Abstract
Background
Severe hemorrhagic shock and resuscitation initiates a dysfunctional systemic inflammatory response leading to end-organ injury. Clinical evidence supports the transfusion of high ratios of plasma and packed red blood cells (pRBCs) in the treatment of hemorrhagic shock. The effects of resuscitation with different ratios of fresh blood products on inflammation and organ injury have not yet been characterized.
Materials and Methods
Mice underwent femoral artery cannulation and pressure-controlled hemorrhage for 60 minutes, then resuscitation with fresh plasma and pRBCs collected from donor mice. Plasma alone, pRBCs alone, and ratios of 2:1, 1:1, and 1:2 plasma:pRBCs were used for resuscitation strategies. Mice were sacrificed to determine biochemical and hematologic parameters, serum cytokine concentrations, tissue myeloperoxidase levels, and vascular permeability.
Results
As compared to other resuscitation strategies, mice resuscitated with pRBCs alone exhibited increased hemoglobin levels, while other hematologic and biochemical parameters were not significantly different among groups. Compared to 1:1, mice resuscitated with varying ratios of plasma:pRBCs exhibited increased cytokine concentrations of KC, MIP-lα, and MIP-2, and increased intestinal and lung myeloperoxidase levels. Mice resuscitated with 1:1 had decreased vascular permeability in the intestine and lung as compared to other groups.
Conclusions
Resuscitation with a 1:1 ratio of fresh plasma:pRBCs results in decreased systemic inflammation and attenuated organ injury. These findings support the potential advantage of transfusing blood products in physiologic ratios to improve the treatment of severe hemorrhagic shock.
Keywords: hemorrhage, shock, inflammation, resuscitation
INTRODUCTION
Despite advancements in trauma care, hemorrhage persists as a leading cause of early death following severe injury, and new, emerging resuscitation strategies represent a potential opportunity to further reduce mortality (1). The optimal resuscitative approach for the treatment of hemorrhagic shock has been recently debated, challenging the role of conventional crystalloid based resuscitation with the development of damage control resuscitation strategies. While traditional recommendations call for the initial infusion of crystalloid followed by blood products (2), this approach may exacerbate inflammation following shock(3). Recent clinical evidence supports the early transfusion of blood products in a high ratio of plasma to packed red blood cells (plasma:pRBCs) in patients sustaining life-threatening hemorrhage (4-7). Borgman, et al. defined the critical ratio of plasma:pRBCs associated with improved mortality as 1:1.4, compared to those patients transfused lower ratios of plasma:pRBCs (5). Other studies found mortality differences between those patients who received ratios of plasma to pRBCs greater or less than 1:1.5 (6, 7). A retrospective review of 16 Level 1 trauma centers found improved survival rates when a plasma:pRBCs ratio greater than 1:2 was utilized (4). While a high ratio of plasma:pRBCs appears to be beneficial, the optimal ratio of blood products remains unclear.
The early and increased use of plasma in trauma resuscitation is not without significant controversy. Criticisms of this strategy include a potential association with acute respiratory distress syndrome (ARDS) and multiple organ failure (7, 8). While not completely understood, hemorrhagic shock and subsequent resuscitation are associated with a dysfunctional systemic inflammatory response and excessive neutrophil activation which may contribute to these later complications (9, 10). Even with timely control of hemorrhage and adequate resuscitation, patients may succumb to the sequelae of the systemic inflammatory response following traumatic injury. While damage control resuscitation may decrease early deaths from hemorrhagic shock, the ideal fluid strategy should avoid any iatrogenic resuscitative injury, decreasing the potential for organ failure and mortality.
Although a high ratio of plasma:pRBCs is associated with improved survival clinically, the effect of varying ratios of blood components on the inflammatory response to hemorrhagic shock is unknown. In the present study, we sought to determine the effect of transfusing various ratios of plasma to pRBCs on systemic inflammation and tissue injury following hemorrhagic shock in mice.
MATERIALS AND METHODS
Animal model
Male C57/Bl6 mice weighing 21-30 grams were purchased from Harlan Laboratories (Indianapolis, IN) fed standard laboratory diet and water ad libitum and acclimated for one week in a climate controlled room with a 12-hour light-dark cycle. All experiments were approved by the Institutional Animal Care and Use Committee at the University of Cincinnati.
Hemorrhage model
Mice were anesthetized with intraperitoneal pentobarbital (0.1mg/gram body weight). After clipping and sterile preparation with povidone-iodine solution and alcohol, the femoral vessels were exposed. Femoral artery cannulation was performed using tapered polyethylene catheters connected to pressure transducers for continuous hemodynamic monitoring (Harvard Apparatus, Holliston, MA). Mice were placed on a circulating water blanket maintained at 41°C to preserve body temperature and avoid hypothermia. After an initial 10 minute period of equilibration, blood was withdrawn over 3 minutes until a systolic blood pressure (SBP) of 25 mmHg was achieved. Mice were maintained at a SBP of 25 ± 5 mmHg for 60 minutes by drawing or administering shed blood volume.
Preparation of additives
Citrate phosphate double dextrose (CP2D; 257.6mmol/L of glucose, 105.0mmol/ L of citrate-citric acid, 18.5mmol/L of monosodium phosphate, with a pH of 5.7) was prepared as anticoagulant. AS-3 (Nutricel) (55.5mmol/L of glucose, 70.1mmol/L of sodium chloride, 20mmol/L of sodium phosphate, 12mmol/L of citric acid, and 2.2mmol/L of adenine, with a pH of 5.8) was used as red cell storage media.
Collection of blood components
Donor male mice were anesthetized with intraperitoneal pentobarbital (0.1mg/gram body weight). Whole blood was collected via sterile cardiac puncture with needles and syringes pre-coated with CP2D. CP2D was added to fresh whole blood in a ratio of 1:7, and samples were gently mixed. Anticoagulated whole blood was centrifuged at 1000xg at 4°C for 15 minutes immediately after collection to separate blood components. The plasma layer was removed from each sample and pooled prior to transfusion. AS-3 was added in a ratio of 2:9 to the remaining red cells and buffy coat, and samples were gently mixed. Packed red blood cell (pRBC) samples were pooled, and both plasma and pRBCs were kept warm on a circulating water blanket prior to transfusion.
Resuscitation
Mice were resuscitated with varying ratios of plasma and pRBCs collected from donor animals to a target SBP of 80 ± 5 mmHg. Mice were transfused with plasma alone, pRBCs alone, or ratios of plasma to pRBCs of 2:1, 1:1, or 1:2. To ensure adequate resuscitation, mice were monitored for fifteen minutes, then decannulated and sacrificed at intervals. Sham animals underwent identical femoral arterial cannulation and monitoring for 90 minutes, but were neither hemorrhaged nor resuscitated. The total period of shock and resuscitation was based on a previous study suggesting that this time point allows optimal examination of the inflammatory response to shock and resuscitation (3).
Hematologic and Biochemical Parameters
Thirty minutes after decannulation, blood was drawn from mice via the left ventricle and was immediately analyzed. The following parameters were assayed on each sample: pH, base excess, bicarbonate, lactate, sodium, potassium, and hemoglobin (I-STAT 1 Analyzer, Heska, Loveland, CO).
Cytokine Analysis
Blood was collected via cardiac puncture at 30 minutes following decannulation. Plasma samples were allowed to clot and were centrifuged at 6,800xg to separate the serum from cellular components. ELISA was performed for the following serum chemokines and cytokines: macrophage inflammatory protein-lα (MIP-lα), macrophage inflammatory protein-2 (MIP-2), and keratinocyte derived chemokine (KC), (Quansys Biosciences, Logan, UT).
Myeloperoxidase Activity
Thirty minutes after resuscitation and following exsanguination, intestine, and lung samples were harvested. Whole lung myeloperoxidase (MPO) activity was determined using methods previously described (11). 100 mg of tissue were homogenized and diluted in 50mM potassium phosphate buffer with 0.5% hexadecyltrimethylammonium bromide, pH 6.0. Following sonication and two freeze-thaw cycles, samples were centrifuged at 12,000xg for 5 minutes. The supernatants were reacted with a H2O2 (0.3mM) solution containing tetramethylbenzidine (1.6mM). MPO activity was determined against a standard absorbance curve. Protein concentrations were quantified using the Pierce BCA Protein Assay Kit (Thermo Scientific, Rockford, IL) and MPO activity was expressed in units/gram protein.
Vascular Permeability
After resuscitation but prior to decannulation, 20mg/kg body weight of 4% Evans Blue (Sigma, St. Louis, MO) was injected via the femoral line. After 30 minutes of circulation, animals underwent laparotomy and sternotomy. A needle was inserted into the beating right ventricle and the superior vena cava was divided sharply below the diaphragm. The intravascular space was flushed with 10 mL of heparinized phosphate buffered saline (20 units heparin/mL PBS) at a continual rate of 3 mL/min using a perfusion pump. The ileum, colon, and lung were harvested, washed in heparinized PBS, and placed in 100mg/mL of formamide (Sigma, St. Louis, MO). All samples were incubated at 37°C for 7 days. Evans Blue concentration in each sample was determined by measuring the absorbance at 620 nm against a standard curve.
Statistics
Results are reported as the mean plus or minus standard error of the mean (SEM). Two-tailed Student t test or analysis of variance (ANOVA) with Tukey’s test was used where appropriate to determine significance. Statistical analysis was performed using SigmaPlot 10 software (Systat Software, Chicago, IL). p values less than or equal to 0.05 were determined to be significant.
RESULTS
Mean systolic blood pressure (SBP) was not different among groups of mice resuscitated with varying ratios of blood products during the initial equilibration, hemorrhagic shock, or resuscitation periods (Figure 1). The mean SBP of all hemorrhaged groups were significantly different from sham animals during hemorrhage, but did not differ significantly from sham mice at the end of resuscitation (Figure 1). Neither the shed blood volume nor the volume required to resuscitate to the target SBP were significantly different between groups of hemorrhaged animals (Figure 2). This data indicates that the different resuscitation strategies utilized here did not significantly differ in their hemodynamic effects.
Figure 1.
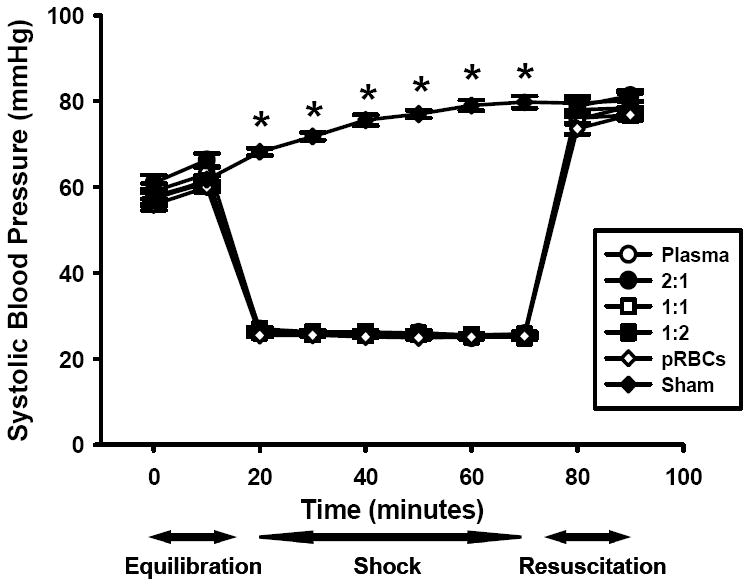
Systolic blood pressure (SBP) of mice undergoing hemorrhage and resuscitation with plasma, packed red blood cells (pRBCs), and ratios or plasma to pRBCs including 2:1, 1:1, and 1:2 compared to sham mice. *p<0.001 vs. other groups, n=20 for each group.
Figure 2.
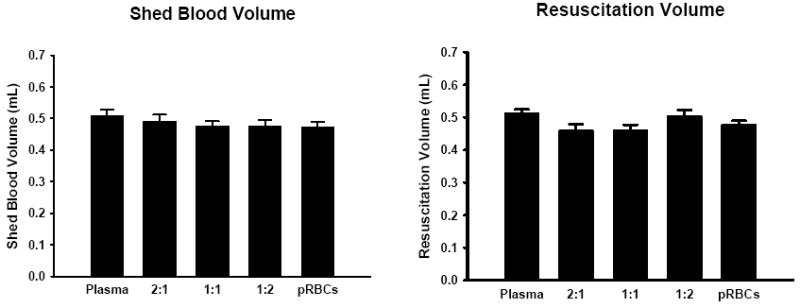
Shed blood volume and resuscitation fluid volume for mice resuscitated with plasma, pRBCs, and plasma:pRBCs ratios of 2:1, 1:1, and 1:2. No significant differences were seen between groups, n=20 for each group.
Mice resuscitated with plasma alone exhibited lower hemoglobin levels compared to mice resuscitated with only pRBCs or a 1:2 ratio of plasma:pRBCs (Table 1). In addition, mice resuscitated with a 1:1 ratio of plasma:pRBCs demonstrated lower hemoglobin levels compared to mice resuscitated with pRBCs alone or a 1:2 ratio of plasma:pRBCs (Table 1). No differences were seen among any resuscitation strategy in bicarbonate, lactate, sodium, or potassium levels drawn from mice 30 minutes after resuscitation (Table 1). While no differences were seen in base excess, mice resuscitated with plasma alone were slightly more alkalotic as determined by pH compared to all other groups (Table 1).
TABLE 1.
Hematologic and biochemical parameters of resuscitated mice
| Resuscitation Strategy | pH | Base Excess | Bicarbonate (mmol/L) | Lactate (mmol/L) | Sodium (mmol/L) | Potassium (mmol/L) | Hemoglobin (grams) |
|---|---|---|---|---|---|---|---|
| Plasma | 7.48±0.02* | 1.2±1.5 | 24.6±1.4 | 1.84±0.27 | 146.0±0.4 | 4.2±0.1 | 9.83±0.33** |
| 2:1 plasma:pRBCs | 7.29±0.02 | 1.5±0.6 | 28.2±0.7 | 0.78±0.06 | 146.3±0.7 | 4.4±0.2 | 11.22±0.73 |
| 1:1 plasma:pRBCs | 7.35±0.02 | -0.2±1.2 | 23.2±1.2 | 1.62±0.68 | 146.2±1.6 | 4.1±0.2 | 10.28±0.92** |
| 1:2 plasma:pRBCs | 7.33±0.04 | 0.8±1.1 | 24.8±1.3 | 1.33±0.26 | 145.3±0.7 | 4.0±0.3 | 13.20±0.60 |
| pRBcs | 7.29±0.01 | -1.0±0.4 | 26.1±0.5 | 2.06±0.69 | 145.2±0.8 | 3.9±1.9 8 | 13.08±0.58 |
p<0.05 vs. all other groups,
p<0.05 vs. pRBCs and 1:2 plasma:pRBCs.
When we evaluated the circulating levels of proinflammatory cytokines after shock, we found that mice resuscitated with a 1:1 ratio of plasma:pRBCs exhibited decreased levels of macrophage inflammatory protein-lα (MIP-lα), compared to mice resuscitated with either plasma or pRBCs alone (Figure 3A). Compared to all other groups, mice resuscitated with a 1:1 ratio of plasma:pRBCs demonstrated significantly lower serum levels of macrophage inflammatory protein-2 (MIP-2; Figure 3B). Mice resuscitated with a 1:1 ratio of plasma:pRBCs exhibited decreased levels of keratinocyte-derived chemokine (KC) compared to mice resuscitated with pRBCs alone, or a 1:2 ratio of plasma:pRBCs (Figure 3C).
Figure 3.
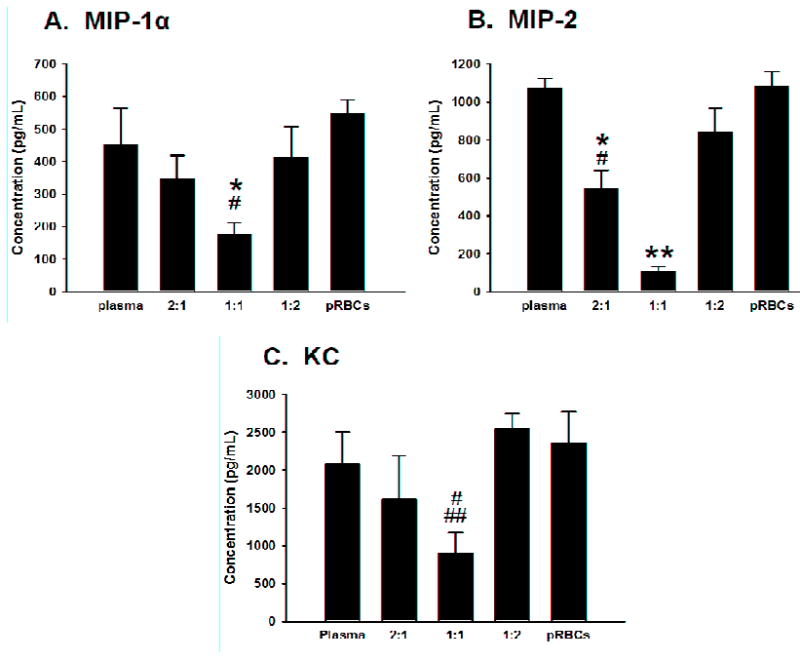
Serum levels of MIP-lα, MIP-2, and KC after hemorrhage and resuscitation with plasma alone, pRBCs alone, and plasma combined with pRBCs in ratios of 2:1, 1:1, and 1:2. *p<0.05 vs. plasma alone, #p<0.05 vs. pRBCs alone, ##p<0.05 vs. 1:2 ratio of plasma:pRBCs, **p<0.05 vs. all other groups
Recruitment of neutrophils into intestine and lung was determined by tissue myeloperoxidase activity. Although not statistically significant, mice resuscitated with plasma alone displayed a trend of increased MPO activity in the ileum compared to mice resuscitated with a 1:1 ratio of plasma:pRBCs (Figure 4A). Compared to mice resuscitated with a 1:1 ratio of plasma:pRBCs, mice resuscitated with plasma alone exhibited increased MPO activity in the colon (Figure 4B). Mice resuscitated with pRBCs alone demonstrated increased MPO activity in the lung compared to mice resuscitated with a 1:1 ratio of plasma:pRBCs (Figure 4C).
Figure 4.

Myeloperoxidase levels (MPO) of ileum, colon, and lung after hemorrhage and resuscitation with plasma, 1:1, or pRBCs 30minutes after resuscitation. **p<0.05 vs. all other groups.
To assess the level of microvascular permeability present in the intestine and lung, tissue leakage of Evans Blue was determined. Ileum and colon samples harvested from mice transfused with a 1:1 ratio of plasma:pRBCs showed significantly decreased vascular permeability compared to mice resuscitated with plasma alone (Figure 5A and 5B). Compared to mice resuscitated with pRBCs alone, mice treated with a 1:1 ratio of plasma:pRBCs showed decreased pulmonary vascular leak (Figure 5C).
Figure 5.

Concentration of Evans Blue in ileum, colon, and lung after hemorrhage and resuscitation with plasma, 2:1, 1:1, 1:2, and pRBCs. *p<0.05 vs. plasma. #p<0.05 vs. pRBCs.
DISCUSSION
In the present study, we evaluated the effects of transfusing different ratios of fresh blood products on the early inflammatory response following hemorrhagic shock. The conventional strategy of resuscitation from hemorrhagic shock, extrapolated from Shires’ initial work supporting the use of isotonic salt solutions (12), has since been shown to exacerbate the inflammatory response to hemorrhage and is associated with increased mortality (3). With the advent of damage control resuscitation strategies utilizing high ratios of plasma:pRBCs, retrospective evidence supports a survival advantage to increased use of ratios of plasma to red blood cells (4-7). These findings are based on clinical experience, without prior assessment of the pro- or anti-inflammatory effects of transfusing a high ratio of plasma:pRBCs In this series of experiments, we sought to characterize how the inflammatory response to hemorrhage is modulated by different ratios of blood products, and to determine the optimal ratio for resuscitation from severe hemorrhagic shock from this aspect.
Organ injury following trauma and hemorrhagic shock is associated with systemic and localized inflammation, accumulation of activated neutrophils, and increased microvascular permeability (13). Chemokines including IL-8 and macrophage inflammatory protein-lα (MIP-lα) have been shown to play crucial roles in regulating neutrophil migration in human and animal injury models (14-16). An initial injury such as hemorrhage may lead to the activation and extravasation of neutrophils and other inflammatory cells into tissues, rendering these organs vulnerable to secondary insults(17). Theoretically, a resuscitation strategy which minimizes excessive chemokine expression and neutrophil recruitment may decrease susceptibility to organ dysfunction and injury following hemorrhagic shock.
IL-8 is a human chemokine increased in plasma and lung tissue in patients with inflammatory pulmonary diseases including adult respiratory distress syndrome (ARDS) (18, 19). Increased levels of IL-8 have been isolated in bronchoalveolar lavage samples in patients with acute lung injury (20), and blockage of IL-8 has reduced neutrophil-mediated reperfusion injury in the lung in a rabbit model (21). While no identical chemokine exists in mice, keratinocyte-derived chemokine (KC) and macrophage inflammatory protein-2 (MIP-2) are murine homologues of human IL-8, with divergent roles in mediating inflammation (22). In a two-hit model of hemorrhagic shock followed by sepsis, antibody neutralization of MIP-2 resulted in decreased MPO activity and less histologic injury in the lung (16). Intra-tracheal anti-MIP-2 decreased pulmonary vascular permeability and neutrophil influx in a model of immune complex mediated alveolitis (23). In a murine model of traumatic injury and hemorrhage, blockade of KC was associated with decreased MPO activity in the liver and lung, as well as decreased organ edema (24). Similarly, in this study, resuscitation with a 1:1 ratio of plasma:pRBCs demonstrated a marked reduction in MIP-2 levels, which correlated with reduced MPO activity and decreased vascular leak in an organ-specific manner.
MIP-1α belongs to the subfamily of C-C chemokines (25). This family of chemokines is vitally important in attracting cells of monocyte/macrophage lineage to sites of injury, as opposed to neutrophil recruitment (26). More recent evidence, however, has found that MIP-1α is both a potent autocrine stimulator of macrophages, as well as an essential chemoattractant of polymorphonuclear cells (PMNs) (14). In a rat model of lung injury, these two important roles of MIP-lα were delineated. Neutralization of MIP-lα decreased production of inflammatory cytokines by macrophages and neutrophil chemotaxis to the lung, with improved pulmonary vascular permeability (14). In second animal model of hemorrhagic shock,, MIP-lα deficient mice exhibited less cytokine production, tissue edema, and neutrophil infiltration compared to wild type mice (27). In our model, we achieved a reduction in MIP-lα levels with decreased neutrophil activity and improved vascular leak by transfusing a physiologic ratio of fresh blood products in a 1:1 fashion.
Hemorrhagic shock alone can serve as an initial priming event to activate neutrophils (16), rendering organs susceptible to subsequent tissue injury. Activation, migration, and translocation of PMNs to sites of injury may then increase oxidative damage to the tissues, causing edema and increased capillary permeability (28). In the present study, we found that the use of varying ratios of pRBCs: plasma for resuscitation led to variations in neutrophil accumulation in colon and lung. Resuscitation with purely plasma resulted in the greatest neutrophil accumulation in colon and resuscitation with pRBCs alone resulted in increased neutrophil accumulation in lung. These data suggest that the inflammatory response to hemorrhage and resuscitation as well as the resulting cellular injury and release of chemoattractants varies among organs as well as with resuscitation strategy.
If mediated by neutrophils, chemoattractants including KC, MIP-2, and MIP-lα may play vital roles in organ injury following hemorrhage. Our data suggest that damage control resuscitation strategies used to treat hemorrhagic shock may several advantages over more traditional resuscitation strategies. Transfusion of a 1:1 ratio of plasma: pRBCs may prove to modulate the inflammatory response, reducing neutrophil recruitment to the intestine and lung and subsequently minimizing organ injury. In this series of experiments, we showed that transfusion ratios other than a 1:1 ratio of plasma: pRBCs during resuscitation from hemorrhagic shock increased systemic levels of vital neutrophil chemoattractants, tissue neutrophil accumulation, and vascular leak in vital organs.
Our data are not without limitations. Damage control resuscitation protocols typically involve the use of at least a small amount of crystalloid fluid in addition to blood and blood products. Our previous experiments indicate that the use of crystalloid fluids, specifically lactated Ringer’s solution, increases the inflammatory response during resuscitation (3). In the present experiments, we excluded all crystalloid fluids in order to allow us to directly examine the effects of varying ratios of packed red blood cells to plasma on the inflammatory response.
Clinically, most traumatic injuries involve both hemorrhage and tissue injury. In the experimental setting, the addition of tissue injury to hemorrhage alters the inflammatory response ((29); Makley et al, unpublished observations 2009). In the present study, we utilized a pure model of severe hemorrhage in order to allow us to evaluate different damage control resuscitation strategies on the inflammatory response to blood loss without the potential confounding factor of additional tissue trauma. Thus, our results should not be over interpreted. Further experiments utilizing varied models of hemorrhagic shock as well as combined hemorrhage and soft tissue injury models will be important in order to address the effect of varying ratios of plasma: pRBCs under these circumstances.
In conclusion, recent retrospective evidence supports a survival advantage and decreased organ injury when a high ratio of plasma:pRBCs are transfused in the setting of severe hemorrhagic shock. The current study suggest that “damage control resuscitation” with a 1:1 ratio of plasma to pRBCs results in diminished systemic inflammation compared to other ratios. The precise mechanism(s) by which this particular ratio of products limits inflammation remains to be determined, but our data suggest that the benefits of damage control resuscitation extend beyond its ability to correct defects in coagulation.
Acknowledgments
This work was supported in part by awards from the National Institutes of Health (GM008478 and GM088589), United States Army (W81XWH-09-1-0625), and United States Air Force (FA8650-10-2-6B01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006 Jun;60(6 Suppl):S3–11. doi: 10.1097/01.ta.0000199961.02677.19. [DOI] [PubMed] [Google Scholar]
- 2.American College of Surgeons. Advanced Trauma Life Support for Doctors. 8. Chicago, IL: American College of Surgeons; 2002. [Google Scholar]
- 3.Makley AT, Goodman MD, Friend LA, Deters JS, Johannigman JA, Dorlac WC, Lentsch AB, Pritts TA. Resuscitation with fresh whole blood ameliorates the inflammatory response after hemorrhagic shock. J Trauma. Feb;68(2):305–11. doi: 10.1097/TA.0b013e3181cb4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holcomb JB, Wade CE, Michalek JE, Chisholm GB, Zarzabal LA, Schreiber MA, Gonzalez EA, Pomper GJ, Perkins JG, Spinella PC, Williams KL, Park MS. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg. 2008 Sep;248(3):447–58. doi: 10.1097/SLA.0b013e318185a9ad. [DOI] [PubMed] [Google Scholar]
- 5.Borgman MA, Spinella PC, Perkins JG, Grathwohl KW, Repine T, Beekley AC, Sebesta J, Jenkins D, Wade CE, Holcomb JB. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007 Oct;63(4):805–13. doi: 10.1097/TA.0b013e3181271ba3. [DOI] [PubMed] [Google Scholar]
- 6.Gunter OL, Jr, Au BK, Isbell JM, Mowery NT, Young PP, Cotton BA. Optimizing outcomes in damage control resuscitation: identifying blood product ratios associated with improved survival. J Trauma. 2008 Sep;65(3):527–34. doi: 10.1097/TA.0b013e3181826ddf. [DOI] [PubMed] [Google Scholar]
- 7.Sperry JL, Ochoa JB, Gunn SR, Alarcon LH, Minei JP, Cuschieri J, Rosengart MR, Maier RV, Billiar TR, Peitzman AB, Moore EE. An FFP:PRBC transfusion ratio >/=1:1.5 is associated with a lower risk of mortality after massive transfusion. J Trauma. 2008 Nov;65(5):986–93. doi: 10.1097/TA.0b013e3181878028. [DOI] [PubMed] [Google Scholar]
- 8.Watson GA, Sperry JL, Rosengart MR, Minei JP, Harbrecht BG, Moore EE, Cuschieri J, Maier RV, Billiar TR, Peitzman AB. Fresh frozen plasma is independently associated with a higher risk of multiple organ failure and acute respiratory distress syndrome. J Trauma. 2009 Aug;67(2):221–7. doi: 10.1097/TA.0b013e3181ad5957. discussion 8-30. [DOI] [PubMed] [Google Scholar]
- 9.Giannoudis PV. Current concepts of the inflammatory response after major trauma: an update. Injury. 2003 Jun;34(6):397–404. doi: 10.1016/s0020-1383(02)00416-3. [DOI] [PubMed] [Google Scholar]
- 10.Bahrami S, Zimmermann K, Szelenyi Z, Hamar J, Scheiflinger F, Redl H, Junger WG. Small-volume fluid resuscitation with hypertonic saline prevents inflammation but not mortality in a rat model of hemorrhagic shock. Shock. 2006 Mar;25(3):283–9. doi: 10.1097/01.shk.0000208808.03148.ea. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki K, Ota H, Sasagawa S, Sakatani T, Fujikura T. Assay method for myeloperoxidase in human polymorphonuclear leukocytes. Anal Biochem. 1983 Jul 15;132(2):345–52. doi: 10.1016/0003-2697(83)90019-2. [DOI] [PubMed] [Google Scholar]
- 12.Shires T, Coln D, Carrico J, Lightfoot S. Fluid Therapy in Hemorrhagic Shock. Arch Surg. 1964 Apr;88:688–93. doi: 10.1001/archsurg.1964.01310220178027. [DOI] [PubMed] [Google Scholar]
- 13.Lomas-Neira JL, Chung CS, Grutkoski PS, Miller EJ, Ayala A. CXCR2 inhibition suppresses hemorrhage-induced priming for acute lung injury in mice. J Leukoc Biol. 2004 Jul;76(1):58–64. doi: 10.1189/jlb.1103541. [DOI] [PubMed] [Google Scholar]
- 14.Shanley TP, Schmal H, Friedl HP, Jones ML, Ward PA. Role of macrophage inflammatory protein-1 alpha (MIP-1 alpha) in acute lung injury in rats. J Immunol. 1995 May 1;154(9):4793–802. [PubMed] [Google Scholar]
- 15.Lee BH, Lee TJ, Jung JW, Oh DJ, Choi JC, Shin JW, Park IW, Choi BW, Kim JY. The role of keratinocyte-derived chemokine in hemorrhage-induced acute lung injury in mice. J Korean Med Sci. 2009 Oct;24(5):775–81. doi: 10.3346/jkms.2009.24.5.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lomas JL, Chung CS, Grutkoski PS, LeBlanc BW, Lavigne L, Reichner J, Gregory SH, Doughty LA, Cioffi WG, Ayala A. Differential effects of macrophage inflammatory chemokine-2 and keratinocyte-derived chemokine on hemorrhage-induced neutrophil priming for lung inflammation: assessment by adoptive cells transfer in mice. Shock. 2003 Apr;19(4):358–65. doi: 10.1097/00024382-200304000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Lomas-Neira J, Chung CS, Perl M, Gregory S, Biffl W, Ayala A. Role of alveolar macrophage and migrating neutrophils in hemorrhage-induced priming for ALI subsequent to septic challenge. Am J Physiol Lung Cell Mol Physiol. 2006 Jan;290(1):L51–8. doi: 10.1152/ajplung.00028.2005. [DOI] [PubMed] [Google Scholar]
- 18.Chollet-Martin S, Montravers P, Gibert C, Elbim C, Desmonts JM, Fagon JY, Gougerot-Pocidalo MA. High levels of interleukin-8 in the blood and alveolar spaces of patients with pneumonia and adult respiratory distress syndrome. Infect Immun. 1993 Nov;61(11):4553–9. doi: 10.1128/iai.61.11.4553-4559.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Broaddus VC, Hebert CA, Vitangcol RV, Hoeffel JM, Bernstein MS, Boylan AM. Interleukin-8 is a major neutrophil chemotactic factor in pleural liquid of patients with empyema. Am Rev Respir Dis. 1992 Oct;146(4):825–30. doi: 10.1164/ajrccm/146.4.825. [DOI] [PubMed] [Google Scholar]
- 20.Lamblin C, Gosset P, Tillie-Leblond I, Saulnier F, Marquette CH, Wallaert B, Tonnel AB. Bronchial neutrophilia in patients with noninfectious status asthmaticus. Am J Respir Crit Care Med. 1998 Feb;157(2):394–402. doi: 10.1164/ajrccm.157.2.97-02099. [DOI] [PubMed] [Google Scholar]
- 21.Sekido N, Mukaida N, Harada A, Nakanishi I, Watanabe Y, Matsushima K. Prevention of lung reperfusion injury in rabbits by a monoclonal antibody against interleukin-8. Nature. 1993 Oct 14;365(6447):654–7. doi: 10.1038/365654a0. [DOI] [PubMed] [Google Scholar]
- 22.Hol J, Wilhelmsen L, Haraldsen G. The murine IL-8 homologues KC, MIP-2, and LIX are found in endothelial cytoplasmic granules but not in Weibel-Palade bodies. J Leukoc Biol Mar. 87(3):501–8. doi: 10.1189/jlb.0809532. [DOI] [PubMed] [Google Scholar]
- 23.Shanley TP, Schmal H, Warner RL, Schmid E, Friedl HP, Ward PA. Requirement for C-X-C chemokines (macrophage inflammatory protein-2 and cytokine-induced neutrophil chemoattractant) in IgG immune complex-induced lung injury. J Immunol. 1997 Apr 1;158(7):3439–48. [PubMed] [Google Scholar]
- 24.Frink M, Hsieh YC, Hsieh CH, Pape HC, Choudhry MA, Schwacha MG, Chaudry IH. Keratinocyte-derived chemokine plays a critical role in the induction of systemic inflammation and tissue damage after trauma-hemorrhage. Shock. 2007 Nov;28(5):576–81. doi: 10.1097/shk.0b013e31814b8e0d. [DOI] [PubMed] [Google Scholar]
- 25.Scapini P, Lapinet-Vera JA, Gasperini S, Calzetti F, Bazzoni F, Cassatella MA. The neutrophil as a cellular source of chemokines. Immunol Rev. 2000 Oct;177:195–203. doi: 10.1034/j.1600-065x.2000.17706.x. [DOI] [PubMed] [Google Scholar]
- 26.Wang JM, Sherry B, Fivash MJ, Kelvin DJ, Oppenheim JJ. Human recombinant macrophage inflammatory protein-1 alpha and -beta and monocyte chemotactic and activating factor utilize common and unique receptors on human monocytes. J Immunol. 1993 Apr 1;150(7):3022–9. [PubMed] [Google Scholar]
- 27.Hsieh CH, F M, Hsieh YC, et al. The role of MIP-1 alpha in the development of systemic inflammatory response and organ injury following trauma hemorrhage. J Immunol. 2008;181(4):2806–12. doi: 10.4049/jimmunol.181.4.2806. [DOI] [PubMed] [Google Scholar]
- 28.Shanley TP, Davidson BA, Nader ND, Bless N, Vasi N, Ward PA, Johnson KJ, Knight PR. Role of macrophage inflammatory protein-2 in aspiration-induced lung injury. Crit Care Med. 2000 Jul;28(7):2437–44. doi: 10.1097/00003246-200007000-00041. [DOI] [PubMed] [Google Scholar]
- 29.Chesebro BB, Rahn P, Carles M, Esmon CT, Xu J, Brohi K, Frith D, Pittet JF, Cohen MJ. Increase in activated protein C mediates acute traumatic coagulopathy in mice. Shock. 2009 Dec;32(6):659–65. doi: 10.1097/SHK.0b013e3181a5a632. [DOI] [PMC free article] [PubMed] [Google Scholar]


