Abstract
Successful behavior demands motor learning to be transferable in some cases (e.g., adjusting walking patterns as we develop and age) and context specific in others (e.g., learning to walk in high heels). Here we investigated differences in motor learning transfer in people learning a new walking pattern on a split-belt treadmill, where the legs move at different speeds. We hypothesized that transfer of the newly acquired walking pattern on the treadmill to natural over ground walking might depend on the pattern of errors experienced during learning. Error patterns within a person's natural range might be experienced as endogenous (i.e., produced by the body), encouraging general adjustments that transfer across contexts. On the other hand, larger errors might be experienced as exogenous (i.e., produced by the environment), indicating unusual conditions requiring context-specific learning. To test this, we manipulated the distribution of errors experienced during learning to lie either within or outside the normal distribution of walking errors. We found that restriction of errors to the natural range produced transfer of the new walking pattern from the treadmill to natural walking off the treadmill, while larger errors prevented transfer. This result helps explain how transfer of motor learning is controlled, and it offers an important strategy for clinical rehabilitation, where transfer of motor learning to other contexts is essential.
Keywords: generalization, human, kinematics, locomotion, motor control
the nervous system has the ability to adapt movements to compensate for changes in the environment or the body. Many studies have demonstrated that robots and treadmills can be used to induce motor learning (i.e., adaptation) by creating new environmental demands (see, e.g., Deubel et al. 1986; Kagerer et al. 1997; Lackner and DiZio 1994; Reisman et al. 2005; Shadmehr and Mussa-Ivaldi 1994; Tremblay et al. 2003). Moreover, “device-induced” learning could be used to rehabilitate subjects with motor deficiencies (Bastian 2008), but it is critical that the learning transfers to “real-world” situations. Unfortunately, it has been demonstrated that a single session of “device-induced” learning only transfers partially and transiently to natural movements (Cothros et al. 2006; Reisman et al. 2009; Reynolds and Bronstein 2004). In walking, we recently demonstrated that removing visual context cues during split-belt walking adaptation partially improves the transfer of treadmill learning to over ground walking, since only the adaptation of temporal—but not of spatial—gait features is transferred (Torres-Oviedo and Bastian 2010). Here we ask whether errors during adaptation might have a more general effect in the specificity of device-induced learning in locomotion.
The notion of credit assignment, which we define as the ability to assign errors to the environment or the body, may be important for understanding transfer of learning (Berniker and Kording 2008). If the source of error that drives learning were estimated to be the environment, one would ideally adapt and apply the learning only to that particular situation. Conversely if the source of error were estimated to be our own faulty movements, one would ideally adapt and apply the learning to any other movement. Consistent with this idea, a promising study has demonstrated that learning induced by a robot transfers to unconstrained reaching movements when subjects experience small errors during gradual perturbations (Kluzik et al. 2008), possibly because small errors can be more easily attributed to subjects' own movements as opposed to the device.
Therefore, the history of errors normally experienced and their similarity to those experienced with the devices might determine the transfer of device-induced learning. Recent studies have demonstrated that the magnitude of errors (Körding and Wolpert 2004; Wei and Körding 2009) and their variability during training can affect the rate of learning (Burge et al. 2008; Korenberg and Ghahramani 2002; Wei and Körding 2010) or what we learn (i.e., a force to counteract a predictable perturbation vs. a force to counteract the average of variable perturbations experienced) (for review, see Davidson and Wolpert 2003). Here we ask whether the error size and variance affect the transfer of learning to movements without the device. To test this question, we manipulated the error that subjects experienced while learning a new walking pattern with a split-belt treadmill. We reasoned that errors out of the ordinary would be assigned to the device—leading to poor transfer—whereas errors usually experienced while walking would be assigned to subjects' natural movement—leading to larger transfer. Therefore we manipulated independently the error variability or size during locomotor adaptation and tested the transfer of learning from these adaptation conditions to natural over ground walking.
METHODS
General Paradigm
Subjects adapted their walking pattern on a split-belt treadmill, and we tested the transfer of this learning to over ground walking (i.e., off the treadmill). Locomotor adaptation was achieved with a split-belt treadmill (Woodway USA, Waukesha, WI) that drove the speed of each leg independently. This paradigm has been demonstrated to induce the storage of a modified walking pattern that is expressed as an aftereffect in regular walking conditions and must be deadapted to return to normal walking (Reisman et al. 2005). The Institutional Review Board at the Johns Hopkins University School of Medicine approved the experimental protocol, and all subjects gave informed consent prior to testing.
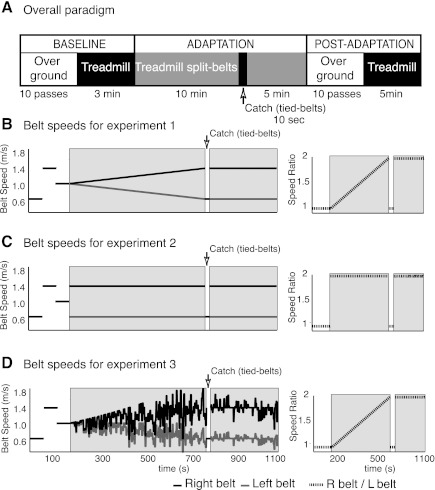
In all experiments, we recoded the subjects' motor behavior during baseline, adaptation, and postadaptation periods off and on the treadmill (Fig. 1A). We collected a baseline period prior to adaptation in which subjects walked with the belts moving together (i.e., “tied”) at three different speeds, a slow (0.75 m/s), a fast (1.5 m/s), and an intermediate (1.125 m/s) speed, for 1 min each. Subjects were then exposed for a total of 15 min to an adaptation period in which the belts on the treadmill were moving at different speeds (i.e., “split”). The belt speed asymmetry was different across experimental groups to alter the errors during adaptation (see experiment descriptions). After 10 min of split-belt adaptation, we collected a 10-s “catch” period during which both belts were moving together at the same speed as in the slow baseline period (0.75 m/s). The recordings during this catch period allowed us to assess storage of the adaptation effects (i.e., aftereffects) on the treadmill. Subjects then walked in the split-belt condition for an additional 5 min to readapt the walking pattern. The treadmill was stopped and restarted again at every speed transition. After the entire adaptation period, subjects were transported on a wheelchair from the treadmill to a 6-m walkway where the over ground transfer was tested. Subjects walked on the walkway for 10 back-and-forth passes to test for transfer to over ground walking of aftereffects due to the split-belt treadmill adaptation. Subjects were asked not to step when sitting in the wheelchair and when standing up in order to record as many of their initial steps after split-belt treadmill adaptation as possible. The self-selected walking speeds in all subjects ranged between 0.8 and 1.2 m/s. After over ground transfer was assessed, subjects returned to the treadmill and walked for 5 min in the tied-belts condition at 0.75 m/s. This last period allowed us to test for washout of the treadmill aftereffects due to over ground walking. We choose to assess aftereffects on the treadmill during catch and washout periods at the slow belt speed since it has been shown to induce the largest aftereffects (Vasudevan and Bastian 2010).
Fig. 1.
General paradigm and perturbation speeds. A: in all groups, baseline behavior was recorded over ground and subsequently on the treadmill. Subjects were then adapted for a total of 15 min. A 10-s catch trial was introduced when subjects had been adapted for 10 min. Subjects were readapted for 5 more min before they were asked to walk over ground, where we tested the transfer of treadmill adaptation to natural walking. Finally, subjects returned to the treadmill, where they walked for 5 min to determine the washout of learning specific to the treadmill from the remaining aftereffects. During baseline and postadaptation periods over ground subjects walked multiple back-and-forth passes on a 6-m walkway. B: belt speed time course and belt speed ratio for experiment 1 (gradual adaptation). During baseline period both belts moved at 0.75 m/s, then at 1.5 m/s, and finally at 1.125 m/s. During the adaptation period belts started moving at 1.125 m/s, then the speed of the belt under the right foot was gradually increased to 1.5 m/s, and the belt under the left foot was gradually decreased to 0.75 m/s. Consequently, the belts' speed ratio gradually changed from 1:1 to 2:1. The 2 belts moved at the same speed (0.75 m/s) during the 10-s catch trial. Subjects were then readapted by maintaining the 2:1 speed ratio. C: belt speed time course and ratio for experiment 2 (abrupt adaptation). Baseline speeds were the same as in experiment 1. During the adaptation period, the belt under the right foot moved at 1.5 m/s and the belt under the left foot moved at 0.75 m/s. Thus the belts' speed ratio abruptly changed from 1:1 to 2:1. Belt speeds during catch and readaptation were the same as in experiment 1. D: belt speed time course and ratio for experiment 3 (noisy adaptation). Belt speeds were variable during adaptation. However, mean speeds during both baseline and adaptation were the same as in experiment 1. Thus the belts' speed ratios during adaptation and readaptation in experiment 3 were the same as in experiment 1.
Experimental Design
To determine whether errors during adaptation can be manipulated to improve transfer of walking adaptation on a treadmill to natural walking, we designed three experiments. In experiment 1, error variability and magnitude during adaptation were kept small by perturbing subjects gradually (Fig. 1B). In experiment 2, error variability during adaptation was kept small, but error magnitude was increased by perturbing subjects abruptly (Fig. 1C). Finally, in experiment 3 error variability during adaptation was increased, but error magnitude was kept small by perturbing subjects gradually but with variable belt speeds (Fig. 1D).
Twenty-four healthy adults participated in this study. Eight subjects (3 men and 5 women; mean age 28.3 ± 7.9 yr) participated in experiment 1, eight subjects (3 men and 5 women; mean age 25.3 ± 4.5 yr) participated in experiment 2, and eight subjects (5 men and 3 women; mean age 25.2 ± 5.9 yr) participated in experiment 3. Since data recordings were noisy in one of the subjects in experiment 1, we excluded this subject from our data analysis.
Experiment 1: gradual adaptation.
Subjects were adapted gradually to keep their error size and error variability during adaptation small. During the first 10 min of adaptation, the belt under the left leg linearly decreased its speed from 1.125 m/s to 0.75 m/s and the belt under the right leg linearly increased its speed from 1.125 m/s to 1.5 m/s. After the catch period (when both belts moved at the same speed), the belts were maintained at a 2-to-1 ratio for an additional 5 min to readapt the walking pattern before testing the learning transfer to over ground walking (Fig. 1B, left: belt speed ratio, right: belt speed time course).
Experiment 2: abrupt adaptation.
Subjects were adapted with an abrupt perturbation to manipulate their error size during adaptation without changing their error variability. During the entire 15 min of the adaptation period belts were maintained at a 2-to-1 ratio (Fig. 1C, left). The belt under the left leg moved at 0.75 m/s, and the belt under the right leg moved at 1.5 m/s (Fig. 1C, right).
Experiment 3: gradual noisy adaptation.
Subjects were adapted with variable speeds on a mean gradual perturbation to manipulate their error variability without changing their error size. The belt speed ratio during the 15 min of adaptation was the same as in experiment 1 (Fig. 1D, right). The speed ratio was linearly increased from 1:1 to 2:1 during the first 10 min of adaptation and then maintained at 2:1 during the 5 min of readaptation. However, we added Gaussian white noise with a constant mean and variance (mean = 0, variance = 0.03) to the treadmill speeds while maintaining the same speed ratio. Speeds were changed every 3 s, and they ranged from 0.3 m/s to 1.8 m/s (Fig. 1D, left).
Adaptation, transfer, and washout of these three groups (i.e., gradual, abrupt, and noisy) were compared to determine the effect of error size and error variability in learning and generalization.
Data Collection
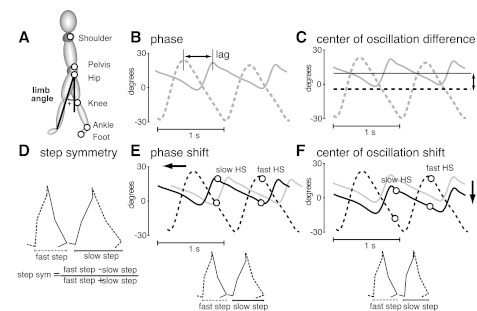
Kinematic data were collected at 100 Hz with Optotrak (Northern Digital). Infrared-emitting markers were placed bilaterally over the following joints: foot (fifth metatarsal head), ankle (lateral malleolus), knee (lateral femoral epicondyle), hip (greater trochanter), pelvis (iliac crest), and shoulder (acromion process). Marker location is indicated in Fig. 2A. The times of heel strike and toe-off (i.e., when the foot contacts and lifts off the ground) were recorded by foot switches placed on the bottom of the shoes or were estimated from the ankle kinematic data. In all experiments subjects were instructed to walk with their arms crossed to allow for data collection without occlusion of hip and pelvis markers.
Fig. 2.
A: diagram of marker locations. Limb angle convention is shown on the stick figure. B: limb angle trajectories plotted as a function of time in early split-belt adaptation; 2 cycles are shown. Limb angles are positive when the limb is in front of the trunk (flexion). Phase quantifies the lag producing the largest cross-correlation between the 2 legs. When the legs move in antiphase, the lag of 0.5 leads to the largest cross-correlation. C: limb angle trajectories are shown in gray, and angle axes about which each leg oscillates are shown in black. In this example, the slow limb (solid line) oscillates more forward with respect to the vertical axis than the fast limb (dashed line). The center of oscillation quantifies the difference in where the legs oscillate, illustrated by the distance between 2 black lines. Since the center of oscillation is dependent upon where the foot is placed at heel strike and where it is lifted off at toe-off, this measure reflects spatial locomotor control. D: an example of kinematic data of 2 consecutive steps is shown. Kinematic data for every 2 steps were used to calculate step symmetry, defined as the difference in step lengths normalized by the step length sum. E: gray trajectory represents the movement in the slow limb in early adaptation. Two time points are marked: slow heel strike (HS) in black and fast HS in gray. The spread between the limb angles is directly proportional to the step lengths shown at bottom. Step lengths can be equalized by changing the timing of foot landing, as shown by the change in phase of the slow limb from the gray trajectory (early adaptation) to the black trajectory. This purely temporal strategy is known as phase shift since subjects equalize step lengths by changing the timing of foot landings with respect to each other. F: step lengths can also be equalized by changing the position of the foot at landing (i.e., the “spatial” placement of the foot). This spatial strategy is known as a shift in the center of oscillation difference since subjects change the midpoint angle around which each leg oscillates with respect to the other leg.
Data Analysis
Learning, transfer, and washout indexes.
We quantified the magnitude of adaptation on the treadmill (TMlearning), its transfer to over ground walking (OGtransfer and %OGtransfer), and subsequent washout of the adaptation when returning to the treadmill (TMwashout and %TMwashout) in the following manner. TMlearning was defined as the size of the catch trial, corrected for any baseline biases (Eq. 1). OGtransfer and TMwashout were similarly corrected for baseline (Eqs. 2 and 3). %OGtransfer and %TMwashout were the OGtransfer and TMwashout values expressed as a percentage of TMlearning (Eqs. 4 and 5):
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
OGbaseline and TMbaseline are the mean of all strides in the over ground and treadmill baseline periods, respectively. TMcatch is the average aftereffect per step during the catch trial period. In other words, TMcatch is the sum of aftereffects divided by the total number of steps taken during the catch trial period. We chose this measure to assess the overall aftereffects experienced on the treadmill after adaptation. OGafter and TMafter are the mean aftereffect of the first three strides during postadaptation periods when subjects walked off and on the treadmill, respectively.
We quantified the learning (TMcatch), transfer (OGtransfer and %OGtransfer), and washout (TMwashout and %TMwashout) temporal and spatial gait features separately, because we have recently shown that the learning (Malone and Bastian 2010) and transfer (Torres-Oviedo and Bastian 2010) of these features can be modulated differently.
To quantify temporal gait features we used phase shift between the two legs. To this end, we computed the cross-correlation between limb angle trajectories during one full step cycle for each leg. Limb angle was defined as the angle between the vertical and the vector from hip to foot ankle on the x-y plane (Fig. 2A). Phase shift was the lag or lead time for a maximum correlation between limb angle trajectories (Fig. 2B). A phase shift value of 0.5 would indicate that legs are moving in antiphase. To correct for subjects' biases, we subtracted the phase shift during the baseline period from all other periods. Consequently, a value of 0 indicates that legs are moving in antiphase, positive phase shifts indicate that the fast leg is phase advanced relative to the slow leg, and negative phase shifts indicate that the fast leg is lagging the slow leg.
To quantify spatial gait features we used center of oscillation difference, which is defined as the difference between angles of oscillation of each leg (Fig. 2C). The angle of oscillation is defined as the angle between the vertical axis (0° axis in Fig. 2C) and the axis about which the leg is oscillating—illustrated by the black dashed and solid lines in Fig. 2C. A center of oscillation value of 0 would indicate that both legs are oscillating about the same axis, a positive value would indicate that the leg on the fast belt is oscillating about an axis that is forward to the one of the leg on the slow belt, and a negative value would indicate that the leg on the slow belt is oscillating about an axis that is forward to the one of the leg on the fast belt (Vasudevan et al. 2011).
We also quantified step length symmetry—defined as the difference between step lengths of the two legs [step length = distance between 2 ankle markers at time of foot contact (heel strike) of leading leg] (Fig. 2D). This difference was normalized by the step length sum to account for step length differences across subjects. A step length symmetry value of 0 would indicate that step lengths are equal, a positive value would indicate that the leg on the fast belt is taking longer steps, and a negative value would indicate that the leg on the slow belt is taking longer steps.
Error distribution analysis.
In all groups, we characterized the error during locomotor adaptation with step length symmetry. We chose step symmetry as a measure of error because it is a global parameter that characterizes temporal and spatial gait asymmetries (Malone and Bastian 2010). We have recently shown that subjects can combine the adaptation of phase shift and center of oscillation shift to equalize step lengths (Malone and Bastian 2010; also shown in Fig. 2, E and F). In other words, step symmetry values depend on when they place the foot on the ground and where they place it.
We used step symmetry values to quantify three error distribution features in every subject: 1) percentage of errors out of the normal range of walking, 2) error mean size, and 3) error variability.
To quantify the percentage of errors out of the normal range of walking (ErrorsOut), we first defined the normal range of errors by computing the 95% confidence interval (CI) of errors (i.e., ±2 × standard deviation of error values) during baseline walking on the treadmill. We did not use baseline values over ground since we did not have enough samples to assume normality. In addition, all subjects were naive to walking on a split-belt treadmill before adaptation, so we assumed that their steps on the treadmill during baseline were similar to those normally experienced over ground. ErrorsOut was computed by counting the number of errors during adaptation that were larger or smaller than the limits specified by CI. ErrorsOut was expressed as a percentage of the total number of errors experienced during adaptation (i.e., all step symmetry values during adaptation). We also calculated ErrorsOut during baseline walking over ground to verify that CI was a good representation of errors normally experienced.
To compute the mean of the error distribution during adaptation for each subject, we calculated the mean step symmetry error over the entire adaptation period. To compute the variance of the error distribution during adaptation for each subject, we calculated the variance around the step symmetry adaptation curve. To do so, we first removed the mean of the adaptation curve (e.g., see Fig. 3). Since the mean changed at a slow rate, we subtracted it by high-pass filtering the data at 0.05 strides/s with a 3rd-order Butterworth. After this filtering procedure the mean for all the groups was not significantly different from zero (all t-values >0.87, P = 0.39). Then we computed the sum of squared residuals and the variance from these data points with zero mean.
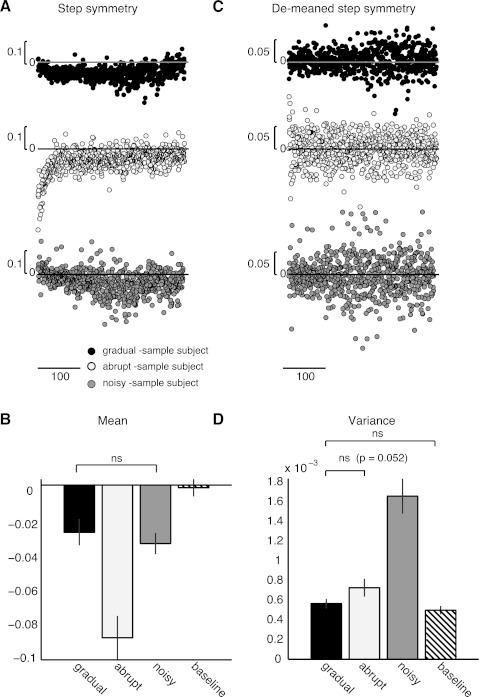
Fig. 3.
A: error (i.e., step symmetry) during adaptation for sample subjects of the gradual (black), abrupt (white), and noisy (gray) groups. Subjects adapted gradually (gray and black dots) (i.e., noisy and gradual groups) maintained step symmetry values close to zero, but subject adapted abruptly had initially larger errors (white dots). Scale bar indicates 100 steps. B: mean errors during adaptation compared with baseline over ground walking for all groups. The mean error for the noisy and gradual groups was not significantly different (ns; P = 0.97). However, the mean error that the abrupt group experienced was significantly larger than that of the noisy (P < 0.001) and gradual (P < 0.001) groups. Mean errors experienced during adaptation in all groups were significantly larger than the errors experienced over ground (hatched bar) (P < 0.001). C: error variability (i.e., high-pass filtered step symmetry time course) during adaptation for sample subjects of the gradual (black), abrupt (white), and noisy (gray) groups. Variability in the step-by-step data of the subject in the noisy group (gray dots) is larger than that in the subjects of the other groups (black and white dots), as indicated by the spread in gray dots compared with black and white dots. D: averaged error variance that subjects experienced during adaptation in each group compared with baseline over ground walking. Error variance in the noisy group was significantly larger than that in the gradual (P = 0.03) and abrupt (P = 0.04) groups and during over ground walking (P < 0.001). However, error variance normally experienced during over ground walking was similar to that during adaptation in the gradual (P = 0.37) and abrupt (P = 0.25) groups.
We also computed error size and variability normally experienced by using the step symmetry values during the baseline walking period on the treadmill. The error size was the mean step symmetry error over the entire baseline period. The variability was the variance of the step symmetry error after subtracting the overall mean during the baseline period (i.e., we assumed the mean at each point was constant). To validate our baseline variance values we also calculated the variance of errors during baseline as we did for the adaptation data using the high-pass filtering method described above. Error variance values were similar with both methods [F(1,44) = 0.9, P = 0.76].
Statistical Analysis
One-way ANOVA was used to compare error size, error variability, learning, transfer, and washout across experimental groups; post hoc analyses were performed with Fisher's least significant difference (LSD) test.
We also performed a stepwise multiple regression to test how transfer (%OGtransfer) was affected by three factors: experimental group (Gadapt), ErrorsOut, and magnitude of initial error (InitialE). InitialE was calculated as the average of the absolute step symmetry values during the first 10 steps. The categorical regressor Gadapt was set to 1 when subjects were trained gradually, 2 when subjects were trained abruptly, and 3 when subjects were trained with variable perturbations. The predicted transfer values were obtained as the linear combination of ErrorsOut, InitialE and Gadapt.
Stated formally:
where
We used P < 0.05 as a measure of significance for all statistical analyses, which were completed with Statistica (StatSoft, Tulsa, OK) software.
RESULTS
Error Size
We found that adaptation to abrupt perturbations leads to larger mean error size than adaptation to gradual perturbations. Figure 3A shows single-subject examples of step-by-step data during adaptation from the gradual, abrupt, and noisy groups for step symmetry. When subjects were adapted gradually, the error is maintained near zero for the entire adaptation. When subjects were adapted abruptly, we observed a larger initial error that is gradually decreased as subjects learn a new walking pattern. We observed differences in the absolute errors experienced during adaptation across groups [F(3,42) = 26.77, P < 0.001; Fig. 3B]. The mean error size that subjects experienced when adapted to abrupt perturbations was larger than when they were adapted to gradual perturbations (P < 0.001). On the other hand, subjects in the gradual and noisy groups experienced similar mean errors during adaptation (P = 0.64). Figure 3B also shows that mean error size experienced during adaptation is also significantly larger than during baseline walking before adaptation (P < 0.04).
Error Variability
We found that adaptation to variable perturbations leads to larger error variability than adaptation to perturbations at consistent speeds. Figure 3C shows single-subject examples of step-by-step data from the gradual, abrupt, and noisy groups for step symmetry after high-pass filtering (see methods). When subjects were adapted gradually but with variable speeds, the error variability—illustrated by the spread in errors (Fig. 3C)—is larger than when subjects were adapted gradually or abruptly. Consequently, the error variance that subjects experienced when adapted to variable perturbations is larger than when they were adapted to gradual or abrupt perturbations [F(3,42) = 11.45, P < 0.001; Fig. 3D]. Post hoc tests showed that subjects in the noisy group had greater error variance than the gradual (P < 0.001) and abrupt (P = 0.02) groups. Also, the variability in errors normally experienced during baseline walking was similar to that experienced in the gradual group (P = 0.97) but larger than in the abrupt (P = 0.01) and variable (P < 0.001) perturbations.
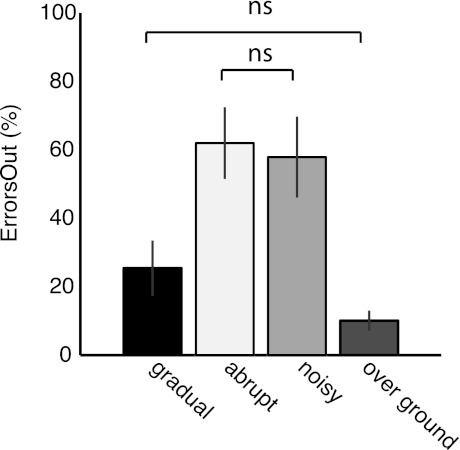
We next tested whether the responses to gradual perturbations, which had the lowest and least variable errors, fell within the normal baseline range more often than the responses to abrupt or noisy perturbations. Figure 4 shows that there is a significant effect of adaptation condition on the percentage of errors out of the normal range (ErrorsOut) [F(3,42) = 21.38, P < 0.001]. Subjects in the gradual group, who experienced during adaptation smaller and less variable errors than the other two groups, showed less ErrorsOut than the abrupt (P < 0.001) and noisy (P = 0.004) groups. In addition, the percentage of ErrorsOut in the gradual group was similar to errors made when walking over ground (P = 0.11).
Fig. 4.
Errors out of the normal range (ErrorsOut) for all groups during adaptation and during over ground walking. Bar height indicates the averaged ErrorsOut across subjects ± SE. Subjects in the noisy and abrupt groups had significantly more ErrorsOut than those in the gradual group (P < 0.004). In addition, the extent of ErrorsOut in the gradual group was similar to the extent of ErrorsOut during over ground walking prior to adaptation (P = 0.1).
Large or Variable Errors During Adaptation Increase Learning
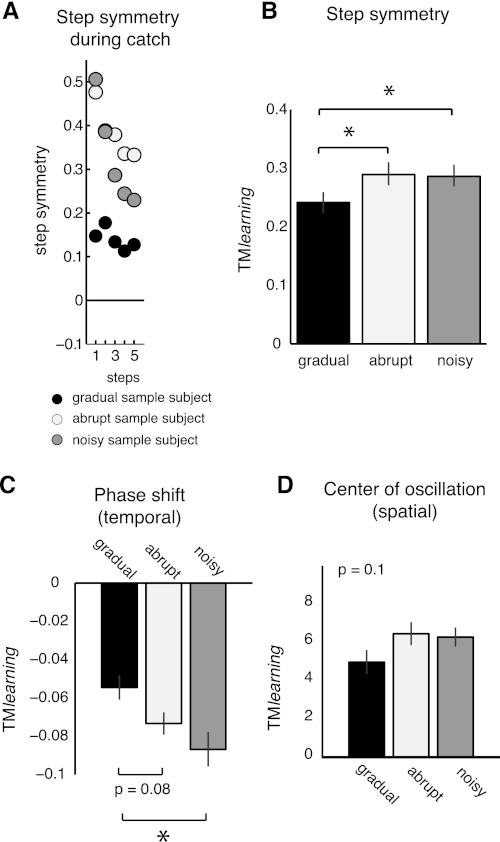
We found that error size and error variability during adaptation modulate how much is learned during split-belt walking adaptation. Recall that learning was assessed with the magnitude of adaptation aftereffects on treadmill during catch trials. We observed differences in learning across groups. These differences are, for example, illustrated in Fig. 5A, showing single-subject examples of step-by-step data from the gradual, abrupt, and noisy groups. When subjects experienced larger errors during adaptation (abrupt group) or were more variable (noisy group), we observed larger aftereffects during catch trials on the treadmill than in the gradual group. We found a significant effect of condition on treadmill learning [F(2,20) = 3.73, P = 0.04; Fig. 5B]. Subjects in the gradual group, who experienced smaller and less variable errors than the other two groups, showed smaller treadmill learning (i.e., TMlearning) than the abrupt (P = 0.02) and noisy (P = 0.04) groups.
Fig. 5.
Error statistics affect treadmill learning. A: examples of subject's step-by-step step symmetry during the catch trial on the treadmill for all groups. The subject in the gradual (black) group experienced small errors and low variability during adaptation. This subject had smaller aftereffects on the treadmill during the catch trial than subjects in the noisy (small error, high variability; gray) and abrupt (large error, low variability; white) groups. B: mean aftereffects on the treadmill during the catch trial for all groups. Bar height indicates the averaged TMlearning across subjects ± SE. Adaptation aftereffects on the treadmill in the gradual (black bar) group were significantly smaller than those in the abrupt (white bar; P = 0.02) and noisy (gray bar; P = 0.04) groups. Asterisks over the bars indicate the statistical significant differences (P < 0.05). C: aftereffects during the catch trial for phase shift (temporal parameter). Subjects in the gradual group had significantly fewer phase shift aftereffects (i.e., less learning) than subjects in the abrupt and noisy groups—experiencing larger or more variable errors, respectively. D: aftereffects during the catch trial for center of oscillation (spatial parameter). A trend similar to phase shift is observed for center of oscillation: smaller aftereffects in the gradual group than in the other 2 groups.
A similar trend was observed in the more specific parameters characterizing temporal and spatial gait features (Fig. 5, C and D). We found a significant effect of condition on timing adaptation {i.e., phase shift [F(2,20) = 4.78, P = 0.02]}, and a similar trend was found in the magnitude of spatial adaptation effects {i.e., center of oscillation [F(2,20) = 1.88, P = 0.1]}. For the temporal parameter, the gradual group showed smaller treadmill learning (i.e., TMlearning) than the noisy group (P = 0.006) and a similar trend was observed compared with the abrupt group (P = 0.08) (Fig. 5C). A similar trend was also observed for the spatial parameter (Fig. 5D).
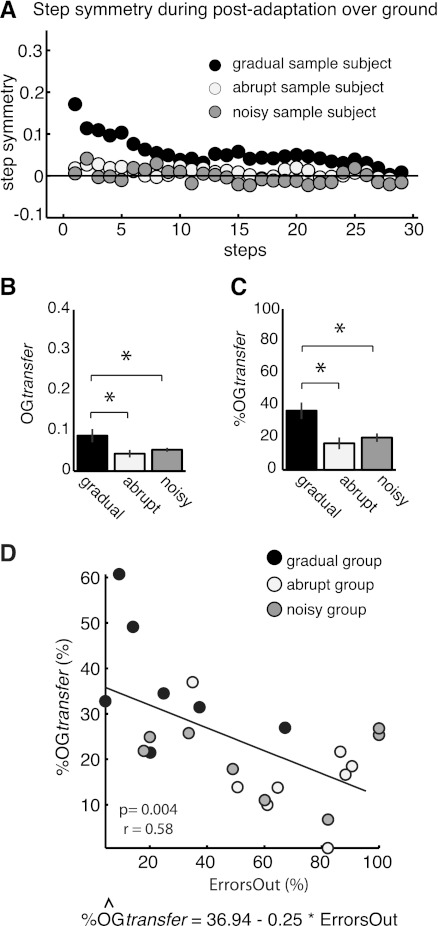
Out-of-the-Ordinary Errors Reduce Transfer
Over ground transfer in the gradual group was larger than in the abrupt and noisy groups, as illustrated in the single-subject examples shown in Fig. 6A. Figure 6B shows group data for the absolute amount of transfer. We saw a significant effect of adaptation condition on the aftereffects when subjects walked over ground, with the gradual group showing much more transfer [F(2,20) = 4.64, P = 0.02]. Recall that subjects in the gradual group also learned slightly less on the treadmill (see Fig. 5), so Fig. 6C shows over ground transfer for all groups as a percentage of learning on the treadmill. Here again there was a significant effect of condition [F(2,20) = 7.53, P = 0.003], and the gradual group showed larger %OGtransfer values compared with the other two groups.
Fig. 6.
Error statistics affect transfer of treadmill learning to over ground walking. A: examples of subject's step-by-step step symmetry when subjects walk off the device (i.e., over ground walking) after adaptation in all groups. The subject in the gradual (black) group, with small errors and low variability, had larger aftereffects over ground after adaptation than the other groups, as illustrated by the larger step symmetry values. B: mean aftereffects during first 3 steps over ground after adaptation in all groups. Bar height indicates the averaged OGtransfer across subjects ± SE. Asterisks over the bars indicate the statistically significant differences (P < 0.05). Transfer of adaptation effects to over ground walking was larger in the gradual (black bar) group than in the abrupt (white bar; P = 0.002) group, and a similar trend was observed compared with the noisy (gray bar; P = 0.06) group. C: normalized transfer (%OGtransfer) expressed as % of treadmill learning. Bar height indicates the averaged %OGtransfer across subjects ± SE. Asterisks below the bars indicate the statistically significant differences (P < 0.05). Transfer of adaptation effects to over ground walking improved significantly when subjects experienced small errors during adaptation (i.e., gradual group). This is indicated by the significantly larger %OGtransfer values in the gradual group compared with the abrupt (white bar) and noisy (gray bar) groups (P < 0.001). D: scatterplots showing the relationship between ErrorsOut and %OGtransfer. Black, white, and gray dots indicate the different adaptation groups. ErrorsOut was the only significant factor that predicted the transfer of adaptation effects to over ground walking (regression equation is shown). The magnitude of transfer was negatively related to the extent of ErrorsOut during adaptation in each subject: the more ErrorsOut of the normal range during adaptation, the less transfer of learning.
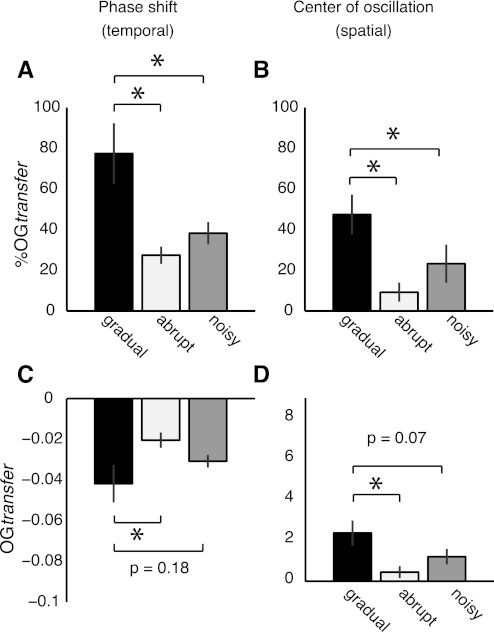
This was also true for the phase shift (temporal parameter) [F(2,20) = 8.38, P = 0.002; Fig. 7A] and center of oscillation (spatial parameter) [F(2,20) = 5.46, P = 0.01; Fig. 7B]. The gradual group had a significant larger transfer of temporal (P < 0.005) and spatial (P < 0.05) %OGtransfer values compared with the other groups. Similar results were observed in the absolute temporal [F(2,20) = 3.5, P = 0.049; Fig. 7C] and spatial [F(2,20) = 4.8, P = 0.02; Fig. 7D] OGtransfer values. Taken together, these results suggest that when subjects experience more ordinary errors, as in the gradual group, there is more temporal and spatial transfer of learning to natural movements.
Fig. 7.
Transfer of temporal and spatial adaptation effects to over ground walking. A: transfer of aftereffects for phase shift expressed as % of treadmill learning. More transfer of adaptation aftereffects to over ground walking is observed in subjects adapted gradually (gradual group) than in subjects adapted abruptly (abrupt group) or with variable speeds (noisy group). B: transfer of aftereffects for center of oscillation expressed as % of treadmill learning. Similar to the results for the temporal parameter, more transfer of adaptation aftereffects to over ground walking is observed in the gradual group than in the other 2 groups—experiencing larger or more variable errors during adaptation. C: aftereffects when subjects walked over ground for phase shift. Aftereffects over ground are significantly larger in the gradual group than in the abrupt group (P = 0.01), and a similar trend is observed between the gradual group and the noisy group (P = 0.18). D: aftereffects when subjects walked over ground for center of oscillation. Similar to the results for the temporal parameter, aftereffects for the spatial parameter are significantly larger in the gradual group than in the abrupt group (P = 0.006), and a similar trend is observed between the gradual group and the noisy group (P = 0.07).
Finally, we performed a stepwise regression analysis to determine whether the group assignment, ErrorsOut, or InitialE predicted the transfer to over ground walking. We found that ErrorsOut was a significant regressor (P = 0.004), while InitialE (P = 0.27) and condition (P = 0.12) were not. Figure 6D shows the predicted %OGtransfer as a function of ErrorsOut for each subject. This result indicates that transfer is not limited by experiencing sudden errors at the beginning of the adaptation or by the experimental group assignment. Transfer of treadmill aftereffects has to do with the extent of ErrorsOut.
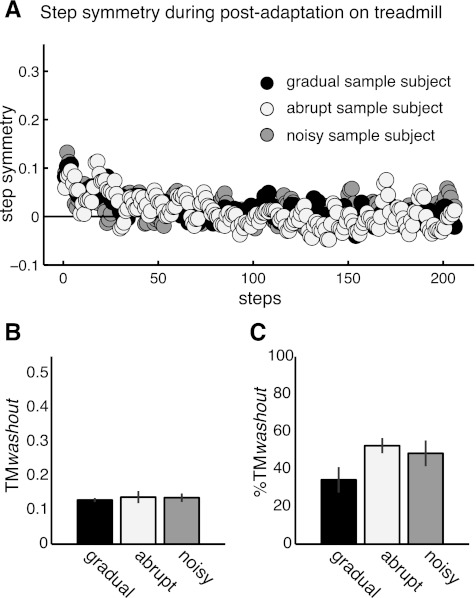
Washout of Treadmill Adaptation
We found that error size and variability during adaptation did not affect the remaining aftereffects when subjects returned to the treadmill after walking over ground. Figure 8A shows single-subject examples of step-by-step data from the gradual, abrupt, and noisy groups for step symmetry values on the treadmill during postadaptation walking. We observed an overlap in the postadaptation curves of the subjects from the three groups (Fig. 8A). Therefore, the adaptation condition did not have a significant effect on the washout of treadmill learning after walking over ground [F(2,20) = 0.11, P = 0.89; Fig. 8B]. Similarly, there was not an effect of error size and variability on the normalized washout index, %TMwashout [F(2,20) = 2.42, P = 0.1; Fig. 8C], suggesting that the over ground experience had a similar effect on the treadmill-specific learning across groups.
Fig. 8.
Effect of error statistics on washout. A: examples of subject's step-by-step step symmetry when subjects returned to the treadmill after over ground walking in all groups. Similar adaptation aftereffects remained when subjects returned to the treadmill in all groups, as indicated by the overlapping step symmetry values in all groups. B: mean aftereffects on treadmill during first 3 steps in postadaptation after over ground walking in all groups. Bar height indicates the averaged adaptation washout (TMwashout) across subjects ± SE. Washout of adaptation effects specific to the treadmill were the same in all groups (P = 0.89). C: normalized washout (%TMwashout) expressed as % of treadmill learning. Bar height indicates the averaged %TMwashout across subjects ± SE. Similar to TMwashout values presented in B, we observe there was not a significant effect of adaptation condition on the normalized washout index.
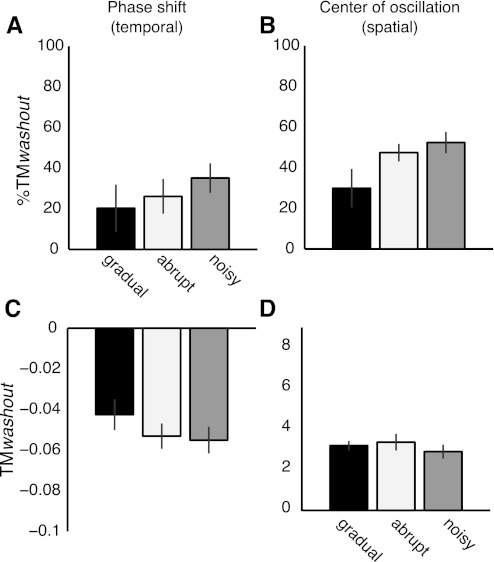
This held true for temporal and spatial adaptation effects. There was no effect of adaptation condition in %TMwashout for phase shift [F(2,20) = 0.67, P = 0.52; Fig. 9A], and there was a trend for center of oscillation [F(2,20) = 3.19, P = 0.06; Fig. 9B]. The trend is likely due to the differences in the normalization factor (TMlearning) across groups (Fig. 6), since the remaining aftereffects TMwashout were similar across groups when washout values were not normalized [F(2,20) = 0.95, P = 0.4 for phase shift, F(2,20) = 0.47, P = 0.63 for center of oscillation; Fig. 9, C and D]. Therefore, these results suggest that error size and variability did not have an effect on the washout of treadmill-specific learning.
Fig. 9.
Washout of spatial and temporal adaptation effects after over ground walking. A: washout of aftereffects for phase shift expressed as % of treadmill learning. There is not a significant effect of adaptation condition on washout of treadmill aftereffects (P = 0.5), suggesting that over ground walking washed out the treadmill learning similarly across groups. B: washout of aftereffects for center of oscillation expressed as % of treadmill learning. Similar to the results for the temporal parameter, over ground walking washed out the treadmill learning similarly across groups (P = 0.06). Therefore, error size or error variability during adaptation does not have an effect on the washout of learning specific to the treadmill. C: aftereffects when subjects returned to the treadmill for phase shift. Although the size of remaining aftereffects for the gradual group is smaller than in the other groups, there is not a significant effect of adaptation condition on aftereffect size (P = 0.4). D: aftereffects when subjects returned to the treadmill after walking over ground for center of oscillation. Similar to the results for the temporal parameter, there is not a significant effect of adaptation condition on aftereffect size (P = 0.6).
DISCUSSION
Our results demonstrate that the type of errors experienced during treadmill adaptation strongly affects the transfer to natural walking. Errors that fall within a subject's normal repertoire (Fig. 4) lead to an adapted walking pattern that transfers to natural over ground walking. In contrast, large errors that fall outside the normal range result in an adapted pattern that does not transfer, despite stronger learning on the treadmill. These results are important because our previous work has suggested that transfer is limited by the prevailing differences in contextual cues between the training and the testing settings (Torres-Oviedo and Bastian 2010). Instead, we may be able to facilitate transfer simply by changing how we introduce new perturbations or environments.
We also found that our manipulation of error did not modulate the washout of treadmill aftereffects following over ground walking. In other words, there is a component of the adapted pattern that remained even after natural walking, and is thus linked to the context of the split-belt treadmill. We therefore conclude that the similarity of errors during adaptation to those normally experienced during natural movements promotes the transfer of learning to natural movements, although there also remains a residual neural representation for the treadmill device.
These findings are an important step in our work to understand how motor learning can be made general versus context specific. We previously showed that reaching adaptation to gradual versus abrupt forces improves the transfer of a learned pattern to a similar reaching task (Kluzik et al. 2008). In that study, the context was nearly identical in the learning and transfer periods, and the reaching movement was not natural in either case (i.e., horizontal planar reaching to projected targets while holding a connected or disconnected robot handle; Kluzik et al. 2008). We were also unable to fully explain what element of the gradual adaptation was responsible for the transfer. Here we found that split-belt walking transferred to a much more natural movement (i.e., over ground walking) done in an entirely different context (i.e., off the treadmill in another room). Importantly, we have also found that learning from errors within a natural range is what predicts transfer of the adapted pattern to a more natural context. Finally, we have extended our studies of reaching to a whole body task like walking and find even clearer results of transfer. Again, this may be because we created situations where errors were more natural.
Error Magnitude or Error Variability During Adaptation Strengthens Learning
Subjects in the abrupt and noisy groups had larger aftereffects during the catch trial on the treadmill (i.e., more learning) than subjects in the gradual group. We suggest that these differences in learning may be due to 1) the magnitude of errors driving the adaptation, 2) the sensitivity to those errors, and 3) the adaptation dose.
The first interpretation is supported by the idea that trial-by-trial learning results from updating movement parameters at each trial to minimize the errors caused by self-generated or externally generated perturbations (Baddeley et al. 2003; Donchin et al. 2003; Fine and Thoroughman 2007). Thus, in a single trial, subjects in the abrupt group might have learned more than subjects in the gradual group because at each trial the former subjects experienced a larger perturbation and updated their movement parameters more to reduce the larger errors (Fig. 2). This proportional relationship between errors and motor learning when errors are not too large has been observed previously in the adaptation of upper body movements (Körding and Wolpert 2004; Wei and Körding 2009) and in locomotion (Green et al. 2010). Here we demonstrate that error magnitude has also an effect in the learning of new walking patterns upon split-belt adaptation.
Second, how much we learn from errors depends on the nervous system's sensitivity to errors during adaptation (Burge et al. 2008; Korenberg and Ghahramani 2002; Wei and Körding 2010). Consequently, we think that subjects in the noisy group learned more because the variable perturbations might have increased the sensitivity to their errors. Namely, the sensitivity of spindles, encoding errors for locomotor adaptation (Bunday and Bronstein 2009), increases when the attention to movements is higher (Hospod et al. 2007) during unstable walking (Prochazka et al. 1988)—as in the noisy group. Therefore, subjects in the noisy group might have learned more from their errors because their stepping was more variable. This is consistent with our previous study showing that learning increases when subjects are more variable during adaptation (Torres-Oviedo and Bastian 2010).
Finally, differences in magnitude of aftereffects may result from differences in the accumulation of trial-by-trial learning. One idea is that trial-by-trial learning is cumulative; therefore greater adaptation effects may result when subjects experience sustained perturbations for a longer versus a shorter period of time. Although all of our groups were exposed to the same period of adaptation, we think the abrupt group had effectively greater adaptation dosage, since subjects in this group experienced the sustained perturbation of 1:2 speed ratio for 15 min, whereas subjects in the other groups did not. Therefore, the adaptation effects of the abrupt group might have been greater than those in the gradual group because the former had a greater adaptation dose than the latter over the same period of time.
Errors During Adaptation Determine the Transfer of Learning
Our results demonstrate that the transfer of treadmill learning to natural walking increases when subjects are adapted gradually. Importantly, this finding is not determined by how much was learned to begin with—the gradual group showed the least adaptation on the treadmill yet the most transfer to walking off the treadmill. This was true for the absolute amount transferred and the percentage transferred. This is consistent with our recent reaching study showing greater transfer to movements without the device when subjects were adapted gradually versus abruptly (Kluzik et al. 2008). Thus adaptation to gradual perturbations facilitates the transfer of device-induced learning to natural movements for different behaviors. We think that this increase in transfer during gradual perturbations is due to the similarity of errors during gradual adaptation to those normally experienced. This explanation is supported by our regression analysis showing that the percentage of transfer is best explained by the amount of errors out of the normal range—the more ErrorsOut during adaptation, the less transfer of learning to natural movements. Therefore, we think that transfer is not limited by experiencing sudden errors at the beginning of the adaptation, such as in the abrupt group, but that errors out of the ordinary anytime during the adaptation would affect the transfer. A recent theoretical study proposed that the generalization of learning is mediated by the ability of the nervous system to assign errors to the environment or the body (i.e., credit assignment) (Berniker and Kording 2008). Here we suggest that out-of-the-ordinary errors are assigned to the environment (e.g., the treadmill), and consequently the acquired learning is linked to that particular context. On the other hand, errors falling within the natural variability of our movements are assigned to the body, and consequently the acquired learning is transferred across contexts. In conclusion, prior experience plays an important role in the generalization of learning (Krakauer et al. 2006). Although reaching and walking are very different behaviors, the similarity between our findings across studies suggests that the ability of the nervous system to classify errors and assign learning based on errors normally experienced is a general principle for motor learning.
Another possible explanation for our results is that different mechanisms are involved in gradual versus abrupt motor adaptation, as suggested by patient and behavioral studies. For example, we have found that cerebellar patients adapt much more normally to gradual perturbations than abrupt perturbations (Criscimagna-Hemminger et al. 2010). This might suggest that extracerebellar mechanisms are more involved in gradual adaptation. Other behavioral studies have shown differences in learning from small errors during gradual and consistent perturbations. For example, there have been demonstrations of longer-lasting aftereffects (Hatada et al. 2006; Kagerer et al. 1997) and better retention (Huang and Shadmehr 2009; Klassen et al. 2005) when subjects are adapted to gradual perturbations in prism and reaching tasks. Therefore, it is possible that more aftereffects are observed in over ground walking because treadmill adaptation to small errors is subserved by a different neural process and leads to more enduring aftereffects.
A Neural Representation for the Device Is Maintained Regardless of the Generalization of Learning
We expected the washout of treadmill aftereffects following natural walking to follow a pattern related to the amount that was transferred (i.e., greater transfer would result in greater washout), since we hypothesized that the extent of transfer and washout of adaptation effects reflects the overlap in the neural representations that are being used across contexts. However, the same aftereffects remained across groups when subjects returned to the device, indicating that although errors during adaptation had an effect on transfer they did not affect the washout of aftereffects specific to the treadmill. We think that when learning is encoded on the treadmill with contextual information specific to this environment there is retention of context-specific learning on the treadmill that is difficult to wash out. This is consistent with previous findings in saccades (Herman et al. 2009; Shelhamer et al. 2005; Shelhamer and Clendaniel 2002), wrist movements (Osu et al. 2004), reaching (Cothros et al. 2009; Gandolfo et al. 1996; Howard et al. 2010), and walking (Bunday and Bronstein 2008; Torres-Oviedo and Bastian 2010), showing that one can only wash out context-specific aftereffects when learning is acquired without contextual cues.
Clinical Implications
Our results suggest that transfer of device-induced learning to natural movements is strongly mediated by the similarity of errors experienced on the device to those normally experienced. Thus populations that normally make larger and more variable errors would tend to assign larger errors on the device more to themselves than to the environment, and consequently will generalize more than subjects who are more precise in their natural movements. This prediction is consistent with recent work demonstrating that split-belt treadmill aftereffects in stroke survivors transfer more to over ground walking than in healthy control subjects (Reisman et al. 2009). In that study, treadmill adaptation was induced with the use of an abrupt perturbation, and stroke survivors had larger and more variable stepping errors than control subjects. We expect that gradual perturbations may promote even better generalization in these patient populations. In addition, we showed improvement in the transfer of all aspects of gait (i.e., temporal and spatial gait features) that were adapted. This has significant relevance for rehabilitation, since our previous work suggested that the adaptation of spatial gait features was tightly linked to the device and could not be altered (Reisman et al. 2009; Torres-Oviedo and Bastian 2010). Our results indicate that small errors may encode learning that is general, which could help to improve gait in patients beyond the clinical setting.
GRANTS
This work was supported by National Institute of Child Health and Human Development Grant HD-048741.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: G.T.O and A.J.B. conception and design of research; G.T.O and A.J.B. interpreted results of experiments; G.T.O prepared figures; G.T.O. drafted manuscript; G.T.O and A.J.B. edited and revised manuscript; A.J.B. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors acknowledge N. H. Bhanpuri for insightful conversations during the preparation of this manuscript.
REFERENCES
- Baddeley RJ, Ingram HA, Miall RC. System identification applied to a visuomotor task: near-optimal human performance in a noisy changing task. J Neurosci 23: 3066–3075, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastian AJ. Understanding sensorimotor adaptation and learning for rehabilitation. Curr Opin Neurol 21: 628–633, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berniker M, Kording K. Estimating the sources of motor errors for adaptation and generalization. Nat Neurosci 11: 1454–1461, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunday KL, Bronstein AM. Visuo-vestibular influences on the moving platform locomotor aftereffect. J Neurophysiol 99: 1354–1365, 2008 [DOI] [PubMed] [Google Scholar]
- Bunday KL, Bronstein AM. Locomotor adaptation and aftereffects in patients with reduced somatosensory input due to peripheral neuropathy. J Neurophysiol 102: 3119–3128, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burge J, Ernst MO, Banks MS. The statistical determinants of adaptation rate in human reaching. J Vis 8: 20.21–20.19, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cothros N, Wong J, Gribble PL. Visual cues signaling object grasp reduce interference in motor learning. J Neurophysiol 102: 2112–2120, 2009 [DOI] [PubMed] [Google Scholar]
- Cothros N, Wong JD, Gribble PL. Are there distinct neural representations of object and limb dynamics? Exp Brain Res 173: 689–697, 2006 [DOI] [PubMed] [Google Scholar]
- Criscimagna-Hemminger SE, Bastian AJ, Shadmehr R. Size of error affects cerebellar contributions to motor learning. J Neurophysiol 103: 2275–2284, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson PR, Wolpert DM. Motor learning and prediction in a variable environment. Curr Opin Neurobiol 13: 232–237, 2003 [DOI] [PubMed] [Google Scholar]
- Deubel H, Wolf W, Hauske G. Adaptive gain control of saccadic eye movements. Hum Neurobiol 5: 245–253, 1986 [PubMed] [Google Scholar]
- Donchin O, Francis JT, Shadmehr R. Quantifying generalization from trial-by-trial behavior of adaptive systems that learn with basis functions: theory and experiments in human motor control. J Neurosci 23: 9032–9045, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine MS, Thoroughman KA. Trial-by-trial transformation of error into sensorimotor adaptation changes with environmental dynamics. J Neurophysiol 98: 1392–1404, 2007 [DOI] [PubMed] [Google Scholar]
- Gandolfo F, Mussa-Ivaldi FA, Bizzi E. Motor learning by field approximation. Proc Natl Acad Sci USA 93: 3843–3846, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DA, Bunday KL, Bowen J, Carter T, Bronstein AM. What does autonomic arousal tell us about locomotor learning? Neuroscience 170: 42–53, 2010 [DOI] [PubMed] [Google Scholar]
- Hatada Y, Rossetti Y, Miall RC. Long-lasting aftereffect of a single prism adaptation: shifts in vision and proprioception are independent. Exp Brain Res 173: 415–424, 2006 [DOI] [PubMed] [Google Scholar]
- Herman JP, Harwood MR, Wallman J. Saccade adaptation specific to visual context. J Neurophysiol 101: 1713–1721, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hospod V, Aimonetti JM, Roll JP, Ribot-Ciscar E. Changes in human muscle spindle sensitivity during a proprioceptive attention task. J Neurosci 27: 5172–5178, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard IS, Ingram JN, Wolpert DM. Context dependent partitioning of motor learning in bimanual movements. J Neurophysiol 104: 2082–2091, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang VS, Shadmehr R. Persistence of motor memories reflects statistics of the learning event. J Neurophysiol 102: 931–940, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagerer FA, Contreras-Vidal JL, Stelmach GE. Adaptation to gradual as compared with sudden visuo-motor distortions. Exp Brain Res 115: 557–561, 1997 [DOI] [PubMed] [Google Scholar]
- Klassen J, Tong C, Flanagan JR. Learning and recall of incremental kinematic and dynamic sensorimotor transformations. Exp Brain Res 164: 250–259, 2005 [DOI] [PubMed] [Google Scholar]
- Kluzik J, Diedrichsen J, Shadmehr R, Bastian AJ. Reach adaptation: what determines whether we learn an internal model of the tool or adapt the model of our arm? J Neurophysiol 100: 1455–1464, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Körding KP, Wolpert DM. The loss function of sensorimotor learning. Proc Natl Acad Sci USA 101: 9839–9842, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenberg A, Ghahramani Z. A Bayesian view of motor adaptation. Curr Psychol Cogn 21: 537–564, 2002 [Google Scholar]
- Krakauer JW, Mazzoni P, Ghazizadeh A, Ravindran R, Shadmehr R. Generalization of motor learning depends on the history of prior action. PLoS Biol 4: e316, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner JR, DiZio P. Rapid adaptation to Coriolis force perturbations of arm trajectory. J Neurophysiol 72: 299–313, 1994 [DOI] [PubMed] [Google Scholar]
- Malone LA, Bastian AJ. Thinking about walking: effects of conscious correction versus distraction on locomotor adaptation. J Neurophysiol 103: 1954–1962, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osu R, Hirai S, Yoshioka T, Kawato M. Random presentation enables subjects to adapt to two opposing forces on the hand. Nat Neurosci 7: 111–112, 2004 [DOI] [PubMed] [Google Scholar]
- Prochazka A, Hulliger M, Trend P, Durmuller N. Dynamic and static fusimotor set in various behavioural contexts. In: Mechanoreceptors: Development, Structure and Function, edited by Hink T, Soukup R, Vejsada R, Zelena J. London: Plenum, 1988, p. 417–430 [Google Scholar]
- Reisman DS, Block HJ, Bastian AJ. Interlimb coordination during locomotion: what can be adapted and stored? J Neurophysiol 94: 2403–2415, 2005 [DOI] [PubMed] [Google Scholar]
- Reisman DS, Wityk R, Silver K, Bastian AJ. Split-belt treadmill adaptation transfers to overground walking in persons poststroke. Neurorehabil Neural Repair 23: 735–744, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds RF, Bronstein AM. The moving platform aftereffect: limited generalization of a locomotor adaptation. J Neurophysiol 91: 92–100, 2004 [DOI] [PubMed] [Google Scholar]
- Shadmehr R, Mussa-Ivaldi FA. Adaptive representation of dynamics during learning of a motor task. J Neurosci 14: 3208–3224, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelhamer M, Aboukhalil A, Clendaniel R. Context-specific adaptation of saccade gain is enhanced with rest intervals between changes in context state. Ann NY Acad Sci 1039: 166–175, 2005 [DOI] [PubMed] [Google Scholar]
- Shelhamer M, Clendaniel RA. Context-specific adaptation of saccade gain. Exp Brain Res 146: 441–450, 2002 [DOI] [PubMed] [Google Scholar]
- Torres-Oviedo G, Bastian AJ. Seeing is believing: effects of visual contextual cues on learning and transfer of locomotor adaptation. J Neurosci 30: 17015–17022, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay S, Shiller DM, Ostry DJ. Somatosensory basis of speech production. Nature 423: 866–869, 2003 [DOI] [PubMed] [Google Scholar]
- Vasudevan EVL, Bastian AJ. Split-belt treadmill adaptation shows different functional networks for fast and slow human walking. J Neurophysiol 103: 183–191, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan EVL, Torres-Oviedo G, Morton SM, Yang JF, Bastian AJ. Younger is not always better: development of locomotor adaptation from childhood to adulthood. J Neurosci 31: 3055–3065, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei K, Körding K. Relevance of error: what drives motor adaptation? J Neurophysiol 101: 655–664, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei K, Körding K. Uncertainty of feedback and state estimation determines the speed of motor adaptation. Front Comput Neurosci 4: 11, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]