Abstract
We describe the real-time movements of the last of the marine mega-vertebrate taxa to be satellite tracked – the giant manta ray (or devil fish, Manta birostris), the world's largest ray at over 6 m disc width. Almost nothing is known about manta ray movements and their environmental preferences, making them one of the least understood of the marine mega-vertebrates. Red listed by the International Union for the Conservation of Nature as ‘Vulnerable’ to extinction, manta rays are known to be subject to direct and incidental capture and some populations are declining. Satellite-tracked manta rays associated with seasonal upwelling events and thermal fronts off the Yucatan peninsula, Mexico, and made short-range shuttling movements, foraging along and between them. The majority of locations were received from waters shallower than 50 m deep, representing thermally dynamic and productive waters. Manta rays remained in the Mexican Exclusive Economic Zone for the duration of tracking but only 12% of tracking locations were received from within Marine Protected Areas (MPAs). Our results on the spatio-temporal distribution of these enigmatic rays highlight opportunities and challenges to management efforts.
Introduction
Satellite tracking has yielded key information about the life history of marine vertebrates, many of which engage in long migrations (travelling thousands of kilometres) [1] and make deep dives [2], beyond the temporal and logistical abilities of researchers to follow them. The insights afforded by such tracking have provided structure around which conservation frameworks and regulations can be built [3] and an understanding of spatial ecology around which marine protected areas (MPAs) can be established (e.g. [4]). Satellite tracking has further provided parameters for models of distribution to enable forecasting of effects of, e.g. climate change, to marine vertebrates (e.g. [5], [6]).
Manta rays (or devil fish, Manta birostris) are the world's largest batoid fish (reaching a measured disc width of 7.1 m), with slow growth and low fecundity, birthing only one or two live ‘pups’ every one to two years following a gestation period of 12 months [7]. They are listed by the International Union for Conservation of Nature (IUCN) as “Vulnerable” to extinction [7] and included on Appendix I and II of the Convention on Migratory Species of Wild Animals. Recently Manta rays were found to encompass a second species Manta alfredi that ranges throughout the Central Eastern Atlantic and Indo-Pacific and possibly a third species constrained to the Gulf of Mexico and the Caribbean [8]. They are known to be purposefully and accidentally captured in fisheries operations and populations in the Pacific, Indian Ocean and Caribbean are apparently declining [7]. Critical information for conservation planning, such as knowledge on their movements and ecology, are however lacking. Indeed, the manta rays may be the least understood of the marine mega-vertebrate groups, and one of the last to be satellite tracked.
Manta rays are most often reported in coastal areas and continental shelves, near seamounts and in upwelling zones [9], [10], [11]. From unpublished reports and popular media, it would appear that manta rays are known to congregate in enormous numbers (up to hundreds of individuals) in some areas (e.g. Mexico, Mozambique, Maldives, Hawaii and Micronesia) for courtship, breeding and to visit cleaning stations. While manta rays are thought to remain resident to some areas [7], particularly the smaller and more coastally-constrained M. alfredi [10], in other areas they are thought to make seasonal long-distance migrations away from breeding areas, although non-breeding sites are not known [12].
Here, we describe the use of real-time satellite telemetry to gather insights into manta ray movements, allowing us to begin to generate environmental parameters for their distribution and assess the extent to which manta rays occur in protected areas.
Materials and Methods
We deployed six towed satellite transmitting position-only tags (Wildlife Computers SPOT5; http://wildlifecomputers.com/spot.aspx) on manta rays, in the southern Gulf of Mexico near Mexico's Yucatan peninsula over the duration of a 13-day research cruise. The research was carried out under permit from the Mexican federal government agency (OFICIO NÚM/SGPA/DGVS/05241). Tags were programmed to record ambient temperature (in asynchronous binning intervals selected to maximise recording around crespuscular periods, at 00:00, 05:00, 11:00, 12:00, 17:00 and 23:00) and to transmit continuously at the sea surface. The tags' position was determined by the Argos System (www.argos-system.org). Tags were attached while swimming behind and above the animal, using a small percutaneous nylon umbrella dart attached to a 1 m long 1/16″ (1.59 mm) stainless steel cable containing a mid-line swivel, inserted into the lower left or lower right quadrant shoulder musculature using a 2 m pole spear. The tags were covered with dark blue antifouling paint to minimize bio-fouling. Manta ray body size, or disc width, the distance between the two unfurled wingtips ±50 cm, was estimated by comparing the ray to a 2 m tagging pole or a snorkeler of known height. Sex was determined by the presence of ‘claspers’ (male sexual organs) [13]. Despite their size, manta rays are cryptic and rarely encountered, we thus applied tags to all the manta rays we were able to encounter during the 13-day sampling period.
Tag-derived ambient temperature data were expressed as a proportion of time spent within predetermined temperature ranges by local night and day periods. These were calculated using the NOAA sunrise/sunset calculator (http://www.srrb.noaa.gov/highlights/sunrise/calcdetails.html) with manta ray latitudes and longitudes, custom coded into MATLAB.
Argos data was filtered to only include location classes (LC) A, B, 0, 1, 2 and 3 for which location accuracy has been determined [14], [15]; locations with LC Z were removed. Unrealistic locations were also removed (swimming speeds greater than 20 km/hr). A behaviourally switching state-space model (SSM) was applied to Argos tracking data to handle observation error, improve data retention, and infer animal behavioural state (referred to as ‘transiting’ and ‘foraging’) from the movement patterns [16]. We used the model originally described by Jonsen et al [17] and refined by Breed et al [16], which has been successfully applied to a number of marine species including pinnipeds [16], [18], sea turtles [19], [20], [21] and cetaceans [22]. The SSM was generated using the software packages R and WinBUGS, and we estimated locations at five-hour intervals, reflecting the average number of Argos locations we received per day [16]. Model parameters were estimated using Markov Chain Monte Carlo (MCMC) estimation from two MCMC chains. We used 10,000 iterations after a burn-in of 5,000 and thinned by five to give the mean and variance for each location and behavioural parameter. Behaviour was discriminated into the two states based on the mean turning angle (γ) and autocorrelation in speed and direction (θ). We observed a lack of overlap between the parameters representing the opposing behavioural states, which indicated a true differentiation in movement patterns.
Movement metrics, describing transit speed and distance travelled, and coincident environmental data (sea surface temperature, chlorophyll-α, bathymetry and sea surface currents) were determined for each position. Environmental data were downloaded from the GODAE High Resolution Sea Surface Temperature Pilot Project (SST, http://ghrsst-pp.metoffice.com/, at ∼1 km resolution), NASA Goddard space flight centre Ocean colour (chlorophyll-α, http://oceancolor.gsfc.nasa.gov/SeaWiFS/,∼4 km product), the General Bathymetric Chart of the Oceans, GEBCO (bathymetry, http://www.gebco.net/, at 30 seconds arc resolution) and CLS AVISO OceanObs (sea surface currents, http://www.aviso.oceanobs.com/, at a resolution of 0.3° at the equator). Locations were also overlaid with the World Database of Protected Areas (http://www.unep-wcmc.org/wdpa) to assess the proportion of locations that were received from within Marine Protected Areas (MPAs).
Areas of high use by manta rays were determined using a quartic kernelling approach [23]. Data were first resolved to the best daily location per individual (if >1 highest location classes were received, the earliest was used) and data from all individuals was grouped for analysis. A utilisation distribution was subsequently created from the satellite tracking data using a smoothing parameter, h, of 10 km (which best represented the underlying spatial architecture of the location data) on a 1×1 km grid and percent volume contours (25, 50 and 75%) were created from the resulting raster.
Results
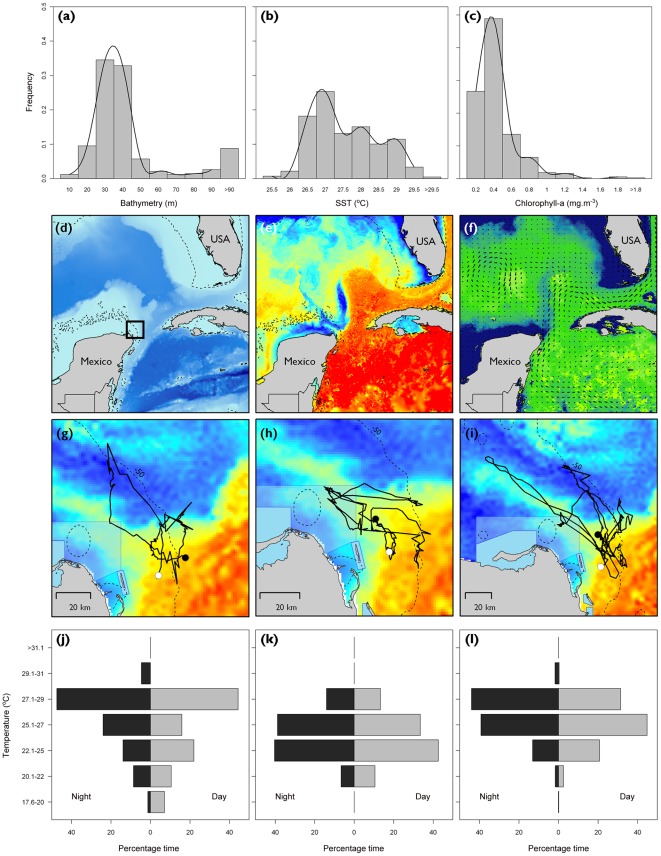
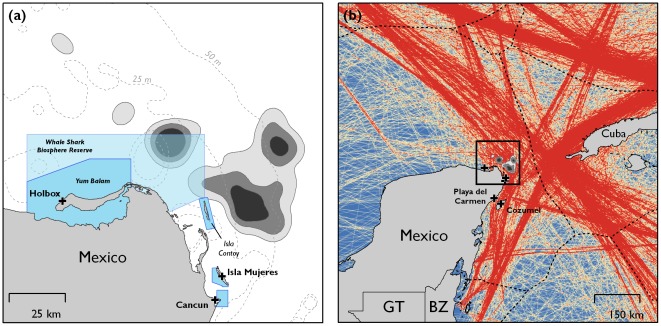
Tags provided data for a mean of 27 days (±21.6 s.d., range 2 to 64 days, Table 1). Tagged animals (n = four females, one male and one juvenile ray of indeterminate sex) remained in frontal zones off the Yucatan peninsula, traversing them repeatedly. Tracks showed strong separation between the state-space model behavioural parameters (γ and θ). Manta rays were in ‘foraging’ state for 97.7% of the locations received from the Argos System, moving at 1.2 km.h−1 (grand median of medians per individual, range 0.9 to 1.7 km.h−1) with animals covering as much 1,151 km before transmission ceased (Fig. 1, cumulative straight line distance between locations, mean track length 368±425 km s.d.). Manta rays moved up to 116 km away from their tag attachment locations and remained within Mexico's territorial jurisdiction for the duration of tracking. Most manta ray locations occurred further than 20 km offshore (92% of all locations) and only 11.5% locations occurred within MPAs (Fig. 2a). Areas with high relative densities of manta ray locations overlapped with dominant shipping routes within the region (Fig. 2b). There were no apparent differences in movement patterns by sex or body size (Table 1), or with ambient water-column temperature (Fig. 1j–l).
Table 1. Deployment metrics for six manta rays.
| Manta Ray | Location | Deployed | Detached | Duration | Sex | DW (cm) |
| 1 | Oligotrophic | 10-Sep-10 | 10-Oct-10 | 32 | F | 400 |
| 2 | Eutrophic | 20-Jul-10 | 10-Aug-10 | 19 | F | 300 |
| 3 | Eutrophic | 21-Jul-10 | 24-Jul-10 | 4 | F | 450 |
| 4 | Oligotrophic | 08-Sep-10 | 10-Sep-10 | 2 | F | 400 |
| 5 | Oligotrophic | 09-Sep-10 | 14-Oct-10 | 41 | UNK | 400 |
| 6 | Oligotrophic | 08-Sep-10 | 29-Oct-10 | 64 | M | 350 |
| Mean (range) | 27 (2–64) | 383 (350–450) |
Figure 1. Movements of manta rays in the western Caribbean and south-east Gulf of Mexico.
Frequency histograms of (a) bathymetry, (b) SST and (c) chlorophyll-α determined from the locations of all satellite tracked manta rays. Regional mapping of (d) bathymetry, (e) SST and (f) Chlorophyll-α imagery with geostrophic currents for 10th Oct 2010. (g–i) Tracks of three of the six manta rays (one female, one male and the juvenile manta ray, mantas 1, 5 and 6, Table 1) are shown with SST imagery (10th October). (j–l) Mean percentage time at temperature plots during night and day (temperature recorded by animal-borne tags) for the same individuals.
Figure 2. Utilisation distribution of manta ray locations (a) (quartic kernelling; grey polygons showing 25%, 50%, 75%, from darkest to lightest grey).
Blue polygons show marine protected areas, tourism ports are indicated (black crosses). Commercial shipping activity, showing transit of boats belonging to the World Meteorological Organisation Voluntary Observing Ship Scheme (b) (red showing higher density of ship transit) from [41]. Core manta ray foraging areas are indicated, with Mexican tourism ports (Holbox, Isla Mujeres, Cancun, Playa del Carmen and Cozumel).
Satellite-tracked manta rays were rarely located in water deeper than 50 m (83% of all locations from waters shallower than 50 m, with 92% of all locations received from waters between 5 and 100 m deep, Fig. 1). Manta rays foraged in waters with sea surface temperatures ranging from 25.1 to 30.0°C, with 95% of all locations occurring in waters warmer than 26.1°C. The majority of manta ray locations occurred in waters with surface chlorophyll-α values between 0.14 and 0.76 mg.m−3 (5th to 95th percentiles, median 0.28 mg.m−3), and geostrophic current speeds of 8.4 to 94.0 cm.sec−1 (5th to 95th percentiles, median 76.6 cm.sec−1).
During our 13 days of boat surveying, including the period in which satellite tags were deployed, we made opportunistic plankton tows (using a 212 µm mesh, 50 cm diameter net) when we observed manta rays ram filter feeding at the surface and sub-surface to identify the prey species they were consuming. Manta rays were observed feeding in both oligotrophic waters during a seasonal spawning event of little tunny (Euthynnus alletteratus) and in eutrophic waters where a seasonal upwelling event (lasting between May and September) gave rise to significant concentrations of zooplankton. Our survey thus enabled us to confirm that manta rays were likely consuming sergestid shrimp and calanoid copepods, as well as chaetognaths and fish eggs.
Discussion
Effective establishment of marine protected areas for the conservation of species of concern depends on a robust understanding of their spatio-temporal distribution [24], [25], [26], [27]. Such understanding has now been gained for many marine species, including some that make basin-wide migrations [28], [29], [30], [31], [32], [33]. With technological improvements, the accuracy with which marine species can be localised has improved more than ten-fold [15], [34], [35] and a suite of ancillary data is now often collected as well as location to inform on migratory and foraging strategies [36], [37], [38].
Models of the spatio-temporal distribution of marine mega-vertebrates may enable both site- based conservation, such as the design and siting of marine protected areas, and the forecasting of climate change effects that may inform future mitigation measures (13). The Whale Shark Biosphere Reserve, declared in 2010, was intended to specifically enhance protection of whale sharks foraging off the Yucatan peninsula; however, it does not encompass the movements of manta rays tracked in this study [39], [40]. Further, it seems that manta ray aggregations coincide with some of the Caribbean's busiest shipping lanes [41], whose impact on manta rays is as yet unknown. Despite legal protection in Mexican waters (Norma Oficial Mexicana NOM-029-PESC-2006, 14 Feb 2007 Diario Oficial), occasional targeted and bycatch capture of manta rays still takes place (Anonymous Fishermen from Quintana Roo, Pers. Obs.) to be used for food and as bait in the shark fishery. There is also a growing demand in Asia for their gill rakers, which are used in traditional medicine [42]. The greatest impact on the aggregation in the next decade, however, may come from the region's expanding and largely unregulated marine megafauna tourism industry.
Acoustic tracking and photo-identification work have suggested strong site fidelity by manta rays to foraging areas in Indonesia [11], Hawaii [13] and Mozambique [43]. Our data add to this picture for the Atlantic Ocean; however, the capacity of manta rays for undertaking long-range migrations still remains uncertain. Without depth recording tags or detailed knowledge on the vertical structure of the water-column, we cannot confirm whether manta rays in this study, like many other planktivores, exhibited diel vertical migration, where animals track their diel migrating planktonic prey through the water column [44], [45]. Manta rays in this study likely foraged on three major prey types: (i) copepods (occurring in eutrophic waters), (ii) chaetognaths (known predators of copepods, influencing their distribution [46]) and (iii) fish eggs (spawned in oligotrophic waters where larval transport is optimised). However, manta 3, tagged in eutrophic waters (observed foraging on copepods), was re-sighted 57 days later foraging on fish spawn in oligotrophic waters, demonstrating that mantas can switch between habitat and prey types. Such plasticity in diet is worthy of further investigation.
Our data suggest that manta rays are foraging over large spatial scales (∼100 km long), too far offshore and too wide ranging to be included within existing MPA networks. Nevertheless, our data highlight significant site fidelity and association with frontal zones, which could be used to assess current biosphere reserve boundaries or to establish new dynamic protected areas overlaying the frontal region. The use of spatial data sets encompassing longer tracking periods are desirable to better inform manta ray management.
We provide a detailed description of the movements and environmental preferences of manta rays, highlighting what are likely foraging movements in shallow waters, in broad thermal fronts off an upwelling zone. We emphasise that few locations are received from protected areas and that manta rays may be subject to anthropogenic threats throughout their putative foraging range. While the broader migratory movements of manta rays are still not known, it is clear that satellite tracking technology has the potential to offer great inroads into understanding movements and contextualising spatially explicit threats to this species.
Acknowledgments
We are grateful to Mexico's Comisión Nacional de Áreas Naturales Protegidas and the tour guides of Holbox for supporting tagging efforts in the field.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was funded by the The Summit Foundation (http://www.summitfdn.org/ and by two other foundations who wish to remain anonymous. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Gore MA, Rowat D, Hall J, Gell FR, Ormond RF. Transatlantic migration and deep mid-ocean diving by basking shark. Biology Letters. 2008;4:395–398. doi: 10.1098/rsbl.2008.0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Graham RT, Roberts CM, Smart JC. Diving behaviour of whale sharks in relation to a predictable food pulse. Journal of the Royal Society, Interface. 2006;3:109–116. doi: 10.1098/rsif.2005.0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pressey RL, Cabeza M, Watts ME, Cowling RM, Wilson KA. Conservation planning in a changing world. Trends in Ecology & Evolution. 2007;22:583–592. doi: 10.1016/j.tree.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Hyrenbach KD, Forney KA, Dayton PK. Marine protected areas and ocean basin management. Aquatic Conservation: Marine and Freshwater Ecosystems. 2000;10:437–458. [Google Scholar]
- 5.Witt MJ, Hawkes LA, Godfrey MH, Godley BJ, Broderick AC. Predicting the impacts of climate change on a globally distributed species: the case of the loggerhead turtle. J Exp Biol. 2010;213:901–911. doi: 10.1242/jeb.038133. [DOI] [PubMed] [Google Scholar]
- 6.Hawkes LA, Witt MJ, Broderick AC, Coker JW, Coyne MS, et al. Home on the range: spatial ecology of loggerhead turtles in Atlantic waters of the USA. Diversity and Distributions. 2011;17:624–640. [Google Scholar]
- 7.Marshall A, Bennett MB, Kodja G, Hinojosa-Alvarez S, Galvan-Magana F, et al. Manta birostris. 2011. IUCN 2011. IUCN Red List of Threatened Species. Version 2011.2. Available: < www.iucnredlist.org>. Accessed: 03 April 2012. http://www.iucnredlist.org/apps/redlist/details/198921/0.
- 8.Marshall AD, Compagno LJV, Bennett MB. Redescription of the genus Manta with resurrection of Manta alfredi (Krefft, 1868) (Chondrichthyes; Myliobatoidei; Mobulidae). Zootaxa. 2009;2301:1–28. [Google Scholar]
- 9.Anderson RC, Adam MS, Goes JI. From monsoons to mantas: seasonal distribution of Manta alfredi in the Maldives. Fisheries Oceanography. 2011;20:104–113. [Google Scholar]
- 10.Couturier LIE, Jaine FRA, Townsend KA, Weeks SJ, Richardson AJ, et al. Distribution, site affinity and regional movements of the manta ray, Manta alfredi (Krefft, 1868), along the east coast of Australia. Marine and Freshwater Research. 2011;62:628–637. [Google Scholar]
- 11.Dewar H, Mous P, Domeier M, Muljadi A, Pet J, et al. Movements and site fidelity of the giant manta ray, Manta birostris, in the Komodo Marine Park, Indonesia. Marine Biology. 2008;155:121–133. [Google Scholar]
- 12.Hoolihan JP, Luo J, Abascal FJ, Campana SE, De Metrio G, et al. Evaluating post-release behaviour modification in large pelagic fish deployed with pop-up satellite archival tags. ICES Journal of Marine Science. 2011;68:880–889. [Google Scholar]
- 13.Deakos MH, Baker JD, Bejder L. Characteristics of a manta ray Manta alfredi population off Maui, Hawaii, and implications for management. Marine Ecology-Progress Series. 2011;429:245–260. [Google Scholar]
- 14.Costa DP, Robinson PW, Arnould JPY, Harrison A-L, Simmons SE, et al. Accuracy of ARGOS Locations of Pinnipeds at-Sea Estimated Using Fastloc GPS. PLos ONE. 2010;5:e8677. doi: 10.1371/journal.pone.0008677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Witt MJ, Åkesson S, Broderick AC, Coyne MS, Ellick J, et al. Assessing accuracy and utility of satellite-tracking data using Argos-linked Fastloc-GPS. Animal Behaviour. 2010;80:571–581. [Google Scholar]
- 16.Breed GA, Jonsen ID, Myers RA, Bowen WD, Leonard ML. Sex-specific, seasonal foraging tactics of adult grey seals (Halichoerus grypus) revealed by state-space analysis. Ecology. 2009;90:3209–3221. doi: 10.1890/07-1483.1. [DOI] [PubMed] [Google Scholar]
- 17.Jonsen ID, Flemming JM, Myers RA. Robust state-space modeling of animal movement data. Ecology. 2005;86:2874–2880. [Google Scholar]
- 18.Maxwell SM, Frank J, Breed G, Robinson P, Simmons S, et al. Marine Mammal Science; 2012. Benthic foraging on seamounts as a specialized foraging behavior by a deep diving marine mammal. [Google Scholar]
- 19.Bailey H, Shillinger G, Palacios D, Bograd S, Spotila J, et al. Identifying and comparing phases of movement by leatherback turtles using state-space models. Journal of Experimental Marine Biology and Ecology. 2008;356:128–135. [Google Scholar]
- 20.Maxwell SM, Breed GA, Nickel BA, Makanga-Bahouna J, Pemo-Makaya E, et al. Using Satellite Tracking to Optimize Protection of Long-Lived Marine Species: Olive Ridley Sea Turtle Conservation in Central Africa. PLos ONE. 2011;6:e19905. doi: 10.1371/journal.pone.0019905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hart KM, Lamont MM, Fujisaki I, Tucker AD, Carthy RR. Common coastal foraging areas for loggerheads in the Gulf of Mexico: Opportunities for marine conservation. Biological Conservation. 2012;145:185–194. [Google Scholar]
- 22.Bailey H, Mate B, Palacios D, Irvine L, Bograd S, et al. Behavioural estimation of blue whale movements in the Northeast Pacific from state-space model analysis of satellite tracks. Endangered Species Research. 2009;10:93–106. [Google Scholar]
- 23.Witt M, McGowan A, Blumenthal J, Broderick A, Gore S, et al. Inferring vertical and horizontal movements of juvenile marine turtles from time-depth recorders. Aquatic Biology. 2010;8:169–177. [Google Scholar]
- 24.Gruss A, Kaplan DM, Guenette S, Roberts CM, Botsford LW. Consequences of adult and juvenile movement for marine protected areas. Biological Conservation. 2011;144:692–702. [Google Scholar]
- 25.Game ET, Grantham HS, Hobday AJ, Pressey RL, Lombard AT, et al. Pelagic protected areas: the missing dimension in ocean conservation. Trends in Ecology & Evolution. 2009;24:360–369. doi: 10.1016/j.tree.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 26.Cañadas A, Sagarminaga R, De Stephanis R, Urquiola E, Hammond PS. Habitat preference modelling as a conservation tool: proposals for marine protected areas for cetaceans in southern Spanish waters. Aquatic Conservation: Marine and Freshwater Ecosystems. 2005;15:495–521. [Google Scholar]
- 27.Gaston KJ, Jackson SF, Cantu-Salazar L, Cruz-Pinon G. The Ecological Performance of Protected Areas. Annual Review of Ecology, Evolution, and Systematics. 2008;39:93–113. [Google Scholar]
- 28.Block BA, Jonsen ID, Jorgensen SJ, Winship AJ, Shaffer SA, et al. Tracking apex marine predator movements in a dynamic ocean. Nature. 2011;475:86–90. doi: 10.1038/nature10082. [DOI] [PubMed] [Google Scholar]
- 29.Hammerschlag N, Gallagher AJ, Lazarre DM. A review of shark satellite tagging studies. Journal of Experimental Marine Biology and Ecology. 2011;398:1–8. [Google Scholar]
- 30.Godley BJ, Blumenthal J, Broderick AC, Coyne MS, Godfrey MH, et al. Satellite tracking of sea turtles: where have we been and where do we go next? Endangered Species Research. 2008;3:3–22. [Google Scholar]
- 31.Hart MK, Hyrenbach KD. Satellite telemetry of marine megavertebrates: the coming of age of an experimental science. Endangered Species Research. 2009;10:9–20. [Google Scholar]
- 32.Sims DW. Tracking and analysis techniques for understanding free-ranging shark movements and behaviour. In: Sharks Biologyof, Relatives their, Biodiversity VolII, Physiology Adpative., editors. and Conservation. Boca Raton, USA: CRC Press; 2010. pp. 351–392. [Google Scholar]
- 33.Witt MJ, Augowet Bonguno E, Broderick AC, Coyne MS, Formia A, et al. Tracking leatherback turtles from the world's largest rookery: assessing threats across the South Atlantic. Proceedings of the Royal Society B: Biological Sciences. 2011;278:2338–2347. doi: 10.1098/rspb.2010.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hazel J. Evaluation of fast-acquisition GPS in stationary tests and fine-scale tracking of green turtles. Journal of Experimental Marine Biology and Ecology. 2009;374:58–68. [Google Scholar]
- 35.Teo SLH, Boustany A, Blackwell S, Walli A, Weng KC, et al. Validation of geolocation estimates based on light level and sea surface temperature from electronic tags. Marine Ecology-Progress Series. 2004;283:81–98. [Google Scholar]
- 36.Bograd SJ, BA B, Costa DP, Godley BJ. Biologging technologies: new tools for conservation. Introduction. Endangered Species Research. 2010;10:1–7. [Google Scholar]
- 37.Kooyman G. Animal-Borne instrumentation systems and the animals that bear them: then (1939) and now (2007). Marine Technology Society Journal. 2007;41:4–6. [Google Scholar]
- 38.Hooker SK, Biuw M, McConnell BJ, Miller PJO, Sparling CE. Bio-logging science: logging and relaying physical and biological data using animal-attached tags. Deep-Sea Research II. 2007;54:177–182. [Google Scholar]
- 39.Graham RT. Whale sharks of the Western Caribbean: an overview of current research and conservation efforts and future needs for effective management of the species. Gulf and Caribbean Research. 2007;19:149–159. [Google Scholar]
- 40.de la Parra Venegas R, Hueter R, Gonzalez Cano J, Tyminski J, Gregorio Remolina J, et al. An Unprecedented Aggregation of Whale Sharks, Rhincodon typus, in Mexican Coastal Waters of the Caribbean Sea. PLos ONE. 2011;6:e18994. doi: 10.1371/journal.pone.0018994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Halpern BS, Walbridge S, Selkoe KA, Kappel CV, Micheli F, et al. A Global Map of Human Impact on Marine Ecosystems. Science. 2008;319:948–952. doi: 10.1126/science.1149345. [DOI] [PubMed] [Google Scholar]
- 42.White W, Giles J, Dharmadi, Potter I. Data on the bycatch fishery and reproductive biology of mobulid rays (Myliobatiformes) in Indonesia. Fisheries Research. 2006;82:65–73. [Google Scholar]
- 43.Marshall AD, Dudgeon CL, Bennett MB. Size and structure of a photographically identified population of manta rays Manta alfredi in southern Mozambique. Marine Biology. 2011;158:1111–1124. [Google Scholar]
- 44.Sims DW, Southall EJ, Tarling GA, Metcalfe JD. Habitat-specific normal and reverse diel vertical migration in the plankton-feeding basking shark. Journal of Animal Ecology. 2005;74:755–761. [Google Scholar]
- 45.Canese S, Cardinali A, Romeo T, Giusti M, Salvati E, et al. Diving behavior of the giant devil ray in the Mediterranean Sea. Endangered Species Research. 2011;14:171–176. [Google Scholar]
- 46.Ohman M, Frost B, Cohen E. Reverse diel vertical migration: an escape from invertebrate predators. Science. 1983;220:1404–1407. doi: 10.1126/science.220.4604.1404. [DOI] [PubMed] [Google Scholar]