Abstract
Riluzole is the only FDA-approved drug to treat amyotrophic lateral sclerosis, but its long-term effects on motoneurons are unknown. Therefore, we treated primary mouse spinal cord cultures with 2 μM riluzole for 4–9 days and then used whole cell patch clamp to record the passive and active properties of both wild-type and SOD1G93A motoneurons. At this concentration, riluzole blocks >50% of the sodium component of a persistent inward current that plays a major role in determining motoneuron excitability. Prolonged riluzole treatment significantly decreased the amplitude of the persistent inward current. This effect was specific for SOD1G93A motoneurons, where the amplitude decreased by 55.4%. In addition, prolonged treatment hyperpolarized the resting membrane potential as well as the voltage onset and voltage maximum of the persistent inward current (∼2–3 mV in each case). These effects appeared to offset one another and resulted in no change in the firing properties. In a subset of cells, acute reapplication of 2 μM riluzole during the recording decreased repetitive firing and the persistent inward current, which is consistent with the normal effects of riluzole. The downregulation of the persistent inward current in response to prolonged riluzole administration is in contrast to the strong upregulation of this same current after descending neuromodulatory drive to the cord is lost following spinal injury. This dichotomy suggests that decreased activation of G protein-coupled pathways can induce upregulation in the persistent inward current but that direct channel block is ineffective.
Keywords: motoneuron excitability, amyotrophic lateral sclerosis, superoxide dismutase 1
amyotrophic lateral sclerosis (ALS) is a multifactorial disease that prematurely kills motoneurons by both cell autonomous and nonautonomous mechanisms (Boillee et al. 2006; Ilieva et al. 2009). Riluzole, the only FDA-approved drug to treat ALS, extends survival by 2–3 mo but does not confer lasting protection (Bensimon et al. 1994; Lacomblez et al. 1996; Traynor et al. 2003). The effects of acute riluzole administration in animal preparations at therapeutically relevant doses include decreasing persistent voltage-gated sodium currents, potentiation of a calcium-dependent potassium current, and inhibition of neurotransmitter release (Bellingham 2011). These effects all act to decrease motoneuron excitability, and thus riluzole may act by limiting excitotoxicity (Bellingham 2011; Van Damme et al. 2005; Van Den Bosch et al. 2006).
It is unclear, however, how or if motoneurons adapt when riluzole is present for days or weeks during treatment. In other dissociated culture systems, decreases in network excitability lead to compensatory synaptic scaling (Ehlers 2003; Turrigiano et al. 1998) or the appearance of intrinsic bursting (Turrigiano et al. 1995). Furthermore, in spinal cord-injured (SCI) rats, motoneurons initially have diminished excitability due to loss of the descending monoaminergic input that is essential for facilitating the motoneuron persistent inward current (PIC) (Harvey et al. 2006b). With time, however, both the sodium and calcium components of the PIC (NaPIC and CaPIC, respectively) are reestablished and motoneuron excitability increases (Harvey et al. 2006b; Li and Bennett 2003). Therefore, we hypothesized that, although acute application of riluzole decreases motoneuron excitability, prolonged treatment with 2 μM riluzole may lead to compensatory mechanisms that reestablish motoneuron excitability. An important consideration, however, is riluzole's mechanism of action. Riluzole suppresses the NaPIC by binding directly to the sodium channel (Benoit and Escande 1991; Hebert et al. 1994), whereas the loss of monoaminergic input stops PIC facilitation via a failure of the monoamines to activate G protein-coupled receptors. The ability for the PIC to adapt during direct channel block versus loss of monoaminergic input may therefore be very different.
We investigated how prolonged exposure to riluzole affects motoneuron NaPICs and other intrinsic properties. If the PIC is very plastic and adapts whenever it is inhibited, we would expect to see an upregulation of the current after prolonged riluzole exposure, which could be responsible for the loss of riluzole's therapeutic action in ALS. Our results however, showed that the PIC amplitude remained the same [wild type (wt)] or decreased (SOD1G93A) after prolonged riluzole administration, suggesting that the NaPIC's capacity for adaptation may be largely confined to its neuromodulatory control system via G protein-coupled receptors and that direct channel block is not effective.
MATERIALS AND METHODS
All procedures were approved by the Northwestern University Animal Care and Use Committee.
Mice.
All mice were bred and maintained in barrier facilities. Nontransgenic female mice were mated with transgenic males expressing the human superoxide dismutase 1 protein with a glycine to alanine mutation at codon 93 (SOD1) (high expressor line). Embryos were genotyped for SOD1 expression with standard PCR protocols after data collection and analysis (Rosen et al. 1993). Briefly, 20–25 mg of tissue was used for the DNA extraction. The primers for amplification were SOD1P7: CAT CAG CCC TAA TCC ATC TGA and SOD1P8: CGC GAC TAA CAA TCA AAG TGA. All breeding, maintenance, and genotyping were done by the Siddique laboratory.
Cell culture.
Spinal cords were removed from embryonic day 12–14 mice and dissociated as previously described (Kuo et al. 2005). Dissociated cells were plated on glass coverslips coated with poly-d-lysine (Sigma, St. Louis, MO). The basic medium contained Neurobasal A medium (Invitrogen, Carlsbad, CA), 2 ml of B27 supplement (Invitrogen), 1 mM l-glutamine (Sigma), 1 ml of penicillin-streptomycin (Invitrogen), and 2 mg/ml glucose (Sigma). The plating medium contained the basic medium plus 15% heat-inactivated horse serum. The plating medium was changed after 2 days to the maintenance medium, which contained the basic medium plus 20 ng/ml nerve growth factor (Invitrogen). The maintenance medium was changed every 2–3 days. In the riluzole drug condition, 2 μM riluzole, dissolved in DMSO (final concentration 0.005%), was added to the maintenance medium after 7 days in culture. Riluzole is resistant to degradation in extreme heat and changes in pH. It is susceptible to oxidation and was degraded by 20% after 12 h in 1% H2O2, but we view this as a very extreme condition not encountered during this study (Kumari et al. 2009). Cells were recorded from between 12 and 16 days in culture. Vehicle control cells treated only with DMSO (N = 16) showed no significant differences and were included in the control group.
Recording solutions.
Electrodes were between 3 and 5 MΩ in resistance and contained (mM) 145 K-gluconate, 0.1 CaCl2, 1.1 EGTA, 5.0 HEPES, 2.0 MgCl2, and 5.0 ATP-Mg2+ with a pH of 7.3 (all from Sigma). Artificial cerebrospinal fluid (aCSF) contained (mM) 126 NaCl, 26.2 NaHCO3, 1.0 NaH2PO4, 3.0 KCl, 1.5 MgSO4, 2.5 CaCl2, and 10 glucose. In all experiments 0.1 mM picrotoxin (Sigma), 0.01 mM strychnine (Sigma), and either 0.01 mM 2,3-dioxo-6-nitro-1,2,3,4,-tetrahydrobenzo[f]quinoxaline-7-sulfonamide disodium salt (NBQX) (Tocris, Ellisville, MO) and 0.05 mM d-(−)-2-amino-5-phosphonopentanoic acid (AP5) (Tocris) or 1.0 mM kynurenic acid (Sigma) were added to block synaptic currents. The aCSF was adjusted to a pH of 7.4 when bubbled with 95% O2-5% CO2. To block sodium channels, TTX was added to the aCSF as a 1 μM solution. In most experiments the aCSF did not contain riluzole. However, in some experiments riluzole was added to the aCSF as a 2 μM solution after all other measurements were recorded. All aCSF solutions were bath applied.
Whole cell recordings.
Cells were transferred from the incubator to a recording chamber, and residual maintenance medium was washed out with riluzole-free aCSF for a minimum of 15 min before recording started. Selected neurons were large (>20-μm diameter), multipolar, and had triangular cell bodies. Neurons identified by these selection criteria are consistently labeled by SMI-32, peripherin, acetylcholine, choline acetyltransferase, and calcitonin gene-related peptide, all markers of motoneurons in vivo (Carriedo et al. 1995, 1996). These criteria preferentially include large motoneurons while excluding small motoneurons. In the spinal cord, motoneurons are by far the largest neurons in vivo; nevertheless, it is possible that a small percentage of large interneurons are included in the sample. The exact developmental stage of these neurons is not known; however, they are thought to be relatively immature since they have not yet developed the CaPIC present in the late neonatal stage (Quinlan et al. 2011). Recordings were made at room temperature with the Multiclamp 700B amplifier (Axon Instruments, Union City, CA). In voltage-clamp mode, fast, slow, and whole cell capacitance transients were compensated for with the capacitance compensation in Multiclamp. Cells were discarded if the initial action potential (AP) overshoot was less than 20 mV, if the resting membrane potential (RMP) was greater than −40 mV or changed more than 5 mV, if access resistance was greater than 30 MΩ, or if input conductance changed more than 30%.
For riluzole to affect the cultured neurons, spontaneous activity must be present in the culture system. Therefore, in a number of experiments, spontaneous firing was recorded in aCSF without synaptic blockers. aCSF with synaptic blockers was used for all subsequent measures. In current-clamp mode, triangular current ramps were used to elicit repetitive firing (10 s total duration) and to produce firing frequency-current (FI) relationships. Depolarizing current steps were also used in some cells to determine the effects on firing behavior when riluzole was added to the aCSF. In voltage-clamp mode, current-voltage (IV) relationships were assessed with very slow triangular voltage ramps (9.4 mV/s) from −90 to −10 mV.
At the end of some whole cell recordings (N = 2), it was verified that riluzole could be washed out during the initial 15-min washout period at the beginning of the experiment. First, 2 μM riluzole was added to the aCSF, and firing was abolished or decreased to steady levels. The riluzole was then washed out of the recording chamber with normal aCSF, and the time until normal firing returned was recorded.
Data analysis.
All data were collected at 20 kHz with a Power1401 (Cambridge Electronics Design). Cell capacitance was measured with the Multiclamp automatic whole cell capacitance compensation. All other data were analyzed off-line with Signal 4.01 software (Cambridge Electronics Design) with custom scripts. Measurements were determined from the average of four traces. In current-clamp mode, AP overshoot (above 0 mV) and voltage and current recruitment thresholds were measured for the first AP elicited during a triangular current ramp. Voltage threshold was defined as the point when the voltage rate of rise exceeded 10 V/s. Current threshold was defined as the current input at the voltage threshold.
The FI relationship was obtained by plotting the instantaneous firing frequencies versus the intensity of the injected current during the ascending and descending portions of the current ramp. The ascending and descending FI gain were determined from the slope of the FI relationship. The difference between the current at AP recruitment (Ion) and derecruitment (Ioff) (ΔI = Ion − Ioff) was also measured.
In voltage-clamp mode, the input (leak) conductance was determined from the slope of the linear portion of the IV relationship taken between −80 and −70 mV. All other measurements were taken after leak subtraction. The PIC voltage onset, amplitude, and max voltage were measured on the ascending phase of the voltage ramp. Voltage onset was defined as the first negative current deflection. PIC amplitude was the maximum negative current, and the PIC max voltage was the voltage at this point.
The PIC is the result of an interaction between a voltage-gated sodium-based persistent inward current and TTX-insensitive outward currents. To determine how the sodium persistent inward current (NaPIC) and the TTX-insensitive current contribute to the PIC amplitude, 1 μM TTX was added to block voltage-gated sodium channels. The resulting IV relationship was labeled TTX insensitive (TTX-ins). After leak subtraction, the voltage onset of the TTX-ins outward current as well as its amplitude at the PIC max voltage were measured. The TTX-ins amplitude was measured at the PIC max voltage to determine how the TTX-ins current affected the PIC amplitude. The NaPIC voltage onset, amplitude, and max voltage could then be determined after subtracting the TTX-ins trace from the original PIC trace.
To determine whether riluzole retained the ability to decrease motoneuron repetitive firing and NaPIC amplitude after prolonged riluzole exposure, 2 μM riluzole was bath applied. The ability of the motoneuron to fire during triangular current ramps as well as during current steps was assessed. Because 2 μM riluzole has been shown to block ∼50% of the NaPIC amplitude (Urbani and Belluzzi 2000), we wanted to compare the NaPIC amplitude before and after 2 μM riluzole was applied. After the initial measurements, 2 μM riluzole was applied and the resulting current trace was labeled +2 Ril. TTX (1 μM) was subsequently added to the aCSF to completely block the sodium component of the PIC (see Fig. 4C). The NaPIC amplitude remaining after 2 μM riluzole was applied (+2Ril NaPIC) could then be determined by subtracting the TTX-ins current trace from the +2Ril current trace (see Fig. 4, C and D).
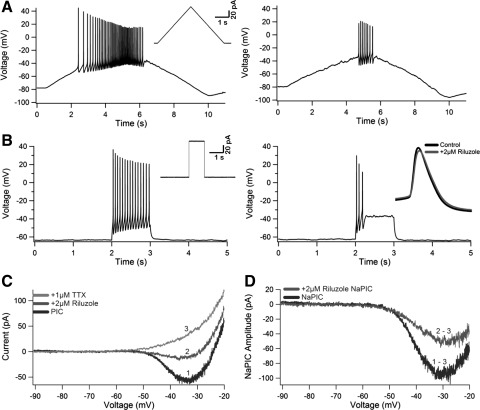
Fig. 4.
Reapplication of riluzole. Riluzole was reapplied to both wt and SOD1 neurons previously exposed to the chronic riluzole treatment. For A and B, all responses before reapplication of riluzole are on left; responses during reapplication are on right. Insets on left for A and B show the current input. All traces are from the same neuron. A: voltage response to a current ramp. Note the decrease in firing, higher current threshold, and smaller action potential (AP) height. B: voltage response to a current step. Note the decrease in firing rate and AP failure. Inset on right shows that the height of the initial AP is not altered. C and D: current response to a voltage ramp. C: current trace before riluzole (black, trace 1), after riluzole application (gray, trace 2), and after TTX application (light gray, trace 3). D: NaPIC amplitudes before and after riluzole application: NaPIC (black; from C: trace 1 − trace 3) and +2 Riluzole NaPIC (gray; from C: trace 2 − trace 3).
Statistics.
All statistics were performed with SPSS 17.0 (SPSS, Chicago, IL). For all measures, 2 (drug: control, 2 μM riluzole) × 2 (genotype: wt, SOD1) factorial ANOVAs were used to determine significance (P > 0.05). Two measures, PIC amplitude and NaPIC onset, were nonparametric. For these measures, the natural log was taken, followed by an ANOVA. Since the statistical results were nearly identical to the nonnormalized data, the nonnormalized means and standard deviations are listed in Table 2. Individual t-tests with Bonferroni adjustments were used to determine pairwise differences if interactions were significant.
Table 2.
PIC, NaPIC, and TTX-ins measures
| Measure | Significance | Direction | wt 0 μΜ | SOD1 0 μΜ | wt 2 μΜ | SOD1 2 μΜ |
|---|---|---|---|---|---|---|
| PIC | N = 46 | N = 25 | N = 51 | N = 30 | ||
| Onset, mV | Drug** | 0 > 2 | −42.5 ± 5.0 | −45.5 ± 3.0 | −46.1 ± 5.1 | −46.2 ± 3.9 |
| Genotype* | wt > SOD1 | |||||
| Max voltage, mV | Drug**** | 0 > 2 | −28.5 ± 4.7 | −29.0 ± 2.6 | −32.0 ± 4.1 | −32.1 ± 3.1 |
| Amplitude, pA | Drug** | 0 < 2 | −36.8 ± 28.6 | −57.9 ± 58.7 | −33.8 ± 25.3 | −25.8 ± 18.9 |
| Interaction** | SOD1: 0 < 2 | |||||
| SOD1 0 < wt 2 | ||||||
| NaPIC | N = 35 | N = 20 | N = 39 | N = 22 | ||
| Onset, mV | Drug* | 0 > 2 | −48.6 ± 6.6 | −49.2 ± 3.9 | −51.5 ± 3.5 | −50.4 ± 4.4 |
| Max voltage, mV | Drug* | 0 > 2 | −24.5 ± 4.8 | −26.0 ± 3.8 | −26.7 ± 4.4 | −28.0 ± 5.4 |
| Amplitude, pA | Ns | −74.7 ± 45.3 | −73.9 ± 41.8 | −68.3 ± 25.4 | −56.6 ± 27.7 | |
| TTX-ins | N = 30 | N = 20 | N = 38 | N = 21 | ||
| Onset, mV | Genotype** | wt < SOD1 | −53.4 ± 12.9 | −45.7 ± 8.8 | −54.0 ± 11.0 | −48.1 ± 13.4 |
| Amp, PIC max | Genotype* | wt > SOD1 | 43.9 ± 30.3 | 28.7 ± 14.5 | 45.1 ± 49.2 | 32.1 ± 23.0 |
Means ± SD for each of the 4 individual groups are presented separately (genotype:drug): wt control (0 μM), SOD1 control (0 μN), wt riluzole (2 μM), and SOD1 riluzole (2 μM). The number of cells per group (N) is above each series of measurements. PIC, persistent inward current; NaPIC, sodium PIC; TTX-ins, TTX-insensitive current; Amp, amplitude. Significant differences for drug, genotype and interactions are listed in a separate column. Significance: not significant (ns),
0.05 ≥ P > 0.01,
0.01 ≥ P > 0.001,
P ≤ 0.0001. The direction of each significant difference is listed in a separate column. The direction is based on actual, not absolute, values.
RESULTS
Wild type versus SOD1.
Previous studies using a similar preparation have shown that SOD1 motoneurons are hyperexcitable compared with wt cells (Kuo et al. 2005; Pieri et al. 2009; van Zundert et al. 2008). We also saw signs of increased excitability. For instance, the AP current threshold was significantly decreased in SOD1 neurons (wt 27.5 ± 48.4 pA vs. SOD1 13.7 ± 34.2 pA), indicating that less excitatory input was needed to initiate AP firing in SOD1 neurons. There was also a significant decrease in input conductance (wt 2.8 ± 1.6 nS vs. SOD1 2.3 ± 1.3 nS) and hyperpolarization of the PIC onset (wt −44.3 ± 5.3 mV vs. SOD1 −45.9 ± 3.5 mV) for SOD1 neurons.
Both PIC onset and input conductance can affect AP current threshold. To determine whether the difference in AP current threshold between wt and SOD1 neurons was due to the difference in one or both of these parameters, AP current threshold was first normalized by PIC onset and input conductance separately and reanalyzed. A significant difference in AP current threshold remained between wt and SOD1 neurons when normalized by either parameter. We then normalized AP current threshold by both PIC onset and input conductance and reanalyzed. After normalization by both measures, AP current threshold was no longer significantly different between wt and SOD1 neurons. Therefore the difference in AP current threshold was dependent on both measures.
We also observed a novel difference between wt and SOD1 motoneurons in the remaining outward current after TTX application (hereafter referred to as TTX-ins). In SOD1 neurons, the TTX-ins outward current had a more depolarized onset and smaller amplitude compared with wt neurons (for example, Fig. 1A). The onset of the TTX-ins outward current was nearly 7 mV more depolarized in SOD1 neurons (wt −53.7 ± 11.8 mV vs. SOD1 −46.9 ± 11.4 mV), and the TTX-ins amplitude measured at the PIC max voltage was significantly decreased by 13 pA. The difference in the TTX-ins amplitude, however, was not significant when normalized by conductance. This indicates that the smaller TTX-ins amplitude was a result of the lower SOD1 input conductance.
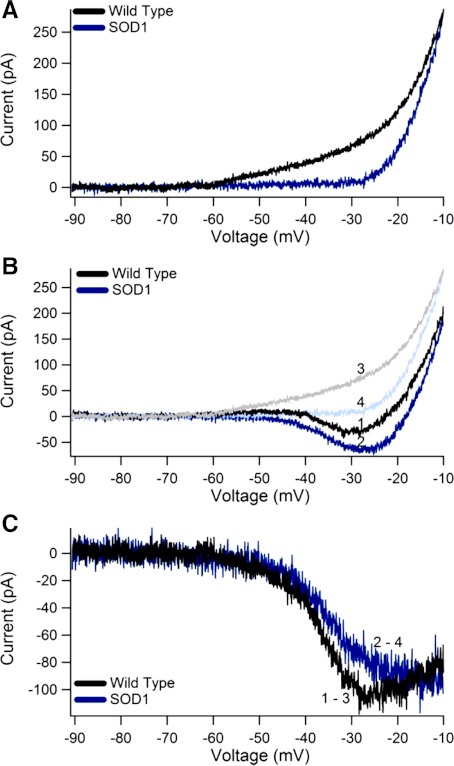
Fig. 1.
Wild-type (wt) and superoxide dismutase 1 mutant (SOD1) current-voltage (IV) relationships. A: TTX-insensitive (TTX-ins) currents for both a wt (black) and a SOD1 (blue) neuron. Note that the SOD1 TTX-ins current has a more depolarized voltage onset and smaller amplitude. B: comparison of the persistent inward current (PIC) traces for the same wt (black, trace 1) and SOD1 (blue, trace 2) neurons as in A. Compare to the TTX-ins traces from the wt (light gray, trace 3) and SOD1 (light blue, trace 4). C: sodium PIC (NaPIC) for the same neurons: wt NaPIC (from B: trace 1 − trace 3). and SOD1 NaPIC (from B: trace 2 − trace 4). Note that the NaPIC amplitudes are nearly identical.
The genotype differences in the AP current threshold, PIC onset, input conductance, and TTX-ins current indicate an increase in the excitability of SOD1 motoneurons. In particular, these changes would increase the likelihood for a motoneuron to reach firing threshold during excitatory synaptic input, consistent with other studies showing an increase in neuron excitability (Kuo et al. 2005; Pieri et al. 2009; van Zundert et al. 2008). However, other measures of excitability such as the NaPIC and FI gain were unchanged (Table 1 and Table 2).
Table 1.
Passive and firing properties
| Measure | Significance | Direction | wt 0 μΜ | SOD1 0 μΜ | wt 2 μΜ | SOD1 2 μΜ |
|---|---|---|---|---|---|---|
| Cell size | N = 57 | N = 32 | N = 59 | N = 40 | ||
| Diameter, μm | ns | 25.7 ± 4.9 | 25.2 ± 3.5 | 26.0 ± 4.3 | 26.6 ± 4.4 | |
| Passive properties | N = 57 | N = 32 | N = 59 | N = 40 | ||
| RMP, mV | Drug* | 0 > 2 | −50.5 ± 6.4 | −49.2 ± 5.5 | −52.7 ± 7.0 | −52.0 ± 7.4 |
| Capacitance, pF | Ns | 30.4 ± 17.5 | 34.0 ± 16.6 | 33.5 ± 14.3 | 31.2 ± 10.6 | |
| N = 47 | N = 25 | N = 52 | N = 30 | |||
| Leak conductance, nS | Genotype* | WT > SOD1 | 2.8 ± 1.3 | 2.6 ± 1.3 | 2.9 ± 1.6 | 2.1 ± 1.0 |
| AP and FI data | N = 57 | N = 31 | N = 59 | N = 38 | ||
| Height (>0 mV) | Ns | 32.4 ± 6.8 | 32.8 ± 6.7 | 32.9 ± 8.0 | 32.2 ± 7.0 | |
| Voltage threshold, mV | Ns | −31.7 ± 3.8 | −32.7 ± 5.1 | −33.4 ± 4.5 | −32.1 ± 3.6 | |
| N = 56 | N = 25 | N = 56 | N = 37 | |||
| Current threshold, pA | Genotype* | WT > SOD1 | 26.8 ± 41.6 | 5.4 ± 32.0 | 28.2 ± 54.5 | 19.3 ± 34.9 |
| N = 56 | N = 30 | N = 54 | N = 35 | |||
| ΔI, pA | Ns | −29.7 ± 18.3 | −24.3 ± 13.7 | −31.4 ± 21.9 | −27.7 ± 13.9 | |
| Ascending gain, Hz/pA | Ns | 0.240 ± 0.09 | 0.251 ± 0.09 | 0.233 ± 0.12 | 0.227 ± 0.08 | |
| N = 50 | N = 28 | N = 51 | N = 31 | |||
| Descending gain, Hz/pA | Ns | 0.382 ± 0.15 | 0.366 ± 0.15 | 0.346 ± 0.15 | 0.370 ± 0.13 | |
| N = 19 | N = 20 | N = 8 | N = 7 | |||
| Spont. activity, AP/min | 71.5 ± 152 | 176.6 ± 197 | 139.5 ± 193 | 71.2 ± 152 |
Means ± SD for each of the 4 individual groups are presented separately (genotype:drug): wild type (wt) control (0 μM), superoxide dismutase 1 mutant (SOD1) control (0 μM), wt riluzole (2 μM), and SOD1 riluzole (2 μM). The number of cells per group (N) is above each series of measurements. RMP, resting membrane potential; AP, action potential; FI, frequency-current; ΔI, difference between current at AP recruitment and derecruitment; Spont., spontaneous. Significant differences for drug, genotype, and interactions are listed in a separate column. Significance: not significant (ns),
0.05 ≥ P > 0.01. The direction of each significant difference is listed in a separate column. The direction is based on actual, not absolute, values.
Influence of TTX-ins current on PIC.
In cultured motoneurons, the PIC is a reflection of both the NaPIC and outward TTX-ins current. When the NaPIC is larger than the TTX-ins current, a downward current deflection is evident in the IV trace and the PIC can be measured. The amplitude of the PIC is therefore dependent on the balance between the NaPIC and TTX-ins amplitudes. In control conditions, the SOD1 motoneurons had a greater mean PIC amplitude than wt neurons, although this was not quite significant after Bonferroni adjustment (wt, control −36.8 ± 28.6 pA vs. SOD1, control −57.9 ± 58.7 pA) (for example, Fig. 1B, traces 1 and 2). Nearly two-thirds of this difference, however, could be accounted for by the smaller TTX-ins current in SOD1 motoneurons, indicating that the NaPIC was unchanged in SOD1 motoneurons. As expected, the NaPIC amplitude was nearly identical in wt and SOD1 motoneurons (wt, control −74.7 ± 45.3 pA vs. SOD1, control −73.9 ± 41.8 pA) (for example, Fig. 1C). In conclusion, the TTX-ins current had an impact on the negative slope area of the PIC, but the total sodium component of the PIC was not altered in SOD1 versus wt motoneurons.
Control versus riluzole treatment.
To test whether chronic riluzole treatment paradoxically increases intrinsic motoneuron excitability in response to prolonged hypoexcitability, we treated our cells with 2 μM riluzole for 4–9 days before recording their electrical properties. For riluzole to have an effect, it was necessary for our cell culture system to be spontaneously active. To ensure that spontaneous firing was present in our cell culture system, aCSF without synaptic blockers was bath applied for a subset of neurons (N = 54) and spontaneous activity was recorded. Spontaneous firing was present in 41 of 54 neurons, and excitatory and/or inhibitory postsynaptic currents were visible in 11 of 13 of the neurons in which no APs were present. There was no difference in spontaneous activity between wt and SOD1 or between drug-treated and untreated neurons (Table 1).
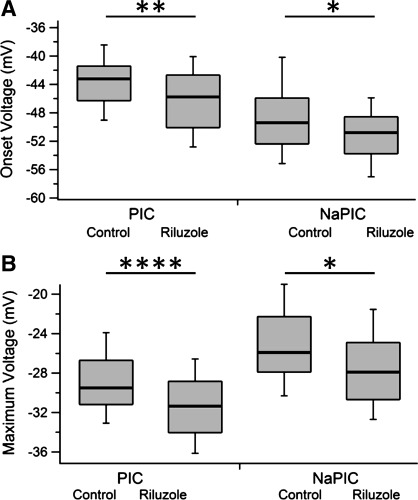
Prolonged treatment with 2 μM riluzole had several main effects on both the passive and active properties of motoneurons, but these changes did not result in intrinsic hyperexcitability as measured by FI gain, FI ΔI, or AP threshold measures (Table 1). The RMP, PIC and NaPIC onset voltage, as well as PIC and NaPIC max voltage were all significantly hyperpolarized by 2–3 mV in the 2 μM riluzole condition (Fig. 2, A and B, respectively). Hyperpolarization of the RMP would indicate hypoexcitability, while hyperpolarization of the PIC and NaPIC onsets and max voltage would lead to hyperexcitability. Unexpectedly, the PIC amplitude was significantly decreased 25% in the 2 μM condition (control −39.8 ± 35 pA vs. 2 μM −28.9 ± 19 pA), and there was a nonsignificant 13% decrease in the NaPIC amplitude. Therefore, chronic exposure to riluzole did not induce a compensatory upregulation of the PIC or NaPIC, contrary to our predictions (Fig. 3, A and B, respectively).
Fig. 2.
PIC and NaPIC onset and max voltages by drug condition. A: onset voltages for the PIC (left) and the NaPIC (right) by drug condition. B: max voltage for the PIC (left) and NaPIC (right) by drug condition. Each is presented as a box plot with median (thick line), 25% (top line), 75% (bottom line), 10% (top whiskers), and 90% (bottom whiskers). Box plots instead of means and SDs were used because the NaPIC onset voltage was nonparametric and to better show the actual distributions. Data shown are pooled across wt and SOD1 neurons as there was no significant main effect of genotype. See Table 2 for data separated by genotype and drug condition (4 groups) and ANOVA results. Significance: *0.05 ≥ P > 0.01, **0.01 ≥ P > 0.001, ****P ≤ 0.0001.
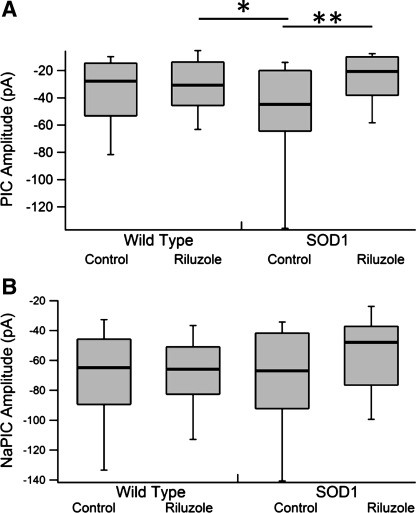
Fig. 3.
PIC and NaPIC amplitudes by drug condition and genotype. A: PIC amplitude with genotype [wt (left) and SOD1 (right)] by drug condition with pairwise differences. Note the decrease in PIC amplitude in SOD1 neurons treated with riluzole. B: NaPIC amplitude with genotype [wt (left) and SOD1 (right)] by drug condition. Note the lack of significant pairwise differences. Each is presented as a box plot with median (thick line), 25% (top line), 75% (bottom line), 10% (top whiskers), and 90% (bottom whiskers). Box plots instead of means and SDs were used because the PIC amplitude was nonparametric and to better show the actual distributions. See Table 2 for ANOVA results. Significance: *0.05 ≥ P > 0.01, **0.01 ≥ P > 0.001.
Although not the main aim of this study, extrinsic excitability did not appear to increase in the riluzole-treated cells. In the absence of synaptic blockers, spontaneous firing was not increased (Table 1) and there was no evidence for the development of bursting behavior. Synaptic scaling, however, was not directly tested and remains a possibility.
Interactions between genotype and drug treatment.
Although acute riluzole application has similar effects on wt and SOD1 neurons (Cao et al. 2002; Kuo et al. 2005), prolonged riluzole treatment produced differential effects. In addition to the main effect of drug treatment on PIC amplitude, there was a significant interaction between genotype and drug treatment. Post hoc analysis revealed that the PIC amplitude between control and riluzole-treated SOD1 neurons was significantly decreased by 55.4%, but there was no change between control and riluzole-treated wt neurons (Fig. 3A; Table 2). Likewise, the NaPIC amplitude between control and riluzole-treated SOD1 neurons was decreased 23%, although this was not significant (Fig. 3B; Table 2). The decrease in PIC amplitude did not, however, lead to decreases in FI gain or changes in other firing parameters.
Reapplication of riluzole.
Although chronic riluzole treatment did not have major effects on motoneuron output, it was important to test whether riluzole could still decrease motoneuron excitability or if neurons had become desensitized to it. For 11 neurons chronically treated with riluzole, 2 μM riluzole was added to the aCSF after initial measurements were taken. Subsequently, TTX (1 μM) was added to the external solution, allowing the comparison between the NaPIC amplitude before and after reapplication of 2 μM riluzole (+2Ril NaPIC) (Fig. 4, C and D). For two neurons, riluzole was washed out of the aCSF before TTX was added.
The reapplication of 2 μM riluzole either decreased or stopped AP firing during current ramps for both wt and SOD1 neurons (N = 9/9; wt N = 5, SOD1 N = 4) (Fig. 4A). During 1-s current steps (N = 8/8; wt N = 4, SOD1 N = 4), the neurons fired repetitive APs but at a slower rate and AP failure occurred before the end of the step (Fig. 4B). However, there was little to no change in the initial AP height during the current step (Fig. 4B, inset). The NaPIC amplitude was decreased an average of 61.5% when 2 μM riluzole was reapplied (wt N = 7, SOD1 N = 4) (Fig. 4D), which is similar to the acute effects of 2 μM riluzole in other studies (Beltran-Parrazal and Charles 2003; Del Negro et al. 2005; Kononenko et al. 2004; Kuo et al. 2006; Ptak et al. 2005; Urbani and Belluzzi 2000; Wu et al. 2004; Zhong et al. 2007). Therefore neurons did not become desensitized to riluzole after prolonged exposure.
It is unlikely that the initial reduction in PIC amplitude for chronically drug-treated SOD1 neurons was due to residual effects of riluzole in the aCSF. First, in aCSF without synaptic blockers, there was no significant difference between spontaneous firing rates for drug-treated and untreated neurons (Table 1). Second, in two of two neurons (wt N = 1, SOD1 N = 1), riluzole was quickly washed out of the bath (within 7 min) after reapplication and repetitive firing returned to normal (data not shown). Both findings indicate that riluzole is easily washed out of the external solution. Although a number of explanations may explain a decrease in PIC amplitude, it is not clear why SOD1 neurons were more affected by prolonged riluzole treatment than wt neurons.
DISCUSSION
Comparisons to past studies: SOD1 versus wt.
Early electrical abnormalities are apparent in SOD1 motoneurons. Previous studies have shown that the NaPIC amplitude was increased in SOD1 spinal (G93A, high expressor line) (Kuo et al. 2005; Quinlan et al. 2011), hypoglossal (G93A, high expressor line) (van Zundert et al. 2008), and corticospinal (G93A, high expressor line) (Pieri et al. 2009) motoneurons. In some of these studies there was also an increase in FI gain (G93A, high expressor line) (Kuo et al. 2005; Pieri et al. 2009; van Zundert et al. 2008). Other studies, however, found either no change in firing behavior (Quinlan et al. 2011) or a decrease in FI gain (G85R and G93A, low expressor line) (Bories et al. 2007; Pambo-Pambo et al. 2009) coupled with changes in motoneuron conductance (G85R and G93A, high expressor line) (Bories et al. 2007; Quinlan et al. 2011) and size (G85R) (Amendola and Durand 2008).
In this study a number of measures (AP current threshold, PIC onset, conductance, and TTX-ins current) indicated an increase in SOD1 motoneuron excitability. We did not, however, see an increase in NaPIC amplitude or FI gain. In control cells, the PIC amplitude appeared larger in SOD1 motoneurons but the pairwise difference between wt control and SOD1 control cells did not quite reach significance (P = 0.069). Some of the diversity between studies is likely due to differences in motoneuron type, preparation, age, and SOD1 mouse model, but all of the studies support the presence of early electrical abnormalities in SOD1 motoneurons.
The most direct comparison to this work is the study by Kuo et al. (2005), which came from our laboratory and used similar cell culture procedures. In Kuo et al. (2005), the NaPIC was increased and an increase in the TTX-ins current was present in low-input conductance neurons (cutoff: <3.25 nS). These results are in contrast to this work, which found no change in the NaPIC and a decrease in the TTX-ins current. The techniques for measuring the amplitude of the NaPIC and TTX-ins current in the present study were very different from those in Kuo et al. (2005). Here we used much slower voltage ramps to decrease the occurrence of breakthrough spikes, which occur because of loss of clamp control over the rapidly activating sodium channels. The slower voltage ramp, however, also increases the effects of channel inactivation. These slower ramps enabled us to directly measure the NaPIC peak amplitude. The TTX-ins amplitude was then measured at the PIC peak voltage. In contrast, Kuo et al. (2005) measured the NaPIC and TTX-ins amplitude as the integral of the curve in a voltage range near firing threshold. Together, the different voltage ramp speeds and measuring techniques likely account for the variation in the NaPIC and TTX-ins current between the two studies. The very slow voltage ramps and smaller dendritic tree are also likely responsible for the lower overall NaPIC amplitudes in this study compared with Quinlan et al. (2011).
Intrinsic motoneuron excitability and riluzole.
In ALS patients, riluzole decreases the rate of disease progression, but this effect is temporary even with continued use (Bensimon et al. 1994; Traynor et al. 2003). The purpose of this study was to determine whether prolonged riluzole treatment caused compensatory upregulation of the NaPIC and subsequently reestablished motoneuron excitability. Instead, the results showed that the NaPIC amplitude was decreased, although not significantly, and that motoneuron intrinsic excitability did not change. Furthermore, motoneurons did not become desensitized to riluzole. It is therefore unlikely that compensatory increases in motoneuron intrinsic excitability cause riluzole to lose its therapeutic value. These results are in sharp contrast to the work of the Bennett lab, which showed compensatory increases in both the NaPIC and the CaPIC after monoaminergic drive to the cord is lost and the PIC is diminished (Harvey et al. 2006c; Li and Bennett 2003).
In the normal state, the NaPIC and the CaPIC can be modulated by affecting the channels directly or by activating G protein-coupled 5-HT2 and NE α1 receptors. Riluzole directly inhibits the NaPIC by blocking inactivated sodium channels (Benoit and Escande 1991; Hebert et al. 1994). In contrast, the loss of monoaminergic drive decreases the PIC because the 5-HT2 and NE α1 receptors are no longer activated. However, when monoaminergic drive is lost, the PIC amplitude gradually increases because the 5-HT2, and possibly NE α1, receptors become constitutively active (Murray et al. 2010; Rank et al. 2011) and supersensitive (Harvey et al. 2006a; Li et al. 2007; Rank et al. 2007). This suggests an interesting speculation, that the loss of monoamines but not direct channel block can result in strong compensatory increases in the PIC amplitude. However, compensatory mechanisms may be impaired or altered during disease and injury and are therefore difficult to compare.
Riluzole produces a stronger effect on the SOD1 PIC.
It was somewhat unexpected that riluzole treatment would have differential effects on wt versus SOD1 motoneurons. Prolonged riluzole treatment, however, decreased the PIC amplitude in SOD1 but not wt motoneurons. This result remained even when conductance was controlled for and cannot be attributed to changes in the TTX-ins current. Although the decrease in PIC amplitude did not change motoneuron firing behavior to injected current, it may have more pronounced effects on synaptic input because of the dendritic location of many PIC channels (Bennett et al. 1998; Lee and Heckman 2000). The specificity of the result suggests that the sodium channels underlying the PIC in SOD1 motoneurons have an altered response to riluzole that could result in greater channel internalization or stabilization of the channel in an inactive state.
Riluzole's transience: alternative hypotheses.
In this study the NaPIC was not upregulated after prolonged (5–9 days) riluzole exposure. In ALS patients, however, riluzole treatment is assessed over months, not days. Therefore it is possible that the exposure to riluzole was too short to produce compensatory changes in excitability. In a number of studies, however, compensatory changes in excitability have been reported after 48 h (Koch et al. 2010) and 3–4 days (Schonfeld-Dado et al. 2009) of exposure to TTX (1 μM) and after 48 h of exposure to prostaglandin E2 (Koch et al. 2010).
An alternative hypothesis is that although prolonged riluzole exposure does not increase intrinsic excitability, it may increase extrinsic motoneuron excitability through synaptic scaling. We saw no increase in spontaneous firing when synaptic blockers were absent, but this study did not directly test synaptic scaling, which can occur during periods of hypoexcitability. It has been repeatedly demonstrated that activity-deprived neurons die off (Baker and Ruijter 1991; Ramakers and Boer 1991; Ruijter et al. 1991) because of an increased sensitivity to glutamate-mediated postsynaptic responses (Fishbein and Segal 2007) and/or increased calcium entry through GluR2-lacking AMPA receptors (Schonfeld-Dado et al. 2009). In these studies, however, synaptic activity was completely blocked through chronic application of TTX (1 μM or higher). In this study, 2 μM riluzole was used, which does not completely block APs. Therefore its capacity to produce synaptic scaling is unknown; however, there was no sign of obvious cellular death between control and cells treated with 2 μM riluzole.
Finally, riluzole may be unable to permanently slow motoneuron death regardless of its effect on motoneuron excitability. Clinical intervention for ALS patients begins well after disease onset, and because of the array of cell autonomous and nonautonomous dysfunctions at this point, it is unlikely that one drug will affect enough pathways to produce lasting results. Drug combinations that include riluzole but affect additional pathways may therefore provide greater protection (Del Signore et al. 2009; Kriz et al. 2003; Waibel et al. 2004).
Conclusion.
Prolonged riluzole treatment had minimal effects on motoneuron firing behavior and remained a potent inhibitor of the PIC and repetitive firing when reapplied. It therefore seems possible that riluzole continues to decrease motoneuron excitability but its therapeutic effects are eventually overwhelmed by other pathologies associated with ALS.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grants NS-050162 and NS-03482 to C. J. Heckman and an individual NRSA F31 NS-060532 to J. E. Schuster.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.E.S., R.F., T.S., and C.J.H. conception and design of research; J.E.S. performed experiments; J.E.S. and C.J.H. analyzed data; J.E.S. and C.J.H. interpreted results of experiments; J.E.S. prepared figures; J.E.S. drafted manuscript; J.E.S. and C.J.H. edited and revised manuscript; J.E.S. and C.J.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Drs. Erdong Liu and Hasan Arrat for their technical support.
REFERENCES
- Amendola J, Durand J. Morphological differences between wild-type and transgenic superoxide dismutase 1 lumbar motoneurons in postnatal mice. J Comp Neurol 511: 329–341, 2008 [DOI] [PubMed] [Google Scholar]
- Baker RE, Ruijter JM. Chronic blockade of bioelectric activity in neonatal rat neocortex in vitro: physiological effects. Int J Dev Neurosci 9: 321–329, 1991 [DOI] [PubMed] [Google Scholar]
- Bellingham MC. A review of the neural mechanisms of action and clinical efficiency of riluzole in treating amyotrophic lateral sclerosis: what have we learned in the last decade? CNS Neurosci Ther 17: 4–31, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltran-Parrazal L, Charles A. Riluzole inhibits spontaneous Ca2+ signaling in neuroendocrine cells by activation of K+ channels and inhibition of Na+ channels. Br J Pharmacol 140: 881–888, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DJ, Hultborn H, Fedirchuk B, Gorassini M. Synaptic activation of plateaus in hindlimb motoneurons of decerebrate cats. J Neurophysiol 80: 2023–2037, 1998 [DOI] [PubMed] [Google Scholar]
- Benoit E, Escande D. Riluzole specifically blocks inactivated Na channels in myelinated nerve fibre. Pflügers Arch 419: 603–609, 1991 [DOI] [PubMed] [Google Scholar]
- Bensimon G, Lacomblez L, Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N Engl J Med 330: 585–591, 1994 [DOI] [PubMed] [Google Scholar]
- Boillee S, Vande Velde C, Cleveland DW. ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron 52: 39–59, 2006 [DOI] [PubMed] [Google Scholar]
- Bories C, Amendola J, Lamotte d'Incamps B, Durand J. Early electrophysiological abnormalities in lumbar motoneurons in a transgenic mouse model of amyotrophic lateral sclerosis. Eur J Neurosci 25: 451–459, 2007 [DOI] [PubMed] [Google Scholar]
- Cao YJ, Dreixler JC, Couey JJ, Houamed KM. Modulation of recombinant and native neuronal SK channels by the neuroprotective drug riluzole. Eur J Pharmacol 449: 47–54, 2002 [DOI] [PubMed] [Google Scholar]
- Carriedo SG, Yin HZ, Lamberta R, Weiss JH. In vitro kainate injury to large, SMI-32+ spinal neurons is Ca2+ dependent. Neuroreport 6: 945–948, 1995 [DOI] [PubMed] [Google Scholar]
- Carriedo SG, Yin HZ, Weiss JH. Motor neurons are selectively vulnerable to AMPA/kainate receptor-mediated injury in vitro. J Neurosci 16: 4069–4079, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Negro CA, Morgado-Valle C, Hayes JA, Mackay DD, Pace RW, Crowder EA, Feldman JL. Sodium and calcium current-mediated pacemaker neurons and respiratory rhythm generation. J Neurosci 25: 446–453, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Signore SJ, Amante DJ, Kim J, Stack EC, Goodrich S, Cormier K, Smith K, Cudkowicz ME, Ferrante RJ. Combined riluzole and sodium phenylbutyrate therapy in transgenic amyotrophic lateral sclerosis mice. Amyotroph Lateral Scler 10: 85–94, 2009 [DOI] [PubMed] [Google Scholar]
- Ehlers MD. Activity level controls postsynaptic composition and signaling via the ubiquitin-proteasome system. Nat Neurosci 6: 231–242, 2003 [DOI] [PubMed] [Google Scholar]
- Fishbein I, Segal M. Miniature synaptic currents become neurotoxic to chronically silenced neurons. Cereb Cortex 17: 1292–1306, 2007 [DOI] [PubMed] [Google Scholar]
- Harvey PJ, Li X, Li Y, Bennett DJ. 5-HT2 receptor activation facilitates a persistent sodium current and repetitive firing in spinal motoneurons of rats with and without chronic spinal cord injury. J Neurophysiol 96: 1158–1170, 2006a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PJ, Li X, Li Y, Bennett DJ. Endogenous monoamine receptor activation is essential for enabling persistent sodium currents and repetitive firing in rat spinal motoneurons. J Neurophysiol 96: 1171–1186, 2006b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PJ, Li Y, Li X, Bennett DJ. Persistent sodium currents and repetitive firing in motoneurons of the sacrocaudal spinal cord of adult rats. J Neurophysiol 96: 1141–1157, 2006c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert T, Drapeau P, Pradier L, Dunn RJ. Block of the rat brain IIA sodium channel alpha subunit by the neuroprotective drug riluzole. Mol Pharmacol 45: 1055–1060, 1994 [PubMed] [Google Scholar]
- Ilieva H, Polymenidou M, Cleveland DW. Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J Cell Biol 187: 761–772, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch H, Huh SE, Elsen FP, Carroll MS, Hodge RD, Bedogni F, Turner MS, Hevner RF, Ramirez JM. Prostaglandin E2-induced synaptic plasticity in neocortical networks of organotypic slice cultures. J Neurosci 30: 11678–11687, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kononenko NI, Shao LR, Dudek FE. Riluzole-sensitive slowly inactivating sodium current in rat suprachiasmatic nucleus neurons. J Neurophysiol 91: 710–718, 2004 [DOI] [PubMed] [Google Scholar]
- Kriz J, Gowing G, Julien JP. Efficient three-drug cocktail for disease induced by mutant superoxide dismutase. Ann Neurol 53: 429–436, 2003 [DOI] [PubMed] [Google Scholar]
- Kumari KS, Satyanarayana B, Nagaswari A, Raj S. Development and validation of a novel stability-indicating LC method for the determination of riluzole in bulk drug and tablets. Chromatographia 69: 513–517, 2009 [Google Scholar]
- Kuo JJ, Lee RH, Zhang L, Heckman CJ. Essential role of the persistent sodium current in spike initiation during slowly rising inputs in mouse spinal neurones. J Physiol 574: 819–834, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo JJ, Siddique T, Fu R, Heckman CJ. Increased persistent Na+ current and its effect on excitability in motoneurones cultured from mutant SOD1 mice. J Physiol 563: 843–854, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacomblez L, Bensimon G, Leigh PN, Guillet P, Powe L, Durrleman S, Delumeau JC, Meininger V. A confirmatory dose-ranging study of riluzole in ALS. ALS/Riluzole Study Group-II. Neurology 47: S242–S250, 1996 [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Adjustable amplification of synaptic input in the dendrites of spinal motoneurons in vivo. J Neurosci 20: 6734–6740, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Murray K, Harvey PJ, Ballou EW, Bennett DJ. Serotonin facilitates a persistent calcium current in motoneurons of rats with and without chronic spinal cord injury. J Neurophysiol 97: 1236–1246, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Bennett DJ. Persistent sodium and calcium currents cause plateau potentials in motoneurons of chronic spinal rats. J Neurophysiol 90: 857–869, 2003 [DOI] [PubMed] [Google Scholar]
- Murray KC, Nakae A, Stephens MJ, Rank M, D'Amico J, Harvey PJ, Li X, Harris RL, Ballou EW, Anelli R, Heckman CJ, Mashimo T, Vavrek R, Sanelli L, Gorassini MA, Bennett DJ, Fouad K. Recovery of motoneuron and locomotor function after spinal cord injury depends on constitutive activity in 5-HT2C receptors. Nat Med 16: 694–700, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pambo-Pambo A, Durand J, Gueritaud JP. Early excitability changes in lumbar motoneurons of transgenic SOD1G85R and SOD1G93A-Low mice. J Neurophysiol 102: 3627–3642, 2009 [DOI] [PubMed] [Google Scholar]
- Pieri M, Carunchio I, Curcio L, Mercuri NB, Zona C. Increased persistent sodium current determines cortical hyperexcitability in a genetic model of amyotrophic lateral sclerosis. Exp Neurol 215: 368–379, 2009 [DOI] [PubMed] [Google Scholar]
- Ptak K, Zummo GG, Alheid GF, Tkatch T, Surmeier DJ, McCrimmon DR. Sodium currents in medullary neurons isolated from the pre-Botzinger complex region. J Neurosci 25: 5159–5170, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan K, Schuster J, Fu R, Siddique T, Heckman C. Altered postnatal maturation of electrical properties in spinal motoneurons in an ALS mouse model. J Physiol 589: 2245–2260, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakers GJ, Boer GJ. Chronic suppression of bioelectric activity and cell survival in primary cultures of rat cerebral cortex: biochemical observations. Eur J Neurosci 3: 154–161, 1991 [DOI] [PubMed] [Google Scholar]
- Rank MM, Li X, Bennett DJ, Gorassini MA. Role of endogenous release of norepinephrine in muscle spasms after chronic spinal cord injury. J Neurophysiol 97: 3166–3180, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rank MM, Murray KC, Stephens MJ, D'Amico J, Gorassini MA, Bennett DJ. Adrenergic receptors modulate motoneuron excitability, sensory synaptic transmission and muscle spasms after chronic spinal cord injury. J Neurophysiol 105: 410–422, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O'Regan JP, Deng HX, Rahmani Z, Krizus A, McKenna-Yasek D, Cayabyab A, Gaston SM, Berger R, Tanzi RE, Halperin JJ, Herzfeldt B, Van Den Bergh R, Hung WY, Bird T, Deng G, Mulder DW, Smyth C, Laing NG, Soriano E, Pericak-Vance MA, Haines J, Rouleau GA, Gusella JS, Horvitz HR, Brown RH., Jr Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 362: 59–62, 1993 [DOI] [PubMed] [Google Scholar]
- Ruijter JM, Baker RE, De Jong BM, Romijn HJ. Chronic blockade of bioelectric activity in neonatal rat cortex grown in vitro: morphological effects. Int J Dev Neurosci 9: 331–338, 1991 [DOI] [PubMed] [Google Scholar]
- Schonfeld-Dado E, Fishbein I, Segal M. Degeneration of cultured cortical neurons following prolonged inactivation: molecular mechanisms. J Neurochem 110: 1203–1213, 2009 [DOI] [PubMed] [Google Scholar]
- Traynor BJ, Alexander M, Corr B, Frost E, Hardiman O. An outcome study of riluzole in amyotrophic lateral sclerosis—a population-based study in Ireland, 1996–2000. J Neurol 250: 473–479, 2003 [DOI] [PubMed] [Google Scholar]
- Turrigiano G, LeMasson G, Marder E. Selective regulation of current densities underlies spontaneous changes in the activity of cultured neurons. J Neurosci 15: 3640–3652, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature 391: 892–896, 1998 [DOI] [PubMed] [Google Scholar]
- Urbani A, Belluzzi O. Riluzole inhibits the persistent sodium current in mammalian CNS neurons. Eur J Neurosci 12: 3567–3574, 2000 [DOI] [PubMed] [Google Scholar]
- Van Damme P, Dewil M, Robberecht W, Van Den Bosch L. Excitotoxicity and amyotrophic lateral sclerosis. Neurodegener Dis 2: 147–159, 2005 [DOI] [PubMed] [Google Scholar]
- Van Den Bosch L, Van Damme P, Bogaert E, Robberecht W. The role of excitotoxicity in the pathogenesis of amyotrophic lateral sclerosis. Biochim Biophys Acta 1762: 1068–1082, 2006 [DOI] [PubMed] [Google Scholar]
- van Zundert B, Peuscher MH, Hynynen M, Chen A, Neve RL, Brown RH, Jr, Constantine-Paton M, Bellingham MC. Neonatal neuronal circuitry shows hyperexcitable disturbance in a mouse model of the adult-onset neurodegenerative disease amyotrophic lateral sclerosis. J Neurosci 28: 10864–10874, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waibel S, Reuter A, Malessa S, Blaugrund E, Ludolph AC. Rasagiline alone and in combination with riluzole prolongs survival in an ALS mouse model. J Neurol 251: 1080–1084, 2004 [DOI] [PubMed] [Google Scholar]
- Wu N, Enomoto A, Tanaka S, Hsiao CF, Nykamp DQ, Izhikevich E, Chandler SH. Persistent sodium currents in mesencephalic V neurons participate in burst generation and control of membrane excitability. J Neurophysiol 93: 2710–2722, 2004 [DOI] [PubMed] [Google Scholar]
- Zhong G, Masino MA, Harris-Warrick RM. Persistent sodium currents participate in fictive locomotion generation in neonatal mouse spinal cord. J Neurosci 27: 4507–4518, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]