Abstract
We report x-ray crystallographic structures of three inhibitors bound to dehydrosqualene synthase from Staphylococcus aureus: 1 (BPH-651), 2 (WC-9) and 3 (SQ-109). Compound 2 binds to the S2 site with its –SCN group surrounded by 4 hydrogen bond donors. With 1, we report two structures: in both, the quinuclidine head group binds in the allylic (S1) site with the side-chain in S2, but in the presence of PPi and Mg2+, the quinuclidine’s cationic center interacts with PPi and 3 Mg2+, mimicking a transition state involved in diphosphate ionization. With 3, there are again two structures. In one, the geranyl side-chain binds to either S1 or S2 and the adamantane head-group binds in S1. In the second, the side-chain binds to S2, while the headgroup binds to S1. These results provide structural clues for the mechanism and inhibition of the head-to-head prenyl transferases and should aid future drug design.
Keywords: isoprenoid, prenyl tranferase, x-ray crystallography, catalysis, drug discovery
INTRODUCTION
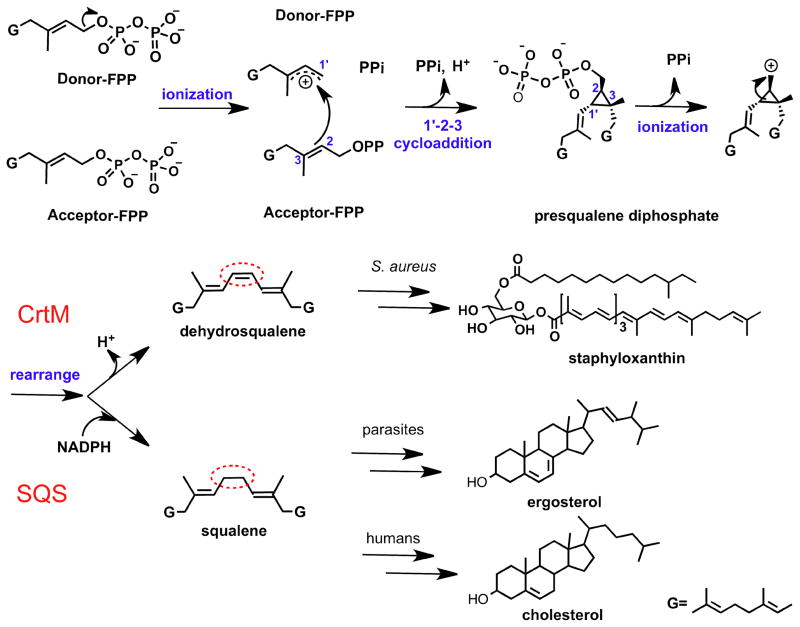
There is currently considerable interest in the structure, function and inhibition of the “head-to-head” class of prenyl tranferase enzymes that condense farnesyl diphosphate to squalene or dehydrosqualene.1 These reactions are carried out by squalene synthase (SQS) in humans and in some protozoa such as Trypanosoma cruzi, or by dehydrosqualene synthase (CrtM) in Staphylococcus aureus (SaCrtM) (Figure 1). Inhibiting SQS in protozoa is of interest since it blocks formation of the essential membrane sterol, ergosterol, while blocking human SQS (HsSQS) lowers cholesterol levels, plus, it results in formation of anti-bacterial neutrophil extra-cellular traps (NETs)2. Also of interest is the observation that inhibiting SaCrtM inhibits biofilm formation3 with S. aureus, in addition to preventing formation of the virulence factor, staphyloxanthin (Figure 1), leading to immune system based bacterial killing.4 There is thus interest in the development of compounds that might inhibit more than one target (e.g. combining NETs/biofilm/STX activity, or direct protozoal killing + NETs formation), and here, we present crystal structures of three SQS/CrtM inhibitors bound to CrtM, together with mechanistic insights into the SQS/CrtM mode of action. The inhibitors investigated are of interest since two are known to inhibit SQS while the third has an unknown mechanism of action but has been used in clinical trials and here, we show that it can serve as a lead for inhibiting both CrtM and SQS.
Figure 1.
Schematic illustration of the first and second half reactions catalyzed by CrtM and SQS leading to formation of dehydrosqualene and squalene, and later conversion of these products to staphyloxanthin, ergosterol and cholesterol.
RESULTS AND DISCUSSION
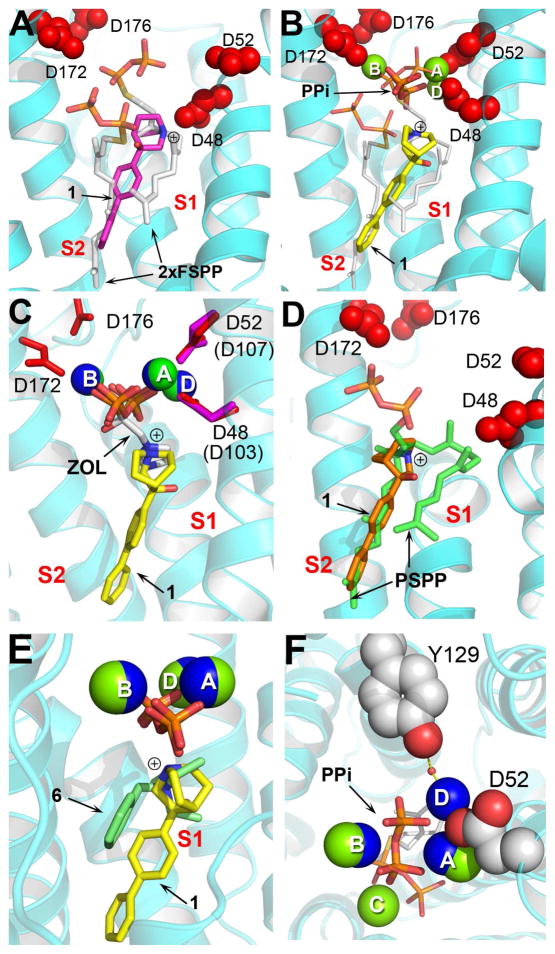
In the case of the quinuclidine 15–7 (Figure 2), a known potent squalene synthase inhibitor, we obtained two new structures of 1 bound to SaCrtM. Electron density results are shown in Figure S1A, B. In the first, Figure 3A (PDB ID code 4E9Z), 1 binds with its cationic head-group in the S1 (cationic/donor) site, while the biphenyl side-chain binds very close to S2. The cationic center is ~1.9Å from C2 in the S1 farnesyl side-chain, Figure 3A. In the second structure, Figure 3B (PDB ID code 4EA0), the side-chain occupies a site between the S1 and S2 FSPP binding sites, while the cationic center is now 2.5Å from C1, 1.7Å from C2 and 0.7Å from C3 in the (overlaid, white) S1 FSPP, Figure 3B. Both structures strongly suggest the quinuclidine is acting as an isostere for the S1 farnesyl carbo-cation. In the second structure (PDB ID code 4EA0) there is also a PPi group as well as three Mg2+, with the (inorganic) PPi being close to the position occupied by the diphosphate group in the S1 FSPP structure,4 plus, two of the three Mg2+ (MgA2+, MgB2+) seen in the FSPP structure are observed, Figure 3B. These results are consistent with the proposal that the initial FPP ionization step occurs in the S1 site,8 and are reminiscent of the observation that cationic bisphosphonate inhibitors of other prenyl synthases, such as FPP synthase (FPPS), have their cationic, anionic and Mg2+ located in very similar positions, as shown in the 4/FPPS9 superimposition shown in Figure 3C. Notably, this S1/cationic site structure is quite distinct from that reported previously for 1 (via soaking, as opposed to co-crystallization), in which the quinuclidine carbo-cation feature was located in essentially the same position as the cyclopropane ring in PSPP, Figure 3D, where it mimics a second transition state. So, the quinuclidine (1) can block either the S1 or S2 head-group sites, opening up new possibilities for inhibitor design.
Figure 2.
Chemical structures of small molecule inhibitors.
Figure 3.
X-ray crystallographic structures of 1 bound to CrtM. (A) CrtM-1 (no Mg2+, PPi; PDB ID code 4E9Z). Cationic (N+) center (blue) is close to S1 FPP C3. FSPP superimposed in white. (B) CrtM-1 + Mg2+ + PPi (PDB ID code 4EA0). Cationic center again close to putative S1 allylic carbocation; N+ also close to PPi group. (C) As (B) but superimposed on zoledronate/Mg2+ ligand from FPPS structure (PDB ID code 2F8C). (D) 1 occupying S2 carbocation center (PDB ID code 3ACW), superimposed on PSPP structure (PDB ID code 3NPR). (E) Structure from B superimposed on 6/Mg2+/PPi from epi-isozizaene synthase (PDB ID 3KB9). (F) Top view of B showing Y129-H2O-MgD2+ and PPi-Mg2+ interactions. The ligand is s-thiolo farnesyl diphosphate.
The 1-PPi-[Mg2+]3 structure (Figure 3B) is also of considerable interest since it bears a strong resemblance - in terms of placement of the cationic center, PPi and [Mg2+]3 groups – to the recently reported structure10 of the terpene cyclase, epi-isozizaene (5) synthase, containing a bound inhibitor, 6 (benzyltriethylammonium, Figure 2). As can be seen in the superposition shown in Figure 3E, both structures lack the [MgC2+] seen in the CrtM/FSPP structure4 and contain instead, a new Mg2+, MgD2+, Figures 3E, F. The rmsd of the N+, PPi and 3 Mg2+ in the CrtM and epi-isozizaene structures is only 0.35Å, supporting the idea that diphosphate ionization of FPP in the head-to-head prenyl transferases, as well as in the terpene cyclase, is dominated by the same driving force, a [Mg2+]3-PPi interaction. The results obtained with the 1-PPi-[Mg2+]3 structure are also of interest since they help clarify the role of Y129 in CrtM (Y171 in HsSQS), which is among the most essential residues needed for catalytic activity (based on mutagenesis8, 11 and a SCORECONS analysis12). In earlier work, it was thought that this residue (in HsSQS) might be involved in stabilizing the farnesyl cation via a cation-π interaction, however, this residue is ~8.5Å from the proposed cationic center. In the 1-PPi-[Mg2+] structure, we now see that the Tyr-OH is hydrogen bonded to a water molecule that coordinates to one of the Mg2+ seen in the x-ray structure, MgD2+, Figure 3F (in blue). This suggests that Y129 may help stabilize and/or facilitate removal of the diphosphate group, rather than directly stabilizing the S1 carbo-cation.
We show in Figure 4A, B the single crystal x-ray crystallographic structure of the thiocyanate 213 bound to CrtM. 2 is a potent SQS inhibitor of interest in treating Chagas disease.14, 15 Electron density results are shown in Figure S1C and full crystallographic data acquisition and structure refinement results are in Table 1. The molecule inhibits CrtM with a Ki= 1.5 μM (Figure S2A) and binds with its diphenyl ether side-chain occupying the S2 site normally occupied by the acceptor FPP, or one of the PSPP side-chains, as seen in the 2 (cyan) – PSPP (yellow) superposition in Figure 4B. This side-chain binding site can also be occupied by several other inhibitors, including the phosphonosulfonates,4 and is completely hydrophobic. The question then arises as to the nature of the interactions undergone by the thiocyanate group. Unlike the quinuclidine inhibitors, the thiocyanate group cannot be charged, however, alkyl thiocyanates can act as proton acceptors, due to the following resonance scheme:
and there is a ΔH = −6.5 kJ mol−1 interaction between phenol and CH3SCN.16 In the 2/CrtM crystal structure (PDB ID code 4E9U), there are four polar residues close to the thiocyanate nitrogen (Y41, Q165, N168 and Y248) with, on average, an SCN-protein distance of ~3.2Å, Figure 4B. Since all of these amino-acid side-chains are polar, it seems likely that they will contribute to ligand binding via electrostatic (hydrogen bonding) interactions, in much the same way that e.g. phenol interacts with the thiocyanate group in liquid MeSCN.16
Figure 4.
X-ray crystallographic structure of 2 bound to CrtM (PDB ID code 4E9U). (A) 2 binds to a buried, hydrophobic S2 site. (B) 2 (in cyan) superimposed on the PSPP reaction intermediate (yellow) bound to CrtM.
Table 1.
Data collection and refinement statistics for CrtM with 1, 2, and 3
| Crystal (PDB ID) | 2/CrtM (soaking) (4E9U) |
1/CrtM (co-crystal) (4E9Z) |
1/CrtM -PPi- Mg2+ (co-crystal) (4EA0) |
3/CrtM (co-crystal) (4EA1) |
3/CrtM (soaking) (4EA2) |
|---|---|---|---|---|---|
| Data collection | |||||
| Radiation source | APS 21-ID-F | APS 21-ID-F | APS 21-ID-F | APS 21-ID-F | APS 21-ID-F |
| Wavelength (Å) | 0.97857 | 0.97857 | 0.97857 | 0.97857 | 0.97857 |
| Space group | P3221 | P32 2 1 | P31 2 1 | P32 2 1 | P32 2 1 |
| a(Å) | 80.4 | 80.6 | 80.3 | 80.6 | 80.1 |
| b(Å) | 80.4 | 80.6 | 80.3 | 80.6 | 80.1 |
| c (Å) | 90.8 | 91.8 | 180.8 | 91.6 | 90.9 |
| Resolution (Å)a | 50.0-2.10 (2.14-2.10) | 50.0-2.06 (2.12-2.06) | 50.0-2.12 (2.17-2.12) | 50.0-2.46 (2.50-2.46) | 50.0-2.02 (2.09-2.05) |
| No. of reflections | 20218 (966) | 21572 (1041) | 37084 (1169) | 12795 (583) | 21470 (858) |
| Completeness (%) | 99.9 (98.9) | 99.9 (100) | 94.5 (61.1) | 99.2 (91.0) | 98.5 (80.9) |
| Redundancy | 10.1 (4.90) | 12.7 (12.7) | 8.8 (6.1) | 9.5 (5.4) | 10.1 (5.0) |
| Rmerge (%) | 10.1 (65.2) | 7.30 (45.0) | 7.60 (59.7) | 8.80 (44.1) | 6.5 (68.9) |
| I/s(I) | 21.3 (1.56) | 44.0 (5.04) | 27.7 (1.52) | 2.69 (1.74) | 41.8 (1.5) |
| Refinement | |||||
| Resolution (Å) a | 36.8-2.1 (2.21-2.10) | 30.0-2.08 (2.12-2.06) | 50.0-2.12 (2.17-2.12) | 30.0-2.46 (2.17-2.12) | 34.7-2.05 (2.10-2.05) |
| No. of reflections | 19540 (2320) | 20373 (1228) | 35058 (1687) | 12091 (819) | 20270 (1246) |
| Rwork (%) | 18.9 (21.8) | 19.1 (20.8) | 22.2 (31.5) | 23.2 (34.8) | 20.6 (27.2) |
| Rfree (%) | 23.6 (26.5) | 24.1 (24.7) | 27.8 (36.3) | 31.4 (34.8) | 26.0 (31.7) |
| Geometry deviations | |||||
| Bond lengths (Å) | 0.007 | 0.015 | 0.005 | 0.006 | 0.014 |
| Bond angles (°) | 0.962 | 1.331 | 0.692 | 0.912 | 1.234 |
| Mean B-values (Å2) / Number of non-H atoms | |||||
| All refined atoms | 38.5 / 2568 | 34.0 / 2602 | 46.2 / 5046 | 42.3 / 2456 | 48.76 / 2467 |
| Compound atoms | 49.7 / 19 | 58.1 / 21 | 38.4 / 42 | 34.9 / 48 | 71.8 / 24 |
| Mg ions | - | - | 28.5 / 6 | - | 75.6 / 1 |
| Water molecules | 38.5 / 156 | 40.8 / 184 | 41.6 / 186 | 40.6 / 47 | 52.4 / 63 |
| Ramachandran plot (%) | |||||
| Most favored | 98.6 | 98.2 | 96.6 | 96.4 | 96.8 |
| Additionally allowed | 1.06 | 1.07 | 2.84 | 1.8 | 2.2 |
| Generously allowed | 0.35 | 0.71 | 0.53 | 1.8 | 1.0 |
Values in the parentheses are for the highest resolution shells.
The question then arises as to whether 2 binds to Trypanosoma cruzi SQS (TcSQS) in the same manner as it does to CrtM. To date, there are no structures of TcSQS. However, there are 11 residues in CrtM (F22, Y41, A134, V137, G138, L141, A157, G161, L164, Q165 and N168) that are close (< 4Å) to 2 in the CrtM structure, and these residues are totally conserved in both HsSQS as well as TcSQS. This strongly suggests that the ligand will bind into the same S2 pocket in TcSQS, with the same polar interactions with the thiocyanate group as in CrtM.
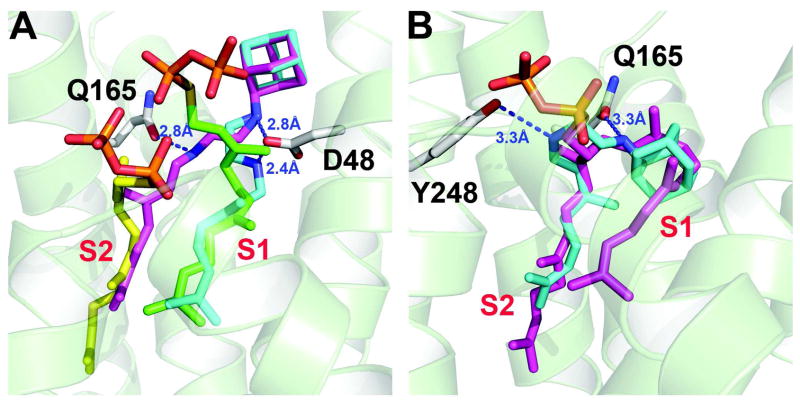
Finally, we determined two structures of 3 bound to CrtM. 3 is a novel, di-alkylated ethylendiamine with an adamantyl “head-group” and a geranyl “side-chain” that potently inhibits the growth of Candida albicans, Aspergillus fumigatus, Mycobacterium tuberculosis and Helicobacter pylori and has progressed through Phase Ia clinical trials.17 The actual enzyme targets involved have not been reported. Nevertheless, based on the general similarities (large hydrophobic group--cation center--small hydrophobic group) between the quinuclidine (1) and 3 structures it seemed possible that 3 might inhibit CrtM and SQS. This is the case, with Ki values of 0.36 μM (CrtM), 0.74 μM (hSQS), and 1.2 μM (TcSQS), Figure S2. We show in Figure 5A two crystallographic structures of 3 bound to CrtM, one obtained by co-crystallization (PDB ID code 4EA1), the other by soaking (PDB ID code 4EA2). Electron density results are shown in Figure S1D, E. In the co-crystal structure, we found two alternative conformations, with the geranyl side-chain binding to either the S1 or S2 sites, the ethylenediamine linker interacting with either D48 or Q165, and the adamantane head-group being solvent exposed (Figures S3, 4). In the structure obtained by soaking, 3 adopts a “bent” conformation with the adamantane head-group binding to the S1 site, close to the position occupied by the quinuclidine head-group in 1, and the geranyl side-chain is located in the S2 site, Figure 5B and S5. In this structure, the hydrophobic head-group occupies the same pocket as occupied by the “bend” in presqualene diphosphate (or FSPP), as can be seen in the superimposition shown in Figure 5B (PSPP in magenta). Since 3 has been successfully tested in humans, the observation that it inhibits both human and T. cruzi SQS as well as S. aureus CrtM clearly makes it an interesting potential lead for further development, as an SQS/CrtM inhibitor.
Figure 5.
X-ray crystallographic structure of 3 bound to CrtM. (A) Structure obtained by co-crystallization (PDB ID code 4EA1) showing geranyl side-chain can occupy both S1 and S2 sites (cyan, S1; magenta, S2), superimposed on the FSPP structures (green, yellow; PDB ID code 2ZCP). (B) Structure obtained by soaking (PDB ID code 4EA2). The inhibitor 3 (cyan) adopts a bent conformation with the adamantane head-group occupying the same site as the “bend” in the PSPP (magenta) intermediate, in S1 (PDB ID code 3NPR).
CONCLUSIONS
The results presented here are of interest for several reasons. First, we have obtained the structure of the potent anti-parasitic thiocyanate drug lead 2, that inhibits squalene synthase, bound here to a dehydrosqualene synthase (Figure 4). The biphenyl ether side-chain binds to the S1 site and the thiocyanate group is involved in an extensive series of electrostatic interactions with protein residues. There are 11 residues in close contact with the ligand and these residues are all conserved in S. aureus CrtM, human SQS as well as T. cruzi SQS, supporting binding of this inhibitor to the S2 pocket the SQS enzymes as well. Second, we report two structures of the quinuclidine (1), bound to dehydrosqualene synthase. One structure (1+PPi/Mg) is of particular interest since it mimics a transition state in which the cationic center formed on FPP (or PSPP) ionization is in S1, while the exiting PPi binds to a new [Mg2+]3 cluster, with one Mg2+ close to the highly conserved Y129, which we propose is positioned to facilitate PPi removal. Third, we find that the anti-infective drug 3 inhibits both CrtM as well as SQS (Figure S2). We obtained two CrtM structures. In one, the geranyl side-chain binds to either the S1 or S2 sites, the ethylenediamine interacts with essential protein residues, and the adamantane group is solvent exposed (Figure 5A). In the second (Figure 5B), the geranyl side-chain occupies the S2 site, while the adamantane group is now buried, and occupies the same position as the “bend” in the S1 PSPP sidechain.8
Overall, these results are of significance since they provide new structural information on the mechanism of action and inhibition of the head-to-head prenyl transferases that are of interest as novel anti-infective drug targets. Such inhibitors may target not only direct pathogen killing,6, 13, 18 but also innate immune system-based killing, via virulence factor inhibition,4 NET formation2 and bio-film inhibition.3
EXPERIMENTAL SECTION
Materials
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO) or Alchem Laboratories Corp. and were used as provided. BL-21 (DE3) competent cells were purchased from Stratagene (La Jolla, CA). Syntheses of 1–3 (Figure 2) were as reported previously.15, 19, 20 The CrtM plasmid was provided by Andrew H.-J. Wang’s group. The human SQS (HsSQS) and Trypanosoma cruzi SQS (TcSQS) plasmids were provided by Dolores Gonzalez-Pacanowska.
Protein Expression, Purification and Crystallization
Protein expression, purification and crystallization were as described previously.4, 6, 8
Inhibition Assays
CrtM, TcSQS and hSQS inhibition assays were as described previously.4, 8
Crystallographic Aspect
Structures were obtained as described in detail previously.4, 8 Structure Figures were made by using PyMOL21 and ligand-protein interaction Figures were made using MOE.22
Supplementary Material
Acknowledgments
This work was supported by NIH Grant AI-074233 to EO. We thank A. H.-J. Wang and C.-I. Liu for providing their CrtM plasmid and D. Gonzalez-Pacanowska for hSQS and TcSQS plasmids. We thank Y. Gao for his assistance with protein crystallization. Use of the Advanced Photon Source was supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract DE-AC02-06CH111357. Use of the Life Science Collaborative Access Team Sector 21 was supported by the Michigan Economic Development Corporation and the Michigan Technology Tri-Corridor (Grant 085P1000817).
The abbreviations used are
- CrtM
dehydrosqualene synthase
- SQS
squalene synthase
- FPP
farnesyl diphosphate
- FPPS
farnesyl diphosphate synthase
- FSPP
S-thiolo-farnesyl diphosphate
- PSPP
presqualene diphosphate
- PPi
diphosphate
- BTAC
benzyltrimethylammonium
- NET
neutrophil extra-cellular trap
Footnotes
Supporting Information Available: electron density maps for inhibitors bound to CrtM (Figure S1), dose-response curves for CrtM inhibition (Figure S2), and protein-ligand interactions for CrtM-3 (Figures S3-S5). This material is available free of charge via the Internet at http://pubs.acs.org.
PDB ID Codes
Data deposition: The atomic coordinates and structure factors have been deposited in the RCSB Protein Data Bank for CrtM complexed with 2 (PDB ID 4E9U), 1 (PDB ID 4E9Z), 1/PPi-Mg2+ (PDB ID 4EA0), and 3 (PDB ID 4EA1 and 4EA2).
References
- 1.Oldfield E, Lin F-Y. Terpene Biosynthesis: Modularity Rules. Angew Chem Int Ed. 2011;50:2–16. doi: 10.1002/anie.201103110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chow OA, von Kockritz-Blickwede M, Bright AT, Hensler ME, Zinkernagel AS, Cogen AL, Gallo RL, Monestier M, Wang Y, Glass CK, Nizet V. Statins enhance formation of phagocyte extracellular traps. Cell Host Microbe. 2010;8:445–54. doi: 10.1016/j.chom.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopez D, Kolter R. Functional microdomains in bacterial membranes. Genes Dev. 2010;24:1893–902. doi: 10.1101/gad.1945010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu CI, Liu GY, Song Y, Yin F, Hensler ME, Jeng WY, Nizet V, Wang AH, Oldfield E. A cholesterol biosynthesis inhibitor blocks Staphylococcus aureus virulence. Science. 2008;319:1391–4. doi: 10.1126/science.1153018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandes Rodrigues JC, Concepcion JL, Rodrigues C, Caldera A, Urbina JA, de Souza W. In vitro activities of ER-119884 and E5700; two potent squalene synthase inhibitors, against Leishmania amazonensis: antiproliferative, biochemical, and ultrastructural effects. Antimicrob Agents Antimicrob Agents Chemother. 2008;52:4098–114. doi: 10.1128/AAC.01616-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sealey-Cardona M, Cammerer S, Jones S, Ruiz-Perez LM, Brun R, Gilbert IH, Urbina JA, Gonzalez-Pacanowska D. Kinetic characterization of squalene synthase from Trypanosoma cruzi: selective inhibition by quinuclidine derivatives. Antimicrob Agents Chemother. 2007;51:2123–9. doi: 10.1128/AAC.01454-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Urbina JA, Concepcion JL, Caldera A, Payares G, Sanoja C, Otomo T, Hiyoshi H. In vitro and in vivo activities of E5700 and ER-119884; two novel orally active squalene synthase inhibitors, against Trypanosoma cruzi. Antimicrob Agents Chemother. 2004;48:2379–87. doi: 10.1128/AAC.48.7.2379-2387.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin FY, Liu CI, Liu YL, Zhang Y, Wang K, Jeng WY, Ko TP, Cao R, Wang AH, Oldfield E. Mechanism of action and inhibition of dehydrosqualene synthase. Proc Natl Acad Sci U S A. 2010;107:21337–42. doi: 10.1073/pnas.1010907107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rondeau JM, Bitsch F, Bourgier E, Geiser M, Hemmig R, Kroemer M, Lehmann S, Ramage P, Rieffel S, Strauss A, Green JR, Jahnke W. Structural basis for the exceptional in vivo efficacy of bisphosphonate drugs. ChemMedChem. 2006;1:267–73. doi: 10.1002/cmdc.200500059. [DOI] [PubMed] [Google Scholar]
- 10.Aaron JA, Lin X, Cane DE, Christianson DW. Structure of epi-isozizaene synthase from Streptomyces coelicolor A3(2), a platform for new terpenoid cyclization templates. Biochemistry (Mosc) 2010;49:1787–97. doi: 10.1021/bi902088z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gu P, Ishii Y, Spencer TA, Shechter I. Function-structure studies and identification of three enzyme domains involved in the catalytic activity in rat hepatic squalene synthase. J Biol Chem. 1998;273:12515–25. doi: 10.1074/jbc.273.20.12515. [DOI] [PubMed] [Google Scholar]
- 12.Valdar WS. Scoring residue conservation. Proteins. 2002;48:227–41. doi: 10.1002/prot.10146. [DOI] [PubMed] [Google Scholar]
- 13.Urbina JA, Concepcion JL, Montalvetti A, Rodriguez JB, Docampo R. Mechanism of action of 4-phenoxyphenoxyethyl thiocyanate (WC-9) against Trypanosoma cruzi, the causative agent of Chagas’ disease. Antimicrob Agents Chemother. 2003;47:2047–50. doi: 10.1128/AAC.47.6.2047-2050.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cinque GM, Szajnman SH, Zhong L, Docampo R, Schvartzapel AJ, Rodriguez JB, Gros EG. Structure-activity relationship of new growth inhibitors of Trypanosoma cruzi. J Med Chem. 1998;41:1540–54. doi: 10.1021/jm970860z. [DOI] [PubMed] [Google Scholar]
- 15.Elhalem E, Bailey BN, Docampo R, Ujvary I, Szajnman SH, Rodriguez JB. Design, synthesis, and biological evaluation of aryloxyethyl thiocyanate derivatives against Trypanosoma cruzi. J Med Chem. 2002;45:3984–99. doi: 10.1021/jm0201518. [DOI] [PubMed] [Google Scholar]
- 16.Igarashi K, Watari F, Aida K. Hydrogen bonding between phenol and alkyl thiocyanates. Spectrochim Acta A. 1969;25:1743. [Google Scholar]
- 17.Rivers EC, Mancera RL. New anti-tuberculosis drugs in clinical trials with novel mechanisms of action. Drug Discov Today. 2008;13:1090–8. doi: 10.1016/j.drudis.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Cammerer SB, Jimenez C, Jones S, Gros L, Lorente SO, Rodrigues C, Rodrigues JC, Caldera A, Ruiz Perez LM, da Souza W, Kaiser M, Brun R, Urbina JA, Gonzalez Pacanowska D, Gilbert IH. Quinuclidine derivatives as potential antiparasitics. Antimicrob Agents Chemother. 2007;51:4049–61. doi: 10.1128/AAC.00205-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orenes Lorente S, Gomez R, Jimenez C, Cammerer S, Yardley V, de Luca-Fradley K, Croft SL, Ruiz Perez LM, Urbina J, Gonzalez Pacanowska D, Gilbert IH. Biphenylquinuclidines as inhibitors of squalene synthase and growth of parasitic protozoa. Bioorg Med Chem. 2005;13:3519–29. doi: 10.1016/j.bmc.2005.02.060. [DOI] [PubMed] [Google Scholar]
- 20.Onajole OK, Govender P, van Helden PD, Kruger HG, Maguire GE, Wiid I, Govender T. Synthesis and evaluation of SQ109 analogues as potential anti-tuberculosis candidates. Eur J Med Chem. 2010;45:2075–9. doi: 10.1016/j.ejmech.2010.01.046. [DOI] [PubMed] [Google Scholar]
- 21.The PyMol molecular graphic system. Vol. 1.3. Schrödinger, LLC; [Google Scholar]
- 22.Molecular Operating Environment (MOE) 2009.10. Chemical Computing Group, Inc; [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.