Abstract
CTLA-4 blockade has demonstrated antitumor efficacy in human clinical trials. The antitumor mechanism is presumably mediated in part by the expansion of tumor-specific T cells. Androgen deprivation, the cornerstone of treatment for patients with metastatic prostate cancer, has been shown to elicit prostate tissue apoptosis and lymphocytic inflammation. We hypothesized that treatment with androgen deprivation, followed by an anti-CTLA-4 antibody, could augment a tumor-specific immune response elicited by androgen deprivation. We report here the results of a phase I trial evaluating a humanized monoclonal antibody targeting CTLA-4, CP-675,206 (tremelimumab), in combination with androgen deprivation using an antiandrogen. Eligible patients were those with PSA-recurrent prostate cancer after primary surgery and/or radiation therapy, not previously treated with androgen deprivation, and without radiographic evidence of metastatic disease. Subjects were treated in two cycles, 3 months apart, in which they received bicalutamide 150 mg daily days 1–28 and tremelimumab on day 29. The primary endpoint of the trial was safety. Secondary endpoints included measures of PSA kinetics and identification of a maximum tolerated dose. Eleven patients were enrolled and completed at least 1 year of follow-up. Dose-limiting toxicities included grade 3 diarrhea and skin rash. No favorable changes in PSA doubling time were observed in a period shortly after completing treatment; however, three patients experienced a prolongation in PSA doubling time detectable several months after completing treatment. The identification of delayed, prolonged favorable changes in serum PSA suggests that future studies could explore this combination in studies evaluating time to disease progression.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-011-1193-1) contains supplementary material, which is available to authorized users.
Keywords: Tremelimumab, Anti-CTLA-4 monoclonal antibody, Bicalutamide, Prostate cancer, Clinical trial
Introduction
Prostate cancer is the most common tumor among men, and the second leading cause of male cancer-related death in the United States [1]. Despite advances in screening and early detection, over 30,000 men are estimated to die in the United States as a result of prostate cancer in 2011 [1]. Treatment with surgery and/or radiation is effective for presumed organ-confined disease; however, approximately one-third of these patients will have recurrent and/or metastatic disease at 10 years [2]. A retrospective review of patients with prostate cancer treated with prostatectomy demonstrated that with evidence of a rise in serum PSA after definitive therapy, so-called “stage D0” disease, patients developed radiographically apparent metastatic disease within a median of 8 years [3]. In this situation, the rate of rise of the serum PSA blood test (PSA doubling time, PSA DT) may be the most important prognostic indicator. Several retrospective reports have highlighted that patients with rapid PSA DT in this stage of disease have a markedly shorter time to the development of metastases and death [4–7]. Androgen deprivation (AD) is often used in this situation; however, there is no consensus as to the optimal time for beginning this therapy. Given the side effects associated with AD, surveillance, with periodic radiographic imaging to determine when to initiate therapy once metastases develop, is also a standard approach. Consequently, this clinical stage D0 disease represents a high-risk population of patients, particularly those with rapid PSA DT, for whom there is no standard therapy. Given the prevalence of this stage of disease, new treatments capable of delaying disease progression, and without the side effects associated with continuous AD, are desirable.
Immune-based therapies theoretically have the potential to eradicate micrometastatic disease and prevent the progression from limited-stage disease to metastatic disease, or to at least slow this progression. Within the last year, two immune-based therapies have been approved by FDA as standard therapies for the treatment of cancer. One autologous vaccine product, sipuleucel-T, was approved as a therapy for patients with advanced, castrate-resistant metastatic prostate cancer [8]. This therapy aims to elicit immune responses to the prostate-specific prostatic acid phosphatase (PAP) tumor antigen. Another therapy, ipilimumab, was approved as a therapy for patients with metastatic melanoma [9]. Ipilimumab is a monoclonal antibody that blocks the action of the cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) regulatory receptor on the surface of T cells. CTLA-4 competes with CD28 for the binding of CD80 or CD86 on professional antigen-presenting cells and provides a suppressive signal blocking T-cell activation and proliferation [10]. Blockade of CTLA-4 has been shown in several murine models to enhance antitumor immunity and to enhance T-cell immunity induced specifically by antitumor vaccines [11–13]. Ipilimumab was approved as a monotherapy for the treatment of metastatic melanoma on the basis of improved survival for patients with treatment-refractory disease [9]. A more recent study has shown a similar benefit in patients with previously untreated metastatic melanoma [14]. Clinical trials have also been conducted in patients with prostate cancer, including studies with ipilimumab alone or in combination with vaccines or GM-CSF, all demonstrating some evidence of efficacy [15–17]. At the time of this writing, a randomized, double-blind, phase III trial is currently underway evaluating ipilimumab versus placebo in patients with early metastatic, castrate-resistant prostate cancer (NCT01057810). Consequently, the further evaluation of CTLA-4-blocking antibodies alone, or in combination with other immune-active therapies, is a logical and promising direction for future anticancer therapies, and prostate cancer in particular.
The primary treatment for patients with metastatic prostate cancer is AD, either by surgical castration or “chemical” castration with gonadotropin-releasing hormone (GnRH) agonists/antagonists and/or nonsteroidal antiandrogens. It has been known for several decades from studies in rats that castration leads to prostate tissue involution and inflammation [18, 19]. Sandford et al. [20] identified that castration of rats followed by intermittent testosterone replacement resulted in sequential waves of prostate tissue apoptosis and regrowth. Hormonally induced prostate inflammation has been used as a model for the study of human non-infectious prostatitis [18, 21–23]. Furthermore, adoptive transfer of lymphocytes from treated animals demonstrated that this prostatitis is an autoimmune process that could be transferred to naïve animals [23]. Several groups have demonstrated that AD also elicits apoptosis and inflammation in normal and malignant human prostate tissue [24, 25]. In particular, Mercader and colleagues reported that short-term AD elicits prostate tissue lymphocytic inflammation dominated by an oligoclonal CD4+ T-cell response [26]. We have further demonstrated that AD in patients leads to increased circulating naïve T cells and elicits IgG responses to proteins expressed in prostate tissue [27]. Drake and colleagues have demonstrated that AD could augment antitumor immune responses, resulting from a whole cell prostate cancer vaccine in a murine model of prostate cancer [28]. A clinical trial using nilutamide followed by a viral vaccine targeting PSA has similarly suggested an immunological and clinical benefit to a combination of AD with immunotherapy [29, 30]. Together, these findings have suggested that prostate cancer immunotherapies might be most effective in the setting of patients being concurrently treated with AD, and/or that AD might be used to elicit a tissue-disruptive response eliciting prostate inflammation/destruction that could be further augmented with immunomodulatory agents [31].
The current study was designed to evaluate the safety of combining treatment with AD and an anti-CTLA-4 monoclonal antibody as treatment for patients with stage D0 prostate cancer. We hypothesized that short-term AD would elicit prostate cancer-associated, T-cell-mediated tissue destruction that might be augmented with an immunomodulatory monoclonal antibody blocking CTLA-4, and that this approach might have therapeutic benefit in patients with recurrent prostate cancer. Patients were treated with high-dose bicalutamide (150 mg per day) for days 1–28 in each of two 3-month cycles. Bicalutamide was used as an alternative to standard LHRH agonist treatment, given that it does not result in long-term and/or variable testosterone depletion. CP-275,206 (tremelimumab, anti-CTLA-4 monoclonal antibody, Pfizer Corporation) was administered on day 29 of each cycle in a dose-escalation design. Endpoints of the study included safety/toxicity assessment, immune response evaluation, and changes in PSA kinetic parameters as measured by effects on PSA DT.
Materials and methods
Study agent and regulatory information
CP-675,206 (tremelimumab) is a humanized monoclonal IgG2 antibody specific for CTLA-4 and was provided by Pfizer Corporation. The study protocol was reviewed and approved by the Institutional Review Board of the University of Wisconsin and FDA, and all patients gave written informed consent for participation.
Patient population
Male patients with a histological diagnosis of adenocarcinoma of the prostate and biochemical (serum PSA) recurrence after definitive surgery and/or radiation therapy were eligible, provided there was no evidence of suspected lymph node, bone, or visceral metastatic disease on bone scans or CT scans. Patients could not have been previously treated with AD therapy, including LHRH agonist or nonsteroidal antiandrogens. Inclusion criteria required that patients have an Eastern Cooperative Oncology Group performance score of <2 and normal bone marrow, liver, and renal function as defined by a WBC ≥3,000/μl, hematocrit ≥30%, platelet count ≥100,000/μl, total bilirubin ≤2.0 mg/dl, and serum creatinine <1.5 mg/dl or a creatinine clearance ≥60 ml/min. Patients were excluded if they had been treated with immunosuppressive therapy, including chemotherapy, corticosteroids, or extensive radiation therapy, within 6 months of study entry, or were on medications with possible anticancer or hormonal effects. All patients were also required to have at least four serum PSA values collected over a 3- to 6-month period of time prior to study entry, all obtained from the same clinical laboratory, to determine a pretreatment PSA DT.
Study design
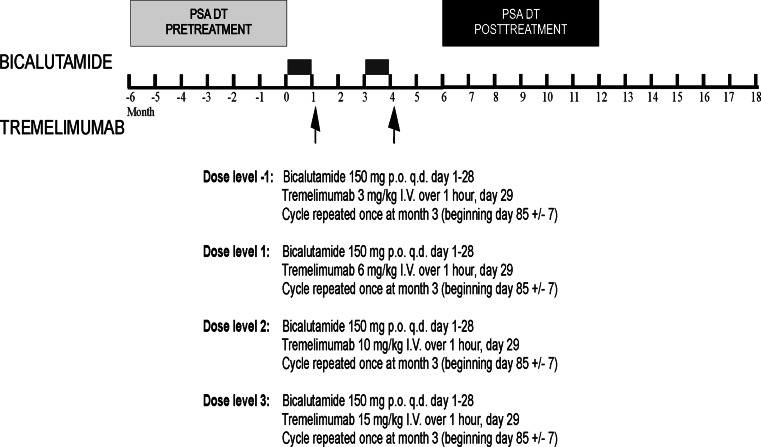
The study was an open-label, dose-escalation, single-institution phase I trial using a dose-escalation schedule with sequential cohorts receiving increasing doses of tremelimumab in combination with bicalutamide (Fig. 1). Given previous clinical trial experience with tremelimumab in other patient populations identifying potential adverse events, and in order to gather more biological response data and identify potential adverse events distinct for the combination of tremelimumab in combination with bicalutamide, six subjects were accrued per dose level. Escalation to the next dose level was permitted if <2 dose-limiting toxicities (DLT) were observed at the preceding dose level. A DLT was defined as any adverse event ≥grade 3 occurring within 28 days of first treatment with tremelimumab and given an attribution of at least possibly related to this agent. The MTD was defined as the dose level preceding a level at which more than one DLT was observed. Adverse events attributed to treatment with bicalutamide were collected and used for dose modification, but were not used for defining DLT.
Fig. 1.
Study schema and dose treatment level. Shown is the treatment and surveillance schema, and planned dose treatment levels
Study procedures
Patients were treated in two 3-month cycles with bicalutamide administered orally, 150 mg per day, for days 1–28 of each cycle, and tremelimumab administered intravenously on day 29 of each cycle. The planned dose of tremelimumab ranged from 3–10 mg/kg and was given as a 60-min infusion without premedication (Table 1). Patients were then followed with surveillance only, and treatment/surveillance was continued until one of the following occurred: (1) radiographic disease progression, (2) intercurrent illness preventing further administration, (3) unacceptable adverse events, (4) patient decision to withdraw from study, or (5) physician discretion that other therapies were warranted. All patients receiving at least one dose of tremelimumab were considered evaluable for adverse event assessment.
Table 1.
Patient demographics
| N | Dose level 1 (N = 5) | Dose level −1 (N = 6) | Combined (N = 11) | |
|---|---|---|---|---|
| Age | ||||
| Range | 11 |
65.2 ± 9.5 55–77 |
64.5 ± 4.2 59–71 |
64.8 ± 6.7 55–77 |
| Race | ||||
| AA | 1 | 0% | 17% | 9% |
| Caucasian | 10 | 100% | 83% | 91% |
| BSA (m2) | 11 | 2.14 ± 0.29 | 2.07 ± 0.10 | 2.10 ± 0.2 |
| ECOG PS | ||||
| 0 | 10 | 80% | 100% | 91% |
| 1 | 1 | 20% | 0% | 9% |
| Gleason score | ||||
| ≤6 | 4 | 1 | 3 | 4 |
| 7 | 4 | 2 | 2 | 4 |
| 8 | 3 | 2 | 1 | 3 |
| Primary therapy | ||||
| Surgery | 9 | 4 | 5 | 9 |
| Radiation | 2 | 1 | 1 | 2 |
| Salvage therapy | ||||
| Radiation | 4 | 4 | 0 | 4 |
| Pretreatment PSA (ng/ml) median (range) | 11 | 8.2 (2.5–29) | 4.5 (2.3–73) | 5.9 (2.3–73) |
| Pretreatment PSA DT (months) median (range) | 11 | 6.3 (3.8–10.2) | 6.7 (2.7–11.3) | 6.3 (2.7–11.3) |
Adverse event monitoring
All patients were evaluated monthly during treatment up to month 5 and then quarterly for 1 year after treatment with the last dose of tremelimumab, for symptomatic evidence of adverse events. Blood tests included complete blood counts, serum electrolytes, creatinine, alkaline phosphatase, glucose, amylase, TSH, and liver function tests. In addition, urinalysis and antinuclear antibody testing were performed prior to study and at study conclusion. Patients were evaluated by a physician at least monthly. All adverse events were graded according to the National Cancer Institute Common Terminology Criteria Grading System, version 3, and assigned an attribution of unrelated, unlikely, possible, probable, or definite in relation to treatment with bicalutamide or tremelimumab.
Clinical response evaluation
Clinical response was not the primary endpoint of this study. However, all subjects were assessed with CT of the abdomen and pelvis and bone scintigraphy within 4 weeks prior to the first dose of bicalutamide, and at yearly intervals or as clinically indicated. Serum PSA was evaluated monthly after beginning the treatment. PSA DT was calculated using all serum PSA values available from the same clinical laboratory for the specified period and using a minimum of four PSA values by the formula ln(2)/b, where b denotes the least square estimator of the linear regression model of the log-transformed PSA values on time. For the pretreatment PSA DT, a period of 4–6 months was used prior to treatment, up to and including day 1 of treatment. The posttreatment PSA DT was determined using all PSA values from month 6 to 12 (or off study if off study prior to month 12), beginning 2 months after completing the treatment with bicalutamide, and additionally from month 12 to 18 for those individuals remaining in surveillance and not being treated with other therapies for prostate cancer.
Immunological evaluation
Serum was collected prior to treatment, and at months 1, 3, 4, 5, and off study for the evaluation of IgG responses. Sera were stored at −80°C in aliquots until use. Peripheral blood mononuclear cells were collected prior to treatment and at month 5 for T-cell analysis. Phage immunoblot was performed to detect IgG responses to specific antigens as we have previously described, using lambda phage encoding 126 unique antigens previously identified as prostate-associated antigens [32–34]. Membranes were scanned, and the digital format was assessed visually, with individual plaques scored positive by independent observers, blinded to the treatment, timing of sample acquisition and membrane layout, as previously reported [32–35]. All of the membranes were scored by the same observers at the same time. Heatmap Builder software (Version 1.1, Stanford University) was used to generate heatmaps displaying changes (gain, loss, or no change) of antibody immune responses following treatment. Confirmatory enzyme-linked immunosorbent assay (ELISA) studies were performed to evaluate IgG specific for PSA or PAP as previously reported [36].
Statistical methods
Demographical variables were summarized by dose level in terms of frequencies and percentages for categorical variables and means ± SD for variables measured on a continuous scale. PSA doubling times were summarized in terms of medians and ranges. Absolute changes in PSA DT from pretreatment (month −6 to baseline) to posttreatment (month 6–12) and follow-up (month 12–18) were evaluated using a nonparametric Wilcoxon signed rank test and displayed graphically using waterfall plots.
Results
Patient population
As shown in Table 1, 11 patients were enrolled on this study between September 2008 and September 2009 at the University of Wisconsin Carbone Cancer Center. The median age for all patients was 63 years (range 55–77 years). The median PSA DT prior to treatment was 6.3 months (range 2.7–10.2 months), and the median pretreatment serum PSA was 5.9 ng/ml (range 2.3–73 ng/ml).
Course of study
Patients were assigned to defined dose levels as shown in Fig. 1. Three dose levels were initially planned, and accrual began at dose level 1 (6 mg/kg tremelimumab). The first patient on study experienced grade 3 diarrhea 13 days after the first dose of tremelimumab. While this episode resolved within 24 h and did not recur with retreatment in cycle 2, the possibility of its relation to tremelimumab treatment could not be excluded, defining this event as a DLT. At this same dose level, patient #5 experienced a grade 3 rash on day 20 after the first dose of tremelimumab. This second DLT resulted in the first dose level closing, and subsequent patients being enrolled at dose level −1 (3 mg/kg tremelimumab). One patient (subject #6) in dose level −1 experienced a grade 3 colitis requiring hospitalization and discontinuation of treatment 22 days after his first dose of tremelimumab. No other DLTs were detected at this dose level in the remaining 5 subjects, defining the 3 mg/kg dose as the MTD for this trial. Patients were followed with clinical and laboratory examinations for 12 months after completing the last dose of tremelimumab to identify any possible long-term adverse events.
Adverse events
Table 2 shows all adverse events occurring in either course of treatment or up to 12-month follow-up that were felt to be at least possibly attributable to study treatment. In this table, the highest grade of an event per individual patient is recorded. As can be seen, this combination was generally well tolerated with the exception of the grade 3 adverse events described above. The major adverse events noted were low-grade constitutional symptoms (primarily fatigue), gastrointestinal symptoms (nausea, diarrhea, and abdominal pain), dermatologic reactions (rash and pruritus), and endocrine reactions (hot flashes and gynecomastia). Rash and gastrointestinal symptoms were expected events attributed to treatment with tremelimumab, having been previously observed in >10% of patients treated on other clinical trials [37, 38]. Endocrine reactions were attributed to bicalutamide as known adverse events. Of note, no unexpected adverse events were observed. Two subjects required steroid treatment for the management of adverse events (subject #5 for rash and subject #6 for colitis). Of the three individuals with grade 3 events, two were re-treated without recurrence of gastrointestinal symptoms or rash. No subject developed adverse events following the second infusion of tremelimumab. In addition, while the total number of subjects treated at each dose was low, there were no obvious differences in the frequency or severity of toxicities with respect to tremelimumab dose (Table 2).
Table 2.
Adverse events
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |||||
|---|---|---|---|---|---|---|---|---|
| 6 (mg/kg) | 3 (mg/kg) | 6 (mg/kg) | 3 (mg/kg) | 6 (mg/kg) | 3 (mg/kg) | 6 (mg/kg) | 3 (mg/kg) | |
| Gastrointestinal | ||||||||
| Diarrhea | 1 | 1 | 1 | |||||
| Constipation | 2 | |||||||
| Nausea | 2 | 1 | ||||||
| Mucositis/stomatitis | 1 | 1 | ||||||
| Colitis | 1 | |||||||
| Distension/bloating | 1 | 1 | ||||||
| Abdominal pain NOS | 1 | 1 | 1 | |||||
| Hemorrhage | 1 | |||||||
| Dermatology/skin | ||||||||
| Rash | 2 | 1 | 1 | |||||
| Pruritus/itching | 2 | 1 | ||||||
| Alopecia | 1 | |||||||
| Dry skin | 1 | |||||||
| Constitutional | ||||||||
| Fatigue | 1 | 4 | 1 | 1 | ||||
| Weight loss | 1 | |||||||
| Anorexia | 2 | |||||||
| Flu-like syndrome | 1 | |||||||
| Rigors/chills | 1 | |||||||
| Endocrine | ||||||||
| Hot flashes/flushes | 1 | 3 | ||||||
| Gynecomastia/breast pain | 3 | 3 | 1 | |||||
| Cardiovascular | ||||||||
| Hypotension | 1 | |||||||
| Neurology | ||||||||
| Dizziness | 1 | |||||||
| Metabolic/laboratory | ||||||||
| Amylase | 1 | |||||||
| Pulmonary | ||||||||
| Dyspnea | 1 | |||||||
| Ocular/visual | ||||||||
| Dry eyes | 1 | |||||||
This table shows all adverse events by grade and by treatment dose level (DL) that were believed to be at least possibly related to treatment. The numbers represent the number of patients (out of 5 or 6 per dose level) experiencing a particular event at any point during multiple cycles of treatment, with the highest grade reported for any single individual
Clinical response
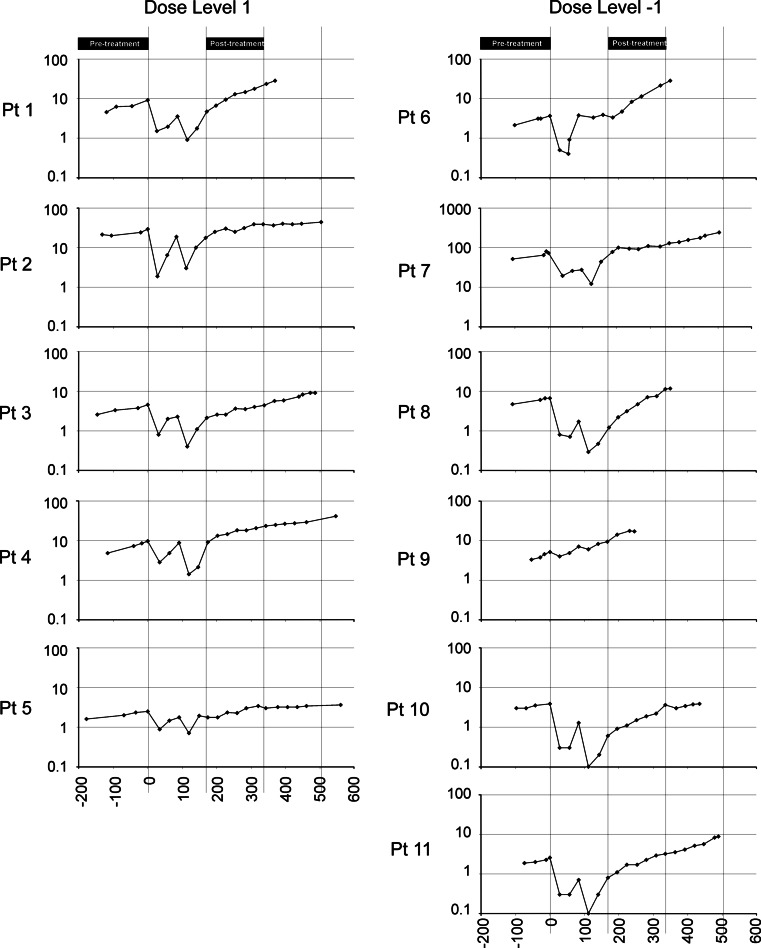
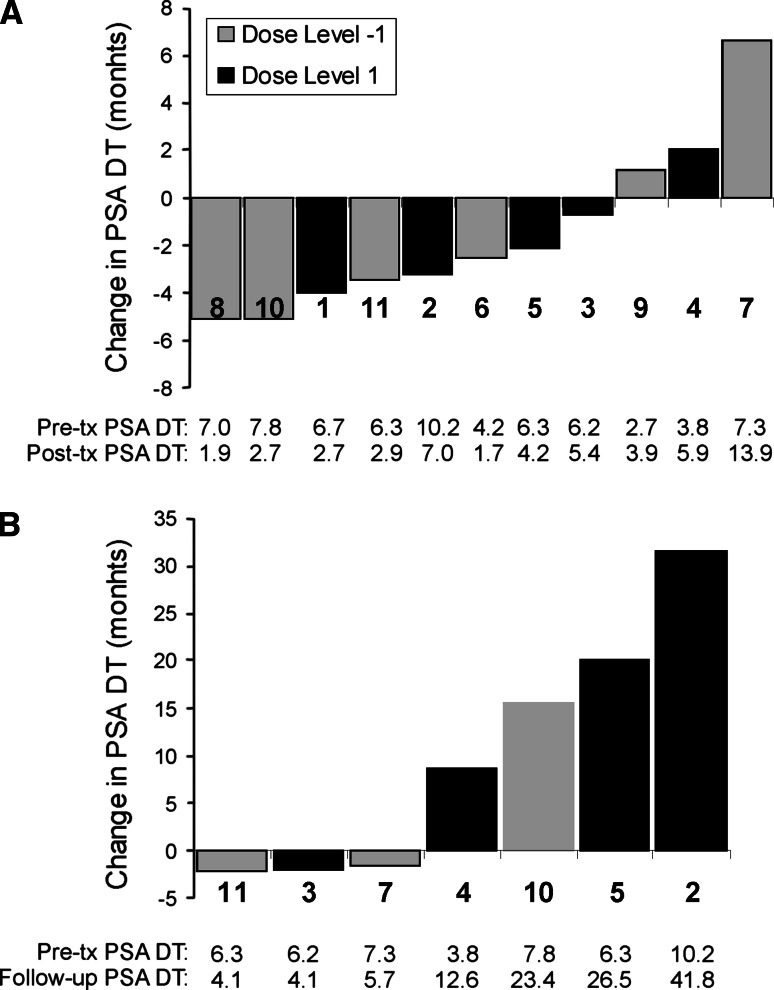
No subjects had symptomatic disease progression; however, two (patients 1 and 6) were found to have evidence of metastases at month 12 on scheduled imaging studies, and a third patient (#9) was found to have metastases at month 8 obtained due to rapidly rising PSA. These patients came off study at these time points, and a fourth (patient #8) came off surveillance at month 12 due to rapidly rising PSA. Serum PSA values were obtained at monthly intervals (Fig. 2). No significant increases in PSA doubling time were detected from pretreatment (month −6 to baseline) to posttreatment (month 6–12), as shown in Fig. 3a. In fact, a decrease in PSA doubling time was found for most patients during this time period. This was likely due to the proximity in time with treatment with bicalutamide, with the PSA measurements beginning 2 months after bicalutamide was stopped and the likely re-equilibration of serum testosterone during this period (compare Fig. 2). Consequently, as many patients continued to be followed in surveillance without other treatment intervention, a later posttreatment term period was also evaluated from months 12 to 18, and 8 months after the bicalutamide was discontinued. Three individuals were observed to have substantial increases in PSA doubling time in this later follow-up period (month 12–18), ranging from 23.4 to 41.8 months (Fig. 3b). The PSA doubling time changes from pre- to posttreatment, or pretreatment to later follow-up, were not statistically different when comparing dose level 1 with dose level −1.
Fig. 2.
Serum PSA levels. Serum PSA values are shown for all patients (Y axis = ng/ml, log scale) with respect to the day of treatment (X axis). Day 0 = day of first treatment with bicalutamide. The period of time used for determining PSA doubling times is shown
Fig. 3.
PSA doubling times. Waterfall plots showing absolute changes in PSA doubling times from pretreatment (month −6 to baseline) to posttreatment (month 6–12) (a) and from pretreatment (month −6 to baseline) to follow-up (month 12–18) (b). The numbers immediately below the columns indicate the individual subjects, corresponding to Fig. 2. In addition, below the columns are shown the calculated PSA doubling times (in months) for the pre-, posttreatment, or follow-up periods for each patient
Immunological analysis
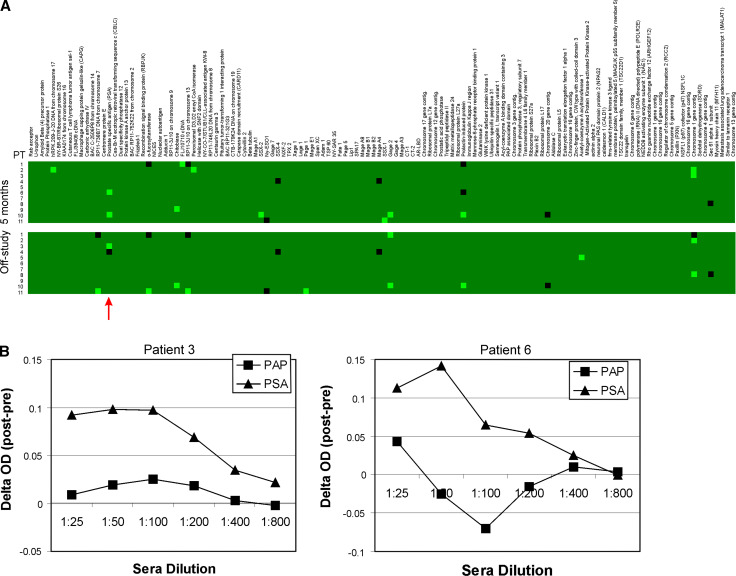
Sera obtained from patients prior to treatment, at 5, and at 12 months were evaluated for IgG responses to a panel of 126 prostate-associated antigens (example in Supplemental Figure 1). Overall, several IgG responses were detectable at 5 and 12 months after treatment that were not detectable pretreatment, suggesting the possibility that treatment elicited prostate antigen-associated immune responses (Fig. 4a). In particular, IgG responses to PSA were detectable in three of 11 subjects, responses which were confirmed in two individuals by indirect ELISA (Fig. 4b). Responses were also observed specific for several cancer-testis antigens (SSX-2, PAGE-1, and GAGE-2), and in three individuals to a protein encoded on chromosome 1. Two of three patients with increased PSA doubling times developed IgG responses to three or more antigens. However, given the small number of patients evaluated, no significant associations were detected in individuals experiencing a prolonged PSA DT with respect to the number of proteins recognized by IgG, or specific proteins recognized.
Fig. 4.
IgG responses to prostate-associated antigens. a Sera were obtained from all patients at month 5 (upper group) or at end of study (lower group) and used to identify IgG responses to a panel of prostate cancer-associated antigens as previously described [32, 33]. The green boxes represent gain of response compared with responses observed from sera obtained at pretreatment, and black boxes represent loss of response compared with responses observed from sera obtained at pretreatment. The red arrow highlights responses observed to PSA. b IgG responses to PSA and PAP were further evaluated by ELISA. Sera obtained from patient 3 (left plot) and 6 (right plot) obtained at pretreatment and at end of study were evaluated for IgG specific for PAP or PSA by ELISA. Shown is the ΔOD (posttreatment optical density–pretreatment optical density) at multiple sera concentrations specific for either protein, and normalized to background
Discussion
We report here a phase I dose-escalation trial using tremelimumab in combination with high-dose bicalutamide in patients with biochemically recurrent prostate cancer. In general, the adverse events observed (primarily rash and GI symptoms) were similar to what have been reported in other clinical trials with anti-CTLA-4 monoclonal antibodies [9, 37–39]. While this was a small trial, no new unanticipated adverse events were detected with this combination. It is perhaps noteworthy that the most significant toxicity observed, which required hospitalization and discontinuation of treatment, occurred in an individual treated once at the lowest dose. This is important, because while 3 mg/kg was defined by the protocol as the maximum tolerated dose in combination with bicalutamide, it is not clear that toxicities were truly more frequent or of greater magnitude at the higher dose. In fact, 15 mg/kg tremelimumab administered as a single agent on an every 3-month schedule had previously been observed to demonstrate fewer adverse events [38]. However, it is particularly relevant to note that our population of patients with early recurrent prostate cancer have no symptoms referable to their disease and generally have a long life expectancy. In this healthy population, there is even more of a need to minimize risk, as opposed to patients with more advanced disease and a shorter life expectancy in which more risk from therapy might be tolerated. Notwithstanding, while this treatment did carry risk, all adverse events observed were manageable, and the frequency and severity of adverse events were not higher than what has been observed with some other immune-based therapies, such as lenalidomide, being evaluated in this stage of disease [40].
While PSA doubling time itself may be associated with prostate cancer progression and death from prostate cancer [5, 7], changes in PSA doubling time following treatment have not been validated as a clinically useful endpoint. Nonetheless, because serum biomarkers such as PSA remain the only measure of disease in this population, it has been recommended by a consensus panel that changes in PSA kinetics be evaluated for treatments that might modify disease behavior [41, 42]. All patients in this trial had a pretreatment PSA doubling time <1 year, a situation in which the median time to developing radiographically apparent metastases is 19 months [5]. Three patients treated were observed to have an increase in PSA doubling time detectable many months after treatment. We do not believe this effect to have been mediated by bicalutamide alone, since this agent works as an antiandrogen and does not suppress the hypothalamic-gonadal axis as do LHRH agonists. Hence, it is not expected to have persistent antitumor effect after it has been discontinued. Immune-based treatments might be expected to demonstrate benefit over time, and hence, it is plausible that this change observed in a small number of subjects was due to the addition of tremelimumab. In fact, at the time of this writing, the two individuals treated at the higher dose of tremelimumab who experienced the greatest increase in PSA DT continue to have a stable prolonged PSA DT, without metastases, and have not received any additional therapy for prostate cancer for 2 years following the last dose of tremelimumab. The identification of increases in PSA doubling time suggests that this approach, using an anti-CTLA-4 monoclonal antibody in combination with bicalutamide, could be evaluated in future clinical trials specifically evaluating the time to radiographic disease progression as a clinical endpoint.
We and others have previously reported that various treatments for prostate cancer can elicit immune responses to prostate-associated proteins [27, 33, 34, 43]. In particular, PSA itself has been reported as a protein commonly recognized in patients with prostate cancer and prostatitis, and a protein to which immune responses are occasionally detected following treatment with immune-modulating treatments [33, 34, 36, 44, 45]. In the current study, we demonstrated that IgG responses were elicited to PSA in at least two of 11 individuals treated. At this point, it is unknown whether the generation of PSA-specific antibodies affected the serum PSA level, but this appears unlikely as the PSA DT did not appear to be affected in patients in whom antibodies to PSA were detected by ELISA (Figs. 2, 4). Moreover, IgG responses were elicited to several cancer-testis antigens, antigens not typically recognized following treatment with AD therapy alone [33]. The recognition of other cancer-testis antigens following treatment with ipilimumab suggests that this may have specifically been the result of treatment with tremelimumab [17, 46]. While these results do not demonstrate antitumor activity of the approach, they do suggest that treatment did result in immune responses to proteins expressed by prostate cancer, the proposed underlying mechanism of this combination approach. Future studies could thus similarly explore AD and CTLA-4 blockade with specific antigen-targeted approaches; antitumor vaccines targeting PSA and several cancer-testis antigens are already underway [47, 48].
In summary, we report that the combination of high-dose bicalutamide (150 mg daily) with tremelimumab was feasible in this small study of patients with early recurrent (clinical stage D0) prostate cancer. The combination did elicit expected adverse events, predominantly rash and gastrointestinal adverse events, as have been seen in other trials with anti-CTLA-4 monoclonal antibodies. Three of 11 patients experienced a delayed change in PSA doubling time, suggesting that future exploration of this combination could evaluate ongoing cyclic treatment in patients with high risk for recurrence, specifically evaluating the time to metastatic disease progression.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Figure 1: Example of High-Throughput Immunoblot Analysis: Sera were obtained from a subject (#2) pretreatment and at study conclusion and were used to probe a panel of phage-encoded antigens, as previously described [32, 33]. The red arrows indicate immunoreactive proteins recognized by IgG after treatment that were not present prior to treatment. Supplementary material 1 (TIFF 6,559 kb)
Acknowledgments
This work was supported by funding from Pfizer Corporation, for Heath A. Smith by DOD W81XWH-10-1-0495, and for Joshua M. Lang by NIH T32CA009614.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Oefelein MG, Smith ND, Grayhack JT, Schaeffer AJ, McVary KT. Long-term results of radical retropubic prostatectomy in men with high grade carcinoma of the prostate. J Urol. 1997;158:1460–1465. doi: 10.1016/S0022-5347(01)64243-5. [DOI] [PubMed] [Google Scholar]
- 3.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–1597. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 4.Freedland SJ, Humphreys EB, Mangold LA, Eisenberger M, Dorey FJ, Walsh PC, Partin AW. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294:433–439. doi: 10.1001/jama.294.4.433. [DOI] [PubMed] [Google Scholar]
- 5.Slovin SF, Wilton AS, Heller G, Scher HI. Time to detectable metastatic disease in patients with rising prostate-specific antigen values following surgery or radiation therapy. Clin Cancer Res. 2005;11:8669–8673. doi: 10.1158/1078-0432.CCR-05-1668. [DOI] [PubMed] [Google Scholar]
- 6.Lee AK, Levy LB, Cheung R, Kuban D. Prostate-specific antigen doubling time predicts clinical outcome and survival in prostate cancer patients treated with combined radiation and hormone therapy. Int J Radiat Oncol Biol Phys. 2005;63:456–462. doi: 10.1016/j.ijrobp.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 7.Freedland SJ, Humphreys EB, Mangold LA, Eisenberger M, Dorey FJ, Walsh PC, Partin AW. Death in patients with recurrent prostate cancer after radical prostatectomy: prostate-specific antigen doubling time subgroups and their associated contributions to all-cause mortality. J Clin Oncol. 2007;25:1765–1771. doi: 10.1200/JCO.2006.08.0572. [DOI] [PubMed] [Google Scholar]
- 8.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 9.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 12.Kwon ED, Hurwitz AA, Foster BA, Madias C, Feldhaus AL, Greenberg NM, Burg MB, Allison JP. Manipulation of T cell costimulatory and inhibitory signals for immunotherapy of prostate cancer. Proc Natl Acad Sci USA. 1997;94:8099–8103. doi: 10.1073/pnas.94.15.8099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hurwitz AA, Foster BA, Kwon ED, Truong T, Choi EM, Greenberg NM, Burg MB, Allison JP. Combination immunotherapy of primary prostate cancer in a transgenic mouse model using CTLA-4 blockade. Cancer Res. 2000;60:2444–2448. [PubMed] [Google Scholar]
- 14.Robert C, Thomas L, Bondarenko I, O’Day S, DJ M, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ, Davidson N, Richards J, Maio M, Hauschild A, Miller WH, Jr, Gascon P, Lotem M, Harmankaya K, Ibrahim R, Francis S, Chen TT, Humphrey R, Hoos A, Wolchok JD. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 15.Thompson RH, Allison JP, Kwon ED. Anti-cytotoxic T lymphocyte antigen-4 (CTLA-4) immunotherapy for the treatment of prostate cancer. Urol Oncol. 2006;24:442–447. doi: 10.1016/j.urolonc.2005.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Small EJ, Tchekmedyian NS, Rini BI, Fong L, Lowy I, Allison JP. A pilot trial of CTLA-4 blockade with human anti-CTLA-4 in patients with hormone-refractory prostate cancer. Clin Cancer Res. 2007;13:1810–1815. doi: 10.1158/1078-0432.CCR-06-2318. [DOI] [PubMed] [Google Scholar]
- 17.Fong L, Kwek SS, O’Brien S, Kavanagh B, McNeel DG, Weinberg V, Lin AM, Rosenberg J, Ryan CJ, Rini BI, Small EJ. Potentiating endogenous antitumor immunity to prostate cancer through combination immunotherapy with CTLA4 blockade and GM-CSF. Cancer Res. 2009;69:609–615. doi: 10.1158/0008-5472.CAN-08-3529. [DOI] [PubMed] [Google Scholar]
- 18.Helminen HJ, Ericsson JL. Ultrastructural studies on prostatic involution in the rat. Evidence for focal irreversible damage to epithelium, and heterophagic digestion in macrophages. J Ultrastruct Res. 1972;39:443–455. doi: 10.1016/S0022-5320(72)90112-8. [DOI] [PubMed] [Google Scholar]
- 19.Helminen HJ, Ericsson JL, Niemi M. Lysosomal changes during castration-induced prostatic involution in the rat. Acta Pathol Microbiol Scand [A] 1970;78:493–494. [PubMed] [Google Scholar]
- 20.Sandford NL, Searle JW, Kerr JF. Successive waves of apoptosis in the rat prostate after repeated withdrawal of testosterone stimulation. Pathology. 1984;16:406–410. doi: 10.3109/00313028409084731. [DOI] [PubMed] [Google Scholar]
- 21.Naslund MJ, Strandberg JD, Coffey DS. The role of androgens and estrogens in the pathogenesis of experimental nonbacterial prostatitis. J Urol. 1988;140:1049–1053. doi: 10.1016/s0022-5347(17)41924-0. [DOI] [PubMed] [Google Scholar]
- 22.Robinette CL. Sex-hormone-induced inflammation and fibromuscular proliferation in the rat lateral prostate. Prostate. 1988;12:271–286. doi: 10.1002/pros.2990120310. [DOI] [PubMed] [Google Scholar]
- 23.Seethalakshmi L, Bala RS, Malhotra RK, Austin-Ritchie T, Miller-Graziano C, Menon M, Luber-Narod J. 17 Beta-estradiol induced prostatitis in the rat is an autoimmune disease. J Urol. 1996;156:1838–1842. doi: 10.1016/S0022-5347(01)65548-4. [DOI] [PubMed] [Google Scholar]
- 24.Staack A, Kassis AP, Olshen A, Wang Y, Wu D, Carroll PR, Grossfeld GD, Cunha GR, Hayward SW. Quantitation of apoptotic activity following castration in human prostatic tissue in vivo. Prostate. 2003;54:212–219. doi: 10.1002/pros.10179. [DOI] [PubMed] [Google Scholar]
- 25.Civantos F, Soloway MS, Pinto JE. Histopathological effects of androgen deprivation in prostatic cancer. Semin Urol Oncol. 1996;14:22–31. [PubMed] [Google Scholar]
- 26.Mercader M, Bodner BK, Moser MT, Kwon PS, Park ES, Manecke RG, Ellis TM, Wojcik EM, Yang D, Flanigan RC, Waters WB, Kast WM, Kwon ED. T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Proc Natl Acad Sci USA. 2001;98:14565–14570. doi: 10.1073/pnas.251140998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morse MD, McNeel DG. Prostate cancer patients treated with androgen deprivation therapy develop persistent changes in adaptive immune responses. Hum Immunol. 2010;71:496–504. doi: 10.1016/j.humimm.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Drake CG, Doody AD, Mihalyo MA, Huang CT, Kelleher E, Ravi S, Hipkiss EL, Flies DB, Kennedy EP, Long M, McGary PW, Coryell L, Nelson WG, Pardoll DM, Adler AJ. Androgen ablation mitigates tolerance to a prostate/prostate cancer-restricted antigen. Cancer Cell. 2005;7:239–249. doi: 10.1016/j.ccr.2005.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arlen PM, Gulley JL, Todd N, Lieberman R, Steinberg SM, Morin S, Bastian A, Marte J, Tsang KY, Beetham P, Grosenbach DW, Schlom J, Dahut W. Antiandrogen, vaccine and combination therapy in patients with nonmetastatic hormone refractory prostate cancer. J Urol. 2005;174:539–546. doi: 10.1097/01.ju.0000165159.33772.5b. [DOI] [PubMed] [Google Scholar]
- 30.Madan RA, Gulley JL, Schlom J, Steinberg SM, Liewehr DJ, Dahut WL, Arlen PM. Analysis of overall survival in patients with nonmetastatic castration-resistant prostate cancer treated with vaccine, nilutamide, and combination therapy. Clin Cancer Res. 2008;14:4526–4531. doi: 10.1158/1078-0432.CCR-07-5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aragon-Ching JB, Williams KM, Gulley JL. Impact of androgen-deprivation therapy on the immune system: implications for combination therapy of prostate cancer. Front Biosci. 2007;12:4957–4971. doi: 10.2741/2441. [DOI] [PubMed] [Google Scholar]
- 32.Maricque BB, Eickhoff JC, McNeel DG. Antibody responses to prostate-associated antigens in patients with prostatitis and prostate cancer. Prostate. 2011;71:134–146. doi: 10.1002/pros.21229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith HA, Maricque BB, Eberhardt J, Petersen B, Gulley JL, Schlom J, McNeel DG. IgG responses to tissue-associated antigens as biomarkers of immunological treatment efficacy. J Biomed Biotech. 2011;2011:454861. doi: 10.1155/2011/454861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zabransky DJ, Smith HA, Thoburn CJ, Zahurak M, Keizman D, Carducci M, Eisenberger MA, McNeel DG, Drake CG, Antonarakis ES (2011) Lenalidomide modulates IL-8 and anti-prostate antibody levels in men with biochemically recurrent prostate cancer. Prostate (in press) [DOI] [PMC free article] [PubMed]
- 35.Dunphy EJ, Eickhoff JC, Muller CH, Berger RE, McNeel DG. Identification of antigen-specific IgG in sera from patients with chronic prostatitis. J Clin Immunol. 2004;24:492–501. doi: 10.1023/B:JOCI.0000040920.96065.5a. [DOI] [PubMed] [Google Scholar]
- 36.McNeel DG, Nguyen LD, Storer BE, Vessella R, Lange PH, Disis ML. Antibody immunity to prostate cancer-associated antigens can be detected in the serum of patients with prostate cancer. J Urol. 2000;164:1825–1829. doi: 10.1016/S0022-5347(05)67114-5. [DOI] [PubMed] [Google Scholar]
- 37.Chung KY, Gore I, Fong L, Venook A, Beck SB, Dorazio P, Criscitiello PJ, Healey DI, Huang B, Gomez-Navarro J, Saltz LB. Phase II study of the anti-cytotoxic T-lymphocyte-associated antigen 4 monoclonal antibody, tremelimumab, in patients with refractory metastatic colorectal cancer. J Clin Oncol. 2011;28:3485–3490. doi: 10.1200/JCO.2010.28.3994. [DOI] [PubMed] [Google Scholar]
- 38.Camacho LH, Antonia S, Sosman J, Kirkwood JM, Gajewski TF, Redman B, Pavlov D, Bulanhagui C, Bozon VA, Gomez-Navarro J, Ribas A. Phase I/II trial of tremelimumab in patients with metastatic melanoma. J Clin Oncol. 2009;27:1075–1081. doi: 10.1200/JCO.2008.19.2435. [DOI] [PubMed] [Google Scholar]
- 39.Hodi FS, Mihm MC, Soiffer RJ, Haluska FG, Butler M, Seiden MV, Davis T, Henry-Spires R, MacRae S, Willman A, Padera R, Jaklitsch MT, Shankar S, Chen TC, Korman A, Allison JP, Dranoff G. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci USA. 2003;100:4712–4717. doi: 10.1073/pnas.0830997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keizman D, Zahurak M, Sinibaldi V, Carducci M, Denmeade S, Drake C, Pili R, Antonarakis ES, Hudock S, Eisenberger M. Lenalidomide in nonmetastatic biochemically relapsed prostate cancer: results of a phase I/II double-blinded, randomized study. Clin Cancer Res. 2010;16:5269–5276. doi: 10.1158/1078-0432.CCR-10-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arlen PM, Bianco F, Dahut WL, D’Amico A, Figg WD, Freedland SJ, Gulley JL, Kantoff PW, Kattan MW, Lee A, Regan MM, Sartor O. Prostate specific antigen working group guidelines on prostate specific antigen doubling time. J Urol. 2008;179:2181–2185. doi: 10.1016/j.juro.2008.01.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scher HI, Eisenberger M, D’Amico AV, Halabi S, Small EJ, Morris M, Kattan MW, Roach M, Kantoff P, Pienta KJ, Carducci MA, Agus D, Slovin SF, Heller G, Kelly WK, Lange PH, Petrylak D, Berg W, Higano C, Wilding G, Moul JW, Partin AN, Logothetis C, Soule HR. Eligibility and outcomes reporting guidelines for clinical trials for patients in the state of a rising prostate-specific antigen: recommendations from the prostate-specific antigen working group. J Clin Oncol. 2004;22:537–556. doi: 10.1200/JCO.2004.07.099. [DOI] [PubMed] [Google Scholar]
- 43.Nesslinger NJ, Sahota RA, Stone B, Johnson K, Chima N, King C, Rasmussen D, Bishop D, Rennie PS, Gleave M, Blood P, Pai H, Ludgate C, Nelson BH. Standard treatments induce antigen-specific immune responses in prostate cancer. Clin Cancer Res. 2007;13:1493–1502. doi: 10.1158/1078-0432.CCR-06-1772. [DOI] [PubMed] [Google Scholar]
- 44.Alexander RB, Brady F, Leffell MS, Tsai V, Celis E. Specific T cell recognition of peptides derived from prostate-specific antigen in patients with prostate cancer. Urology. 1998;51:150–157. doi: 10.1016/S0090-4295(97)00480-9. [DOI] [PubMed] [Google Scholar]
- 45.Alexander RB, Brady F, Ponniah S. Autoimmune prostatitis: evidence of T cell reactivity with normal prostatic proteins. Urology. 1997;50:893–899. doi: 10.1016/S0090-4295(97)00456-1. [DOI] [PubMed] [Google Scholar]
- 46.Yuan J, Gnjatic S, Li H, Powel S, Gallardo HF, Ritter E, Ku GY, Jungbluth AA, Segal NH, Rasalan TS, Manukian G, Xu Y, Roman RA, Terzulli SL, Heywood M, Pogoriler E, Ritter G, Old LJ, Allison JP, Wolchok JD. CTLA-4 blockade enhances polyfunctional NY-ESO-1 specific T cell responses in metastatic melanoma patients with clinical benefit. Proc Natl Acad Sci USA. 2008;105:20410–20415. doi: 10.1073/pnas.0810114105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kantoff PW, Schuetz TJ, Blumenstein BA, Glode LM, Bilhartz DL, Wyand M, Manson K, Panicali DL, Laus R, Schlom J, Dahut WL, Arlen PM, Gulley JL, Godfrey WR. Overall survival analysis of a phase II randomized controlled trial of a poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28:1099–1105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gnjatic S, Altorki NK, Tang DN, Tu SM, Kundra V, Ritter G, Old LJ, Logothetis CJ, Sharma P. NY-ESO-1 DNA vaccine induces T-cell responses that are suppressed by regulatory T cells. Clin Cancer Res. 2009;15:2130–2139. doi: 10.1158/1078-0432.CCR-08-2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Example of High-Throughput Immunoblot Analysis: Sera were obtained from a subject (#2) pretreatment and at study conclusion and were used to probe a panel of phage-encoded antigens, as previously described [32, 33]. The red arrows indicate immunoreactive proteins recognized by IgG after treatment that were not present prior to treatment. Supplementary material 1 (TIFF 6,559 kb)