Abstract
Acute chest syndrome (ACS) is defined as fever, respiratory symptoms and a new pulmonary infiltrate in an individual with sickle cell disease (SCD). Nearly half of ACS episodes occur in SCD patients already hospitalized, potentially permitting pre-emptive therapy in high-risk patients. Simple transfusion of red blood cells may abort ACS if given to patients hospitalized for pain who develop fever and elevated levels of secretory phospholipase A2 (sPLA2). In a feasibility study (PROACTIVE; ClinicalTrials.gov NCT00951808), patients hospitalized for pain who developed fever and elevated sPLA2 were eligible for randomization to transfusion or observation; all others were enrolled in an observational arm. Of 237 enrolled, only 10 were randomized; one of the four to receive transfusion had delayed treatment. Of 233 subjects receiving standard care, 22 developed ACS. A threshold level of sPLA2 ≥ 48 ng/ml gave optimal sensitivity (73%), specificity (71%) and accuracy (71%), but a positive predictive value of only 24%. The predictive value of sPLA2 was improved in adults and patients with chest or back pain, lower haemoglobin concentration and higher white blood cell counts; and those receiving less than two-thirds maintenance fluids. The hurdles identified in PROACTIVE should facilitate design of a larger, definitive, phase 3 randomized controlled trial.
Keywords: Acute chest syndrome, sPLA2, transfusion, vaso-occlusive episode, pulmonary fat embolism
Introduction
Acute chest syndrome (ACS), characterized by fever, respiratory symptoms and a new pulmonary infiltrate, is responsible for much of the morbidity of sickle cell disease (SCD), is the second most common cause of hospitalization, and is the leading cause of death (Platt, et al 1994, Sprinkle, et al 1986, Vichinsky 1991). A majority of patients will experience at least one episode of ACS, and repeated episodes can result in progressive lung disease (Powars, et al 1988, Weil, et al 1993). Fifty percent of patients who develop ACS are initially admitted with other diagnoses, such as acute pain episodes and only subsequently develop ACS (Vichinsky, et al 2000). Transfusion is widely used to treat ACS and leads to rapid clinical improvement, especially when used early after onset (Mallouh and Asha 1988)
The aetiology of ACS is multifactorial, but the various causes initiate a similar cascade of events that can rapidly cause serious lung injury (Castro, et al 1994, Johnson and Verdegem 1988, Kolquist, et al 1996, Vichinsky, et al 2000, Vichinsky, et al 1997, Vichinsky, et al 1994). The exact pathophysiology of ACS is not known but several studies have implicated secretory phospholipase A2 (sPLA2) in its pathogenesis (Ballas, et al 2006, Kuypers and Styles 2004, Kuypers, et al 1999, Naprawa, et al 2005, Styles, et al 2000, Styles, et al 2007, Styles, et al 1996). Type IIa sPLA2 is a calcium-dependent protein that cleaves phospholipids to generate non-esterified fatty acids and lysophospholipids. sPLA2 is secreted by many cells within the body and is induced by inflammatory signals, such as tumour necrosis factor (TNF) and interleukin-1 (IL1) (Pfeilschifter, et al 1989). By generating important intermediates, such as arachidonic acid or lysoplatelet activating factor, sPLA2 appears to play an important role as a mediator between the proximal and distal effectors of inflammation (Henderson 1987, Lewis, et al 1990). Elevations of sPLA2 have been reported in many disease states in which inflammation is prominent, including sepsis and acute respiratory distress syndrome (ARDS) (Anderson, et al 1994, Baur, et al 1989, Green, et al 1991, Koike, et al 2000, Nevalainen 1993, Smith, et al 1992).
Previous studies of sPLA2 in SCD have shown that sPLA2 is elevated in ACS; sPLA2 levels correlate with clinical severity and levels often rise in the 24 to 48 h before ACS is diagnosed clinically. In preliminary studies, a threshold of 100ng/ml best predicted impending ACS (Ballas, et al 2006, Styles, et al 2000, Styles, et al 2007, Styles, et al 1996). A subsequent pilot intervention trial provided evidence that ACS could be prevented by transfusion, when using sPLA2 levels and fever to predict those at risk for ACS (Styles, et al 2007). The Sickle Cell Disease Clinical Research Network (SCDCRN) initiated the feasibility study “Preventing Acute Chest Syndrome with Transfusion Trial” (PROACTIVE) to better establish the accuracy of sPLA2 as a predictor of ACS and to confirm results of previous studies. This report documents the results of this study.
Methods
PROACTIVE was a feasibility study to determine if elevation of serum levels of sPLA2 could be used along with transfusion to prevent ACS. This study had two primary objectives. The first was to assess the feasibility of recruiting a sufficient number of eligible subjects to a randomized interventional trial of transfusion vs. standard care such that: a) consent to the trial and randomization occurred within 8 h of eligibility determination; b) initiation of the experimental transfusion occurred within 14 h of eligibility determination; and c) 40 subjects could be randomized in 12 months. The second objective was to optimize the utility of serum sPLA2 levels in predicting imminent ACS. Twenty-six centres were contracted to enroll patients to PROACTIVE. All sites obtained Human Subjects approval with local Institutional Review Boards. All patients consented to participation in the study prior to enrollment.
Study design
This feasibility study for PROACTIVE included two design components: Component 1: The PROACTIVE randomized controlled interventional trial protocol with a target enrollment of 40 subjects (20 in each treatment group); and Component 2: An observational cohort consisting of an estimated 300 subjects ineligible for the interventional trial, either because they failed to meet randomization criteria or developed ACS prior to randomization. The trial duration was to be 13 months, with an expected recruitment of 40 randomized participants in 12 months.
Study Flow
All SCD patients (Hb SS, SC or S-β0 or S-β+ thalassaemia) aged ≥ 2 years who were admitted to the hospital with acute pain and no evidence of ACS were asked to participate in the screening/observational protocol. Subjects were excluded for medical conditions for the which the physician felt a transfusion was likely, transfusions in the last 60 days, or conditions making transfusion unacceptable or difficult (such as multiple alloantibodies). A maximum of three daily SPLA2 levels were determined and comprehensive clinical and laboratory data were collected. Those with fever on admission or who developed a fever during the screening phase received a clinically indicated chest X-ray (CXR); if positive for infiltrate they remained in the observational protocol if other randomization criteria had not been met. Subjects who had three daily sPLA2 levels < 100 ng/ml also remained in the observational protocol and a CXR was required at 72 h or at the time of discharge if discharge occurred prior to 72 h.
Subjects who had a positive screening sPLA2 (>100 ng/ml) had a study-mandated CXR as soon as the sPLA2 elevation was detected. To be eligible for randomization, subjects required all of the following at the time of randomization:
sPLA2 level > 100 ng/ml within the same 24-h window as fever (temperature> 38.0° C) and a CXR that was negative for new pulmonary infiltrate. If a negative CXR was not done within the 12 h preceding randomization, a repeat CXR was required to confirm eligibility.
Hb <100 g/dl
Signed consent/assent for participation in the randomized interventional trial.
ACS was defined as having a new infiltrate on a CXR as read by the local site. Reported positive CXR results were later compared with study forms to confirm the diagnosis of ACS. If a CXR was reported positive but a diagnosis of ACS was not made, these CXR reports were retrieved from the sites and blindly and independently reviewed by Drs. Styles and Miller to determine whether or not ACS was present.
Transfusion arm subjects received a single transfusion of 7–13 ml/kg packed red blood cells (PRBC) within 14 h of eligibility determination. In addition to ABO/Rh, PRBC transfusions were to be, at a minimum, phenotypically matched for the E, C, and K1 antigens. Subjects not randomized to transfusion and those in the observational cohort received standard care.
Quantitative sPLA2 protein measurements
SPLA2 measurements were determined every 24 h. The measurements were performed by enzyme-linked immunosorbent assay (ELISA; Cayman Chemicals, Ann Arbor, MI, USA) using serum in the following manner: Strip wells were pre-coated with a mouse capture antibody and blocked with a proprietary formulation of proteins, both obtained from Cayman Chemicals. An eight-point standard curve of sPLA2 (13 ng/ml to 600 ng/ml) was supplied. High, mid, and low quality control (QC) standards were supplied at 200, 100, and 50 ng/ml, respectively
The linear range for quantitation in the assay was claimed by the manufacturer to be 25 – 400 ng/mL. The assay acceptance criteria required that all three QC standards have accuracy between 80% and 120%, with special provisions for accepting values between 25 – 100 or 100 – 400 if only the high QC standard or the low QC standard failed, respectively. The assay was validated in the manufacturer’s laboratory to have accuracy and precision (%CV) within 15%.
Statistical Analyses
The analysis subset included subjects that had no transfusions and at least one sPLA2 value during the study prior to either discharge or ACS diagnosis. The primary predictor variable was the maximum sPLA2 value prior to ACS diagnosis or discharge.
In a preliminary analysis, potential threshold levels of sPLA2 were evaluated with regard to their impact on the sensitivity (true positive rate; TPR), false positive rate (FPR; 1 – specificity), accuracy (ACC; number of correct classifications), and positive predictive value (PPV) of developing ACS. The area under the curve (AUC) for the Receiver Operating Characteristic (ROC) was examined to assess whether maximum sPLA2 had an overall adequate predictive ability across the range of candidate thresholds. The optimal threshold level for sPLA2 was defined to be that which provided the largest difference between the TPR and the FPR. The analysis was repeated for paediatric and adult subgroups, as well as for subjects with and without fever.
The data were further analysed to determine if the threshold levels of other clinical variables would enhance the predictive ability for ACS diagnosis. Both continuous and discrete variables were analysed, including fever, respiratory and heart rate, reported sites of pain, breath signs (rales, decreased breath sounds, shortness of breath, cough, or wheezing), oxygen saturation, whether incentive spirometry was ordered, IV rate, specific drugs taken (including hydroxycarbamide), number of previous ACS hospitalizations reported, and laboratory findings, including red and white blood cell counts, reticulocytes, platelets, and haemoglobin. For each of these variables, both baseline and maximum/minimum or any/none during the study period was considered, and three types of binary classification analyses were conducted: Univariate analyses determined the optimal threshold level for each independent variable in predicting ACS. Composite analyses determined the optimal threshold levels for sPLA2 in combination with one or two other variables in predicting ACS. Two-stage screening analyses determined the optimal threshold levels for predicting ACS when the data was first partitioned into two subgroups by a threshold level for a (non-sPLA2) clinical sign or symptom, then partitioned by the optimal threshold level for sPLA2 within each data subgroup. The goal, determined a priori, was to identify predictive combinations (possibly within subgroups) that yielded a PPV ≥ 40%, while still maintaining a TPR ≥ 70% and FPR ≤ 20%.
Finally, classification trees (Breiman 1984) were constructed to identify subgroups of subjects that had high prevalence of ACS. Using this methodology, the study population was recursively partitioned into two groups based upon a splitting rule that effectively maximized the prevalence for ACS within one of the subgroups (without regard for the sensitivity and specificity). To enhance the validity of the trees, the candidate variables considered for splitting were restricted to those pre-determined to have a high predictive ability of ACS, based upon a conditional forest analysis (Strobl, et al 2008).
All analyses were done for the combined adult/paediatric population, as well as separately for adults (age ≥ 18 years) and children (age < 18 years).
Results
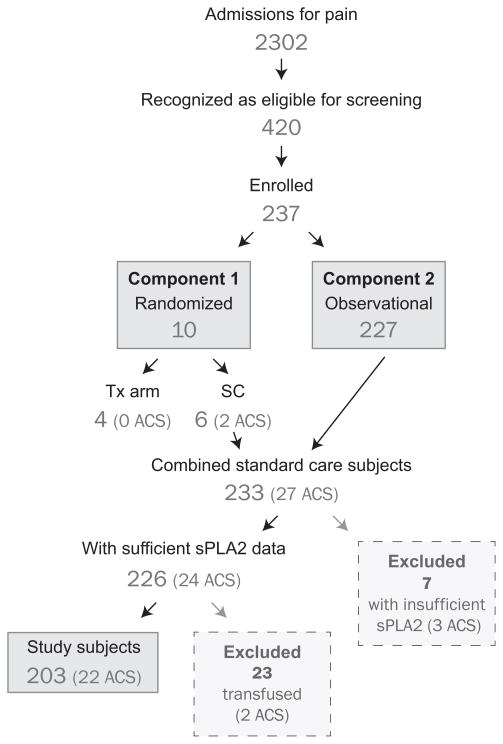
During the study period, there were 2302 SCD admissions for pain at all participating institutions. Of these, 420 patients were recognized as eligible for enrollment and 237 were enrolled (Fig 1). The reasons patients were recognized as eligible but excluded from enrollment are summarized in Table I. Of the 233 subjects receiving standard care, 30 were excluded from the analysis subset; seven had insufficient sPLA2 data and 23 received clinically indicated transfusions prior to an ACS diagnosis or discharge. Descriptive characteristics for the remaining 203 subjects are shown in Table II. Children made up a greater percentage of enrolled subjects. Of the 23 subjects initially reported to have a new infiltrate on CXR, six lacked a corresponding diagnosis of ACS on the daily study form; on review, five had ACS and one did not. Although adults had lower overall sPLA2 levels, they had similar rates of developing ACS (11.5% vs. 10.3%). Management and clinical course of enrolled subjects are described elsewhere (Miller, et al 2012); no deaths occurred during the trial.
Fig 1. Disposition of Patients in Study.
Tx, transfusion, SC, standard care; sPLA2, secretory phospholipase A2; ACS, acute chest syndrome.
Table I.
Reasons screened subjects were excluded from enrollment
| Number Screened | 420 |
|---|---|
| • Did not meet inclusion criteria | 42 |
| ○ Acute chest syndrome present at screening | 31 |
| ○ Lack of acute pain diagnosis | 10 |
| ○ Prior Enrollment in PROACTIVE Feasiblity Study | 1 |
| • Met at least one exclusion criterion | 120 |
| ○ RBC transfusion within last 60 days | 46 |
| ○ History of alloimmunization | 20 |
| ○ Transfusion-dependent coexisting medical condition | 17 |
| ○ Diagnosis of pulmonary infiltrate | 7 |
| ○ Various combinations of above | 22 |
| ○ Pregnant | 3 |
| ○ Objection to transfusion | 3 |
| ○ Treated with systemic steroids in previous week | 2 |
| • Eligible Subjects | 258 |
| ○ No signed consent | 21 |
| ○ Enrolled | 237 |
PROACTIVE, Preventing Acute Chest Syndrome with Transfusion Trial; RBC, red blood cell
Table II.
Baseline demographic characteristics
| N (%) | Overall (N=203) | Age group
|
|
|---|---|---|---|
| Paediatric (N=107) | Adult (N=96) | ||
| Gender (male) | 102 (50%) | 58 (54%) | 44 (46%) |
| Race (black) | 198 (98%) | 103 (96%) | 95 (99%) |
| Range of maximum sPLA2 value | |||
| >0 – 25 | 106 (52%) | 43 (40%) | 63 (66%) |
| >25 – 50 | 34 (17%) | 18 (17%) | 16 (17%) |
| >50 – 100 | 22 (11%) | 16 (15%) | 6 (6%) |
| >100 – 200 | 13 (6%) | 9 (8%) | 4 (4%) |
| >200 – 800 | 28 (14%) | 21 (20%) | 7 (7%) |
| Acute Chest Syndrome | 22 (11%) | 11 (10%) | 11 (11%) |
sPLA2, secretory phospholipase A2
Only 10 subjects met the criteria for randomization into the interventional trial; four were randomized to the transfusion arm and six to standard care. All 10 subjects were randomized within 8 h of eligibility (mean: 3.4 h, range: 0.4–7.5 h). Three of four subjects randomized to transfusion were transfused within the mandated six hours from randomization (2.9, 4.3, and 5.9 hours) and within 14 hours of eligibility (3.3, 7.9, and 13.3 hours). One subject was transfused 23.9 h after eligibility and 20.8 h after randomization due to unavailability of an inpatient hospital bed required for a study-indicated transfusion. None of the four subjects randomized to the transfusion arm developed ACS, while two of the six subjects (33%) randomized to standard care developed ACS.
Threshold of sPLA2 prior to ACS diagnosis
The analysis subset included 203 subjects, of which 22 developed ACS during the study and 181 did not. A cross-tabulation of ACS status with the predetermined sPLA2 threshold of 100 ng/ml showed that 13 of 161 patients below this threshold developed ACS (8%) and 9 of 42 at or above this threshold developed ACS (21%); this threshold corresponded to a sensitivity of 41%, specificity of 82%, accuracy of 77%, and PPV of 21%.
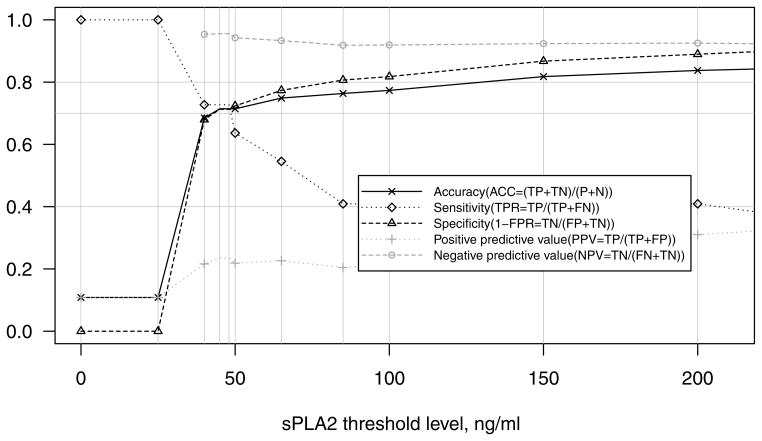
The analyses of all potential threshold levels in the combined adult and paediatric study population (Fig 2) suggested that sPLA2 ≥ 48 ng/ml provided the optimal trade-off between sensitivity (73%), specificity (71%), and accuracy (71%); however, this threshold yielded a PPV of only 24%. The ROC curve demonstrated adequate overall predictive ability of sPLA2 (AUC=0.72). The presence of fever within 24 h of the maximum sPLA2 did not improve the predictive ability (data not shown). Analysis of the paediatric (n=107) and adult (n=96) subgroups demonstrated that sPLA2 was an adequate predictor of ACS in both subgroups (AUC=0.72 for both). The threshold of sPLA2 ≥ 48 ng/ml was also optimal in both age groups; however, in adults, it yielded a higher PPV of 37% and lower sensitivity of 64%, whereas in children it yielded a higher sensitivity of 82% and lower PPV of 18%.
Fig 2. Classification Rates for predicting ACS in adults and children.
For each threshold level of sPLA2, true positives (TP), true negatives (TN), false positives (FP), and false negatives (FN) were tabulated and used to compute the following rates: accuracy (ACC = (TP+TN)/(TP+TN+FP+FN)), sensitivity (TPR = TP/(TP+FN)), specificity (1 – FPR = TN/(FP+TN)), positive predictive value (PPV = TP/(TP+FP)), and negative predictive value (NPV = TN/(FN+TN)).
In adults, the univariate analyses identified that haemoglobin concentration ≤ 70 g/l yielded a PPV of 40% (TPR=73%, FPR=15%); 20 adults (21%) fell below that threshold. No other univariate potential predictors of ACS achieved PPV > 40% while maintaining adequate sensitivity and specificity for either the combined population or the paediatric subgroup.
The composite analyses identified that having both sPLA2 ≥ 48 ng/ml and baseline white blood cell count (WBC) ≥ 10.4 × 109/l yielded a PPV of 38% (TPR=73%, FPR=15%) in the combined adult/paediatric population. In the adult subgroup, no composite predictors achieved adequate (≥ 70%) sensitivity. In children, the combination of sPLA2 ≥ 31 ng/ml and baseline haemoglobin ≤ 74 g/l yielded a PPV of 44% (TPR=73%, FPR=11%). A three-way composite analysis of these important predictors identified that having sPLA2 ≥ 31 ng/ml combined with baseline WBC ≥ 10.4 × 109/l, and baseline haemoglobin ≤ 93 g/l yielded a PPV of 43% (TPR=73%, FPR=15%) in the combined adult/paediatric population; 37 subjects met this criteria (16 developed ACS). The two-stage screening analyses identified that sPLA2 ≥ 77 ng/ml yielded a PPV of 42% (TPR=73%, FPR=14%) among all subjects who reported back pain at baseline, and sPLA2 ≥ 55 ng/ml yielded a PPV of 41% (TPR=75%, FPR=19%) among all subjects who reported chest pain on any day during the study.
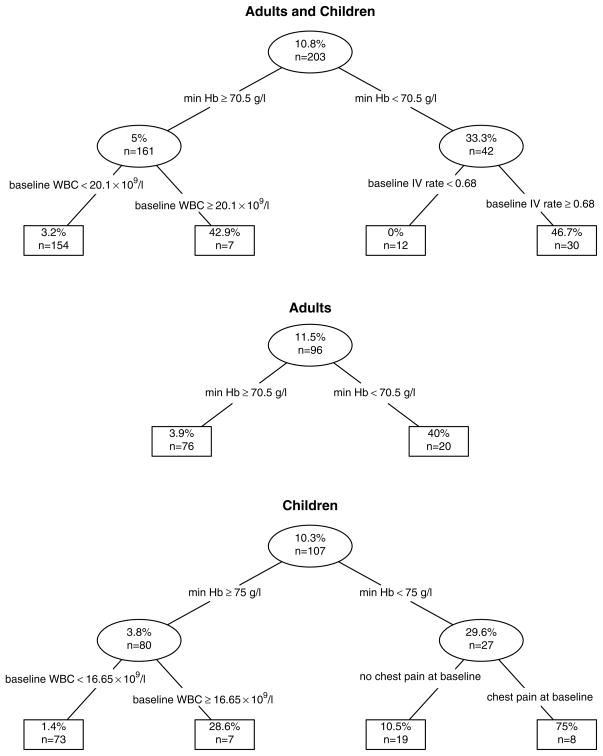
The classification tree analysis (Fig 3) confirmed the other findings. In the combined adult/paediatric population, the first split of minimum haemoglobin < 71 g/l at any time during hospital stay before ACS or discharge yielded 40 subjects that had 35% prevalence of ACS. The subsequent split of baseline intravenous infusion rate ≥ 68% of maintenance yielded a group of 28 subjects that had 50% prevalence of ACS. There were no subjects with baseline IV rate < 68% and minimum haemoglobin < 71 g/l that developed ACS; and the subsequent split on the left side of the tree yielded 7 subjects with minimum haemoglobin > 70 g/l and baseline WBC ≥ 20.1 × 109/l of which 3 (43%) developed ACS. In adults, the first split was identical to that of the combined adult/paediatric population: 20 adults had a minimum haemoglobin ≤ 70 g/l, of which eight (40%) developed ACS. In children, the first split of minimum haemoglobin < 75 g/l yielded a group of 27 subjects that had a 30% prevalence of ACS, and the subsequent split yielded 8 children with baseline chest pain and minimum haemoglobin < 75 g/l of which 6 (75%) subsequently developed ACS.
Fig 3. Classification trees.
Within each age group, data was recursively partitioned into two groups based upon a splitting rule that effectively optimized the prevalence within one of the subgroups. The candidate splits included all possible threshold levels for clinical predictors that were determined to have high predictive ability in this data. The number of subjects and the rate of ACS are reported within each node.
Discussion
Regardless of aetiology, the course of ACS is often severe, with multiple lobe involvement, hypoxia and rapid progression despite aggressive intervention (Castro, et al 1994, Vichinsky, et al 2000, Vichinsky, et al 1997). ACS is frequently associated with vaso-occlusive pain episodes and these ACS events are particularly severe (Vichinsky, et al 2000). Even one episode of ACS leads to a shortened life expectancy (Platt, et al 1994). Given the morbidity and consequences of ACS, there is considerable interest in identifying efficacious treatments and, ultimately, in preventing its occurrence altogether.
Blood transfusion was chosen as the best potential therapy to prevent ACS based on its demonstrated therapeutic effects. Transfusion early in the course of ACS has been found to hasten resolution (Mallouh and Asha 1988), has beneficial effects on oxygenation (Emre, et al 1995, Vichinsky, et al 2000), and appears to reduce occurrence if given preventively(Hankins, et al 2005, Miller, et al 2001). It has been speculated that transfusion may impact by down-regulating vascular cellular adhesion molecule 1 (Liem, et al 2004, Sakhalkar, et al 2004, Stuart and Setty 1999) and/or perhaps by providing Duffy antigen, which is largely lacking in sickle cell populations and, at least in vitro, modifies the effects of cytokine release, most notably IL-8 (Abboud, et al 2000, Afenyi-Annan, et al 2008, Hadley and Peiper 1997). It is not known whether transfusion has any direct effect upon the production, activity or metabolism of sPLA2. A pilot study utilizing sPLA2 as a predictor for ACS demonstrated that transfusion prior to clinically apparent ACS reduced its occurrence (Styles, et al 2007) and, based on this, the SCDCRN designed the PROACTIVE trial.
Given that large controlled trials have historically been difficult to undertake in SCD, especially in the inpatient setting, PROACTIVE was redesigned as a feasibility trial. It is of some concern that our goal of randomizing 40 subjects in 12 months was not achieved, even with the participation of 26 centres. Of 2302 patients hospitalized for pain, less than 10% were enrolled for possible randomization. Difficulties with staffing at sites, limited or non-availability of the sPLA2 assay on weekends, and inability to get consent under the rigorous time constraints of the study were common reasons for failure to enroll patients. These are important factors that will have to be addressed prior to proceeding with larger trials.
The often subtle presentation and subsequent rapidly progressive course of ACS and uncertainty regarding the time needed for potential therapeutic impact of transfusion mandated a small window between ascertainment of eligibility (including a lack of ACS) and administration of treatment; that transfusion therapy cannot be safely blinded compounds these potential issues. The window of 14 h between eligibility and initiation of treatment was a compromise between this need for expeditious therapy and anticipated impediments to prompt randomization and preparation for transfusion. As one of four subjects randomized to transfusion experienced inordinate delay of therapy, our limited data suggest an eligibility-to-treatment interval similar to that chosen for PROACTIVE may be difficult to achieve; additional potential subjects may have been excluded from enrollment because timely transfusion at the site clearly could not have been accomplished. For future studies, a longer interval between randomization and transfusion may be necessary; however, the maximum duration in the PROACTIVE of 26 hours between the eligibility-establishing negative chest radiograph and initiation of transfusion therapy is already generous given the (sometimes) rapid progression of ACS. The small numbers of patients enrolled in PROACTIVE precluded any assessment of the effectiveness of transfusion.
Data from the patients enrolled in the observational component of the trial allowed us to evaluate the pre-established sPLA2 threshold of 100 ng/mL. This threshold showed good specificity, but inadequate sensitivity. The paucity of ACS events in this study population contributed to the low PPV for all thresholds of sPLA2 considered. From a clinical standpoint, a higher PPV would reduce the number of patients “unnecessarily” transfused due to a spurious high-risk screening level; we chose a PPV of 40% as a reasonable clinical threshold. Of the several other clinical and laboratory variables examined, only low haemoglobin in adults (≤ 70 g/l) performed better than sPLA2 in prediction of ACS in our overall study population. A threshold of 48 ng/ml provided better sensitivity while preserving adequate accuracy and minimal reduction in specificity. The sPLA2 assay had similar overall predictive ability in adults versus children as well as the same optimal threshold in the two age groups, but the accuracy and positive predictive value was better in adults and the sensitivity was better in children. In general, children achieved higher sPLA2 values than adults. It is not known why the assay was more predictive in adults; adults do tend to have more severe ACS and a higher mortality rate (Vichinsky 1997, Vichinsky, et al 2000), and perhaps a difference in aetiology of ACS between adults and children could impact sPLA2 values.
The pre-established threshold of 100 ng/ml for sPLA2 was effective in a small pilot study (Styles, et al 2000) and validated in a larger, multi-institutional cohort (Ballas, et al 2006). sPLA2 assays used in previous clinical studies have included both activity of sPLA2 and protein concentration of sPLA2. Correlation between sPLA2 concentration and activity has been excellent so this does not appear to explain the differences in results (Styles, et al 1996). The ELISA-based assay used in this study to measure sPLA2 protein was developed separately from that used in previous trials. Given the disparity between the ELISA results from this study and that of previous reports, it would be wise to initiate a comparison of both ELISA assays with known samples to determine if there are differences between the two assays themselves or if the differences are the result of operator variation. In addition, due to the recognition of sPLA2 as a potential risk factor for coronary artery disease (Koenig, et al 2009), several commercial assay kits have recently become available. It will be particularly important for future studies to define an optimal threshold for whatever methodology is utilized.
Fever had previously been identified as a clinical indicator that improved the accuracy of the sPLA2 assay by eliminating many false positives. However, when the optimal threshold analysis was repeated for patients with and without fever, the presence of fever did not improve accuracy of the sPLA2 assay in predicting ACS. The univariate analyses identified only that in adults a low haemoglobin concentration (at or below 70 g/l) was helpful in predicting ACS independent of other variables. The composite analysis identified the combination of low haemoglobin level and an elevated sPLA2 as being predictive in children; no composite variables were identified to predict ACS in the adult group. A two-stage screening analysis revealed that in subjects with back pain or chest pain, it was possible to achieve adequate predictive ability (PPV ≥ 40%) using sPLA2 as a diagnostic criterion; chest pain may have been reflective of impending ACS. In addition, both chest and back pain may have led to splinting, which may contribute to the development of ACS (Bellet, et al 1995, Gelfand, et al 1993, Needleman, et al 2002, Rucknagel 2001). In the overall study population, the combination of elevated WBC and elevated sPLA2 was predictive; a better PPV (43%) was obtained when low haemoglobin concentration was added to the criteria. Finally, the classification tree analysis confirmed the findings in the other analyses by identifying that low haemoglobin and elevated WBC led to a higher rate of ACS in patients; it is of interest that a previous study of occurrence of ACS in hospitalized children with sickle cell disease identified high WBC and low haemoglobin levels were associated with nosocomial ACS (Buchanan, et al 2005).
The classification tree analysis also revealed that IV fluid infusion rates above 2/3 maintenance were associated with a higher rate of ACS. In light of the clinical and radiological findings consistent with pulmonary oedema in many SCD patients with ACS, this finding suggests that hydration should be used judiciously in patients admitted with pain (Ardiles and Dark 2007, Girard 1979, Haynes and Allison 1986). Many investigators in PROACTIVE were restrictive in fluid administration during the study(Miller, et al 2012); further restriction of fluid provision among clinical centres might further reduce the incidence of nosocomial ACS. The impact of fluid administration on the course of a pain episode is unknown.
In conclusion, the PROACTIVE study suffered from many of the difficulties seen in other studies in SCD and other rare diseases, including challenges in meeting enrollment goals. Adjustments in availability of study personnel may be required to optimize recruitment, and focusing on those patients at highest risk, i.e. with lower haemoglobin, higher WBC count and chest or back pain, might reduce projected numbers of randomized patients needed to assess treatment efficacy. SPLA2 levels accurately predicted ACS but further clarification of optimal sPLA2 methodology and thresholds is needed to assess definitively its potential clinical role. ACS continues to be an important problem in SCD, and efforts to improve treatment and prevention must continue. The hurdles identified in PROACTIVE should facilitate design of a larger, definitive, Phase 3 randomized controlled trial to determine whether transfusion can indeed prevent ACS in selected patients hospitalized for pain.
PROACTIVE Acknowledgements
This publication was made possible by Grant Number U10HL083721 from the National Heart, Lung, and Blood Institute, National Institutes of Health. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
The manuscript was drafted by a core writing group that consisted of Dr. Styles, Dr. Miller, and Dr. Wager. Dr. Wager conducted the statistical analysis. All other authors contributed substantially to the design, acquisition and interpretation of data, critical revisions of the manuscript, and approval of the final versions.
In addition to the authors of this manuscript, the following individuals were instrumental in the planning, conduct and/or care of patients enrolled in this study at each of the participating institutions as follows:
Emory University, AFLAC Cancer Center and Blood Disorders Service, Atlanta, GA: Carlton D. Dampier, MD
Aflac Cancer Center and Blood Disorders Service, Emory University School of Medicine and Children’s Healthcare of Atlanta, Atlanta, GA: Peter A. Lane, MD, Tamara N. New, MD, Terrell Faircloth, CCRC
Boston Medical Center, Boston, MA:, Asif I. Qureshi, MD
Children’s Hospital Boston, Boston, MA: Matthew M Heeney, M.D, Debra L. Weiner, M.D., Ph.D., Meredith Anderson; For GCRC-supported studies: “This project was funded in part by grant MO1-RR02172 from the National Center for Research Resources, National Institutes of Health, to the Children’s Hospital Boston General Clinical Research Center.” Abbreviations may include NCRR, NIH, and GCRC.
Children’s Hospital of Philadelphia, Philadelphia, PA: Kwaku Ohene-Frempong, MD, Clinical and Translational Research Center, Children’s Hospital of Philadelphia, CTRC grant number UL1-RR-024134
St. Christopher’s Hospital for Children, Philadelphia, PA: Norma B. Lerner, MD, MPH, Michele Cahill, RN, MaryLou MacDermott, CRNP, Maureen Meier, RN, CCRC
Division of Pediatric Hematology/Oncology, AI DuPont Hospital for Children, Wilmington, DE: Lynn Marrs, BS, RN, CCRC
Division of Pediatric Hematology/Oncology, University of Louisville, Louisville, KY: Salvatore Bertolone, MD, Ashok B. Raj, MD
Center for Sickle Cell Disease and Department of Medicine, Howard University, Washington, DC: Victor R. Gordeuk, MD, Georgetown-Howard Universities Center for Clinical and Translational Science and supported by the National Institutes of Health National Center for Research Resources, Grant U54 RR026076
Children’s Hospital & Research Center, Oakland, CA: Mark Walters, MD;
University of Illinois at Chicago, Chicago, IL: Richard J. Labotka, MD, Robert Molokie, MD, Sandra Gooden, RN, Daisy Pacelli, MPH, RN, Lani Krauz, RN
Virginia Commonwealth University, Richmond, VA: Wally R. Smith, MD, Kamar Godder, MD, MPH, Susan D. Roseff, MD
Johns Hopkins University School of Medicine, Baltimore, MD: James F. Casella, MD, Jeffrey Keefer, MD, PhD, Sophie Lanzkron MD, MHS, Cedron Williams, Phillip Seaman; Johns Hopkins University: Johns Hopkins Institute for Clinical and Translational Research; grant # U10 HL083721.
University of North Carolina at Chapel Hill, Chapel Hill, NC: Susan K. Jones, RN, Dell Strayhorn, FNP, MPH, Teresa Etscovitz; UNC Clinical and Translational Science Award,” grant # UL1RR025747
University of Mississippi Medical Center, Jackson, MS: Mary Gail Smith, MD, Carolyn Bigelow, MD, Suvankar Majumdar, MD, Glenda Thomas, RN, Arleen Anderson, RN
Medical College of Georgia, Atlanta, GA: Abdullah Kutlar, MD, Leigh Wells, RN, MSN, Latanya Bowman, Pritam Bora
Wayne State University, Detroit, MI: Paul Swerdlow, MD
Duke University Medical Center, Durham, NC: Marilyn J. Telen, MD, Laura M. De Castro, MD, Shital Kamble, PhD, Shelby Reed, PhD, Courtney D. Thornburg, MD, Hai Huang, Jude Jonassaint, RN
New York Methodist Hospital, Brooklyn, NY: Emmely M. Colon, Herold Duroseau, MD, Deepak Kilari, MD, Charlene Webb, LPN
Interfaith Medical Center, Brooklyn, NY: Edouard Guillaume, MD, Rafat Ahmed, MD, Miren Blackwood, Huguette Souffrant, Hossam Awad
Cincinnati Children’s Hospital Medical Center, Cincinnati, OH: Karen Kalinyak, MD, Clinton H. Joiner, MD, PhD
Ohio State University, Adult Sickle Cell Program Columbus, OH: Eric H. Kraut, MD, Leslie Witkoff, RN
Nationwide Children’s Hospital, Columbus OH: Melissa M. Rhodes, MD, Kami Perdue, CRA
New England Research Institutes, Watertown, MA: Sonja M. McKinlay, PhD, Beatrice Files, MD, Hae-Young Kim, DrPH, David Brazier, PMP, Margaret C. Bell, MS, MPH, Dianne Gallagher, MPH
National Heart, Lung, and Blood Institute, Bethesda, MD: Harvey Luksenburg, MD, Henry Chang, MD, Liana Harvath, PhD, Myron Waclawiw, PhD, Erin Smith, Ellen M. Werner, PhD
Data and Safety Monitoring Board Members: (Chair) Ted Wun, MD, FACP, Amy Becker, MD, Lennette Benjamin, MD, Susan Claster, MD, Michael Farrell, MD, Allison A. King, MD, MPH, Jeannette Y. Lee, PhD, Robert P. McMahon, PhD, Julie A. Panepinto, MD, MSPH
Finally, the SCDCRN thanks the individuals who enrolled in the trial and their families.
Footnotes
Financial Disclosures
| Lori Styles, MD | Consultant, Eli Lilly |
| Carrie G. Wager, PhD | none |
| Richard J. Labotka, MD | none |
| Kim Smith-Whitley, MD | none |
| Alexis A. Thompson, MD MPH | Research support: Novartis, Baxter, Pfizer |
| Peter A. Lane MD | none |
| Lillian E.C. McMahon MD | none |
| Robin Miller MD | none |
| Susan D Roseff MD | none |
| Rathi V. Iyer MD | none |
| Lewis Hsu MD, PhD | Research Support: Glycomimetics, NIH Consultant, Eli Lilly |
| Oswaldo Castro MD | none |
| Kenneth I. Ataga MD | Research support: HemaQuest, Eli Lilly |
| Onyinye Onyekwere MD MS FAAP | none |
| Maureen Okam MD MPH | none |
| Rita Bellevue MD | none |
| Scott T. Miller, MD | none |
References
- Abboud MR, Taylor EC, Habib D, Dantzler-Johnson T, Jackson SM, Xu F, Laver J, Ballas SK. Elevated serum and bronchoalveolar lavage fluid levels of interleukin 8 and granulocyte colony-stimulating factor associated with the acute chest syndrome in patients with sickle cell disease. Br J Haematol. 2000;111:482–490. doi: 10.1046/j.1365-2141.2000.02358.x. [DOI] [PubMed] [Google Scholar]
- Afenyi-Annan A, Kail M, Combs MR, Orringer EP, Ashley-Koch A, Telen MJ. Lack of Duffy antigen expression is associated with organ damage in patients with sickle cell disease. Transfusion. 2008;48:917–924. doi: 10.1111/j.1537-2995.2007.01622.x. [DOI] [PubMed] [Google Scholar]
- Anderson BO, Moore EE, Banerjee A. Phospholipase A2 regulates critical inflammatory mediators of multiple organ failure. J Surg Res. 1994;56:199–205. doi: 10.1006/jsre.1994.1032. [DOI] [PubMed] [Google Scholar]
- Ardiles T, Dark D. Pulmonary complications of sickle cell disease in adults. Mo Med. 2007;104:250–254. [PubMed] [Google Scholar]
- Ballas SK, Files B, Luchtman-Jones L, Benjamin L, Swerdlow P, Hilliard L, Coates T, Abboud M, Wojtowicz-Praga S, Kuypers FA, Michael Grindel J. Secretory phospholipase A2 levels in patients with sickle cell disease and acute chest syndrome. Hemoglobin. 2006;30:165–170. doi: 10.1080/03630260600642260. [DOI] [PubMed] [Google Scholar]
- Baur M, Schmid TO, Landauer B. Role of phospholipase A in multiorgan failure with special reference to ARDS and acute renal failure (ARF) Klinische Wochenschrift. 1989;67:196. doi: 10.1007/BF01711353. [DOI] [PubMed] [Google Scholar]
- Bellet PS, Kalinyak KA, Shukla R, Gelfand MJ, Rucknagel DL. Incentive spirometry to prevent acute pulmonary complications in sickle cell diseases. N Engl J Med. 1995;333:699–703. doi: 10.1056/NEJM199509143331104. [DOI] [PubMed] [Google Scholar]
- Breiman L. Classification and regression trees. Wadsworth International Group; Belmont, CA, USA: 1984. [Google Scholar]
- Buchanan ID, Woodward M, Reed GW. Opioid selection during sickle cell pain crisis and its impact on the development of acute chest syndrome. Pediatr Blood Cancer. 2005;45:716–724. doi: 10.1002/pbc.20403. [DOI] [PubMed] [Google Scholar]
- Castro O, Brambilla DJ, Thorington B, Reindorf CA, Scott RB, Gillette P, Vera JC, Levy PS. The acute chest syndrome in sickle cell disease: incidence and risk factors. Blood. 1994;84:643–649. [PubMed] [Google Scholar]
- Emre U, Miller ST, Gutierez M, Steiner P, Rao SP, Rao M. Effect of transfusion in acute chest syndrome of sickle cell disease. J Pediatr. 1995;127:901–904. doi: 10.1016/s0022-3476(95)70025-0. [DOI] [PubMed] [Google Scholar]
- Gelfand MJ, Daya SA, Rucknagel DL, Kalinyak KA, Paltiel HJ. Simultaneous occurrence of rib infarction and pulmonary infiltrates in sickle cell disease patients with acute chest syndrome. J Nucl Med. 1993;34:614–618. [PubMed] [Google Scholar]
- Girard W. Case report: postoperative pulmonary edema and sickle cell crisis. Clin Notes Respir Dis. 1979;17:13–14. [PubMed] [Google Scholar]
- Green JA, Smith GM, Buchta R, Lee R, Ho KY. Circulating phospholipase A2 activity associated with sepsis and septic shock is indistinguishable from that associated with rheumatoid arthritis. Inflammation. 1991;15:335. doi: 10.1007/BF00917352. [DOI] [PubMed] [Google Scholar]
- Hadley TJ, Peiper SC. From malaria to chemokine receptor: the emerging physiologic role of the Duffy blood group antigen. Blood. 1997;89:3077–3091. [PubMed] [Google Scholar]
- Hankins J, Jeng M, Harris S, Li CS, Liu T, Wang W. Chronic transfusion therapy for children with sickle cell disease and recurrent acute chest syndrome. J Pediatr Hematol Oncol. 2005;27:158–161. doi: 10.1097/01.mph.0000157789.73706.53. [DOI] [PubMed] [Google Scholar]
- Haynes J, Jr, Allison RC. Pulmonary edema. Complication in the management of sickle cell pain crisis. Am J Med. 1986;80:833–840. doi: 10.1016/0002-9343(86)90624-8. [DOI] [PubMed] [Google Scholar]
- Henderson WR., Jr Eicosanoids and lung inflammation. Am Rev Respir Dis. 1987;135:1176–1185. doi: 10.1164/arrd.1987.135.5.1176. [DOI] [PubMed] [Google Scholar]
- Johnson CS, Verdegem TD. Pulmonary complications of sickle cell disease. Seminars in Respiratory Medicine. 1988;9:287–296. [Google Scholar]
- Koenig W, Vossen CY, Mallat Z, Brenner H, Benessiano J, Rothenbacher D. Association between type II secretory phospholipase A2 plasma concentrations and activity and cardiovascular events in patients with coronary heart disease. Eur Heart J. 2009;30:2742–2748. doi: 10.1093/eurheartj/ehp302. [DOI] [PubMed] [Google Scholar]
- Koike K, Yamamoto Y, Hori Y, Ono T. Group IIA phospholipase A2 mediates lung injury in intestinal ischemia-reperfusion. Ann Surg. 2000;232:90–97. doi: 10.1097/00000658-200007000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolquist KA, Vnencak-Jones CL, Swift L, Page DL, Johnson JE, Denison MR. Fatal fat embolism syndrome in a child with undiagnosed hemoglobin S-beta+ thalassemia: A complication of acute parvovirus B19 infection. Pediatric Pathology and Laboratory Medicine. 1996;16:71–82. [PubMed] [Google Scholar]
- Kuypers F, Styles LA. The role of secretory phospholipase A2 in acute chest syndrome. Cellular and Molecular Biology. 2004;50:87–94. [PubMed] [Google Scholar]
- Kuypers FA, Larkin SA, Styles L, de Jong K. Secretory phospholipase A2 induced endothelial damage: a possible role in acute chest syndrome. Blood. 1999;94:420a. [Google Scholar]
- Lewis RA, Austen KF, Soberman RJ. Leukotrienes and other products of the 5-lipoxygenase pathway. Biochemistry and relation to pathobiology in human diseases. N Engl J Med. 1990;323:645–655. doi: 10.1056/NEJM199009063231006. [DOI] [PubMed] [Google Scholar]
- Liem RI, O’Gorman MR, Brown DL. Effect of red cell exchange transfusion on plasma levels of inflammatory mediators in sickle cell patients with acute chest syndrome. Am J Hematol. 2004;76:19–25. doi: 10.1002/ajh.20054. [DOI] [PubMed] [Google Scholar]
- Mallouh AA, Asha M. Beneficial effect of blood transfusion in children with sickle cell chest syndrome. Am J Dis Child. 1988;142:178–182. doi: 10.1001/archpedi.1988.02150020080034. [DOI] [PubMed] [Google Scholar]
- Miller ST, Wright E, Abboud M, Berman B, Files B, Scher CD, Styles L, Adams RJ. Impact of chronic transfusion on incidence of pain and acute chest syndrome during the Stroke Prevention Trial (STOP) in sickle-cell anemia. J Pediatr. 2001;139:785–789. doi: 10.1067/mpd.2001.119593. [DOI] [PubMed] [Google Scholar]
- Miller ST, Kim HY, Weiner D, Wager CG, Gallagher D, Styles L, Dampier CD. Inpatient management of sickle cell pain: A ‘snapshot’ of current practice. Am J Hematol. 2012;87:333–336. doi: 10.1002/ajh.22265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naprawa JT, Bonsu BK, Goodman DG, Ranalli MA. Serum biomarkers for identifying acute chest syndrome among patients who have sickle cell disease and present to the emergency department. Pediatrics. 2005;116:e420–425. doi: 10.1542/peds.2004-2107. [DOI] [PubMed] [Google Scholar]
- Needleman JP, Benjamin LJ, Sykes JA, Aldrich TK. Breathing patterns during vaso-occlusive crisis of sickle cell disease. Chest. 2002;122:43–46. doi: 10.1378/chest.122.1.43. [DOI] [PubMed] [Google Scholar]
- Nevalainen T. Serum phospholipase A2 in inflammatory diseases. Clin Chem. 1993;39:2453. [PubMed] [Google Scholar]
- Pfeilschifter J, Pignat W, Vosbeck K. Interleukin 1 and tumor necrosis factor synergistically stimulate prostaglandin synthesis and phospholipase A2 release from rat renal mesangial cells. Biochem Biophys Res Comm. 1989;159:385. doi: 10.1016/0006-291x(89)90003-x. [DOI] [PubMed] [Google Scholar]
- Platt OS, Brambilla DJ, Rosse WF, Milner PF, Castro O, Steinberg MH, Klug PP. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med. 1994;330:1639–1644. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- Powars D, Weidman JA, Odom-Maryon T, Niland JC, Johnson C. Sickle cell chronic lung disease: prior morbidity and the risk of pulmonary failure. Medicine. 1988;67:66–76. [PubMed] [Google Scholar]
- Rucknagel DL. The role of rib infarcts in the acute chest syndrome of sickle cell diseases. Pediatr Pathol Mol Med. 2001;20:137–154. [PubMed] [Google Scholar]
- Sakhalkar VS, Rao SP, Weedon J, Miller ST. Elevated plasma sVCAM-1 levels in children with sickle cell disease: impact of chronic transfusion therapy. Am J Hematol. 2004;76:57–60. doi: 10.1002/ajh.20016. [DOI] [PubMed] [Google Scholar]
- Smith GM, Ward RL, McGuigan L, Rajkovic IA, Scott KF. Measurement of human phospholipase A2 in arthritis plasma using a newly developed sandwich ELISA. Br J Rheumatol. 1992;31:175–178. doi: 10.1093/rheumatology/31.3.175. [DOI] [PubMed] [Google Scholar]
- Sprinkle RH, Cole T, Smith S, Buchanan GR. Acute chest syndrome in children with sickle cell disease. American Journal of Pediatric Hematology/Oncology. 1986;8:105–110. [PubMed] [Google Scholar]
- Strobl C, Boulesteix AL, Kneib T, Augustin T, Zeileis A. Conditional variable importance for random forests. BMC Bioinformatics. 2008;9:307. doi: 10.1186/1471-2105-9-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart MJ, Setty BN. Sickle cell acute chest syndrome: pathogenesis and rationale for treatment. Blood. 1999;94:1555–1560. [PubMed] [Google Scholar]
- Styles LA, Schalkwijk CG, Aarsman AJ, Vichinsky EP, Lubin BH, Kuypers FA. Phospholipase A2 levels in acute chest syndrome of sickle cell disease. Blood. 1996;87:2573–2578. [PubMed] [Google Scholar]
- Styles LA, Aarsman AJ, Vichinsky EP, Kuypers FA. Secretory phospholipase A(2) predicts impending acute chest syndrome in sickle cell disease. Blood. 2000;96:3276–3278. [PubMed] [Google Scholar]
- Styles LA, Abboud M, Larkin S, Lo M, Kuypers FA. Transfusion prevents acute chest syndrome predicted by elevated secretory phospholipase A2. Br J Haematol. 2007;136:343–344. doi: 10.1111/j.1365-2141.2006.06409.x. [DOI] [PubMed] [Google Scholar]
- Vichinsky E. Comprehensive care in sickle cell disease: its impact on morbidity and mortality. Seminars in Hematology. 1991;28:220. [PubMed] [Google Scholar]
- Vichinsky E. Mortality of acute chest syndrome in sickle cell disease. Blood. 1997;90:264a. [Google Scholar]
- Vichinsky EP, Williams R, Das M, Earles AN, Lewis N, Adler A, McQuitty J. Pulmonary fat embolism: A distinct cause of severe acute chest syndrome in sickle cell anemia. Blood. 1994;83:3107–3112. [PubMed] [Google Scholar]
- Vichinsky EP, Styles LA, Colangelo LH, Wright EC, Castro O, Nickerson B. Acute chest syndrome in sickle cell disease: clinical presentation and course. Cooperative Study of Sickle Cell Disease. Blood. 1997;89:1787–1792. [PubMed] [Google Scholar]
- Vichinsky EP, Neumayr LD, Earles AN, Williams R, Lennette ET, Dean D, Nickerson B, Orringer E, McKie V, Bellevue R, Daeschner C, Manci EA. Causes and outcomes of the acute chest syndrome in sickle cell disease. National Acute Chest Syndrome Study Group. N Engl J Med. 2000;342:1855–1865. doi: 10.1056/NEJM200006223422502. [DOI] [PubMed] [Google Scholar]
- Weil JV, Castro O, Malik AB, Rodgers G, Bonds DR, Jacobs TP. Pathogenesis of lung disease in sickle hemoglobinopathies. American Review of Respiratory Disease. 1993;148:249–256. doi: 10.1164/ajrccm/148.1.249. [DOI] [PubMed] [Google Scholar]