Abstract
Serotonergic neurons in the raphe nuclei constitute one of the most prominent neuromodulatory systems in the brain. Projections from the dorsal and median raphe nuclei provide dense serotonergic innervation of the glomeruli of olfactory bulb. Odor information is initially processed by glomeruli, thus serotonergic modulation of glomerular circuits impacts all subsequent odor coding in the olfactory system. The present study discloses that serotonin (5-HT) produces excitatory modulation of external tufted (ET) cells, a pivotal neuron in the operation of glomerular circuits. The modulation is due to a transient receptor potential (TRP) channel-mediated inward current induced by activation of 5-HT2A receptors. This current produces membrane depolarization and increased bursting frequency in ET cells. Interestingly, the magnitude of the inward current and increased bursting inversely correlate with ET cell spontaneous (intrinsic) bursting frequency: slower bursting ET cells are more strongly modulated than faster bursting cells. Serotonin thus differentially impacts ET cells such that the mean bursting frequency of the population is increased. This centrifugal modulation could impact odor processing by: 1) increasing ET cell excitatory drive on inhibitory neurons to increase presynaptic inhibition of olfactory sensory inputs and postsynaptic inhibition of mitral/tufted cells; and/or 2) coordinating ET cell bursting with exploratory sniffing frequencies (5–8 Hz) to facilitate odor coding.
Keywords: intrinsic excitability, transient receptor potential channels, olfaction, glomerular circuits, excitatory modulation
sensory systems detect and process environmental cues to help shape adaptive behavioral responses to external events. Sensory processing varies with behavioral state. This is mediated, in part, by neuromodulatory systems. Neuromodulators, released in specific behavioral contexts (e.g., fear, arousal, and reward expectation outcome), alter the response properties and synaptic efficacies of neurons and local circuits to modify how sensory signals are processed (Hurley et al. 2004).
Serotonin (5-HT) is a prominent neuromodulator (Jacobs and Azmitia 1992). Serotonergic innervation of the forebrain including the olfactory bulb (OB) derives from neurons primarily in the dorsal and median raphe nuclei of the midbrain (Jacobs and Azmitia 1992; McLean and Shipley 1987). These 5-HT neurons project widely throughout the forebrain and have been implicated in a variety of central nervous system functions including aggression, drug abuse, anxiety, respiration, sleep patterns, stress, feeding, motor control, motivated behaviors, and sensory processing (Hurley et al. 2004; Jones and Blackburn 2002; Ptak et al. 2009). The use of 5-HT reuptake inhibitors to treat anxiety and depression suggests that tonic levels of 5-HT play a key role in maintaining appropriate affective tone (Jones and Blackburn 2002). The discharge activity of serotonergic neurons in the raphe nuclei increases during transition from sleep to awake/arousal state, during oral-buccal movements, and when animals await a delayed reward (Jacobs and Azmitia 1992). These findings indicate that 5-HT modulation of target cells and circuits varies with behavioral state.
Sensory modulation by 5-HT is well-documented in the visual and auditory systems at the thalamic and cortical levels (Hurley et al. 2004). 5-HT fibers from the dorsal and medial raphe nuclei project to the OB with especially dense innervation of the glomeruli (McLean and Shipley 1987), the initial circuitry that processes input from primary sensory neurons. Stimulation of the dorsal raphe nucleus produces a GABAB receptor-mediated presynaptic inhibition of the olfactory sensory neuron (OSN) axonal terminals (Petzold et al. 2009). This is thought to reflect direct 5-HT excitation of GABAergic periglomerular (PG) neurons via 5-HT2C receptors, consistent with an earlier study that showed a 5-HT2C receptor-mediated direct depolarization in ∼30% of tested glomerular neurons (Hardy et al. 2005). Knowledge of glomerular circuitry has increased greatly in recent years (Wachowiak and Shipley 2006). Glomerular neurons are classified into three subpopulations: PG, external tufted (ET), and short-axon (SA) cells (Pinching and Powell 1971a; Price and Powell 1970; Wachowiak and Shipley 2006). PG cells are GABAergic, ET cells are glutamatergic, and SA cells express both GABA and dopamine (Hayar et al. 2004a; Kiyokage et al. 2010). The synapses linking these three populations of neurons form several distinct intra- and interglomerular circuits (Aungst et al. 2003; Shao et al. 2009; Wachowiak and Shipley 2006). Thus 5-HT modulation of these three cell types may differentially impact the operation of glomerular circuits to alter the glomerular input-output function.
The present study focuses on 5-HT modulation of ET cells, which play a pivotal role in multiple glomerular circuits. ET cells spontaneously generate rhythmic bursts of action potentials (Hayar et al. 2004b; Liu and Shipley 2008). Bursting is autonomous, generated by multiple ET cell intrinsic conductances (Liu and Shipley 2008), many of which are targets of 5-HT modulation in other neurons (Bickmeyer et al. 2002; Carr et al. 2002; Li et al. 2008). ET cells receive monosynaptic sensory input and provide direct excitatory outputs to the majority (∼70%) of GABAergic PG and SA cells (Hayar et al. 2004a; Shao et al. 2009). ET cells also provide excitatory input to mitral/tufted cells (De Saint Jan et al. 2009; Gire and Schoppa 2009). Thus 5-HT modulation of ET cells can influence multiple glomerular circuits and impact sensory coding in all downstream olfactory networks.
METHODS
Slice preparation.
All experimental procedures were carried out in accordance with protocols submitted to and approved by the University of Maryland Institutional Animal Care and Use Committee and were in accordance with National Institutes of Health (NIH) guidelines. Male C57BL/6 mice (6–8 wk old) were anesthetized with saturated isoflurane and decapitated. The OBs were dissected, and horizontal slices (350-μm thickness) were prepared with a Vibratome 1000 (Technical Products International, St. Louis, MO) or Leica VT1000S (Nussloch, Germany) in ice-cold oxygenated (95% O2-5% CO2) sucrose-based artificial cerebrospinal fluid (ACSF) containing (in mM): 205 sucrose, 2.5 KCl, 0.5 CaCl2, 5 MgSO4, 10 glucose, 26 NaHCO3, and 5 BES. Slices recovered at 30°C for 30 min in normal ACSF (in mM: 124 NaCl, 2.5 KCl, 1.3 CaCl2, 1.3 MgSO4, 10 glucose, 26 NaHCO3, and 5 BES) continuously bubbled with 95% O2-5% CO2 and were held at room temperature (22°C) until recording.
Identification of ET cells.
ET cells were initially reported in Golgi anatomy studies (Macrides and Schneider 1982; Pinching 1970; Pinching and Powell 1971a,b; Ramón y Cajal 1911). Recently, ET cells were more rigorously characterized by correlating their morphological and electrophysiological properties (Antal et al. 2006; Hayar et al. 2004b). Based on these studies, we identify ET cells by three criteria: 1) spontaneous intrinsic burst firing that persists even when fast synaptic transmitter (AMPA, NMDA and GABAA) receptors are blocked (Hayar et al. 2004b; Liu and Shipley 2008); 2) “pear”-shaped cell body located in the deep half of the glomerular layer when viewed in near-infrared differential interference contrast (IR-DIC) optics; and 3) an apical dendrite with extensively ramified tuft confined to the glomerulus and absence of lateral dendrites in the external plexiform layer (EPL). A second type of tufted cells (∼30%) have lateral dendrites in the EPL and require depolarizing current to exhibit bursting (Antal et al. 2006). We recognize cells with these features (Hayar et al. 2004b) but refer to them as “superficial tufted cells” (ST cells) to avoid confusion with ET cells as defined above. Juxtaglomerular neurons with “plateau potentials” that can support bursts of action potentials have been also reported (McQuiston and Katz 2001; Zhou et al. 2006), but it is not known whether these cells support spontaneous bursting, a key feature of ET cells.
Electrophysiology.
Slices in the recording chamber were continuously perfused at 3 ml/min with normal ACSF oxygenated with 95% O2-5% CO2 and warmed to 30°C. Cells were visualized under a BX51WI microscope equipped with near-IR-DIC optics, ×40 water immersion objective (Olympus Optical, Tokyo, Japan), and a CCD 100 camera connected to a HR-120 monochrome monitor (Dage, Stamford, CT).
Recording pipettes (4.5–6 MΩ) were pulled from 1.5-mm outer diameter filamented borosilicate glass with a Flaming/Brown P-97 puller. Pipette solution consisted of (in mM): 120 K-gluconate, 5 MgCl2, 10 HEPES, 3 Na2ATP, 0.3 Na3GTP, 3.0 EGTA, 0.16 CaCl2, 10 di-Tris phosphocreatine, and 0.1% biocytin (pH 7.3, 290–300 mosM). In Cs+-based pipette solution, K-gluconate was replaced by 120 mM Cs-gluconate, and pH was adjusted with CsOH.
Voltage and current patch-clamp recordings were made with a MultiClamp 700A or 700B amplifier (Molecular Devices, Foster City, CA). Voltage or current records were low-pass filtered at 4 kHz and sampled at 10 kHz with a DIGIDATA 1322A 16-bit analog-to-digital converter (Molecular Devices) using Clampex 9.2 (Molecular Devices). Data were analyzed with Clampfit 9.2 (Molecular Devices) and Origin 7.5 or 8.5 (OriginLab, Northampton, MA). Numerical data are expressed as means ± SE. All statistical analyses were performed with Origin 8.5.
For all voltage-clamp experiments, the membrane potential was held at −55 mV unless otherwise stated. From this holding potential, hyperpolarizing and depolarizing voltage steps were applied to determine current characteristics and current-voltage relationship (I-V) plots for each cell. For hyperpolarization-activated cation current (Ih), the instantaneous current measured immediately after the onset of each hyperpolarizing step was subtracted from the mean steady-state current (450–500 ms into the hyperpolarization) to calculate the Ih. Junction potentials were calculated (Nernst equation) as 14.4 mV (Ag/AgCl reference electrode) and 15.1 mV (KCl reference electrode). Recorded potentials were not corrected for junction potential. For the measurement of the 5-HT currents, traces were filtered at 10 Hz, and the means of 5-s baseline before 5-HT (or agonist) application and 5 s during the maximum 5-HT effect were calculated and subtracted to give the magnitude of the 5-HT current. ET cell membrane potential varies continuously during their spontaneous activity, particularly in high-frequency bursting cells. Thus, to assess 5-HT effect on membrane potential, the minimal membrane potential occurring at the end of each burst was measured (Liu and Shipley 2008). Statistical significance of population responses were calculated by using Student's t-test or ANOVA with Bonferroni post hoc comparisons. Significance of individual cell bursting frequency changes was assessed with the Kolmogorov-Smirnov (K-S) test.
Chemical and drug application.
d,l-2-amino-5-phosphonopentanoic acid (AP-5), 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide (NBQX) disodium salt, SR-95531 (gabazine), (2S)-3-[[(1S)-1-(3,4-dichlorophenyl)ethyl]amino-2-hydroxypropyl](phenylmethyl) phosphinicacidhydrochlorid (CGP 55845), TTX citrate, 4-ethylphenylamino-1,2-dimethyl-6-methylaminopyrimidinium (ZD 7288) chloride, 8-[5–2,4-dimethoxy-5-(4-trifluoromethylphenylsulphonamido)phenyl-5-oxopentyl]-1,3,8-triazaspiro[4.5]decane-2,4-dione (RS 102221), 4-(4-fluorobenzoyl)-1-(4-phenylbutyl)piperidine (4F 4PP), 2-aminoethoxydiphenylborane (2-APB), and N-(1-methyl-1H-indolyl-5-yl)-N′′-(3-methyl-5-isothiazolyl)urea (SB 204741) were from Tocris Cookson (Ellisville, MO). N,N,N,N-tetraethylammonium (TEA), 5-HT hydrochloride, α-methyl-5-HT (α-Me-5-HT), nifedipine, mibefradil, and all other chemicals were from Sigma (St. Louis, MO). ω-Conotoxin GVIA and ω-agatoxin IVA were from Alomone Labs. (1R,4aR,11R,12aS,13S,16aS,23R,24aS)-eicosahydro-5H,17H-1,23:11,13-diethano-2H,14H-[1,11]dioxacycloeicosino[2,3-b:12,13-b′]dipyridine (Xestospongin C; XeC) was purchased from A. G. Scientific.
To prevent serotonin oxidation and breakdown, sodium metabisulfite was included in the 5-HT solutions at a concentration of 10:1 sodium metabisulfite:5-HT (Carr et al. 2002) in the initial experiments (Fig. 1, A and B, and Fig. 2). After confirming that sodium metabisulfite was not necessary if 5-HT was prepared freshly, the use of this chemical was discontinued so that vast majority of our data (Fig. 1, C and D, and Figs. 3–6) were collected without sodium metabisulfite. When using BaCl2, the normal ACSF was modified to substitute an equimolar concentration of MgCl2 for MgSO4 to prevent BaSO4 precipitation. All other chemicals were bath-applied.
Fig. 1.
5-HT induces an apparent inward current (I) and reduces membrane resistance (Rm). A: 5-HT induces a dose-dependent apparent inward current in an external tufted (ET) cell voltage-clamped at −55 mV. B: pooled data showing the dose dependence of 5-HT-induced apparent inward current. C: membrane responses to a 5-mV (30-ms duration) hyperpolarizing voltage step in the absence (syn. blockers) and presence of 5-HT (20 μM) show a reduction in membrane resistance by 5-HT. Inset: a blowup of the last 2 ms of steady-state response used for the resistance calculation. D: pooled data showing that 5-HT reduction of membrane resistance is reversed on washout (n = 5; ***P < 0.001 compared with syn. blockers). Syn. blockers represent synaptic transmission blockers including 10 μM NBQX, 50 μM APV, 10 μM gabazine, and 10 μM CGP 55845.
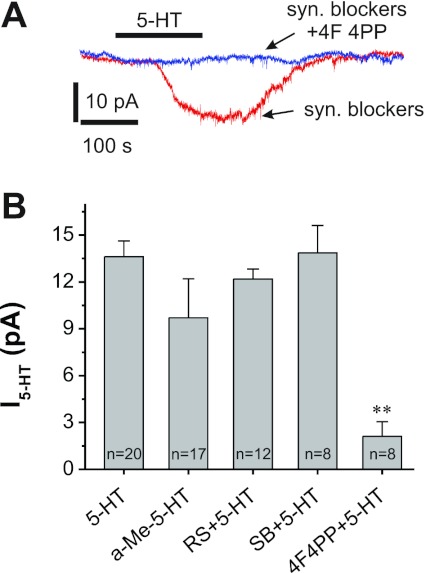
Fig. 2.
5-HT2A receptors mediate the 5-HT-induced apparent inward current (I5-HT). A: traces from an ET cell showing the 5-HT (20 μM)-induced inward current is abolished by 4F 4PP (5 μM), a selective 5-HT2A receptor antagonist. B: pooled data showing α-methyl-5-HT (a-Me-5-HT; 10 μM), a selective 5-HT2 receptor agonist, induces an inward current similar to 5-HT. The antagonist 4F 4PP (5 μM) blocks 5-HT currents (**P < 0.01), whereas the selective 5-HT2B receptor antagonist SB 204741 (SB; 30 μM) and selective 5-HT2C receptor antagonist RS 102221 (RS; 10 μM) have no effect on the 5-HT-induced inward current.
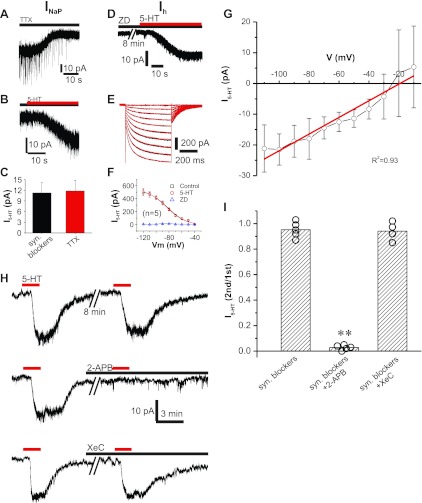
Fig. 3.
5-HT-induced inward current is a nonselective cation current mediated by transient receptor potential (TRP) channels. A: TTX (1 μM) blocks a tonic inward current at −55 mV, indicating that persistent Na+ current (INaP) is active at resting membrane potential (Vm). B: 5-HT (20 μM) induces an inward current in the presence of TTX (1 μM). C: pooled data show that TTX has no effect on 5-HT-induced inward current. D: 5-HT (20 μM) induces an inward current in the presence of ZD 7288 (ZD; 20 μM), a selective Ih channel blocker. E: superimposed traces showing the hyperpolarizing voltage step-activated inward current (Ih) in the absence (black) or the presence (red) of 5-HT (20 μM). F: pooled data show that 5-HT (20 μM) has no effect on Ih. G: population data showing the current-voltage relationship (I-V) of 5-HT-induced inward current (black) with a linear least-squares best fit (red) indicating reversal potential at −19.2 mV. H: repeated exposure to 5-HT (20 μM) induces identical inward currents (top trace). The inward current is abolished by 2-APB (100 μM), a broad-spectrum TRP channel blocker (middle trace). The inositol-1,4,5-trisphosphate (IP3) signaling pathway inhibitor Xestospongin C (XeC; 5 μM) does not alter 5-HT-induced inward current. I: population bar graph showing the 5-HT-induced inward current is blocked by 2-APB (**P < 0.001) but not by XeC.
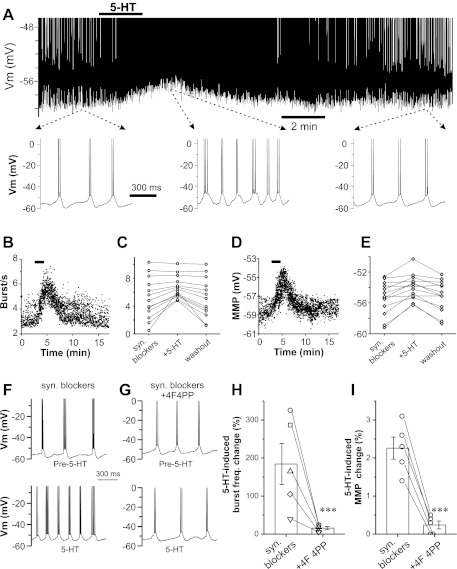
Fig. 4.
5-HT increases spontaneous burst frequency (freq.) via 5-HT2A receptors. A: current-clamp recording from an ET cell showing that 5-HT reversibly produces membrane depolarization and increases spontaneous burst frequency. Bottom traces show an expanded time base before (left), during (middle), and after (right) 5-HT. B: scatterplot showing instantaneous frequency of each burst before and after 5-HT (20 μM). C: population data showing spontaneous bursting frequencies of 13 ET cells before (syn. blockers) and during 5-HT (20 μM) application (5-HT) and following 5-HT washout. D: scatterplot of the minimum membrane potential (MMP) occurring at the end of each burst before and after 5-HT (20 μM). E: population data of 13 ET cells showing the MMP before (syn. blockers) and during 5-HT (20 μM) application and following 5-HT washout. F and G: current-clamp traces showing the 5-HT (20 μM) effects on spontaneous burst frequency in the absence (F; syn. blockers) or presence of 4F 4PP (5 μM; G; syn. Blockers+4F4PP), a selective antagonist of 5-HT2A receptors. H and I: scatterplot showing the 5-HT-induced change in bursting frequency (H) and MMP (I) in 5 ET cells in the absence (syn. blockers) and presence of 4F 4PP (***P < 0.001).
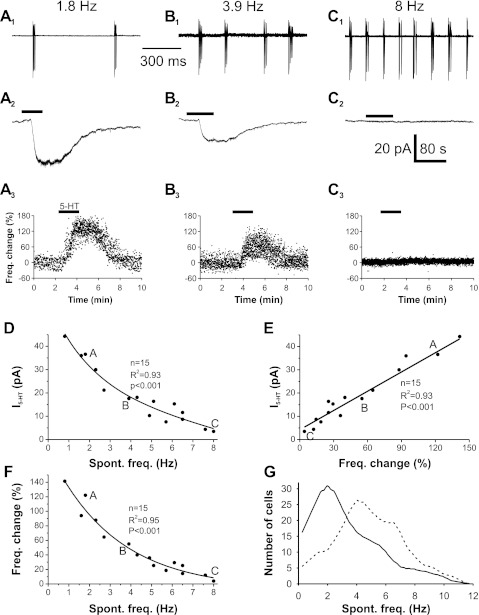
Fig. 5.
5-HT-induced inward current and increase in spontaneous burst frequency negatively correlates with intrinsic bursting frequency. A1, B1, and C1: cell-attached recording of 3 different ET cells exhibiting low (A1), intermediate (B1), and high (C1) spontaneous bursting frequency. A2, B2, and C2: voltage-clamp recording showing 5-HT-induced inward currents in the low- (A2), intermediate- (B2), and high- (C2) frequency bursting cells shown in A1, B1, and C1. A3, B3, and C3: scatterplot of the percentage difference from mean of the instantaneous burst frequency of each burst before, during, and after 5-HT in the same cells shown in A1, B1, and C1. D: scatterplot showing the correlation between 5-HT-induced inward current and cell intrinsic burst frequency. This distribution best fits to a single exponential (r2 = 0.93). “A,” “B,” and “C” represent data from cells shown in A1, B1, and C1, respectively. E: scatterplot of the relationship between 5-HT-induced current and change in bursting frequency. The black line is a least-squares linear best fit (r2 = 0.93) F: scatterplot of the relationship between 5-HT-induced increase in spontaneous (Spont.) burst frequency and the ET cell intrinsic burst frequency. This distribution best fits to a single exponential (r2 = 0.95). G: distribution of spontaneous bursting frequency recorded from a population of 288 ET cells (solid line) and predicted distribution of bursting frequencies from the same population of ET cells in the presence of 5-HT (dashed line) extrapolated with the exponential equation (y = 190.8569e−0.3542x) from F.
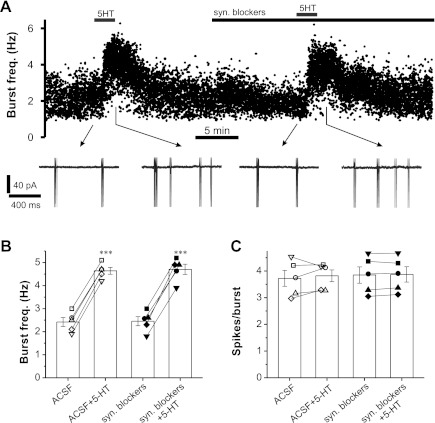
Fig. 6.
5-HT increases spontaneous burst frequency of ET cells in the absence of synaptic transmission blockers. A: scatterplot of spontaneous burst frequency in a representative ET cell showing the 5-HT (20 μM) effects in the absence (1st dose) and presence (2nd dose) of synaptic transmission blockers, including 10 μM NBQX, 50 μM APV, 10 μM gabazine, and 10 μM CGP 55845. B and C: pooled data (n = 5 cells) showing the effect of 5-HT on spontaneous burst frequency (B; ***P < 0.001) and spikes per burst (C) in 5 ET cells in the absence [artificial cerebrospinal fluid (ACSF) + 5-HT] or presence of synaptic transmission blockers (syn. blockers+5-HT). Different symbol shapes represent different cells.
Unless otherwise indicated, synaptic transmission blockers [including 10 μM NBQX, 50 μM dl-2-amino-5-phosphonovaleric acid (APV), 10 μM gabazine, and 10 μM CGP 55845] were present in the ACSF throughout recordings.
RESULTS
5-HT produces a dose-dependent inward current in ET cells.
To investigate potential modulation of ET cells, 5-HT was bath-applied while cells were voltage-clamped at −55 mV in the presence of 10 μM NBQX, 50 μM APV, 10 μM gabazine, and 10 μM CGP 55845 to eliminate circuit effects. As shown in Fig. 1, 5-HT produced an apparent inward current (Fig. 1A). This current persisted in TTX (1 μM), which blocks action potential-dependent circuits, confirming a direct effect of 5-HT on ET cells. The current was dose-dependent over the range tested from 0.2 to 40 μM 5-HT (Fig. 1B).
This current may be due to activation of inward currents, inhibition of outward currents, or both. We used small (5-mV), brief (30-ms) hyperpolarization voltage steps to measure membrane resistance and tested the effect of 5-HT. As shown in Fig. 1C, the hyperpolarizing voltage step evoked a larger steady-state inward current in the presence of 5-HT (20 μM), reflecting a reduction of membrane resistance. Measurement from five cells revealed a 5-HT-induced reversible reduction of membrane resistance from 262.0 ± 15.2 to 217.6 ± 14.7 MΩ (n = 5; P < 0.001; Fig. 1D). This decrease in membrane resistance indicates that 5-HT results in opening of channels passing inward current rather than closing of channels passing outward current.
5-HT-induced inward current is mediated by 5-HT2A receptors.
Serotonin receptors are divided into seven types (5-HT1-5-HT7) based on structural, transduction pathways and pharmacology (Hoyer et al. 2002). 5-HT1, 5-HT2, 5-HT3, and 5-HT5 receptors are present in the OB (McLean et al. 1995; Tecott et al. 1993; Whitaker-Azmitia et al. 1993); 5-HT2 receptors predominate in the glomerular layer (Morilak et al. 1993). To investigate the potential role of 5-HT2 receptors in 5-HT-induced current in ET cells, we tested the effect of the 5-HT2 receptor agonist, α-Me-5-HT (Ismaiel et al. 1990). As shown in Fig. 2B, bath-applied α-Me-5-HT (20 μM) produced an apparent inward current with amplitude of 9.71 ± 2.49 pA (n = 17), which is statistically indistinguishable (P = 0.595) from the 20 μM 5-HT-induced inward current (13.62 ± 1.00 pA, n = 14), indicating that 5-HT2 receptor activation induces currents similar to 5-HT. There are three 5-HT2 subtype receptors: 5-HT2A, 5-HT2B, and 5-HT2C (Hoyer et al. 2002). The 5-HT2C subtype mediates membrane depolarization in a subset of unidentified glomerular neurons (Hardy et al. 2005). Thus we used 5-HT2 subtype-specific antagonists to investigate which 5-HT2 receptor subtypes mediate the 5-HT current in ET cells. After slices were treated with the selective 5-HT2C antagonist RS 102221 (Bonhaus et al. 1997; 10 μM) for 10 min, 5-HT (20 μM) still produced an inward current (Fig. 2B; 12.19 ± 0.64 pA, n = 12) indistinguishable (P = 0.24) from 5-HT alone (13.62 ± 1.00 pA, n = 20). Similar results (P = 0.27 compared with 13.62 ± 1.00 pA in 5-HT alone, n = 20) were observed with a second selective 5-HT2C antagonist, SB 242084 (Kennett et al. 1997; 20 μM, 12.67 ± 0.52 pA, n = 5, data not shown), indicating that 5-HT2C receptors do not mediate the inward current in ET cells. Similarly, the 5-HT current was not affected (P = 0.57 compared with 13.62 ± 1.00 pA in 5-HT alone, n = 20) by the selective 5-HT2B receptor antagonist SB 204741 (Forbes et al. 1995; 30 μM, 13.9 ± 1.8 pA, n = 8). However, the 5-HT current was abolished by the selective 5-HT2A receptor antagonist 4F 4PP (Acuna-Castillo et al. 2002; 5 μM, 2.1 ± 0.9 pA, n = 8, P < 0.001 compared with 13.6 ± 1.0 pA in 5-HT alone, n = 20). These pharmacological results demonstrate that the 5-HT-induced inward current in ET cells is mediated by 5-HT2A receptors.
5-HT induces a nonselective cation current in ET cells.
What is the basis of this 5-HT2A receptor-mediated current in ET cells? Activation of protein G-coupled 5-HT2A receptors leads to production of inositol-1,4,5-trisphosphate (IP3) and diacylglycerol (DAG) via PLC (Hoyer et al. 2002). DAG activates PKC, and IP3 activates calmodulin kinase II (CaMKII) by releasing Ca2+ from endoplasmic reticulum (ER; Hoyer et al. 2002). The downstream targets of these transduction pathways include a number of cellular membrane conductances, e.g., Ih (Bickmeyer et al. 2002), persistent Na+ (INaP) current (Carr et al. 2002), l- and T-type Ca2+ current (Li et al. 2008), and K+ current (Zhang et al. 2008). All of these currents play a role in ET cell spontaneous bursting. Therefore, we next investigated the effect of 5-HT on each of these intrinsic ET cell conductances.
As shown in Fig. 3A, the voltage-gated Na+-channel blocker TTX (1 μM) induced an apparent outward current (19.81 ± 3.49 pA, n = 8) in ET cells held at −55 mV, indicating block of INaP (Hayar et al. 2004b; Liu and Shipley 2008). This is consistent with our previous study showing that INaP in ET cells is active at resting membrane potential (Hayar et al. 2004b; Liu and Shipley 2008). However, TTX did not alter the 5-HT-induced inward current (11.82 ± 3.00 pA in TTX vs. 11.28 ± 2.85 pA in control, n = 8; Fig. 3, B and C), indicating that INaP does not play a role in the 5-HT effect.
ET cells have a prominent Ih that is required for spontaneous bursting. Ih is subject to PKC modulation, and 5-HT has been reported to modulate Ih in hippocampal pyramidal neurons (Bickmeyer et al. 2002) and trigeminal motor neurons (Larkman and Kelly 2001). Therefore, we tested the potential role of Ih in the 5-HT-induced current. As shown in Fig. 3D, in the presence of the selective Ih channel blocker ZD 7288 (20 μM), 5-HT still produced an inward current (14.6 ± 3.1 pA, n = 9) indistinguishable (P = 0.587) from control (13.7 ± 2.9 pA, n = 9) in ET cells held at −55 mV. We further explored this conductance by applying a series of hyperpolarizing voltage steps (500 ms) in cells held at −40 mV. 5-HT (20 μM) had no effect on Ih over the tested range of −50 to −120 mV (Fig. 3, E and F). Taken together, these results indicate that 5-HT does not modulate Ih in ET cells.
As activation of 5-HT2 receptors induces nonselective cation currents in some neurons (Hardy et al. 2005; Stanford and Lacey 1996), we tested whether the 5-HT current in ET cells was a nonselective cation current. Known voltage-activated conductances in ET cells (Liu and Shipley 2008) were pharmacologically blocked or antagonized. With a Cs+-based intracellular solution that blocks most potassium currents, ET cells were identified by bursting activity in the cell-attached configuration before going whole cell. Once whole cell status was achieved, synaptic antagonists (10 μM NBQX, 50 μM APV, 10 μM gabazine, and 10 μM CGP 55845), 1 μM TTX (Na+-channel blocker), 10 mM TEA-Cl (K+-channel blocker), 100 μM ZD 7288 (Ih blocker), 3 mM CsCl (Ih and K+-channel blocker), and 100 μM CdCl2 (high-voltage Ca2+-channel blocker) were added to the bath. Under these conditions, 40 μM 5-HT still produced an inward current of 18.33 ± 4.26 pA (n = 4), which is statistically indistinguishable (P = 0.297) from 40 μM 5-HT in ACSF containing the same synaptic antagonists without Cs+ in the pipette (17.8 ± 3.6 pA, n = 12). This suggests that 5-HT activates a nonselective cation current. To see whether the reversal potential for 5-HT current is consistent with nonselective cation currents, we further blocked the remaining calcium currents with a cocktail of blockers (5 μM nifedipine, 1 μM ω-conotoxin GVIA, 1 μM mibefradil, and 30 nM ω-agatoxin IVA) and 0 mM external Ca2+. Under this condition, we then measured I5-HT for 40 μM 5-HT at different holding potentials (Fig. 3G). Linear I-V curve fitting indicates reversal potential at −19.2 mV (r2 = 0.93, P < 0.001; n = 9; Fig. 3G), consistent with a nonselective cation current (Hardy et al. 2005). In summary, 5-HT induced-currents in the presence of multiple antagonists, blockers, and ion substitutions indicate that activation of 5-HT2A receptors modulate a nonselective cation current.
The 5-HT current in ET cells is mediated by TRP channels.
Transient receptor potential (TRP) channels are a superfamily of nonselective cation channels that play critical roles in nociception, hearing, taste, olfaction, vision, and thermal sensation (Venkatachalam and Montell 2007). TRP channels are divided into two groups: group 1 TRPs including five family members (TRPC, TRPV, TRPM, TRPN, and TRPA) and group 2 including two family members (TRPP and TRPML). In the OB, TRPC1, TRPC3, and TRPC6 are expressed in the glomerular layer (Otsuka et al. 1998), although the cell types expressing these channels and their physiological roles are unknown. We investigated the potential role of TRP channels in the 5-HT-induced nonselective cation current with the broad-spectrum TRP (including TRPC1, TRPC3, TRPC5, TRPC6, TRPV6, TRPM3, TRPM7, TRPM8, and TRPP2) channel blocker 2-APB (100 μM, 10 min; Iwasaki et al. 2001). As shown in Fig. 3, H and I, 2-APB completely abolished the 5-HT-induced inward current, indicating that the current is mediated by TRP channels. In addition to blocking TRP channels, 2-APB also antagonizes IP3 receptors (Iwasaki et al. 2001), which are present in the ER membrane and constitute one of the downstream transduction pathways targeted by activation of G protein-coupled 5-HT2A receptors (Hoyer et al. 2002). Several TRP channels including TRPC1 and TRPC3 can be activated by IP3-induced intracellular Ca2+ release from ER depending on cell types and species (Venkatachalam and Montell 2007). Therefore, we tested whether the 2-APB block of 5-HT current was due to the shutdown of IP3 signal transduction by using XeC, a potent and selective inhibitor of IP3-mediated Ca2+ release from ER (Oka et al. 2002). As shown in Fig. 3, H and I, XeC (5 μM, 10 min) had no effect on the 5-HT inward current. These results indicate that the 5-HT current is mediated by TRP channels that are independent of IP3 signal transduction.
5-HT depolarizes and increases spontaneous bursting frequency in ET cells.
ET cells burst spontaneously. Spontaneous bursting frequency is voltage-dependent, i.e., it is increased by depolarization and decreased by hyperpolarization (Hayar et al. 2004b). As 5-HT produces an inward current in ET cells, it should depolarize ET cells and increase spontaneous burst frequency. To test this, we examined 5-HT effects on spontaneous bursting in ET cells in current-clamp with synaptic blockers NBQX, APV, gabazine, and CGP 55845 in the bath to eliminate circuit effects. As shown in Fig. 4, A–E, 5-HT (20 μM) reversibly and significantly depolarized the membrane and increased spontaneous bursting frequency. The magnitudes of both the membrane depolarization and increased spontaneous burst frequency depended on the intrinsic bursting frequency of the cells: the slower the spontaneous burst frequency, the greater increase in membrane depolarization and burst frequency. Serotonin increased spontaneous bursting frequency only in cells bursting at lower intrinsic bursting frequencies (0.5–6.2 Hz, n = 9, K-S test) and not in cells with higher frequencies (7.1–10.3 Hz, n = 4, K-S test). In the slower bursting cells (range 0.5–6.2 Hz, n = 9; Fig. 4, C and E), 5-HT (20 μM) increased mean burst frequency from 3.6 ± 0.6 to 5.4 ± 0.4 Hz (P < 0.01) and caused a depolarization from −56.1 ± 0.7 to −54.0 ± 0.6 mV (P < 0.001) with no effect on spikes per burst (3.7 ± 0.5 in control compared with 3.6 ± 0.8 in 5-HT, n = 9; P = 0. 341). In faster bursting ET cells (7.1–10.3 Hz, n = 4), 5-HT did not alter burst frequency (8.7 ± 0.7 Hz in control compared with 8.7 ± 0.5 Hz in 5-HT; P = 0.873), membrane potential (−53.8 ± 0.5 to −53.7 ± 0.6 mV; P = 0.275), or spikes per burst (3.2 ± 0.7 to 3.3 ± 0.9; P = 0.218).
As the effect of 5-HT on inward current is mediated by 5-HT2A receptors (Fig. 2), we reasoned that its effect on bursting frequency should be eliminated by 5-HT2A receptor antagonists. To test this, 4F 4PP, a selective 5-HT2A receptor antagonist (which itself showed no effect on spontaneous bursting in each of 8 ET cells with bursting frequency range from 1.2 to 9.5 Hz), was tested on cells with low-to-intermediate bursting frequency (1.2–5.8 Hz, n = 5) that exhibited a significant 5-HT effect (20 μM). As shown in Fig. 4, F–I, 4F 4PP (5 μM) treatment for 10 min completely blocked the 5-HT (20 μM) effects on both bursting frequency (Fig. 4, G and H) and membrane potential (Fig. 4, G and I). This shows that increased frequency of slower bursting ET cells is due to 5-HT2A receptor-mediated depolarizing current.
The previous experiments showed differential effects of 5-HT based on the ET cell intrinsic bursting frequency. This could reflect two populations of ET cells, low intrinsic bursting with strong 5-HT responsiveness and high intrinsic bursting frequency with weak 5-HT responsiveness. Alternatively, ET cells could be a continuous population spanning a range of intrinsic burst frequencies that inversely correlates with the magnitude of the effects of 5-HT. To test this, we correlated 5-HT responses with I5-HT and change in bursting frequency across a population of ET cells with different intrinsic bursting frequencies. In cell-attached mode, the intrinsic bursting frequency of the cell was measured, and the impact of 20 μM 5-HT on bursting was tested. The same cell was then ruptured to whole cell mode, and the 5-HT-induced current was measured. In a low intrinsic bursting frequency cell (1.8 Hz), 5-HT increased spontaneous bursting frequency by 122% and caused a 36.6-pA inward current (Fig. 5, A1–A3). In a cell with an intermediate burst frequency (3.9 Hz), 5-HT induced a smaller (55%) increase in burst frequency and a smaller inward current (17.6 pA; Fig. 5, B1–B3). In a high-frequency (8 Hz) bursting cell (Fig. 5C1), 5-HT had little effect on either bursting frequency or inward current (Fig. 5, C2 and C3). Analysis across a population of 15 ET cells revealed a single distribution of neurons with a negative correlation between 5-HT-induced inward current and intrinsic bursting frequencies of the ET cells (single exponential fit, r2 = 0.93; P < 0.001; Fig. 5D). Since depolarization of ET cells increases bursting, this predicts that the magnitude of the 5-HT-induced inward current should exhibit a positive correlation with the 5-HT-induced change in bursting frequency. Indeed, in the same 15 ET cells, the magnitude of 5-HT inward current highly correlates with burst-frequency change (linear best fit, r2 = 0.93; P < 0.001; Fig. 5E). Thus 5-HT-induced changes in bursting frequency should negatively correlate to the ET cell intrinsic bursting frequency. As shown in Fig. 5F, a negative correlation between 5-HT-induced bursting change and ET cell intrinsic bursting frequency is observed across the 15 ET cells (single exponential fit, r2 = 0.95; P < 0.001; n = 15; Fig. 5F).
Each glomerulus is surrounded by ET cells that spontaneously burst at different intrinsic frequencies ranging from 0.15 to 11 Hz (mean 3.3 ± 0.14, n = 288 cells; Fig. 5G). 5-HT input should shift the ET cell population mean bursting frequency toward higher rates. We calculated the predicted 5-HT-induced change in bursting frequency for each of 288 cells in which we measured intrinsic bursting frequency. This calculation predicts that across the ET cell population, 5-HT shifts the mean bursting frequency of the population from 3.3 ± 0.14 to 4.8 ± 0.14 Hz (P < 0.001; n = 288; Fig. 5G). This suggests that 5-HT inputs from the raphe nuclei allow different behavioral states to modulate the population bursting profile of ET cells and regulate sensory coding at the initial stage of synaptic processing in the olfactory system.
Serotonergic modulation of ET cells and glomerular circuits.
The preceding experiments demonstrate that 5-HT has a direct depolarizing action that increases the burst frequency of ET cells. These actions were observed when ET cells were isolated from circuit effects by pharmacological block of fast neurotransmitters. However, in normal conditions, circuit effects may influence net 5-HT actions on ET cells. For example, ET cells provide monosynaptic excitatory input to approximately ⅔ of all GABAergic PG cells, and PG cells provide inhibitory synaptic feedback to ET cells (Shao et al. 2009). By increasing ET cell burst frequency, 5-HT could increase inhibitory feedback that counteracts its direct excitatory action on ET cells. In addition, serotonin acting via 5-HT2C receptors has been reported to depolarize directly some PG cells (Petzold et al. 2009); if these PG cells synapse back onto ET cells, they might further oppose direct excitatory action on ET cells. Thus both direct and indirect (via ET cells) 5-HT-induced elevation of GABA release from PG cells might override direct enhancement of ET cell bursting. To investigate whether such circuit effects play a role, we tested the action of 5-HT on ET cells first in ACSF and, following washout, retested 5-HT in the presence of fast synaptic blockers. ET cells bursting in the frequency range of 1.9–3.0 Hz were analyzed because the effects of 5-HT are maximal in these slower bursting cells, thus they are most likely to exhibit GABA-mediated feedback counteraction of 5-HT excitation.
As shown in Fig. 6, A and B, 5-HT (20 μM) significantly increased spontaneous burst frequency from 2.4 ± 0.19 to 4.6 ± 0.15 Hz (n = 5; P < 0.001) in normal ACSF lacking synaptic blockers. Following 5-HT washout and addition of fast synaptic blockers, burst frequencies again increased from 2.5 ± 0.44 to 4.7 ± 0.49 Hz (n = 5; P < 0.001) when 5-HT was reapplied. This indicates that, although there may be increased GABA output from elevated ET cell bursting in the presence of 5-HT, the resulting feedback inhibition does not offset the direct excitatory actions of 5-HT on ET cells. A previous study had reported that bath application of the GABAA agonist, isoguvacine, did not significantly influence the bursting frequency of ET cells but did reduce the number of spikes per burst (Hayar and Ennis 2007). However, in our experiments, 5-HT, which would be expected to increase endogenous release of GABA, had no effect on the number of spikes per burst in ET cells (Fig. 6C).
Taken together, the present results indicate that 5-HT modulation increases ET cell output. This should increase excitatory drive on GABAergic PG cells, which, taken with the direct depolarizing action of 5-HT on PG cells, might increase presynaptic inhibition of olfactory sensory input and postsynaptic inhibition of mitral/tufted cells.
DISCUSSION
Centrifugal serotonergic inputs preferentially target the glomerular layer in the OB (McLean and Shipley 1987). The present findings demonstrate that 5-HT has a direct action on ET cells. Specifically, 5-HT activates a TRP channel-mediated, nonselective cation current via 5-HT2A receptors. The resulting depolarization increases spontaneous burst frequency in ET cells. Intriguingly, the strength of this 5-HT action is inversely proportional to the intrinsic burst frequency of a given ET cell, i.e., 5-HT produces a greater depolarization and a larger increase in burst frequency in slower than faster bursting ET cells. As a result, 5-HT causes the population of ET cells to burst in a higher frequency range. As ET cells receive monosynaptic sensory input and provide excitatory input to mitral cells and most glomerular inhibitory circuits (De Saint Jan et al. 2009; Hayar et al. 2004a; Shao et al. 2009; Wachowiak and Shipley 2006), 5-HT can potently influence sensory signaling at the first stage of odor processing in the brain.
Serotonin produces a variety of cellular and circuit actions in the brain through different 5-HT receptor subtypes (Jacobs and Azmitia 1992). Several 5-HT receptor subtypes are present in the OB (McLean et al. 1995; Petzold et al. 2009; Tecott et al. 1993). 5-HT2A receptor mRNA and protein are heavily expressed in the glomerular layer (Morilak et al. 1993), and activation of 5-HT2A receptors mediates excitation in unidentified glomerular neurons and mitral cells (Hardy et al. 2005). By contrast, in vivo studies have reported that 5-HT state-dependently suppresses spontaneous firing in mitral cells (Bloom et al. 1964; Von Baumgarten et al. 1963). Thus there is a seeming paradox: direct activation of 5-HT2A receptors on mitral cells produces excitation, but at the circuit level 5-HT appears to inhibit mitral cells. This might be due to a direct excitatory action of 5-HT on mitral cells and indirect inhibition via activation of OB GABAergic interneurons. Consistent with this possibility, Petzold et al. (2009) recently reported that serotonin excites some GABAergic PG neurons via 5-HT2C receptors. The present findings demonstrate that 5-HT directly excites glutamatergic ET cells. ET cells provide strong monosynaptic excitatory drive to the majority of GABAergic PG cells (Shao et al. 2009), which generate postsynaptic inhibition of mitral cells. The direct excitatory effect of 5-HT on ET cells was not influenced by feedback inhibition despite the reported direct excitation of 5-HT on PG cells and the increased ET cell drive on PG cells (Hardy et al. 2005; Hayar et al. 2004a; Petzold et al. 2009). This is consistent with a previous report that ET cell bursting is not significantly altered by GABAA receptor antagonists (Hayar and Ennis 2007). It remains to be seen whether the direct excitation of PG cells via 5-HT and indirect excitation via ET cells generate circuit level inhibition sufficient to generate net inhibition of mitral cells.
Ionic mechanisms of 5-HT2A action on ET cells.
Serotonin produced an inward current in ET cells pharmacologically isolated from known circuit effects. As 5-HT modulates most of the known ET cell voltage-gated conductances in other brain neurons, we tested its effect on these conductances. The 5-HT-mediated inward current persisted when each of these conductances was blocked. Further experiments disclosed that 5-HT activated a nonselective cation current. Nonselective cation currents are linked to 5-HT2 receptors in other brain areas including neurons in the substantia nigra pars reticulata, rostral ventrolateral medulla, and possibly OB mitral cells (Hardy et al. 2005; Stanford and Lacey 1996). Additional experiments indicate that the 5-HT-induced nonselective cation current in ET cells is mediated by TRP channels, specifically those that are independent of the IP3 signaling pathway. Consistent with this, mRNA encoding multiple TRP channels (TRPC1, TRPC3, and TRPC6) is present in glomerular neurons (Otsuka et al. 1998).
Functional impact of 5-HT on glomerular circuits.
As ET cells receive monosynaptic sensory input, the depolarizing action of 5-HT could enhance ET cell sensitivity to weak sensory inputs by depolarizing the membrane potential closer to spike threshold. ET cells in the same glomerulus are electrically coupled, and their bursting is highly coordinated (Gire and Schoppa 2009; Hayar et al. 2005); thus 5-HT could increase the overall sensitivity of the glomerulus to sensory inputs. This is consistent with a report showing that serotonin increased sensitivity in a Bombyx mori moth glomerulus that responds to female pheromones (Gatellier et al. 2004).
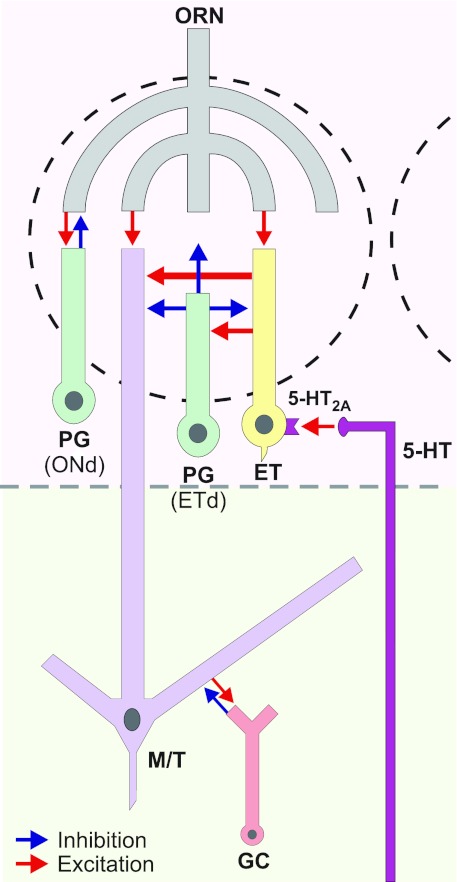
Alternatively, serotonin may attenuate the impact of sensory inputs as it does in the visual and auditory systems (Chen and Regehr 2003; Hurley et al. 2004; Rhoades et al. 1994). Spontaneous ET cell bursting provides tonic excitatory drive on PG cells, causing GABA release that generates tonic presynaptic inhibition of OSN terminals and tonic postsynaptic inhibition of mitral cells (Pirez and Wachowiak 2008; Shao et al. 2009). By enhancing ET cell bursting, 5-HT may increase excitatory drive on PG cells, further augmenting spontaneous GABA release and increasing tonic postsynaptic inhibition of mitral cells as well as presynaptic inhibition of sensory terminals (Fig. 7). ET cells also provide monosynaptic excitatory input to SA cells (Shao et al. 2009) that lead to inhibition of mitral cells in distant glomeruli (Aungst et al. 2003). Thus 5-HT may enhance inter- as well as intraglomerular inhibition.
Fig. 7.
Schematic diagram shows the 5-HT action on ET cells and its impact on the glomerular circuitry. Serotonin released from centrifugal fibers activates 5-HT2A receptors on ET cells. This excitation leads to increased ET cell bursting and enhancement of ET cell output to GABAergic periglomerular (PG) cells and mitral/tufted (M/T) cells. This may increase presynaptic inhibition of olfactory receptor neuron (ORN) terminals and postsynaptic inhibition of M/T cells. Previous studies (Shao et al. 2009) show that there are 2 classes of PG cells: ⅔ are driven most effectively by ET cells (ETd, ET cell-driven), and ⅓ are driven by monosynaptic olfactory nerve input (ONd, olfactory nerve-driven). GC, granule cell.
Could serotonin coordinate ET cells with sniffing?
An intriguing finding is that the magnitude of the action of 5-HT varies inversely with the intrinsic burst frequency in ET cells. Serotonin produces a stronger depolarization and a larger increase in bursting frequency in slow- than in fast-bursting ET cells. This differential cellular effect may reflect different levels of 5-HT innervation, 5-HT2A receptors, or TRP channels (or some combination of these) that are inversely related to the intrinsic burst frequencies of ET cells. The normal variation in the intrinsic burst frequency for each ET cell is narrow (Hayar et al. 2004b), but across the ET cell population the spectrum of intrinsic burst frequencies spans from 0.15 to 11 Hz (Fig. 5G; Hayar et al. 2004b), similar to spectrum of sniffing frequencies in rodents (Verhagen et al. 2007).
Serotonergic neurons in the raphe nuclei exhibit behavior-dependent firing. Phasic and repetitive patterns of auditory and visual stimuli excite dorsal raphe neurons followed by a period of inhibition (Heym et al. 1982). Activation of raphe neurons is associated with increased motor activity, especially in the repetitive or central pattern generator mode, such as chewing and biting, licking, grooming, and whisking of the vibrissae (Jacobs and Azmitia 1992). Serotonin modulates neurons in brainstem respiratory centers (Depuy et al. 2011; Ptak et al. 2009). It is not known whether increased sniffing frequency is associated with increased activity of 5-HT raphe neurons, but sniffing is associated with elevated 5-HT level in mouse hippocampus, lateral septum, and caudate putamen, which receive inputs from the dorsal and medial raphe nuclei (Beekman et al. 2005). Rodents respond to novel odors with high-frequency sniffing (5–10 Hz; Adrian 1950; Verhagen et al. 2007; Welker 1964). By coordinately acting on respiratory centers and shifting the frequency spectrum of ET cell spontaneous bursting to a higher level, serotonin might synchronize ET cells with exploratory sniffing to optimize glomerular activity to sniff frequencies that drive phasic olfactory input. In vivo experiments are needed to test this prediction.
GRANTS
Research was funded by NIH National Institute on Deafness and Other Communication Disorders (NIDCD) Grant 5-R01-DC-005676-13.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.L., J.L.A., and M.T.S. conception and design of research; S.L. and J.L.A. performed experiments; S.L. and J.L.A. analyzed data; S.L. and J.L.A. interpreted results of experiments; S.L., J.L.A., and A.C.P. prepared figures; S.L. drafted manuscript; S.L., A.C.P., and M.T.S. edited and revised manuscript; S.L., J.L.A., A.C.P., and M.T.S. approved final version of manuscript.
ACKNOWLEDGMENTS
Present address of J. L. Aungst: Food and Drug Administration, Riverdale, MD 20770 (e-mail: jason.aungst@fda.hhs.gov).
REFERENCES
- Acuna-Castillo C, Villalobos C, Moya PR, Saez P, Cassels BK, Huidobro-Toro JP. Differences in potency and efficacy of a series of phenylisopropylamine/phenylethylamine pairs at 5-HT(2A) and 5-HT(2C) receptors. Br J Pharmacol 136: 510–519, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian ED. The electrical activity of the mammalian olfactory bulb. Electroencephalogr Clin Neurophysiol 2: 377–388, 1950 [DOI] [PubMed] [Google Scholar]
- Antal M, Eyre M, Finklea B, Nusser Z. External tufted cells in the main olfactory bulb form two distinct subpopulations. Eur J Neurosci 24: 1124–1136, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aungst JL, Heyward PM, Puche AC, Karnup SV, Hayar A, Szabo G, Shipley MT. Centre-surround inhibition among olfactory bulb glomeruli. Nature 426: 623–629, 2003 [DOI] [PubMed] [Google Scholar]
- Beekman M, Flachskamm C, Linthorst AC. Effects of exposure to a predator on behaviour and serotonergic neurotransmission in different brain regions of C57bl/6N mice. Eur J Neurosci 21: 2825–2836, 2005 [DOI] [PubMed] [Google Scholar]
- Bickmeyer U, Heine M, Manzke T, Richter DW. Differential modulation of I(h) by 5-HT receptors in mouse CA1 hippocampal neurons. Eur J Neurosci 16: 209–218, 2002 [DOI] [PubMed] [Google Scholar]
- Bloom FE, Costa E, Salmoiraghi GC. Analysis of individual rabbit olfactory bulb neuron responses to the microelectrophoresis of acetylcholine, norepinephrine and serotonin synergists and antagonists. J Pharmacol Exp Ther 146: 16–23, 1964 [PubMed] [Google Scholar]
- Bonhaus DW, Weinhardt KK, Taylor M, DeSouza A, McNeeley PM, Szczepanski K, Fontana DJ, Trinh J, Rocha CL, Dawson MW, Flippin LA, Eglen RM. RS-102221: a novel high affinity and selective, 5-HT2C receptor antagonist. Neuropharmacology 36: 621–629, 1997 [DOI] [PubMed] [Google Scholar]
- Carr DB, Cooper DC, Ulrich SL, Spruston N, Surmeier DJ. Serotonin receptor activation inhibits sodium current and dendritic excitability in prefrontal cortex via a protein kinase C-dependent mechanism. J Neurosci 22: 6846–6855, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Regehr WG. Presynaptic modulation of the retinogeniculate synapse. J Neurosci 23: 3130–3135, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Saint Jan D, Hirnet D, Westbrook GL, Charpak S. External tufted cells drive the output of olfactory bulb glomeruli. J Neurosci 29: 2043–2052, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depuy SD, Kanbar R, Coates MB, Stornetta RL, Guyenet PG. Control of breathing by raphe obscurus serotonergic neurons in mice. J Neurosci 31: 1981–1990, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes IT, Jones GE, Murphy OE, Holland V, Baxter GS. N-(1-methyl-5-indolyl)-N′-(3-methyl-5-isothiazolyl)urea: a novel, high-affinity 5-HT2B receptor antagonist. J Med Chem 38: 855–857, 1995 [DOI] [PubMed] [Google Scholar]
- Gatellier L, Nagao T, Kanzaki R. Serotonin modifies the sensitivity of the male silkmoth to pheromone. J Exp Biol 207: 2487–2496, 2004 [DOI] [PubMed] [Google Scholar]
- Gire DH, Schoppa NE. Control of on/off glomerular signaling by a local GABAergic microcircuit in the olfactory bulb. J Neurosci 29: 13454–13464, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy A, Palouzier-Paulignan B, Duchamp A, Royet JP, Duchamp-Viret P. 5-Hydroxytryptamine action in the rat olfactory bulb: in vitro electrophysiological patch-clamp recordings of juxtaglomerular and mitral cells. Neuroscience 131: 717–731, 2005 [DOI] [PubMed] [Google Scholar]
- Hayar A, Ennis M. Endogenous GABA and glutamate finely tune the bursting of olfactory bulb external tufted cells. J Neurophysiol 98: 1052–1056, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayar A, Karnup S, Ennis M, Shipley MT. External tufted cells: a major excitatory element that coordinates glomerular activity. J Neurosci 24: 6676–6685, 2004a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayar A, Karnup S, Shipley MT, Ennis M. Olfactory bulb glomeruli: external tufted cells intrinsically burst at theta frequency and are entrained by patterned olfactory input. J Neurosci 24: 1190–1199, 2004b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayar A, Shipley MT, Ennis M. Olfactory bulb external tufted cells are synchronized by multiple intraglomerular mechanisms. J Neurosci 25: 8197–8208, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heym J, Trulson ME, Jacobs BL. Raphe unit activity in freely moving cats: effects of phasic auditory and visual stimuli. Brain Res 232: 29–39, 1982 [DOI] [PubMed] [Google Scholar]
- Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav 71: 533–554, 2002 [DOI] [PubMed] [Google Scholar]
- Hurley LM, Devilbiss DM, Waterhouse BD. A matter of focus: monoaminergic modulation of stimulus coding in mammalian sensory networks. Curr Opin Neurobiol 14: 488–495, 2004 [DOI] [PubMed] [Google Scholar]
- Ismaiel AM, Titeler M, Miller KJ, Smith TS, Glennon RA. 5-HT1 and 5-HT2 binding profiles of the serotonergic agents alpha-methylserotonin and 2-methylserotonin. J Med Chem 33: 755–758, 1990 [DOI] [PubMed] [Google Scholar]
- Iwasaki H, Mori Y, Hara Y, Uchida K, Zhou H, Mikoshiba K. 2-Aminoethoxydiphenyl borate (2-APB) inhibits capacitative calcium entry independently of the function of inositol 1,4,5-trisphosphate receptors. Receptors Channels 7: 429–439, 2001 [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev 72: 165–229, 1992 [DOI] [PubMed] [Google Scholar]
- Jones BJ, Blackburn TP. The medical benefit of 5-HT research. Pharmacol Biochem Behav 71: 555–568, 2002 [DOI] [PubMed] [Google Scholar]
- Kennett GA, Wood MD, Bright F, Trail B, Riley G, Holland V, Avenell KY, Stean T, Upton N, Bromidge S, Forbes IT, Brown AM, Middlemiss DN, Blackburn TP. SB 242084, a selective and brain penetrant 5-HT2C receptor antagonist. Neuropharmacology 36: 609–620, 1997 [DOI] [PubMed] [Google Scholar]
- Kiyokage E, Pan YZ, Shao Z, Kobayashi K, Szabo G, Yanagawa Y, Obata K, Okano H, Toida K, Puche AC, Shipley MT. Molecular identity of periglomerular and short axon cells. J Neurosci 30: 1185–1196, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkman PM, Kelly JS. Modulation of the hyperpolarisation-activated current, Ih, in rat facial motoneurones in vitro by ZD-7288. Neuropharmacology 40: 1058–1072, 2001 [DOI] [PubMed] [Google Scholar]
- Li S, Geiger JD, Lei S. Neurotensin enhances GABAergic activity in rat hippocampus CA1 region by modulating l-type calcium channels. J Neurophysiol 99: 2134–2143, 2008 [DOI] [PubMed] [Google Scholar]
- Liu S, Shipley MT. Multiple conductances cooperatively regulate spontaneous bursting in mouse olfactory bulb external tufted cells. J Neurosci 28: 1625–1639, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrides F, Schneider SP. Laminar organization of mitral and tufted cells in the main olfactory bulb of the adult hamster. J Comp Neurol 208: 419–430, 1982 [DOI] [PubMed] [Google Scholar]
- McLean JH, Darby-King A, Paterno GD. Localization of 5-HT2A receptor mRNA by in situ hybridization in the olfactory bulb of the postnatal rat. J Comp Neurol 353: 371–378, 1995 [DOI] [PubMed] [Google Scholar]
- McLean JH, Shipley MT. Serotonergic afferents to the rat olfactory bulb: I. Origins and laminar specificity of serotonergic inputs in the adult rat. J Neurosci 7: 3016–3028, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuiston AR, Katz LC. Electrophysiology of interneurons in the glomerular layer of the rat olfactory bulb. J Neurophysiol 86: 1899–1907, 2001 [DOI] [PubMed] [Google Scholar]
- Morilak DA, Garlow SJ, Ciaranello RD. Immunocytochemical localization and description of neurons expressing serotonin2 receptors in the rat brain. Neuroscience 54: 701–717, 1993 [DOI] [PubMed] [Google Scholar]
- Oka T, Sato K, Hori M, Ozaki H, Karaki H. Xestospongin C, a novel blocker of IP3 receptor, attenuates the increase in cytosolic calcium level and degranulation that is induced by antigen in RBL-2H3 mast cells. J Pharmacol 135: 1959–1966, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka Y, Sakagami H, Owada Y, Kondo H. Differential localization of mRNAs for mammalian trps, presumptive capacitative calcium entry channels, in the adult mouse brain. Tohoku J Exp Med 185: 139–146, 1998 [DOI] [PubMed] [Google Scholar]
- Petzold GC, Hagiwara A, Murthy VN. Serotonergic modulation of odor input to the mammalian olfactory bulb. Nat Neurosci 12: 784–791, 2009 [DOI] [PubMed] [Google Scholar]
- Pinching AJ. Synaptic connexions in the glomerular layer of the olfactory bulb. J Physiol 210: 14P–15P, 1970 [PubMed] [Google Scholar]
- Pinching AJ, Powell TP. The neuron types of the glomerular layer of the olfactory bulb. J Cell Sci 9: 305–345, 1971a [DOI] [PubMed] [Google Scholar]
- Pinching AJ, Powell TP. The neuropil of the periglomerular region of the olfactory bulb. J Cell Sci 9: 379–409, 1971b [DOI] [PubMed] [Google Scholar]
- Pirez N, Wachowiak M. In vivo modulation of sensory input to the olfactory bulb by tonic and activity-dependent presynaptic inhibition of receptor neurons. J Neurosci 28: 6360–6371, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Powell TP. The mitral and short axon cells of the olfactory bulb. J Cell Sci 7: 631–651, 1970 [DOI] [PubMed] [Google Scholar]
- Ptak K, Yamanishi T, Aungst J, Milescu LS, Zhang R, Richerson GB, Smith JC. Raphe neurons stimulate respiratory circuit activity by multiple mechanisms via endogenously released serotonin and substance P. J Neurosci 29: 3720–3737, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramón y Cajal S. Histologie du Système Nerveux de l'Homme et des Vertébrés. Paris: A. Maloine, 1911 [Google Scholar]
- Rhoades RW, Bennett-Clarke CA, Shi MY, Mooney RD. Effects of 5-HT on thalamocortical synaptic transmission in the developing rat. J Neurophysiol 72: 2438–2450, 1994 [DOI] [PubMed] [Google Scholar]
- Shao Z, Puche AC, Kiyokage E, Szabo G, Shipley MT. Two GABAergic intraglomerular circuits differentially regulate tonic and phasic presynaptic inhibition of olfactory nerve terminals. J Neurophysiol 101: 1988–2001, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford IM, Lacey MG. Differential actions of serotonin, mediated by 5-HT1B and 5-HT2C receptors, on GABA-mediated synaptic input to rat substantia nigra pars reticulata neurons in vitro. J Neurosci 16: 7566–7573, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecott LH, Maricq AV, Julius D. Nervous system distribution of the serotonin 5-HT3 receptor mRNA. Proc Natl Acad Sci USA 90: 1430–1434, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem 76: 387–417, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhagen JV, Wesson DW, Netoff TI, White JA, Wachowiak M. Sniffing controls an adaptive filter of sensory input to the olfactory bulb. Nat Neurosci 10: 631–639, 2007 [DOI] [PubMed] [Google Scholar]
- Von Baumgarten R, Bloom FE, Oliver AP, Salmoiraghi GC. Response of individual olfactory nerve cells to microelectrophoretically administered chemical substances. Pflügers Arch 277: 125–140, 1963 [Google Scholar]
- Wachowiak M, Shipley MT. Coding and synaptic processing of sensory information in the glomerular layer of the olfactory bulb. Semin Cell Dev Biol 17: 411–423, 2006 [DOI] [PubMed] [Google Scholar]
- Welker WI. Analysis of sniffing of the albino rat. Behaviour 22: 223–244, 1964 [Google Scholar]
- Whitaker-Azmitia PM, Clarke C, Azmitia EC. Localization of 5-HT1A receptors to astroglial cells in adult rats: implications for neuronal-glial interactions and psychoactive drug mechanism of action. Synapse 14: 201–205, 1993 [DOI] [PubMed] [Google Scholar]
- Zhang P, Yang C, Delay RJ. Urine stimulation activates BK channels in mouse vomeronasal neurons. J Neurophysiol 100: 1824–1834, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Xiong W, Masurkar AV, Chen WR, Shepherd GM. Dendritic calcium plateau potentials modulate input-output properties of juxtaglomerular cells in the rat olfactory bulb. J Neurophysiol 96: 2354–2363, 2006 [DOI] [PubMed] [Google Scholar]