Abstract
We have addressed the source and nature of the persistent neural activity that bridges the stimulus-free gap between the conditioned stimulus (CS) and unconditioned stimulus (US) during trace eyelid conditioning. Previous work has demonstrated that this persistent activity is necessary for trace eyelid conditioning: CS-elicited activity in mossy fiber inputs to the cerebellum does not extend into the stimulus-free trace interval, which precludes the cerebellar learning that mediates conditioned response expression. In behaving rabbits we used in vivo recordings from a region of medial prefrontal cortex (mPFC) that is necessary for trace eyelid conditioning to test the hypothesis that neurons there generate activity that persists beyond CS offset. These recordings revealed two patterns of activity during the trace interval that would enable cerebellar learning. Activity in some cells began during the tone CS and persisted to overlap with the US, whereas in other cells, activity began during the stimulus-free trace interval. Injection of anterograde tracers into this same region of mPFC revealed dense labeling in the pontine nuclei, where recordings also revealed tone-evoked persistent activity during trace conditioning. These data suggest a corticopontine pathway that provides an input to the cerebellum during trace conditioning trials that bridges the temporal gap between the CS and US to engage cerebellar learning. As such, trace eyelid conditioning represents a well-characterized and experimentally tractable system that can facilitate mechanistic analyses of cortical persistent activity and how it is used by downstream brain structures to influence behavior.
Keywords: single units, medial prefrontal cortex, classical conditioning, pontine nuclei, dextran tracer
the ability to use prior associative learning to predict events and guide actions is essential to survival. Because events are often separated in time, the neural mechanisms of forming associations between events that do not overlap in time are of particular interest. Several forebrain regions appear to be specialized for this purpose, because they contain neurons capable of generating activity that persists beyond the termination of one stimulus to overlap in time with an associated second stimulus. These persistent responses, such as those observed in primate prefrontal cortex during delayed-response tasks, are hypothesized to contribute to working memory and other cognitive processes by maintaining spike activity between a cue and the later reinforcement that it predicts (Curtis 2006; Frank and Brown 2003; Fuster 2001; Goldman-Rakic 1995). These findings highlight the need to understand how cortical persistent activity is used by downstream brain regions to influence behavior.
Two variants of eyelid conditioning, delay and trace, represent experimentally tractable instances of forming associations between temporally overlapping or nonoverlapping events, respectively. In delay eyelid conditioning, where a conditioned stimulus (CS; a tone) overlaps with a reinforcing unconditioned stimulus (US; a stimulus to the eye), the cerebellum mediates learning and the expression of conditioned responses (CRs) relatively independently of forebrain input (Halverson and Freeman 2010; Mauk and Thompson 1987; Steinmetz et al. 1989). In contrast, both cerebellum and forebrain are required for trace eyelid conditioning (Clark et al. 2002; Kalmbach et al. 2009; Takehara et al. 2003; Woodruff-Pak and Disterhoft 2008), where a stimulus-free trace interval separates the CS and US. Recent studies have demonstrated that cerebellar learning requires CS- and US-driven inputs to overlap in time (or nearly so), and thus trace eyelid conditioning requires an input to the cerebellum that persists beyond CS offset to occur in close temporal proximity with the US (Kalmbach et al. 2009, 2010).
Several forebrain regions, including medial prefrontal cortex (mPFC), hippocampus, and striatum, are necessary for trace eyelid conditioning (Flores and Disterhoft 2009; Kalmbach et al. 2009; Kim et al. 1995; Moyer et al. 1990; Powell et al. 2001; Takehara et al. 2003; Weible et al. 2000) and are candidate sources of this persistent activity. Although multiple lesion and recording studies have demonstrated a role for the hippocampus in trace eyelid conditioning, the hippocampus lacks direct projections to the pons/cerebellum and activity capable of bridging the trace interval during trace eyelid conditioning. In contrast, the mPFC sends direct projections to the pontine nuclei (Buchanan et al. 1994; Schmahmann and Pandya 1997; Weible et al. 2007; Wiesendanger and Wiesendanger 1982) and is therefore in a unique position to provide the cerebellum with a persistent input that bridges the stimulus-free temporal gap during trace conditioning.
We tested the hypothesis that neurons in rabbit mPFC generate persistent activity that extends beyond CS offset during trace eyelid conditioning. We used in vivo single-unit recordings from trained animals and anterograde tracing to test whether mPFC persistent activity can be conveyed to the cerebellum via the pontine nuclei to support trace eyelid conditioning. Recordings revealed two patterns of activity in the mPFC and pontine nuclei that occurred during the trace interval in close temporal proximity with the US. In the pontine nuclei, persistent activity was observed in regions that showed dense labeling after mPFC injections of an anterograde tracer. These results suggest that mPFC neurons bridge the temporal gap between CS and US during trace eyelid conditioning and therefore enable the cerebellar learning that is necessary for expression of conditioned eyelid responses.
METHODS
Subjects and surgical procedures.
All surgical and experimental procedures were approved by The University of Texas at Austin Institutional Animal Care and Use Committee and were in accordance with the National Institutes of Health guidelines. A total of 13 New Zealand albino rabbits (2.5–3 kg) were implanted with custom microdrives housing 6–16 independently movable tetrodes (gold-plated to 0.5–1.25 MΩ impedance at 1 kHz) to record single-unit activity in the medial agranular cortex (AGm) and dorsocaudal anterior cingulate cortex (dACC; collectively referred to as shoulder cortex) regions of the mPFC (n = 8) or the pontine nuclei (n = 5). Tetrode bundles were 1.5–2 mm in diameter. Rabbits were prepared for surgery by subcutaneous injection of a ketamine (45 mg/kg) and acepromazine (1.5 mg/kg) cocktail, mounted in a specially designed stereotaxic apparatus (with lambda 1.5 mm below bregma), and maintained under deep anesthesia with 1–3% isoflurane. A midline incision was made along the skull and the skin retracted. A craniotomy was made above the target region (ranging between +3.0 and 4.0 mm from bregma and 1.0–1.5 mm lateral from the midline for mPFC, −9.5 to −10.5 from bregma and 2 mm lateral from the midline for pontine nuclei). The microdrive was lowered such that tetrodes just entered the brain (mPFC) or were slowly lowered 10 mm ventral to the brain surface (pontine nuclei), and the craniotomy was filled with Kwiksil (WPI). Recording electrodes and a ground screw placed in the posterior skull were connected to an electrode interface board (Neuralynx), and the microdrive was permanently fixed to the skull with screws and dental cement. Rabbits were additionally prepared with a head bolt fixed in dental cement over the anterior skull (to hold the eyelid detector during conditioning), and a set of two stainless steel stimulating electrodes were implanted anterior and posterior to the eye contralateral to the microdrive implant for the US.
Four additional rabbits were anesthetized as described above but were used for pressure injection of the fluorescent anterograde tracers FluoroRuby or Alexa 594-conjugated dextran (Molecular Probes; 10,000 MW) in the mPFC. A 1-μl Hamilton syringe was loaded with a 10–15% solution of dextran tracer in sterile artificial cerebral spinal fluid and fixed in a stereotaxic apparatus. The syringe tip was lowered 1.25 mm into the brain and was left in place for 10 min to allow the tissue to settle, after which tracer was injected at 0.05 μl/min for 20 min (1 μl total). The needle was left in place for another 20 min to allow tracer to diffuse before the syringe needle was retracted. The craniotomy was filled with bone wax or Kwiksil, and the overlying skin was sutured. A 10- to 14-day survival time was used to allow tracer to move through the axons to reach the pons and fill terminals.
Behavioral training and analysis. Rabbits were gently restrained in a custom-built Plexiglas restrainer and placed in shielded, noise-attenuating chambers. Pure tones (1.3 kHz, 80 dB, 5-ms rise time) delivered through a speaker connected to an audio source module (Coulbourne Instruments) served as the CS. (One rabbit was trained using a 9.5-kHz tone and did not show any differences in the acquisition or expression of learning, or in patterns of single-unit responses, relative to the other rabbits.) A 50-ms train of current pulses (100 Hz, 1-ms pulse width; A-M Systems model 2100 stimulator) delivered through the electrodes positioned near the eye served as the US. Eyelid stimulation was calibrated for each rabbit to be just suprathreshold for eliciting full eyelid closure (typically between 1 and 3 mA). Stimulus presentation was controlled via custom-designed software. Trace conditioning trials consisted of a 500-ms tone CS followed by a 500-ms stimulus-free interval and terminated with the US (Fig. 1A). Daily training sessions consisted of 12 blocks of 9 trials, the first of which was a CS-only probe trial followed by 8 CS-US paired presentations (108 trials total, 30 ± 10-s intertrial interval).
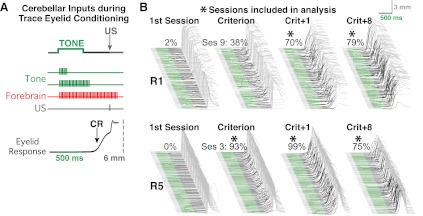
Fig. 1.
A schematic representation of cerebellar inputs during trace eyelid conditioning and examples of eyelid responses during behavioral training sessions from 2 rabbits. A: trace conditioning trials involved presenting a 50-ms periorbital stimulation (unconditioned stimulus, US) 1,000 ms after the onset of a 500-ms tone (conditioned stimulus, CS), resulting in a 500-ms stimulus-free trace interval after the tone (top). Rabbits learned to make anticipatory eyelid responses as a result of training (bottom trace, upward deflection indicates eyelid closure). Inputs to the cerebellum that enable learning include tone-activated mossy fibers (green) and US-activated climbing fibers (gray). Because cerebellar learning requires temporal overlap (within 100–200 ms) between CS-activated and US-activated inputs, an additional input from the forebrain (red) that persists into the trace interval to produce sufficient overlap with the US is hypothesized. B: example behavioral sessions from 2 rabbits (R1 and R5). Each waterfall plot shows trials from 1 session, with each sweep showing 1 trial with the first trial at the bottom. The green portion of each sweep denotes the time of CS presentation (500 ms), and the black portion denotes the stimulus-free trace interval (500 ms). The light gray portion of each sweep on the right shows the reflex responses to the US (calibrated to 6 mm). Note that any response beginning in the green or black portions is a conditioned (learned) response (CR). The percentages indicated above each waterfall plot represent the percentage of trials in which a CR was made. During the first training session (left), rabbits made few if any CRs. With continued training, rabbits met the learning criterion (8 CRs out of any block of 9 consecutive trials); however, only sessions in which the CR rate exceeded 50% were included in analyses for the current study (asterisks).
External eyelid position was measured beginning 200 ms before CS onset through 2,300 ms after CS onset using an infrared emitter/detector attached to the subject's head bolt. Eyelid responses were digitized at 1 kHz and analyzed off-line with custom software. These measurements were calibrated assuming a full eyelid closure of 6 mm. A CR was defined as an eyelid closure of at least 0.3 mm that occurred between CS and US onsets. CR onset was determined using an algorithm that detects the initial inflection of the eyelid sweep from baseline during trials in which the CR criterion was reached. Trials with eyelid deflections >0.3 mm during the 200-ms pretrial baseline were excluded from behavioral and spike analysis (typically, only 2–5 trials are excluded per session due to the low spontaneous blink rate in rabbits). We used the well-established learning criterion of the first instance of eight CRs out of nine consecutive trials. Only neural data from postcriterion sessions in which the CR rate exceeded 50% were included in analysis (e.g., Figs. 1B and 2A).
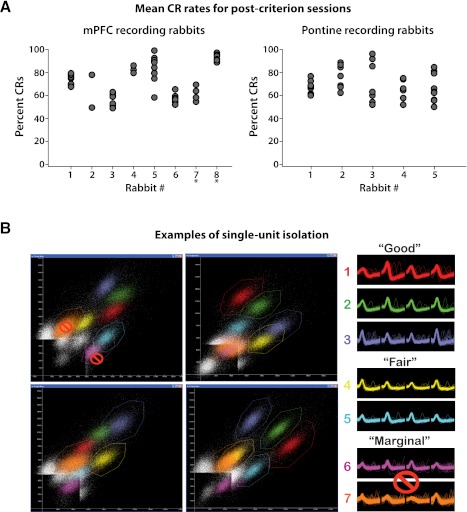
Fig. 2.
CR rates for each rabbit included in analyses and example single-unit isolation for neural recordings. A: likelihood of CRs for each session included in analyses is given for each rabbit. Note that a range of performance rates was observed both within and across rabbits for postcriterion sessions, representing the variability in performance levels typically observed. Rabbits for which histology could not be recovered are denoted with asterisks (see methods). mPFC, medial prefrontal cortex. B: example peak-by-peak scatter plots created from tetrode recordings used to isolate single-unit activity (left) and examples of the rating system applied regarding isolation quality (right). In this example, units 1–3 were rated “good” due to high signal-to-noise ratios and little or no overlap with other unit activity, whereas units 4 and 5 were rated “fair” due to <10% overlap with background or another unit's activity. Units 6 and 7 were rated “marginal” due to >10% overlap with background or another unit's activity. Only single units rated as having good or fair isolation were included in analyses.
Single-unit recordings and analysis.
To ensure that the activity from a given neuron was not included more than once in analysis, we included the single-unit activity from a given tetrode only if the tetrode was moved a minimum of 160 μm from a site of previous single-unit activity that was included in analysis. An exception was made if at least 80 μm of movement was made during which no isolated activity was observed during an intermediate session. Single-unit activity was recorded throughout training sessions with a Digital Lynx acquisition system (Neuralynx). Neural signals from each wire of a tetrode were fed to a multichannel, unity-gain head stage, amplified and bandpass filtered between 600 and 6,000 Hz, digitized at 32 kHz, and stored on a personal computer for off-line analysis. Neural data were synchronized with the presentation of training stimuli by triggering the Digital Lynx I/O port with the same TTL pulse used to trigger CS and US presentations.
Single-unit activity was isolated off-line using interactive cluster-cutting software (WinClust; Knierim JJ and Mauk MD, adapted from Wilson MA). In brief, the waveform parameters of digitized neural events recorded from the four closely spaced wires of a tetrode were displayed on scatter plots for one wire versus another (e.g., Fig. 2B). Because many waveform parameters vary as a function of distance from the recording site, the activity of a single neuron can be discriminated from that of other isolated neurons and from background neural activity by identifying the boundaries of clusters of points on the various scatter plots. Spike height was the parameter used to initially identify cluster boundaries, with “energy” (a parameter particularly sensitive to waveform shape, defined as the area under the depolarization phase divided by the area above the hyperpolarization phase) and valley used as secondary parameters. The timestamps from isolated clusters of neural events were extracted and identified as the activity of a single unit. The quality of isolation for each identified cell was rated from 1 (very good) to 4 (marginal) before the activity for trace conditioning-associated responses was examined (Siegel et al. 2008; Yoganarasimha et al. 2006). Units rated as “good” or better displayed high signal-to-noise ratios and little or no overlap with other unit activity (Fig. 2B, units 1–3). Units rated as “fair” met minimal signal-to-noise criteria, with <10% overlap with background or another unit's activity (Fig. 2B, units 4 and 5). Units were rated as “marginal” due to >10% overlap with background or another unit's activity (Fig. 2B, units 6 and 7). Marginally isolated cells were excluded from analysis.
For each cell the spikes were sorted into 100-ms bins for the purposes of constructing peristimulus histograms and statistical analysis. Analysis included 10 pre-CS bins (the 1-s period before the CS), 5 bins during the CS, 5 bins during the trace interval, and the 30 bins (3 s) following the US, resulting in 40 trial-associated bins. For analysis purposes the average counts for the 10 pre-CS bins were determined for every trial. The statistical reliability of changes in activity during trials over a session was determined using separate paired t-tests for each of the 40 bins following the CS, with the variability across trials as the error term (Furtak et al. 2007; Quintana et al. 1988; Weible et al. 2003; Zhou et al. 2007). Each paired t-test compared the counts within a given time bin for each trial with the average counts of the pre-CS bins for that same trial. A Bonferroni correction was used to produce an overall P value of 0.05 for each cell (α = 0.05/40 = 0.00125 for each individual comparison). Trials excluded from behavioral analysis were also excluded from spike analysis.
We defined eight categories of response type, depending on which time bins showed significant trial-related changes. 1) “Persistently active” neurons showed increased activity to the tone (at least 1 significant tone bin) that persisted into (was contiguous with) at least two trace interval bins (i.e., within 300 ms before US onset). This criterion was based on observations that cerebellar learning requires that US-driven climbing fiber input arrive no more than 300–400 ms after the offset of mossy fiber input (Kalmbach et al. 2009). Therefore, neurons with activity that persisted into the trace interval for at least 200 ms could in theory contribute to learning and the ongoing expression of CRs mediated by the cerebellum for the trace conditioning paradigm used here. 2) “Tone” neurons showed changes in activity for at least one bin during the tone. 3) “US” neurons showed changes in activity in at least one of the five bins following US presentation. 4) “Trace interval” neurons showed changes in activity during at least one trace interval bin. 5) “Tone + US” neurons shown significant changes for at least one tone and one US bin. 6) “Tone + trace” neurons showed significant changes for at least one tone bin and at least one noncontiguous trace interval bin but did not meet the criteria for persistent activity. 7) “Trace + US” neurons showed significant changes for at least one trace interval and one US bin. 8) “Tone + trace + US” neurons showed significant changes for at least one tone bin plus at least one noncontiguous trace bin plus at least one US bin.
Identification of fluorescent labeling.
The mPFC injection site and terminals in the pontine nuclei were visualized using either a manual Olympus BH-2 microscope (for FluoroRuby) or a fully automated Zeiss Imager Z2 microscope (for Alexa 594). For FluoroRuby-injected animals, sections containing the pontine nuclei were examined for axons and terminal labeling between ∼9.0 and 11.0 mm posterior to bregma by manually scanning using a ×10 objective. Labeled axons and clustered terminals were easily identified using this procedure. A ×20 objective was used to verify any sparse labeling that was observed and to more accurately identify the apparent edges of terminal fields. Occasionally, the ×20 objective was used in an apparently unlabeled area to verify the apparent absence of mPFC terminals. The location, size, and general shape and orientation of terminal fields were estimated and plotted on images of representative coronal sections relative to the immediate surrounding structures, such as the pyramidal tract and medial lemniscus, the ventral or lateral surface of the brain, and the lateral or medial pontine nuclei. For Alexa 594 labeling, the entire rostral-caudal extent of the pons was imaged in serial 50-μm sections using an appropriate filter set (Zeiss filter set 71, excitation BP 592/24; dichroic FT 615; emission BP 675/100). Typically, the pontine nuclei and surrounding structures was imaged by creating a mosaic of 66 individual 902 × 676-μm images, with single images compiled using AxioVision. Images of background autofluorescence were also obtained by acquiring images using a GFP filter-set (Zeiss filter set 38, excitation BP 470/40; dichroic FT 495; emission BP 525/50). In a subset of sections, putative axon terminals were visually identified from volume images obtained using a Leica SP-RS two-photon scanning laser microscope. Excitation was achieved using a mode-locked femtosecond-pulse titanium-sapphire laser (Mai-Tai; Spectra Physics, Mountain View, CA) at 860 nm. Images were obtained by collecting reflected photons using non-descanned external detectors. Signals were separated using a DXCR 565 dichroic mirror and HQ 645/100 and HQ 525/50 filters for red and green, respectively. Terminal fields typically could be followed across multiple sections and became smaller and more sparse as they progressed through the rostral-caudal axis. The number of sections in which a terminal field was observed was used to estimate approximate size in the rostral-caudal axis. The terminal fields plotted in Fig. 8 represent discrete clusters, varying from 100 to 1,500 μm2. Post hoc image processing was performed using ImageJ (National Institutes of Health, Bethesda, MD).
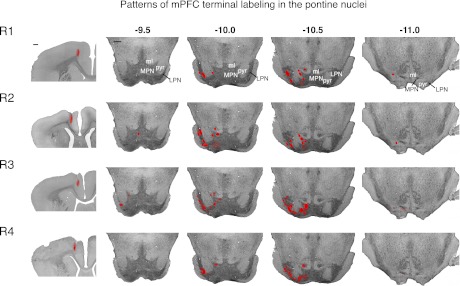
Fig. 8.
Injection sites for each of the 4 rabbits that received injections of anterograde tracer in the mPFC and the resulting labeled pontine terminal fields (plotted on representative coronal sections of the pons, red ellipses) are shown. Most terminal fields, particularly the largest and densest, were observed in the pontine nuclei surrounding the descending pyramidal cell tract. Note that decreased opacity denotes sparser terminal labeling (see Fig. 7 for example). Scale bar, 1 mm.
Histology.
After experiments were completed, recording rabbits received marker lesions through a subset of tetrodes in the recording bundle (2 mA for 15–20 s). At least 48 h later, rabbits were given a lethal intravenous injection of Euthasol (0.3 ml/kg) and perfused through the heart with 0.9% physiological saline followed by 10% formalin. The brains were extracted and fixed further for a minimum of 2 wk before being cryoprotected in a 30% sucrose solution for 48–72 h. Brains were sectioned on a freezing microtome (40- or 50-μm slices) and mounted on gel-subbed or Microfrost Plus slides (Fisher Scientific). Sections were stained with cresyl violet to aid in visualizing electrode tracks, marker lesion gliosis, and mPFC structures. The identities of tetrode tracks observed histologically were determined based on relative location within the recording bundle, utilizing the subset of lesioned tetrodes to help disambiguate tetrode track damage observed in the tissue. Approximate recording locations of single-unit activity were determined by measuring the distance moved from the day of the recording session to the tetrode's maximum depth, from the tip of the tetrode verified histologically. Recording sites were plotted on exemplar coronal sections relative to the surrounding structures. Two of the rabbits were shipped to Woods Hole after the completion of experiments for teaching purposes, and so the tissue from those rabbits could not be recovered (68 mPFC cells from 11 sessions).
Rabbits injected with anterograde tracer were euthanized as described above and perfused with cold artificial cerebrospinal fluid and 4% paraformaldehyde in 0.1 mM phosphate buffer. The brains were extracted and given 2–3 wk of postfixation followed by 2–3 days of cryoprotection. Brains were sectioned on a freezing microtome (50-μm slices), mounted in 0.9% saline, and dried overnight. Slides were briefly washed in 50% ethanol (1 min) and then in 100% ethanol (1 min), cleared in xylene (10 min), and coverslipped with DPX mounting medium.
RESULTS
To test the hypothesis that neurons in mPFC generate persistent activity during trace eyelid conditioning, rabbits were implanted with multiple tetrodes to record single-unit activity from the region of mPFC previously shown to be necessary for the acquisition and expression of trace eyelid responses (AGm and dACC, sometimes referred to as shoulder cortex; Kalmbach et al. 2009; Kronforst-Collins and Disterhoft 1998; Weible et al. 2000). Recordings were obtained during trace conditioning sessions that consisted of 108 training trials with a 500-ms tone CS and a 500-ms stimulus-free trace interval (Fig. 1A; see methods). For the present purpose, analysis was limited to recordings from sessions after an initial performance criterion was met (8 CRs out of any block of 9 consecutive trials) and the session response rate was at least 50% (Figs. 1B and 2A).
A total of 299 single units were recorded from the mPFCs of 8 rabbits over 52 sessions, ranging from 3 to 13 sessions per rabbit. The majority of these neurons were located in the deep layers (5 and 6) of the mPFC (Fig. 3A). Raster plots and cumulative peristimulus histograms of spike activity constructed for each session revealed that 81% of recorded cells displayed a significant change in activity of some type during the trace conditioning trials. Several response profiles were identified, including responses confined to the tone epoch (22 cells that increased and 35 that decreased activity) and responses to US onset (42 cells increased and 68 decreased), or combinations thereof (Fig. 4). However, these responses alone would not engage the cerebellar mechanisms that support the acquisition and expression of trace conditioned responses. (Kalmbach et al. 2009, 2010).
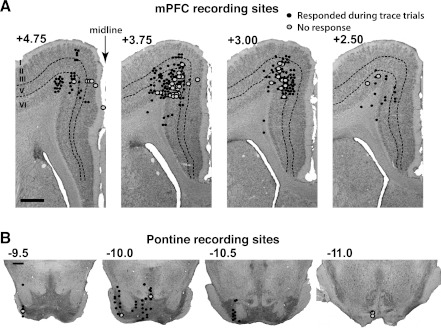
Fig. 3.
Estimated recording sites of single-unit activity included for analysis from the mPFC (A; with the exception of 68 cells from 2 animals, see methods) and pontine nuclei (B). Black circles show the locations of single units that showed significant changes in activity during trace conditioning trials; gray circles show the locations of units that did not. The anterior-posterior coordinate of each section relative to bregma (in mm) is indicated by the numbers. Scale bars, 1 mm. A: estimated recording locations of mPFC cells included for analysis. Most cells were in the deeper layers of mPFC regions previously shown to be necessary for the expression of trace eyelid conditioned responses [the agranular cortex (AGm) and dorsocaudal anterior cingulate cortex (dACC) regions; see text]. B: estimated recording locations of all pontine cells included for analysis. Recordings were targeted toward the rostral pons where previous inactivation studies showed a selective effect on the expression of trace CRs.
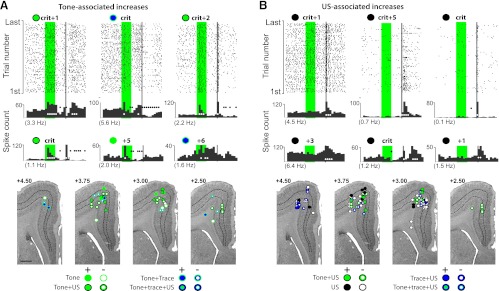
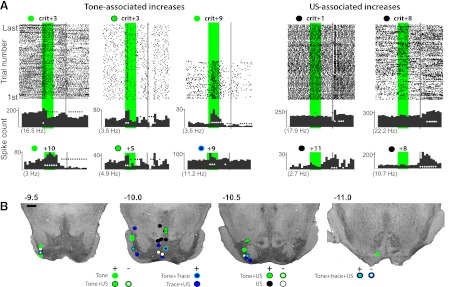
Fig. 4.
Examples of tone- and US-associated increases in single-unit activity observed in mPFC and corresponding estimated recording locations. Scale bar, 1 mm. A: raster plots (top) and the associated cumulative peristimulus histograms (top histograms) from 3 of the cells that displayed tone-evoked responses that did not persist to overlap with the US, plus cumulative histograms from 3 additional example cells (bottom histograms). In this and subsequent figures, the green bar represents the tone (500 ms) and the gray bar represents the US (50 ms). Each row in the raster plot represents time during 1 trial, and each dot represents a spike. The bottom row is the first trial. One second of pretrial baseline activity is shown, with average pre-CS baseline activity indicated below each histogram in parentheses. White circles indicate bins (100 ms) showing significantly increased activity; black circles indicate bins with significantly decreased activity. The number of sessions relative to criterion from which recordings were taken is indicated above (e.g., +5 = fifth session after criterion). Estimated recording sites are given for this cell type (bottom) and are coded relative to whether cells increased (solid circles) or decreased activity (open circles) in response to tone onset (green symbols) and whether the cell also showed US-related (black) or trace interval activity (blue). B: raster plots and cumulative histograms for mPFC cells displaying US-associated responses and estimated recording sites as described in A.
mPFC neurons show tone onset persistent activity.
We observed two patterns of activity associated with trace eyelid conditioning that would be capable of providing the cerebellum with an input that overlaps with the US. The first pattern of activity began during the tone CS (within 50–300 ms after tone onset) and persisted into the trace interval, whereas for the second pattern, activity changes occurred during the stimulus-free trace interval. For convenience, we refer to these response patterns as “tone onset persistent activity” and “trace interval activity,” respectively. A total of 74 cells showed significant tone onset persistent activity whereby 17 of the cells showed an increase in activity and 57 showed decreased activity (e.g., Fig. 5). We defined persistent activity (both increases and decreases) as a significant change in activity that persisted beyond tone offset at least 200 ms into the trace interval, because previous results suggest this to be the minimal temporal proximity to the US necessary to support the cerebellar expression of CRs (Kalmbach et al. 2009). Most cells displaying tone onset persistent activity showed significant changes throughout the entire trial, often lasting for 1–3 s beyond the US before returning to baseline activity rates. This was true whether a given neuron displayed increased or decreased activity (e.g., Fig. 5, A and B). Cells that displayed tone onset persistent responses were observed in sessions in which rabbits had just met criterion, as well as in sessions recorded from well-trained animals (ranging from 4 to 11 sessions postcriterion; Fig. 5, A and B; behavior accompanying each raster plot is given in Fig. 5C). Most of the tone onset persistent activity neurons were located between 3.0 and 3.75 mm anterior to bregma and were rarely (if at all) observed in the superficial cortical layers (Fig. 5D; the locations of 2 cells were estimated to be at the layer 5/3 border, which, given the inherent error in estimating recording sites, may or may not have been in deep layer 3). The location of the recording electrode contributed to the likelihood of observing persistent activity more than other factors, such as CR rate or the session in which criterion was reached (between the 3rd and 10th sessions across animals). Persistent activity changes were observed in each of the rabbits recorded from for this study.
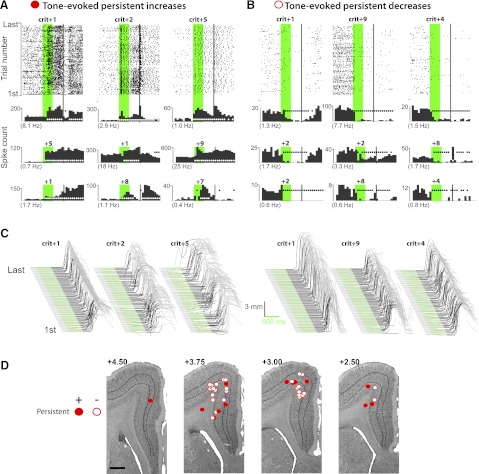
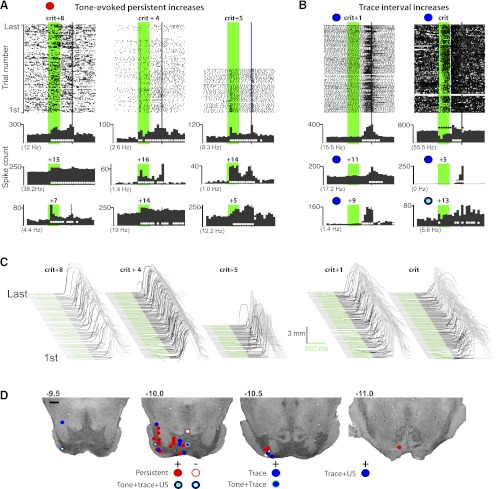
Fig. 5.
Example raster plots and cumulative histograms from 9 of the mPFC cells that displayed tone-evoked persistent increases (A) and 9 of the cells that showed persistent decreases in activity (B) during trace conditioning, and the estimated recording locations of these cells (D; scale bar, 1 mm). Raster plots and histograms are as described in Fig. 4, except that red symbols represent activity that persisted to overlap. A: cells that displayed increased spike activity that persisted to overlap (or nearly overlap) with the US. B: cells that displayed persistent decreases in activity. C: raw eyelid response data for the sessions corresponding to the raster plots given in A and B, as described in Fig. 1. D: cells that showed persistent changes in activity were found exclusively in the deep (output) layers of the mPFC.
Significant increases and decreases in activity were also observed with onsets during the stimulus-free trace interval (21 cells showed increased activity and 63 showed decreased activity). In most cases this activity persisted to overlap with the US (e.g., Fig. 6, A and B; 2 exceptions are also given). Although the onsets for some of these neurons occurred early enough within the trace interval to have been in response to tone offset, the majority of trace interval responses occurred during the latter half of the 500-ms stimulus-free interval during the time in which rabbits made CRs (Fig. 6, A and B; behavior accompanying each raster plot is given in Fig. 6C; session averages of the onset of CRs was 785 ± 10 ms across sessions/rabbits). For these neurons the changes in activity may have been driven by behavioral feedback, although a delayed response to tone onset and/or offset cannot be ruled out. Trace interval responses were observed in all cortical layers, but the locus of such responses appeared to be more anterior in the mPFC relative to tone onset persistent activity, approximately +4.0 mm from bregma (Fig. 6D). Again, neither a rabbit's level of performance nor its previous acquisition rate was related to the likelihood of observing trace interval activity.
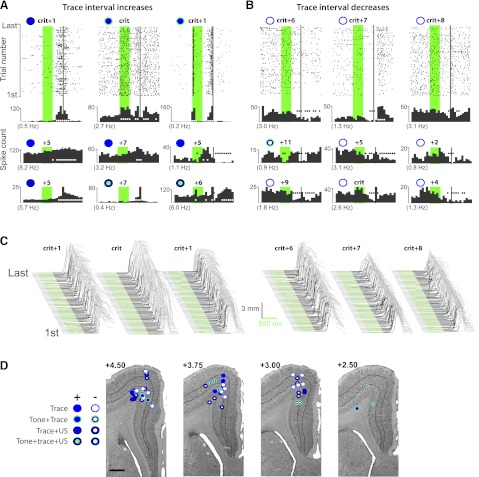
Fig. 6.
Example raster plots and cumulative histograms from 9 of the mPFC cells that displayed trace interval increases (A) and 9 of the cells that showed trace interval decreases in activity (B) during trace conditioning, and the estimated recording locations of those cells (D; scale bar, 1 mm). Raster plots and histograms are as described in Fig. 4. A: cells that displayed increased spike activity during the trace interval. Often the activity displayed a similar profile to that observed for the behavioral conditioned eyelid responses (top left, top right, and middle bottom histograms; see text). For other neurons, the onset of increased activity occurred before or after CR onset but was less frequently observed (e.g., middle left, middle right, and bottom right histograms). B: significant decreases in spike activity during the trace interval were also observed. C: raw eyelid response data for the sessions corresponding to the raster plots given in A and B, as described in Fig. 1. D: cells showing trace interval changes in activity were observed in both the more superficial input layers and the deep, output layers of mPFC. The overall locus of cells that displayed trace interval activity changes tended to be more rostral than cells showing tone onset persistent activity changes.
Both response types, tone onset persistent activity and trace interval activity, represent input patterns that occur in close enough temporal proximity with the US to support cerebellar learning (Kalmbach et al. 2009, 2010).
Anatomical projection from the mPFC to the pontine nuclei.
On the basis of the recording locations identified above, we next tested the hypothesis that mPFC neurons provide input to neurons in the pons, whose mossy fiber axons would in turn provide the cerebellum with an input that bridges the stimulus-free trace interval and allow the cerebellum to acquire and express adaptive responses. To test this hypothesis, we used injection of an anterograde tracer into the specific regions of mPFC where tone-evoked persistent or trace interval activity was observed to determine whether neurons there project to the pontine nuclei.
Either the fluorescent anterograde tracer FluoroRuby (n = 2) or Alexa 594-conjugated dextran (n = 2) was pressure injected into the mPFC region of four rabbits at locations where tone onset persistent activity and trace interval activity were observed (e.g., Figs. 7A and 8). This same region also has been shown to be necessary for the acquisition and expression of trace eyelid responses in rabbit (Kalmbach et al. 2009; Kronforst-Collins and Disterhoft 1998; Weible et al. 2000). Clearly labeled axons were observed in the descending corticospinal pyramidal tract ipsilateral to the injection site throughout the extent of the pons (−9.0 to −11.0 from bregma, e.g. Fig. 7B). As the pyramidal tract descended into the vicinity of the pons, axons were observed to exit the pyramidal tract into the surrounding neuropil, both medially and laterally to the tract. Both small spherical (50–100 μm in diameter) and relatively large and more irregularly shaped terminal plexuses (covering an area of 150–1,500 μm2) were observed just adjacent to the pyramidal tract, sometimes forming elongated clouds of labeled terminals that extended along the medial (in the medial pontine nucleus) and/or lateral edges (in the lateral pontine nucleus) of the tract (e.g., Fig. 7B, insets). Dense terminal plexuses were observed along the lateral edge of the lateral pontine nucleus in all four rabbits (e.g., Fig. 7B, inset 2, asterisk; examples of dense vs. sparser terminal labeling are given in Fig. 7, C and D, respectively). Only in rare cases (2 of the 156 sections from Alexa dextran injections) was obvious terminal labeling observed in the pontine nuclei contralateral to the injection site. Once the bottom of the ipsilateral pyramidal tract approached the ventral extent of the brain, terminal plexuses were infrequently observed and tended to be small and sparse, although labeled axons were still clearly observed in the pyramidal tract. The pattern of terminal labeling was quite consistent in all four rabbits (Fig. 8). These anatomical observations suggest that the caudal AGm and dACC heavily innervate specific regions of the pontine nuclei in the rabbit and that output from the deep layers of these mPFC regions can influence the activity of pontine cells.
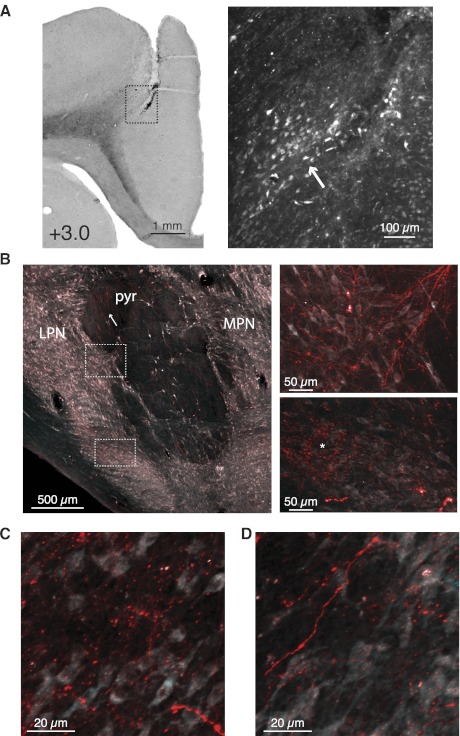
Fig. 7.
Injections of anterograde tracers (Alexa 594 or FluoroRuby) in the mPFC revealed axonal projections and regions of putative axon terminals in the ipsilateral pons. A: a representative injection site of anterograde tracer in mPFC is shown. Uptake of tracer by layer 5 projection neurons (arrow) was restricted to a subset of neurons near the injection site. B: labeled axons (arrow) were observed in the pyramidal tract ipsilateral to the injection site (pyr) and could be seen exiting the tract and traversing the neuropil (top inset). Examples of representative “dense” and “sparse” terminal fields are shown and were observed lateral to (bottom inset, asterisk), medial to, and ventral to the pyramidal tract (not shown). C and D: examples of dense and sparser terminal labeling, respectively.
Pontine neurons show tone onset persistent activity.
To test whether pontine neurons display tone onset persistent activity and/or trace interval activity similar to that observed in mPFC, we implanted five rabbits with tetrodes and recorded single-unit activity from the pontine nuclei during trace eyelid conditioning. A total of 54 single units were recorded from the pontine nuclei of 5 rabbits during the expression of trace eyelid conditioning (data from 40 postlearning sessions in total, ranging from 6 to 10 sessions per rabbit; Fig. 2A). Raster plots and cumulative histograms of spike activity constructed for each session revealed that 91% of recorded pontine cells displayed significant changes in activity during trace conditioning trials. In general, pontine cells showed patterns of activity similar to those observed for mPFC neurons. Figure 9 shows examples of pontine neurons that displayed changes in activity confined to the tone epoch (6 cells increased and 0 cells decreased activity), in response to the US (5 cells increased and 3 cells decreased activity), or both (3 cells increased and 2 cells decreased activity). Based on the findings of previous studies, this activity is insufficient to support the learning and expression of cerebellum-mediated CRs during trace conditioning (Kalmbach et al. 2009, 2010).
Fig. 9.
Examples of tone- and US-associated increases in single-unit activity observed in the pontine nuclei and the corresponding estimated recording locations. A: raster plots and cumulative histograms from 3 of the cells that displayed tone-evoked responses that did not persist to overlap with the US, plus cumulative histograms from 3 additional example cells (left) and from 3 of the cells that displayed US-evoked increases, plus cumulative histograms from 2 additional examples (right). B: recording locations for tone- and US-associated responses were sometimes observed near the descending pyramidal tract but were also observed at sites farther than anterogradely labeled mPFC terminal fields were observed. Scale bar, 1 mm.
The most common response pattern observed for pontine cells was tone onset persistent increases in activity (16 rate-increasing cells, Fig. 10A; 2 rate-decreasing cells, data not shown). The onset of this activity was similar to that observed in mPFC cells (100–200 ms), although a few showed strong short-latency increases followed by more moderate persistent activity (e.g. Fig. 10A). Trace interval increases in activity were also observed for pontine cells (e.g. Fig. 10B), although less frequently than tone onset persistent responses (11 rate-increasing cells and 1 rate-decreasing cell; behavior accompanying each raster plot is given in Fig. 10C).
Fig. 10.
Example raster plots and cumulative histograms from 9 of the pontine cells that displayed tone-evoked persistent increases (A) and 6 of the cells that showed trace interval increases in activity (B) during trace conditioning, and the estimated recording locations of those cells (D). Both response types were also observed in the mPFC. A: pontine tone-evoked persistent increases in activity displayed a similar onset to that observed in mPFC cells. B: also similar to mPFC cells, pontine cells displayed trace interval increases in activity that most often was similar to the overall temporal profile of behavioral CRs. C: raw eyelid response data for the sessions corresponding to the raster plots given in A and B. D: the recording locations of tone onset persistently active pontine cells were most often observed near the descending pyramidal tract, whereas cells showing trace interval activity were observed both near and at more distant sites relative to the pyramidal tract. Scale bar, 1 mm.
The majority of tone onset persistent activity in the pontine nuclei was located in close proximity to the descending pyramidal tract and along the lateral extent of the lateral pontine nucleus (Fig. 10D). Trace interval activity was observed near the pyramidal tract but was also observed at more distant sites from the pyramidal tract. Both tone onset persistent and trace interval activity were most prevalent around 10 mm posterior to bregma. The colocalization of persistent activity responses with mPFC-labeled terminals in the pontine nuclei (Fig. 11) suggests that this pattern of activity observed in the deep layers of the mPFC could be conveyed to the cerebellum via the pontine nuclei.
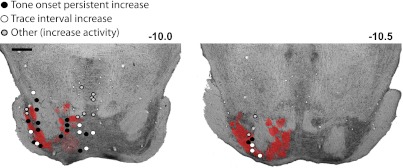
Fig. 11.
The terminal fields observed for the 4 rabbits that received injections of anterograde tracer in the mPFC are overlaid on representative coronal sections of the pons (red), along with the estimated recording locations of cells that showed tone onset persistent increases in activity (black circles), trace interval increases (white circles), and other patterns of increased activity (gray circles). The recording locations of tone onset persistently active pontine cells more closely corresponded with the locations of mPFC terminal fields observed with anterograde labeling than did pontine cells that showed trace interval or other patterns of trial-associated activity. Scale bar, 1 mm.
DISCUSSION
We have tested the hypothesis that, within a region of rabbit mPFC that is necessary for trace eyelid conditioning (Kalmbach et al. 2009; Kronforst-Collins and Disterhoft 1998; Weible et al. 2000), neurons generate activity that persists long enough into the stimulus-free trace interval to engage cerebellar learning. Although a variety of response types were observed, many neurons showed increases in activity that 1) began during the tone CS and persisted into the trace interval or 2) was confined to the trace interval proper, either of which could engage the cerebellar mechanisms that mediate expression of learned responses by providing an input that overlaps with the US. Injections of anterograde anatomical tracers identified projections from this region of mPFC to the region of pons that is necessary for trace eyelid conditioning in rabbit (Kalmbach et al. 2009). Recordings from this pontine region also revealed neurons that displayed persistent activity or trace interval activity to a tone CS during trace conditioning trials. Together, these observations suggest a source of activity that bridges the stimulus-free trace interval during trace eyelid conditioning and a pathway via the pons that could convey this activity to the cerebellum. Previous work using direct stimulation of mossy fiber inputs to the cerebellum (Kalmbach et al. 2010) suggests that either of these activity patterns, combined with activity in other mossy fibers that ends at tone offset, is sufficient for the cerebellum to acquire and express trace eyelid responses.
Implications for trace eyelid conditioning.
Pavlov (1927) coined the term “trace conditioning” from the assumption that the ability to learn despite the stimulus-free trace interval implies the existence of a stimulus memory trace within the brain that outlasts the CS and overlaps in time with the US. Together with previous work (Clark et al. 2002; ; Kalmbach et al. 2009, 2010; Kronforst-Collins and Disterhoft 1998; Powell et al. 2001; Takehara et al. 2003; Weible et al. 2000; Woodruff-Pak and Disterhoft 2008), our results suggest that persistent activity in mPFC neurons instantiates a stimulus memory trace that is necessary for trace eyelid conditioning.
In addition to supporting Pavlov's original insight, this suggests a relatively simple explanation for the necessity of forebrain structures for trace but not delay eyelid conditioning (Clark et al. 2002; Mauk and Thompson 1987; Flores and Disterhoft 2009; Kalmbach et al. 2009; Oakley and Russell 1972; Powell et al. 2001; Solomon et al. 1986; Steinmetz et al. 1989; Takehara et al. 2003; Weible et al. 2000). Both forms of learning are abolished by lesions of the cerebellum (Kalmbach et al. 2009; Takehara et al. 2003; Woodruff-Pak et al. 1985), whereas trace eyelid conditioning has attracted considerable interest following observations that it is impaired by lesions of the hippocampus or mPFC (Clark et al. 2002; Kalmbach et al. 2009; Kim et al. 1995; Kronforst-Collins and Disterhoft 1998; Moyer et al. 1990; Powell et al. 2001; Solomon et al. 1986; Takehara et al. 2003; Weible et al. 2000). Delay eyelid conditioning, by comparison, is largely unaffected by the same lesions (Clark et al. 2002; Kalmbach et al. 2009; Kronforst-Collins and Disterhoft 1998; Mauk and Thompson 1987; Oakley and Russell 1972; Powell et al. 2001; Solomon et al. 1986; Takehara et al. 2003; Weible et al. 2000) and is mediated relatively directly by the cerebellum via CS-activated mossy fiber inputs and US-activated climbing fiber inputs to the cerebellum (Aitkin and Boyd 1978; Campolattaro et al. 2011; Hesslow 1994; Hesslow et al. 1999; Nicholson and Freeman 2003; Steinmetz et al. 1989). The differential effects of forebrain lesions on delay and trace conditioning were the basis for previous suggestions that the forebrain influences the mossy fiber input to the cerebellum during trace conditioning. Weiss and Disterhoft (1996) suggested that forebrain input “enhances” mossy fiber inputs. Clark et al. (2002) suggested more specifically that the forebrain extends mossy fiber activity into the trace interval. Observations that mossy fiber and climbing fiber inputs must overlap in time to engage cerebellar learning (Kalmbach et al. 2009) provided evidence consistent with the idea that the forebrain enables cerebellar learning and the expression of conditioned responses by providing another mossy fiber input to the cerebellum, one that does not terminate with the CS but instead persists into the trace interval to overlap with the US (Clark et al. 2002; Kalmbach et al. 2009, 2010). Our data suggest that the final output of forebrain processing in trace eyelid conditioning is reflected in persistent activity in deep layer neurons of mPFC. This general pattern of activity was previously observed in ensemble analyses during trace eyelid conditioning in the rat prelimbic cortex (Takehara et al. 2008) and was suggested recently by multiunit recordings in rabbit (Darling et al. 2011). Direct projections to pons, demonstrated in the present study and previously in rabbit and other species (Buchanan et al. 1994; Schmahmann and Pandya 1997; Weible et al. 2007; Wiesendanger and Wiesendanger 1982), would translate this persistent activity into a mossy fiber input that overlaps with the US-activated climbing fiber input to enable cerebellar learning and support the ongoing expression of conditioned responses.
Our data do not address whether the persistent activity seen in mPFC neurons is learned or reflects a learning-independent process. Although we recorded single units from the rabbits during the 3–10 days of acquisition, adjustments to the depth of the recording tetrodes were often made during this period in an effort to maximize the number of cells recorded each day (a common practice). However, the data reported presently strongly suggest that such procedures would introduce a bias toward observing an increase in the proportion of cells showing persistent activity across training sessions. We observed persistent activity in the deeper layers of mPFC, so simply moving the recording tetrodes deeper over training could result in an increased probability of observing such activity independent of learning. To appropriately address whether persistent activity responses are learned requires recording mPFC neurons from the outset of training to the point where conditioned responses appear without adjusting the depth of the tetrodes. An alternative approach would be to compare recordings from trained animals with those from pseudoconditioned animals given only unpaired presentations of CS and US. Previous studies have shown that mPFC single units show decreased responses to tone presentation within the first 10 trials of pseudoconditioning, which then remained similar to baseline activity levels for 10 additional sessions (Weible et al. 2003). The single-unit recordings reported presently were obtained from the first session following the appearance of conditioned responses through many sessions later when the animals were overtrained, so we can only conclude that persistent activity is present in mPFC and pontine neurons at these times. Note that we did not observe obvious changes in the likelihood of encountering any pattern of trace-conditioned associated activity in the mPFC as the animals became overtrained. Although this latter observation differs from previous recordings of mPFC neurons during trace conditioning (Weible et al. 2003), the results are not in conflict because the recordings were from slightly different mPFC regions and more from different layers (superficial input layers vs. deeper output layers as reported presently).
Persistent activity within deep layers of mPFC.
Although stimulus-evoked persistent activity has been reported in the mPFC of several species during associative tasks that include a temporal gap (Bodner et al. 1996; Funahashi et al. 1989; Fuster et al. 2000; Narayanan and Laubach 2006; Pasupathy and Miller 2005), including prelimbic recordings during trace conditioning in rats (Takehara-Nishiuchi and McNaughton 2008), it was not observed in previous single-unit recording studies of the rabbit mPFC (Weible et al. 2003). Previous recordings in rabbit were focused in the medial wall of the ACC and may have included substantially more layer 2/3 cells than reported in the present study. Our recordings reveal a locus of persistently responsive cells centered around the shoulder cortex (AGm and dACC) and apparently restricted to the deep cortical layers. However, this does not exclude a potentially important role for layer 2/3 mPFC neurons in trace eyelid conditioning. For example, neurons from layer 2/3 may be involved in the acquisition or modulation of activity in layer 5 neurons.
Although our data suggest that the final output of mPFC from deep layer neurons includes activity that persists well into the trace interval, the data are correlational and provide only an initial basis for addressing whether tone onset persistent and/or trace interval-associated activity changes are intrinsic to mPFC or are relayed from other brain regions. Additional experiments are required to test the true origin and necessity of such activity. For example, inactivation of the deep cerebellar nucleus can be used to block the expression of trace conditioned responses (Kalmbach et al. 2009; Pakaprot et al. 2009) and determine whether the trace interval activity observed might reflect motor feedback regarding the conditioned response, as previously suggested (Weiss and Disterhoft 1996). In addition, the absence of either persistent or trace interval-associated activity during learning in hippocampal lesioned animals would suggest that such activity requires hippocampal input to the mPFC. In such a case, persistent and/or trace interval activity during learning may be the result of a collaboration between hippocampus/parahippocampal cortices and prefrontal cortex, as previously suggested (Hasselmo and Stern 2006; Hyman et al. 2010, 2011). Indeed, local field potential recordings from mPFC and the cerebellum have shown increased coherence to hippocampal theta during trace conditioning trials, suggesting that these areas are coordinated with hippocampal activity during learning (Darling et al. 2011; Hoffman and Berry 2009; Wikgren et al. 2010).
Finally, persistent activity states in cortical neurons can be modulated in vitro and in vivo by various neurotransmitters, such as dopamine (Vijayraghavan et al. 2007; Williams and Goldman-Rakic 1995), acetylcholine (Dembrow et al. 2010; Egorov et al. 2002), and norepinephrine (Wang et al. 2007). The ability to abolish the expression of trace conditioned responses by using in vivo infusions to pharmacologically block persistent activity states in mPFC neurons during trace conditioning would provide strong evidence that tone onset persistent activity is necessary for the cerebellar expression of conditioned responses when the CS and US are separated in time.
Although cells that displayed increased activity during trace conditioning trials that overlapped with the US (tone onset persistent and trace interval activity) were the focus of this study, we observed neurons with a variety of response properties. For example, the majority of mPFC cells displayed trace conditioning-associated decreases in activity, as has been previously reported (Weible et al. 2003). Based on their recordings, Weible et al. (2003) suggested that stimuli-evoked decreases in activity could reflect mPFC network responses to excitatory input and effectively increase the signal-to-noise of trace conditioning-associated excitatory responses by decreasing the activity of a substantial proportion of the network during that time. Heterogeneity in response properties could also reflect heterogeneity in the types of cells recorded (pyramidal vs. interneuron) or heterogeneity in pyramidal cell types. Recent evidence suggests that the intrinsic cellular properties of layer 5 pyramidal neurons in mPFC depend on whether they project to the pons or elsewhere, such as the contralateral cortex or striatum (Dembrow et al. 2010; Morishima and Kawaguchi 2006). Furthermore, corticopontine neurons were more easily induced into persistent activity states in response to cholinergic agonists in vitro (Dembrow et al. 2010). Based on these observations, one possibility is that mPFC neurons that project to the pons display tone-evoked persistent activity during trace eyelid conditioning, whereas neurons that display other types of responses may project elsewhere. This possibility is supported indirectly by our observation that the most common response type observed in areas of pons receiving axons from trace conditioning-related areas of mPFC was a tone-evoked persistent increase in activity.
The colocalization of pontine cells that displayed significant trace conditioning-related responses with the terminal fields observed in this and previous studies (Buchanan et al. 1994; Weible et al. 2007) suggests that mPFC activity influences pontine neuron activity directly. The identification of persistent activity in this monosynaptic circuit makes it possible to directly address whether activity observed in pontine neurons does indeed reflect mPFC excitatory input. For example, pontine single-unit recordings paired with inactivation of the mPFC would reveal whether trace conditioning-associated activity observed in the pons is the result of mPFC inputs. Antidromic stimulation of the pons in combination with mPFC single-unit recordings can be used to investigate the identity of mPFC neurons displaying tone-evoked persistent activity during trace conditioning.
Trace eyelid conditioning, delay cells, and working memory.
The tone-evoked persistent activity responses we observed in rabbit mPFC during trace conditioning are quite similar to the so-called “delay cell” responses seen in primate dorsolateral prefrontal cortex (dlPFC) during delayed response tasks (Bodner et al. 1996; Funahashi et al. 1989; Fuster et al. 2000; Fuster 2001; Goldman-Rakic 1995; Vijayraghavan et al. 2007; Wang et al. 2007). To the extent that delay cells in dlPFC (and other cortical regions) of primate reflect processing related to working memory, our data suggest the same is true for persistent activity in mPFC, trace eyelid conditioning, and working memory. In retrospect, perhaps this should not be surprising given that the delay response tasks commonly employed in the primate studies and trace eyelid conditioning are procedurally similar. In the typical delayed-response task, a reinforcing stimulus is delivered a few seconds after the offset of a cue stimulus. Although the terminology is confusing (the stimulus-free periods are called the “delay interval” for delay tasks and the “trace interval” for trace conditioning), the two tasks share in common the computational requirement of associating a cue and a reinforcing stimulus that do not overlap in time. Our results suggest that trace eyelid conditioning engages the same persistent activity mechanisms in the mPFC of rabbit as that reported for monkey during working memory delayed-response tasks. This interpretation is further supported by evidence from lesion studies showing that damage to various regions of mPFC in rat produces deficits in classic spatial working memory tasks (Bailey and Mair 2004; Horst and Laubach 2009; Ragozzino et al. 1998, 2002; Taylor et al. 2003). Thus trace eyelid conditioning may represent an experimentally tractable means to address important and unresolved questions regarding cortical persistent activity, including whether it is learned or regulated, and if so, how?
GRANTS
This work was supported by National Institute of Mental Health Grants MH74006 and MH46904 and by the McKnight Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.J.S., B.K., and M.D.M. conception and design of research; J.J.S. and B.K. performed experiments; J.J.S., B.K., and R.A.C. analyzed data; J.J.S., B.K., R.A.C., and M.D.M. interpreted results of experiments; J.J.S., B.K., and R.A.C. prepared figures; J.J.S., B.K., and R.A.C. drafted manuscript; J.J.S., B.K., R.A.C., and M.D.M. edited and revised manuscript; J.J.S., B.K., R.A.C., and M.D.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Tobin Davis for assistance with mPFC recordings, Frank Riusech for additional technical assistance, and Daniel Johnston for useful suggestions.
REFERENCES
- Aitkin LM, Boyd J. Acoustic input to the lateral pontine nuclei. Hear Res 1: 67–77, 1978 [DOI] [PubMed] [Google Scholar]
- Bailey KR, Mair RG. Dissociable effects of frontal cortical lesions on measures of visuospatial attention and spatial working memory in the rat. Cereb Cortex 14: 974–985, 2004 [DOI] [PubMed] [Google Scholar]
- Bodner M, Kroger J, Fuster JM. Auditory memory cells in dorsolateral prefrontal cortex. Neuroreport 7: 1905–1908, 1996 [DOI] [PubMed] [Google Scholar]
- Buchanan SL, Thompson RH, Maxwell BL, Powell DA. Efferent connections of the medial prefrontal cortex in the rabbit. Exp Brain Res 100: 469–483, 1994 [DOI] [PubMed] [Google Scholar]
- Campolattaro MM, Kashef A, Lee I, Freeman JH. Neuronal correlates of cross-modal transfer in the cerebellum and pontine nuclei. J Neurosci 31: 4051–4062, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RE, Manns JR, Squire LR. Classical conditioning, awareness, and brain systems. Trends Cogn Sci 6: 524–531, 2002 [DOI] [PubMed] [Google Scholar]
- Curtis CE. Prefrontal, and parietal contributions to spatial working memory. Neuroscience 139: 173–180, 2006 [DOI] [PubMed] [Google Scholar]
- Darling RD, Takatsuki K, Griffin AL, Berry SD. Eyeblink conditioning contingent on hippocampal theta enhances hippocampal and medial prefrontal responses. J Neurophysiol 105: 2213–2224, 2011 [DOI] [PubMed] [Google Scholar]
- Dembrow NC, Chitwood RA, Johnston D. Projection-specific neuromodulation of medial prefrontal cortex neurons. J Neurosci 30: 16922–16937, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egorov AV, Hamam BN, Fransen E, Hasselmo ME, Alonso AA. Graded persistent activity in entorhinal cortex neurons. Nature 420: 173–178, 2002 [DOI] [PubMed] [Google Scholar]
- Flores LC, Disterhoft JF. Caudate nucleus is critically involved in trace eyeblink conditioning. J Neurosci 29: 14511–14520, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank LM, Brown EN. Persistent activity and memory in the entorhinal cortex. Trends Neurosci 26: 400–401, 2003 [DOI] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey's dorsolateral prefrontal cortex. J Neurophysiol 61: 331–349, 1989 [DOI] [PubMed] [Google Scholar]
- Furtak SC, Allen TA, Brown TH. Single-unit firing in rat perirhinal cortex caused by fear conditioning to arbitrary and ecological stimuli. J Neurosci 27: 12277–12291, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex—an update: time is of the essence. Neuron 30: 319–333, 2001 [DOI] [PubMed] [Google Scholar]
- Fuster JM, Bodner M, Kroger JK. Cross-modal and cross-temporal association in neurons of frontal cortex. Nature 405: 347–351, 2000 [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. Neuron 14: 477–485, 1995 [DOI] [PubMed] [Google Scholar]
- Halverson HE, Freeman JH. Medial auditory thalamic input to the lateral pontine nuclei is necessary for auditory eyeblink conditioning. Neurobiol Learn Mem 93: 92–98, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Stern CE. Mechanisms underlying working memory for novel information. Trends Cogn Sci 10: 487–493, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesslow G. Correspondence between climbing fibre input, and motor output in eyeblink-related areas in cat cerebellar cortex. J Physiol 476: 229–244, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesslow G, Svensson P, Ivarsson M. Learned movements elicited by direct stimulation of cerebellar mossy fiber afferents. Neuron 24: 179–185, 1999 [DOI] [PubMed] [Google Scholar]
- Hoffman LC, Berry SD. Cerebellar theta oscillations are synchronized during hippocampal theta-contingent trace conditioning. Proc Natl Acad Sci USA 106: 21371–21376, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horst NK, Laubach M. The role of rat dorsomedial prefrontal cortex in spatial working memory. Neuroscience 164: 444–456, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman JM, Hasselmo ME, Seamans JK. What is the functional relevance of prefrontal cortex entrainment to hippocampal theta rhythms? Front Neurosci 5: 24, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman JM, Zilli EA, Paley AM, Hasselmo ME. Working memory performance correlates with prefrontal-hippocampal theta interactions but not with prefrontal neuron firing rates. Front Integr Neurosci 4: 2, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmbach BE, Ohyama T, Mauk MD. Temporal patterns of inputs to cerebellum necessary and sufficient for trace eyelid conditioning. J Neurophysiol 104: 627–640, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmbach BE, Ohyama T, Kreider JC, Riusech F, Mauk MD. Interactions between prefrontal cortex, and cerebellum revealed by trace eyelid conditioning. Learn Mem 16: 86–95, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Clark RE, Thompson RF. Hippocampectomy impairs the memory of recently, but not remotely, acquired trace eyeblink conditioned responses. Behav Neurosci 109: 195–203, 1995 [DOI] [PubMed] [Google Scholar]
- Kronforst-Collins MA, Disterhoft JF. Lesions of the caudal area of rabbit medial prefrontal cortex impair trace eyeblink conditioning. Neurobiol Learn Mem 69: 147–162, 1998 [DOI] [PubMed] [Google Scholar]
- Mauk MD, Thompson RF. Retention of classically conditioned eyelid responses following acute decerebration. Brain Res 403: 89–95, 1987 [DOI] [PubMed] [Google Scholar]
- McEchron MD, Disterhoft JF. Sequence of single neuron changes in CA1 hippocampus of rabbits during acquisition of trace eyeblink conditioned responses. J Neurophysiol 78:1030–1044, 1997 [DOI] [PubMed] [Google Scholar]
- McEchron MD, Weible AP, Disterhoft JF. Aging and learning-specific changes in single-neuron activity in CA1 hippocampus during rabbit trace eyeblink conditioning. J Neurophysiol 86: 1839–1857, 2001 [DOI] [PubMed] [Google Scholar]
- Morishima M, Kawaguchi Y. Recurrent connection patterns of corticostriatal pyramidal cells in frontal cortex. J Neurosci 26: 4394–4405, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer JR, Jr, Deyo RA, Disterhoft JF. Hippocampectomy disrupts trace eye-blink conditioning in rabbits. Behav Neurosci 104: 243–252, 1990 [DOI] [PubMed] [Google Scholar]
- Narayanan NS, Laubach M. Top-down control of motor cortex ensembles by dorsomedial prefrontal cortex. Neuron 52: 921–931, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson DA, Freeman JH., Jr Addition of inhibition in the olivocerebellar system and the ontogeny of a motor memory. Nat Neurosci 6: 532–537, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley DA, Russell IS. Neocortical lesions and Pavlovian conditioning. Physiol Behav 8: 915–926, 1972 [DOI] [PubMed] [Google Scholar]
- Pakaprot N, Kim S, Thompson RF. The role of the cerebellar interpositus nucleus in short and long term memory for trace eyeblink conditioning. Behav Neurosci 123: 54–61, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasupathy A, Miller EK. Different time courses of learning-related activity in the prefrontal cortex and striatum. Nature 433: 873–876, 2005 [DOI] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned Reflexes: an Investigation of the Physiological Activity of the Cerebral Cortex, edited and translated by Anrep GV. London: Oxford University Press, 1927 [Google Scholar]
- Powell DA, Skaggs H, Churchwell J, McLaughlin J. Posttraining lesions of the medial prefrontal cortex impair performance of Pavlovian eyeblink conditioning but have no effect on concomitant heart rate changes in rabbits (Oryctolagus cuniculus). Behav Neurosci 115: 1029–1038, 2001 [DOI] [PubMed] [Google Scholar]
- Quintana J, Yajeya J, Fuster JM. Prefrontal representation of stimulus attributes during delay tasks. I. Unit activity in cross-temporal integration of sensory and sensory-motor information. Brain Res 474: 211–221, 1988 [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Adams S, Kesner RP. Differential involvement of the dorsal anterior cingulate and prelimbic-infralimbic areas of the rodent prefrontal cortex in spatial working memory. Behav Neurosci 112: 293–303, 1998 [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Detrick S, Kesner RP. The effects of prelimbic and infralimbic lesions on working memory for visual objects in rats. Neurobiol Learn Mem 77: 29–43, 2002 [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. Anatomic organization of the basilar pontine projections from prefrontal cortices in rhesus monkey. J Neurosci 17: 438–458, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JJ, Neunuebel JP, Knierim JJ. Dominance of the proximal coordinate frame in determining the locations of hippocampal place cell activity during navigation. J Neurophysiol 99: 60–76, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon PR, Vander Schaaf ER, Thompson RF, Weisz DJ. Hippocampus and trace conditioning of the rabbit's classically conditioned nictitating membrane response. Behav Neurosci 100: 729–744, 1986 [DOI] [PubMed] [Google Scholar]
- Steinmetz JE, Lavond DG, Thompson RF. Classical conditioning in rabbits using pontine nucleus stimulation as a conditioned stimulus and inferior olive stimulation as an unconditioned stimulus. Synapse 3: 225–233, 1989 [DOI] [PubMed] [Google Scholar]
- Takehara K, Kawahara S, Kirino Y. Time-dependent reorganization of the brain components underlying memory retention in trace eyeblink conditioning. J Neurosci 23: 9897–9905, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takehara-Nishiuchi K, McNaughton BL. Spontaneous changes of neocortical code for associative memory during consolidation. Science 322: 960–963, 2008 [DOI] [PubMed] [Google Scholar]
- Taylor CL, Latimer MP, Winn P. Impaired delayed spatial win-shift behaviour on the eight arm radial maze following excitotoxic lesions of the medial prefrontal cortex in the rat. Behav Brain Res 147: 107–114, 2003 [DOI] [PubMed] [Google Scholar]
- Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AF. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci 10: 376–384, 2007 [DOI] [PubMed] [Google Scholar]
- Wang M, Ramos BP, Paspalas CD, Shu Y, Simen A, Duque A, Vijayraghavan S, Brennan A, Dudley A, Nou E, Mazer JA, McCormick DA, Arnsten AF. Alpha2A-adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell 129: 397–410, 2007 [DOI] [PubMed] [Google Scholar]
- Weible AP, McEchron MD, Disterhoft JF. Cortical involvement in acquisition and extinction of trace eyeblink conditioning. Behav Neurosci 114: 1058–1067, 2000 [DOI] [PubMed] [Google Scholar]
- Weible A, Oreilly J, Weiss C, Disterhoft J. Comparisons of dorsal, and ventral hippocampus cornu ammonis region 1 pyramidal neuron activity during trace eyeblink conditioning in the rabbit. Neurosci 141: 1123–1137, 2006 [DOI] [PubMed] [Google Scholar]
- Weible AP, Weiss C, Disterhoft JF. Activity profiles of single neurons in caudal anterior cingulate cortex during trace eyeblink conditioning in the rabbit. J Neurophysiol 90: 599–612, 2003 [DOI] [PubMed] [Google Scholar]
- Weible AP, Weiss C, Disterhoft JF. Connections of the caudal anterior cingulate cortex in rabbit: neural circuitry participating in the acquisition of trace eyeblink conditioning. Neuroscience 145: 288–302, 2007 [DOI] [PubMed] [Google Scholar]
- Weiss C, Disterhoft JF. Eyeblink conditioning, motor control, and the analysis of limbic-cerebellar interactions. Behav Brian Sci 19: 479–504, 1996 [Google Scholar]
- Wiesendanger R, Wiesendanger M. The corticopontine system in the rat. I. Mapping of corticopontine neurons. J Comp Neurol 208: 215–226, 1982 [DOI] [PubMed] [Google Scholar]
- Wikgren J, Nokia MS, Penttonen M. Hippocampo-cerebellar theta band phase synchrony in rabbits. Neurosci 165: 1538–1545, 2010 [DOI] [PubMed] [Google Scholar]
- Williams GV, Goldman-Rakic PS. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature 376: 572–575, 1995 [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Disterhoft JF. Where is the trace in trace conditioning? Trends Neurosci 31: 105–112, 2008 [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Lavond DG, Thompson RF. Trace conditioning: abolished by cerebellar nuclear lesions but not lateral cerebellar cortex aspirations. Brain Res 348: 249–260, 1985 [DOI] [PubMed] [Google Scholar]
- Yoganarasimha D, Yu X, Knierim JJ. Head direction cell representations maintain internal coherence during conflicting proximal and distal cue rotations: comparison with hippocampal place cells. J Neurosci 26: 622–631, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou YD, Ardestani A, Fuster JM. Distributed and associative working memory. Cereb Cortex 17: i77–i87, 2007 [DOI] [PubMed] [Google Scholar]