Abstract
There is an urgent need for the development of novel therapies to treat pancreatic cancer, which is among the most lethal of all cancers. KRAS activating mutations, which are found in >90% of pancreatic adenocarcinomas, drive tumor dependency on the Ras/MAPK and Akt signaling pathways. Radiation is currently being explored as a component of the standard treatment regimen for pancreatic cancer. This study’s purpose was to test the hypothesis that MEK inhibitors will offer clear therapeutic benefit when integrated into radiotherapy treatment regimens for treatment of this disease. We explored the activation of the MAPK and Akt pathways in response to radiation in multiple pancreatic tumor cell lines. Small molecule inhibitors of MEK (PD0325901) and Akt (API-2) were subsequently evaluated for their radiosensitizing potential alone and in combination. In vivo efficacy was tested in subcutaneous MIA-PaCa2 xenografts. Phosphorylated levels of ERK-1/2 and Akt were found to increase in response to radiation treatment in our pancreatic tumor cell line panel. MEK inhibitor-induced radiosensitization was observed in vitro and in vivo. The further addition of an Akt inhibitor to the MEK inhibitor/radiation regimen resulted in enhanced therapeutic gain as determined by increased radiosensitization and tumor cell death. In conclusion, MEK inhibition results in growth arrest, apoptosis, and radiosensitization of multiple preclinical pancreatic tumor models, and the effects can be enhanced by combination with an Akt inhibitor. These results provide rationale for further testing of a treatment regimen in pancreatic cancer that combines MEK inhibition with radiation, optimally in conjunction with Akt inhibition.
Keywords: MEK-1/2, Akt, PI3-kinase, Radiation, Pancreatic Cancer
Introduction
Aberrant KRAS signaling is a hallmark of the vast majority of pancreatic cancers, which exhibit an especially high incidence (>90%) of KRAS mutations. Consequently these cancers display activation of the RAF/MEK/MAPK (ERK) signaling cascade. Phosphorylation of these kinases drives proliferation of pancreatic cancer cells and impacts their survival and metastatic spread (1, 2). Consequently, a growing number of MEK inhibitors have now entered clinical testing against a variety of solid tumor types, including pancreatic cancer (3–7). However, the large number of genetic aberrations in pancreatic cancer makes it unlikely that single agent therapy will produce meaningful therapeutic benefit to this patient population.
Multiple, potentially attractive strategies exist for combining MEK inhibitors with other therapies. Specifically, combined targeting of both MEK and PI3K has attracted much interest for the treatment of KRAS driven tumors (8, 9). Oncogenic KRAS drives activation of both the MAPK as well as PI3K/Akt pathways, which are important for proliferation, survival, and tumorigenesis. Compensatory signaling arising from crosstalk between these pathways can reduce the therapeutic effectiveness of targeting either pathway alone. Specifically, PI3K-Akt pathways have been implicated in mediating resistance to MEK inhibitors (9–11). Conversely, inhibition of Akt/mTOR signaling in human cancer cells can lead to ERK pathway activation through a PI3K-dependent mechanism (12).
Co-targeting both the MAPK and PI3K/Akt pathways is also potentially advantageous in the radiotherapy setting. Numerous lines of evidence point to hyperactivation of either of these pathways leading to the development of radioresistance (13–17). These findings have led to the discovery that MEK and Akt inhibitors as single agents possess radiosensitizing properties in a broad spectrum of human tumors (18–20).
Molecularly-targeted approaches that enhance the effectiveness of radiation are particularly attractive for the treatment of pancreatic cancer. There are presently few therapeutic options for patients diagnosed with this disease. Approximately 80% of patients are diagnosed with locally-advanced or metastatic disease that precludes surgical intervention. Radiation therapy significantly improves local control and is considered a standard of care for patients with locally-advanced pancreatic cancer. Thus, strategies aimed at improving radiation efficacy could play a major role in the design of improved therapies for this disease.
We hypothesized that activation of PI3K/Akt signaling would compromise the full potential of MEK inhibitors to sensitize pancreatic cancer cells to the lethal effects of radiation. The purpose of this study was to explore the response of a panel of pancreatic tumor models to MEK inhibition with concurrent radiation treatment. We show here that radiation and MEK inhibition independently upregulate Akt activity and that co-targeting both the MAP kinase and PI3K/Akt pathways results in improved radiosensitization and tumor control both in vitro and in vivo.
Materials and Methods
Antibodies, Chemicals, and Cell Culture
Akt, phospho-Akt (Ser473), ERK-1/2, phospho-ERK-1/2 (Thr202/Tyr204), and cleaved PARP (Asp214), antibodies were purchased from Cell Signaling Technology (Danvers, MA). Ki-67 antibody was purchased from Dako (Carpinteria, CA). API-2/Triciribine was purchased from Tocris (Ellisville, MO). PD0325901 was purchased from LC Laboratories (Woburn, MA). The structures for PD0325901 and API-2 are shown in Figure 1. MIA-PaCa2 (DMEM and 10% FBS), Panc-1 (RPMI-1640 and 10% FBS), BxPC-3 (RPMI-1640 + 10% FBS), Capan-1 (Iscove’s Modified Dulbecco’s medium + 20% FBS), Capan-2 (McCoy’s 5a Modified medium + 10% FBS), AsPC-1 (RPMI-1640 + 10% FBS), and HepG2 (DMEM:F12 + 10% FBS) cells were purchased from ATCC (Manassas, VA), expanded upon receipt and numerous vials of low passage cells were banked in liquid nitrogen. Cells were never passaged more than 3 months. Cells were grown in a 37°C incubator with 5% CO2.
Figure 1.
Chemical structures of PD0325901 (A) and API-2 (B).
Immunoblotting
Cell lysates were prepared immediately in RIPA lysis buffer (1% NP-40, 150mM NaCl, 50mM Tris-HCL pH 7.4, 0.25% Na-deoxycholate, 1 mM EDTA) supplemented with 1x protease inhibitor (cOmplete, Roche Applied Science) and phosphatase inhibitors (PhosSTOP, Roche Applied Science). Protein concentration was determined with a Dc Protein Assay Kit (BioRad, Hercules, CA). Proteins were resolved by SDS/PAGE and transferred to nitrocellulose membranes. Primary antibodies were allowed to bind for 2 hours at room temperature, and used at a dilution of 1:500–2,000, except for GAPDH which was used at 1:10,000. After washing in TBS-Tween, membranes were incubated with horseradish peroxidase-conjugated secondary antibodies diluted 1:10,000 for 1 hour. Membranes were washed with TBS-Tween and incubated for 1 minute with enhanced chemiluminescence reagent (Amersham Pharmacia, Uppsala, Sweden) before exposing film.
Clonogenic Survival Assays
Cells were trypsinized to generate single cell suspensions and cells were seeded into six-well or 60 mm tissue culture plates (in triplicate). After allowing 6 h for adherence, cells were incubated with DMSO, PD0325901 (10 or 100 nM), or various concentrations of API-2 (0.1 to 1 μM) for one hour before irradiation. 10–14 days after seeding, colonies were stained with 0.5% crystal violet, and the number of colonies containing at least 50 cells were determined. Plating efficiency, survival fractions, and dose enhancement ratios (DER) were calculated according to previously described methods (21). For each condition, six wells were plated in replicate for experiments performed in a six-well plate, and in triplicate for experiments performed in 60 mm culture plates. Experiments were repeated multiple, independent times.
Tumor Xenograft Studies
All animal procedures were approved by the University of Michigan Committee for Use and Care of Animals. 4–6 week-old athymic CD-1 female mice were obtained from Charles River Laboratories (Wilmington, MA) and acclimatized for at least one week before use. The mice were injected subcutaneously with 5x106 MIA-PaCa-2 cells in 100 μl serum-free RPMI per flank. Tumors were allowed to grow to the size of approximately 100 mm3, as measured by magnetic resonance imaging (MRI), before randomization to groups consisting of treatment with vehicle, PD0325901 (10 mg/kg daily via oral gavage), API-2 (1 mg/kg daily injected intraperitoneally), and/or radiation (2 Gy) for a total of 10 days (days 1–10). PD0325901 was prepared in 0.2% Tween-80 with 0.5% hydroxypropylmethlcellulose in sterile water, while API-2 was prepared in 15% DMSO in 0.9% sterile saline. Baseline (pre-treatment) MRI scans were conducted on day 0, days 4 and 7 (during treatment), day 11 (one day after completion of treatment), followed by weekly thereafter (day 18, 25, 32, etc.). Mice were weighed on the day of each MRI scan to monitor for toxicity.
Experimental Radiation
Radiation was performed at 320 kVp, 10 mA using a IC-320 orthovoltage irradiator (Kimtron Medical, CT). For in vitro experiments, a 20x24 cm cone was used at a source-to-surface distance (SSD) of 50cm at a dose rate of ~434 cGy/min. For animal irradiation, a 6x8 cm cone was used at an SSD of 40cm, at a dose rate of ~138 cGy/min. Dosimetry was carried out using an ionization chamber connected to an electrometer system that is directly traceable to a National Institute of Standards and Technology (NIST) calibration. Mice were anesthetized with isoflurane and placed in cardboard restraints. Flank irradiation was carried out using a custom cut lead secondary collimator.
Xenograft Tumor Volume
During MRI examinations, animals were anesthetized with 1–2% isoflurane/air, with body temperature maintained at 37°C, using an Air-Therm air heater (World Precision Instruments, Sarasota, FL). MRI scans were performed immediately prior to first treatment (day 0), days 4, 7, and 11, and weekly thereafter using a 9.4T, 16cm horizontal bore Agilent Direct Drive system (Palo Alto, CA) with a quadrature rat head coil (Doty Scientific, Inc., Columbia, SC). Axial T2-weighted images were acquired using a fast spin-echo sequence with the following parameters: repetition time (TR)/effective echo time (TE), 4000/60 ms; echo spacing, 15 ms; echo train length, 4; field of view (FOV), 30 x 30 mm; matrix, 256x128; slice thickness, 1 mm ; and 25 contiguous slices. Tumor regions of interest (ROI) were contoured on T2-weighted images and used for volumetric analysis. Image post-processing and analysis was performed using “in-house” program developed in Matlab (MathWorks, Natick, MA).
Immunohistochemistry
Tumors were harvested and fixed in 10% neutral-buffered formalin for at least 48 hours (n=4 per group). Tumors were sectioned and paraffin-embedded and 5 micron sections were cut onto slides. Paraffin was removed in xylene and slides were rehydrated through gradually decreasing alcohol concentrations 2 min per step before ending in tap water (100% ethanol, 95% ethanol, 70% ethanol, water). Antigen retrieval was performed by microwaving slides for 10 min in pH 6.0 citrate buffer, followed by a 10 min cooling period, and a 10 min running water wash. Immunoperoxidase staining was performed on a DAKO AutoStainer at room temperature by applying peroxidase block (5 min), buffer rinse, primary antibody (30 min), buffer rinse, secondary antibody (EnVision + anti-rabbit) 30 min, buffer rinse, DAB 5 min, buffer rinse, followed by hematoxylin counterstain (2 seconds), and water rinse. Slides were then dehydrated through gradually decreasing alcohol concentrations (70%% ethanol, 95% ethanol, 100% ethanol, 2 min each), 3 xylene washes (2 min each), and followed by placement of a coverslip. Images were captured on an Olympus BX-51 microscope (20X magnification).
Data Analysis
Data is represented as the mean ± standard error of the mean (s.e.m.) for clonogenic survival and xenograft tumor growth experiments. Statistical comparisons were made using the unpaired two-tailed Student’s t-test with p-values <0.05 being judged significant.
Results
PD0325901, a potent MEK inhibitor, radiosensitizes pancreatic cancer cells
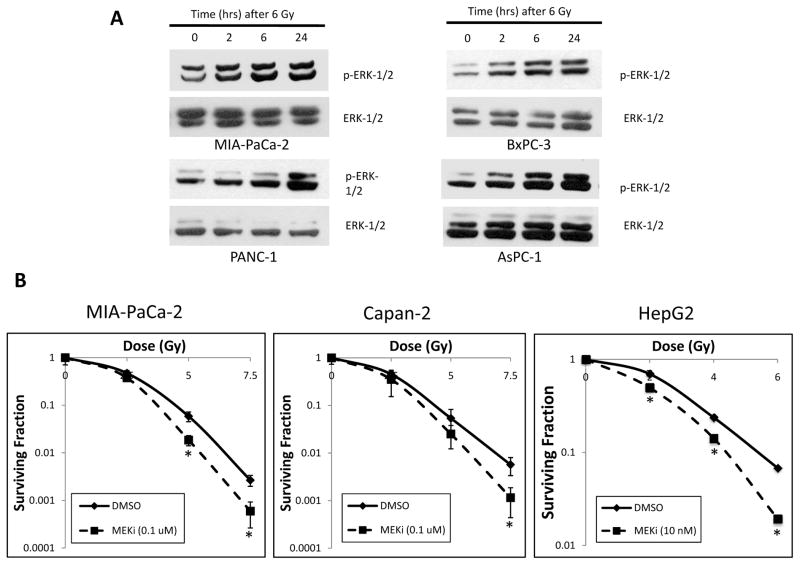
The impact of radiation on MAPK pathway activation was determined in a panel of six human pancreatic adenocarcinoma cell lines (MIA-PaCa-2, BxPC-3, PANC-1, AsPC-1, Capan-1, and Capan-2), and a hepatocellular carcinoma cell line (HepG2). A time-dependent increase in phospho-ERK-1/2 (pERK) activity in response to radiation was observed in every model. Representative data for four of the cell lines are shown in Figure 2A. Some cell lines demonstrated activation of ERK-1/2 as early as 2 hours, but all cells showed activation by 24 hrs. These effects were also observed at a lower radiation dose of 3 Gy (data not shown).
Figure 2. Radiation up-regulates ERK-1/2 activity, and a MEK-1/2 inhibitor radiosensitizes multiple cell lines.
A) Six pancreatic cancer cell lines were irradiated including MIA-PaCa-2, BxPC-3, PANC-1, AsPC-1, Capan-1, and Capan-2 (first 4 cell lines shown). p-ERK-1/2 expression was determined by immunoblotting at various time points after 6 Gy of irradiation (0, 2, 6, and 24 hours). Irradiation resulted in increased p-ERK-1/2 in all cell lines, maximal at 24 hours. Total ERK-1/2 levels shown as an equal loading control.
B) Clonogenic survival assays were performed by pre-treating cells with DMSO or MEKi (100 nM for MIA-PaCa-2, 10 nM for HepG2) for 1 hour prior to increasing doses of radiation, then changing the medium 24 hours later, and allowing the cell lines to form colonies over 1–2 weeks. MEKi treatment resulted in substantial radiosensitization in MIA-PaCa-2, Capan-2, and HepG2 cells. Asterisks indicate p-value < 0.05 compared to DMSO-treated cells. Error bars represent s.e.m.
Clonogenic assays were carried out to test the radiosensitivity of these cell lines under conditions where ERK activation is suppressed by MEK inhibitor treatment. Cells were pre-treated with the MEK inhibitor PD0325901 followed by irradiation in the continued presence of the MEK inhibitor. The concentration of PD0325901 employed in these studies (10 to 100 nM) was previously determined to result in near complete loss of detectable pERK activity by 3 hrs in all cell lines tested, and as early as 1 hour in the majority of the cell lines studied (Supplementary data, Figure S1).
As shown in Fig. 2B, treatment with PD0325901 resulted in significant radiosensitization in multiple pancreatic cancer cell lines, including MIA-PaCa-2 andCapan-2 cell lines, with dose enhancement factors of 1.34, and 1.25, respectively. Since these cell lines are KRAS mutant, we also tested HepG2 cells, an NRAS mutant cell line, in order to determine whether PD0325901-mediated radiosensitization was dependent on RAS isoform or tissue of origin (i.e. pancreas versus liver). We again observed significant radiosensitization, at a dose sufficient for target inhibition, with a dose enhancement factor of 1.51. As expected, radiation induced G2/M arrest at 24 hours, (Table 1). However, radiation did not induce a substantial increase in the sub-G1 fraction at 48 hours relative to that found in control or PD0325901-treated cells, consistent with the concept that radiation predominantly functions by inducing post-mitotic arrest/death. The G1 block observed under conditions of MEK inhibition is consistent with previous reports (22, 23).
Table 1.
Cell cycle analysis on pancreatic cancer cell lines.
Cell cycle analysis performed by treating pancreatic cancer cell lines with combinations of DMSO, MEKi (100 nM), API-2 (8 μM), and RT (6 Gy), and determining subG1, G1, S, and G2/M phase fractions at 24 and 48 hours.
| MIA-PaCa-2 | 24 hours | 48 hours | ||
|---|---|---|---|---|
| G1 (%) | S (%) | G2/M (%) | sub-G1 fraction | |
| DMSO | 65.2 | 26.3 | 8.6 | 1.2 |
| MEKi | 90.6 | 6.8 | 2.6 | 8.1 |
| API-2 | 72.3 | 19.1 | 8.6 | 0.0 |
| MEKi + API-2 | 95.4 | 4.1 | 0.5 | 18.4 |
| DMSO + RT | 54.3 | 26.5 | 19.2 | 0.0 |
| MEKi + RT | 91.4 | 5.4 | 3.2 | 10.3 |
| API-2 +RT | 63.8 | 13.8 | 22.4 | 5.3 |
| MEKi + API-2 +RT | 85.5 | 7.4 | 7.1 | 13.3 |
| BxPC-3 | 24 hours | 48 hours | ||
| G1 (%) | S (%) | G2/M (%) | sub-G1 fraction | |
| DMSO | 46.2 | 24.7 | 29.1 | 7.0 |
| MEKi | 60.3 | 10.9 | 28.8 | 17.9 |
| API-2 | 56.2 | 14.9 | 29.0 | 16.7 |
| MEKi + API-2 | 55.7 | 12.0 | 32.3 | 26.0 |
| DMSO + RT | 44.0 | 18.1 | 38.0 | 9.9 |
| MEKi + RT | 32.3 | 10.8 | 56.9 | 21.5 |
| API-2 +RT | 44.8 | 13.3 | 41.9 | 17.5 |
| MEKi + API-2 +RT | 37.6 | 14.5 | 48.0 | 20.0 |
| PANC-1 | 24 hours | 48 hours | ||
| G1 (%) | S (%) | G2/M (%) | sub-G1 fraction | |
| DMSO | 37.7 | 44.2 | 18.1 | 0.4 |
| MEKi | 61.0 | 22.4 | 16.6 | 2.5 |
| API-2 | 45.5 | 40.9 | 13.7 | 0.0 |
| MEKi + API-2 | 69.7 | 16.1 | 14.3 | 3.4 |
| DMSO + RT | 43.4 | 33.5 | 23.1 | 1.0 |
| MEKi + RT | 61.0 | 17.0 | 22.1 | 3.8 |
| API-2 +RT | 48.5 | 29.1 | 22.3 | 1.5 |
| MEKi + API-2 +RT | 65.6 | 11.5 | 23.0 | 5.2 |
Concurrent treatment with PD0325901 and radiation improves therapeutic response in vivo
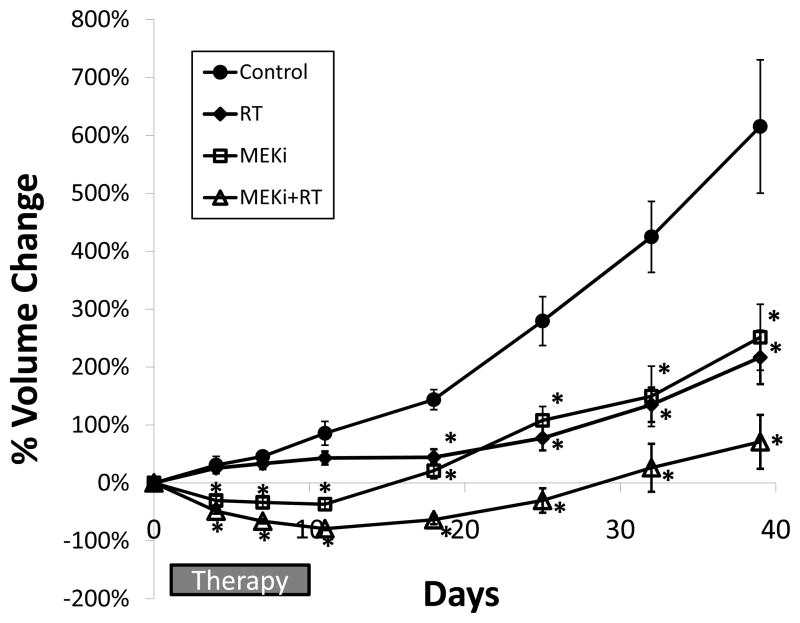
MIA-PaCa-2 cells were subcutaneously implanted in athymic nude mice and tumors allowed to reach a size of approximately 100 mm3 before mice were randomized to one of four groups: (1) control (vehicle), (2) RT, (3) PD0325901, and (4) PD0325901 + RT. For radiation, 2 Gy per day was chosen as the daily dose, similar to standardly used clinical practice guidelines. Treatments occurred daily for ten consecutive days (days 1–10). Baseline MRI scans were performed on days 0, days 4 & 7 (during treatment), day 11 (at end of therapy), and then weekly thereafter (days 18, 25, 32, etc.). As demonstrated in Fig. 3, control tumors continued to grow at a rapid pace after randomization. RT-treated tumors grew initially, but then experienced essentially no change in tumor volume throughout therapy, consistent with induction of growth arrest and post-mitotic death. PD0325901-treated tumors experienced rapid regressions during treatment, with the nadir corresponding to a ~35% reduction in volume at day 11 and resumed rapid growth immediately after treatment was discontinued. Tumors treated concurrently with PD0325901 and RT exhibited the greatest therapeutic response with approximately an 80% reduction in tumor volume by day 11. Given that volume reductions were not observed in the RT single modality arm, these results provide evidence that concurrent MEK inhibition and radiation treatment results in therapeutic sensitization. Mice, weighed twice weekly and monitored closely during therapy administration, had no significant toxicity with only a maximum 6% decline in body weight (Supplementary Fig. S2).
Figure 3. Combination MEKi and radiation induces maximal decreases in tumor volume, correlating with therapeutic efficacy, in a pancreatic cancer xenograft model.
MIA-PaCa-2 cells were injected into the flanks of athymic nude mice, and tumors were allowed to reach a size of approximately 100 μL (by serial MRI scans), before randomization to: (1) control (vehicle), (2) RT (2 Gy x 10 days), (3) MEKi (10 mg/kg once daily x 10 days), and (4) MEKi + RT (MEKi delivered 2–3 hours prior to RT). Treatments occurred from days 1–10. MRI sequences were obtained on days 0, 4, 7, 11, 18, 25, and weekly thereafter until study endpoint. Curves represents percent change in tumor volume during study period by treatment group: (1) control (n=16 tumors, 8 mice), (2) RT (n=16 tumors, 9 mice), (3) MEKi (n=12 tumors, 6 mice), (4) MEKi + RT (n=14 tumors, 8 mice). Error bars represent s.e.m, and asterisk represents p < 0.05 compared to the control group.
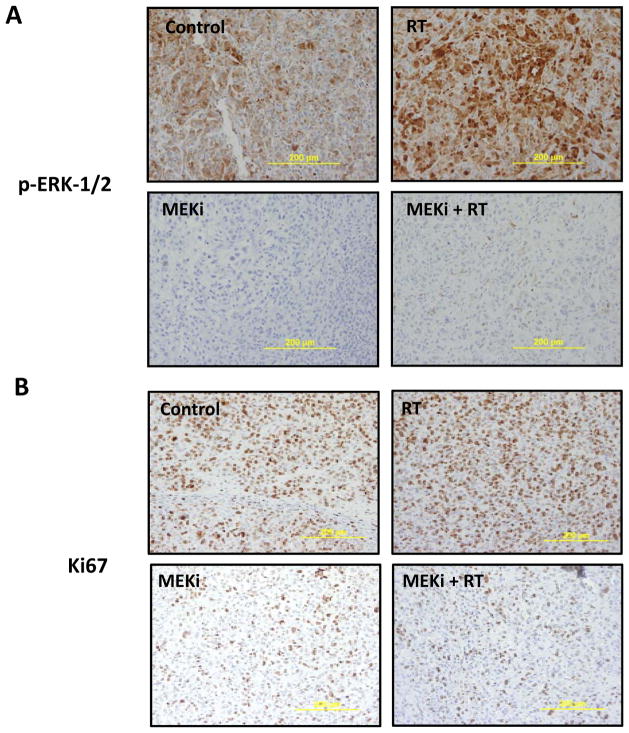
Immunohistochemical staining was carried out on tumors excised after four days of treatment. As shown in Fig. 4A, radiation produced marked up-regulation of ERK-1/2 activity compared to control tumors. PD0325901 treatment resulted in a profound loss of pERK activity, confirming effective target inhibition of MEK. Less than 3% of cells demonstrated any pERK expression in both MEK inhibitor-treated groups. Tumors from the combination arm further exhibited a significant decrease in cellularity, consistent with the improved efficacy of this treatment regimen relative to single agent/modality treatment alone. To analyze the functional impact of reduced pERK expression, Ki67 staining was also carried out. Surprisingly, despite the enhanced reduction in cellular density induced by concurrent radiation and MEK inhibitor treatment, the proliferative index appeared to be comparable for cells treated with the combination versus MEK inhibitor alone (Figure 4B). This led us to explore whether activation of the PI3K pathway could be compromising overall effectiveness of MEK inhibitor-based radiotherapy regimens.
Figure 4. Radiation up-regulates ERK-1/2 activity, which is abrogated by MEKi treatment, and MEKi treatment results in substantially decreased Ki67 staining within tumors.
Immunohistochemical staining on tumors isolated at day 4 of treatment from the four groups of mice were stained with p-ERK-1/2 (A) or Ki67 (B). 3–4 tumors from 2 mice from each group were fixed, embedded, and stained. Scale (yellow line) is 200 μm.
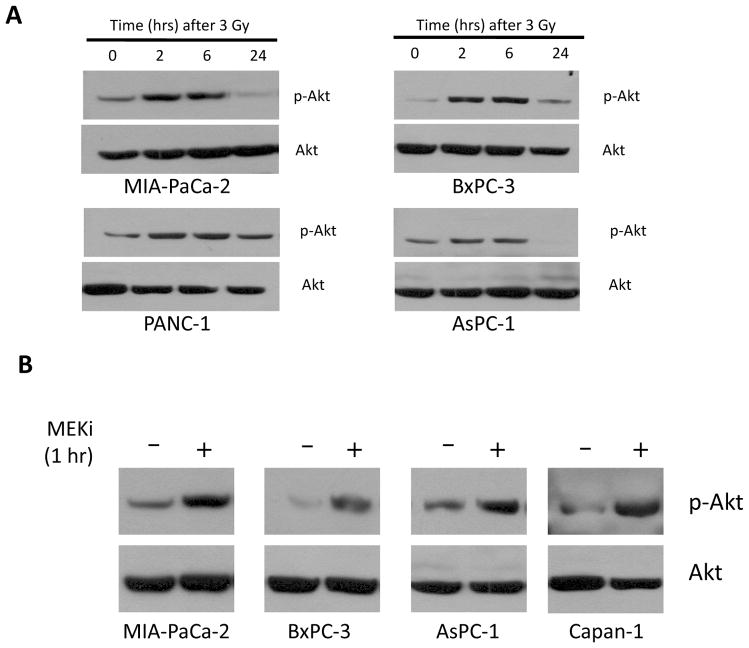
Radiation and PD0325901 independently up-regulate Akt activity
As shown in Fig. 5A, radiation induces a rapid and transient activation of Akt in five of six pancreatic cancer cell lines tested beginning within 2 hours after radiation that is maintained for at least 6 hours. By 24 hours after radiation, pAkt levels have returned to their pre-irradiation levels. It is interesting to note that Akt activation occurs earlier than ERK activation (Fig. 2A). We also examined the effect of PD0325901 treatment on PI3K/Akt activation. In Figure 5B, one hour of MEK inhibitor treatment produced a significant increase in pAkt expression. The amount of pAkt returned to control levels by 6 hours (data not shown). Taken together, treatment of pancreatic cancer cells with either radiation or MEK inhibitor induces activation of Akt, perhaps suggesting that these cells activate pro-survival mechanism(s) in response to cellular damage or stress.
Figure 5. Akt is activated by radiation or MEKi.
A) Immunoblots demonstrating activation of Akt as measured by phospho-Akt in response to radiation (3 Gy) in a time-dependent fashion in multiple pancreatic cancer cell lines.
B) Immunoblots demonstrating rapid activation of Akt after 1 hour of MEKi treatment in multiple pancreatic cancer cell lines.
For A and B, total Akt is shown as an equal loading control.
Dual inhibition of MEK and Akt inhibition promotes apoptosis in multiple pancreatic tumor models
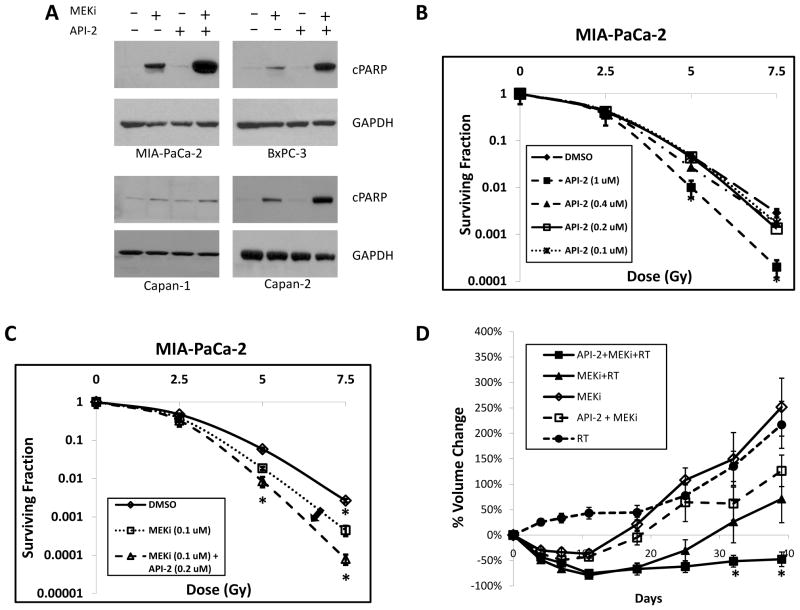
Based on the above results, we hypothesized that Akt inhibition could potentially sensitize cells to MEK-1/2 inhibition and radiation. Consequently, a panel of four pancreatic tumor cell lines (MIA-PaCa-2, BxPC-3, Capan-1, Capan-2) were treated with API-2, a selective Akt inhibitor (24). Treatment with API-2 for 1 hour resulted in greater than 95% reduction in pAkt levels at doses of 8 μM and higher, which occurred regardless of the presence or absence of PD0325901 (Supplementary Fig S3). We next treated these pancreatic cancer cell lines with PD0325901 and API-2, either alone or in combination. Twenty four hours after treatment, we performed immunoblotting to detect cleaved PARP (a marker of cells undergoing apoptosis). In all but one cell line, combination treatment with PD0325901 and API-2 produced a striking degree of enhanced apoptosis compared to that elicited by either agent alone (Figure 6A). Flow cytometry assessment of cell viability showed clear evidence that combination therapy resulted in the highest proportion of non-viable cells in the sub-G1 fraction (Table 1). This result is consistent with the immunoblotting data showing a significant hyper-activation of apoptotic pathways. These data led us to further explore the impact on overall therapeutic effectiveness of co-targeting both of these major signaling pathways in the radiation setting.
Figure 6. Combination of API-2 and MEKi induces activation of apoptotic pathways, radiosensitizes pancreatic cancer cells, and maximizes therapeutic efficacy in a xenograft model.
A) Various cell lines were treated with various combinations of API-2 (8 μM) and/or MEKi (100 nM) for 24 hrs, lysed, and then subjected to immunoblotting for cleaved PARP, as a measure of activation of the apoptotic pathway. GAPDH shown as an equal loading control.
B) Clonogenic survival assay was performed by pre-treating cells with DMSO or increasing concentrations of API-2 for 1 hour prior to increasing doses of radiation, then changing the medium 24 hours later, and allowing the cell lines to form colonies over 1–2 weeks. API-2 treatment resulted in substantial radiosensitization in MIA-PaCa-2 cells at 1 μM, but not at lower concentrations.
C) Clonogenic survival assay was performed by pre-treating cells with DMSO, MEKi (100 nM), or the combination of MEKi and API-2 (0.2 μM). The addition of a lower, sub-effective dose of API-2 resulted in further radiosensitization of MIA-PaCa-2 cells (arrow).
D) MIA-PaCa-2 xenograft model experiments were performed with various combinations of API-2, MEKi, and RT. Percent change in tumor volume during study period by treatment group: (1) MEKi (n=12 tumors, 6 mice), (2) MEKi + RT (n=14 tumors, 7 mice), (3) API-2 + MEKi (n=9 tumors, 5 mice), (4) API-2 + MEKi + RT (n=12 tumors, 6 mice), (5) RT (n=16 tumors, 9 mice). The percent change in tumor volume during study period by treatment group is shown.
Error bars in (A) and (B) represent s.e.m, and asterisks indicate p-value < 0.05 compared to DMSO-treated cells. Error bars in (D) represent s.e.m. and asterisks represent differences with a p-value < 0.05 when comparing MEKi + RT group to API-2 + MEKi + RT group. All four treatment groups in (D) had p < 0.05 compared to the control (no-treatment) group at all time points.
Akt inhibition further improves therapeutic efficacy of radiation administered concurrently with PD0325901
The same panel of four models tested in Figure 5 was also treated with radiation alone or in combination with PD0325901 and/or API-2. None of the models exhibited a significant increase in cPARP levels in response to radiation treatment (Supplementary Figure S4). This result is consistent with prior evidence showing that RT does not induce apoptosis by 24 hours, and predominantly exerts anti-neoplastic effects by inducing growth arrest and post-mitotic death.
Clonogenic assays were then carried out to explore the ability of API-2 to radiosensitize cells. A dose of 1μM was found to elicit a significant degree of radiosensitization (Figure 6B). Furthermore, a subeffective dose of API-2 (0.2 μM) when combined with PD0325901 further enhanced the degree of radiosensitization compared to the MEK inhibitor alone (Figure 6C).
We next tested whether Akt inhibition in vivo would further enhance the tumor inhibitory effects of MEK inhibition and radiation. Mice bearing MIA-PaCa-2 xenografts that reached ~100 mm3 in size were irradiated after dosing of either PD0325901 or API-2 alone versus co-administration of both agents. API-2 was administered daily for 10 consecutive days at a dose that previously has been shown to be effective in other tumor models (1 mg/kg i.p. daily) (24). However, this dose of API-2 proved to be ineffective at retarding the growth of MIA-PaCa-2 tumors as reflected by a delayed and modest reduction in tumor volume relative to the vehicle treated controls (Supplementary Figure S5). In contrast, API-2 when administered along with PD0325901 and concurrent radiotherapy produced a significant delay in tumor growth (Figure 6D). The added therapeutic activity of crippling both MEK and Akt became evident after the cessation of treatment (day 18). Statistically significant differences between the PD0325901/radiation and PD0325901/API-2/radiation groups did not occur until day 39 and continued until the end of the study (day 60). As before, there were no remarkable clinical signs of toxicity in any of the groups and weight loss never exceeded 6% (Supplementary Figure S2).
Discussion
It is well established that KRAS is mutated in over 90% of pancreatic cancers, and the high frequency of this genetic aberration is virtually unique to pancreatic cancer (25, 26). The high frequency of KRAS mutations in pancreatic cancer makes the RAS/MAPK pathway an attractive target for intervention. The emergence of highly potent and selective small molecule inhibitors of MEK, a critical downstream player in the RAS/ERK pathway, enables effective pathway suppression to produce meaningful therapeutic activity in a broad spectrum of human tumors (27).
Preclinical data suggest that roughly half of KRAS mutant tumors are susceptible to MEK inhibitor-based therapy and the subset of these tumors most sensitive to MEK inhibition are wild type for PIK3CA (9). Effective use of MEK inhibitors to treat pancreatic cancer will need to address activation of the PI3K pathway, which tracks with the aggressiveness of this disease. Indeed, activated Akt and PI3K/p110γ overexpression bear importance for pancreatic cancer progression and survival (28–30). Collectively, these findings provide strong impetus to design treatment regimens that block signaling through both the MEK/ERK and PI3K/Akt pathways.
There is a growing body of evidence demonstrating substantial cross-talk between the Ras/ERK and PI3K/Akt pathways, and that compensatory activation of either pathway mediates resistance to inhibition of the other pathway (9–12, 31). Our results show that MEK inhibition activates the PI3K/Akt pathway in multiple pancreatic models. Our findings further show that a combination approach targeting both pathways results in an enhancement of apoptosis and is highly efficacious in MIA-PaCa-2 tumors.
As radiation is an important component of local therapy for locally advanced pancreatic cancer, we have further explored the concept of combining MEK and Akt inhibitors to enhance the effects of radiotherapy. We found that radiation results in time-dependent activation of ERK in vitro and in vivo, and that upstream MEK inhibition results in significant radiosensitization in multiple pancreatic cancer cell lines. Importantly, the radiosensitizing potential of MEK inhibition was confirmed in vivo. Recently, other groups have demonstrated that another MEK inhibitor (AZD6244) also radiosensitizes cancer cell lines with a broad range of histologies (18, 19). Ongoing studies in our laboratory are exploring the mechanistic basis of MEK inhibitor-induced radiosensitization and early results suggest that the mechanism may be related to inability to promote or repair DNA damage. It has also been proposed that a reduction in HIF-1 signaling under hypoxic conditions occurs in response to MEK inhibition thereby circumventing hypoxia-induced radioresistance (18, 32).
Focusing exclusively on pancreatic cancer models, we show that radiation activates both Ras/MAPK and PI3K/Akt signaling, providing a strong rationale for investigating radiotherapy regimens that incorporate agents targeting both pathways. Our subsequent in vitro and in vivo combination studies provide further evidence that this is a viable approach warranting further investigation. Combination of PD0325901 with API-2 and concurrent radiotherapy produced a statistically-significant enhancement in radiosensitization in clonogenic survival assays, and in tumor reduction compared to all other treatment arms, and occurred without additional toxicity. We believe that this data argues that ERK-1/2 and Akt activation after radiation serve as survival mechanisms to correct the DNA-damaging effects of radiation. In a similar fashion, radiation activates Akt, and blockade of signaling through Akt with API-2 also radiosensitizes cells. Likewise, there is evidence in the literature that hyperactivation of the Ras/MAPK or Akt pathways makes cells more resistant to the effects of radiation, therefore providing more evidence that these pathways are important for radiation survival, and not a bystander effect of radiation damage. Our unifying hypothesis is that cells have compensatory signaling pathways, which promote resistance not only to the effects of chemotherapy or targeted agents, but also to radiation. Radiation activates both PI3K/Akt and Ras/MAPK pathways, independently promoting cell survival through different pathways. However, there is evidence emerging for considerable cross-talk occurring between the PI3K/Akt and Ras/MAPK pathways, such that blockage of one pathway with a targeted agent results in compensatory activation of the other. We have also shown that this likely occurs in the context of radiation, since the combination of MEK-1/2 and Akt inhibition further radiosensitizes cells beyond MEK-1/2 inhibition alone. Furthermore, the earlier activation of Akt compared with ERK-1/2 activation after radiation may have important implications for the proper sequencing and design of treatments incorporating targeted agents in combination with radiation.
The in vivo studies reported here have relied on the use of subcutaneously-implanted xenografts. There are divergent views on the relative values of subcutaneous and orthotopic models in predicting clinical outcome (33). One position is that subcutaneous models when properly used and interpreted are immensely valuable. An example is the encouraging clinical activity seen with MEK inhibitors in BRAF mutated tumors, an outcome predicted on the basis of subcutaneous models, which further predicted diminished or no activity of these agents in BRAF/KRAS wild type tumors (23). Another position is that orthotopic models are superior based on their recapitulation of the tumor microenvironment and their utility for studying site-specific effects of therapy. Pancreatic tumors, in particular are poorly perfused and poorly vasucularized (34). However, orthotopic pancreatic xenografts have not exhibited the diminished vascularity seen in transgenic mouse models of pancreatic cancer and human tumors (34). This result has implications for whether orthotopic xenograft models will necessarily be any more predictive than subcutaneous xenografts in predicting response to chemotherapy as well as radiation. Studies are now underway in our laboratory to address the therapeutic potential of co-targeting MAP kinase and PI3K signaling with concurrent radiation in orthotopic pancreatic xenografts. We are encouraged by data obtained thus far with MIA-PaCa-2 orthotopic tumors showing that PD0325901 in combination with PI3K pathway inhibition results in enhanced efficacy over the single agent arms as reflected by a 2 to 4 fold increase in % T/C value, calculated as the tumor burden on the last day of treatment of the treated group relative to the vehicle control group (Sebolt-Leopold, unpublished data). With regard to the consideration of model systems, the effect of PD0325901 alone in these orthotopic xenografts was comparable to that observed in the present study with subcutaneous MIA-PaCa-2 xenografts.
In summary, we have demonstrated that radiation activates both ERK and PI3K/Akt signaling. Inhibition of either pathway can result in radiosensitization of pancreatic tumor cells. However, combined treatment with agents targeting both pathways produces the greatest degree of therapeutic effect as measured by increased dose enhancement factor in vitro and tumor reduction in vivo. Our results provide rationale for exploring a regimen combining MEK inhibition and radiation, optimally in conjunction with PI3K/Akt inhibition for the treatment of pancreatic cancer.
Supplementary Material
Acknowledgments
We would like to thank Swaroop Bhojani and Meredith Morgan for technical support and advice.
Grant Support:
This work was supported by the following grants: NIH P01 CACA85878 (A.R., B.R.), NIH P50CA093990 (A.R., B.R.), and a RSNA Research Resident/Fellow Grant (T.W.). T. Williams has been designated a B. Leonard Holman Pathway Fellow by the American Board of Radiology.
References
- 1.Balmanno K, Cook SJ. Tumour cell survival signalling by the ERK1/2 pathway. Cell Death Differ. 2009;16:368–77. doi: 10.1038/cdd.2008.148. [DOI] [PubMed] [Google Scholar]
- 2.Campbell PM, Der CJ. Oncogenic Ras and its role in tumor cell invasion and metastasis. Semin Cancer Biol. 2004;14:105–14. doi: 10.1016/j.semcancer.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 3.Lorusso PM, Adjei AA, Varterasian M, Gadgeel S, Reid J, Mitchell DY, et al. Phase I and pharmacodynamic study of the oral MEK inhibitor CI-1040 in patients with advanced malignancies. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:5281–93. doi: 10.1200/JCO.2005.14.415. [DOI] [PubMed] [Google Scholar]
- 4.Tolcher AW, Bendell JC, Patnaik A, Papadopoulos K, Bellew KM, Cox DS, et al. A phase Ib study of the MEK inhibitor GSK1120212 combined with gemcitabine in patients with solid tumors: Interim results. J Clin Oncol. 2011;29:Abstract 278. [Google Scholar]
- 5.Sebolt-Leopold JS. Advances in the development of cancer therapeutics directed against the RAS-mitogen-activated protein kinase pathway. Clin Cancer Res. 2008;14:3651–6. doi: 10.1158/1078-0432.CCR-08-0333. [DOI] [PubMed] [Google Scholar]
- 6.Wang JY, Wilcoxen KM, Nomoto K, Wu S. Recent advances of MEK inhibitors and their clinical progress. Curr Top Med Chem. 2007;7:1364–78. doi: 10.2174/156802607781696837. [DOI] [PubMed] [Google Scholar]
- 7.Gilmartin AG, Bleam MR, Groy A, Moss KG, Minthorn EA, Kulkarni SG, et al. GSK1120212 (JTP-74057) is an inhibitor of MEK activity and activation with favorable pharmacokinetic properties for sustained in vivo pathway inhibition. Clin Cancer Res. 2011 doi: 10.1158/1078-0432.CCR-10-2200. [DOI] [PubMed] [Google Scholar]
- 8.Engelman JA, Chen L, Tan X, Crosby K, Guimaraes AR, Upadhyay R, et al. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med. 2008;14:1351–6. doi: 10.1038/nm.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halilovic E, She QB, Ye Q, Pagliarini R, Sellers WR, Solit DB, et al. PIK3CA mutation uncouples tumor growth and cyclin D1 regulation from MEK/ERK and mutant KRAS signaling. Cancer research. 2010;70:6804–14. doi: 10.1158/0008-5472.CAN-10-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wee S, Jagani Z, Xiang KX, Loo A, Dorsch M, Yao YM, et al. PI3K pathway activation mediates resistance to MEK inhibitors in KRAS mutant cancers. Cancer Res. 2009;69:4286–93. doi: 10.1158/0008-5472.CAN-08-4765. [DOI] [PubMed] [Google Scholar]
- 11.Balmanno K, Chell SD, Gillings AS, Hayat S, Cook SJ. Intrinsic resistance to the MEK1/2 inhibitor AZD6244 (ARRY-142886) is associated with weak ERK1/2 signalling and/or strong PI3K signalling in colorectal cancer cell lines. Int J Cancer. 2009;125:2332–41. doi: 10.1002/ijc.24604. [DOI] [PubMed] [Google Scholar]
- 12.Carracedo A, Ma L, Teruya-Feldstein J, Rojo F, Salmena L, Alimonti A, et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest. 2008;118:3065–74. doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sklar MD. The ras oncogenes increase the intrinsic resistance of NIH 3T3 cells to ionizing radiation. Science. 1988;239:645–7. doi: 10.1126/science.3277276. [DOI] [PubMed] [Google Scholar]
- 14.Bernhard EJ, McKenna WG, Hamilton AD, Sebti SM, Qian Y, Wu JM, et al. Inhibiting Ras prenylation increases the radiosensitivity of human tumor cell lines with activating mutations of ras oncogenes. Cancer Res. 1998;58:1754–61. [PubMed] [Google Scholar]
- 15.McKenna WG, Muschel RJ, Gupta AK, Hahn SM, Bernhard EJ. The RAS signal transduction pathway and its role in radiation sensitivity. Oncogene. 2003;22:5866–75. doi: 10.1038/sj.onc.1206699. [DOI] [PubMed] [Google Scholar]
- 16.Shimura T, Kakuda S, Ochiai Y, Kuwahara Y, Takai Y, Fukumoto M. Targeting the AKT/GSK3beta/cyclin D1/Cdk4 survival signaling pathway for eradication of tumor radioresistance acquired by fractionated radiotherapy. International journal of radiation oncology, biology, physics. 2011;80:540–8. doi: 10.1016/j.ijrobp.2010.12.065. [DOI] [PubMed] [Google Scholar]
- 17.Deng R, Tang J, Ma JG, Chen SP, Xia LP, Zhou WJ, et al. PKB/Akt promotes DSB repair in cancer cells through upregulating Mre11 expression following ionizing radiation. Oncogene. 2011;30:944–55. doi: 10.1038/onc.2010.467. [DOI] [PubMed] [Google Scholar]
- 18.Shannon AM, Telfer BA, Smith PD, Babur M, Logie A, Wilkinson RW, et al. The mitogen-activated protein/extracellular signal-regulated kinase kinase 1/2 inhibitor AZD6244 (ARRY-142886) enhances the radiation responsiveness of lung and colorectal tumor xenografts. Clin Cancer Res. 2009;15:6619–29. doi: 10.1158/1078-0432.CCR-08-2958. [DOI] [PubMed] [Google Scholar]
- 19.Chung EJ, Brown AP, Asano H, Mandler M, Burgan WE, Carter D, et al. In vitro and in vivo radiosensitization with AZD6244 (ARRY-142886), an inhibitor of mitogen-activated protein kinase/extracellular signal-regulated kinase 1/2 kinase. Clin Cancer Res. 2009;15:3050–7. doi: 10.1158/1078-0432.CCR-08-2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chautard E, Loubeau G, Tchirkov A, Chassagne J, Vermot-Desroches C, Morel L, et al. Akt signaling pathway: a target for radiosensitizing human malignant glioma. Neuro Oncol. 2010;12:434–43. doi: 10.1093/neuonc/nop059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morgan MA, Parsels LA, Zhao L, Parsels JD, Davis MA, Hassan MC, et al. Mechanism of radiosensitization by the Chk1/2 inhibitor AZD7762 involves abrogation of the G2 checkpoint and inhibition of homologous recombinational DNA repair. Cancer research. 2010;70:4972–81. doi: 10.1158/0008-5472.CAN-09-3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sebolt-Leopold JS, Dudley DT, Herrera R, Van Becelaere K, Wiland A, Gowan RC, et al. Blockade of the MAP kinase pathway suppresses growth of colon tumors in vivo. Nature medicine. 1999;5:810–6. doi: 10.1038/10533. [DOI] [PubMed] [Google Scholar]
- 23.Solit DB, Garraway LA, Pratilas CA, Sawai A, Getz G, Basso A, et al. BRAF mutation predicts sensitivity to MEK inhibition. Nature. 2006;439:358–62. doi: 10.1038/nature04304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang L, Dan HC, Sun M, Liu Q, Sun XM, Feldman RI, et al. Akt/protein kinase B signaling inhibitor-2, a selective small molecule inhibitor of Akt signaling with antitumor activity in cancer cells overexpressing Akt. Cancer research. 2004;64:4394–9. doi: 10.1158/0008-5472.CAN-04-0343. [DOI] [PubMed] [Google Scholar]
- 25.Almoguera C, Shibata D, Forrester K, Martin J, Arnheim N, Perucho M. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell. 1988;53:549–54. doi: 10.1016/0092-8674(88)90571-5. [DOI] [PubMed] [Google Scholar]
- 26.DeVita VT, Hellman S, Rosenberg SA. Cancer, principles & practice of oncology. 7. Philadelphia, PA: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 27.Sebolt-Leopold JS, Herrera R. Targeting the mitogen-activated protein kinase cascade to treat cancer. Nat Rev Cancer. 2004;4:937–47. doi: 10.1038/nrc1503. [DOI] [PubMed] [Google Scholar]
- 28.Yamamoto S, Tomita Y, Hoshida Y, Morooka T, Nagano H, Dono K, et al. Prognostic significance of activated Akt expression in pancreatic ductal adenocarcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2004;10:2846–50. doi: 10.1158/1078-0432.ccr-02-1441. [DOI] [PubMed] [Google Scholar]
- 29.Schlieman MG, Fahy BN, Ramsamooj R, Beckett L, Bold RJ. Incidence, mechanism and prognostic value of activated AKT in pancreas cancer. Br J Cancer. 2003;89:2110–5. doi: 10.1038/sj.bjc.6601396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edling CE, Selvaggi F, Buus R, Maffucci T, Di Sebastiano P, Friess H, et al. Key role of phosphoinositide 3-kinase class IB in pancreatic cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16:4928–37. doi: 10.1158/1078-0432.CCR-10-1210. [DOI] [PubMed] [Google Scholar]
- 31.Mirzoeva OK, Das D, Heiser LM, Bhattacharya S, Siwak D, Gendelman R, et al. Basal subtype and MAPK/ERK kinase (MEK)-phosphoinositide 3-kinase feedback signaling determine susceptibility of breast cancer cells to MEK inhibition. Cancer Res. 2009;69:565–72. doi: 10.1158/0008-5472.CAN-08-3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ciuffreda L, Del Bufalo D, Desideri M, Di Sanza C, Stoppacciaro A, Ricciardi MR, et al. Growth-inhibitory and antiangiogenic activity of the MEK inhibitor PD0325901 in malignant melanoma with or without BRAF mutations. Neoplasia. 2009;11:720–31. doi: 10.1593/neo.09398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sausville EA, Burger AM. Contributions of human tumor xenografts to anticancer drug development. Cancer Res. 2006;66:3351–4. doi: 10.1158/0008-5472.CAN-05-3627. discussion 4. [DOI] [PubMed] [Google Scholar]
- 34.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–61. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.