Abstract
Background
Anaphylaxis management guidelines recommend the use of intramuscular adrenaline in severe reactions, complemented by antihistamines and corticoids; secondary prevention includes allergen avoidance and provision of self-applicable first aid drugs. Gaps between recommendations and their implementation have been reported, but only in confined settings. Hence, we analysed nation-wide data on the management of anaphylaxis, evaluating the implementation of guidelines.
Methods
Within the anaphylaxis registry, allergy referral centres across Germany, Austria and Switzerland provided data on severe anaphylaxis cases. Based on patient records, details on reaction circumstances, diagnostic workup and treatment were collected via online questionnaire. Report of anaphylaxis through emergency physicians allowed for validation of registry data.
Results
2114 severe anaphylaxis patients from 58 centres were included. 8% received adrenaline intravenously, 4% intramuscularly; 50% antihistamines, and 51% corticoids. Validation data indicated moderate underreporting of first aid drugs in the Registry. 20% received specific instructions at the time of the reaction; 81% were provided with prophylactic first aid drugs at any time.
Conclusion
There is a distinct discrepancy between current anaphylaxis management guidelines and their implementation. To improve patient care, a revised approach for medical education and training on the management of severe anaphylaxis is warranted.
Introduction
Background
Severe anaphylaxis is an acute and life-threatening IgE-mediated hypersensitivity reaction [1], [2]. A particular cause such as insect venom, food items or drugs is traced in two out of three cases [3]. Beyond skin and gastrointestinal symptoms, airway constriction and circulatory collapse can be fatal.
Due to differences in recognition, diagnosis and reporting of anaphylactic reactions [4], estimates of lifetime prevalence range between 0.05% and 2% [5]. As with other allergic diseases, several surveys suggest a rising incidence of anaphylaxis [6], [7].
Primary prevention strategies have not been established yet, stressing the need for defined first aid management. Different practice parameters have been published to guide initial treatment and secondary prevention of anaphylaxis.
Management Recommendations
International attempts have repeatedly been made to compile available insight on the diagnosis and management of anaphylaxis [8], complemented through a European initiative focussing on children [9]. On a national scale, the Resuscitation Council (from the United Kingdom) has agreed on a widely implemented guideline [10] giving detailed instructions on the use of emergency drugs and other treatment options.
There is expert agreement [11] to apply adrenaline intramuscularly as first line treatment in all potentially life-threatening anaphylactic reactions in the field, despite inconclusive evidence to support this recommendation [12]. On account of tachyarrhythmia side effects, intravenous application is limited to management involving specialised health care providers such as anaesthetists or emergency physicians.
The use of antihistamines and corticoids is subject to controversy as an ancillary option, the latter supposed to be of particular value in asthmatic individuals. There is no support through controlled trials for both treatment options [13], [14].
The mainstay of secondary prevention is patient education focussing on avoidance of known or suspected allergens and early symptom recognition. Self-injectable adrenaline devices as well as oral antihistamines and corticoids are commonly provided to the patient [15].
Besides the lack of data supporting management recommendations, their use has been only sporadically evaluated.
Current Implementation
A recent systematic review on studies reporting gaps in anaphylaxis management traced a profound discrepancy between guidelines and their implementation in a widespread variety of settings [16]. Lack of knowledge about diagnosis and treatment was identified to hamper correct management on a professional level, leading to infrequent and delayed use of intramuscular adrenaline and failure to prescribe auto-injectors. Patient instruction on avoidance and proper self-application of first aid drugs was often reported to be insufficient. However, studies on anaphylaxis management generally focussed on a subset of treatment options (e.g. adrenaline) and rely on unique settings such as emergency departments or schools only.
Anaphylaxis Registry
Hence, to provide sound figures for the current implementation of guidelines, we present the first large-scale analysis of initial treatment and secondary prevention data including all ages and settings, from the transnational anaphylaxis registry, covering the general population of Germany, Austria and Switzerland [17]. This will enable us to spot gaps and target interventional strategies and to improve patient care in severe anaphylaxis.
Methods
Design and Subjects
The anaphylaxis registry consecutively recorded incident cases of severe anaphylaxis, first occurrence and recurrent disease. It was aimed to obtain well defined, standardized data of affected patients in Germany, Austria and Switzerland.
Following an acute reaction, patients are being referred to specialized outpatient clinics for further allergological evaluation, usually related to dermatology or paediatric departments of tertiary hospitals. 83 centres participating in the anaphylaxis registry were included in this analysis. At these visits, all individuals reporting respiratory or circulatory symptoms in conjunction with the initial reaction, indicating severe anaphylaxis, were invited to participate and asked to give written informed consent, for children by a guardian.
The study was approved by the ethics committee at Charité University Medical Centre Berlin, Germany.
Measurement
All information was retrieved from patient records, and if available complemented by emergency physicians’ protocol. Data was acquired anonymously, and entered by trained health professionals in allergy centres into an online questionnaire covering symptoms, diagnostic workup, cause, co-morbidities, and treatment details.
Since speculation about the cause of anaphylaxis is known to be misleading, causes were limited to confirmed cases of insect sting, food or drugs only. Grading of severity was based on symptoms recorded, categorized according to [1]. Information on the person having carried out first aid treatment was pooled in 5 clusters to reflect assumed level of training in emergency handling, professionals in 3 (emergency, hospital or registered physicians) and lay helpers in 2 (first aid drugs lay- or self-administered).
Quality Management
At time of inclusion, centres received training to assure quality standards. An independent expert committee updated the questionnaire annually, based on e.g. double entry congruency. To account for heterogeneity of anaphylactic reactions, closed questions were complemented by free text manually.
Representativeness and accuracy of the anaphylaxis registry were assured through data collected directly from emergency physicians (EPs) in a sample catchment area. EPs were asked to complete a condensed version of the original questionnaire used for the Registry immediately after first aid treatment.
Analysis
Independent evaluation was performed by two trained epidemiologists, using SAS 9.2 (SAS Institute Inc., Cary, U.S.). The cross-sectional information in the Registry allowed for the calculation of frequencies (of categories) only. Basic stratification was used to identify subgroup differences. Confidence intervals of prevalence measures were calculated using the standard Wald procedure. Weighting of EPs’ validation data was performed using a logistic regression model including age, cause and severity to match distribution of cases in anaphylaxis registry, Berlin catchment area. Missing data was minimized by individual queries involving the referral centres, if unavailable analyses were restricted to complete cases.
Results
Of 83 referral centres participating in the anaphylaxis registry, 58 entered valid data between 2006 and 2010, 27 centres on 10 or more patients (17 dermatology, 9 paediatrics, 1 other). 2114 patients sought further evaluation of severe anaphylaxis, more than 85% within 6 months after the incident. In accord with population sizes, distribution of country membership (Germany 75.8%, Austria 9.1%, Switzerland 15.1%) and sex (female 47.2%) was well-balanced, with about one-fifth below legal age (20.2%). The most common assured cause of anaphylaxis was insect sting (47.9%), followed by food (16.0%) and drugs (9.4%, table 1).
Table 1. Patients in anaphylaxis registry, first aid treatment stratified by general characteristics and reaction circumstances.
| First aid treatment | |||||||
| Emergency physician | Physician in hospital | Physician in med. practice | Drugs lay-administered | Drugs self-administered | |||
| n | (%) | % | % | % | % | % | |
| Total | 2114 | (100.0) | 34.5 | 23.6 | 14.0 | 5.3 | 4.7 |
| Country | |||||||
| Germany | 1602 | (75.8) | 39.1 | 21.8 | 13.0 | 4.2 | 4.1 |
| Austria | 193 | (9.1) | 29.5 | 29.5 | 19.7 | 2.1 | 4.7 |
| Switzerland | 319 | (15.1) | 14.7 | 28.8 | 15.7 | 12.5 | 7.5 |
| Sex | |||||||
| Female | 998 | (47.2) | 37.1 | 23.4 | 13.7 | 6.3 | 3.6 |
| Male | 1116 | (52.8) | 32.3 | 23.7 | 14.2 | 4.3 | 5.6 |
| Age | |||||||
| <18 years | 428 | (20.2) | 20.8 | 26.6 | 15.2 | 18.7 | 3.0 |
| 18–64 years | 1401 | (66.3) | 36.5 | 23.3 | 13.5 | 2.0 | 5.4 |
| >64 years | 285 | (13.5) | 45.3 | 20.7 | 14.7 | 1.1 | 3.9 |
| Location of anaphylactic reaction | |||||||
| Hospital, medical practice | 224 | (10.6) | 5.4 | 48.7 | 29.5 | 1.3 | 0.9 |
| Work place, school | 105 | (5.0) | 34.3 | 22.9 | 14.3 | 10.5 | 2.9 |
| Restaurant | 84 | (4.0) | 33.3 | 29.8 | 2.4 | 8.3 | 9.5 |
| At home | 524 | (24.8) | 36.1 | 19.8 | 13.7 | 9.9 | 5.0 |
| Outdoors (nature) | 452 | (21.4) | 40.9 | 19.9 | 19.0 | 2.2 | 4.9 |
| Outdoors (city) | 185 | (8.8) | 44.3 | 19.5 | 10.3 | 6.5 | 5.4 |
| Cause (only confirmed) | |||||||
| Insect sting | 1012 | (47.9) | 43.1 | 19.9 | 14.5 | 2.2 | 4.2 |
| Food | 339 | (16.0) | 24.8 | 21.5 | 10.3 | 16.5 | 6.2 |
| Drugs | 198 | (9.4) | 20.7 | 35.9 | 21.7 | 3.0 | 3.0 |
| Severity (following [1] ) | |||||||
| Severe reaction (II) | 587 | (27.8) | 24.5 | 27.1 | 15.8 | 7.5 | 5.5 |
| Shock (III) | 1448 | (68.5) | 37.2 | 22.0 | 13.8 | 4.5 | 4.6 |
| Resp./circulatory arrest (IV) | 64 | (3.0) | 59.4 | 29.7 | 3.1 | 3.1 | 1.6 |
First aid Treatment
One in three reactions were initially handled by an emergency physician (EP, 34.5%), 37.6% by other physicians, and 10.0% received first aid treatment through non-professionals (self- or lay-administered). However, Switzerland reported a lower frequency of EP treatment (14.7%) and a higher for lay-administered drugs (12.5%). Children and adolescents were also more likely to receive first aid treatment by lay helpers (18.7 vs. 1.9% in adults), especially at pre-school age (33.2%).
Of all anaphylactic reactions taking place in a hospital or medical practice (n = 224), 61.3% were caused by drugs and were most commonly treated by physicians on site (79.1%). Food items were responsible for 77.5% of severe anaphylaxis cases in restaurants (n = 84), and more often treated with self-administered first aid drugs than in other reactions (10.2 vs. 4.5%).
Severest cases with respiratory and/or circulatory arrest (n = 64) were more often treated by EPs (59.4 vs. 33.5%).
Recurrent disease accounted for 32.2% of registered reactions. Among these, first aid drugs were more commonly self- (13.1 vs. 1.6%) or lay-administered (8.5 vs 2.9%) compared to first occurrence cases.
First Aid Drugs
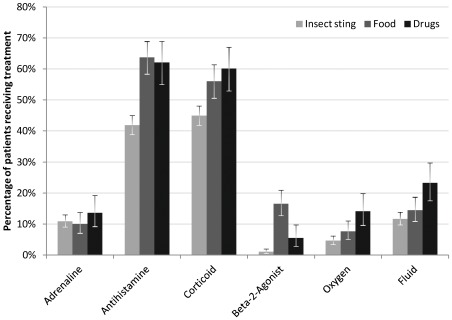
13.0% received adrenaline, irrespective of the cause. Application of antihistamines (50.1%) and corticoids (51.3%) was both less frequent in insect venom reactions compared to other elicitors. Beta-2-agonists were mainly given in food-induced anaphylaxis (5.9% in all reactions). Treatment with oxygen (6.3%) and fluids (13.9%) was more common in drug reactions than in insect venom or food-induced anaphylaxis (figure 1).
Figure 1. Drugs used for emergency treatment of anaphylaxis, by cause.
Only assured cases. All application routes, error bars indicate 95% confidence intervals.
Application Routes
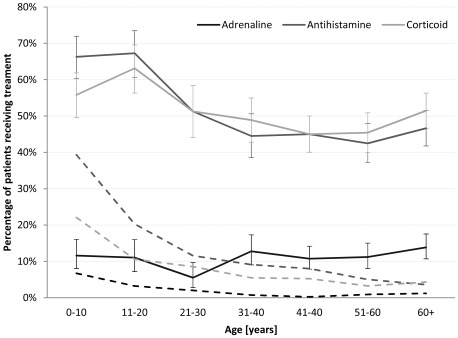
Adrenaline was applied mainly intravenously (7.6%), compared to the recommended intramuscular route (3.9%), especially in Austria (18.7 vs. 3.1%). Only centres from Switzerland reported a frequent use of the intramuscular route (6.9%). EPs were more likely to apply adrenaline intravenously (11.9%), where self- and lay-administration was mainly intramuscular (23.2% and 12.6%). Less than half cases suffering respiratory and/or circulatory arrest received adrenaline (48.0%, table 2). With a steady age distribution for all application routes, adrenaline per inhalation was confined to the first 2 decades of life and decreased thereafter (figure 2).
Table 2. Application routes of drugs used for emergency treatment, stratified by country, first aid treatment and severity.
| Adrenaline | Antihistamine | Corticoid | Beta-2-Agonist | Oxygen | Fluid | ||||||
| IM | IV | inhal. | IV | PO | IV | PO | inhal. | ||||
| n | % | % | % | % | % | % | % | % | % | % | |
| Total | 2114 | 3.9 | 7.6 | 2.3 | 38.1 | 13.8 | 42.9 | 8.7 | 5.9 | 6.3 | 13.9 |
| Country | |||||||||||
| Germany | 1602 | 3.4 | 6.4 | 1.9 | 34.9 | 11.0 | 40.8 | 6.1 | 5.5 | 5.7 | 12.2 |
| Austria | 193 | 3.1 | 18.7 | 2.6 | 54.4 | 6.7 | 58.5 | 2.6 | 3.6 | 11.4 | 32.6 |
| Switzerland | 319 | 6.9 | 6.6 | 4.1 | 44.2 | 31.7 | 44.2 | 25.4 | 9.1 | 6.3 | 11.0 |
| Inital treatment by | |||||||||||
| Emergency physician | 730 | 1.5 | 11.9 | 2.6 | 48.1 | 3.7 | 54.0 | 2.5 | 2.7 | 8.5 | 17.8 |
| Physician in hospital | 499 | 3.4 | 7.8 | 2.8 | 51.3 | 10.0 | 57.7 | 5.4 | 7.0 | 6.6 | 15.6 |
| Physician in med. practice | 296 | 4.1 | 8.4 | 2.4 | 43.9 | 14.9 | 50.7 | 9.5 | 9.8 | 8.1 | 19.3 |
| Drugs lay-administered | 111 | 12.6 | 1.8 | 3.6 | 15.3 | 72.1 | 18.0 | 40.5 | 22.5 | 5.4 | 9.9 |
| Drugs self-administered | 99 | 23.2 | 1.0 | 0.0 | 6.1 | 66.7 | 6.1 | 53.5 | 8.1 | 1.0 | 1.0 |
| Severity (following [1] ) | |||||||||||
| Severe reaction (II) | 587 | 4.3 | 2.7 | 2.2 | 38.5 | 19.4 | 43.6 | 12.1 | 10.2 | 3.6 | 6.1 |
| Shock (III) | 1448 | 3.9 | 8.0 | 2.1 | 38.0 | 11.9 | 42.5 | 7.5 | 4.4 | 6.4 | 16.4 |
| Resp./circ. arrest (IV) | 64 | 1.6 | 42.2 | 6.3 | 40.6 | 4.7 | 51.6 | 4.7 | 0.0 | 29.7 | 31.3 |
IM - intramuscular, IV - intravenous, PO – oral, inhal. - per inhalation.
Figure 2. Drugs used for emergency treatment of anaphylaxis, by age.
Dashed lines indicate proportion of patients having received inhalation (adrenaline) or oral (antihistamine, corticoid) treatment only, error bars indicate 95% confidence intervals.
Antihistamines and corticoids were given intravenously in most cases receiving that agent (38.1% and 42.9%), oral application was common in non-professional first aid treatment (table 2). Overall use of antihistamines and corticoids was highest in childhood and adolescence, mainly due to a high proportion of oral application, which declined in higher age groups (figure 2).
Inhalation of beta-2-agonists was confined to less severe reactions and most frequently lay-administered (22.5%).
Compared to other countries report of oxygen and fluid therapy was highest in Austria (11.4/32.6%). Only 29.7 and 31.3% of the most severe reactions (°IV) received oxygen and fluids (table 2).
Validation Data
To account for reporting error in the anaphylaxis registry, we collected firsthand information on treatment of 218 severe anaphylaxis cases from EPs in the catchment area Berlin, Germany. Baseline characteristics were similarly distributed, except for an underrepresentation of females in the Anaphylaxis Registry (59.8 vs. 28.9%). Besides a lower frequency of drug-related anaphylaxis (18.8 vs. 7.8%) occurring mainly in hospitals (13.1 vs. 3.2%), reaction circumstances such as location, cause and severity recorded in the anaphylaxis registry resemble EP’s firsthand data (table 3).
Table 3. Baseline characteristics and reaction circumstances of severe anaphylaxis patients treated by emergency physicians.
| Emergency Physicians | Anaphylaxis Registry# | |||||
| Berlin* (n = 218) | Berlin* (n = 90) | all centres (n = 730) | ||||
| n | (%) | n | (%) | n | (%) | |
| Sex | ||||||
| Female | 128 | (59.8) | 26 | (28.9) | 370 | (50.7) |
| Male | 86 | (40.2) | 64 | (71.1) | 360 | (49.3) |
| Age | ||||||
| <18 years | 14 | (6.4) | 1 | (1.1) | 89 | (12.2) |
| 18–64 years | 154 | (70.6) | 77 | (85.6) | 512 | (70.1) |
| >64 years | 50 | (22.9) | 12 | (13.3) | 129 | (17.7) |
| Location of anaphylactic reaction | ||||||
| Hospital, medical practice | 28 | (13.1) | 2 | (3.2) | 12 | (2.2) |
| Work place, school | 11 | (5.1) | 6 | (9.7) | 36 | (6.5) |
| Restaurant | 6 | (2.8) | 10 | (16.1) | 28 | (5.1) |
| At home | 119 | (55.6) | 22 | (35.5) | 189 | (34.2) |
| Outdoors (nature) | 9 | (4.2) | 10 | (16.1) | 185 | (33.5) |
| Outdoors (city) | 34 | (15.9) | 9 | (14.5) | 82 | (14.9) |
| Cause (only confirmed) | ||||||
| Insect sting | 49 | (22.5) | 23 | (25.6) | 436 | (59.7) |
| Food | 31 | (14.2) | 18 | (20.0) | 84 | (11.5) |
| Drugs | 41 | (18.8) | 7 | (7.8) | 41 | (5.6) |
| Severity (following [1] ) | ||||||
| Severe reaction (II) | 33 | (15.1) | 25 | (27.8) | 144 | (19.7) |
| Shock (III) | 176 | (80.7) | 60 | (66.7) | 539 | (73.8) |
| Resp./circulatory arrest (IV) | 9 | (4.1) | 5 | (5.6) | 38 | (5.2) |
Reported by emergency physicians in Berlin, Germany vs. self report in anaphylaxis registry.
comparable catchment areas, # only those initally treated by emergency physician.
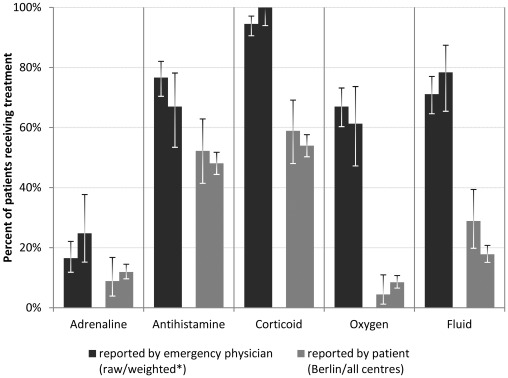
Frequency of adrenaline administered parenterally was lower in the registry compared to Berlin EPs’ direct report, even after accounting for differences in baseline characteristics and reaction circumstances. Comparison of antihistamines and corticoids indicated a similar proportion of underreporting in the anaphylaxis registry. Failure to trace non-drug treatments became apparent matching data on oxygen and fluid therapy (figure 3).
Figure 3. Drugs used by emergency physicians for initial treatment of anaphylaxis.
Firsthand report (EPs) vs. self report (anaphylaxis registry). Parenteral application routes only. * Weighted for age, cause and severity distribution in anaphylaxis registry, Berlin catchment area.
Instruction and Immunotherapy
61.5% of all cases were given general advice on anaphylaxis, including avoidance recommendation and delivery of allergy IDs. 75.1% received information on the use of first aid drugs in case of recurrent disease. Only a small proportion was instructed at the time of the initial reaction (11.6 and 19.6% respectively).
Children and adolescents were more likely to receive instructions (78.2/86.5%). Furthermore, cases of food-induced anaphylaxis were commonly given general and specific information on first aid drugs immediately following the incident (21.0/27.7%). 68.2/57.7% of patients with the most severe reactions (°IV) were instructed at any time (table 4).
Table 4. Patient instruction and specific immunotherapy after severe anaphylaxis, stratified by general characteristics and reaction circumstances.
| General instructions (avoidance, allergy ID) | Instructions for emergency medication | SIT | ||||||
| at initial reaction | In between | at referral centre | at initial reaction | In between | at referral centre | |||
| n | % | % | % | % | % | % | % | |
| Total | 2043 | 11.6 | 7.6 | 51.6 | 19.6 | 14.5 | 54.4 | 43.9 |
| Children (<18 years) | 410 | 27.1 | 9.5 | 58.0 | 31.2 | 10.2 | 62.7 | 24.6 |
| Country | ||||||||
| Germany | 1545 | 12.9 | 8.6 | 47.8 | 18.6 | 17.2 | 51.7 | 45.6 |
| Austria | 190 | 3.2 | 5.3 | 53.2 | 7.9 | 8.4 | 61.1 | 50.5 |
| Switzerland | 308 | 10.7 | 3.9 | 69.8 | 31.5 | 5.2 | 63.6 | 31.2 |
| Type of referral centre | ||||||||
| Dermatology | 1644 | 7.9 | 7.2 | 49.1 | 17.2 | 15.8 | 53.4 | 48.4 |
| Paediatrics | 320 | 32.5 | 10.6 | 62.2 | 34.4 | 9.1 | 63.1 | 16.9 |
| Internal medicine/ENT | 78 | 5.1 | 2.6 | 60.3 | 9.0 | 10.3 | 39.7 | 60.3 |
| Cause (only confirmed) | ||||||||
| Insect sting | 989 | 8.9 | 9.2 | 45.0 | 21.7 | 20.0 | 60.6 | 82.6 |
| Food | 328 | 21.0 | 8.8 | 67.1 | 27.7 | 12.2 | 68.3 | 4.0 |
| Drugs | 187 | 16.0 | 5.9 | 79.1 | 6.4 | 3.2 | 22.5 | 0.5 |
| Most severe reactions (IV) | 56 | 17.9 | 3.6 | 58.9 | 16.1 | 12.5 | 39.3 | 37.5 |
SIT: Specific immunotherapy, ENT: Ear, nose and throat/Otolaryngology.
43.9% underwent specific immunotherapy, with the highest frequency in anaphylaxis caused by insect venom (82.6%).
Prophylaxis
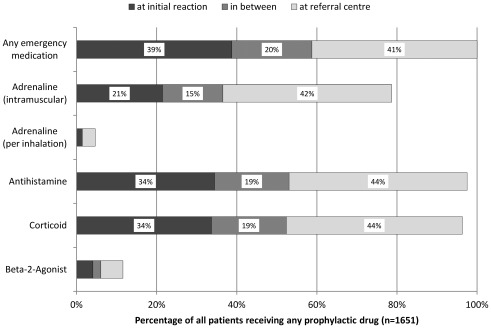
80.8% were given prophylactic first aid drugs at any time. Adrenaline auto-injectors were prescribed in 64.2%, oral antihistamines and corticoids in 79.1/78.0%. Of those receiving any prophylaxis, only one in five was given adrenaline and one in three antihistamines and corticoids immediately after the anaphylactic reaction (figure 4).
Figure 4. First time receiving prophylactic first aid drugs following severe anaphylaxis.
Discussion
Key Results
Severe anaphylaxis in the field was generally handled by professionals, involvement of lay helpers was only frequent in children and adolescents. Overall, less than 1 in 6 received adrenaline, even worse, only half of patients with respiratory or circulatory arrest. Though, application of antihistamines and corticoids was reported for the majority of anaphylactic reactions. Against current guidelines, adrenaline was applied intravenously by health professionals of any background in many cases.
Only 1 in 5 was provided with general information and adrenaline auto-injectors immediately after the incident, most patients received their first instructions at the referral centre visit. About 1 in 6 did not receive specific immunotherapy following severe insect venom anaphylaxis.
The distinct underuse of adrenaline is in line with several prior surveys of anaphylaxis management, for example from US emergency departments [18] or a questionnaire-based approach targeting German paediatricians [19]. The preference for intravenous application as seen in other settings (e.g. [20]) is not supported by guidelines or original literature [21], [22]. Yet, this analysis provides the first transnational and population-based survey of first aid treatment and secondary prevention of severe anaphylaxis. With a general perspective we demonstrated severe under- and misuse of adrenaline and failure to provide adequate patient instructions in the acute setting.
Strengths and Weaknesses
All data in the anaphylaxis registry is derived from medical records in specialized referral centres, supplemented by emergency physician’s on-site documentation, if available. Transcription from the records is carried out by trained professionals and is shown to be accurate by double entry comparison. But content of medical records on the other hand is non-standardised, its integrity limited by the patient’s failure to spot and recall treatment details. Comparison with data collected directly from EPs fortunately revealed only a moderate underreporting of adrenaline, antihistamine and corticoid use in the anaphylaxis registry, not to the extent to change interpretation of the data. Only the use of oxygen and fluids is shown to be highly underreported.
To keep time and effort for the participating centres workable, we limited the set of items covered in the database and omitted several aspects such as dosing and timing of drugs or details of patient education. Patients were not traced prospectively to cover recidivism or impact of management modalities. Furthermore, our approach is generally limited to information conveyed through the patients, impeding statements concerning knowledge, degree of training or attitude towards different treatment options of the initial caregiver. For patients were identified at referral centres, this type of registry does not allow for inferring case fatality. However, up to date 6 deaths were reported to the registry via allergists.
Our target population comprises all individuals having experienced a severe anaphylactic reaction recently, living in a participating country. Our registry is not exhaustive as not all patients are referred to or follow the recommendation to present to a specialised allergy centre. Selection maybe influenced by socio-demographic background, perceived severity of anaphylaxis or other health-related attitudes. Furthermore, not all referral centres were traced or included in the study, rising concern about differential selection. Fortunately, general patient characteristics and circumstances of anaphylaxis based on data directly collected from EPs were comparable to our study population.
We assume to base the following interpretation on a highly standardized and valid set of primary data drawn from a sample representative of the general population.
Implications
In light of established guidelines for the management of anaphylaxis, our survey confirmed known major gaps in their implementation on a transnational scale.
Failure to apply adrenaline timely and correctly in unquestionable severe anaphylaxis is most striking, in the field as well as in professional settings. In addition, only a minority of cases receive early and thorough patient education and preventive first aid drugs. We suppose a lack of knowledge and practical training to be responsible for these drawbacks. Our study identified not only EPs but all medical professionals as the target audience for continuing education, they are accountable for more than 90% of initial treatments, the main setting to advance management.
Recommendations
To improve treatment of anaphylaxis, we strongly recommend revision of medical education and practical training, targeting a broad range of professionals [23]. This approach could foster a high coverage of guideline-conform management. We propose a close collaboration of physicians in primary care settings such as EPs and specialised allergists for the development of interventional strategies. With our strong data at hand, there is no reason to delay implementation of educational programs on a national or even transnational scale.
The European Academy of Allergy and Clinical Immunology (EAACI) is currently putting together an updated guideline for the management of anaphylaxis in children and adults (personal communication). Yet, to achieve sufficient implementation of current and future recommendations, a new approach for the dissemination of guidelines and continuing medical education is inevitable.
The future role of the anaphylaxis registry, currently embracing other European countries, is to monitor trends and evaluate interventional strategies aiming to improve patient care and other public health goals, concerning aspects of anaphylaxis occurrence, natural history of disease and management [24]. Alongside these given aims of our survey, comparison of treatment options might prove helpful to settle the longstanding debate about the effectiveness of first aid drugs, especially adrenaline, not yet resolved by interventional trials.
Acknowledgments
We are most indebted to patients and staff at the allergy centres, members of the anaphylaxis registry, who provided data for this project: U. Rabe, Clinic of Pneumology, Johanniter-Hospital Treuenbrietzen, Germany; M. Henzgen, Pneumology/Allergology of the Department of Internal Medicine I, Friedrich-Schiller-University Jena, Germany; E. Coors, Department of Dermatology and Venerology, University Medical Center Hamburg-Eppendorf, Germany; W. Aberer, Department of Dermatology, Medical University of Graz, Austria; L. Lange, Children’s Hospital, St. Marien-Hospital Bonn, Germany; B. Wedi, Department of Dermatology, Hannover Medical School, Germany; H. Dickel, Department of Dermatology and Allergology, Ruhr-University Bochum, Germany; P. Schmid-Grendelmeier, Department of Dermatology, University Hospital of Zurich, Switzerland; T. Reese, Children’s Hospital, Mathias-Spital Rheine, Germany; G. Hansen, Department of Paediatric Pneumology and Neonatology, Hannover Medical School, Germany; T. Kinaciyan, Department of Dermatology and Centre of Physiology and Pathophysiology, Medical University of Vienna, Austria; P. Eng, Paediatric Pulmonology and Allergy/Immunology, Children’s Hospital Lucerne, Switzerland; T. Bieber and J. Hanfland, Department of Dermatology, University Hospital Bonn, Germany; K. Beyer, Department of Paediatric Pneumology and Immunology, University Hospital Charité Berlin, Germany; P. Eng, Paediatric Pulmonology and Allergy/Immunology, Children’s hospital Aarau, Switzerland; M. Rett, Children’s Hospital, Clinic Itzehoe, Germany; N. Hunzelmann, Department of Dermatology and Venerology, University Hospital of Cologne, Germany; J. Fischer, Department of Dermatology, University of Tuebingen, Germany; M. Kopp, Department of Dermatology and Allergy, University of Luebeck; H. Merk, Department of Dermatology, University Hospital Aachen, Germany; T. Fuchs, Department of Dermatology, Venerology and Allergy, Georg-August-University Goettingen, Germany; E. Varga, Department of Paediatrics, Medical University of Graz, Austria; F. Riffelmann, Allergology Unit, Hospital Kloster Grafschaft/Schmallenberg, Germany; A. Nordwig, Children’s Hospital Dresden-Neustadt, Dresden, Germany; Z. Szepfalusi, Department of Paediatrics, Medical University of Vienna, Austria; R. Bruns, Department of Paediatrics, Ernst-Moritz-Arndt-University of Greifswald, Germany; R Guggenheim, Department of Paediatrics, Triemli City Hospital Zurich, Switzerland; I. Yildiz, Children’s Hospital, Friedrich-Ebert-Hospital Neumuenster, Germany; S. Thies, Department of Dermatology and Allergy, Asklepios Hospital Uckermark Schwedt, Germany; T. Spindler, Waldburg-Zeil Hospitals, Department of Paediatrics, Clinics Wangen im Allgäu, Germany; B. Schilling, Paediatric Pulmonology and Allergy, Children’s Hospital III. Orden, Passau, Germany; T. Schinkel, Departement of Paediatrics and Adolescent Medicine, Clinic Barnim, Werner Forßmann Hospital Eberswalde, Germany; I. Neustädter, Department for Children and Adolescents, Hospital Fuerth, Germany; I. Bauhaus, Department of Paediatrics, Rehab-clinic Graal-Müritz, Germany; U. Wiencke-Graul and S. Plank-Habibi, Department of Dermatology, Clinic Alzenau, Germany; S. Schweitzer-Krantz, Children’s Hospital, Evangelic Hospital Duesseldorf, Germany; M. Polz, Children’s Hospital, GPR Hospital Ruesselsheim, Germany; K. Tenbrock and S. Lehmann, Department of Paediatrics, University Hospital Aachen, Germany; A. Heinzmann, Paediatrics and Adolescent Medicine, University of Freiburg, Germany; B. Kreft, Department of Dermatology and Venerology, Martin-Luther-University Halle-Wittenberg, Germany; L. Klimek and O. Pfaar, Department of Otorhinolaryngology, Head and Neck, University Hospital Mannheim, Germany; E. Rietschel, Children’s Hospital, University of Cologne, Germany; J. Schmitt, Department of Dermatology, University Hospital Carl Gustav Carus, Technical University of Dresden, Germany; F. Friedrichs, Private paediatric practice Laurensberg Aachen, Germany; A. Henschel, Department of Dermatology and Allergy, Clinic Spandau Berlin, Germany; S. Volkmuth, Department of Paediatrics, Hospital Niederberg Velbert, Germany; M. Bücheler, Department of Otorhinolaryngology, Head and Neck Surgery, Allergology, Evangelical Hospital Bonn, Germany; U. Hillen, Department of Dermatology, University Hospital Essen, Germany; B. Niggemann, DRK-Kliniken Berlin, Germany.
We wish to thank all participating emergency physicians (AG-Notarzt Berlin) and K. Beyer for collection of the validation data, J. Grünhagen, S. Dölle and S. Claus for their local support of data collection and cleaning and B. Paßmann for quality assessment of the registry, all Charité, Berlin, Germany.
Footnotes
Competing Interests: This work was supported by unrestricted grants from Allergopharma and ALK-Abello. There are no patents, products in development or marketed products to declare. This does not alter the authors’ adherence to all the PLoS ONE policies on sharing data and materials.
Funding: This work was supported by unrestricted grants from Allergopharma, Reinbek, Germany, ALK-Abello, Wedel, Germany and the German Federal Office for Consumer Protection and Food Safety. The funders had no role in the design, management, data collection, analysis or interpretation of the data or in the writing of the manuscript or the decision to submit for publication.
References
- 1.Ring J, Behrendt H. Anaphylaxis and anaphylactoid reactions. Classification and pathophysiology. Clin Rev Allergy Immunol. 1999;17:387–399. doi: 10.1007/BF02737644. [DOI] [PubMed] [Google Scholar]
- 2.Johansson SG, Bieber T, Dahl R, Friedmann PS, Lanier BQ, et al. Revised nomenclature for allergy for global use: Report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J Allergy Clin Immunol. 2004;113:832–836. doi: 10.1016/j.jaci.2003.12.591. [DOI] [PubMed] [Google Scholar]
- 3.Alves B, Sheikh A. Age specific aetiology of anaphylaxis. Archives of disease in childhood. 2001;85:348. doi: 10.1136/adc.85.4.348b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simons FE, Sampson HA. Anaphylaxis epidemic: fact or fiction? J Allergy Clin Immunol. 2008;122:1166–1168. doi: 10.1016/j.jaci.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 5.Lieberman P, Camargo CA, Bohlke K, Jick H, Miller RL, et al. Epidemiology of anaphylaxis: findings of the American College of Allergy, Asthma and Immunology Epidemiology of Anaphylaxis Working Group. Ann Allergy Asthma Immunol. 2006;97:596–602. doi: 10.1016/S1081-1206(10)61086-1. [DOI] [PubMed] [Google Scholar]
- 6.Gupta R, Sheikh A, Strachan D, Anderson HR. Increasing hospital admissions for systemic allergic disorders in England: analysis of national admissions data. BMJ. 2003;327:1142–1143. doi: 10.1136/bmj.327.7424.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Decker WW, Campbell RL, Manivannan V, Luke A, St Sauver JL, et al. The etiology and incidence of anaphylaxis in Rochester, Minnesota: a report from the Rochester Epidemiology Project. J Allergy Clin Immunol. 2008;122:1161–1165. doi: 10.1016/j.jaci.2008.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sampson HA, Munoz-Furlong A, Campbell RL, Adkinson NF, Bock SA, et al. Second symposium on the definition and management of anaphylaxis: summary report–Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006;117:391–397. doi: 10.1016/j.jaci.2005.12.1303. [DOI] [PubMed] [Google Scholar]
- 9.Muraro A, Roberts G, Clark A, Eigenmann PA, Halken S, et al. The management of anaphylaxis in childhood: position paper of the European academy of allergology and clinical immunology. Allergy. 2007;62:857–871. doi: 10.1111/j.1398-9995.2007.01421.x. [DOI] [PubMed] [Google Scholar]
- 10.Soar J, Pumphrey R, Cant A, Clarke S, Corbett A, et al. Emergency treatment of anaphylactic reactions–guidelines for healthcare providers. Resuscitation. 2008;77:157–169. doi: 10.1016/j.resuscitation.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Alrasbi M, Sheikh A. Comparison of international guidelines for the emergency medical management of anaphylaxis. Allergy. 2007;62:838–841. doi: 10.1111/j.1398-9995.2007.01434.x. [DOI] [PubMed] [Google Scholar]
- 12.Sheikh A, Shehata YA, Brown SG, Simons FE. Cochrane Database Syst Rev; 2008. Adrenaline (epinephrine) for the treatment of anaphylaxis with and without shock.CD006312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheikh A, ten Broek V, Brown SG, Simons FE. Cochrane Database Syst Rev; 2007. H1-antihistamines for the treatment of anaphylaxis with and without shock.CD006160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choo KJ, Simons FE, Sheikh A. Cochrane database of systematic reviews; 2010. Glucocorticoids for the treatment of anaphylaxis.CD007596. [DOI] [PubMed] [Google Scholar]
- 15.Simons FE. Anaphylaxis, killer allergy: long-term management in the community. The Journal of allergy and clinical immunology. 2006;117:367–377. doi: 10.1016/j.jaci.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Kastner M, Harada L, Waserman S. Gaps in anaphylaxis management at the level of physicians, patients, and the community: a systematic review of the literature. Allergy. 2010;65:435–444. doi: 10.1111/j.1398-9995.2009.02294.x. [DOI] [PubMed] [Google Scholar]
- 17.Hompes S, Kohli A, Nemat K, Scherer K, Lange L, et al. Pediatric allergy and immunology: official publication of the European Society of Pediatric Allergy and Immunology; 2011. Provoking allergens and treatment of anaphylaxis in children and adolescents - data from the anaphylaxis registry of German-speaking countries. [DOI] [PubMed] [Google Scholar]
- 18.Gaeta TJ, Clark S, Pelletier AJ, Camargo CA. National study of US emergency department visits for acute allergic reactions, 1993 to 2004. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2007;98:360–365. doi: 10.1016/S1081-1206(10)60883-6. [DOI] [PubMed] [Google Scholar]
- 19.Mehl A, Wahn U, Niggemann B. Anaphylactic reactions in children–a questionnaire-based survey in Germany. Allergy. 2005;60:1440–1445. doi: 10.1111/j.1398-9995.2005.00909.x. [DOI] [PubMed] [Google Scholar]
- 20.Haymore BR, Carr WW, Frank WT. Anaphylaxis and epinephrine prescribing patterns in a military hospital: underutilization of the intramuscular route. Allergy and asthma proceedings : the official journal of regional and state allergy societies. 2005;26:361–365. [PubMed] [Google Scholar]
- 21.Simons FE, Gu X, Simons KJ. Epinephrine absorption in adults: intramuscular versus subcutaneous injection. The Journal of allergy and clinical immunology. 2001;108:871–873. doi: 10.1067/mai.2001.119409. [DOI] [PubMed] [Google Scholar]
- 22.McLean-Tooke AP, Bethune CA, Fay AC, Spickett GP. Adrenaline in the treatment of anaphylaxis: what is the evidence? BMJ. 2003;327:1332–1335. doi: 10.1136/bmj.327.7427.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grouhi M, Alshehri M, Hummel D, Roifman CM. Anaphylaxis and epinephrine auto-injector training: who will teach the teachers? The Journal of allergy and clinical immunology. 1999;104:190–193. doi: 10.1016/s0091-6749(99)70134-x. [DOI] [PubMed] [Google Scholar]
- 24.Worm M, Timmermans F, Moneret-Vautrin A, Muraro A, Malmheden Y II, et al. Towards a European registry of severe allergic reactions: current status of national registries and future needs. Allergy. 2010;65:671–680. doi: 10.1111/j.1398-9995.2010.02332.x. [DOI] [PubMed] [Google Scholar]