Abstract
Edwardsiella bacteria are leading fish pathogens causing huge losses to aquaculture industries worldwide. E. tarda is a broad-host range pathogen that infects more than 20 species of fish and other animals including humans while E. ictaluri is host-adapted to channel catfish causing enteric septicemia of catfish (ESC). Thus, these two species consist of a useful comparative system for studying the intricacies of pathogen evolution. Here we present for the first time the phylogenomic comparisons of 8 genomes of E. tarda and E. ictaluri isolates. Genome-based phylogenetic analysis revealed that E. tarda could be separate into two kinds of genotypes (genotype I, EdwGI and genotype II, EdwGII) based on the sequence similarity. E. tarda strains of EdwGI were clustered together with the E. ictaluri lineage and showed low sequence conservation to E. tarda strains of EdwGII. Multilocus sequence analysis (MLSA) of 48 distinct Edwardsiella strains also supports the new taxonomic relationship of the lineages. We identified the type III and VI secretion systems (T3SS and T6SS) as well as iron scavenging related genes that fulfilled the criteria of a key evolutionary factor likely facilitating the virulence evolution and adaptation to a broad range of hosts in EdwGI E. tarda. The surface structure-related genes may underlie the adaptive evolution of E. ictaluri in the host specification processes. Virulence and competition assays of the null mutants of the representative genes experimentally confirmed their contributive roles in the evolution/niche adaptive processes. We also reconstructed the hypothetical evolutionary pathway to highlight the virulence evolution and niche adaptation mechanisms of Edwardsiella. This study may facilitate the development of diagnostics, vaccines, and therapeutics for this under-studied pathogen.
Introduction
The genus Edwardsiella, consisting of three species Edwardsiella tarda, Edwardsiella ictaluri and Edwardsiella hoshinae, was firstly described in 1965 by Ewing et al [1] to designate a distinct taxa within the family Enterobacteriaceae. E. hoshinae is sometimes isolated from animals but its ability to cause disease has not been established and relatively little is known regarding its habitats. E. ictaluri is a notorious fish pathogen causing enteric septicemia exclusively in channel catfish (ESC) [1]–[4]. E. tarda is the most predominant species as it is a common inhabitant of animals including fish, reptiles, amphibians, chickens, other warm-blooded animals and humans [1], [4], [5], [6]. E. tarda is also the etiological agent of edwardsiellosis, characterized by systemic hemorrhagic septicemia, internal abscesses, and skin lesions leading to mass mortality outbreaks in more than 20 species of freshwater and marine fish, causing devastating economic losses in worldwide aquaculture [1], [4]. Moreover, E. tarda is also associated with opportunistic infections in humans, most commonly gastroenteritis and wound infections, and sporadic septicemia, meningitis and liver abscess [6], [7], raising a concern that E. tarda is becoming a significant zoonotic pathogen that warrants extensive investigation.
The diversity of E. tarda isolates in terms of natural niches, geographical dissemination, biochemical and physiological features, and pathogenic properties have been examined using a variety of techniques, including phenotypic analysis, serovar grouping [1], [8], total, extracellular and outer membrane protein profiling [9], plasmids, production of fatty acid methyl esters and antibiotic resistance patterns [10]. PCR-based genetic analysis based on gyrB or virulence determinants [11], [12], restriction fragment length polymorphism (RFLP) PCR of 16S rDNA [10], rep-PCR [12]–[15], and PCR ribotyping of 16S-23S spacer genes in rRNA operons were also performed in attempts to group various E. tarda isolates [15]. These analytical methods are useful in assessing relatedness of strains but are limited in their resolution between pathogenic strains and environmental isolates, and in their ability to define genetic variances that relate to pathogenicity and phylogenetic significance and offer greater potential for development of practical and reliable diagnostics, vaccines, and therapeutics.
To comprehensively and systematically explore the genetic diversity and virulence evolution of Edwardsiella strains, a genome wide profiling is needed. The complete genome sequences of E. tarda EIB202 [16], FL6-60 [17], and E. ictaluri 93–146 [18] (Table 1) can be used as the reference for comparative genomic analysis. Here we report the sequencing of the genomes of one eel-isolated virulent E. tarda strain (080813), one human feces-isolated E. tarda type strain (ATCC15947), one freshwater fish-isolated E. tarda strain (DT), and one E. ictaluri type strain (ATCC33202) using next generation sequencing methods, including Roche 454 and Illumina Solexa (Table 1). We also used the published draft genome sequence of E. tarda strain ATCC23685 isolated from human feces for comparative analysis. High-resolution genetic fingerprinting of bacterial isolates will be a valuable tool for distinguishing relapses from new infections, and identifying environmental reservoirs. Furthermore, we performed a genomic survey of gene drifts and positive selection in Edwardsiella strains and reconstructed the hypothetical evolutionary pathway to highlight their virulence evolution and niche adaptation mechanisms.
Table 1. Strains used in this study and general sequence information of different Edwardsiella strains.
| Organism | Strain | Classificationa | Status | Size (Mbp) | ORFS | GC (%) | Originb | Plasmid | Platform | Accession No. |
| E. tarda | EIB202 | EdwGI | Complete | 3.760 | 3,563 | 59.7 | Turbot in Yantai, China (2008) [16], [25] | 1 | 454 | CP001135 |
| E. tarda | FL6-60 | EdwGI | Complete | 3.684 | 3,194 | 59.8 | Striped bass in Maryland, U.S.A (1994) [22] | 1 | 454 | CP002154 |
| E. tarda | ATCC23685 | EdwGII | Draft | 3.631 | 3,397 | 57 | Human feces in U.S.A (1959) [21] | NAc | 454 | ADGK00000000 |
| E. tarda | 080813 | EdwGI | Draft | 4.296 | 4,146 | 58.3 | Japanese eel in Fujian, China (2008) [12] | >1 | 454 | AFJH00000000 |
| E. tarda | ATCC15947 | EdwGII | Draft | 3,694 | 3,351 | 57.1 | Human feces in Kentucky, U.S.A (1959) [19] | NA | Solexa | AFJG00000000 |
| E. tarda | DT | EdwGII | Draft | 3.759 | 3,460 | 57 | Oscar fish in Guangzhou, China (2007) [12] | NA | 454 | AFJJ00000000 |
| E. ictaluri | 93–146 | Complete | 3.812 | 3,783 | 57.4 | Catfish in Louisiana, U.S.A (1993) [24] | 0 | 454 | CP001600 | |
| E. ictaluri | ATCC33202 | Draft | 3.703 | 3,617 | 57.7 | Catfish in Georgia, U.S.A (1976) [20] | NA | 454 | AFJI00000000 |
E. tarda strains were classified into EdwGI and EdwGII clades according to their sequence similarity and ANI value as detailed in the related text.
The isolation time of Edwardsiella strain are shown within brackets.
NA indicated that plasmids were not investigated in this study.
Results
Selection and phenotypes of Edwardsiella strains
With the aim to investigate genome diversity of Edwardsiella strains from various natural habitats, we selected four strains isolated from different hosts and different geographic locations of the world and sequenced their genomes with the next generation sequencing methods (Table 1). E. tarda 080813 was isolated from diseased Japanese eel in Fujian, China [12]. E. tarda DT was isolated from Oscar (Astronotus ocellatus) in Guangzhou, China [12]. E. tarda ATCC15947 is the type strain of E. tarda isolated from human feces in Kentucky, USA [19] and E. ictaluri ATCC33202, the type strain of E. ictaluri, was isolated from diseased channel catfish in Georgia, USA [20]. Three other published Edwardsiella genomes were also used in this study, including E. tarda ATCC23685 isolated from human feces [21], E. tarda FL6-60, a highly virulent strain isolated from a striped bass in Maryland, USA [22], and E. ictaluri 93–146, isolated from a commercial catfish pond in Louisiana, USA [23], [24]. The published genome of E. tarda strain EIB202, isolated from a diseased turbot (Scophthalmus maximus) in Shandong, China, was also included as the reference genome in this study [16], [25].
We assessed the biochemical and growth characteristics of the sequenced Edwardsiella strains. While the growth rate of E. ictaluri ATCC33202 was markedly lower than that of E. tarda strains, there is no significant variation in growth rate among the different strains of E. tarda in LB rich medium (data not shown). Based on the API 20E test, E. tarda is an easily recognizable species as it produces H2S (H2S), ornithine decarboxilase (ODC) and generates indole from tryptophan (IND), while E. ictaluri ATCC33202 was negative in these tests as previously described (Table S1) [1].
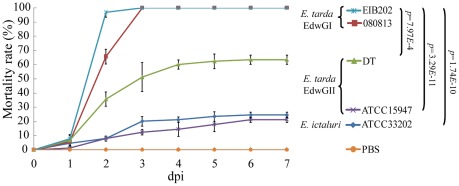
We used zebrafish as the animal model to investigate virulence characteristics of these strains (Figure 1). Fish injected with 5 µl 1×105 cfu/ml of E. tarda 080813 and EIB202 showed 100% cumulative mortality rate at 3 days post infection (dpi), while significant lower mortality rates were obtained for E. tarda DT (66%, p = 7.97E-4), E. tarda ATCC15947 (21%, p = 3.29E-11) and E. ictaluri ATCC33202 (24%, p = 1.74E-10) at 7 dpi. Mortalities due to Edwardsiella infection in adult zebra fish began 1 dpi and continued through 5 dpi, after which there were no further deaths. The majority of the mortalities occurred between 1 and 3 dpi. The fish infected by E. tarda 080813 and EIB202 exhibited typical symptoms of edwardsiellosis [25], i.e. bleeding in the injection sites, ulceration and necrosis in internal organs and a high bacterial load in the organs as examined by plate count on DHL selection agar. E. tarda ATCC15947 and DT as well as E. ictaluri ATCC 33202 displayed no or slight clinical signs of infection. Control fish treated with 5 µl PBS showed no mortality or signs of disease over a period of 7 dpi. The group of zebra fish challenged with E. ictaluri showed the lowest mortality rate in all these Edwardsiella strains, which might be a manifestation of the fact that E. ictaluri is almost exclusively associated with ictalurid fish [1].
Figure 1. Pathogenic characteristics of Edwardsiella strains.
Cumulative mortality in zebra fish i.m. challenged with the indicated Edwardsiella strains. No mortality was observed after 7 days of observation (data not shown). Error bars showed the standard deviations calculated from three individual experiments.
General features of sequenced genomes
Sequenced genomes generated 23 to 36-fold coverage (averaged read length ranging from 399 to 428 bp) with 83–117 large contigs (longer than 500 bp) for Roche 454 samples and 80-fold coverage and 159 assembled contigs for the Illumina Solexa sample, respectively (Table 1). The predicted median genome size of sequenced strains is 3,819,423 bp and the average G+C content ranged from 57% to 58.4%, which is similar to that of EIB202 (59.7%). E. tarda 080813 contained a higher G+C content (58.38%) than that of E. tarda ATCC15947 (57.11%), DT (57.03%) and E. ictaulut ATCC33202 (57.56%). RAST subsystem-based annotation identified 3,460 predicted coding sequences (CDSs) in the draft genome of DT, 3,617 in ATCC33202, 3,351 in ATCC15947, and 4,146 in 080813, respectively (Tables 1 and S2). Thus the genome of 080813 stands so far as the largest genome in the sequenced Edwardsiella strains. Approx. 20% of CDSs in Edwardsiella species were annotated as hypothetical proteins. The overall subsystem category distributions of E. tarda strains and E. ictaluri strains were similar (Table S2).
Genomic plasticity of Edwardsiella strains
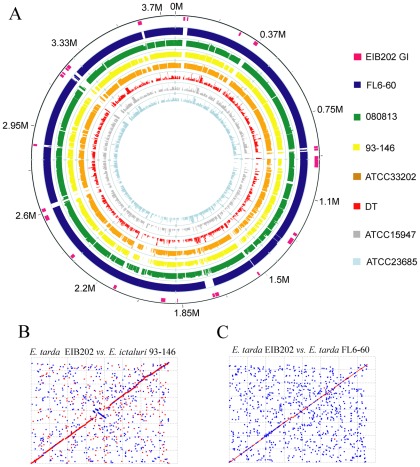
Global pairwise genomic alignment revealed 8 Edwardsiella strains could be easily classified into 3 groups as of EIB202-like strains, ATCC15947-like strains, and E. ictaluri strains (Figure 2). Nucleotide sequence alignments showed high sequence homology between EIB202 and other EIB202-like E. tarda strains (e.g. 080813, FL6-60) (≥94% average sequence identity) (Table S3). EIB202 also showed high sequence similarities with E. ictaluri strains (e.g. 93–146, ATCC33202) (92.24%). Sequence alignment revealed 85%∼88% average sequence identity between EIB202-like strains and ATCC15947-like strains (e.g. ATCC15947, ATCC23685, DT) (Figure 2A, Table S3), which was much lower than sequence identity between EIB202 and EIB202-like E. tarda strains or between EIB202 and E. ictaluri strains. The results showed significant difference among inter-group strains (EIB202-like strains, E. ictaluri strains and ATCC15947-like strains) while the intra-group strains did not show significant difference when used p<1E-3 as threshold (Tables S3). Given the genome-wide diversity between these two types of E. tarda strains, in this paper, we termed E. tarda EIB202- like strains (EIB202, FL6-60, 080813) as E. tarda genotype I (EdwGI) and E. tarda ATCC15947-like strains (e.g. ATCC15947, ATCC23685, DT) as E. tarda genotype II (EdwGII), respectively.
Figure 2. Schematic comparison of the Edwardsiella genomes.
(A) The outside circle represents E. tarda EIB202 GIs (pink). The next 7 circles ranging from outside to inside show the coordinated mapping of 2 complete genomes (E. tarda FL6-60 and E. ictaluri 93–146) and 5 contig sets of genomes against E. tarda EIB202 reference genome sequence. (B and C) Dot plot comparison of MUMmer nucmer output between E. tarda EIB202 (x-axis) and E. ictaluri 93–146 (y-axis) (B), or between E. tarda EIB202 (x-axis) and E. tarda FL6-60 (y-axis) (C). Red and blue plot means forward and reverse matches, respectively.
EIB202 has been established to harbor 24 genomic islands (GIs) [16]. We identified 11 GIs in FL6-60 and 31 GIs in E. ictaluri 93–146, respectively (Table S4). Comparison of the GI sequences of EIB202 to that of other EdwGI strains showed that FL6-60 shared most of GI sequences with E. tarda EIB202 except GI2, GI12, and GI23, which appear to encode prophage and/or transposase genes (Table S4) [16]. Interestingly, the plasmid pFL6-60 of E. tarda FL6-60 contained many (9/63) prophage genes which show high similarity to the prophage and mobile genetic elements in EIB202 chromosome, suggesting that pFL6-60 might be released from the chromosome. E. tarda 080813 shared more than half of the GI-like sequences with EIB202, including GI7 that encodes a type III secretion system (T3SS) gene cluster, and GI17 encoding a type VI secretion system (T6SS) gene cluster. Two E. ictaluri strains shared most of the GIs between themselves and showed high sequence divergence to E. tarda strains in terms of GI content except the GIs for T3SS and T6SS (Table S4).
Thirteen families of IS elements were identified in the sequenced genomes of Edwardsiella (Table S4). The most abundant IS elements among these sequenced strains included ISKpn2, IS102, IS200, ISEc30 and partial ISSaen1, which are common in the Enterobacteriaceae [26]. There are clearly different types of IS distributed among E. tarda and E. ictaluri species, while the two E. ictaluri strains show the same IS profile. There are 33 complete copies of the IS1414 element in E. ictaluri 93–146 while only one copy of IS1414 (contain a nonsense mutation TAC to TAA in codon 10 of tnpA gene) in E. tarda EIB202, which might account for the dormant state of IS1414 in EIB202. Sequencing results showed that intact IS1414 is also present in the E. ictaluri ATCC33202 draft genome. A partial of IS1414 is found in E. tarda 080813 contigs and the draft sequences of all E. tarda EdwGII strains showed no homology to this mobile element, indicating that IS1414 sequence may exist in ancestral E. trada EdwGI and E. ictaluri strains.
Taken together, the variance distribution of GI and IS elements in different E. tarda strains corresponds to the broad host range properties of E. tarda while the conversed GI and IS elements profiles in E. ictaluri strains imply that the genomes of different E. ictaluri might be kept less modified in relatively fixed hosts.
Phylogenetic relationships of Edwardsiella strains
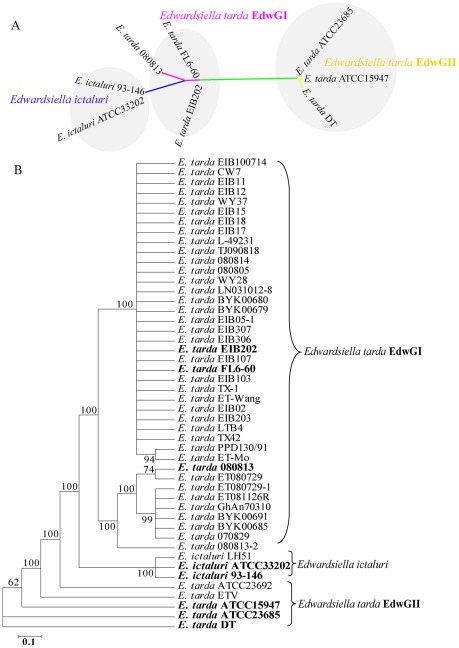
The specific taxonomic position of Edwardsiella bacterium in Enterobacteriaceae was previously reached with 44 house-keeping genes [16]. The same method was applied to the sequenced 8 genomes. The result indicated that the 3 EdwGI strains clustered tightly together with the 2 E. ictaluri strains, forming a distinct branch and the 3 E. tarda EdwGII strains are clustered into another branch (Figure S1). In this study, a genome-wide SNP-based maximum likelihood tree was further constructed using all high confidence SNP sites among the 8 Edwardsiella strains. The result demonstrated that E. tarda EdwGI and EdwGII strains and E. ictaluri strains are clustered into 3 distinct clades and E. tarda EdwGI strains are more closely related to E. ictaluri strains than to E. tarda EdwGII strains (Figure 3A).
Figure 3. Phylogenetic tree of Edwardsiella species.
(A) Maximum likelihood phylogeny based on all filtered SNPs across 8 Edwardsiella genomes. Branches are colored according to the main phylogeographic lineages of Edwardsiella bacteria. (B) NJ tree of 48 Edwardsiella strains inferred from concatenated alignments of partial coding sequences of glyA, mdh, pgi, fusA, aspA and tpi genes with 100 bootstrap iterations. Strains investigated in this study are indicated in bold font.
Multilocus sequence analysis (MLSA) of 48 collected Edwardsiella strains (Table S5) isolated from various hosts at different time also showed that E. tarda strains isolated from diseased fish were clustered tightly together with the E. tarda EdwGI strains EIB202 and FL6-60, and the majority of E. tarda strains from diseased eel were grouped with the E. tarda EdwGI strain 080813, forming a larger branch (Figure 3B). The E. ictaluri strains could be closely classified as a unique group, while E. tarda EdwGII strains are clustered into another distant branch (Figure 3B). All these phylogenetics/phylogenomics relationship of 8 sequenced Edwardsiall strains indicating that the genetic relationship of EdwGI E. tarda and E. ictaluri are closer to each other than that between E. tarda strains of EdwGI and EdwGII.
We then estimated the last common ancestor between each pair of genomes based on the pairwise synonymous substitution frequency (Ds) values of ∼1,000 house-keeping genes shared by the 8 genomes. The estimated Ds value was 0.0004 between E. tarda EIB202 and FL6-60, 0.0005 between E ictaluri ATCC33202 and 93–146, 0.18 between EIB202 and E ictaluri, and 0.48 between E. tarda EdwGI and EdwGII strains (Table S6). Mirroring the nucleotide-based phylogeny results (Figure 3), E. tarda EdwGI and EdwGII strains split from a common ancestor much longer than that for EdwGI E. tarda and E. ictaluri strains, suggesting a common ancestor might exist for E. tarda EdwGI strains and E. ictaluri strains.
We further took advantage of the widely used average nucleotide identity (ANI) method introduced by Konstantinidis and Tiedje [27], [28] which transform the ANI values derived from genome sequences into DNA-DNA hybridization (DDH) values traditionally used in species definition. We used the ANI data of the 8 Edwardsiella genomes to split the three groups of isolates by using the 94% ANI criterion (equal to 70% DDH value) for assignment of strains to species (Table S6) [28]. The results showed that ANI value of the 3 EdwGI strains were higher than 94% while that between E. tarda EdwGI strains and E. ictaluri species were ∼92%, demonstrating their close phylogenomic relationships (Table S6). ANI analysis indicated that E. tarda EdwGII strains showed more distant relationships to E. tarda EdwGI strains (∼82%) and to E. ictaluri species (∼82%). Furthermore, the averaged Ds values derived from the housekeeping genes in each pair of the Edwardsiella strains showed a tight correspondence to their ANI values (R2 = 0.9949) (Table S6, Figure S2), demonstrating that both methods are valid to distinguish different species of Edwardsiella.
Taken together, these data support the following phylogenetic inferences. Firstly, 8 Edwardsella strains could be grouped into three distinct major lineages. E. tarda EdwGI strains form a monophyletic lineage which is the sister clade of E. ictaluri strains. Secondly, although some of the E. tarda strains isolated from humans (EdwGII) and marine fish (EdwGI) were classified into the same species, these strains might diverge from a common ancestor before the E. tarda EdwGI and E. ictaluri strains split from each other.
Distribution of orthologs and specific genes in Edwardsiella strains
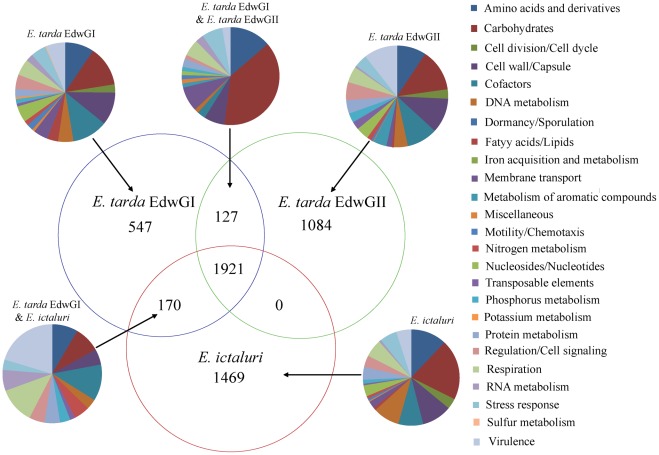
Comparison of the genome sequences revealed that 1,921 distinct genes were shared by the 8 Edwardsiella strains (Figure 4, Table S7). Another 844 orthologs were identified in 3 E. tarda EdwGI strains. Other 1,211 orthologs were shared by 2 E. ictaluri strains and 1,639 homologs in 3 E. tarda EdwGII strains, respectively (Figure 4, Table S7). Hence, the core gene set (1,921) may represent about 36.1% of all distinct genes identified in the 8 genomes (Figure 4). The following genes were highlighted to pertain to different clusters of Edwardsiella strains.
Figure 4. The Venn diagram illustrating the number of genes unique or shared between two Edwardsiella lineages.
The associated pie charts showed the functional groups assigned for CDSs in relevant sections of the Venn diagram. The strains used for comparison were E. tarda EIB202, FL6-60, and 080813 in EdwGI lineage, DT, ATCC15947, and ATCC23685 in EdwGII lineage, and E. ictaluri ATCC33202 and 93–146.
E. tarda contain 127 genes whose sequences are absent in the genome of E. ictaluri strains (Figure 4, Table S7). These genes include tnaA and tnaB for indole production which is one of the differential phenotypes for E. tarda and E. ictaluri (Table S1) [1]. The genes also include pvsA, pvsD, pvsE, and pvuA, encoding siderophore vibrioferrin biosynthesis and transport related proteins that play essential roles in a unique iron acquisition system originally identified in marine bacteria Vibrio parahaemolyticus, V. alginolyticus, and V. splendidus [29], [30], presumably endowing E. tarda species survival and propagation advantages in the marine environment and other iron-restricted environments. Several genes encoding two component system (TCS) are specific to E. tarda, including yehT/yehU involved in deoxycholate and crystal violet resistance [31], a potential pleC/pleD system involved in intracellular infection [32], and the lytR/lytS system implicated to be involved in bacterial stress responses [33]. These lineage-specific genes might underlie the differentiation of the host-adaptation processes of E. tarda and E. ictaluri.
E. tarda EdwGI strains and E. ictaluri strains shared a wide range of genes involved in host interaction and virulence, including T3SS and T6SS (Table S8). Previous reports showed that E. tarda T3SS and T6SS gene clusters consist of 32 and 16 genes, respectively [16], [34], [35]. The T3SS and T6SS genes in EdwGI strains and E. ictaluri strains are highly homologous to the previously described counterparts in E. tarda strain PPD130/91 [18], [36], [37]. Examination of T3SS and T6SS homologs in EdwGI strains and E. ictaluri strains showed the same genetic organization and shared 78%–100% amino acid sequence identity (Figure S3, Table S8). T6SS secreted protein EvpP [18], [36], [37] displayed the highest genetic diversity (78%–91% amino acid sequence identity) among the EdwGI strains and E. ictaluri strains (Table S8). Notably, E. tarda EdwGII strains lost most of the T3SS and T6SS orthologs (Figures S3, Table S8), indicated that some important virulence factors were missing in E. tarda EdwGII strains ATCC15947, DT, and ATCC23685. The NJ-based tree of 6 Edwardsiella isolates (3 EdwGI strains, 2 E. icatluri strains and E. tarda PPD130/91) (Figure S3C) and the MLSA result (Figure 3B) indicated that PPD130/91 could be classified into EdwGI. Encoded in the T3SS gene locus, EsrA/EsrB was established to be responsible for regulation of the T3SS and T6SS in E. tarda [34], [36], [37]. Sequence analysis of EsrA/EsrB genes of Edwardsiella strains showed the same phylogeny topology to that of the phylogenetic tree inferred by house-keeping genes (Figures 3 and S3D).
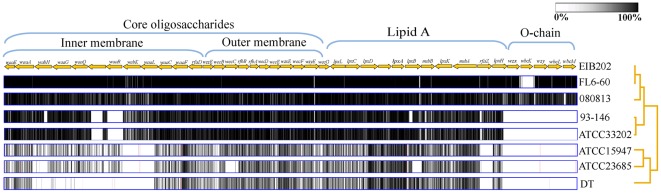
Previous serotyping schemes have recognized more than 61 O groups and 45 H antigens in E. tarda [38] while E. ictaluri isolates from enteric septicemia of catfish (ESC) outbreaks are all of the same serotype [39]. The genetic distance of the predicted LPS genes of the Edwardsiella strains (Figure 5) was largely consistent with their phylogenetic tree (Figure 3). E. tarda EdwGI strains and E. ictaluri strains share a majority of LPS genes except waaR, encoding a core glycosyl transferase and some genes involved in O-antigen synthesis (wzx, wbcK, wzy, wbcL, and wbcM) (Figure 5, Table S9) [40], implying a genetic basis for LPS or O-serotype variations between the host-specific and broad host-range strains. Moreover, high sequence diversity was observed in the genes for inner core oligosaccharides and O-chain between E. tarda EdwGI and EdwGII strains (Figure 5). These variable regions include the waaL gene required for production of high molecular weight O-antigen side chains in E. tarda [41] (Table S9). Interestingly, the O-antigen gene cluster of E. tarda EdwGII strains (ATCC15947 and ATCC23685) isolated from human feces showed more sequence similarities to E. coli than to other Edwardsiella strains, suggesting a putative human gut adaptation process of these bacteria (Table S9).
Figure 5. LPS related genes of Edwardsiella strains.
Mauve progressive alignment of the concatenated coding sequences of 8 sequenced Edwardsiella strains using E. tarda EIB202 as reference. Arrows indicate the gene coding orientation in EIB202 genome. The dendrogram is derived from NJ analysis of concatenated amino acid sequences of LPS biosynthesis genes with 1,000 bootstrap iterations. Gradient bar indicated the sequence similarity of LPS coding sequences of Edwardsiella strains to those of EIB202.
Polymorphisms and positive selection in Edwardsiella core genomes
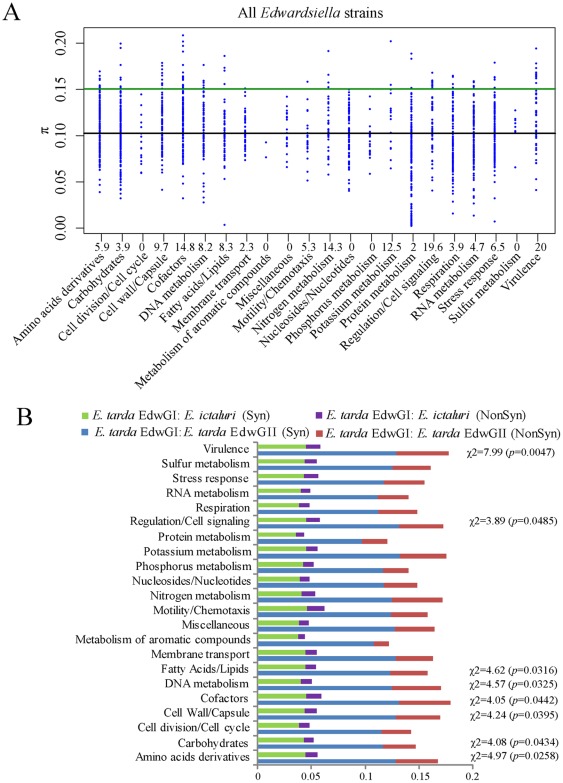
To understand the level and nature of nucleotide variation among all 8 sequenced Edwardsiella genomes, nucleotide diversity (π) of 1921 aligned orthologous sequences were calculated (Figure 6A, Table S10) [42], [43]. Although these 8 genomes were clustered into 3 distinct phylogenetic clades, most of the orthologs involved in cell cycle, membrane transport and nucleotides/RNA metabolisms showed a high degree of conservation and less than 5% orthologs displayed significantly greater (>1.5 standard deviation (σ)) π values than the mean π value among these lineages (Figure 6A). In contrast, high percentage of homologous genes related to the RAST-defined functions in cell wall and capsule (9.7%), cofactors (14.8%), nitrogen metabolism (14.3%), regulation and cell signaling (19.6%), and virulence (20%) exhibited significantly high nucleotide diversity (>1.5σ above the mean π value) among these Edwardsiella genomes (Figure 6A). Nucleotide diversity calculation was also performed with EdwGI/EdwGII (Figure S4A) and with EdwGI/E. ictaluri (Figure S4B) orthologs. The results indicated that EdwGI and EdwGII genomes share significantly high diversity (>1.5σ) in cell wall and capsule (10.6%), cofactors (12.9%), regulation and cell signaling (14.8%), and virulence (21.1%) (Figure S4A) while that between EdwGI strains and E. ictaluri mainly focus on membrane transport (11.4%), motility and chemotaxis (17.4%), nitrogen metabolism (14.3%), regulation and cell signaling (11.1%), and virulence (12.3%) (Figure S4B).
Figure 6. Genome-wide nucleotide variations among the orthologs of sequenced Edwardsiella strains.
(A) Nucleotide diversity (π) for 8 Edwardsiella strains. The black line represents the average π value of all orthologs. Green line indicates π values above 1.5σ (standard deviation) from the average π values of all orthologs, respectively. The percent of genes with π values large than 1.5σ from the average π value in each function category are shown under x axis. (B) Analysis of the ratio of nonsynonymous (NonSyn) to synonymous (Syn) SNP rates according to the RAST-annotated categories. The set of genes which contain significant high ratios (p<0.05) of nonsynonymous (NonSyn) SNPs than synonymous (Syn) SNPs between E. tarda EdwGI and E. tarda EdwGII strains were as indicated.
We further compared the proportion of nonsynonymous (NonSyn) changes in different functional groups of gene sets between EdwGI/E. ictaluri and EdwGI/EdwGII strains [42], [43]. When use EIB202 as reference, we found that the ratios of NonSyn changes between EdwGI and EdwGII strains were significantly different in some function categories (Figure 6B), including cell wall and capsule (p = 0.0395), regulation and cell signaling (p = 0.0485), and virulence (p = 0.0047), which were consistent with the detected categories with high nucleotide diversity (Figures 6A and S4A).
A molecular adaptation analyses was performed with 1,921 Edwardsiella orthologs to detect gene displaying features of differential selective pressure (positive selection) using two different positive selection models (Branch and Site models) in PAML package [44]. 136 and 129 genes were shown to be under positive selection when used E. tarda EdwGI and E. ictaluri strains as foreground branches, respectively (p<0.05, likelihood ratio test, LRT) (Tables 2 and S11). In particular, thirteen iron uptake and utilization related genes, which were classified as virulence related genes according to the RAST function catalogs, were significantly enriched in gene set (p = 2.18E-13, FDR q value = 5.67E-12) in E. tarda EdwGI strains. These genes included hemX, hemC, hemD, hemM, hemN, hemS, hemT and ETAE_1794, a ChuX-like heme iron utilization protein [45], ETAE_2768-2770, an iron transport related ABC transporter system, as well as fur and basS, two genes involved in iron uptake [46] (Table 2). Another group of genes subjected to high selection pressure in EdwGI strains (Table 2) are genes required for responses to environmental stresses including phoR (response to phosphate starvation) [47], gor (oxidative stress response) [48], envZ (osmolarity stress regulation) [49], and pspF (involved in responses to ethanol, osmotic shock, and heat shock) [50]. The widespread presence of positive selection sites in iron acquisition-related genes and signal response-related genes indicated their essential roles for the E. tarda EdwGI strains to inhabit different environment niches. Similarly, a significantly large number of surface structure (cell wall and capsule/motility and chemotaxis) related genes under positive selection were enriched in E. ictaluri (n = 14, p = 9.91E-8, FDR q value = 8.54E-6), including flagellar biosynthesis genes flhB, flhA, motA, fliG, and fliR and the LPS assembly related gene imp, membrane associated proteins such as Tol-Pal system-related genes tolB/tolC [51], the penicillin-binding protein encoded by mrcB, and the outer membrane protein gene ompW [52]. The selection of these surface related structures might have specifically contributed to the adaptation processes of the bacterium to the channel catfish host.
Table 2. Representative genes with high diversity or under positive selection.
| CDS | Gene | RAST catalog | Pia | E. tarda EdwGI | E. ictaluri | Annotation | |
| Chib | LRTc | LRT | |||||
| ETAE_0116 | hemX | Virulence | * | * | Uroporphyrinogen III C-methyltransferase | ||
| ETAE_0117 | hemD | Virulence | 0.153 | * | * | Uroporphyrinogen-III synthase | |
| ETAE_0118 | hemC | Virulence | * | ** | Porphobilinogen deaminase | ||
| ETAE_0271 | hemN | Virulence | * | ** | Fe-S oxidoreductases | ||
| ETAE_1404 | hemM | Virulence | ** | Outer membrane lipoprotein | |||
| ETAE_1798 | hemS | Virulence | * | Hemin transport protein | |||
| ETAE_1799 | hmuT | Virulence | 0.168 | * | ** | Hemin-binding periplasmic protein | |
| ETAE_1794 | Virulence | 0.157 | * | * | Heme iron utilization protein | ||
| ETAE_2610 | fur | Virulence | * | Ferric uptake regulator | |||
| ETAE_2768 | Virulence | * | ABC transporter, substrate binding protein | ||||
| ETAE_2769 | Virulence | * | ABC transporter, permease protein | ||||
| ETAE_2770 | Virulence | * | ABC transporter, ATP-binding protein | ||||
| ETAE_0393 | basS | Regulation and cell signaling | 0.154 | * | Sensor protein BasS/PmrB | ||
| ETAE_1081 | phoR | Other | * | Phosphate regulon sensor protein | |||
| ETAE_3367 | gor | Stress response | * | Glutathione-disulfide reductase | |||
| ETAE_3278 | envZ | Other | * | Osmolarity sensor protein | |||
| ETAE_1242 | pepN | Stress response | * | Aminopeptidase N | |||
| ETAE_1868 | pspF | Stress response | ** | Phage shock protein F | |||
| ETAE_2912 | Regulation and cell signaling | 0.159 | ** | Transcriptional regulator, LysR family | |||
| ETAE_2767 | emrB | Other | ** | Multidrug resistance protein B | |||
| ETAE_1219 | flhB | Motility and chemotaxis | ** | Flagellar biosynthetic protein | |||
| ETAE_1220 | flhA | Motility and chemotaxis | * | Flagellar biosynthesis protein | |||
| ETAE_1338 | motA | Motility and chemotaxis | * | Flagellar motor protein | |||
| ETAE_2143 | fliG | Motility and chemotaxis | * | Flagellar motor switch protein | |||
| ETAE_2154 | fliR | Motility and chemotaxis | * | Flagellar biosynthesis pathway component | |||
| ETAE_0191 | tolC | Virulence | ** | Outer membrane protein | |||
| ETAE_2573 | tolB | Virulence | * | Translocation protein | |||
| ETAE_1528 | ompW | Other | * | Outer membrane protein W | |||
| ETAE_0263 | mltC | Cell wall and capsule | * | Murein transglycosylase C | |||
| ETAE_0382 | yjfG | Cell wall and capsule | ** | UDP-N-acetylmuramate | |||
| ETAE_0603 | imp | Cell wall and capsule | * | LPS-assembly protein | |||
| ETAE_0695 | mrcB | Cell wall and capsule | * | Penicillin-binding protein | |||
| ETAE_1032 | Cell wall and capsule | ** | Fimbrial usher protein | ||||
| ETAE_1126 | amiA | Cell wall and capsule | ** | N-Acetylmuramoyl-L-alanine amidase | |||
Nucleotide diversity value (π) of orthologs of sequenced Edwardsiella strains [43]; Representative genes which differed by above 1.5 σ from the average π value of all orthogolous were listed.
χ2 test of nonsynonymous (NonSyn) changes of E. tarda EdwGI strains/E. ictaluri and E. tarda EdwGI/EdwGII lineages.
LRT test of branch and site models in PAML package [44].
p<0.05;
p<0.01.
The detailed p value and other information were shown in Tables S10 and S11.
Strain specific and positively selected genes contribute to virulence and adaptation
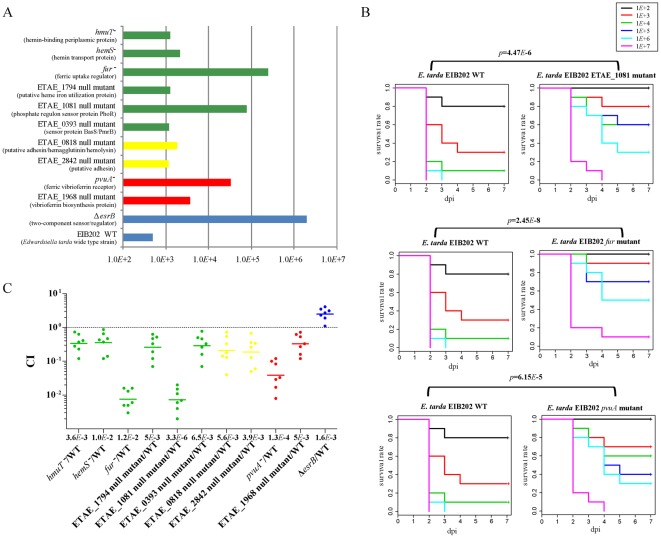
We were intrigued by the possibility that the strain specific genes and the positively selected genes might contribute to the colonization and virulence towards the hosts. Previous findings have demonstrated that the T3SS and T6SS are essential for the virulence of E. tarda and E. ictaluri [34], [35], [37], [53]. To evaluate if other strain-specific genes and positively selected genes might be involved in the host virulence and colonization, we selected 10 representative genes (2 E. tarda specific genes, 2 E. tarda EdwGI strain-specific genes, 6 positively selected genes in EdwGI strains) (Figure 7, Tables 2 and S7) and generated isogenic E. tarda mutant strains to test the LD50 values and competition index in zebra fish model. Compared to the parental E. tarda EIB202, all the mutants exhibited 2.3 to 504 fold attenuation in virulence (Figure 7A). The cumulative mortality of the mutant strains with the gene disruption in ETAE_1081, fur, and pvuA showed significantly decreased virulence when compared with that of parental E. tarda EIB202 (p<0.01) (Figure 7B), indicating that these genes play critical roles in the invasion process in fish. All the mutant strains displayed significantly decreased growth competition against the wild-type strain (Figure 7C). The ΔesrB mutant strain, which was found to inhibit the expression of T3SS and T6SS while activate hemolysin EthA production, displayed 4000-fold virulence attenuation and transitorily slightly enhanced competition index [37], [54] (Figure 7C). These results indicated that a subset, if not all, of the diversified and positively selected genes may influence virulence evolution and adaptation processes in Edwardsiella.
Figure 7. Contributive roles of representative diversified or positive selection genes in the virulence and colonization in zebra fish.
(A) LD50 values of the wild-type EIB202 (WT) and the null mutants of the indicated genes. LD50 is calculated by the method described elsewhere [36]. (B) Virulence comparison of parental E. tarda EIB202 with the mutants with gene disruption in ETAE_1081, fur, and pvuA, respectively. Graphs show survival curves of zebra fish following injected with varying dosages of E. tarda strains. All mutant strains are significantly attenuated compared to parental EIB202 strain (p<0.01, Mantel-Haenszel Chi-squared test). (C) Competitive indexes of the indicated strains against WT in zebra fish at 24 h after inoculation. WT was differentiated from the mutant strain based upon GFP label or Km resistance on DHL agar plates as detailed in the Materials and Methods [37]. The strain ΔesrB with significantly attenuated virulence while transitorily enhanced CI was included in the experiments as a control [36], [37]. The p value of the decreased growth competition of the mutants against WT are shown under x axis (p<0.01, one sample t-test).
Discussion
In this study, we presented for the first time a genome-wide comparative analysis of various Edwardsiella isolates pertaining to E. tarda EdwGI and EdwGII and E. ictaluri lineages as evaluated relative to their genome sequences. The genomic comparison and positive selection model analysis between E. tarda EdwGI and EdwGII strains, and E. ictaluri strains help to explain the differences in host range and pathogenesis among these three groups of closely related organisms and show potential key gene contents facilitating adaptation in different lineage of Edwardsiella strains. The low level of virulence in the E. tarda EdwGII lineage could be explained by the missing of some important virulence associated gene clusters such as T3SS and T6SS (Figures S3A and S3B, Table S8), as observed in the previous work where low virulence phenotypes were associated with deletions or other mutations in T3SS and/or T6SS [34], [35], [36], [55]. While the high virulence of E. tarda EdwGI strains in zebrafish could be due to the pool of genes involved in host-pathogen interactions, stress responses and adaptation to various hosts (Table S7). Moreover, the function comparison analysis of the genes in E. tarda EdwGI and EdwGII strains revealed a high diversity of cell wall/capsule-, regulation/cell signaling- and virulence-related genes (Figure S4), suggesting that this may constitute a genetic basis for the different niche adaptation characteristics and virulence mechanisms of these two E. tarda lineages. Specifically, many iron scavenging related genes were detected among the virulence genes under positive selection, showing strong signs of adaptive evolution in the E. tarda EdwGI lineages (Table 2). Mutational analysis of these genes really demonstrated their essential roles in virulence and colonization (Figure 7). Taken together, T3SS and T6SS as well as iron scavenging related genes thus fulfilled the criteria of a key evolutionary factor likely facilitating the virulence evolution and adaptation to a broad range of hosts in the E. tarda EdwGI strains.
Compared to the E. tarda strains with a broad host-range, the E. ictaluri strains share the freshwater ictalurid fish as their monomorphic host [1]–[4]. Correspondingly, the gene contents in the E. ictaluri strains are highly conserved (Figures 2 and 4, Tables S3 and S7). The loss of the biosynthetic and uptake gene clusters for the siderophore vibrioferrin, which is specific to the most abundant marine bacteria V. alginolyticus, V. parahaemolyticus and V. splendidus [29], [30], may be an important factor restricting the habitats of E. ictaluri species to freshwater fish. Moreover, evolution selection analysis showed that the genes for surface structures including flagellar biosynthesis and cell wall and capsule are under an adaptive evolution process, which might constitute one of the adaptive traits in E. ictaluri (Table 2).
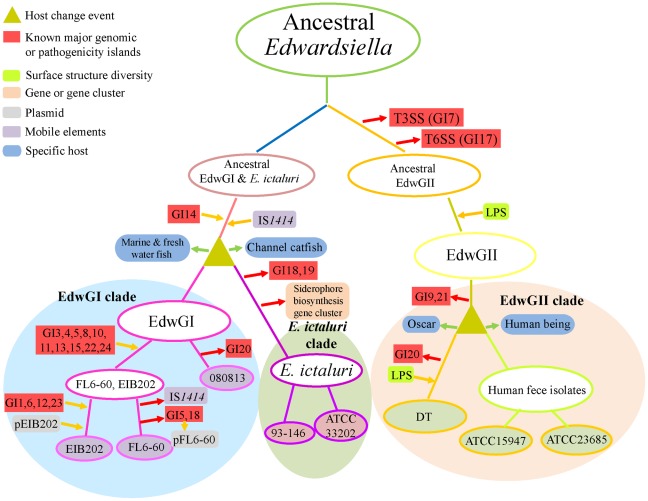
Exploration of the genome content of the strains will definitely provide clues enabling us to track and reconstitute the evolutionary events in Edwardsiella. We proposed hypothetical evolutionary scenarios for the Edwardsiella strains (Figure 8). Over long periods of time, the large scale changes and microevolution events, including genomic island acquisition and deletion, lateral gene transferring, and mutation accumulation in the genomes, have driven the dynamic modifications of the genome content. On the other hand, various environmental factors such as growth temperatures, osmolarity, and iron limitation etc. have served to select and shape the gene contents in the evolution and adaptation processes of Edwardsiella populations. Unknown changes in hosts might have led ancestral Edwardsiella clones to diverge into two major subpopulations, which subsequently developed into two distinct clades (E. tarda EdwGI lineage and E. ictaluri) and one nonpathogenic or environmental clade (E. tarda EdwGII lineage) (Figures 3 and 8).
Figure 8. Proposed hypothetical evolutionary pathway of Edwardsiella species.
Probable insertions, deletions of GIs and gene clusters found in 8 Edwardsiella strains are indicated by yellow and red arrows, respectively. Host change events of different strains are indicated by green arrows. Hypothetical ancestral strains are indicated by open circles.
In conclusion, the widely used next generation sequencing methods make it is possible to rapidly identify new genes, gene loss, lineage-specific sequences, darwinian selection and even bacteria adaptation evolution processes underlying the different virulence or niche adaptation features of pathogens, to reconstitute the genetic series of events associated with pathogen evolution, and to trace a specific kind of etiological agent in epidemic outbreaks. Evolutionary parallelism of Edwardsiella lineages provides a model to study evolutionary diversity processes linked to the virulence divergence and niche adaptation of pathogenic microorganisms. This approach may facilitate the development of reliable and useful diagnostics, vaccines, and therapeutics for less studied pathogens.
Experimental Procedures
Ethics statement
The animal work presented here was approved by the Animal Care Committee, East China University of Science and Technology (approval ID: 2006(272)).
Bacterial strains
All E. tarda strains were grown overnight at 28°C in Luria-Bertani (LB) medium or desoxycholate hydrogen sulfide lactose (DHL) plates. E. ictaluri ATCC33202 was grown for 48 h at 25°C in Brain Heart Infusion (BHI) medium with shaking. For API 20E index experiments (bioMérieux, France, Marcy l'Etoile, France), Edwardsiella colonies were emulsified into 5 ml of sterile 0.9% NaCl and inoculated into strips according to the instructions provided by the manufacturer.
Construction of null mutant strains
Insertional null mutants were generated as previously described [37] in E. tarda EIB202. Internal fragments of the target genes were obtained by PCR using the primers (Table S12) and treated with BglII/SphI restriction enzymes and cloned into the corresponding restrict sites of pNQ705-1 [56] carrying a kanamycin (Km) and chloramphenicol (Cm) resistance genes. The derivative plasmids were conjugated into EIB202 from Escherichia coli SM10 λpir. The insertion of the plasmid into each gene of E. tarda EIB202 was confirmed by PCR analysis with specific primer pairs (Table S12). Stability of the insertion mutation was tested by growth for 30 generations in the absence of Km as previously described [56].
Pathogenicity test
Healthy zebra fish weighing ∼0.25 g were acclimatized for 2 weeks in a laboratory breeding system. Aquaria were supplied with flow-through dechlorinated and continuously aerated water at a rate of ∼0.5 L/min. Water temperature was maintained by a central heater at 22±2°C. The fish were reared with a photoperiod of 12∶12 h (light/dark). Pathogenicity was defined by the mortality rate of infected zebra fish. Three paralleled groups of 30 fish were injected intramuscularly (i.m.) with 5 µl bacterial suspension of 1×105 cfu/ml after being sedated in 100 mg/L tricaine methanesulfonate (MS-222, Sigma). Three paralleled control groups of 30 zebra fish were i.m. injected with 5 µl PBS with the same MS-222 treatment. All injected zebra fish were observed for a period of 14 days. The fish deaths caused by Edwardsiella strains were confirmed by isolation and re-injection of the strains into zebra fish. The LD50 values of all strains were determined in zebra fish as previously described [36]. Competitive index (CI) of the wild-type E. tarda EIB202 (WT) and ΔesrB strain was performed as previously described by using one-half of the EIB202G harboring a GFP reporter and one-half of the ΔesrB strain inoculum (1×105 CFU/ml of each strain) [36], [37]. Seven zebra fish used were sacrificed at 24 h post infection, and were grinded and plated on DHL agar to determine the bacterial loads. WT strain was differentiated from the mutant strain based upon GFP label [37]. The ratios of ΔesrB strain counts to WT were used to determine the competitive index. The CI values of other mutants against WT were determined in the same way except being plated on the DHL plates containing Km or DHL plates only for discrimination of the mutants or WT.
High density sequencing and assembly of genomes
Bacterial genomes were sequenced using the next generation sequencing platforms, Roche 454 (GS FLX Titanium) system and Illumina Solexa Hiseq 2000 system. Large contigs were assembled by using the Newbler de novo assembler package for 454 samples. For each Solexa sample, pair-end reads were assembled using Velvet with various values of “hash length” and “cutoff” set by a local Perl script [57]. The quality recalculation process of contigs was performed with Perl script implemented in Consed package [58].
Genome annotation and comparative genomics
Newly sequenced draft genome sequences were first annotated by using automated prokaryotic annotation pipeline server RAST [59] and then check manually by search against nr protein database using Blastp (E-value cutoff as 1E-10 and 60% minimum amino acid sequence identity). We also evaluated the annotation accuracy by comparison the RAST gene calling result of initial E. tarda EIB202 454 contigs and simulated Solexa reads of EIB202 genome sequence (assembled by Velvet [57]) with published EIB202 CDSs (CP001135), respectively. More than 92% CDSs were shared in all three kinds of sequences. Most of the CDSs (∼7%) lost in RAST annotation result were putative transposon and prophage related genes, which were excluded in this study. Orthologs of 8 strains were determined by using the best bidirectional Blastp search against EIB202 and query sequences with E-value less than 1E-10 and identity more than 60%, matching at least 80% of the length of both query and subject sequences. Genome islands (GIs) and IS elements were predicted by Island Viewer [60] and IS finder [61], respectively. For draft sequences, we identified mobile elements by using IS finder and the absent of these elements in different strains were verified by using PCR method. NUCmer was used for alignment of multiple complete and draft genome sequences with E. tarda EIB202 as the reference genome. Genome comparative circular maps were constructed by using GenomeViz package using the NUCmer-coords result files [62], [63].
SNP calling
MUMmer [62] and NCBI Blastn were used to align large query contigs to the finished EIB202 reference sequence and to generate primary SNP calls. Pseudogene, repetitive sequences, including variable number tandem repeats, single-base insertions or deletions and prophage-related and insertion sequences were excluded from this analysis. SNPs in homopolymeric sequences or Phrap quality low than 40 were also automatically removed by local Perl scripts.
Phylogeny of Edwardsiella species
All filtered SNPs (coding and noncoding SNPs) of 8 Edwardsiella strains were used to infer the phylogenetic relationships of Edwardsiella strains using maximum likelihood method with 100 bootstrap pseudoreplicates for clade supported by PhyML package. MLSA of 48 Edwardsialla isolates (Table S5) was conducted using the concatenated alignment sequences of 6 house-keeping genes (glyA, mdh, pgi, fusA, aspA and tpi) by MEGA5 program with 100 bootstrap iterations for clade support [64]. The ANI values between the query genome and the reference genome were calculated by the Perl script provided by Konstantinos and Tiedje [27].
SNP analysis
SNAP package was used to obtain the observed synonymous (Syn) substitutions and non-synonymous (NonSyn) substitutions [65]. Gene-by-gene genetic diversity (π) among all Edwardsiella strains according to the RAST subsystem category was calculated using Variscan [66]. Omega value (ω = dN/dS, where dN and dS are the nonsynonymous and synonymous substitution rates, respectively) was used to analyze the selective pressures acting on Edwardsiella orthologous genes. We first fitted different evolutionary branch models to analyze the ω value among the E. tarda EdwGI, E. tarda EdwGII and E. ictaluri lineages in the phylogenetic tree generated by PhyML (Figure 3A) using the codoml module implemented by PAML (4.4c) program [44]. We also used the site-model of codeml module in the PAML package to detect positive selection sites for aligned genes by calculating likelihood ratio test (LRT) value of model M2a (positive selection) vs. model M1a (nearly neutral) and M8 (beta & ω) vs. M7 (beta), respectively [44].
Statistical analysis
Chi-squared (χ2) test and Mantel-Haenszel Chi-squared test were used for comparisons of the mortalities of zebra fish infected with E. tarda EIB202, sequenced Edwardsiella strains, and other E. tarda EIB202 mutant strains. Difference of sequence identity was analyzed by one-way ANOVA analysis and Tukey's HSD test. Chi-squared (χ2) test was also used to determine whether the proportion of NonSyn changes in various groups of genes showed significant differences in different Edwardsiella lineages. An independent one-sample t-test was used to determine whether CI values of the mutants against the wild-type strain were significantly different to the log transformation of CI value 0, the expected value implying that there would be no difference between wide-type strain and the mutant strain. Function enrichment was calculated using the hypergeometric distribution at a significance cutoff of ∼5% false discovery rate (FDR). All statistical analysis was performed using R program.
Data Availability
The nucleotide sequence of the draft sequences were submitted to the GenBank database under accession numbers AFJH00000000 (E. tarda 080813), AFJG00000000 (E. tarda ATCC15947), AFJJ00000000 (E. tarda DT) and AFJI00000000 (E. ictaluri ATCC33202), respectively. Sequences used for the multilocus sequence analysis were available under the accession numbers JN709499-JN709721.
Supporting Information
Phylogenetic tree of Edwardsiella species. Phylogenies of Edwardsiella species inferred from concatenated alignments of the protein sequences encoded by 44 house-keeping genes (adk, aroC, dnaA, dnaK, frr, fusA, gapA, gyrA, gryB, infC, nusA, pgk, phoB, phoR, pyrG, recC, rplA, rplB, rplC, rplD, rplE, rplF, rplK, rplL, rplM, rplN, rplP, rplS, rplT, rpmA, rpoA, rpoB, rpoC, rpoE, rpsB, rpsC, rpsE, rpsI, rpsJ, rpsK, rpsM, rpsS, smpB, and tsf) by PhyML program with 100 bootstrap iterations for clade support. Bacillus cereus ATCC14579 was used as the outgroup strain.
(TIF)
Relationships between ANI and synonymous nucleotide substitutions. Each blue square represents the ANI of all genome sequence between two strains (x axes) plotted against (y axes) the average rate of synonymous nucleotide substitutions of housekeeping genes.
(TIF)
Edwardsiella virulence gene clusters of secreted proteins. T6SS (A) and T3SS (B) gene clusters of sequenced E. tarda EdwGI and E. ictaluri strains. All genes with high similarity are indicated in the same color and the gene names are shown below according to the color scheme. (C) NJ-tree of 6 Edwardsialla isolates (3 EdwGI strains, 2 E. icatluri strains and E. tarda PPD130/91) inferred from concatenated T6SS and T3SS aligned sequences. (D) NJ tree of 8 Edwardsiella species inferred from concatenated alignments of the coding sequences of esrA and esrB genes with 1000 bootstrap iterations.
(TIF)
Nucleotide diversity (π) of orthoglous of Edwardsiella . (A) Nucleotide diversity (π) for E. tarda EdwGI and EdwGII. (B) Nucleotide diversity (π) for E. tarda EdwGI and E. ictaluri strains. The blank line represents the average π value of all orthologs. Green line indicates π values above 1.5σ (standard deviation) from the average π values of all orthologs, respectively. The percent of genes with π values large than 1.5σ from the average π value in each function category are shown under x axis.
(TIF)
API-20E test of Edwardsiella strains.
(DOC)
RAST annotation of the genomes of the Edwardsiella strains.
(XLS)
Sequence identity of 8 Edwardsiella strains.
(DOC)
Predicted GI related sequences and IS distribution in the genomes of the Edwardsiella strains.
(XLS)
Multilocus sequence analysis of 48 strains.
(XLS)
ANI value and synonymous substitution frequency of the strains studied.
(DOC)
Ortholog distribution in the genomes of the Edwardsiella strains.
(XLS)
Amino acid sequence identity of the T3SS and T6SS between the Edwardsiella strains.
(XLS)
LPS biosynthesis related genes in the Edwardsiella strains.
(XLS)
Nucleotide variation value pi of the Edwardsiella strains.
(XLS)
positive selected genes.
(XLS)
primer list.
(XLS)
Acknowledgments
We would thank Prof. Dr. Li Sun and Prof. Dr. Zhaolan Mo (Institute of Oceanology, Chinese Academy of Sciences), Prof. Dr. Xiaohua Zhang (Ocean University of China), Prof. Bin Wang, and Dr. Shigen Ye (Dalian Fisheries University), Dr. Hui Gong (Fujian Academy of Agricultural Sciences), Prof. Cunbin Shi and Mr. Fei Zhao (Pearl River Fisheries Research Institute, Chinese Academy of Fishery Science), Mr. Zhuangchun Zhu (North China Coal Medical University), Prof. Xianle Yang (APCCMA, Shanghai Ocean University), Dr. Ka Yin Leung (National University of Singapore) for sharing Edwardsiella strains.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by grants from the National High Technology Research and Development Program of China (2008AA092501), Ministry of Agriculture of China (No. CARS-50), Shanghai Leading Academic Discipline Project (No. B505) as well as the National Special Fund for State Key Laboratory of Bioreactor Engineering (No. 2060204). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Abbott SL, Janda JM. The genus Edwardsiella. Prokaryotes. 2006;6:72–89. [Google Scholar]
- 2.Morrison EE, Plumb JA. Olfactory organ of channel catfish as a site of experimental Edwardsiella ictaluri infection. J Aquat Anim Health. 1994;6:101–109. [Google Scholar]
- 3.Panangala VS, Shoemaker CA, McNulty ST, Arias CR, Klesius PH. Intra and interspecific phenotypic characteristics of fish pathogenic Edwardsiella ictaluri and E. tarda. Aquac Res. 2006;37:49–60. [Google Scholar]
- 4.Mohanty BR, Sahoo PK. Edwardsiellosis in fish: a brief review. J Biosci. 2007;32:1331–1344. doi: 10.1007/s12038-007-0143-8. [DOI] [PubMed] [Google Scholar]
- 5.Bockemühl J, Pan-Urai R, Burkhardt F. Edwardsiella tarda associated with human disease. Pathobiology. 1971;37:393–401. [PubMed] [Google Scholar]
- 6.Kawai T, Kusakabe H, Seki A, Kobayashi S, Onodera M. Osteomyelitis due to trimethoprim/sulfamethoxazole-resistant Edwardsiella tarda infection in a patient with X-linked chronic granulomatous disease. Infection. 2011;39:171–173. doi: 10.1007/s15010-011-0080-1. [DOI] [PubMed] [Google Scholar]
- 7.Golub V, Kim AC, Krol V. Surgical wound infection, tuboovarian abscess, and sepsis caused by Edwardsiella tarda: case reports and literature review. Infection. 2010;38:487–489. doi: 10.1007/s15010-010-0057-5. [DOI] [PubMed] [Google Scholar]
- 8.Castro N, Toranzo AE, Barja JL, Nunez S, Magarinos B. Characterization of Edwardsiella tarda strains isolated from turbot, Psetta maxima (L.). J Fish Dis. 2006;29:541–547. doi: 10.1111/j.1365-2761.2006.00750.x. [DOI] [PubMed] [Google Scholar]
- 9.Kumar G, Rathore G, Sengupta U, Singh V, Kapoor D, et al. Isolation and characterization of outer membrane proteins of Edwardsiella tarda and its application in immunoassays. Aquaculture. 2007;272:98–104. [Google Scholar]
- 10.Acharya M, Maiti NK, Mohanty S, Mishra P, Samanta M. Genotyping of Edwardsiella tarda isolated from freshwater fish culture system. Comp Immunol Microbiol Infect Dis. 2007;30:33–40. doi: 10.1016/j.cimid.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Lan J, Zhang XH, Wang Y, Chen J, Han Y. Isolation of an unusual strain of Edwardsiella tarda from turbot and establish a PCR detection technique with the gyrB gene. J Appl Microbiol. 2008;105:644–651. doi: 10.1111/j.1365-2672.2008.03779.x. [DOI] [PubMed] [Google Scholar]
- 12.Wang YM, Wang QY, Xiao JF, Liu Q, Wu HZ, et al. Genetic relationships of Edwardsiella strains isolated in China aquaculture revealed by rep-PCR genomic fingerprinting and investigation of Edwardsiella virulence genes. J Appl Microbiol. 2011;111:1337–1348. doi: 10.1111/j.1365-2672.2011.05166.x. [DOI] [PubMed] [Google Scholar]
- 13.Castro N, Toranzo AE, Bastardo A, Barja JL, Magariños B. Intraspecific genetic variability of Edwardsiella tarda strains from cultured turbot. Dis Aquat Org. 2011;95:253–258. doi: 10.3354/dao02363. [DOI] [PubMed] [Google Scholar]
- 14.Maiti NK, Mandal A, Mohanty S, Samanta M. Comparative analysis of genome of Edwardsiella tarda by BOX-PCR and PCR-ribotyping. Aquaculture. 2008;280:60–63. [Google Scholar]
- 15.Panangala VS, Santen VL, Shoemaker CA, Klesius PH. Analysis of 16S-23S intergenic spacer regions of the rRNA operons in Edwardsiella ictaluri and Edwardsiella tarda isolates from fish. J Appl Microbiol. 2005;99:657–669. doi: 10.1111/j.1365-2672.2005.02626.x. [DOI] [PubMed] [Google Scholar]
- 16.Wang QY, Yang MJ, Xiao JF, Wu HZ, Wang X, et al. Genome sequence of the versatile fish pathogen Edwardsiella tarda provides insights into its adaptation to broad host ranges and intracellular niches. PLoS ONE. 2009;4:1331–1344. doi: 10.1371/journal.pone.0007646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soest JJ. Infectious disease studies in zebrafish: the fish pathogen Edwardsiella tarda as a model system: Department of Molecular Microbiology and Department of Molecular Cell Biology, Institute of Biology Leiden, Faculty of Science, Leiden University. 2011.
- 18.Dumpala PR, Lawrence ML, Karsi A. Proteome analysis of Edwardsiella ictaluri. Proteomics. 2009;9:1353–1363. doi: 10.1002/pmic.200800652. [DOI] [PubMed] [Google Scholar]
- 19.Ewing WH, McWhorter AC, Escobar MR, Lubin AH. Edwardsiella, a new genus of Enterobacteriaceae based on a new species, E. tarda. Int J Syst Evol Microbiol. 1965;15:33–38. [Google Scholar]
- 20.Hawke JP, McWhorter A, Steigerwalt AG, Brenner NJ. Edwardsiella ictaluri sp. nov., the causative agent of enteric septicemia of catfish. Int J Syst Evol Microbiol. 1981;31:396–400. [Google Scholar]
- 21.Amandi A, Hiu SF, Rohovec JS, Fryer JL. Isolation and characterization of Edwardsiella tarda from fall chinook salmon (Oncorhynchus tshawytscha). Appl Environ Microbiol. 1982;43:1380–1384. doi: 10.1128/aem.43.6.1380-1384.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baya AM, Romalde JL, Green DE, Navarro RB, Evans J, et al. Edwardsiellosis in wild striped bass from the Chesapeake Bay. J Wildl Dis. 1997;33:517–525. doi: 10.7589/0090-3558-33.3.517. [DOI] [PubMed] [Google Scholar]
- 23.Thune RL, Stanley LA, Cooper RK. Pathogenesis of gram-negative bacterial infections in warmwater fish. Annu Rev Fish Dis. 1993;3:37–68. [Google Scholar]
- 24.Thune RL, Fernandez DH, Battista JR. An aroA mutant of Edwardsiella ictaluri is safe and efficacious as a live, attenuated vaccine. J Aquat Anim Health. 1999;11:358–372. [Google Scholar]
- 25.Xiao JF, Wang QY, Liu Q, Wang X, Liu H, et al. Isolation and identification of fish pathogen Edwardsiella tarda from mariculture in China. Aquac Res. 2008;40:13–17. [Google Scholar]
- 26.Partridge SR, Hall RM. The IS1111 family members IS4321 and IS5075 have subterminal inverted repeats and target the terminal inverted repeats of Tn21 family transposons. J Bacteriol. 2003;185:6371–6384. doi: 10.1128/JB.185.21.6371-6384.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Konstantinidis KT, Tiedje JM. Genomic insights that advance the species definition for prokaryotes. Proc Natl Acad Sci U S A. 2005;102:2567–2572. doi: 10.1073/pnas.0409727102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goris J, Konstantinidis KT, Klappenbach JA, Coenye T, Vandamme P, et al. DNA-DNA hybridization values and their relationship to whole-genome sequence similarities. Int J Syst Evol Microbiol. 2007;57:81–91. doi: 10.1099/ijs.0.64483-0. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto S, Okujo N, Yoshida T, Matsuura S, Shinoda S. Structure and iron transport activity of vibrioferrin, a new siderophore of Vibrio parahaemolyticus. J Biochem. 1994;115:868–874. doi: 10.1093/oxfordjournals.jbchem.a124432. [DOI] [PubMed] [Google Scholar]
- 30.Wang QY, Liu Q, Ma Y, Zhou LY, Zhang YX. Isolation, sequencing and characterization of cluster genes involved in the biosynthesis and utilization of the siderophore of marine fish pathogen Vibrio alginolyticus. Arch Microbiol. 2007;188:433–439. doi: 10.1007/s00203-007-0261-6. [DOI] [PubMed] [Google Scholar]
- 31.Hirakawa H, Nishino K, Hirata T, Yamaguchi A. Comprehensive studies of drug resistance mediated by overexpression of response regulators of two-component signal transduction systems in Escherichia coli. J Bacteriol. 2003;185:1851–1856. doi: 10.1128/JB.185.6.1851-1856.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lai TH, Kumagai Y, Hyodo M, Hayakawa Y, Rikihisa Y. The Anaplasma phagocytophilum PleC histidine kinase and PleD diguanylate cyclase two-component system and role of cyclic Di-GMP in host cell infection. J Bacteriol. 2009;191:693–700. doi: 10.1128/JB.01218-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wipat A, Carter N, Brignell SC, Guy BJ, Piper K, et al. The dnaB-pheA (256°-240°) region of the Bacillus subtilis chromosome containing genes responsible for stress responses, the utilization of plant cell walls and primary metabolism. Microbiology. 1996;142:3067–3078. doi: 10.1099/13500872-142-11-3067. [DOI] [PubMed] [Google Scholar]
- 34.Tan YP, Zheng J, Tung SL, Rosenshine I, Leung KY. Role of type III secretion in Edwardsiella tarda virulence. Microbiology. 2005;151:2301–2313. doi: 10.1099/mic.0.28005-0. [DOI] [PubMed] [Google Scholar]
- 35.Zheng J, Leung KY. Dissection of a type VI secretion system in Edwardsiella tarda. Mol Microbiol. 2007;66:1192–1206. doi: 10.1111/j.1365-2958.2007.05993.x. [DOI] [PubMed] [Google Scholar]
- 36.Wang X, Wang QY, Xiao JF, Liu Q, Wu HZ, et al. Edwardsiella tarda T6SS component evpP is regulated by esrB and iron, and plays essential roles in the invasion of fish. Fish Shellfish Immun. 2009;27:469–477. doi: 10.1016/j.fsi.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 37.Wang X, Wang QY, Xiao JF, Liu Q, Wu HZ, et al. Hemolysin EthA in Edwardsiella tarda is essential for fish invasion in vivo and in vitro and regulated by two-component system EsrA-EsrB and nucleoid protein HhaEt. Fish Shellfish Immun. 2010;29:1082–1091. doi: 10.1016/j.fsi.2010.08.025. [DOI] [PubMed] [Google Scholar]
- 38.Tamura K, Sakazaki R, McWhorter AC, Kosako Y. Edwardsiella tarda serotyping scheme for international use. J Clin Microbiol. 1988;26:2343–2346. doi: 10.1128/jcm.26.11.2343-2346.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lobb CJ, Ghaffari SH, Hayman JR, Thompson DT. Plasmid and serological differences between Edwardsiella ictaluri strains. Appl Environ Microbiol. 1993;59:2830–2836. doi: 10.1128/aem.59.9.2830-2836.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang X, Quinn PJ. Lipopolysaccharide: biosynthetic pathway and structure modification. Prog Lipid Res. 2010;49:97–107. doi: 10.1016/j.plipres.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 41.Xu LL, Wang QY, Xiao JF, Liu Q, Wang X, et al. Characterization of Edwardsiella tarda waaL: roles in lipopolysaccharide biosynthesis, stress adaptation, and virulence toward fish. Arch Microbiol. 2010;192:1039–1047. doi: 10.1007/s00203-010-0635-z. [DOI] [PubMed] [Google Scholar]
- 42.Nei M, Li WH. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci U S A. 1979;76:5269–5273. doi: 10.1073/pnas.76.10.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nei M, Gojobori T. Simple methods for estimating the numbers of synonymous and nonsynonymous nucleotide substitutions Mol Biol Evol. 1986;3:418–426. doi: 10.1093/oxfordjournals.molbev.a040410. [DOI] [PubMed] [Google Scholar]
- 44.Yang Z. PAML 4: phylogenetic analysis by maximum likelihood. Mol Biol Evol. 2007;24:1586–1591. doi: 10.1093/molbev/msm088. [DOI] [PubMed] [Google Scholar]
- 45.Stojiljkovic I, Hantke K. Transport of haemin across the cytoplasmic membrane through a haemin specific periplasmic binding protein dependent transport system in Yersinia enterocolitica. Mol Microbiol. 1994;13:719–732. doi: 10.1111/j.1365-2958.1994.tb00465.x. [DOI] [PubMed] [Google Scholar]
- 46.Chamnongpol S, Dodson W, Cromie MJ, Harris ZL, Groisman EA. Fe (III) mediated cellular toxicity. Mol Microbiol. 2002;45:711–719. doi: 10.1046/j.1365-2958.2002.03041.x. [DOI] [PubMed] [Google Scholar]
- 47.Baek JH, Kang YJ, Lee SY. Transcript and protein level analyses of the interactions among PhoB, PhoR, PhoU and CreC in response to phosphate starvation in Escherichia coli. FEMS Microbiol Lett. 2007;277:254–259. doi: 10.1111/j.1574-6968.2007.00965.x. [DOI] [PubMed] [Google Scholar]
- 48.Loprasert S, Whangsuk W, Sallabhan R, Mongkolsuk S. The unique glutathione reductase from Xanthomonas campestris: gene expression and enzyme characterization. Biochem Biophys Res Commun. 2005;331:1324–1330. doi: 10.1016/j.bbrc.2005.04.050. [DOI] [PubMed] [Google Scholar]
- 49.Forst S, Delgado J, Inouye M. Phosphorylation of OmpR by the osmosensor EnvZ modulates expression of the ompF and ompC genes in Escherichia coli. Proc Natl Acad Sci U S A. 1989;86:6052–6056. doi: 10.1073/pnas.86.16.6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jovanovic G, Weiner L, Model P. Identification, nucleotide sequence, and characterization of PspF, the transcriptional activator of the Escherichia coli stress-induced psp operon. J Bacteriol. 1996;178:1936–1945. doi: 10.1128/jb.178.7.1936-1945.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bernadac A, Gavioli M, Lazzaroni JC, Raina S, Lloubes R. Escherichia coli tol-pal mutants form outer membrane vesicles. J Bacteriol. 1998;180:4872–4878. doi: 10.1128/jb.180.18.4872-4878.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu C, Lin X, Ren H, Zhang Y, Wang S, et al. Analysis of outer membrane proteome of Escherichia coli related to resistance to ampicillin and tetracycline. Proteomics. 2006;6:462–473. doi: 10.1002/pmic.200500219. [DOI] [PubMed] [Google Scholar]
- 53.Rogge ML, Thune RL. Regulation of the Edwardsiella ictaluri Type III Secretion System by pH and Phosphate Concentration through EsrA, EsrB, and EsrC. Appl Environ Microbiol. 2011;77:4293–4302. doi: 10.1128/AEM.00195-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lan MZ, Peng X, Xiang MY, Xia ZY, Bo W, et al. Construction and characterization of a live, attenuated esrB mutant of Edwardsiella tarda and its potential as a vaccine against the haemorrhagic septicaemia in turbot, Scophthamus maximus (L.). Fish Shellfish Immun. 2007;23:521–530. doi: 10.1016/j.fsi.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 55.Cheng S, Hu Y, Zhang M, Sun L. Analysis of the vaccine potential of a natural avirulent Edwardsiella tarda isolate. Vaccine. 2010;28:2716–2721. doi: 10.1016/j.vaccine.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 56.Milton DL, Norqvist A, Wolf-Watz H. Cloning of a metalloprotease gene involved in the virulence mechanism of Vibrio anguillarum. J Bacteriol. 1992;174:7235–7244. doi: 10.1128/jb.174.22.7235-7244.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zerbino DR, Birney E. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 2008;18:821–829. doi: 10.1101/gr.074492.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gordon D, Desmarais C, Green P. Automated finishing with Autofinish. Genome Res. 2001;11:614–625. doi: 10.1101/gr.171401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aziz RK, Bartels D, Best AA, DeJongh M, Disz T, et al. The RAST Server: rapid annotations using subsystems technology. BMC Genomics. 2008;9:75. doi: 10.1186/1471-2164-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Langille MGI, Brinkman FSL. IslandViewer: an integrated interface for computational identification and visualization of genomic islands. Bioinformatics. 2009;25:664–665. doi: 10.1093/bioinformatics/btp030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Siguier P, Perochon J, Lestrade L, Mahillon J, Chandler M. ISfinder: the reference centre for bacterial insertion sequences. Nucleic Acids Res. 2006;34:D32–D36. doi: 10.1093/nar/gkj014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ghai R, Hain T, Chakraborty T. GenomeViz: visualizing microbial genomes. BMC Bioinformatics. 2004;5:198. doi: 10.1186/1471-2105-5-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kurtz S, Phillippy A, Delcher A, Smoot M, Shumway M, et al. Versatile and open software for comparing large genomes. Genome Biol. 2004;5:R12. doi: 10.1186/gb-2004-5-2-r12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, et al. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chappey C. Computational analysis of HIV molecular sequences. Computational Genomics: Current Methods. 2007;109 [Google Scholar]
- 66.Vilella AJ, Blanco-Garcia A, Hutter S, Rozas J. VariScan: analysis of evolutionary patterns from large-scale DNA sequence polymorphism data. Bioinformatics. 2005;21:2791–2793. doi: 10.1093/bioinformatics/bti403. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Phylogenetic tree of Edwardsiella species. Phylogenies of Edwardsiella species inferred from concatenated alignments of the protein sequences encoded by 44 house-keeping genes (adk, aroC, dnaA, dnaK, frr, fusA, gapA, gyrA, gryB, infC, nusA, pgk, phoB, phoR, pyrG, recC, rplA, rplB, rplC, rplD, rplE, rplF, rplK, rplL, rplM, rplN, rplP, rplS, rplT, rpmA, rpoA, rpoB, rpoC, rpoE, rpsB, rpsC, rpsE, rpsI, rpsJ, rpsK, rpsM, rpsS, smpB, and tsf) by PhyML program with 100 bootstrap iterations for clade support. Bacillus cereus ATCC14579 was used as the outgroup strain.
(TIF)
Relationships between ANI and synonymous nucleotide substitutions. Each blue square represents the ANI of all genome sequence between two strains (x axes) plotted against (y axes) the average rate of synonymous nucleotide substitutions of housekeeping genes.
(TIF)
Edwardsiella virulence gene clusters of secreted proteins. T6SS (A) and T3SS (B) gene clusters of sequenced E. tarda EdwGI and E. ictaluri strains. All genes with high similarity are indicated in the same color and the gene names are shown below according to the color scheme. (C) NJ-tree of 6 Edwardsialla isolates (3 EdwGI strains, 2 E. icatluri strains and E. tarda PPD130/91) inferred from concatenated T6SS and T3SS aligned sequences. (D) NJ tree of 8 Edwardsiella species inferred from concatenated alignments of the coding sequences of esrA and esrB genes with 1000 bootstrap iterations.
(TIF)
Nucleotide diversity (π) of orthoglous of Edwardsiella . (A) Nucleotide diversity (π) for E. tarda EdwGI and EdwGII. (B) Nucleotide diversity (π) for E. tarda EdwGI and E. ictaluri strains. The blank line represents the average π value of all orthologs. Green line indicates π values above 1.5σ (standard deviation) from the average π values of all orthologs, respectively. The percent of genes with π values large than 1.5σ from the average π value in each function category are shown under x axis.
(TIF)
API-20E test of Edwardsiella strains.
(DOC)
RAST annotation of the genomes of the Edwardsiella strains.
(XLS)
Sequence identity of 8 Edwardsiella strains.
(DOC)
Predicted GI related sequences and IS distribution in the genomes of the Edwardsiella strains.
(XLS)
Multilocus sequence analysis of 48 strains.
(XLS)
ANI value and synonymous substitution frequency of the strains studied.
(DOC)
Ortholog distribution in the genomes of the Edwardsiella strains.
(XLS)
Amino acid sequence identity of the T3SS and T6SS between the Edwardsiella strains.
(XLS)
LPS biosynthesis related genes in the Edwardsiella strains.
(XLS)
Nucleotide variation value pi of the Edwardsiella strains.
(XLS)
positive selected genes.
(XLS)
primer list.
(XLS)
Data Availability Statement
The nucleotide sequence of the draft sequences were submitted to the GenBank database under accession numbers AFJH00000000 (E. tarda 080813), AFJG00000000 (E. tarda ATCC15947), AFJJ00000000 (E. tarda DT) and AFJI00000000 (E. ictaluri ATCC33202), respectively. Sequences used for the multilocus sequence analysis were available under the accession numbers JN709499-JN709721.