Abstract
In recent years, noninvasive brain stimulation techniques like transcranial direct current stimulation (tDCS) have gained immense popularity owing to their effects on modulating cortical activity and consequently motor and cognitive performance. However, the neurophysiology underlying such neuroplastic changes is less understood. This article critically evaluates the contemporary approach of combined tDCS and neuroimaging as a means to provide novel insights in understanding the neurophysiological and neuroplastic processes modulated by this brain stimulation technique. We end by briefly suggesting further lines of inquiry.
Keywords: neuroimaging, functional magnetic resonance imaging, electroencephalography, graph theory
external application of direct electrical current to the head is one of the oldest techniques used to modulate cortical excitability. The noninvasive version of direct current application to the scalp surface is more commonly known as transcranial direct current stimulation (tDCS). tDCS modulates cortical excitability by constantly applying weak electrical current over time to increase (positive polarity) or decrease (negative polarity) cortical excitability (Nitsche et al. 2008). This technique has recently been reintroduced in neuroscience research by virtue of its potential for both the investigation of causal brain-behavior relationships and for the rehabilitation of many diseases. It is worth mentioning here that progress in neuroscience often depends on the convergence of evidence from multiple methods. Since every single technique has its own limitations, there is a clear theoretical advantage in combining different approaches. In the last decade, combined transcranial magnetic stimulation (TMS)-neuroimaging studies have greatly stimulated research in understanding neurophysiological and neuroplastic effects induced by noninvasive brain stimulation (Siebner et al. 2009). In this article, however, we critically evaluate the emergence of the approach to combine tDCS with neuroimaging techniques to understand tDCS-induced neurophysiological effects on whole brain functional networks. Because the majority of evidence has been gained recently from modulation of the primary motor cortex (M1) in healthy subjects, we concentrate on these studies and end by discussing future research directions.
Overview of tDCS administration.
tDCS is typically applied through a bipolar electrode montage. The electrodes, covered by a sponge soaked in a conducting solution like saline or tap water, are attached to the subject's scalp. The anode is the positively charged electrode, and the cathode is the negatively charged electrode. The primary stimulation parameters that are controlled by the experimenter include: 1) electrode size, 2) intensity of stimulation, 3) duration of stimulation, and 4) electrode montage. Typically, large electrode sizes (5 × 5 cm or 5 × 7 cm) are used to maintain a low current density such that the skin sensation of the electrical stimulation is bearable and also to avoid local skin burns. The intensity of electrical current used most commonly range between 1 and 2 mA, with the latter being more common in montages with one electrode being placed on an extra-cephalic location like the arm. Coupled with the large electrode surface area, these low intensities allow maintenance of an optimal, safe current density between 24 and 29 μA/cm2 (Nitsche et al. 2008). When applied for several minutes, tDCS produces lasting effects in the human cortex. The duration of the excitability changes induced by tDCS depends on stimulation duration. These are stable for up to about an hour if tDCS is applied for 9–13 min (Nitsche et al. 2008). Finally, the electrode montage for tDCS administration is determined based on the region being stimulated. Most commonly, for the modulation of the left M1, the active electrode is placed over the representational field of the right hand localized using suprathreshold TMS pulses, whereas the reference electrode is generally placed on the contralateral supraorbital region (Nitsche et al. 2008).
Physiological effects of tDCS.
Early investigations of the physiological effects of tDCS and recent computational models of current induction in the cortical tissue by tDCS suggest that short-term polarization with tDCS can change membrane excitability rather than actually induce action potentials. In this regard, anodal tDCS increases membrane excitability (i.e., increases resting membrane potentials) while cathodal tDCS hyperpolarizes membrane potentials. It is thus important to recognize that tDCS can mediate almost immediate changes in membrane excitability, which impacts the response of the involved neural circuit to any incoming inputs, and also mediate activity-dependent changes in synaptic transmission properties when coupled with some behavioral training (Nitsche et al. 2008). Recently, it was demonstrated that anodal tDCS mediates its physiological effects by long-term synaptic potentiation (LTP) due to activity-dependent release of brain-derived neurotrophic factor (BDNF) (Fritsch et al. 2010). Since BDNF is important for mediating translation and transcription associated with protein synthesis for LTP, it is understandable that offline memory consolidation and long-term retention can be specifically improved by anodal tDCS.
It is also important to highlight the potential interaction between physiological effects of tDCS and homeostatic neural plastic mechanisms because cortical excitability modulation by tDCS may produce some modification in the threshold for LTP and long-term synaptic depression. Synapses that are at a higher level of excitation, likely to occur with conditioning by anodal tDCS, can have a higher threshold for LTP, thus making subsequent LTP induction less probable. On the contrary, synapses at a lower level of baseline excitability, likely to occur with conditioning by cathodal tDCS, can have a lower threshold for LTP, thus making subsequent LTP induction more probable (this is in accordance with the Bienenstock-Cooper-Munroe rule). This form of homeostatic plasticity is an essential mechanism for neurons to prevent an uncontrolled increase in synaptic effectiveness. Moreover, it has important implications regarding the application of tDCS to facilitate neuroplasticity, as related to motor learning, because it determines appropriate modulation of cortical excitability of brain regions involved in the given functional task performance.
Polarity-specific effects of tDCS over the motor cortex on specific neurotransmitters were recently investigated using magnetic resonance spectroscopy (MRS) (Stagg et al. 2009). MRS is an imaging technique that allows the estimation of different neurotransmitter concentration such as gamma amino butyric acid (GABA), glutamate, etc. These results showed that 10 min of anodal tDCS over left M1 specifically reduced GABA levels in the cortex while cathodal tDCS reduced glutamate levels coupled with correlated decreases in GABA levels. Recently, Stagg et al. (2011) also demonstrated that changes in GABA concentration elicited by anodal tDCS over M1 correlates with the amount of motor learning across individuals: the amount of decrease in GABA concentration due to tDCS was positively correlated with the amount of motor learning in a serial reaction time task (SRTT). Interestingly, when the subjects performed the SRTT in a functional magnetic resonance imaging (fMRI) scanner, GABA responsiveness also correlated with the decreases in blood oxygenation-dependent (BOLD) signal in the left M1 (contralateral to performing hand) during the task. Thus, these findings highlight the role of GABAergic modulation by anodal tDCS in facilitating motor learning and are suggestive of a possible relevance of GABA in LTP-like synaptic plasticity in human motor learning. Future studies must investigate the relevance of this GABAergic modulation in motor learning following cathodal tDCS since both anodal and cathodal tDCS modulation decreased GABA concentration in the original study conducted by Stagg et al. (2009).
An emerging theme from the aforementioned introduction is that modulation of seemingly “localized” cortical excitability over M1 results in modulation of complex motor performance suggesting that tDCS affects more widespread brain networks, thus inducing plasticity in behavior.
What can brain network analysis inform about neuroplasticity?
The study of brain networks involves examination and quantifying properties of interactions between the interconnected components of neural circuits, at multiple spatial scales like from the microscale level of networks of a group of synapses or neurons to the macroscale level of networks of various populations of neurons to the networks in the whole brain. Primarily, brain networks are studied based on the hypothesis that most of the complex cognitive behaviors exhibited by animals emerge from the intricate organization of and interaction between the basic neuronal elements, i.e., neurons over and above the functions and properties of the component neurons themselves (Sporns 2010). In fact, in this multiscale brain network architecture, the interaction between networks at different scales plays an integral role in determining global function. In humans, noninvasive neuroimaging techniques offer the best possible means to study brain networks and their properties in humans in present times. By tracking neural activity in real time, directly or indirectly, neuroimaging methods help provide a complex spatiotemporal description of plastic reorganization in humans, which occurs as a result of experience and also following insults or injuries to the nervous system. Particularly, it involves detailed investigations of connectivity between different regions of the brain, or in other words, study of dynamics of networks involving regions that are “connected” functionally and/or structurally.
Thus, studying brain networks may serve as an invaluable tool to study underlying neuroplastic processes influenced by noninvasive brain stimulation techniques such as tDCS. In this context, regional cerebral blood flow (rCBF), BOLD signals and oscillatory dynamics through magnetoencephalography, and electroencephalography (EEG), coupled with large-scale brain network analysis, can help identify noninvasive neural markers of neuroplasticity in the human brain. The timing of tDCS relative to neuroimaging defines which questions can be tackled using a combined tDCS neuroimaging approach. When tDCS is applied “offline” before neuroimaging, these techniques can map the spatio-temporal pattern of functional reorganization induced in the brain by tDCS or the lasting functional impact of tDCS on rest- or task-related neural activity at the systems level. Particularly, this might also help clarify some heterogeneous evidence about homeostatic neural plasticity modulated by tDCS. This offline approach, in which tDCS and neuroimaging are separated in time, is also technically easier to implement than the “online” approach, in which tDCS and neuroimaging overlap in time with tDCS having the possibility to adversely affect data acquisition during neuroimaging. However, this online approach is the only means to use tDCS to test how cortical modulation instantaneously modifies the activity and connectivity within the modulated neural circuits or networks.
Neuroplastic effects of tDCS on brain networks.
While there is sufficient evidence to support the significant influence of tDCS in inducing neuroplastic changes reflected in observable behavioral changes, the exact mechanism of action of tDCS in producing this neuromodulation is not completely clear. Thus, recent efforts to combine tDCS and neuroimaging in experimental paradigms have been undertaken to provide a more methodical characterization of neuroplastic modulation by tDCS through the use of brain network analysis techniques. Baudewig et al. (2001) examined sensorimotor brain activation before and after 5 min of tDCS over left M1, during performance of a sequential finger-opposition task. This work was the first attempt to detect tDCS-induced modulations of brain activity via changes of the BOLD MRI response to a well-defined functional challenge. The authors found that increased excitability associated with anodal tDCS occurred with increased activation in sensorimotor cortical regions while cathodal tDCS led to decreased activation in the same regions. However, the first study to track changes at rest as well as task-related (finger movements) brain activation used positron emission tomography (Lang et al. 2005). The authors studied rCBF changes after application of 20 min of tDCS over left M1. It was found that real tDCS, i.e., both anodal and cathodal, increased rCBF in M1, sensorimotor cortex, frontal cortical regions compared with sham, and these effects persisted for up to 50 min after end of tDCS. Additionally, anodal tDCS also increased rCBF in subcortical brain regions compared with cathodal tDCS. These findings were the first to show experimentally that the presumed local tDCS application over M1 may produce long-lasting neuroplastic alterations in more widespread brain networks over and beyond stimulated M1 cortical regions.
However, methods to analyze and quantify brain network dynamics were less developed at that time, and recent technological advances have facilitated more detailed computations of network dynamics through analysis of complex neuroimaging data sets of high dimensionality and volume. Thus, the application of characterization of brain network dynamics to study neuroplastic modulation by tDCS is a recent development. In this regard, graph theoretical analyses have been applied to characterize brain network changes in EEG (Polanía et al. 2010) as well as fMRI (Polanía et al. 2011) following 10 min of anodal tDCS over left M1. Graph theory is a mathematical approach to quantify the cost of information transfer and processing in a defined brain network by calculating amount of interconnectivity (edges) between different brain regions (nodes) as well as length of connections within that network.
The results showed that 10 min of anodal tDCS modulated high-frequency oscillatory activity in beta (15–30 Hz) and gamma (60–90 Hz) in the functional EEG synchronization-based connectivity metric during performance of a simple finger-tapping motor task not only in electrodes over the stimulated motor cortex, but also in bilateral frontal, parietal, and premotor cortical regions compared with sham stimulation. On the other hand, with the higher spatial resolution of fMRI, it was possible to show that 10 min of anodal tDCS actually increased short range connections from M1 to premotor and parietal cortical regions, while concomitantly increasing interconnectedness in prefrontal cortex in resting brain dynamics (Polania et al. 2011).
Interestingly, recent studies also compared changes in fMRI during simultaneous tDCS application during data acquisition in the MRI scanner (“online” approach, Kwon et al. 2008; Antal et al. 2011). In the first study (Kwon et al. 2008), anodal tDCS was applied over left M1 during grasp-release hand movements using 4 × 21-s stimulation phases (resting-tDCS-tDCS-tDCS-tDCS). No cortical activation was detected in any of the stimulation phases except the fourth tDCS phase. Activation was found not only under the electrode but also in the left supplementary motor cortex and the right posterior parietal cortex. However, in this study, cathodal stimulation was not applied. Therefore, in another study (Antal et al. 2011), the authors addressed the question as to whether anodal and cathodal tDCS result in differential BOLD fMRI signal changes during a rest condition as well as the polarity-specific effects of tDCS on the brain network activated by a voluntary finger-tapping task. Although specific brain network analytical approaches were not used here, neither anodal nor cathodal tDCS over the M1 for 20-s stimulation duration induced a detectable BOLD signal change. However, compared with a voluntary finger-tapping task without stimulation, anodal tDCS during finger tapping resulted in a decrease in the BOLD response in the supplementary motor area (SMA). Cathodal stimulation did not result in significant change in BOLD response in the SMA, although a tendency toward decreased activity could be seen. In the control experiment, in which the electrodes were placed over left and right occipito-temporo-parietal junction, neither cathodal nor anodal stimulation resulted in a significant change of BOLD signal during finger tapping in any brain area including SMA, premotor cortex, and M1.
Together, these findings provide further support for the notion that tDCS applied over a specific cortical region, like M1, induces widespread changes of cerebral activity at cortical and subcortical levels and alters functional connectivity between this cortex and motor association cortices.
Future directions.
While these recent studies provide some insight about potential network-level neuroplastic modulation by localized application of tDCS over M1, it is evident that our understanding of neuroplastic alterations induced by tDCS is far from complete. Future studies are needed to systematically investigate the polarity-specific changes in brain network dynamics induced by tDCS to provide a plausible mechanistic account of neuroplasticity and explain behavioral neurophysiological changes that are modulated by tDCS (see Fig. 1). Furthermore, this approach can help examine homeostatic plasticity induced by tDCS at the level of brain networks, an important phenomenon for the application of tDCS in treating diseases known to have pathological altered cortical excitability (i.e., stroke). An important point to be noted here is that the analytical approach used to describe and quantify brain dynamics significantly influences the inferences that can be drawn from the data. Most of the recent studies described above, including graph theoretical analysis, used connectivity-model based analyses to describe changes in brain network dynamics. However, using model-free, data-driven approaches in the future, like independent component analysis, may also be important to initially identify specific networks modulated by tDCS in an unbiased manner.
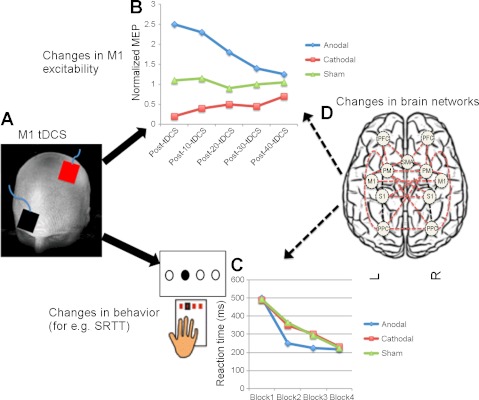
Fig. 1.
A: a schematic summary of transcranial direct current stimulation (tDCS) effects over M1 (bipolar electrode montage with one electrode over M1, and other over contralateral supraorbital region). B: M1 tDCS mediates polarity-specific changes in cortical excitability (shown as motor-evoked potentials, i.e., MEPs expressed as a ratio to baseline) that outlasts duration of stimulation up to 40 min. C also leads to changes in behavior such as motor learning on a serial reaction time task (SRTT). Anodal tDCS over M1 improves motor performance on SRTT more than cathodal and sham tDCS (i.e., greater decrease in reaction time in the early blocks). D: representative cortical brain networks with interacting regional nodes that are likely modulated by tDCS over M1. PFC, prefrontal cortex; SMA, supplementary motor area; PM, premotor cortex; S1, primary somatosensory cortex; M1, primary motor cortex; PPC, posterior parietal cortex. Complex brain network dynamics recorded through neuroimaging could help identify and quantify widespread changes in brain activity and functional connectivity within brain networks to provide more novel insights into neuroplastic mechanisms modulated by tDCS. Note: data shown in graphs are representative of previously published results (Nitsche et al. 2008).
Moreover, brain network analytical approaches are useful tools to noninvasively evaluate not only the functional changes induced by tDCS but also to track the effects of other noninvasive stimulation methods that have also been reported to produce neuroplastic alterations in the human brain (e.g., transcranial random noise stimulation, transcranial alternating current stimulation, and TMS). All these approaches will have important implications in future clinical applications of brain stimulation as well as help define noninvasive markers of neuroplasticity.
In summary, such combined approaches to study and quantify neurophysiological processes associated with neuroplasticity are critical to help identify, monitor, and potentiate neuroplasticity that is crucial for functional recovery in patients suffering from brain lesions like stroke and traumatic brain injury. In fact, in the years to come, methods of characterizing network dynamics and properties using neuroimaging techniques may have significant value in advancing our understanding of the computational processes performed by the brain during human cognition and behavior, and, more importantly, to explain the tremendous plasticity exhibited by the brain in the face of experience and injury.
GRANTS
This work was supported by funding from the Department of Defense in the Center for Neuroscience and Regenerative Medicine to A. Venkatakrishnan and M. Sandrini.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Leonardo G. Cohen for insightful comments and suggestions.
REFERENCES
- Antal A, Polania R, Schmidt-Samoa C, Dechent P, Paulus W. Transcranial direct current stimulation over the primary motor cortex during fMRI. Neuroimage 55: 590–596, 2011 [DOI] [PubMed] [Google Scholar]
- Baudewig J, Nitsche MA, Paulus W, Frahm J. Regional modulation of BOLD MRI responses to human sensorimotor activation by transcranial direct current stimulation. Magn Reson Med 45: 196–201, 2001 [DOI] [PubMed] [Google Scholar]
- Fritsch B, Reis J, Martinowich K, Schambra HM, Ji Y, Cohen LG, Lu B. Direct current stimulation promotes BDNF-dependent synaptic plasticity: potential implications for motor learning. Neuron 66: 198–204, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon YH, Ko MH, Ahn SH, Kim YH, Song JC, Lee CH, Chang MC, Jang SH. Primary motor cortex activation by transcranial direct current stimulation in the human brain. Neurosci Lett 435: 56–59, 2008 [DOI] [PubMed] [Google Scholar]
- Lang N, Siebner HR, Ward NS, Lee L, Nitsche MA, Paulus W, Rothwell JC, Lemon RN, Frackowiak RS. How does transcranial DC stimulation of the primary motor cortex alter regional neuronal activity in the human brain? Eur J Neurosci 22: 495–504, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, Paulus W, Hummel F, Boggio PS, Fregni F, Pascual-Leone A. Transcranial direct current stimulation: state of the art 2008. Brain Stimul 1: 206–223, 2008 [DOI] [PubMed] [Google Scholar]
- Polanía R, Nitsche MA, Paulus W. Modulating functional connectivity patterns and topological functional organization of the human brain with transcranial direct current stimulation. Hum Brain Mapp July 6 doi: 10.1002/hbm.21104, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanía R, Paulus W, Antal A, Nitsche MA. Introducing graph theory to track for neuroplastic alterations in the resting human brain: a transcranial direct current stimulation study. Neuroimage 54: 2287–2296, 2011 [DOI] [PubMed] [Google Scholar]
- Siebner HR, Bergmann TO, Bestmann S, Massimini M, Johansen-Berg H, Mochizuki H, Bohning DE, Boorman ED, Groppa S, Miniussi C, Pascual-Leone A, Huber R, Taylor PC, Ilmoniemi RJ, De Gennaro L, Strafella AP, Kähkönen S, Klöppel S, Frisoni GB, George MS, Hallett M, Brandt SA, Rushworth MF, Ziemann U, Rothwell JC, Ward N, Cohen LG, Baudewig J, Paus T, Ugawa Y, Rossini PM. Consensus paper: combining transcranial stimulation with neuroimaging. Brain Stimul 2: 58–80, 2009 [DOI] [PubMed] [Google Scholar]
- Sporns O. Networks of the Brain. MIT Press, p. 375, 2010 [Google Scholar]
- Stagg CJ, Bachtiar V, Johansen-Berg H. The role of GABA in human motor learning. Curr Biol 21: 480–484, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg CJ, Best JG, Stephenson MC, O'Shea J, Wylezinska M, Kincses ZT, Morris PG, Matthews PM, Johansen-Berg H. Polarity-sensitive modulation of cortical neurotransmitters by transcranial stimulation. J Neurosci 29: 5202–5206, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]