was positioned on the nerve with a remote motorized microdrive (FHC, Bowdoinham, ME). For our recordings we focused on the nerve branch innervating the saccule and lagena (and not on the utricular nerve branch), since most data available in teleosts are from saccular afferents for comparison (saccule: Fay 1984; Fay and Edds-Walton 1997a; Lu et al. 1998; Weeg et al. 2002; lagena: Lu et al. 2003; Meyer et al. 2004; utricle: Fay 1984; Lu et al. 2004).
Neural signals were amplified using a DAM80 Bioamplifier (World Precision Instruments) and bandpass filtered between 300 and 3,000 Hz. Spikes were discriminated using a single voltage criterion, and spike times were recorded with a 0.1-ms sampling period.
Data analysis.
From the spike times recorded during stimulation, period histograms were formed and the coefficient of synchronization (R) was calculated to measure the degree of phase locking (Goldberg and Brown 1969). To minimize misinterpretations of R when having a smaller number of spikes (N), the Rayleigh statistic Z = R2Ns was applied to quantify phase locking (Batschelet 1981; Fay 1984). The Z value represented the response magnitude for all directional afferents in this study, since units strongly phase-locked to the stimulus. To be accepted into the category of strong phase-locking, units had to have a Z value of 20 or more (the probability of obtaining a Z value of 20 by chance is 0.001).
To quantify directional preference of individual afferents, the fish was stimulated along six directions in the horizontal plane and six directions in the midsagittal plane. The Z value as a function of stimulus direction was presented in two polar coordinate plots, corresponding to the two planes. These polar plots, or directional response patterns (DRPs), were plotted independently for different stimulus levels. For each plot, the response data at the six angles were plotted twice, once at the nominal axis angle and then again at the angle plus 180°. This convention was used because the stimulus was an oscillatory motion along the given axis, and neural responses were averaged and therefore do not define a preference for one polarity of oscillation over the other. The best axis of each DRP was determined by placing a line (conceptually representing a y-axis) through the maximum and minimum of each DRP. The DRPs in sturgeon usually followed a nearly cosine shape, or a nearly circular line passing through the origin in polar coordinates.
The best axis (BA) in three dimensions was computed from the BA components measured in the horizontal and vertical planes. Background activity was defined as spontaneous firing in the absence of an intentional stimulus and was measured over eight repetitions of the stimulus interval with no stimulus present.
RESULTS
Spatial arrangement and hair cell orientation of the saccule and lagena.
Although the general morphology of the ear of lake sturgeon bears considerable similarity to ears in teleosts, there are also a few differences. Because these differences are important for the interpretation of the physiological data presented, we will summarize them briefly. The reader should refer to Popper (1978) and Lovell et al. (2005) regarding general morphology and hair cell orientation and to Meyer et al. (2010) regarding innervation and recording site for a more detailed description.
In contrast to the solid otoliths of teleosts, the sensory epithelia of the utricle, saccule, and lagena in lake sturgeon are covered by otoconia embedded in a gelatinous matrix. The utricle lies in the horizontal plane of the fish, whereas the saccule and lagena are both located in one vertically oriented pouch (Fig. 1A). The ciliary bundles on the apical surface of most hair cells on the saccule are dorsoventrally oriented (with the kinocilium pointing up or down in elevation; Fig. 1B), but the anterior part of the saccule bends such that ciliary bundles of that portion are oriented approximately along the anterior posterior axis (kinocilia pointing along the front-back axis of the fish; Fig. 1B). This is typical for the saccule in ancestral bony fish (Mathiesen and Popper 1987; Platt et al. 2004; Popper 1978; Popper and Northcutt 1983). The ciliary bundles on the hair cells of the lagena have more variation in orientation, with some being oriented dorsoventrally and some deviating from this orientation slightly (Fig. 1B). Most areas of the saccular and lagenar epithelia are nearly parallel to the midsagittal plane as determined from computer-assisted tomography (CT) scans that were constructed from cross sections through the head of lake sturgeon (Meyer M and Ketten DR, unpublished observations).
Physiology.
We recorded and analyzed 177 sound-responsive units from 21 animals. All of these units showed directional selectivity and strongly phase-locked to the stimulus waveform (Z ≥ 20). For 170 of these directional units, we also obtained frequency responses (Meyer et al. 2010). The BF of those units ranged between 100 and 330 Hz. Sixty percent of the units had their BF at 100 Hz and about 30% at 141 Hz. Threshold of directional fibers ranged between −23 and 34 dB (mean: 9 dB), thus representing an absolute threshold range of about 60 dB re 1 nm of displacement (Meyer et al. 2010).
Eighty-three additional units (same set of 21 animals) from which we recorded showed no phase-locking. Most of those units were found more dorsally on the nerve branch that innervated the saccule and lagena. We tested frequency and directional responses on a subset of those units (13/83), and some of them (8/13) responded with increased spike rate to the directional stimulus. Those units were analyzed to test the effect of level, frequency, and directionality on spike rate, not on Z.
Directional responses.
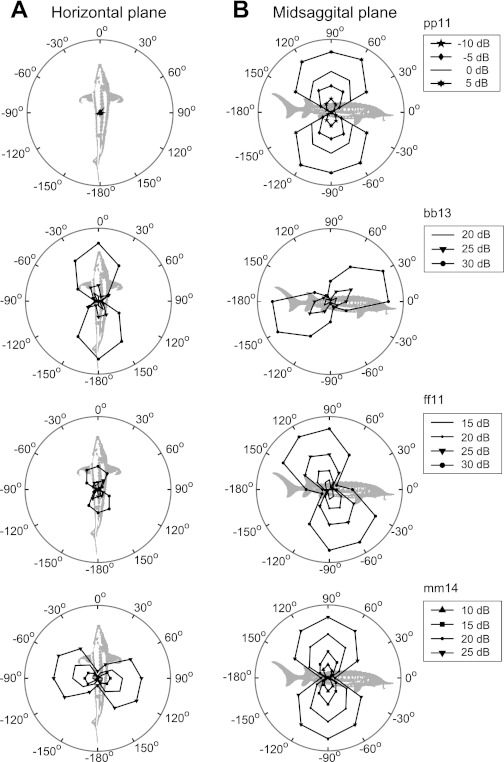
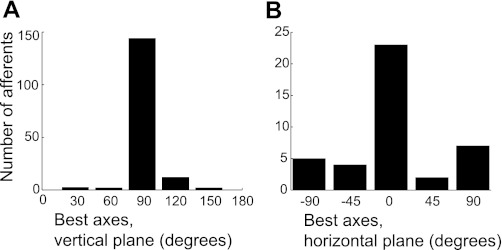
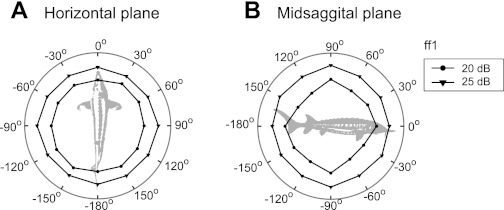
Directionally selective afferents showed DRPs that approximately resembled cosine functions, with a best axis in the vertical and/or horizontal plane indicating the BA of a unit. The DRP shapes shown in Fig. 2 are representative for directionally tuned units in lake sturgeon. Most afferents (76%, 135/177) had their BA near the vertical axis (between 80° and 100°, Fig. 3A) and did not respond at all to horizontal stimulation (e.g., afferent pp11, Fig. 2A). The remainder (24%, 42/177) showed responses resembling a cosine function, also in the horizontal plane (e.g., bb13, ff11, and mm14, Fig. 2).
Fig. 2.
Directional response profiles (DRPs) of 4 afferents using the Z value as a measure of response strength. For each unit, DRPs are obtained for horizontal plane stimuli (A) and midsagittal plane stimuli (B) at various levels. The maximum amplitude (maximum response) and best frequency (BF) for each unit were as follows: pp11: Z = 304, BF = 100 Hz; bb13: Z = 42, BF = 200 Hz; ff11: Z = 278, BF = 141 Hz; mm14: Z = 243, BF = 100 Hz.
Fig. 3.
Distributions of best axes (BAs) in the vertical plane (A; n = 177 afferents, bin width = 30°) and horizontal plane (B; n = 42 afferents, bin width = ±45°). Note that 135 afferents did not show any response to stimuli in the horizontal plane.
Most BAs in the horizontal plane clustered between −28° and 25° azimuth (26/42, Fig. 3). For these afferents, the BAs in the vertical plane usually differed from 90° (e.g., ff11: vertical plane BA = 120°). However, 13 afferents with BAs in the vertical plane at 90° also had BAs at or near ±90° in the horizontal plane (9%, 13/42; e.g., mm14, Fig. 2).
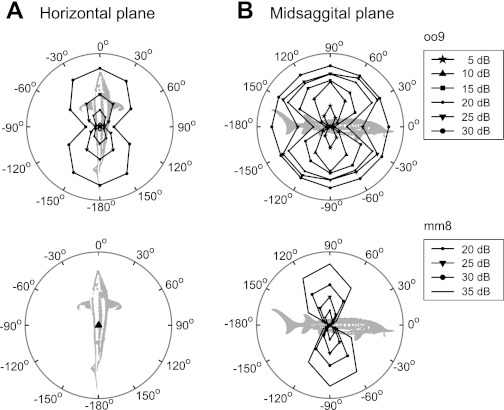
Figure 4 shows two examples of afferents representing extreme cases of DRPs. Afferent oo9 had a narrow vertical plane DRP within its dynamic range (5 and 10 dB) and lost directionality at the highest level (circle at 30 dB, Fig. 4B, top). The response to horizontal plane stimuli (unit oo9 DRP with BA at 0°, Fig. 4A, top) only occurred at the highest levels (at saturation of the unit in response to vertical plane stimuli). The DRP of a unit was called “narrow” when the ratio of the Z value at the BA and the Z value at the stimulus angle deviating ±30° from the BA was larger than one. Six percent (10/177) of afferents had a narrow DRP at lower levels like oo9, but this afferent was the only one becoming omnidirectional (equally responsive to all angles tested) at the highest level tested. In contrast, afferent mm8 also had a narrow DRP in the vertical plane (Fig. 4B, bottom), but the profile remained narrow across all levels as was the case for 3% (6/177) of all afferents.
Fig. 4.
Two afferents represented extreme cases in terms of DRP shape (using the Z value as a measure of response strength). A: responses to horizontal plane stimuli. B: responses to vertical plane stimuli. The maximum amplitude for each unit was as follows: oo9: Z = 415, BF = 100 Hz, mm8: Z = 189, BF = 141 Hz.
Non-phase-locking responses.
Eighty-three units encountered during recordings did not show any phase-locking to the stimulus waveform (Z ≤ 5). Therefore, instead of Z, spike rate was plotted as a function of stimulus angle for a subset of these units (13/83). Eight of these units responded with an increased spike rate to directional stimuli, but they showed approximately the same spike rate to all axis angles tested (Fig. 5) and were therefore classified as omnidirectional. Their spike rate increased with level but remained omnidirectional. In addition, the isolevel frequency response function of these units resembled a low-pass filter with a BF below 100 Hz (not shown).
Fig. 5.
Example of a non-phase-locking unit with Z values <5. Spike rate (instead of the Z value) is plotted as a function of stimulus angle. Spike rate increased with level. A: responses to horizontal plane stimuli. B: responses to vertical plane stimuli. Units with this response pattern have been classified as efferents in teleosts (Edds-Walton et al. 1999; Weeg et al. 2002). Average spike rates were 55 spikes/s at 20 dB, 68.9 spikes/s at 25 dB, and 26 spikes/s at background rate.
Best directions in three dimensions.
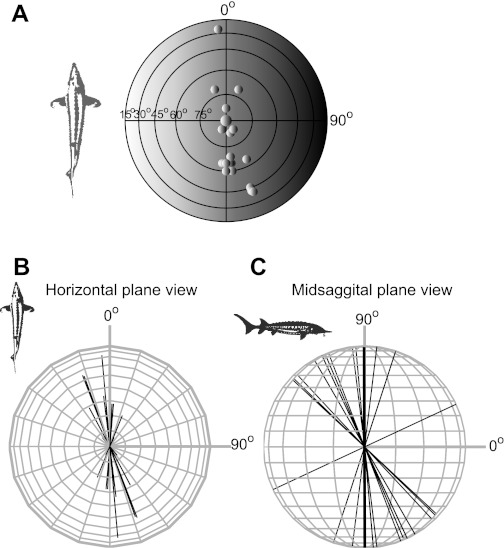
In Fig. 6, the best axis orientations for 156 afferents evaluated for BAs in azimuth and elevation are summarized in spherical space. The majority of afferents had their BA at 90° vertical as represented by the sphere in the center of Fig. 6A and the thicker middle line in Fig. 6C (see also Table 1). The 3-D representations illustrate that there is some spread of BAs in the vertical plane (Fig. 6, A and C) and much less variety in BAs to stimuli in the horizontal plane, since most axes pointed toward 0° azimuth (Fig. 6B, front-back direction).
Fig. 6.
BAs in 3-dimensional (3-D) space for 156 afferents plotted at different perspectives. A: top view of a globe with the fish at the center. Small spheres correspond to angles in elevation (cross point at the center represents 90°, the BA of most units). Each sphere stands for the location on the globe's surface at which one afferent's BA penetrates the surface. The 0° azimuth is in front of the fish; the 90° azimuth is to the right of the fish. B: horizontal plane projection of the 3-D vectors. C: vertical plane projection of the 3-D vectors.
Table 1.
Best axes for azimuth and elevation used for the three-dimensional plots in Fig. 6
| Azimuth, ° | Elevation, ° | No. of Cells |
|---|---|---|
| 22 | 72 | 1 |
| −20 | 72 | 1 |
| 25 | 95 | 1 |
| No response | 90 | 135 |
| −5 | 25 | 1 |
| 5 | 115 | 1 |
| 3 | 115 | 1 |
| −28 | 113 | 2 |
| −7 | 115 | 1 |
| −5 | 115 | 1 |
| −7 | 111 | 1 |
| −7 | 120 | 1 |
| 0 | 115 | 1 |
| 0 | 120 | 1 |
| −20 | 133 | 1 |
| −20 | 136 | 1 |
| −21 | 137 | 1 |
| −20 | 97 | 1 |
| 0 | 90 | 1 |
| −40 | 95 | 1 |
| 0 | 83 | 1 |
Values are for n = 156 afferents.
DISCUSSION
In this study, we characterized the response properties of primary auditory afferents to directional stimulation. Our methods were the same as those used in previous teleost studies, since we intended to make our data directly comparable to findings in teleosts (e.g., Atlantic cod, Gadus morhua, Hawkins and Horner 1981; goldfish, Carassius auratus, Fay 1984; oyster toadfish, Fay and Edds-Walton 1997a; and sleeper goby, Dormitatus latifrons, Lu et al. 1998).
Directional responses.
Directional selectivity was found in all phase-locking afferents as illustrated by the shape of the isolevel response functions to varying directional stimuli. The shape resembled a cosinusoidal function. This was typically found in primary auditory afferents of teleosts (Hawkins and Horner 1981; Fay 1984; Fay and Edds-Walton 1997a; Lu et al. 1998; Weeg et al. 2002) and was expected if afferents innervated a single hair cell or a group of hair cells having similar directional preferences. Peripheral directional encoding occurred at a wide range of stimulus intensities because units varied widely in threshold in lake sturgeon (Meyer et al. 2010). Afferents with sharpened DRPs across all levels (such as unit mm8, Fig. 3B, bottom) or DRPs that flattened and broadened with level have also been described for a few saccular or lagenar afferents in goldfish, sleeper goby, and midshipman fish, Porychthus notatus (Fay 1984; Lu et al. 1998, 2003; Weeg et al. 2002).
Afferents that did not show any phase-locking to the stimulus waveform (Z ≤ 5; Fig. 5) but fired with an increased spike rate to directional stimulation with no directional preference have been reported for the oyster toadfish (Edds-Walton et al. 1999; Weeg et al. 2002) and categorized as responses from efferent fibers. Anatomic studies in the oyster toadfish showed that fibers of this response type innervated a much larger area of the saccular epithelium than units with directional tuning (Edds-Walton et al. 1999). Another possibility is that non-phase-locking units have innervated the posterior crista or the macula neglecta (Meyer et al. 2010), although we would not necessarily expect those fibers to respond to linear acceleration because the posterior crista and macula neglecta are not otolith organs.
BAs in the vertical plane.
Nine percent of all directionally tuned afferents had DRPs with BAs at 90° vertical and nearly 90° in the horizontal plane (unit mm14, Fig. 2). For an afferent with a BA at 90° (vertical plane), one would normally expect to see a null in the horizontal plane if these afferents innervated hair cells that are oriented dorsoventrally (unit pp11, Fig. 2). A response at 90° in the horizontal plane may be explained by hair cells that are located at parts of the epithelia that slightly bend into the horizontal plane (most caudal part of the lagena) or by hair cells that are tilted at parts of the epithelium that are uneven. Thus a better way to determine their exact BAs would be by moving the fish along six axes in the frontal plane. These units were not included in the 3-D representation, since frontal plane stimulation was not used in data collection.
For the majority of afferents (75%), BAs for the vertical plane clustered around 90°. These afferents did not respond to horizontal stimulation, or only at very high levels. Such preference for 90° angles (up-down axis) in the vertical plane is consistent with the vertical orientation of the saccular and lagenar otolithic organs and with the dorsoventral orientation of a large proportion of hair cell cilia on these epithelia (Fig. 1; Lovell et al. 2005; Popper 1978).
Some variety of hair cell orientations in the vertical plane, especially of lagenar hair cells, has been reported in lake sturgeon (Lovell et al. 2005). Thus one would expect to see more afferents with BAs other than 90° vertical. To avoid sampling biases, we employed several techniques, including the use of electrodes with different diameters, positioning electrodes on top of the nerve at multiple locations (at least about 10 different positions per experiment), and advancing through the entire nerve in 1-μm steps in depth during each experiment. During recordings from one animal, uniformity of afferent best responses to vertical plane stimuli at 90° occurred, although these afferents varied a lot in terms of thresholds and spontaneous activity. This observation also reduces the possibility that we recorded from one fiber multiple times during one experiment. Although we attempted to avoid sampling bias, there may still be a subpopulation of afferents that remained uncharacterized.
In the oyster toadfish, the best elevations could not always be predicted by hair cell orientation in the region innervated (Edds-Walton et al. 1999), and curvature of the epithelia and interactions of otoliths with the hair cell layer may have resulted in unpredicted irregularities in directional tuning of afferents (Edds-Walton et al. 1999). The same may apply to afferent responses in lake sturgeon.
In summary, the peripheral encoding of sound source direction in elevation by saccular and lagenar afferents appears to be restricted in lake sturgeon compared with teleosts. Other computational strategies may be used to encode the location of a sound in elevation. For instance, the sturgeon could use a central comparison of the inputs from afferents innervating the horizontally oriented utricle with the inputs from vertically oriented saccule and lagena. Nonauditory strategies, such as directing the head to the area of maximal sensitivity (similar to eye and head movements centering a visual scene onto the fovea) may also be used to compensate the limitations found in lake sturgeon. Directional sensitivity may be emphasized for the vertical plane, since information from the top may be most important for lake sturgeon, being bottom feeders. In addition, the vestibular role of the saccule and lagena provides the animal with gravitational information in the vertical plane.
BAs in the horizontal plane.
The azimuths of those primary afferents that responded to horizontal stimuli clustered around 0° (front-back axis). This can be explained by major areas of the saccule and lagena being oriented parallel to the midsagittal plane of the fish (along the front-back axis of the fish). All findings in teleost fishes studied so far indicate that the best azimuth of primary afferents innervating the saccule or lagena generally is in line with the orientation of the epithelium in the respective species (Fay 1984; Fay and Edds-Walton 1997a; Lu et al. 1998, 2003; Meyer et al. 2004; Weeg et al. 2002).
In teleost fishes, the saccule and lagena deviate from the midline of the fish at angles between 16° and 40° in azimuth (Fay 1984; Edds-Walton and Fay 1997a; Lu et al. 1998, 2003). For any sound source in azimuth, the stimulation of the right and left saccules (or lagenae) leads to differences in response magnitude arising from these epithelia in teleost fishes. This difference in response magnitude could then be used to centrally compute the azimuth of a sound source (first suggested by Sand 1974). Anatomical and physiological evidence for binaural processing has been found in teleosts beginning at the level of the brain stem (reviewed by Fay 2005; Edds-Walton and Fay 2009).
In contrast to teleosts, a comparison between the overall output of the two ears cannot be used in lake sturgeon for the computation of azimuth, since most portions of the saccular and lagenar epithelia are oriented parallel to the midline of the fish and stimulation by a sound source located at the right or left would cause similar response magnitudes in both ears. One alternative mechanism to accomplish source location in azimuth, however, would be through activation of hair cells on the horizontally oriented utricle. In goldfish and sleeper goby, afferents innervating the utricle showed a wide range of BAs to horizontal plane stimuli (Fay 1984; Lu et al. 2004). This was consistent with the widespread morphological polarization of hair cells in the horizontal plane of the utricle in these species. The horizontally oriented utricle of lake sturgeon also has a wide range of hair cell orientations (Lovell et al. 2005). Thus it would be interesting to investigate utricular afferent responses to horizontal plane stimuli in lake sturgeon to test whether the utricle may play a role in encoding the particle motion axis in azimuth similarly to goldfish and sleeper goby.
Adult lake sturgeon can reach a body length up to 164 cm (Dumont et al. 1987). The use of interaural time differences, which occur when sound from the left or right reaches one ear earlier in time than the other (as it applies to land vertebrates), would potentially be possible in adult lake sturgeon because of their large head size (estimated head width of adult lake sturgeon at the level of the ears is 15 cm; for juvenile sturgeon used in this study, minimum distance was 1.5 cm). The estimated distance between the two ears could result in time delays that a central nervous system would be able to compute. For instance, an interaural distance of 10 cm would result in a time delay for underwater sound of 67 μs, and a distance of 1.5 cm in a time delay of 10 μs, between the two ears. Studies in terrestrial vertebrates have shown that time delays of 10 μs can be discriminated (Joris et al. 1998). However, similar data for fish are not available.
Summary and evolutionary considerations.
The shapes of the DRPs resembling a cosine function were very similar to those found in the periphery of teleosts (modern bony fishes), which we know most about. This observation agrees with the finding that many basic physiological characteristics typical for the encoding of sound in teleosts and other vertebrates have been found in lake sturgeon (see also Meyer et al. 2010). However, coding for sound direction in the vertical and horizontal planes seems limited in lake sturgeon. Thus mechanisms for encoding sound direction in the lake sturgeon might either be limited or different from the strategies proposed for teleosts. Certainly, behavioral experiments in lake sturgeon are needed to evaluate the capability of locating a sound source.
The coding strategies for sound direction in lake sturgeon could represent the ancestral condition of all fishes. It should be noted, however, that during the course of its evolution, lake sturgeon might also have developed characteristics in parallel to those of modern fishes. In addition, lake sturgeon may have adapted to certain ecological niches (living at the bottom of rivers and lakes), leading to a constrained ability to localize sounds. As a consequence, a full assessment of ancestral (representing the ancestral condition) versus more modern coding strategies can only be made by comparing more species of the group of nonteleost Actinopterygian bony fishes and by looking at the out group of bony fishes, the elasmobranchs (sharks and rays). One study that involved two species of sharks (spotted and brown-banded bamboo shark) demonstrated that the physiological responses (auditory evoked potentials) to particle acceleration had nearly equal thresholds at all seven directions (Casper and Mann 2007).
Most teleosts species are smaller than sharks and sturgeon (smaller head sizes make the use of sound cues more difficult) and thus may have experienced different environmental pressures, leading to a different or even improved development of mechanisms for sound source localization. However, more data, at the behavioral and physiological level in elasmobranchs as well as in other nonteleost ray-finned bony fishes, are needed to evaluate such a hypothesis.
GRANTS
We thank the Parmly Hearing Institute for providing a Parmly Hearing Scholar fellowship to M. Meyer during a 3-mo stay in Chicago. This research was supported in part by National Institutes of Health (NIH) Grants R01 DC006215 to R. R. Fay and the Parmly Hearing Institute of Loyola University Chicago and P30 DC004664 to the Center of Comparative and Evolutionary Biology of Hearing of the University of Maryland.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.M., A.N.P., and R.R.F. conception and design of research; M.M. performed experiments; M.M. analyzed data; M.M., A.N.P., and R.R.F. interpreted results of experiments; M.M., prepared figures; M.M. drafted manuscript; M.M., A.N.P., and R.R.F. edited and revised manuscript; M.M., A.N.P., and R.R.F. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Kaushik Ghose for help with Matlab. Lake sturgeons were kindly supplied by the Wisconsin Department of Natural Resources.
REFERENCES
- Auer NA. Importance of habitat and migration to sturgeons with emphasis on lake sturgeon. Can J Fish Aquat Sci 53, Suppl 1: 152–160, 1996 [Google Scholar]
- Batschelet E. Circular Statistics in Biology. New York: Academic, 1981 [Google Scholar]
- Bemis WE, Kynard B. Sturgeon rivers: an introduction to acipenseriform biogeography and life history. Environ Biol Fishes 48: 167–183, 1997 [Google Scholar]
- Bruch R, Binkowski FP. Spawning behavior of lake sturgeon (Acipenser fulvescens). J Appl Ichthyol 18: 570–579, 2002 [Google Scholar]
- Casper BM, Mann DA. Dipole hearing measurements in elasmobranch fishes. J Exp Biol 210: 75–81, 2007 [DOI] [PubMed] [Google Scholar]
- Christensen-Daalsgard J, Brandt C, Wilson M, Wahlberg M, Madsen PT. Hearing in the African lungfish (Protopterus annectens): pre-adaptation to pressure-hearing in tetrapods? Biol Lett 7: 139–141, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries HL. The mechanics of the labyrinth otoliths. Acta Otolaryngol (Stockh) 38: 262–273, 1950 [DOI] [PubMed] [Google Scholar]
- Dumont P, Fortin R, Desjardins G, Bernard M. Biology and exploitation of lake sturgeon in the Quebec waters of the Saint-Laurent River. In: Proceedings of a Workshop on the Lake Sturgeon (Acipenser fulvescens), edited by Olver CH. Peterborough, ON: Ontario Ministry of Natural Resources, 1987, p. 57–76 [Google Scholar]
- Edds-Walton PL, Fay RR. Physiological evidence for binaural directional computations in the brainstem of the oyster toadfish (Opsanus tau). J Exp Biol 212: 1483–1493, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edds-Walton PL, Fay RR, Highstein SM. Dendritic arbors and central projections of physiologically characterized auditory fibers from the saccule of the toadfish (Opsanus tau). J Comp Neurol 411: 212–238, 1999 [PubMed] [Google Scholar]
- Fay RR. The goldfish ear codes the axis of particle motion in three dimensions. Science 225: 951–953, 1984 [DOI] [PubMed] [Google Scholar]
- Fay RR. Sound source localization by fishes. In: Sound Source Localization, edited by Popper AN, Fay RR. New York: Springer, 2005, p. 36–66 [Google Scholar]
- Fay RR, Edds-Walton PL. Directional response properties of saccular afferents of the toadfish (Opsanus tau). Hear Res 111: 1–21, 1997a [DOI] [PubMed] [Google Scholar]
- Fay RR, Edds-Walton PL. Diversity in frequency response properties of saccular afferents of the toadfish (Opsanus tau). Hear Res 113: 235–246, 1997b [DOI] [PubMed] [Google Scholar]
- Fay RR, Popper AN. Evolution of hearing in vertebrates: the inner ears and processing. Hear Res 149: 1–10, 2000 [DOI] [PubMed] [Google Scholar]
- Flock A. Structure of the macula utriculi with special reference to directional interplay of sensory responses as revealed by morphological polarization. J Cell Biol 22: 413–431, 1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JM, Brown PB. Response of binaural neurons of dog superior olivary complex to dichotic tonal stimuli: some physiological mechanisms of sound localization. J Neurophysiol 32: 613–636, 1969 [DOI] [PubMed] [Google Scholar]
- Grande L, Bemis WE. Interrelationships of Acipenseriformes, with comments on Chondrostei. In: Interrelationships of Fishes, edited by Stianssny ML, Parenti LR, Johnson GD. San Diego, CA: Academic, 1996, p. 85–115 [Google Scholar]
- Hawkins AD, Horner K. Directional characteristics of primary auditory neurons from the cod ear. In: Hearing and Sound Communication in Fishes, edited by Tavolga WN, Popper AN, Fay RR. New York: Springer, 1981, p. 189–219 [Google Scholar]
- Hudspeth AJ, Corey DP. Sensitivity, polarity and conductance change in the response of vertebrate hair cells to controlled mechanical stimuli. Proc Natl Acad Sci USA 74: 2407–2411, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joris PX, Smith PH, Yin TCT. Coincidence detection in the auditory system: 50 years after Jeffress. Neuron 21: 1235–1238, 1998 [DOI] [PubMed] [Google Scholar]
- Liem KF, Bemis WE, Walker WF, Jr, Grande L. Functional Anatomy of the Vertebrates. An Evolutionary Perspective (3rd ed.). Orlando, FL: Harcourt Brace College, 2001 [Google Scholar]
- Lovell JM, Findlay MM, Moate RM, Nedwell JR, Pegg MA. The inner ear morphology and hearing abilities of the paddlefish (Polyodon spathula) and the lake sturgeon (Acipenser fulvescens). Comp Biochem Physiol A Mol Integr Physiol 142: 286–296, 2005 [DOI] [PubMed] [Google Scholar]
- Lu Z, Popper AN. Neural response directionality correlates of hair cell orientation in a teleost fish. J Comp Physiol A 187: 453–465, 2001 [DOI] [PubMed] [Google Scholar]
- Lu Z, Song J, Popper AN. Encoding of acoustic directional information by saccular afferents of the sleeper goby (Dormitator latifrons). J Comp Physiol A 182: 805–815, 1998 [DOI] [PubMed] [Google Scholar]
- Lu Z, Xu Z, Buchser WJ. Acoustic response properties of lagenar nerve fibers in the sleeper goby (Dormitator latifrons). J Comp Physiol A 189: 889–905, 2003 [DOI] [PubMed] [Google Scholar]
- Lu Z, Xu Z, Buchser WJ. Coding of acoustic particle motion by utricular fibers in the sleeper goby (Dormitator latifrons). J Comp Physiol A 190: 923–938, 2004 [DOI] [PubMed] [Google Scholar]
- Mathiesen C, Popper AN. The ultrastructure and innervation of the ear of the gar (Lepisosteus osseus). J Morphol 194: 129–142, 1987 [DOI] [PubMed] [Google Scholar]
- Meyer M, Popper AN, Fay RR. Frequency tuning and directional preferences in lagenar nerve fibers of the goldfish (Carassius auratus) (Abstract). Abstr Midwinter Res Meet Assoc Res Otolaryngol 27: 325, 2004 [Google Scholar]
- Meyer M, Popper AN, Fay RR. Frequency tuning and intensity coding of the auditory periphery of the lake sturgeon (Acipenser fulvescens). J Exp Biol 213: 1567–1578, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson JS. Fishes of the World (4th ed.). New York: Wiley, 1996 [Google Scholar]
- Platt C, Jorgensen JM, Popper AN. The inner ear of the lungfish (Protopterus). J Comp Neurol 471: 277–278, 2004 [DOI] [PubMed] [Google Scholar]
- Popper AN. Ultrastructure of the auditory regions in the inner ear of the lake whitefish. Science 192: 1020–102, 1976 [DOI] [PubMed] [Google Scholar]
- Popper AN. Scanning electron microscopic study of the otolithic organs in the bichir (Polypterus bichir) and shovel-nose sturgeon (Scaphirhynchus platorynchus). J Comp Neurol 18: 117–128, 1978 [DOI] [PubMed] [Google Scholar]
- Popper AN, Fay RR. The auditory periphery in fishes. In: Comparative Hearing: Fish and Amphibians, edited by Fay RR, Popper AN. New York: Springer, 1999, p. 43–100 [Google Scholar]
- Popper AN, Fay RR. Sound Source Localization. New York: Springer, 2005 [Google Scholar]
- Popper AN, Northcutt RG. Structure and innervation of the inner ear of the bowfin, Amia calva. J Comp Neurol 213: 279–286, 1983 [DOI] [PubMed] [Google Scholar]
- Rafinesque CS. Addition to the observations on the sturgeons of North America. Am Mon Mag 1: 288, 1817 [Google Scholar]
- Retzius G. Das Gehörorgan der Wirbelthiere. Stockholm: Samson and Wallin, 1881, vol. 1 [Google Scholar]
- Rogers P, Cox M. Underwater sound as a biological stimulus. In: Sensory Biology of Aquatic Animals, edited by Atema J, Fay RR, Popper AN, Tavolga WN. New York: Springer, 1988, p. 131–149 [Google Scholar]
- Rogers P, Zeddies DG. Multipole mechanisms for directional hearing in fish. In: Fish Bioacoustics, edited by Webb JF, Popper AN, Fay RR. New York: Springer, 2008, p. 233–252 [Google Scholar]
- Rupp B, Northcutt RG. The diencephalon and pretectum of the white sturgeon (Acipenser transmontanus): a cytoarchitectonic study. Brain Behav Evol 51: 239–262, 1998 [DOI] [PubMed] [Google Scholar]
- Sand O. Directional sensitivity of microphonic potentials from the perch. J Exp Biol 60: 881–899, 1974 [DOI] [PubMed] [Google Scholar]
- Sand Bleckmann H O. Orientation to auditory and lateral line stimuli. In: Fish Bioacoustics, edited by Webb JF, Popper AN, Fay RR. New York: Springer, 2008, p. 183–232 [Google Scholar]
- Schuijf A, Buwalda RJA. On the mechanism of directional hearing in cod (Gadus morhua). J Comp Physiol A 98: 333–344, 1975 [Google Scholar]
- Weeg MS, Fay RR, Bass AH. Directional response and frequency tuning in saccular nerve fibers of a vocal fish, Porychthus notatus. J Comp Physiol A 188: 631–641, 2002 [DOI] [PubMed] [Google Scholar]
- Zeddies DG, Fay RR, Alderks PW, Shaub KS, Sisneros JA. Sound source localization by the plainfin midshipman fish (Porichthys notatus). J Acoust Soc Am 127: 3104–3113, 2010 [DOI] [PubMed] [Google Scholar]