Introduction
Under certain conditions, pathogens like Yersinia secrete high levels of proteins. This ability correlates with cytotoxicity and virulence. The complete inactivity of the secreted proteins when added directly to host cells stimulated research to disclose this “non-AB toxin mechanism” of virulence, and eventually led to the discovery of the type III secretion mechanism and the “injection model” for virulence effector targeting. Recent studies question this model and suggest that the T3SS effector-targeting mechanism may resemble the classical AB toxin delivery mechanism.
The Ca2+ Paradox
Human pathogenic Yersinia species are uniquely dependent on millimolar concentrations of Ca2+ when grown at 37°C. This phenotype is characterized by normal growth at 37°C in the presence of Ca2+, and growth restriction in Ca2+-depleted medium [1]. This unusual Ca2+ requirement has been linked to the presence of a virulence plasmid [2], originally discovered by Zink et al. [3]. The plasmid encoded a set of proteins (Yersinia outer proteins, or Yops) [4] that were secreted into the culture supernatant in massive amounts during growth restriction in the absence of Ca2+ [5], [6]. The coupling of the Yops to the virulence plasmid indicated strongly that they were essential virulence determinants. This hypothesis was further supported when convalescence sera obtained from Yersinia-infected patients were shown to contain antibodies recognizing the secreted Yop proteins [4], leading to the hypothesis that low intracellular Ca2+ inside mammalian cells induced expression of the Yop virulon during infection. The role of calcium was thus paradoxical as conditions promoting Yop secretion resulted in growth restriction.
Polarized Translocation of Yop Effectors
Cavanaugh and Randall were the first to demonstrate that blockage of phagocytosis is crucial for Yersinia to cause disease [7]. The significance of this finding was later strengthened by the finding that the bacteria replicate predominantly at extracellular sites during infection [8]. YopH and YopE were essential to the ability to block phagocytosis [9]. These data demonstrated that Yersinia is an extracellular pathogen and that Yop expression is a prerequisite for virulence, and also raised the question of how the pathogen was able to affect target cells from its extracellular location—an especially intriguing question because, unlike AB toxins, the addition of purified Yops to cultured cells did not cause any obvious effects.
The first important discovery to resolve this issue was the finding that microinjection of purified YopE into target cells induced a cytotoxic response [10], demonstrating that YopE must be translocated into target cells to elicit its biological function. It was also concluded that in addition to YopE, the secreted protein YopD was essential for the delivery of YopE across the target cell membrane by infecting bacteria [10]. Confocal imaging and biochemical fractionation were used to demonstrate that contact between Yersinia and the target cell induces Yop expression accompanied by the translocation of YopE into target cells [11], [12]. The majority of YopE was present in the target cell cytosol and no YopE was detected in the culture medium, indicating that Yop translocation was polarized and occurred only at the zone of contact between the bacterium and the target cell [12]. Translocation of Yop effectors into target cells was later demonstrated by another approach using an elegant reporter system [13].
Does the Type III Secretion System Function as a Microsyringe?
Cornelis and co-workers were the first to demonstrate that the virulence plasmid of pathogenic Yersinia encodes a dedicated Yop secretion system [14]. Some genes identified by Cornelis et al. had homologous counterparts in plant pathogens, and Reeves and Salmond realized that these different pathogens exhibited a common secretion pathway, dubbed “Type III secretion” (T3S) [15]. An additional set of Yersinia genes was identified that displayed remarkable homology to corresponding genes in Salmonella and Shigella [16]. The idea that highly conserved secretory systems could allow heterologous secretion was based on these observations, and corroborated by experimental evidence demonstrating that S. typhimurium secreted YopE via a T3SS-dependent mechanism. Both secretion and cell contact-dependent translocation of effectors into the target cells were functionally conserved among yersiniae, salmonellae, and shigellae [17], which led us to suggest that the T3SS-dependent secretion and translocation of effector proteins were highly conserved among different pathogens and occurred through a “microinjection mechanism.”
That the T3SSs are organized into supra-molecular structures known as needle complexes was first shown in S. typhimurium [18]. This structure, which is believed to be common to all T3SSs, resembles a syringe with a base structure and a needle-like protrusion extending from the surface of the pathogen. The entire needle complex is traversed by a fine, hollow tube that may allow passage of effector proteins across the bacterial cell envelope [19]. Due to the suggestive similarities between the needle complex and a syringe, it has been generally accepted that the effectors travel directly through the needle complex from the bacterial cytosol into the lumen of the eukaryotic target cell. However, no experimental results have been presented to demonstrate that the effectors are secreted through the needle structure.
Are the Effectors Translocated through a Pore Formed in the Host Cell Membrane?
The secreted substrates can be divided into two functional classes: effectors are delivered into the target cell where they elicit a biological response, while translocators facilitate the delivery of effectors across the plasma membrane [10], [12], [13]. A key signature of translocators is the putative membrane spanning domain(s), which has some similarities to pore-forming toxins [20]. The first translocator protein found to exhibit pore-forming and hemolytic activities was Shigella IpaB [21]. Subsequently, the Yersinia homologue YopB was found to be essential for Yop effector translocation; similar to IpaB, YopB also induces erythrocyte hemolysis [22], [23]. The pore-forming ability of YopB correlates with functional translocation, supporting the idea that the effectors are delivered through a pore in the host cell membrane. Recent work has revealed that LcrV (initially thought to be a translocator protein) is localized at the tip of the needle complex [24]. LcrV has also been proposed to be essential for insertion of the hydrophobic translocators YopB and YopD into target cell membranes. These properties of LcrV agree nicely with the “injection model.”
However, all findings are not compatible with this model. For example, Sasakawa and co-workers reported that the Shigella translocators IpaB, IpaC, and IpaD are surface-localized before target cell contact [25]. The majority of these proteins are rapidly released after target cell contact is established, indicating that Shigella senses target cell contact and responds accordingly. Furthermore, latex beads coated with purified Ipa protein complexes are internalized by target cells through a mechanism that resembles the active, T3SS-dependent engulfment of Shigella [26]. The apparent surface localization of these proteins before target cell contact is difficult to reconcile with the injection model. On the other hand, recent studies in Shigella demonstrating that IpaB and subsequently IpaC are recruited to the tip of the needle from its location at the bacterial surface in response to target cell contact lend support to the injection model [27]. Nevertheless, these results do not support the idea that the T3SS forms a conduit that allows both translocators and effectors to be secreted in one step by the same T3SS, since at least the translocators are secreted to the surface before eukaryotic cell contact has been established.
Binary AB Toxin Revisited?
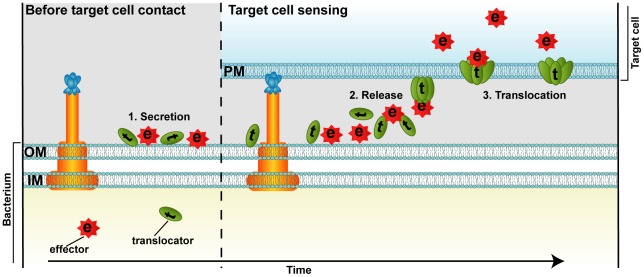
Recent attempts to visualize T3SS substrates during infection using immunogold labeling and transmission electron microscopic analysis revealed that the majority of Yop translocators and effectors are present on the surface of the bacterium before target cell contact [28]. This finding was surprising because the injection model predicts that the T3SS substrates should be present in a secretion competent conformation in the bacterial cytosol before target cell contact. Remarkably, a yopH null mutant coated with purified YopH was translocation competent and complemented the mutant phenotype. The translocation of surface-localized YopH and YopH-β-lactamase reporters is also T3SS dependent and requires functional translocators, as well as a specific translocation domain present in YopH. The N-terminal secretion signal is redundant for translocation of externally added YopH, demonstrating that secretion and translocation are two separate events. Importantly, T3SS-dependent translocation of externally added YopH has also been achieved in S. typhimurium, indicating that the ability to translocate surface-localized effectors is conserved among different T3SS-dependent pathogens. Taken together, these data support a two-step model in which the T3SS substrates are secreted first. After target cell sensing, these surface-localized effectors are translocated across the target cell plasma membrane (Figure 1). The translocation step could superficially be compared to the delivery of binary AB toxins: the hydrophobic T3SS translocators resemble the pore-forming B-moiety that mediates the translocation of the catalytic A-moiety (Yop effectors). In this scenario, tight binding of the bacterium to the target cell provides the micro-environment required for efficient translocation of effectors across the plasma membrane. This requires that the surface-localized T3SS substrates are released when the bacterium senses target cell contact, leading to derepression of the yop regulon via the T3SS [11]. Because increased yop expression is coupled to increased Yop translocation, release may be coupled to the needle complex. Therefore, we suggest a sensory role for the needle complex, thereby explaining the requirement for target cell contact in T3SS-dependent effector delivery. However, our results do not exclude the injection model, because it is still possible for the two processes to operate in parallel. Obviously, more work is needed before the molecular details of the translocation are resolved.
Figure 1. Proposed model for T3SS-dependent protein translocation by a binary AB toxin like mechanism.
T3SS translocators (t) and effectors (e) are secreted by the T3SS across the bacterial envelope (IM and OM) to the surface of the cell before host cell contact (1). Target cell sensing results in release of the surface localized T3SS substrates (2). The translocators (t) assemble into a pore in the target cell plasma membrane (PM) and mediate the translocation of the effectors (e) into the target cell cytoplasm (3).
Footnotes
The authors have declared that no competing interests exist.
This work was supported by the Swedish Research Council (VR), the Kempe Foundation, the Carl Trygger Foundation for Scientific Research, and the Foundation for Medical Research (Insamlingsstiftelsen) at Umeå University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Brubaker RR. The Vwa+ virulence factor of yersiniae: the molecular basis of the attendant nutritional requirement for Ca++. Rev Infect Dis. 1983;5(Suppl 4):S748–S758. doi: 10.1093/clinids/5.supplement_4.s748. [DOI] [PubMed] [Google Scholar]
- 2.Gemski P, Lazere JR, Casey T. Plasmid associated with pathogenicity and calcium dependency of Yersinia enterocolitica. Infect Immun. 1980;27:682–685. doi: 10.1128/iai.27.2.682-685.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zink DL, Feeley JC, Wells JG, Vanderzant C, Vickery JC, et al. Plasmid-mediated tissue invasiveness in Yersinia enterocolitica. Nature. 1980;283:224–226. doi: 10.1038/283224a0. [DOI] [PubMed] [Google Scholar]
- 4.Bolin I, Portnoy DA, Wolf-Watz H. Expression of the temperature-inducible outer membrane proteins of yersiniae. Infect Immun. 1985;48:234–240. doi: 10.1128/iai.48.1.234-240.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heesemann J, Algermissen B, Laufs R. Genetically manipulated virulence of Yersinia enterocolitica. Infect Immun. 1984;46:105–110. doi: 10.1128/iai.46.1.105-110.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Portnoy DA, Moseley SL, Falkow S. Characterization of plasmids and plasmid-associated determinants of Yersinia enterocolitica pathogenesis. Infect Immun. 1981;31:775–782. doi: 10.1128/iai.31.2.775-782.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cavanaugh DC, Randall R. The role of multiplication of Pasteurella pestis in mononuclear phagocytes in the pathogenesis of flea-borne plague. J Immunol. 1959;83:348–363. [PubMed] [Google Scholar]
- 8.Simonet M, Richard S, Berche P. Electron microscopic evidence for in vivo extracellular localization of Yersinia pseudotuberculosis harboring the pYV plasmid. Infect Immun. 1990;58:841–845. doi: 10.1128/iai.58.3.841-845.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosqvist R, Forsberg A, Rimpilainen M, Bergman T, Wolf-Watz H. The cytotoxic protein YopE of Yersinia obstructs the primary host defence. Mol Microbiol. 1990;4:657–667. doi: 10.1111/j.1365-2958.1990.tb00635.x. [DOI] [PubMed] [Google Scholar]
- 10.Rosqvist R, Forsberg A, Wolf-Watz H. Intracellular targeting of the Yersinia YopE cytotoxin in mammalian cells induces actin microfilament disruption. Infect Immun. 1991;59:4562–4569. doi: 10.1128/iai.59.12.4562-4569.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pettersson J, Nordfelth R, Dubinina E, Bergman T, Gustafsson M, et al. Modulation of virulence factor expression by pathogen target cell contact. Science. 1996;273:1231–1233. doi: 10.1126/science.273.5279.1231. [DOI] [PubMed] [Google Scholar]
- 12.Rosqvist R, Magnusson KE, Wolf-Watz H. Target cell contact triggers expression and polarized transfer of Yersinia YopE cytotoxin into mammalian cells. EMBO J. 1994;13:964–972. doi: 10.1002/j.1460-2075.1994.tb06341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sory MP, Cornelis GR. Translocation of a hybrid YopE-adenylate cyclase from Yersinia enterocolitica into HeLa cells. Mol Microbiol. 1994;14:583–594. doi: 10.1111/j.1365-2958.1994.tb02191.x. [DOI] [PubMed] [Google Scholar]
- 14.Michiels T, Wattiau P, Brasseur R, Ruysschaert JM, Cornelis G. Secretion of Yop proteins by Yersiniae. Infect Immun. 1990;58:2840–2849. doi: 10.1128/iai.58.9.2840-2849.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salmond GP, Reeves PJ. Membrane traffic wardens and protein secretion in gram-negative bacteria. Trends Biochem Sci. 1993;18:7–12. doi: 10.1016/0968-0004(93)90080-7. [DOI] [PubMed] [Google Scholar]
- 16.Bergman T, Erickson K, Galyov E, Persson C, Wolf-Watz H. The lcrB (yscN/U) gene cluster of Yersinia pseudotuberculosis is involved in Yop secretion and shows high homology to the spa gene clusters of Shigella flexneri and Salmonella typhimurium. J Bacteriol. 1994;176:2619–2626. doi: 10.1128/jb.176.9.2619-2626.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosqvist R, Hakansson S, Forsberg A, Wolf-Watz H. Functional conservation of the secretion and translocation machinery for virulence proteins of yersiniae, salmonellae and shigellae. EMBO J. 1995;14:4187–4195. doi: 10.1002/j.1460-2075.1995.tb00092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kubori T, Matsushima Y, Nakamura D, Uralil J, Lara-Tejero M, et al. Supramolecular structure of the Salmonella typhimurium type III protein secretion system. Science. 1998;280:602–605. doi: 10.1126/science.280.5363.602. [DOI] [PubMed] [Google Scholar]
- 19.Galan JE, Wolf-Watz H. Protein delivery into eukaryotic cells by type III secretion machines. Nature. 2006;444:567–573. doi: 10.1038/nature05272. [DOI] [PubMed] [Google Scholar]
- 20.Hakansson S, Bergman T, Vanooteghem JC, Cornelis G, Wolf-Watz H. YopB and YopD constitute a novel class of Yersinia Yop proteins. Infect Immun. 1993;61:71–80. doi: 10.1128/iai.61.1.71-80.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.High N, Mounier J, Prevost MC, Sansonetti PJ. IpaB of Shigella flexneri causes entry into epithelial cells and escape from the phagocytic vacuole. EMBO J. 1992;11:1991–1999. doi: 10.1002/j.1460-2075.1992.tb05253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boland A, Sory MP, Iriarte M, Kerbourch C, Wattiau P, et al. Status of YopM and YopN in the Yersinia Yop virulon: YopM of Y.enterocolitica is internalized inside the cytosol of PU5-1.8 macrophages by the YopB, D, N delivery apparatus. EMBO J. 1996;15:5191–5201. [PMC free article] [PubMed] [Google Scholar]
- 23.Hakansson S, Schesser K, Persson C, Galyov EE, Rosqvist R, et al. The YopB protein of Yersinia pseudotuberculosis is essential for the translocation of Yop effector proteins across the target cell plasma membrane and displays a contact-dependent membrane disrupting activity. EMBO J. 1996;15:5812–5823. [PMC free article] [PubMed] [Google Scholar]
- 24.Mueller CA, Broz P, Muller SA, Ringler P, Erne-Brand F, et al. The V-antigen of Yersinia forms a distinct structure at the tip of injectisome needles. Science. 2005;310:674–676. doi: 10.1126/science.1118476. [DOI] [PubMed] [Google Scholar]
- 25.Watarai M, Tobe T, Yoshikawa M, Sasakawa C. Contact of Shigella with host cells triggers release of Ipa invasins and is an essential function of invasiveness. EMBO J. 1995;14:2461–2470. doi: 10.1002/j.1460-2075.1995.tb07243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Menard R, Prevost MC, Gounon P, Sansonetti P, Dehio C. The secreted Ipa complex of Shigella flexneri promotes entry into mammalian cells. Proc Natl Acad Sci U S A. 1996;93:1254–1258. doi: 10.1073/pnas.93.3.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stensrud KF, Adam PR, La Mar CD, Olive AJ, Lushington GH, et al. Deoxycholate interacts with IpaD of Shigella flexneri in inducing the recruitment of IpaB to the type III secretion apparatus needle tip. J Biol Chem. 2008;283:18646–18654. doi: 10.1074/jbc.M802799200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akopyan K, Edgren T, Wang-Edgren H, Rosqvist R, Fahlgren A, et al. Translocation of surface-localized effectors in type III secretion. Proc Natl Acad Sci U S A. 2011;108:1639–1644. doi: 10.1073/pnas.1013888108. [DOI] [PMC free article] [PubMed] [Google Scholar]