Abstract
Degenerin/epithelial Na+ channels (DEG/ENaCs) are voltage-independent Na+ or Na+/Ca2+ channels expressed in many tissues and are needed for a wide range of physiological functions, including sensory perception and transepithelial Na+ transport. In the nervous system, DEG/ENaCs are expressed in both neurons and glia. However, the role of glial vs. neuronal DEG/ENaCs remains unclear. We recently reported the characterization of a novel DEG/ENaC in Caenorhabditis elegans that we named ACD-1. ACD-1 is expressed in glial amphid sheath cells. The glial ACD-1, together with the neuronal DEG/ENaC DEG-1, is necessary for acid avoidance and attraction to lysine. We report presently that knockout of acd-1 in glia exacerbates sensory deficits caused by another mutant: the hypomorphic allele of the cGMP-gated channel subunit tax-2. Furthermore, sensory deficits caused by mutations in Gi protein odr-3 and guanylate cyclase daf-11, which regulate the activity of TAX-2/TAX-4 channels, are worsened by knockout of acd-1. We also show that sensory neurons of acd-1 tax-2(p694) double mutants fail to undergo changes in intracellular Ca2+ when animals are exposed to low concentrations of attractant. Finally, we show that exogenous expression of TRPV1 in sensory neurons and exposure to capsaicin rescue sensory deficits of acd-1 tax-2(p694) mutants, suggesting that sensory deficits of these mutants are bypassed by increasing neuronal excitability. Our data suggest a role of glial DEG/ENaC channel ACD-1 in supporting neuronal activity.
Keywords: degenerin/epithelial sodium channel, chemotaxis, imaging
glia constitute the major cellular component of the vertebrate nervous system. However, until a decade ago researchers thought that glia' s only role was to support and nourish neurons. Recent studies have shown instead that glia are critical for the development of the nervous system and play a key role in neuronal disorders including multiple sclerosis, amyotrophic lateral sclerosis, spinocerebellar ataxia, Parkinson's disease, Huntington's disease, ischemia, neuropathic pain, and epilepsy (reviewed in Iadecola and Nedergaard 2007; Lobsiger and Cleveland 2007; Rossi et al. 2007; Scholz and Woolf 2007). Glial cells can influence neuronal excitability both indirectly and directly. Indirectly, glia regulate neuronal function by controlling the concentration of ions, neurotransmitters, and neuronal modulators in the extracellular space (Cohen et al. 1968; Kuffler 1967). Glial cells also modulate neuronal excitability directly by releasing either transmitters or co-agonists onto neurons (Gourine et al. 2010; Jourdain et al. 2007; Vesce et al. 2007; Volterra and Meldolesi 2005).
Degenerin/epithelial Na+ channels (DEG/ENaCs) are voltage-independent Na+ or Na+/Ca2+ channels composed of three identical or homologous subunits (Jasti et al. 2007) that participate in a large variety of physiological functions, including sensory perception (touch, pain, taste; Chandrashekar et al. 2010; Chalfie 2009; Chen et al. 2002; Price et al. 2000; Price et al. 2001; Sluka et al. 2003), learning and memory (Wemmie et al. 2002), and transepithelial Na+ transport (Hummler et al. 1996). DEG/ENaCs have been found in glia: 1) messenger RNA and protein for DEG/ENaC subunits were found in retinal Muller cells (Brockway et al. 2002), and the contribution of DEG/ENaCs in retinal function has been suggested based on the finding that amiloride, a DEG/ENaC blocker, alters electroretinograms (Brockway et al. 2005). 2) β-ENaC is expressed in Schwann cells associated with Ruffini's endings of the rat incisor (Hitomi et al. 2009), and ASIC2 is expressed in the inner core lamellae (thought to be of glial origin) of the Pacinian corpuscles (Calavia et al. 2010). 3) Several members of the DEG/ENaC family of channels are expressed in astrocytes and gliomas and have been suggested to play a role in glioma malignancy (Berdiev et al. 2003; Vila-Carriles et al. 2006, 2007).
We recently reported the cloning and characterization of a novel DEG/ENaC channel gene from Caenorhabditis elegans that we named acd-1 (Wang et al. 2008). ACD-1 is expressed in amphid sheath cells (and at low levels also in the spermatheca, not shown), a pair of glial cells that ensheath the sensory dendrites of 12 pairs of C. elegans sensory neurons. We found that knockout of acd-1 in glia worsens the reduced sensitivity to acidic environments and lysine of deg-1 null mutants. We undertook this study to test the prediction that knockout of acd-1 in glia worsens sensory deficits caused by mutations in other sensory neurons genes and to shed light on the mechanism of ACD-1-mediated glia regulation of the function of sensory neurons. We show in this study that knockout of acd-1 channel in glia exacerbates sensory deficits of a hypomorphic mutant of tax-2, the β-subunit of a cGMP-gated channel, needed in C. elegans for chemotaxis to odors and tastants (Bargmann et al. 1993; Coburn and Bargmann 1996). We also show that knockout of acd-1 exacerbates sensory deficits caused by mutations in genes that encode signaling molecules, the Gi protein ODR-3 and the guanylate cyclase protein DAF-11, which have been proposed to regulate the activity of the TAX-2/TAX-4 channel (Bargmann 2006). Finally, we show that exogenous depolarization and increase in intracellular Ca2+ of the sensory neurons rescue the sensory deficits of acd-1 tax-2(p694) double-mutant animals. Together, these data show that when acd-1 is knocked out, sensory deficits caused by mutations in sensory neurons genes are exacerbated, suggesting a role of glial ACD-1 in supporting neuronal function. Increasing sensory neurons excitability through another mechanism bypasses the ACD-1 requirement.
MATERIALS AND METHODS
Molecular biology.
The construction of Pvap-1::ACD-1 has been described previously (Wang et al. 2008). We constructed Podr-3::TRPV1 using a previously described construct that drives expression of rat TRPV1 in ASE neurons (Suzuki et al. 2008). To target expression of TRPV1 in AWC neurons, we replaced the promoter of gcy-6, functional in ASEs (Yu et al. 1997), with the promoter of the odr-3 gene (1.5 kb upstream of the start codon) upstream of rat TRPV1 in the pPD115.118 Fire vector. To construct Podr-3::TAX-2, we swapped the TRPV1 sequence with TAX-2 cDNA sequence. TAX-2 cDNA was cloned by RT-PCR using the one-step RT-PCR kit Titanium (Clontech) and gene-specific primers designed according to the tax-2 published sequence (Coburn and Bargmann 1996). Total RNA from a C. elegans mixed-age population was used as template. TAX-2 cDNA was initially cloned in TOPO PCR2.1 vector for sequence verification. To drive expression of GCaMP in amphid sheath glia, we inserted the GCaMP1.0 sequence in pPD95.75 Fire vector downstream of the promoter of the vap-1 gene, which functions in glial amphid sheath cells (Perens and Shaham 2005).
C. elegans strains and growth.
Nematode strains were maintained at 20°C on standard nematode growth medium (NGM) seeded with Escherichia coli strain OP50 (Brenner 1974). Wild-type animals were N2 Bristol. Other strains used were ZB90 acd-1(bz90) I, PR691 tax-2(p691) I, PR694 tax-2(p694) I, CX2205 odr-3(n2150) V, DR47 daf-11(m47) V, BLC20 acd-1(bz90) I tax-2(p694) I, BLC135 acd-1(bz90) I;odr-3(n2150) V, BLC136 acd-1(bz90) I;daf-11(m47) V, BLC139 blcEx67 [Podr-3::VR-1, PT02B11.3::GFP], BLC140 acd-1(bz90) I tax-2(p694) I; blcEx67 [Podr-3::VR-1, PT02B11.3::GFP], BLC145 acd-1(bz90) I tax-2(p694) I; kyEx878 [str-2::GCamP1.0, unc-122::GFP]; blcEx69 [Podr-3::VR-1, pRF4], BLC47 acd-1(bz90) I tax-2(p694) I; blcEx19 [PVAP-1::ACD-1::RFP, Pglr-1::yc2.12], BLC134 acd-1(bz90)I tax-2(p694) I; kyEx878 [str-2::GCamP1.0, unc-122::GFP]; blcEx66 [PVAP-1::ACD-1, pRF4], DA1297 lin-15B(n765) X; adEx1297 [lin-15(+) gcy-6::GFP], BLC188 tax-2(p694) I; adEx1288 [lin-15(+) gcy-6::GFP], BLC167 acd-1(bz90) I; adEx1288 [lin-15(+) gcy-7::GFP], BLC137 acd-1(bz90) I tax-2(p694) I ;adEx1288 [lin-15(+) gcy-6::GFP], BLC167 tax-(p691) I; adEx1288 [lin-15(+) gcy-7::GFP], CX7369 kyEx878 [str-2::GCaMP1.0, unc-122::GFP], BLC55 acd-1(bz90) I; kyEx878 [str-2::GCaMP1.0, unc-122::GFP], BLC56 tax-2(p694) I;kyEx878 [str-2::GCaMP1.0, unc-122::GFP], BLC57 acd-1(bz90) I tax-2(p694) I; kyEx878 [str-2::GCaMP1.0, unc-122::GFP], BLC76 tax-2(p691) I; kyEx878 [str-2::GCaMP1.0, unc-122::GFP], BLC187 kyIs150 IV [tax-2(delta):GFP], BLC115 acd-1(bz90) I; kyIs150 IV [tax-2(delta):GFP], OS1984 lin-15B(n765) X; nsEx1111 [lin-15(+) PVAP-1::GCamMP1.0], and BLC40 acd-1(bz90) I; nsEx1111 [lin-15(+) PVAP-1::GCamMP1.0]. Germline transformation was carried out as described previously (Mello et al. 1991), and double mutants were generated by standard genetic crosses. Mutations were followed through the crosses by PCR. acd-1(bz90) is referred to as “acd-1” in figures and text for simplicity.
Behavioral assays.
For chemotaxis and avoidance assays, we followed previously described procedures (Bargmann et al. 1993; Bargmann and Horvitz 1991; Troemel et al. 1995). For Na-acetate assays, a chunk of agar 1 cm in diameter was removed from 10-cm plates and soaked in the attractant for 3 h. Chunks were put back in the plate overnight to allow equilibration and formation of a gradient. For isoamyl alcohol and trimethylthiazole assays, 1 μl of odor at the indicated dilution was placed on one side of the plate. Thirty worms were then placed between the test spot and a control spot on the opposite side of the plate. Ten microliters of 20 mM NaN3 were placed on both spots to anesthetize animals once they reached the spot. After 1 h, animals on each side of the plate were counted and an attraction index was determined as follows: (number of animals at attractant − number of animals at control)/(total number of animals). When capsaicin was used, 200 μl of 50 μM capsaicin diluted in water were spread on the plate 10 min before the animals were transferred. For octanol avoidance assays, we dipped an eyelash hair glued on a toothpick in octanol and placed it in front of a forward-moving animal on a plate without food. We measured the time it took for the animal to respond to the odor by reversing direction (Troemel et al. 1995). Odors were diluted in ethanol. Statistical significance was determined by ANOVA.
Ca2+ imaging.
Animals were glued on glass coverslips using surgical glue (Gluture or Nexcare 3M) and immediately immersed in M13 buffer. Coverslips were then transferred to a perfusion chamber mounted on a Nikon Eclipse E600FN microscope equipped with green fluorescent protein (GFP) filters. We imaged the cell body of AWCon neurons and of amphid glia (Chalasani et al. 2007). Animals were perfused using a gravity perfusion system at a rate of ∼75 μl/s with S-Basal buffer (100 mM NaCl, 50 mM K-phosphate, pH 6) or S-Basal plus isoamyl alcohol and capsaicin at the indicated dilutions and concentration. Based on the size of the perfusion tubes and chamber, the level of solution in the chamber and the level of solution in the syringes, it took 20–40 s to completely exchange the solution in the bath. Images were acquired using TILLvisION version 3.3 software and the Imago charge-coupled device camera (T.I.L.L. Photonics). Images were acquired at 1 Hz, and typical exposure time was 30–100 ms. The image stacks were then analyzed using ImageJ. First, images were registered using ImageJ TurboReg to correct for movement. The region of interest corresponding to each cell was then selected according to the shape and position of the cells. The fluorescence intensity of the region of interest was measured and output to a data log file. Origin 6.1 was used to plot the data created by ImageJ.
Xenopus oocytes expression and electrophysiology.
acd-1 cRNA synthesis and expression in Xenopus oocytes was achieved as previously described (Wang et al. 2008; Wang and Bianchi 2009). Briefly, capped RNA was synthesized using the T7 mMESSAGE mMACHINE kit (Ambion), purified (Qiagen RNAeasy columns), and run on denaturating agarose gels to check for size and cRNA integrity. cRNA quantification was then performed spectroscopically. Stage V and VI oocytes were selected among multistaged oocytes dissected by 2-h collagenase treatment (2 mg/ml in Ca2+-free OR2 solution) from Xenopus laevis ovaries. Oocytes were incubated in OR2 medium, which consists of 82.5 mM NaCl, 2.5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 1 mM Na2HPO4, 0.5 g/l polyvinyl pyrolidone, and 5 mM HEPES (pH 7.2), supplemented with penicillin and streptomycin (0.1 mg/ml) and 2 mM Na-pyruvate. Oocytes were then injected with 69 nl of cRNA for a final amount of 5 ng/oocyte. Oocytes were incubated in OR2 at 20°C for 2–4 days before recording.
Currents were measured using a two-electrode voltage-clamp amplifier (GeneClamp 500B; Axon Instruments) at room temperature. Electrodes (0.2–0.5 M) were filled with 3 M KCl, and oocytes were perfused with a solution containing (in mM) 100 NaCl, 2 KCl, 1 CaCl2, 2 MgCl2, and 10 HEPES, pH 7.2, plus isoamyl alcohol at the indicated concentrations. We used the pCLAMP suite of programs (Axon Instruments) for data acquisition and analysis. Currents were filtered at 200 Hz and sampled at 1 kHz.
RESULTS
Knockout of glial acd-1 exacerbates sensory deficits caused by a hypomorphic allele of tax-2.
We previously reported the cloning and characterization of ACD-1, a novel DEG/ENaC channel subunit expressed in C. elegans amphid glia (Wang et al. 2008). We found that knockout of acd-1 in glia exacerbates sensory deficits caused by a deg-1 null mutation (Chalfie and Wolinsky 1990). In this study, we tested the prediction that knockout of acd-1 in glia exacerbates sensory deficits caused by other mutations in sensory neuron genes, and we sought to shed light on the mechanism underlying ACD-1 regulation of sensory neuron function.
We thus acquired the tax-2(p694) mutant strain, crossed it with acd-1 knockout to generate acd-1 tax-2(p694) double-mutant animals, and tested the function of sensory neurons. tax-2 encodes for a β-subunit of a cGMP-gated channel expressed in several C. elegans sensory neurons. tax-2, together with the α-subunit gene tax-4, is necessary for chemotaxis to water-soluble attractants and odors, including Na+ and isoamyl alcohol, and for avoidance of repellents (Bargmann et al. 1993; Coburn and Bargmann 1996). tax-2(p694) is a hypomorphic allele in which part of the promoter region and the first exon of tax-2 are deleted (Coburn and Bargmann 1996). Loss of these portions of the tax-2 sequence likely affects tax-2 transcription. Indeed, the expression of a tax-2 GFP transgene that mimics the molecular lesion of tax-2(p694) allele, termed tax-2(delta)::GFP, is strongly reduced in AQR, AFD, ASE and BAG neurons (Coburn and Bargmann 1996).
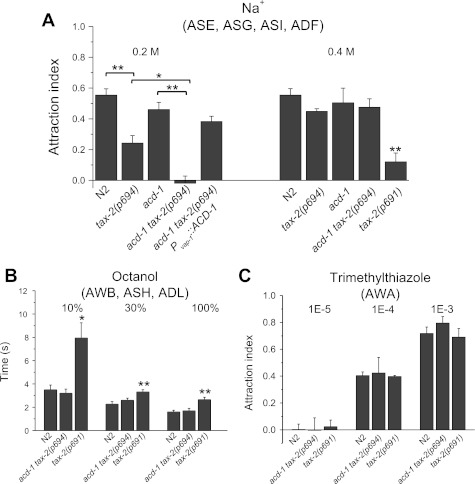
When we assayed attraction to 0.2 M Na+, we found that tax-2(p694) animals were less attracted than wild-type animals to this concentration of Na+ [Fig. 1A, means ± SE (SD): N2, 0.550 ± 0.04 (0.16); tax-2(p694), 0.240 ± 0.04 (0.16); P < 0.01]. This was not surprising, since tax-2(p694) strongly reduces expression of TAX-2 in ASE neurons, the principal chemosensory neurons that drive attraction to Na+ (Bargmann and Horvitz 1991; Suzuki et al. 2008). Knockout of acd-1 exacerbated the reduced sensitivity to Na+ of tax-2(p694) animals. acd-1 tax-2(p694) double-mutant animals were completely insensitive to 0.2 M Na+ [less sensitive than the sum of tax-2(p694) and acd-1 reduced sensitivities] and distributed randomly on a plate in which a Na+ gradient with a peak of 0.2 M was established [Fig. 1A, −0.019 ± 0.040 (0.12); P < 0.05 compared with tax-2(p694); P < 0.01 compared with acd-1]. This phenotype was rescued by expression of ACD-1 in amphid sheath cells of acd-1 tax-2(p694) mutants [Fig. 1A, 0.380 ± 0.03 (0.12)]. At 0.4 M Na+, we found that tax-2(p694), acd-1, and acd-1 tax-2(p694) single- and double-mutant animals were attracted to Na+ similarly to wild-type animals. The strong allele tax-2(p691) had severely impaired chemotaxis to 0.4 M Na+ (Coburn and Bargmann 1996) [Fig. 1A, N2, 0.525 ± 0.04 (0.153); tax-2(p694), 0.418 ± 0.028 (0.076); acd-1, 0.468 ± 0.062 (0.177); acd-1 tax-2(p694), 0.488 ± 0.048 (0.127); tax-2(p691), 0.120 ± 0.057 (0.11); P < 0.01 compared with N2].
Fig. 1.
Knockout of glial channel acd-1 worsens reduced attraction to Na+ of tax-2(p694) mutants. A: chemotaxis to 0.2 and 0.4 M Na+ was determined for wild-type Caenorhabiditis elegans (N2) and for acd-1, tax-2(p694), acd-1 tax-2(p694), acd-1 tax-2(p694); Pvap-1::ACD-1, and tax-2(p691) mutants. Number of assays was 15, 11, 7, 7, and 12 for 0.2 M Na+ and 14, 7, 8, 7, and 4 for 0.4 M Na+, respectively, with 30 animals used in each assay. tax-2(p694) mutant animals were less attracted to 0.2 M Na+ than other animals, and knockout of acd-1 worsened this chemotaxis defect. The chemotaxis index for acd-1 knockout animals is not statistically different from that of wild type. Data are means ± SE. *P < 0.05; **P < 0.01 (ANOVA). Chemotaxis to Na+ is mediated primarily by ASE neurons and to a lesser extent by ASG, ASI, and ADF neurons (Bargmann and Horvitz 1991). B: repulsion by octanol was normal in acd-1 tax-2(p694) double mutants but defective in tax-2(p691) mutants. The time that animals took to respond to octanol at 3 dilutions was recorded. Number of animals tested was 10, 10, and 16 for 10% octanol; 38, 20, and 25 for 30% octanol, and 25, 20, and 26 for 100% octanol. Data are means ± SE. *P < 0.05; **P < 0.01 (ANOVA). Octanol is sensed by AWB, ASH, and ADL neurons (Troemel et al. 1997). C: attraction to trimethylthiazole mediated by AWA neurons was normal in tax-2(p691) and acd-1 tax-2(p694) mutant animals. Attraction to trimethylthiazole at 3 dilutions was assayed. Wild type and mutants did not respond to the most diluted concentration. Number of assays was 4, 3, and 3 for 1E-5 dilution; 6, 3, and 3 for 1E-4 dilution; and 3, 4, and 3 for 1E-3 dilution with 30 animals used in each assay. Data are means ± SE.
tax-2(delta)::GFP is not expressed in AQR, AFD, ASE, and BAG neurons, which normally express TAX-2 (Coburn and Bargmann 1996). However, TAX-2 expression maybe reduced in other sensory neurons in tax-2(p694) mutants. Indeed, the tax-2(delta)::GFP and tax-2::GFP expression levels cannot be strictly compared for small changes because different transgenes could be present in different copy number, thus influencing GFP expression level. We thus considered the possibility that TAX-2 expression level in tax-2(p694) could be reduced in other neurons, resulting in an effect of glial acd-1 knockout on the sensitivity to other sensory cues. For example, TAX-2 is expressed in AWB neurons (Coburn and Bargmann 1996) that mediate avoidance of octanol (together with ASH and ADL neurons that do not express TAX-2) (Troemel et al. 1997). We found that acd-1 tax-2(p694) double-mutant animals responded normally to three different dilutions of this odor. However, tax-2(p691) showed reduced repulsion by octanol [Fig. 1B, in s, at 10%: N2, 3.57 ± 0.040 (1.20); acd-1 tax-2(p694), 3.20 ± 0.35 (1.13); tax-2(p691), 7.93 ± 1.3 (5.23); P < 0.05; at 30%: N2, 2.26 ± 0.21 (1.32); acd-1 tax-2(p694), 2.60 ± 0.18 (0.82); tax-2(p691), 3.32 ± 0.19 (0.94); P < 0.01; at 100%: N2, 1.60 ± 0.14 (0.70), acd-1 tax-2(p694), 1.70 ± 0.20 (0.92); tax-2(p691), 2.65 ± 0.19 (1.00); P < 0.01]. These results show that TAX-2 functions in AWB neurons to mediate response to octanol. They also suggest that glial acd-1 has no effect on the activity of these sensory neurons. As a control, we also tested attraction to the odor trimethylthiazole, which is sensed by AWA neurons (Bargmann 1993). AWA neurons do not express TAX-2 (Coburn and Bargmann 1996). Attraction to three dilutions (1E-5, 1E-4, and 1E-3) of trimethylthiazole was similar to wild type in both acd-1 tax-2(p694) and tax-2(p691) mutants [Fig. 1C, at 1E-5: N2, 0.025 ± 0.040 (0.081); acd-1 tax-2(p694), −0.003 ± 0.093 (0.16); tax-2(p691), 0.023 ± 0.049 (0.086); at 1E-4: N2, 0.40 ± 0.03 (0.06); acd-1 tax-2(p694), 0.42 ± 0.11 (0.20); tax-2(p691), 0.390 ± 0.008 (0.01); at 1E-3: N2, 0.71 ± 0.05 (0.08); acd-1 tax-2(p694), 0.79 ± 0.05 (0.09); tax-2(p691), 0.69 ± 0.06 (0.11)]. In conclusion, we found that knockout of acd-1 in glia worsened the reduced sensitivity of tax-2(p694) animals to 0.2 M Na+ mediated primarily by ASE, where TAX-2 expression level is strongly reduced in tax-2(p694) mutants. However, it did not affect sensory perception mediated by neurons that do not express TAX-2 (AWA). Our data on octanol avoidance also suggest that knockout of acd-1 does not indiscriminately affect the function of all sensory neurons in which TAX-2 is expressed and that are ensheathed by amphid glia, as also shown by Bacaj et al. (2008).
acd-1 tax-2(p694) double mutants have reduced sensitivity to the odor isoamyl alcohol.
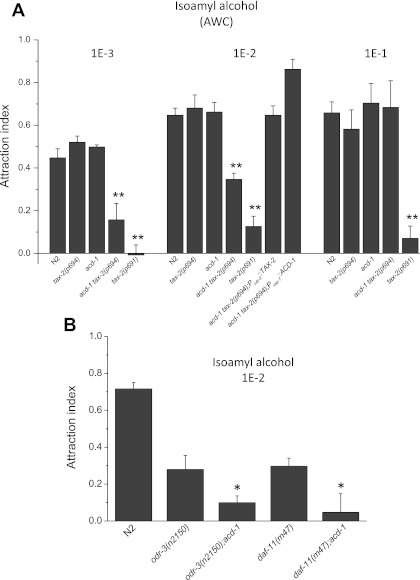
We next tested the sensitivity of tax-2(p694) and acd-1 single- and double-mutants to the odor isoamyl alcohol. AWC sensory neurons sense isoamyl alcohol (Bargmann et al. 1993). tax-2(delta)::GFP is expressed in AWC neurons, suggesting that the p694 allele does not eliminate the expression of TAX-2 in these neurons (Coburn and Bargmann 1996). However, based on the argument made above about GFP reporters, TAX-2 expression in AWC neurons of tax-2(p694) animals could still be reduced, rendering AWC neurons of this mutant susceptible to knockout of acd-1 in glia. We assayed chemotaxis to isoamyl alcohol at three different dilutions: 1E-3, 1E-2, and 1E-1. We found that tax-2(p694) and acd-1 single mutants responded to all the three dilutions of isoamyl alcohol similarly to wild type [Fig. 2A, at 1E-3: N2, 0.446 ± 0.042 (0.120); tax-2(p694), 0.520 ± 0.029 (0.060); acd-1, 0.497 ± 0.010 (0.020); at 1E-2: N2, 0.640 ± 0.033 (0.12); tax-2(p694), 0.680 ± 0.062 (0.170); acd-1, 0.662 ± 0.044 (0.140); at 1E-1: N2, 0.657 ± 0.052 (0.100); tax-2(p694), 0.581 ± 0.090 (0.220); acd-1, 0.703 ± 0.091 (0.150)]. However, acd-1 tax-2(p694) double mutants were less attracted to isoamyl alcohol at the 1E-3 and 1E-2 dilutions compared with wild type and single mutant animals [at 1E-3: 0.157 ± 0.029 (0.270), P < 0.01; at 1E-2: 0.340 ± 0.029 (0.120), P < 0.01]. The severe allele tax-2(p691) had strongly compromised sensitivity to isoamyl alcohol at all the dilutions tested [Fig. 2A, at 1E-3: −0.007 ± 0.046 (0.09), P < 0.01; at 1E-2: 0.120 ± 0.048 (0.120), P < 0.01; at 1E-1: 0.070 ± 0.057 (0.100), P < 0.01]. We conclude that knockout of glial DEG/ENaC channel acd-1 combined with neuronal tax-2(p694) mutation renders animals less sensitive to isoamyl alcohol. Notably, the reduced sensitivity to isoamyl alcohol of acd-1 tax-2(p694) mutants was rescued by expression of ACD-1 under the control of glial promoter vap-1 (Wang et al. 2008) and by expression of TAX-2 under the control of the promoter of the odr-3 gene expressed in AWC in addition to AWA, AWB, ASH, and ADF neurons (Roayaie et al. 1998) [Fig. 2A, acd-1 tax-2(p694); Pvap-1::ACD-1, 0.647 ± 0.044 (0.110); acd-1 tax-2(p694); Podr-3::TAX-2, 0.860 ± 0.047 (0.110)]. These data show that the reduced attraction to diluted isoamyl alcohol observed in acd-1 tax-2(p694) double-mutant animals is due to impaired and lack of function of TAX-2 and ACD-1 in AWC neurons and amphid glia, respectively, in these mutants. To streamline our approach and interpretations, for the remaining of the study we mostly focused on chemotaxis to isoamyl alcohol, which is mediated by only one pair, and not multiple pairs, of sensory neurons, the AWC neurons.
Fig. 2.
Knockout of acd-1 unmasks a chemotaxis defect in tax-2(p694) mutants and worsens sensory deficits caused by mutations in signaling genes odr-3 and daf-11. A: chemotaxis to isoamyl alcohol at 1E-3, 1E-2, and 1E-1 dilutions was determined for wild-type C. elegans (N2) and for acd-1, tax-2(p694), acd-1 tax-2(p694), and tax-2(p691) mutants. At 1E-2 dilution, chemotaxis of acd-1 tax-2(p694); Podr-3::TAX-2 and acd-1 tax-2(p694); Pvap-1::ACD-1 animals was also determined. Number of assays was 9, 4, 4, 13, and 4 for 1E-3 dilution; 14, 8, 19, 17, 6, 7, and 7 for 1E-2 dilution; and 4, 6, 3, 3, and 3 for 1E-1 dilution with 30 animals used in each assay. AWC neurons mediated attraction to isoamyl alcohol. **P < 0.01 compared with wild type (ANOVA). Data are means ± SE. B: attraction to isoamyl alcohol at 1E-2 dilution was assayed as described in A. Number of assays for wild type (N2), odr-3(n2150), acd-1;odr-3(n2150), daf-11(m47), and acd-1;daf-11(m47) animals was 12, 13, 13, 10, and 11, respectively, with 30 animals used in each assay. Data are means ± SE. *P < 0.05 compared with odr-3 and daf-11 single mutants (ANOVA).
Knockout of acd-1 worsens sensory deficits caused by mutations in genes that regulate TAX-2 function.
Genetic studies suggest that the cyclic nucleotide-gated channel encoded by the tax-4 and tax-2 genes is a sensory transduction channel downstream of G protein signaling. The proposed pathway for sensory transduction is that signaling by Gi protein odr-3 regulates the function of either a cGMP phosphodiesterase or a membrane guanylate cyclase encoded by the gene daf-11, resulting in closing or opening of the cGMP-gated channel (Roayaie et al. 1998; Vowels and Thomas 1994). Given that ODR-3 and DAF-11 regulate the activity of TAX-2/TAX-4, we hypothesized that knockout of acd-1 in glia worsens sensory deficits caused by mutations in odr-3 and daf-11. We crossed the acd-1 knockout strain with odr-3(n2150) and daf-11(m47) mutants and assayed attraction to isoamyl alcohol (dilution 1E-2). Both these mutants have strongly reduced but not complete loss of sensitivity to isoamyl alcohol (Roayaie et al. 1998; Vowels and Thomas 1994). We found that the acd-1;odr-3(n2150) and acd-1;daf-11(m47) double-mutant animals displayed an exacerbated phenotype [Fig. 2B, odr-3(n2150), 0.279 ± 0.075 (0.270); acd-1;odr-3(n2150), 0.099 ± 0.037(0.130); P < 0.05; daf-11(m47), 0.297 ± 0.043 (0.140); acd-1;daf-11(m47), 0.047 ± 0.100 (0.330); P < 0.05]. Thus knockout of acd-1 in glia worsens sensory deficits caused not only by mutations in tax-2 but also by mutations in Gi protein gene odr-3 and guanylate cyclase gene daf-11, whose gene products regulate the activity of TAX-2/TAX-4 channels.
TAX-2 expression level and AWC neuron structure are normal in acd-1 tax-2(p694) mutants.
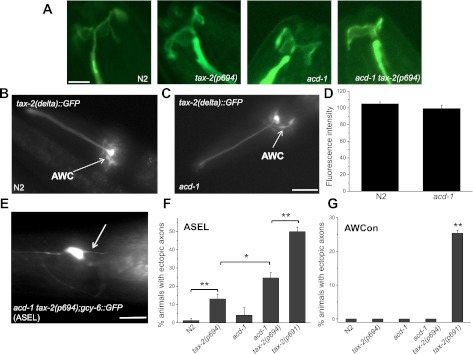
We previously showed that knockout of glial acd-1 does not cause structural defects in the glial amphid sheath cells and ASH neuron dendrites, including the cilia (Wang et al. 2008). However, we wanted to confirm that AWC sensory neurons are intact in tax-2(p694) and acd-1 single and double mutants. We also analyzed tax-2(delta)::GFP in AWC neurons to establish whether knockout of glial acd-1 causes changes in its expression level. In this case the comparison of GFP expression levels is appropriate because the same transgene is analyzed in wild-type and acd-1 knockout genetic backgrounds, so changes in GFP fluorescence are attributable only to the difference in genetic makeup of the strains and not to the number of copies of the transgene. We found that the typical fanlike structure of AWC neuron cilia (Perkins et al. 1986) was preserved in tax-2(p694) and acd-1 as well as in acd-1 tax-2(p694) mutants (Fig. 3A). We also found that the level of expression of tax-2(delta)::GFP was not affected by knockout of acd-1 in glia (Fig. 3, B–D). We conclude that knockout of acd-1 in glia does not cause defects in the structure of AWC sensory neurons or changes in the level of expression of the cGMP-gated channel subunit TAX-2 in AWC neurons.
Fig. 3.
Morphological features of sensory neurons in tax-2 and acd-1 single and double mutants. A: Z-stack projections of confocal microscope photographs of wild type (N2), tax-2(p694), acd-1, and acd-1 tax-2(p694) animals expressing GCaMP1.0 in AWCon neurons. Apparent in all genetic backgrounds is the fan-shaped cilia of the AWC neuron. Scale bar, 4 μm. B and C: fluorescent photographs of wild type (N2) and acd-1 transgenic animals expressing tax-2(delta)::GFP. In both strains, the green fluorescent protein (GFP) construct that represents expression of TAX-2 in tax-2(p694) mutant animals is apparent in AWC neurons (arrows). Scale bar, 100 μm. D: the fluorescence intensity of AWC neurons expressing the tax-2(delta)::GFP transgene in wild type (N2) and acd-1 knockout genetic backgrounds was quantified. Quantification was done using ImageJ on photographs taken using a Leica microscope equipped with GFP filters with the same exposure time (1.5 s). Data are means ± SE. Means are not statistically different; n = 20 and 18 neurons, respectively. E: fluorescent photograph of a acd-1 tax-2(p694) double-mutant animal expressing gcy-6::GFP in the ASE left (ASEL) neuron. ASEL mediates attraction to Na+ (Suzuki et al. 2008). The arrow points to an ectopic axon. Scale bar, 50 μm. F: the percentage of animals showing ectopic axons in ASEL neurons was determined in wild type (N2), tax-2(p694), acd-1, acd-1 tax-2(p694), and tax-2(p691) animals in 3 independent experiments. Total number of animals analyzed was 40, 49, 30, 48, and 42, respectively. G: the percentage of animals showing ectopic axons in AWCon neurons was also quantified in wild type (N2), tax-2(p694), acd-1, acd-1 tax-2(p694), and tax-2(p691) animals. Total number of animals analyzed was 18, 34, 27, 16, and 40, respectively. AWCon neurons express GCamP1.0 under the control of str-2 (Chalasani et al. 2007). Data are means ± SE. *P < 0.05; **P < 0.01 (ANOVA). Note that acd-1 is not statistically different from wild type in ASEL ectopic axons quantification.
Ectopic axons in sensory neurons of acd-1 tax-2(p694) mutants.
It was previously reported that mutations in tax-2 cause abnormal axonal branching of sensory neurons. Ectopic axons were found in different sensory neurons of tax-2(p691) (Coburn et al. 1998). The percentage of animals showing ectopic axons varied depending on the sensory neuron analyzed, with ASJ and ASI neurons being the most and least affected, respectively. It was suggested that ectopic axons may be caused by the reduced activity of these neurons as a result of loss of TAX-2 function (Coburn et al. 1998). We found that 24.6% of the acd-1 tax-2(p694) animals had ectopic ASE left (ASEL) axons, and this percentage was higher (50%) in tax-2(p691) animals [Fig. 3, E and F, acd-1 tax-2(p694), 24.6 ± 2.91 (5), P < 0.05 compared with tax-2(p694); tax-2(p691), 50 ± 2.4 (4), P < 0.01 compared with acd-1 tax-2(p694)] (Coburn et al. 1998). We also found that 13% of tax-2(p694) animals displayed ectopic ASEL axons [13.06 ± 2.54 (4.4)]. We did not detect ectopic axons in AWC of tax-2(p694), acd-1, or acd-1 tax-2(p694) mutants. However, 25.3% of tax-2(p691) animals had ectopic AWC axons [Fig. 3G, 25.3 ± 0.88 (1.5), P < 0.01 compared with N2]. We conclude that neurons that are most functionally affected in acd-1 tax-2(p694) mutants, the ASEL neurons, have ectopic axons that probably reflect their low functionality. Overall, our analysis shows that the severity of the ectopic axons phenotype mirrors the severity of the behavioral phenotypes, corroborating that ectopic axons reflect reduced functionality of the sensory neurons.
In vivo Ca2+ imaging reveals that AWC neurons of acd-1 tax-2(p694) mutants do not respond to diluted isoamyl alcohol.
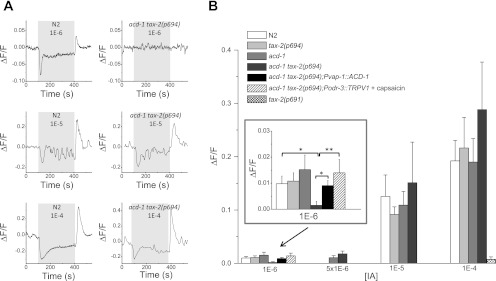
Given the reduced attraction of acd-1 tax-2(p694) double-mutant animals to isoamyl alcohol (Fig. 2A) and the fact that amphid glia ensheath the cilia of AWC sensory neurons, we next sought to determine whether AWC neurons of these mutants signal abnormally in response to exposure to isoamyl alcohol. In wild-type animals, AWC neurons respond to isoamyl alcohol by a change in intracellular Ca2+ concentration (Chalasani et al. 2007). Ca2+ is reduced by exposure to isoamyl alcohol and increases on odor removal. Isoamyl alcohol at 1E-3 dilution produces maximal Ca2+ changes in AWC neurons when perfused onto the nose of C. elegans, suggesting that it may correspond to the straight concentration or the 1E-1 dilution in behavioral assays where the odor is placed 5 cm away (Chalasani et al. 2007; Coburn and Bargmann 1996). Given that acd-1 tax-2(p694) mutants display reduced attraction to isoamyl alcohol at the 1E-3 and 1E-2 dilutions in behavioral assays, we tested 1E-6, 5E-6, 1E-5, and 1E-4 dilutions in Ca2+ imaging experiments. We found that AWC neurons of acd-1 tax-2(p694) mutants responded to isoamyl alcohol at 5E-6, 1E-5, and 1E-4 dilutions similarly to wild type [Fig. 4, A and B, in ΔF/F (change in fluorescence) at 5E-6: N2, 0.0129 ± 0.0037 (0.0145); acd-1 tax-2(p694), 0.017 ± 0.005 (0.0156); at 1E-5: N2, 0.125 ± 0.040 (0.106); tax-2(p694), 0.091 ± 0.014 (0.047); acd-1, 0.109 ± 0.025 (0.063); acd-1 tax-2(p694), 0.151 ± 0.076 (0.170); at 1E-4: N2, 0.192 ± 0.038 (0.018); tax-2(p694), 0.216 ± 0.057 (0.16); acd-1, 0.190 ± 0.04 (0.116); acd-1 tax-2(p694), 0.283 ± 0.089 (0.267)]. However, the majority of AWC neurons of acd-1 tax-2(p694) double mutants failed to respond to the 1E-6 dilution (only 1/22 responded with a ΔF/F of 0.033), whereas 9/20, 7/14, and 7/17 responded in wild-type, tax-2(p694), and acd-1 animals, respectively [Fig. 4B, inset, in ΔF/F: N2, 0.0098 ± 0.0029 (0.0132); tax-2(p694), 0.0108 ± 0.0032 (0.0122); acd-1, 0.0152 ± 0.0056 (0.0234); acd-1 tax-2(p694), 0.0015 ± 0.0015 (0.007); P < 0.05 compared with N2 or single mutants]. We conclude that acd-1 tax-2(p694) reduced attraction to diluted isoamyl alcohol is due to reduced responsiveness of AWC neurons to low concentrations of this attractant. Importantly, Ca2+ transients in response to 1E-6 isoamyl alcohol were restored in acd-1 tax-2(p694) transgenic animals expressing ACD-1 in amphid glia [Fig. 4B, inset, in ΔF/F: 0.0091 ± 0.0019 (0.0061)]. Note that the Ca2+ transients elicited in wild-type animals are smaller than the ones observed by Chalasani et al. (2007). This difference may be related to the different perfusion system used in our experiments. Our data also suggest that the 1E-2 dilution of isoamyl alcohol in chemotaxis assays probably results in a concentration of 1E-6 of this odor at an ∼5-cm distance from the assay spot. Behavioral and AWC Ca2+ defects in acd-1 tax-2(p694) mutants were detected in chemotaxis and Ca2+ imaging assays, respectively, at these two concentrations. As expected, AWC neurons of tax-2(p691) mutants did not respond to the highest concentration of isoamyl alcohol [Fig. 4B, in ΔF/F: 0.0076 ± 0.0045 (0.0155)], supporting the possibility that the lack of attraction of tax-2(p691) mutants to isoamyl alcohol is due to the insensitivity of AWC neurons in this mutant to this odor.
Fig. 4.
Reduced Ca2+ responses on exposure to isoamyl alcohol in AWC neurons of acd-1 tax-2(p694) double-mutant animals. A: examples of Ca2+ responses elicited by perfusion with isoamyl alcohol at 1E-6, 1E-5, and 1E-4 dilutions in AWCon neurons of wild-type (N2) and acd-1 tax-2(p694) double-mutant animals as indicated. Ca2+ decreases on perfusion with the odor and increased on odor removal (Chalasani et al. 2007). Ca2+ changes were detected in only 1 AWCon neuron of acd-1 tax-2(p694) double-mutant animals at 1E-6 out of 22 examined. Gray box indicates the time of perfusion with isoamyl alcohol. Ca2+ changes are reported as changes in GCaMP1.0 fluorescence (ΔF/F) expressed in AWCon neurons (Chalasani et al. 2007). B: average increase of fluorescence, and thus Ca2+, on odor removal in wild-type (N2), tax-2(p694), acd-1, and acd-1 tax-2(p694) mutant animals at different concentrations as indicated. At 1E-4 dilution, Ca2+ changes in AWCon neurons of tax-2(p691) mutants are also shown. Inset represents a larger scale for the data at 1E-6 dilution. At this concentration, Ca2+ changes on odor removal in acd-1 tax-2(p694); Pvap-1::ACD-1 and acd-1 tax-2(p694); Podr-3::TRPV1 animals in the presence of capsaicin (50 μM) are also shown. Number of animals analyzed was 20, 14, 17, 22,10, and 10 for 1E-6 dilution; 15 and 9 for 5E-6 dilution; 7, 11, 6, and 5 for 1E-5 dilution; and 23, 8, 7, 9, and 12 for 1E-4 dilution. Data are means ± SE. *P < 0.05; **P < 0.01 (ANOVA). [IA], isoamyl alcohol concentration.
Glial amphid sheath cells and ACD-1 channels are sensitive to the odor isoamyl alcohol, but at higher concentrations.
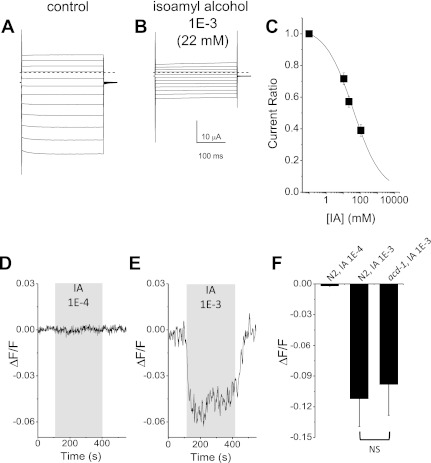
One simple model of how ACD-1 channel expressed in glia may contribute to AWC neurons sensitivity to isoamyl alcohol would be that ACD-1 channels are themselves sensitive to isoamyl alcohol. To test this possibility, we assayed ACD-1 sensitivity to isoamyl alcohol in the Xenopus oocytes expression system. We found that ACD-1 currents were inhibited by perfusion with isoamyl alcohol (Fig. 5, A and B). However, the Ki was 38 mM, corresponding to a dilution of ∼1.7E-3 (Fig. 5C), a concentration that is ∼1,700 times higher than the concentration at which AWC neurons of acd-1 tax-2(p694) mutants fail to respond to isoamyl alcohol (Fig. 4). Do glial amphid sheath cells respond to isoamyl alcohol, and if they do, does ACD-1 channel mediate this response? We looked at Ca2+ responses in glial amphid sheath cells on perfusion of animals with isoamyl alcohol at 1E-4 and 1E-3 dilutions. We found that isoamyl alcohol at 1E-3 dilution caused a reduction in Ca2+ concentration. However, knockout of acd-1 did not significantly alter these Ca2+ changes, suggesting that acd-1 does not contribute to this response [Fig. 5, D–F, in ΔF/F: N2, −0.111 ± 0.03 (0.10); acd-1, −0.098 ± 0.030 (0.08)]. We conclude that glial amphid sheath cells respond to the odor isoamyl alcohol by reduction of intracellular Ca2+ concentration. However, this response is not mediated by ACD-1 channels, and it is elicited by concentrations of the odor that are above the concentrations at which AWC neurons of acd-1 tax-2(p694) mutants fail to respond. The sensitivity of glial amphid sheath cells to odors needs further investigation but does not seem to contribute to the phenotype that we observe in acd-1 tax-2(p694) double-mutant animals.
Fig. 5.
ACD-1 channels and glial amphid sheath cells are inhibited by high concentrations of isoamyl alcohol. A: family of ACD-1 currents in a Xenopus oocyte injected with acd-1 cRNA. Currents were elicited by voltage steps from −160 to + 60 mV in 20-mV increments in an oocyte perfused with a physiological solution. The holding voltage was −30 mV. B: the same oocyte shown in A was perfused with physiological solution plus 1E-3 isoamyl alcohol. C: isoamyl alcohol dose-response curve. Data are means ± SE; n = 17. Data were fitted by a sigmoid curve that indicates a Ki of 38 mM (1.7E-3 dilution). D: example of GCamP1.0 fluorescence that reports intracellular Ca2+ changes in amphid sheath cells of a wild-type animal (N2) during perfusion with 1E-4 isoamyl alcohol. At this concentration, there was no change in intracellular Ca2+ in glial amphid sheath cells. E: same as in D during perfusion of a wild-type animal (N2) with 1E-3 isoamyl alcohol. F: average fluorescence changes in amphid sheath cells of wild-type (N2) and acd-1 mutant animals perfused with isoamyl alcohol at 1E-4 and 1E-3 dilutions as indicated. Data are means ± SE; n = 8, 15, and 8, respectively. NS, not significantly different.
Activation of rat TRPV1 expressed in AWC neurons by capsaicin ameliorates the chemotaxis defects of acd-1 tax-2(p694) mutants.
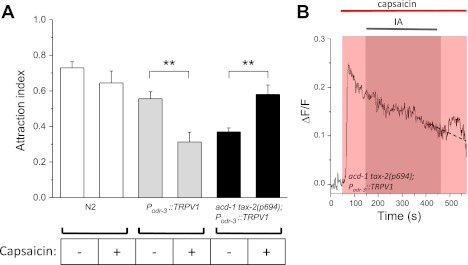
Our results show that knockout of acd-1 in glia worsens sensory deficits caused by mutations in channel subunit genes. One feature of many ion channels is that they are sensitive to the voltage across the plasma membrane. DEG/ENaCs such as DEG-1 and cGMP-gated channels such as TAX-2/TAX-4 are not classical voltage-gated channels, but they often display rectification properties that render their activity sensitive to the voltage across the membrane (Goodman et al. 2002; Komatsu et al. 1999). In addition, voltage-gated channels could be present in AWC neurons, where they could be necessary for amplification of the signal (Suzuki et al. 2003) or to generate action potentials (Mellem et al. 2008). We thus hypothesized that ACD-1 in glia might be involved directly or indirectly in regulating the membrane potential of sensory neurons, therefore resulting in tuning of sensory channels activity. To test this hypothesis, we designed an experiment in which the membrane potential of AWC sensory neurons could be exogenously manipulated. We expressed the rat TRPV1 channel in AWC sensory neurons of wild-type and acd-1 tax-2(p694) animals (Tobin et al. 2002). TRPV1 is a capsaicin-gated channel that conducts cations into the cell, and thus it induces membrane depolarization in the presence of capsaicin (Caterina et al. 1997). We then assayed attraction to isoamyl alcohol at the dilution of 1E-2 in these transgenic animals in the absence and presence of capsaicin. We found that whereas Podr-3::TRPV1 animals were less attracted to isoamyl alcohol in the presence of capsaicin, acd-1 tax-2(p694); Podr-3::TRPV1 animals were more attracted to this odor in the presence of capsaicin [Fig. 6A, P odr-3::TRPV1, 0.555 ± 0.039 (0.10); Podr-3::TRPV1 in the presence of capsaicin, 0.310 ± 0.054 (0.15); P < 0.01; acd-1 tax-2(p694); Podr-3::TRPV1, 0.370 ± 0.022 (0.05); acd-1 tax-2(p694); Podr-3::TRPV1 in the presence of capsaicin, 0.580 ± 0.052 (0.140); P < 0.01]. Activation of TRPV1 in AWC is expected to cause membrane depolarization and an increase in intracellular Ca2+. To confirm this, we imaged AWC neurons of acd-1 tax-2(p694);Podr-3::TRPV1 while perfusing the animal's nose with capsaicin. We found that capsaicin caused a long-lasting increase in intracellular Ca2+ in these neurons (Fig. 6B). When we added isoamyl alcohol (1E-6) to the capsaicin solution, we detected transient elevation of Ca2+ on odor removal [Figs. 6B and 4B, inset, in ΔF/F: acd-1 tax-2(p694); Podr-3::TRPV1 in the presence of capsaicin, 0.013 ± 0.005 (0.016)]. These results suggest that AWC membrane depolarization and/or an increase in basal Ca2+ level produced by activation of TRPV1 exerts a rescuing effect in AWC neurons of acd-1 tax-2(p694) mutants. Together, our data suggest that knockout of acd-1 in glia combined with the hypomorphic mutation tax-2(p694) directly or indirectly causes hyperpolarization of the membrane potential or reduction of intracellular Ca2+ in AWC neurons, which in turn renders these neurons less responsive to isoamyl alcohol. Our data also suggest that there might be an optimal level of membrane potential (or Ca2+) in AWC neurons that ensures an efficient response to odors, as also suggested by Ferkey et al. (2007).
Fig. 6.
Opposite effects of activating TRPV1 in AWC neurons on chemotaxis to isoamyl alcohol in wild type and acd-1 tax-2(p694) double mutants. A: the rat capsaicin receptor TRPV1 was expressed in AWC neurons of wild type and acd-1 tax-2(p694) mutants using the promoter of odr-3. The animals' attraction to 1E-2 isoamyl alcohol in the absence and presence of capsaicin (50 μM) in both genetic backgrounds was then determined. Attraction of wild-type animals (N2) to 1E-2 isoamyl alcohol in the presence of capsaicin was determined as control. Data for wild-type animals in the absence of capsaicin are from Fig. 2B and are shown for comparison. Data are means ± SE. Number of assays was 11, 7, 7, 8, 7, and 8, respectively, with 30 animals used in each assay. **P < 0.01. B: GCaMP1.0 fluorescence in an AWCon sensory neuron of a acd-1 tax-2(p694); Podr-3::TRPV1 transgenic animal during perfusion of the animal with capsaicin (50 μM) and with capsaicin (50 μM) + isoamyl alcohol (1E-6). GCaMP1.0 fluorescence (thus Ca2+) increased when capsaicin was added to the bath and remained elevated to ∼50% of the maximal level throughout the recording. Removal of isoamyl alcohol caused a transient elevation of fluorescence (dashed line indicates the expected fluorescence level based on the decay rate).
DISCUSSION
Glia in C. elegans sensory perception.
Recent work highlights the profound importance of glia in sensory perception in C. elegans (Bacaj et al. 2008; Wang et al. 2008). Bacaj and colleagues ablated amphid glia and found 1) defects in sensory cilia structure in AWA and AWC with reduced chemotaxis to AWA- and AWC-sensed odorants and 2) reduced chemotaxis to sodium without defects in cilia structure of sodium-sensing neurons ASE. This work establishes that glia play a role not only in neuronal development but also in neuronal function. We have shown previously and in the present study that knockout of acd-1 in glia does not compromise the structure of glia or neurons (Wang et al. 2008) but influences the function of sensory neurons. In particular, the fanlike structure of the cilia in AWC neurons is preserved in acd-1 tax-2(p694) mutant animals (Perkins et al. 1986), yet these animals are less sensitive to isoamyl alcohol.
It should be emphasized that our previous work and present data suggest that knockout of acd-1 does not compromise the activity of all sensory neurons. For example, AWB neurons do not seem affected (Fig. 1, C and D). Typically, neuronal function has to be already altered by a mutation in a neuronal gene in order for the knockout of acd-1 to have an impact on the phenotype (Fig. 1, A and B, and Wang et al. 2008). The exception that we found is attraction to isoamyl alcohol mediated by AWC neurons (Fig. 2A). In this case the attraction to isoamyl alcohol is not compromised in tax-2(p694) animals, at least not under our experimental conditions. However, when tax-2(p694) mutation is combined with acd-1 knockout, then a phenotype becomes apparent.
Insight into the role of DEG/ENaC channels in glia/neuron functional interaction.
Our experiments, in which we depolarized the membrane potential and increased intracellular Ca2+ concentrations of AWC neurons by exogenously expressing the rat capsaicin receptor TRPV1 and exposing the animals to capsaicin, suggest that ACD-1 may contribute in a direct or indirect way to keep the membrane potential of sensory neurons relatively depolarized or their basal Ca2+ level relatively high. Our data showing the deleterious effect of depolarizing and increasing intracellular Ca2+ levels in wild-type sensory neurons suggest that the level of Ca2+ or resting potential must remain within a certain range to ensure optimal sensory neurons function, as shown by Ferkey et al. (2007).
How may ACD-1, which is expressed in glia, influence the membrane potential or basal Ca2+ level of sensory neurons? There are several possible mechanisms, including effects of ACD-1 on the expression level, localization, or function of proteins involved in sensory signaling or in maintaining neuronal excitability or basal Ca2+ levels in sensory neurons, effects on the extracellular ionic composition, and effects on the release of glial factors that influence neuronal excitability (Gourine et al. 2010). More experiments are needed to distinguish between these possibilities. However, analogy with a mammalian sensory structure, the Pacinian corpuscle, and similarity of ACD-1 with mammalian ENaCs suggest a possible mechanism that will be further investigated. In some tissues, such as the collecting duct of the kidney, Na+ transport through ENaCs establishes a favorable driving force for K+ excretion through K+ channels. It is possible that glial ENaCs such as ACD-1 facilitate K+ excretion in the microenvironment between the glia and the neurons, therefore setting the resting potential (or resting Ca2+). Such a model would fit with our previously reported finding that ACD-1 is enriched at the end of the amphid sheath cell process where ensheathing of the dendrites occurs (Wang et al. 2008).
Ilyinsky et al. (1976) indeed used integrative ultramicroflame photometry to show that K+ concentration in the fluid between the nerve fiber and the internal lamellae of the Pacinian corpuscle of the cat is higher than in blood and surrounding tissues. Using electrophysiological techniques, they also showed that a concentration of K+ between 6 and 12 mM lowers the mechanical threshold of these mechanosensory structures. Interestingly, K+ concentrations higher than 12 mM lower the sensitivity of Pacinian corpuscles. Thus there is a range of K+ concentration that is optimal for Pacinian corpuscles function. Interestingly, the DEG/ENaC channel ASIC2 is expressed on the internal lamellae (Calavia et al. 2010).
At this point we do not know what proteins in the sensory neurons are affected by glial ACD-1. Voltage-gated channels and channels with rectification properties are influenced by membrane voltage, and many ion channels and signaling proteins are influenced by intracellular Ca2+. Regardless of whether sensory neurons fire regenerative action potentials (Mellem et al. 2008), they are likely to depend on at least one Ca2+- or voltage-sensitive component for sensory transduction.
There are a total of 56 glial cells in the adult C. elegans hermaphrodite and 28 genes in C. elegans that encode for DEG/ENaC channel subunits (Goodman and Schwarz 2003); the expression pattern of 20 of these is still unknown. It will be interesting to establish whether DEG/ENaC subunits that are highly homologous to ACD-1 are expressed in glia and play a role in sensory perception. In mammals, DEG/ENaCs have been found in brain astrocytes (Berdiev et al. 2003; Vila-Carriles et al. 2006, 2007), in Schwann cells associated with Ruffini endings (Hitomi et al. 2009), in Muller cells of the retina (Brockway et al. 2002), and in the internal lamellae of the Pacinian corpuscles (Calavia et al. 2010). However, little is known about the role of DEG/ENaC channels in these cells. It is possible that mammalian DEG/ENaCs expressed in glia play roles similar to C. elegans ACD-1. Future studies using glial-targeted DEG/ENaC knockouts are needed to test this possibility.
GRANTS
This work was supported by American Cancer Society Grant RGS-09-043-01-DDC and National Institute of Neurological Disorders and Stroke (NINDS) Grant NS070969 (to L. Bianchi) and by NINDS Training Grant NS07044–33 (to Y. Wang).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Y.W., G.D., and L.B. performed experiments; Y.W., G.D., and L.B. analyzed data; Y.W., G.D., and L.B. edited and revised manuscript; Y.W., G.D., and L.B. approved final version of manuscript; L.B. conception and design of research; L.B. interpreted results of experiments; L.B. prepared figures; L.B. drafted manuscript.
ACKNOWLEDGMENTS
We thank the C. elegans Genetics Center and the Bargmann and Shaham laboratories for providing nematode strains. We are especially grateful to Grigoris Oikonomou from the Shaham laboratory for sharing the unpublished OS1984 strain. We also thank Junichi Nakai and the Rinken Bank for granting the use of the GCaMP strains for calcium imaging and Stephen Roper, Nirupa Chaudari, and Isabel Perea-Martinez for help with the confocal microscope. We are grateful to Cori Bargmann, Sreekanth H. Chalasani, and Shai Shaham for helpful comments, to Sophia Hassor for technical assistance, and to Gerhard Dahl, Peter Larsson, and Emily Clark Khan for critical reading of the manuscript.
REFERENCES
- Bacaj T, Tevlin M, Lu Y, Shaham S. Glia are essential for sensory organ function in C. elegans. Science 322: 744–747, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann CI. Chemosensation in C. elegans. WormBook: 1–29, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann CI. Genetic and cellular analysis of behavior in C. elegans. Annu Rev Neurosci 16: 47–71, 1993 [DOI] [PubMed] [Google Scholar]
- Bargmann CI, Hartwieg E, Horvitz HR. Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell 74: 515–527, 1993 [DOI] [PubMed] [Google Scholar]
- Bargmann CI, Horvitz HR. Chemosensory neurons with overlapping functions direct chemotaxis to multiple chemicals in C. elegans. Neuron 7: 729–742, 1991 [DOI] [PubMed] [Google Scholar]
- Berdiev BK, Xia J, McLean LA, Markert JM, Gillespie GY, Mapstone TB, Naren AP, Jovov B, Bubien JK, Ji HL, Fuller CM, Kirk KL, Benos DJ. Acid-sensing ion channels in malignant gliomas. J Biol Chem 278: 15023–15034, 2003 [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics 77: 71–94, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockway LM, Benos DJ, Keyser KT, Kraft TW. Blockade of amiloride-sensitive sodium channels alters multiple components of the mammalian electroretinogram. Vis Neurosci 22: 143–151, 2005 [DOI] [PubMed] [Google Scholar]
- Brockway LM, Zhou ZH, Bubien JK, Jovov B, Benos DJ, Keyser KT. Rabbit retinal neurons and glia express a variety of ENaC/DEG subunits. Am J Physiol Cell Physiol 283: C126–C134, 2002 [DOI] [PubMed] [Google Scholar]
- Calavia MG, Montano JA, Garcia-Suarez O, Feito J, Guervos MA, Germana A, Del Valle M, Perez-Pinera P, Cobo J, Vega JA. Differential localization of acid-sensing ion channels 1 and 2 in human cutaneus pacinian corpuscles. Cell Mol Neurobiol 30: 841–848, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389: 816–824, 1997 [DOI] [PubMed] [Google Scholar]
- Chalasani SH, Chronis N, Tsunozaki M, Gray JM, Ramot D, Goodman MB, Bargmann CI. Dissecting a circuit for olfactory behaviour in Caenorhabditis elegans. Nature 450: 63–70, 2007 [DOI] [PubMed] [Google Scholar]
- Chalfie M. Neurosensory mechanotransduction. Nat Rev Mol Cell Biol 10: 44–52, 2009 [DOI] [PubMed] [Google Scholar]
- Chalfie M, Wolinsky E. The identification and suppression of inherited neurodegeneration in Caenorhabditis elegans. Nature 345: 410–416, 1990 [DOI] [PubMed] [Google Scholar]
- Chandrashekar J, Kuhn C, Oka Y, Yarmolinsky DA, Hummler E, Ryba NJ, Zuker CS. The cells and peripheral representation of sodium taste in mice. Nature 464: 297–301, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Zimmer A, Sun WH, Hall J, Brownstein MJ. A role for ASIC3 in the modulation of high-intensity pain stimuli. Proc Natl Acad Sci USA 99: 8992–8997, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coburn CM, Bargmann CI. A putative cyclic nucleotide-gated channel is required for sensory development and function in C. elegans. Neuron 17: 695–706, 1996 [DOI] [PubMed] [Google Scholar]
- Coburn CM, Mori I, Ohshima Y, Bargmann CI. A cyclic nucleotide-gated channel inhibits sensory axon outgrowth in larval and adult Caenorhabditis elegans: a distinct pathway for maintenance of sensory axon structure. Development 125: 249–258, 1998 [DOI] [PubMed] [Google Scholar]
- Cohen MW, Gerschenfeld HM, Kuffler SW. Ionic environment of neurones and glial cells in the brain of an amphibian. J Physiol 197: 363–380, 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferkey DM, Hyde R, Haspel G, Dionne HM, Hess HA, Suzuki H, Schafer WR, Koelle MR, Hart AC. C. elegans G protein regulator RGS-3 controls sensitivity to sensory stimuli. Neuron 53: 39–52, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman MB, Ernstrom GG, Chelur DS, O'Hagan R, Yao CA, Chalfie M. MEC2 regulates C. elegans DEG/ENaC channels needed for mechanosensation. Nature 415: 1039–1042, 2002 [DOI] [PubMed] [Google Scholar]
- Goodman MB, Schwarz EM. Transducing touch in Caenorhabditis elegans. Annu Rev Physiol 65: 429–452, 2003 [DOI] [PubMed] [Google Scholar]
- Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K, Kasparov S. Astrocytes control breathing through pH-dependent release of ATP. Science 329: 571–575, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitomi Y, Suzuki A, Kawano Y, Nozawa-Inoue K, Inoue M, Maeda T. Immunohistochemical detection of ENaCbeta in the terminal Schwann cells associated with the periodontal Ruffini endings of the rat incisor. Biomed Res 30: 113–119, 2009 [DOI] [PubMed] [Google Scholar]
- Hummler E, Barker P, Gatzy J, Beermann F, Verdumo C, Schmidt A, Boucher R, Rossier BC. Early death due to defective neonatal lung liquid clearance in alpha-ENaC-deficient mice. Nat Genet 12: 325–328, 1996 [DOI] [PubMed] [Google Scholar]
- Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci 10: 1369–1376, 2007 [DOI] [PubMed] [Google Scholar]
- Ilyinsky OB, Akoev GN, Krasnikova TL, Elman SI. K and Na ion content in the Pacinian corpuscle fluid and its role in the activity of receptors. Pflügers Arch 361: 279–285, 1976 [DOI] [PubMed] [Google Scholar]
- Jasti J, Furukawa H, Gonzales EB, Gouaux E. Structure of acid-sensing ion channel 1 at 1.9 A resolution and low pH. Nature 449: 316–323, 2007 [DOI] [PubMed] [Google Scholar]
- Jourdain P, Bergersen LH, Bhaukaurally K, Bezzi P, Santello M, Domercq M, Matute C, Tonello F, Gundersen V, Volterra A. Glutamate exocytosis from astrocytes controls synaptic strength. Nat Neurosci 10: 331–339, 2007 [DOI] [PubMed] [Google Scholar]
- Komatsu H, Jin YH, L'Etoile N, Mori I, Bargmann CI, Akaike N, Ohshima Y. Functional reconstitution of a heteromeric cyclic nucleotide-gated channel of Caenorhabditis elegans in cultured cells. Brain Res 821: 160–168, 1999 [DOI] [PubMed] [Google Scholar]
- Kuffler SW. Neuroglial cells: physiological properties and a potassium mediated effect of neuronal activity on the glial membrane potential. Proc R Soc Lond B Biol Sci 168: 1–21, 1967 [DOI] [PubMed] [Google Scholar]
- Lobsiger CS, Cleveland DW. Glial cells as intrinsic components of non-cell-autonomous neurodegenerative disease. Nat Neurosci 10: 1355–1360, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellem JE, Brockie PJ, Madsen DM, Maricq AV. Action potentials contribute to neuronal signaling in C. elegans. Nat Neurosci 11: 865–867, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello CC, Kramer JM, Stinchcomb D, Ambros V. Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J 10: 3959–3970, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perens EA, Shaham S. C. elegans daf-6 encodes a patched-related protein required for lumen formation. Dev Cell 8: 893–906, 2005 [DOI] [PubMed] [Google Scholar]
- Perkins LA, Hedgecock EM, Thomson JN, Culotti JG. Mutant sensory cilia in the nematode Caenorhabditis elegans. Dev Biol 117: 456–487, 1986 [DOI] [PubMed] [Google Scholar]
- Price MP, Lewin GR, McIlwrath SL, Cheng C, Xie J, Heppenstall PA, Stucky CL, Mannsfeldt AG, Brennan TJ, Drummond HA, Qiao J, Benson CJ, Tarr DE, Hrstka RF, Yang B, Williamson RA, Welsh MJ. The mammalian sodium channel BNC1 is required for normal touch sensation. Nature 407: 1007–1011, 2000 [DOI] [PubMed] [Google Scholar]
- Price MP, McIlwrath SL, Xie J, Cheng C, Qiao J, Tarr DE, Sluka KA, Brennan TJ, Lewin GR, Welsh MJ. The DRASIC cation channel contributes to the detection of cutaneous touch and acid stimuli in mice. Neuron 32: 1071–1083, 2001 [DOI] [PubMed] [Google Scholar]
- Roayaie K, Crump JG, Sagasti A, Bargmann CI. The G alpha protein ODR-3 mediates olfactory and nociceptive function and controls cilium morphogenesis in C. elegans olfactory neurons. Neuron 20: 55–67, 1998 [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Brady JD, Mohr C. Astrocyte metabolism and signaling during brain ischemia. Nat Neurosci 10: 1377–1386, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci 10: 1361–1368, 2007 [DOI] [PubMed] [Google Scholar]
- Sluka KA, Price MP, Breese NM, Stucky CL, Wemmie JA, Welsh MJ. Chronic hyperalgesia induced by repeated acid injections in muscle is abolished by the loss of ASIC3, but not ASIC1. Pain 106: 229–239, 2003 [DOI] [PubMed] [Google Scholar]
- Suzuki H, Kerr R, Bianchi L, Frokjar-Jensen C, Slone D, Xue J, Gerstbrein B, Driscoll M, Schafer WR. In vivo imaging of C. elegans mechanosensory neurons demonstrates a specific role for the MEC-4 channel in the process of gentle touch sensation. Neuron 39: 1005–1017, 2003 [DOI] [PubMed] [Google Scholar]
- Suzuki H, Thiele TR, Faumont S, Ezcurra M, Lockery SR, Schafer WR. Functional asymmetry in Caenorhabditis elegans taste neurons and its computational role in chemotaxis. Nature 454: 114–117, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin D, Madsen D, Kahn-Kirby A, Moulder G, Barstead R, Maricq A, Bargmann C. Combinatorial expression of TRPV channel proteins defines their sensory functions and subcellular localization in C. elegans neurons. Neuron 35: 307–318, 2002 [DOI] [PubMed] [Google Scholar]
- Troemel ER, Chou JH, Dwyer ND, Colbert HA, Bargmann CI. Divergent seven transmembrane receptors are candidate chemosensory receptors in C. elegans. Cell 83: .207–218, 1995 [DOI] [PubMed] [Google Scholar]
- Troemel ER, Kimmel BE, Bargmann CI. Reprogramming chemotaxis responses: sensory neurons define olfactory preferences in C. elegans. Cell 91: 161–169, 1997 [DOI] [PubMed] [Google Scholar]
- Vesce S, Rossi D, Brambilla L, Volterra A. Glutamate release from astrocytes in physiological conditions and in neurodegenerative disorders characterized by neuroinflammation. Int Rev Neurobiol 82: 57–71, 2007 [DOI] [PubMed] [Google Scholar]
- Vila-Carriles WH, Kovacs GG, Jovov B, Zhou ZH, Pahwa AK, Colby G, Esimai O, Gillespie GY, Mapstone TB, Markert JM, Fuller CM, Bubien JK, Benos DJ. Surface expression of ASIC2 inhibits the amiloride-sensitive current and migration of glioma cells. J Biol Chem 281: 19220–19232, 2006 [DOI] [PubMed] [Google Scholar]
- Vila-Carriles WH, Zhou ZH, Bubien JK, Fuller CM, Benos DJ. Participation of the chaperone Hsc70 in the trafficking and functional expression of ASIC2 in glioma cells. J Biol Chem 282: 34381–34391, 2007 [DOI] [PubMed] [Google Scholar]
- Volterra A, Meldolesi J. Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci 6: 626–640, 2005 [DOI] [PubMed] [Google Scholar]
- Vowels JJ, Thomas JH. Multiple chemosensory defects in daf-11 and daf-21 mutants of Caenorhabditis elegans. Genetics 138: 303–316, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Apicella A, Jr, Lee SK, Ezcurra M, Slone RD, Goldmit M, Schafer WR, Shaham S, Driscoll M, Bianchi L. A glial DEG/ENaC channel functions with neuronal channel DEG-1 to mediate specific sensory functions in C. elegans. EMBO J 27: 2388–2399, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Bianchi L. Insights into the molecular determinants of proton inhibition in an acid-inactivated degenerins and mammalian epithelial Na+ channel. Biochemistry 48: 10005–10013, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wemmie JA, Chen J, Askwith CC, Hruska-Hageman AM, Price MP, Nolan BC, Yoder PG, Lamani E, Hoshi T, John H, Freeman J, Welsh MJ. The acid-activated ion channel ASIC contributes to synaptic plasticity, learning and memory. Neuron 34: 463–477, 2002 [DOI] [PubMed] [Google Scholar]
- Yu S, Avery L, Baude E, Garbers DL. Guanylyl cyclase expression in specific sensory neurons: a new family of chemosensory receptors. Proc Natl Acad Sci USA 94: 3384–3387, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]