Abstract
Much research has focused on how people stop initiated response tendencies when instructed by a signal. Stopping of this kind appears to have global effects on the motor system. For example, by delivering transcranial magnetic stimulation (TMS) over the leg area of the primary motor cortex, it is possible to detect suppression in the leg when the hand is being stopped (Badry R et al. Suppression of human cortico-motoneuronal excitability during the stop-signal task. Clin Neurophysiol 120: 1717–1723, 2009). Here, we asked if such “global suppression” can be observed proactively, i.e., when people anticipate they might have to stop. We used a conditional stop signal task, which allows the measurement of both an “anticipation phase” (i.e., where proactive control is applied) and a “stopping” phase. TMS was delivered during the anticipation phase (experiment 1) and also during the stopping phase (experiments 1 and 2) to measure leg excitability. During the anticipation phase, we did not observe leg suppression, but we did during the stopping phase, consistent with Badry et al. (2009). Moreover, when we split the subject groups into those who slowed down behaviorally (i.e., exercised proactive control) and those who did not, we found that subjects who slowed did not show leg suppression when they stopped, whereas those who did not slow did show leg suppression when they stopped. These results suggest that if subjects prepare to stop, then they do so without global effects on the motor system. Thus, preparation allows them to stop more selectively.
Keywords: cognitive control, response inhibition, transcranial magnetic stimulation, stop signal task, proactive control
when reacting to changes in the environment, people are able to quickly stop actions that are already in progress. One way to measure this behavior in the laboratory is with the stop signal task (Logan et al. 1984; Verbruggen and Logan 2009). For this task, participants initiate go responses on each trial and occasionally have to try to stop them when a signal occurs. Much research has examined outright stopping of this kind (Verbruggen and Logan 2008). The research suggests that a fast mechanism is used to stop, perhaps via the subthalamic nucleus (STN) of the basal ganglia (Aron et al. 2007; Aron and Poldrack 2006). The STN sends a very broad output to the pallidum and so could have widespread effects on the motor system (Mink 1996). Consistent with this, two studies (Badry et al. 2009; Majid et al. 2011) have observed that if subjects stop the hand, then there is suppression of the task-irrelevant leg. These studies used transcranial magnetic stimulation (TMS) over the leg area in the primary motor cortex to measure corticomotor excitability via concurrent electromyography (see also Coxon et al. 2006; Leocani et al. 2000; Sohn et al. 2002; van den Wildenberg et al. 2010 for further evidence).
Here, we asked if such “global suppression” can be observed proactively, i.e., when people anticipate they might have to stop. To encourage subjects to engage proactive control, adjustments can be made to the basic stop signal paradigm. There are several ways to do this, e.g., by increasing the proportion of stop trials or by comparing mixed go and stop blocks with pure go blocks (Bissett and Logan 2011). The result in each case is a slowing down of reaction time (RT) on trials where stopping is anticipated relative to trials where it is not (Chikazoe et al. 2009; De Jong et al. 1995; Jahfari et al. 2010; Leotti and Wager 2010; Vink et al. 2005; Zandbelt and Vink 2010). Such response slowing could arise from changes in several processes, including 1) prolongation of the decision to respond, 2) lesser facilitation of the motor system, and 3) proactive suppression of the motor system (Jahfari et al. 2010; Leotti and Wager 2010; Verbruggen and Logan 2009). This last process of proactive suppression may contribute to response slowing by dampening response output (Jahfari et al. 2010). However, it is not known if proactive suppression has global or selective effects over the motor system when subjects slow down their responses in anticipation of stopping (Aron 2010).
If preparing to stop involves the proactive recruitment of the same mechanism involved in stopping outright, then preparing to stop might also have a global effect over the motor system. A precedent for this prediction comes from the hold-your-horses theory proposed by Frank and colleagues (Frank 2006; Frank et al. 2007). According to this theory, the presence of conflict between competing responses recruits the STN of the basal ganglia to momentarily withhold all response output. We acknowledge that a competition between going and stopping is conceptually quite different from a competition among alternative responses. Nevertheless, the STN might exert its transient global inhibitory influence over the motor system in the presence of different kinds of conflict, including when there is conflict between the need to respond quickly and the need to possibly stop. Since the STN has been hypothesized to have a broad effect on the motor system during successful stopping (Aron et al. 2007; Aron and Poldrack 2006), we predicted that the corticomotor excitability of the leg should be reduced when there is proactive control of hand responses in anticipation of stopping (i.e., global suppression).
In experiment 1, we tested whether there is global suppression of the motor system during proactive control. We used the conditional stop signal task (De Jong et al. 1995; Jahfari et al. 2010). In this version of the stop signal task, participants are instructed to make responses to left- and right-pointing target arrows and to inhibit responses after an auditory stop signal only if the arrow points in a “critical” direction (e.g., left). Participants are instructed to ignore the stop signal when the arrow points in the “noncritical” direction (e.g., right). Thus, participants can use proactive control if the target is critical, and this results in a slower RT on critical than noncritical trials (De Jong et al. 1995; Jahfari et al. 2010). Here, we refer to this as the “response delay effect” (RDE). The RDE is accompanied by reduced excitability in the responding hand for critical relative to noncritical responses, and this difference has been detected as early as 160 and 200 ms after the go signal (Jahfari et al. 2010). This early reduction of excitability for critical responses may be a marker for the recruitment of a proactive control mechanism. To test whether this putative mechanism influences only the responding hand or the entire motor system, we measured the excitability of a task-irrelevant muscle within the same early response phase during the performance of the same task. We delivered single-pulse TMS over the primary motor cortex and recorded the resulting motor evoked potentials (MEPs) from the leg while participants provided task responses with their hand. Consistent with prior work (Badry et al. 2009; Majid et al. 2011), we treated leg MEPs as the “signature” of global motor suppression.
We delivered TMS stimuli with high temporal precision in the “anticipation” phase and also in the “stopping” phase. For the anticipation phase, we predicted that if participants globally suppress the motor system in anticipation of stopping, then leg MEPs would be suppressed during critical go trials compared with noncritical go trials. During the stopping phase, we predicted leg suppression for successful stop trials, as previously demonstrated (Badry et al. 2009; Majid et al. 2011). Such a replication would serve to validate our methods for detecting global motor suppression.
In experiment 2, we used a very similar setup. The key difference was that leg TMS was only delivered during the stopping phase, at one of three specific time points: 200, 220, or 240 ms after the stop signal. We did this to replicate the results of experiment 1 and to better characterize the timing of global motor suppression during successful stopping and its relationship to proactive control.
METHODS
Subjects
In experiment 1, there were 19 young adult subjects (6 men and 13 women, 3 left handed and 16 right handed, 21.4 ± 2.8 yr of age); in experiment 2, there were 20 young adult subjects (9 men and 11 women, 2 left handed and 18 right handed, 20.7 ± 2.0 yr of age). All subjects provided informed consent according to a protocol approved by the Institutional Review Board of the University of California-San Diego. They also completed a TMS safety screening questionnaire.
Task
We used a modified version of the conditional stop task of Jahfari et al. (2010) (see Fig. 1). Stimuli were presented using PsychToolbox3 (http://www.psychtoolbox.org) running in Matlab (Mathworks, Natick, MA) on an iMac desktop computer (Apple, Cupertino, CA). Each trial began with a blank screen for 1,400 ms followed by a white fixation cross for a variable period of 500–700 ms (steps of 100 ms, mean: 600 ms). A left- or right-pointing arrow stimulus was then presented for 1,000 ms or until a response was registered. Responses were executed with a leftward lateral movement of the right index finger or a downward movement of the right pinky finger.
Fig. 1.
The conditional stop task consisted of critical trials (dark gray, for illustrative purposes) and noncritical trials (light gray). The auditory stop signal was only relevant for critical trials, e.g., when the target arrow pointed left. A: in experiment 1, transcranial magnetic stimulation (TMS) pulses were delivered during a baseline period (200 or 300 ms before the arrow) and at 120 or 200 ms after the arrow. Pulses were also delivered at 100 ms before each subject's critical go reaction time (RT) as measured during practice. B: in experiment 2, TMS pulses were delivered 200, 220, or 240 ms after the stop signal. ITI, intertrial interval.
In every four trials, there were three go trials and one stop trial. On stop trials, an auditory stop signal (500 Hz, 400 ms) sounded at a variable stop signal delay (SSD) after the presentation of the arrow. The stop signal indicated that the subject should try to cancel their response but only if the arrow pointed in the critical direction (e.g., rightward pointing). The critical direction was counterbalanced across subjects and was held constant for an individual subject throughout the experiment. The SSD was dynamically adjusted according to the subject's performance to converge on a 50% stopping rate. If subjects failed to stop, then the SSD was reduced by 50 ms; if they succeeded in stopping, then it was increased by 50 ms (Aron and Poldrack 2006). Two different staircases were used and were yoked to the critical trials. These started with SSD values of 200 and 250 ms.
Before the experiments, subjects were trained on 4 practice blocks of 24 trials during which TMS was not administered.
Electromyography and TMS
Surface electromyography (EMG) recordings were made with 10-mm-diameter Ag-AgCl hydrogel electrodes placed over the tibialis anterior muscle of the leg in a belly-tendon montage and over the lateral portion of the talus bone in the ankle to serve as a ground. In all subjects, EMG recording was done from the left leg only. The EMG signal was amplified using a Grass QP511 Quad alternating current Amplifier System Grass amplifier (Grass Technologies, West Warwick, RI) with a bandpass filter between 30 Hz and 1 kHz and a notch filter at 60 Hz. Data were sampled at 2 kHz with a CED Micro 1401 mk II and were recorded using CED Signal version 4 (Cambridge Electronic Design, Cambridge, UK).
We used a 7 cm figure-eight “Batwing” coil (type no. 15411) and a MagStim 200-2 system (Magstim, Whitland, UK) to deliver single-pulse TMS stimuli over the scalp. The Batwing coil is optimized for stimulating the leg area. We were careful to observe a safe level of stimulation according to TMS guidelines (Wassermann 1998). To locate the representation of the tibialis anterior muscle of the left leg, pulses were first delivered 2 cm anterior of the vertex with the coil angled ∼10° lateral to the midsagittal line and 15° above the vertex plane. The coil was then repositioned incrementally to locate the position that produced the most reliable MEPs in the left leg. This locus was marked on a snug-fitting lycra swim cap, which was worn by the subject throughout the experiment. Resting motor threshold was determined by finding the lowest stimulus intensity that produced MEPs of at least 0.05-mV amplitude on at least 5 of 10 trials (Rossini et al. 1994). The test stimulus intensity was 115% of the resting motor threshold.
MEP Analysis
MEP analysis was performed using custom software in Matlab R2009SV (MathWorks). All MEPs were visually inspected to exclude trials where the MEP was contaminated with EMG noise as well as trials with MEP peak-to-peak amplitudes of <0.05 mV or >2 mV. No trials were excluded due to EMG noise in either experiment, likely because the leg muscle was not necessary for response execution and was at rest. However, 15.2 ± 4.2% of MEPs in experiment 1 and 15.6 ± 8.1% of MEPs in experiment 2 were outside the allowable range. After these MEPs were excluded, the remaining MEPs were Winsorized. Accordingly, MEPs with amplitudes that were >3 SD from the mean for each condition were assigned the value of the nearest MEP amplitude within 3 SD of the mean. Normalization of the data was performed after preprocessing and Winsorizing and is explained in more detail below because it differed between experiments 1 and 2.
Experimental Procedures
Experiment 1.
Experiment 1 consisted of 20 blocks of 24 trials. TMS was delivered at 200 or 300 ms before the go arrow to serve as a baseline and 120 or 200 ms after the arrow (Fig. 1A). TMS was also delivered on stop trials at 100 ms before the mean critical go RT as determined using correct critical go trials from the practice blocks, based on prior methodology (Badry et al. 2009). On TMS trials, only one pulse was delivered at any one of these time points. TMS was delivered on 240 go trials (120 critical) and 80 stop trials (40 critical). The p(TMS) at each time point was equal for critical and noncritical trials. Specifically, TMS was delivered during the baseline period on 40 critical trials and 40 noncritical trials, at 120 ms after the target on 45 critical trials and 45 noncritical trials, at 200 ms after the target on 45 critical trials and 45 noncritical trials, and during the stopping phase (i.e., critical go RT − 100 ms) on 30 critical trials and 30 noncritical stop trials. TMS was not delivered on the remaining 120 go trials (60 critical trials) and 40 stop trials (20 critical trials). To normalize each subject's data, the mean MEP amplitude for each condition was divided by the mean baseline MEP amplitude.
Measures of interest included mean critical go RT, noncritical go RT, failed stop RT, noncritical stop RT, SSD, the probability of successful inhibition [p(inhibit)], RDE (critical go RT − noncritical go RT), and stop signal RT (SSRT). SSRT is an estimate of the speed of stopping, which was calculated using the so-called “integration method” (Logan et al. 1984; Verbruggen and Logan 2009). Because TMS can interfere with task performance, these measures of interest were calculated separately for TMS and no-TMS trials. Separate SSD staircases were used for TMS and no-TMS trials, and these were yoked only to the critical direction.
Experiment 2.
The same conditional stop task and procedures were used as for experiment 1 except that 1) subjects performed 32 blocks of 24 trials during the experiment proper; 2) TMS was delivered on every stop trial and only on stop trials, and it was delivered at 200, 220, or 240 ms after the stop signal (Fig. 1B); 3) for both behavioral and TMS analysis, we performed a median split of the subjects into “RDE” and a “no-RDE” groups based on the RDE measure; and 4) as there was no TMS during the intertrial interval, we used a common average baseline for normalization: for each subject, the mean MEP amplitude for each condition was divided by the average amplitude of all of the MEPs for that subject.
RESULTS
Experiment 1
Stimulation parameters.
Resting motor threshold was 55.8 ± 5.1% of the maximum stimulator output, and the mean test stimulus intensity was 64.2 ± 5.8%. The baseline MEP amplitude was 0.53 ± 0.36 mV.
Task performance.
Table 1 shows a summary of all behavioral data for all TMS and no-TMS trials. Overall, subjects performed satisfactorily on the task.
Table 1.
Behavioral results from experiment 1
| Anticipation Phase |
Stopping Phase |
||||
|---|---|---|---|---|---|
| No TMS | TMS at 120 ms | TMS at 200 ms | Failed stop with no TMS | Failed stop with TMS | |
| Critical trials | |||||
| Mean go RT, ms | 577.8 (84.0) | 576.3 (69.0) | 582.3 (81.7) | 530.8 (73.0) | 518.5 (63.7) |
| Errors, % | 2.5 (2.7) | 3.2 (2.8) | 2.57 (4.6) | ||
| SSRT, ms | 333.8 (56.2) | ||||
| Mean SSD, ms | 210.3 (103.2) | ||||
| p(inhibit) | 0.47 (0.2) | 0.48 (0.1) | |||
| Noncritical trials | |||||
| Mean go RT, ms | 506.1 (38.7) | 509.6 (34.2) | 506.3 (38.0) | 518 (37.0)* | 519 (36.2)* |
| Errors, % | 0.6 (1.4) | 1.5 (3.6) | 0.7 (1.1) | ||
| RDE, ms | 72.7 (73.2) | 66.7 (62.1) | 76.0 (71.9) | ||
Values are means (SD); n = 19 subjects. TMS, transcranial magnetic stimulation; RT, reaction time; SSRT, stop signal RT; SSD, stop signal delay; RDE, response delay effect (critical go RT −noncritical go RT).
Noncritical stop trials are trials in which the subject should never stop.
For no-TMS trials, SSRT was estimated at 334 ms with a mean SSD of 210 ms and p(inhibit) at 0.47. The critical go RT for no-TMS trials was 578 ms, and the noncritical go RT was 506 ms, giving a RDE of 73 ms [t(18) = 4.3, P < 0.001]. For TMS trials, SSRT was estimated at 373 ms with a mean SSD of 206 ms and p(inhibit) at 0.48. The critical go RT for TMS trials was 579 ms, and the noncritical Go RT was 508 ms, giving a RDE of 71 ms [t(18) = 4.6, P < 0.001].
During practice, the critical go RT was 585 ms and the noncritical Go RT was 532 ms. The critical go RT during practice did not differ significantly from the critical go RT for no-TMS trials measured during the experiment proper [t(18) = 0.53, P = 0.60]. This is important because TMS pulse times were determined based on the practice critical go RT. However, noncritical go RT for no-TMS trials was significantly faster during the experiment proper than during practice [t(18) = 5.93, P <0.001]. Practice SSRT was estimated at 360 ms with a mean SSD of 204 ms and p(inhibit) at 0.27. The low p(inhibit) value reflects the fact that there were not many stop trials and thus the staircases did not have time to stabilize around 0.5.
MEP amplitude.
We predicted that if subjects prepare to stop using a global inhibitory mechanism, then there would be suppression of leg MEPs in the anticipation phase for critical versus noncritical go trials. The TMS data are shown in Fig. 2A. We tested our hypothesis with ANOVA that included the factors of condition (critical vs. noncritical) and two time points (120 and 200 ms after the go stimulus). There were no main effects or interactions (all P ≥ 0.10).
Fig. 2.
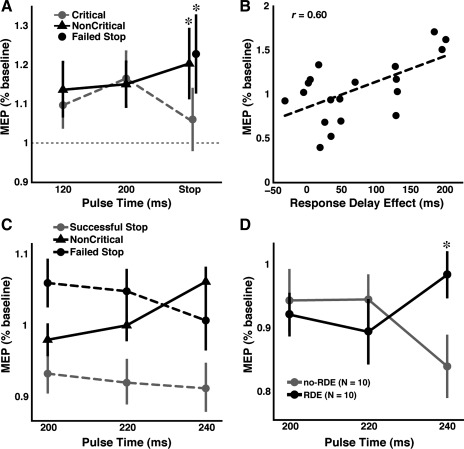
Normalized leg motor evoked potential (MEP) results for experiments 1 and 2. A: in experiment 1, leg MEP amplitudes did not differ between critical and noncritical trials at 120 or 200 ms after the target but were reduced during successful stop trials compared with failed stop and noncritical stop trials. B: in experiment 1, those subjects who demonstrated a greater response delay effect (RDE; critical RT − noncritical RT) also demonstrated greater leg excitability during successful stopping. C: in experiment 2, leg MEP amplitudes were reduced at 200, 220, and 240 ms after the stop signal during successful stop trials compared with failed stop and noncritical stop trials. D: subjects in experiment 2 were divided into those who exhibited a response delay effect (“RDE group”) and those who did not (“no-RDE group”) by performing a median split based on the RDE. During successful stop trials, the no-RDE group demonstrated greater leg suppression than the RDE group at 240 ms but not at 200 or 220 ms after the stop signal. *Statistical significance at P < 0.05.
To validate the leg TMS methodology, we examined MEPs in the stopping phase. There was significantly reduced MEP amplitude for successful versus failed stop trials [t(18) = 2.6, P < 0.05 by two-tailed test] and for successful stop versus noncritical stop trials [t(18) = 2.7, P < 0.05 by two-tailed test], replicating the results of Badry et al. (2009) and Majid et al. (2011). The direction of the critical response (i.e., left or right) did not influence leg MEP amplitude during successful stopping [t(17) = 0.98, P = 0.34].
Thus, while there was a behavioral RDE, we did not detect global suppression of the motor system during the anticipation phase, contrary to our prediction. Nevertheless, we did detect reduced leg excitability during successful stopping. (See the discussion for the implications of these results.) We note that while leg excitability was reduced during successful stopping relative to the other conditions, it was not reduced below our intertrial baseline, unlike previous studies (Badry et al. 2009; Majid et al. 2011). This may relate to different aspects of our experimental design, such as the pulse timing. Previously, the effect of leg suppression associated with stopping was demonstrated to be transient (Majid et al. 2011). Therefore, variability in the timing of the TMS pulse relative to the stop signal in our experiment may have blurred across the moment at which the leg was maximally suppressed. We explore this pulse timing issue in greater detail below.
Interestingly, we noted that some subjects did not slow down on critical go trials at all, whereas others slowed down a lot. This raised the possibility that the degree of slowing might affect the way stopping is carried out. We therefore correlated the size of the RDE (critical go RT − noncritical go RT) for no-TMS trials against the amplitude of the leg MEPs on successful stop trials. There was a significant correlation [r(19) = 0.60, P < 0.01], indicating that those subjects who slowed more for critical versus noncritical trials had less leg suppression when they stopped (Fig. 2B). This suggests that there might be different mechanisms for stopping (global vs. nonglobal), and which one is used depends on the degree of behavioral slowing in anticipation of stopping.
We note a possible confound for this analysis in terms of the relative timing of the TMS pulse for different subjects. The TMS pulse was delivered at the practice period critical go RT − 100 ms and corresponded to a mean time of 279.7 ± 78.2 ms after the stop signal on successful stop trials. The high variability in pulse times across subjects raises the possibility that the pulse timing was systematically different for people who slowed in anticipation of stopping compared with those who did not. However, there was no evidence of a correlation between the timing of the TMS pulse (i.e., the interval between the stop signal and the TMS pulse) and MEP amplitude [r(19) = −0.15, P = 0.55]. Nor was there evidence of a correlation between MEP amplitude and the timing of the TMS pulse relative to the critical go RT from no-TMS trials [r(19) = 0.2, P = 0.41]. While the difference in pulse timing across subjects is very unlikely to explain the observed relationship between the RDE and leg suppression during stopping, we nevertheless performed a second experiment in which the timing of TMS relative to the stop signal was held constant across subjects.
Experiment 2
Stimulation parameters.
Resting motor threshold was 56.8 ± 7.2% of the maximum stimulator output, and the mean test stimulus intensity was 65.3 ± 8.4%. The baseline MEP amplitude was 0.49 ± 0.33 mV.
Task performance.
FOR THE ENTIRE GROUP (N = 20).
Table 2 shows a summary of all behavioral data for all TMS (i.e., stop trials) and no-TMS trials (i.e., go trials). In experiment 2, TMS was delivered on every stop trial. TMS was never delivered on go trials. Overall, the subjects performed similarly to experiment 1. SSRT (TMS trials) was estimated at 307 ms with a mean SSD of 205 ms and p(inhibit) at 0.47. The critical go RT (no-TMS trials) was 563 ms, and the noncritical go RT (no-TMS trials) was 508 ms, giving a RDE of 55 ms [t(19) = 4.0, P < 0.001].
Table 2.
Behavioral results from experiment 2
| TMS |
||||
|---|---|---|---|---|
| No TMS | 200 ms | 220 ms | 240 ms | |
| Critical trials | ||||
| Mean go RT, ms | 563.2 (85.1) | |||
| Failed stop RT, ms | 516.6 (65.9) | 534.6 (71.6) | 520.2 (69.5) | |
| Errors, % | 2.2 (0.9) | |||
| SSRT, ms | 307.2 (104.0) | |||
| Mean SSD, ms | 204.5 (105.9) | |||
| p(inhibit) | 0.47 (0.1) | 0.45 (0.1) | 0.49 (0.1) | 0.47 (0.1) |
| Noncritical trials | ||||
| Mean go RT, ms | 507.9 (45.3) | |||
| Stop RT, ms | 515.8 (42.0) | 525.7 (44.8) | 522.0 (45.7) | |
| Errors, % | 1.4 (1.8) | 1.1 (1.8) | 1.9 (2.9) | 1.2 (2.4) |
| RDE, ms | 55.4 (61.3) | |||
Values are means (SD); n = 20 subjects.
FOR THE RDE (N = 10) AND no-RDE (N = 10) GROUPS.
Experiment 1 showed that some subjects did not slow at all and that there was a correlation between the RDE and leg MEP amplitude when stopping. Accordingly, we split the subject group into a RDE group and a no-RDE group based on a median split of the RDE (critical go RT − noncritical go RT; Table 3). The RDE was 100 ms for the RDE group and 11 ms for no-RDE group, and this was a significant difference [t(18) = 4.8, P < 0.0005]. The RDE in the no-RDE group was not significantly different from zero [t(9) = 1.5, P = 0.16]. The between-group difference in the RDE was predominantly due to a large difference in the critical go RT, which was significantly longer for the RDE group than the no-RDE group [t(18) = 5.4, P < 0.0001].
Table 3.
Behavioral results from experiment 2 for the RDE and no-RDE groups
| TMS |
||||
|---|---|---|---|---|
| No TMS | 200 ms | 220 ms | 240 ms | |
| Critical trials | ||||
| Mean go RT, ms | ||||
| RDE group | 628.3 (62.4) | |||
| No-RDE group | 498.1 (44.4) | |||
| Failed stop RT, ms | ||||
| RDE group | 562.0 (55.2) | 587.5 (51.1) | 568.6 (56.5) | |
| No-RDE group | 471.2 (39.1) | 481.7 (44.5) | 471.8 (42.2) | |
| Errors, % | ||||
| RDE group | 1.8 (0.6) | |||
| No-RDE group | 2.5 (1.1) | |||
| SSRT, ms | ||||
| RDE group | 260.5 (111.8) | |||
| No-RDE group | 353.9 (74.0) | |||
| Mean SSD, ms | ||||
| RDE group | 273.0 (95.6) | |||
| No-RDE group | 136.0 (64.3) | |||
| p(inhibit) | ||||
| RDE group | 0.49 (0.0) | 0.49 (0.1) | 0.50 (0.1) | 0.49 (0.1) |
| No-RDE group | 0.45 (0.1) | 0.42 (0.1) | 0.48 (0.1) | 0.46 (0.1) |
| Noncritical trials | ||||
| Mean go RT, ms | ||||
| RDE group | 528.2 (40.2) | |||
| No-RDE group | 487.5 (41.8) | |||
| Stop RT, ms | ||||
| RDE group | 524.4 (40.7) | 534.1 (41.1) | 536.9 (49.5) | |
| No-RDE group | 507.2 (43.6) | 517.3 (48.8) | 507.1 (38.2) | |
| Errors, % | ||||
| RDE group | 0.9 (1.3) | 0.3 (1.0) | 1.3 (2.2) | 1.2 (3.0) |
| No-RDE group | 1.9 (2.1) | 1.9 (2.2) | 2.5 (3.5) | 1.3 (1.6) |
| RDE, ms | ||||
| RDE group | 100.0 (54.9) | |||
| No-RDE group | 10.7 (22.0) | |||
Values are means (SD); n = 10 subjects/group.
To examine how the RDE might change across time (i.e., experience with the task), we calculated the RDE separately for the training session and for the first and second half of the experiment proper. ANOVA with the factors of session (training, first half, and second half) and group (RDE vs. no-RDE) yielded a significant main effect of group [F(2,18) = 20.2, P < 0.0001] but no significant main effect of session [F(2,18) = 0.5, P = 0.61] or session × group interaction [F(2,18) = 1.72, P = 0.21]. Thus, participants in the two RDE groups showed stable behavioral performance throughout the experiment.
MEP amplitude.
FOR THE ENTIRE GROUP (N = 20).
We predicted that the leg would be suppressed during successful stop trials relative to failed stop and noncritical stop trials at all or one of 200, 220, or 240 ms after the stop signal. The normalized MEP data are shown in Fig. 2C. We were not interested in comparing noncritical with failed stop trials. Therefore, we conducted two separate ANOVAs. The first ANOVA included stop trial condition (successful vs. failed) and the three time points (200, 220, and 240 ms after the stop signal). There was a significant main effect of stop trial condition [F(1,19) = 11.5, P < 0.01], with greater leg suppression for successful than failed stopping but no other significant main effect or interaction. The second ANOVA included stop trial condition (successful vs. noncritical) and the three time points (200, 220, and 240 ms after the stop signal). There was a significant main effect of stop trial condition [F(1,19) = 9.1, P < 0.01], with greater leg suppression for successful than noncritical stop trials but no other significant main effect or interaction. The results from both ANOVAs replicate experiment 1 as well as those of Badry et al. (2009) and Majid et al. (2011). As in experiment 1, the direction of the critical response (i.e., left or right) did not influence leg MEP amplitude during successful stopping [t(18) = 0.31, P = 0.76].
FOR THE RDE (N = 10) AND no-RDE (N = 10) GROUPS.
Based on the findings of experiment 1, we predicted that the no-RDE group would show greater leg suppression during successful stopping than the RDE group at some or all of 200, 220, or 240 ms after the stop signal. We conducted ANOVA with the factors of group (RDE vs. no-RDE) and time point (200, 220, or 240 ms after the stop signal) on the leg MEP data for successful stop trials only. There was a significant group × time point interaction [F(2,17) = 3.9, P < 0.05]. Comparisons between the two groups at each time point revealed that the no-RDE group exhibited greater leg suppression than the RDE group only at the 240-ms time point [t(18) = 2.3, P < 0.0167, Bonferroni corrected for the three comparisons; Fig. 2D].
We note, however, that there was an SSRT difference between the RDE and no-RDE groups. SSRT (with TMS) was 261 ms for the RDE group and 354 ms for the no-RDE group. This was a significant difference [t(18) = 2.2, P < 0.05]. However, SSRT during the practice session (no-TMS) was 264 ms for the RDE group and 300 ms for the no-RDE group, and this was not a significant difference [t(18) = 0.9, P = 0.39]. The groups did not differ with regard to p(inhibit) during the experiment proper [t(18) = 1.7, P = 0.11].
Yet, the difference in SSRT is unlikely to explain the MEP results for two reasons. First, SSRT was shorter for the RDE group than the no-RDE group. If the two groups used the same stopping mechanism, only at different time points, then we might have expected significantly greater leg suppression for the RDE group than the no-RDE group at the early TMS time point of 200 ms after the stop signal (as the SSRT difference was ∼40 ms). But, leg excitability for the RDE group was not significantly reduced compared with the no-RDE group at any time point. Second, it is likely that the SSRT estimate in the TMS phase was affected by the TMS stimuli themselves. TMS has been shown to interfere with task performance, and it may have caused the difference in SSRT to emerge between the two groups. Notably, SSRT measured during practice did not differ between the RDE and no-RDE groups. Another alternative explanation is that the shorter SSRT observed for the RDE group could reflect a potential benefit afforded by the proactive recruitment of a selective stopping mechanism.
Auxiliary Results
We reanalyzed the data from experiment 1 using an average baseline (i.e., average of all pulses not administered during the intertrial interval, to be consistent with experiment 2), and the overall pattern of results remained the same: ANOVA with the factors of condition (critical vs. noncritical) and time points (120 and 220 ms after the go stimulus) yielded no significant main effects or interactions. Leg MEP amplitude was significantly reduced for successful stop versus failed stop trials [t(18) = 2.6, P < 0.05 by two-tailed test] and for successful stop versus noncritical stop trials [t(18) = 3.0, P < 0.01 by two-tailed test]. The correlation between the RDE and MEP amplitude on successful stop trials also remained significant [r(19) = 0.47, P < 0.05].
Additionally, we found evidence of “conflict-induced slowing,” i.e., slowing on noncritical stop trials (with stop signals, but for which stopping is not needed) versus go trials in experiment 1 [t(18) = 2.43, P < 0.05] and in experiment 2 [t(19) = 3.8, P < 0.001], but this did not correlate significantly with leg MEP amplitude during noncritical stop trials across participants in either experiments 1 or 2 [r(19) = −0.03, P = 0.90, and r(20) = 0.13, P = 0.57, respectively]. While this pattern suggests that conflict-induced slowing may not depend on a global inhibition mechanism, we note that in experiment 2 the no-RDE group did exhibit greater conflict-induced slowing than the RDE group [t(18) = 3.6, P < 0.01]. This suggests that the no-RDE group experienced motor inhibition after a stop signal on noncritical trials. Although the TMS methodology we used here may not have been sensitive enough, or timed correctly, to detect a global inhibitory effect at the leg during conflict-induced slowing, the observed behavioral pattern leaves open the possibility that conflict-induced slowing recruits a global inhibitory mechanism.
DISCUSSION
Much research has suggested that outright stopping is achieved by a fast mechanism in the brain that has global effects on the motor system. In experiment 1, we asked if such global suppression can be observed proactively, i.e., when people anticipate they might have to stop. We used a conditional stop signal task and leg TMS to measure whether there was global suppression during an anticipation phase and during a stopping phase. Leg suppression was not observed during the anticipation phase, but it was for the stopping phase. Furthermore, we observed that those subjects who exhibited the most behavioral slowing (i.e., larger RDE) were those who also showed the least leg suppression when stopping. In experiment 2, we used a similar task and setup except that TMS pulses were only delivered in the stopping phase and at specific time points. We found, again, that those subjects who slowed in anticipation of stopping did not have as much leg suppression at the time of stopping as those who did not slow in anticipation of stopping. These results provide further evidence for different modes of stopping, a global mechanism and a selective one, and they clarify the circumstances under which these are used. We specifically show that if subjects do not prepare to stop (manifest in minimal RT slowing), then, if they are required to stop, they resort to using an emergency stopping mechanism with apparently global effects on the motor system.
Does Anticipating the Need to Stop Lead to Global Suppression of the Motor System?
Previous evidence has suggested that the mechanism used to stop a response outright has a global inhibitory effect on the motor system (Badry et al. 2009; Majid et al. 2011). We predicted that if proactive control involves the recruitment of the same or a similar mechanism, then exercising proactive control in anticipation of stopping should also globally suppress the motor system. Although we replicated the finding of global suppression during outright stopping (Badry et al. 2009; Majid et al. 2011), we did not detect global suppression during anticipation of stopping. One potential explanation for this is that the TMS methodology we used is insensitive to global suppression when it occurs proactively. Yet, a recent study (Cai et al. 2011) showed that TMS is sensitive enough to detect the effects of proactive control on the motor system–at least for the hand, and for a paradigm where suppression is targeted at particular response channels before the go stimulus. A second explanation for not finding global suppression during anticipation of stopping is a lack of sufficient statistical power. For experiment 1, we calculated a Cohen's d value of 0.25 for the comparisons of leg MEP amplitudes between critical and noncritical trials at 120 ms after the go stimulus. This is a small effect size, and over 200 subjects would be required to reach significance. A third explanation is that this conditional stop task may not be ideally suited to examine global effects of proactive control because the go stimulus that tells the subject which response to make is the same stimulus that tells the subject whether he/she may need to stop or not. Thus, if the subject uses a proactive control mechanism at all, it may be a selective one that is targeted at the single response that may need to be stopped rather than a global brake. Future studies could address whether there is a global suppression mechanism in the proactive control or hold-your-horses period for other kinds of decision-making tasks that would more clearly require multiple responses to be withheld.
Relation to Neural Systems
We suppose that subjects who slowed down their responses on critical go trials were partially using a proactive suppression mechanism that was selectively targeted at the particular response that might need to be stopped (c.f. Cai et al. 2011). We speculate that this selective mechanism engages the indirect pathway of the basal ganglia, including the striatum (Aron and Verbruggen 2008; Majid et al. 2011). This is consistent with an fMRI study (Jahfari et al. 2010) that compared critical and noncritical go trials and revealed striatal activation (see also Chikazoe et al. 2009; Jahfari et al. 2011; Vink et al. 2005; Vink et al. 2006; Zandbelt and Vink 2010). Additional neuroimaging studies (Forstmann et al. 2010; Forstmann et al. 2008) have implicated the striatum in the tradeoff between response speed versus accuracy, and this tradeoff could depend on the selective proactive control of particular responses when accuracy is favored over speed. Such a striatal mechanism for selective proactive control might establish its influence gradually over particular responses, as opposed to transiently and globally inhibiting the entire motor system.
In contrast to the proposed selective stopping mechanism mediated by the indirect pathway, nonselective stopping may instead be implemented via the hyperdirect pathway, characterized by fast and direct projections from the cortex (i.e., right inferior frontal cortex and presupplementary motor area) to the STN (Aron et al. 2007; Aron and Poldrack 2006). The STN is a deep brain structure with diffuse excitatory projections to output nuclei of the basal ganglia, which, in turn, exert an inhibitory influence over the motor system (Mink 1996; Nambu et al. 2002). Therefore, recruitment of the STN could result in the rapid suppression of activity globally throughout the motor system.
Conclusions and Implications
In each of two experiments, we found that, taking all subjects together, the leg muscle was suppressed when the hand was stopped. In each experiment, we also found that those subjects who slowed down more in anticipation of stopping did not show as much leg suppression during the outright stopping phase as those who did not slow down. This suggests that if subjects do not prepare to stop, then they have to resort to using an emergency stopping mechanism with global effects on the motor system. In contrast, if subjects do prepare to stop, then they evidently stop with lesser global effects. While further research is required to test the neural basis of this distinction, this study provides novel insights into the relationship between preparing to stop and stopping outright.
The results could have practical implications. There is evidently a large variability in whether people bother to prepare to stop or not. It is possible that this individual difference reflects a degree of “motor caution” that could relate to levels of impulsivity. This could be testable in future studies that use personality rating scales. Furthermore, the ability or disposition to prepare to stop in advance may help us to limit our dependence on “emergency” behavioral inhibition mechanisms that could disrupt other ongoing behaviors. For example, a person who stops him or herself from uttering an offensive phrase midsentence may stop speaking altogether, whereas a person who proactively inhibits the urge to utter that offensive phrase may be able to continue speaking without interruption.
GRANTS
This work was supported by National Institute on Drug Abuse Grant DA-026452.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: I.G. and A.R.A. conception and design of research; I.G. and C.L.O. performed experiments; I.G. analyzed data; I.G. and A.R.A. interpreted results of experiments; I.G. prepared figures; I.G. and A.R.A. drafted manuscript; I.G. and A.R.A. edited and revised manuscript; I.G. and A.R.A. approved final version of manuscript.
REFERENCES
- Aron AR. From reactive to proactive and selective control: developing a richer model for stopping inappropriate responses. Biol Psychiatry 69: e55–e68, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Durston S, Eagle DM, Logan GD, Stinear C, Stuphorn V. Converging evidence for a fronto-basal-ganglia network for inhibitory control of action and cognition. J Neurosci 27: 11860–11864, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. Cortical and subcortical contributions to stop signal response inhibition: role of the subthalamic nucleus. J Neurosci 26: 2424–2433, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Verbruggen F. Stop the presses: dissociating a selective from a global mechanism for stopping. Psych Science 19: 1146–1153, 2008 [DOI] [PubMed] [Google Scholar]
- Badry R, Mima T, Aso T, Nakatsuka M, Abe M, Fathi D, Foly N, Nagiub H, Nagamine T, Fukuyama H. Suppression of human cortico-motoneuronal excitability during the stop-signal task. Clin Neurophysiol 120: 1717–1723, 2009 [DOI] [PubMed] [Google Scholar]
- Bissett PG, Logan GD. Balancing cognitive demands: control adjustments in the stop-signal paradigm. J Exp Psychol Learn Mem Cog 37: 392–404, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W, Oldenkamp CL, Aron AR. A proactive mechanism for selective suppression of response tendencies. J Neurosci 31: 5965–5969, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikazoe J, Jimura K, Hirose S, Yamashita Ki, Miyashita Y, Konishi S. Preparation to inhibit a response complements response inhibition during performance of a stop-signal task. J Neurosci 29: 15870–15877, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coxon JP, Stinear C, Byblow WD. Intracortical inhibition during volitional inhibition of prepared action. J Neurophysiol 95: 3371–3383, 2006 [DOI] [PubMed] [Google Scholar]
- De Jong R, Coles MG, Logan GD. Strategies and mechanisms in nonselective and selective inhibitory motor control. J Exp Psychol Hum Percept Perform 21: 498–511, 1995 [DOI] [PubMed] [Google Scholar]
- Forstmann BU, Anwander A, Schäfer A, Neumann J, Brown S, Wagenmakers EJ, Bogacz R, Turner R. Cortico-striatal connections predict control over speed and accuracy in perceptual decision making. Proc Natl Acad Sci USA 107: 15916–15920, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstmann BU, Dutilh G, Brown S, Neumann J, von Cramon DY, Ridderinkhof KR, Wagenmakers EJ. Striatum and pre-SMA facilitate decision-making under time pressure. Proc Natl Acad Sci USA 105: 17538–17542, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ. Hold your horses: a dynamic computational role for the subthalamic nucleus in decision making. Neural Networks 19: 1120–1136, 2006 [DOI] [PubMed] [Google Scholar]
- Frank MJ, Samanta J, Moustafa AA, Sherman SJ. Hold your horses: impulsivity, deep brain stimulation, and medication in parkinsonism. Science 318: 1309–1312, 2007 [DOI] [PubMed] [Google Scholar]
- Jahfari S, Stinear CM, Claffey M, Verbruggen F, Aron AR. Responding with restraint: what are the neurocognitive mechanisms? J Cog Neurosci 22: 1479–1492, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahfari S, Waldorp L, van den Wildenberg WP, Scholte HS, Ridderinkhof KR, Forstmann BU. Effective connectivity reveals important roles for both the hyperdirect (fronto-subthalamic) and the indirect (fronto-striatal-pallidal) fronto-basal ganglia pathways during response inhibition. J Neurosci 31: 6891–6899, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leocani L, Cohen LG, Wassermann EM, Ikoma K, Hallett M. Human corticospinal excitability evaluated with transcranial magnetic stimulation during different reaction time paradigms. Brain 123: 1161–1173, 2000 [DOI] [PubMed] [Google Scholar]
- Leotti LA, Wager TD. Motivational influences on response inhibition measures. J Exp Psychol Hum Percept Perform 36: 430–447, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan GD, Cowan WB, Davis KA. On the ability to inhibit simple and choice reaction time responses: a model and a method. J Exp Psychol Hum Percept Perform 10: 276–291, 1984 [DOI] [PubMed] [Google Scholar]
- Majid A, Cai W, George J, Verbruggen F, Aron AR. Transcranial magnetic stimulation reveals dissociable mechanisms for global versus selective corticomotor suppression underlying the stopping of action. Cereb Cortex; doi:10.1093/cercor/bhr112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol 50: 381–425, 1996 [DOI] [PubMed] [Google Scholar]
- Nambu A, Tokuno H, Takada M. Functional significance of the cortico-subthalamo-pallidal “hyperdirect” pathway. Neurosci Res 43: 111–117, 2002 [DOI] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, Dimitrijević MR, Hallett M, Katayama Y, Lücking CH. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee Electroencephalogr Clin Neurophysiol 91: 79–92, 1994 [DOI] [PubMed] [Google Scholar]
- Sohn YH, Wiltz K, Hallett M. Effect of volitional inhibition on cortical inhibitory mechanisms. J Neurophysiol 88: 333–338, 2002 [DOI] [PubMed] [Google Scholar]
- van den Wildenberg WP, Burle B, Vidal F, van der Molen MW, Ridderinkhof KR, Hasbroucq T. Mechanisms and dynamics of cortical motor inhibition in the stop-signal paradigm: a TMS study. J Cog Neurosci 22: 225–239, 2010 [DOI] [PubMed] [Google Scholar]
- Verbruggen F, Logan G. Response inhibition in the stop-signal paradigm. Trends Cogn Sci 12: 418–424, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F, Logan GD. Proactive adjustments of response strategies in the stop-signal paradigm. J Exp Psychol Hum Percept Perform 35: 835–854, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink M, Kahn RS, Raemaekers M, Van Den Heuvel M, Boersma M, Ramsey NF. Function of striatum beyond inhibition and execution of motor responses. Hum Brain Mapp 25: 336–344, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink M, Ramsey NF, Raemaekers M, Kahn RS. Striatal dysfunction in schizophrenia and unaffected relatives. Biol Psychiatry 60: 32–39, 2006 [DOI] [PubMed] [Google Scholar]
- Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalogr Clin Neurophysiol 108: 1–16, 1998 [DOI] [PubMed] [Google Scholar]
- Zandbelt BB, Vink M. On the role of the striatum in response inhibition. PLos One 5: e13848, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]