Abstract
BACKGROUND
Season of birth has been reported as a risk factor for food allergy, but the mechanisms by which it acts are unknown.
METHODS
Two populations were studied; 5862 children from the National Health and Nutrition Examination Survey (NHANES) III, 1514 well-characterized food allergic children from the Johns Hopkins Pediatric Allergy Clinic (JHPAC). Food allergy was defined as self report of an acute reaction to a food (NHANES), or as milk, egg and peanut allergy. Logistic regression compared fall or non-fall birth between (1) food allergic and non-allergic subjects in NHANES, adjusted for ethnicity, age, income and sex, and (2) JHPAC subjects and the general Maryland population. For NHANES, stratification by ethnicity and for JHPAC, eczema, was examined.
RESULTS
Fall birth was more common among food allergic subjects in both NHANES (OR: 1.91, 95%CI: 1.31–2.77) and JHPAC/Maryland (OR: 1.31, 95%CI: 1.18–1.47). Ethnicity interacted with season (OR 2.34, 95%CI 1.43–3.82 for Caucasians, OR 1.19, 95%CI 0.77–1.86 for non-Caucasians, p=0.04 for interaction), as did eczema (OR 1.47, 95%CI 1.29–1.67 with eczema, OR 1.00, 95%CI 0.80–1.23 without eczema, p=0.002 for interaction).
Conclusions
Fall birth is associated with increased risk of food allergy, and this risk is greatest among those most likely to have seasonal variation in vitamin D during infancy (Caucasians) and those at risk for skin barrier dysfunction (subjects with a history of eczema), suggesting that vitamin D and the skin barrier may be implicated in seasonal associations with food allergy.
Keywords: food allergy, season of birth, eczema, vitamin D
Introduction
Food allergy is common, affecting an estimated 3–8% of children in the US (1–3). Much of the etiology of food allergy is unclear, including the environmental factors which may contribute to risk. Variations in microbial exposures (4, 5), vitamin D exposure(5–8), and diet (9–14) have all been proposed as possible contributors to the development of food allergy. One environmental determinant of food allergy risk, the relative excess of fall (and sometimes winter) birthdays among food allergic patients, may shed light on these potential influences on food allergy. This association has been sporadically reported throughout the world, including the US (15–19), although not from nationally representative data in the US.
The reasons why fall or winter birth might be associated with food allergy are not known. It has been assumed that relative vitamin D deficiency mediates this relationship, although this has not been specifically confirmed (8). Another plausible theory is that decreased UV light, alone or through its effects on vitamin D, results in increased skin inflammation and compromised skin barrier at a critical point for the development of food allergy. Because Caucasians have more seasonal variation in vitamin D than darker skinned populations, a stronger association would be expected in this group if vitamin D is the mechanism. Here we explore these theories by (1) evaluating whether there is a relationship between season of birth and risk of food allergy in two large datasets, (2) examining whether associations with season vary by ethnic group, and (3) examining whether eczema interacts with season to affect the risk of food allergy.
Methods
Study populations
Two study populations were used: the National Health and Nutrition Examination Survey III (NHANES III) and the Johns Hopkins Pediatric Allergy Clinic (JHPAC).
NHANES III
NHANES III is a publicly available, complex, multi-stage survey of the non-institutionalized U.S. population aged 2 months and over that was conducted by the Centers for Disease Control and Prevention (CDC) between 1988 and 1994. Questionnaires were initially administered on visits to the subject’s home, with follow-up laboratory and physical examination in a mobile van. NHANES data collection was approved by the Institutional Review Board of the National Center for Health Statistics, Centers for Disease Control and Prevention, and informed consent was given by all participants. Young children, older persons, black persons and Mexican Americans were over sampled. For this analysis, subjects aged 17 or younger were included. Age, race/ethnicity, family income ratio (the ratio of the family’s income to the poverty level), and gender were included as possible confounders. Data on clinical history of eczema were not collected in this sample.
JHPAC
Data was retrospectively collected from patient charts on pediatric subjects (aged <21 at first clinic visit) as was previously described in three reports on the natural history of food allergy that focused on egg (20), milk (21) and peanut allergy (22). Some subjects overlapped between these studies, but they were only represented once here. Besides history of the relevant allergy, other data collected included sex, age, and history of asthma, allergic rhinitis and eczema. This population is overwhelmingly Caucasian, and race/ethnicity data were not systematically collected. In order to assess how the season of birth of subjects who presented to the allergy clinic with food allergy compared to the general Maryland population, data was taken from the US census’s birth records for Maryland for the years 1990–2005 (23).
Definition of allergy
NHANES III
Food allergy was defined as a positive answer to the following question: “within an hour after eating something has [the subject] ever had a severe reaction, such as itching all over, trouble breathing, flushing, hives, or swelling of the face or hands or feet?” Supporting IgE information was not available.
JHPAC
Milk and egg allergy were defined as a history of IgE mediated symptoms clearly associated with exposure to milk or egg with a positive skin prick test or serum specific IgE (>0.35 kUa/l) test to the respective food. Peanut allergy was defined as a history of IgE mediated symptoms clearly associated with exposure to peanut, or if no history of exposure to peanut, a peanut IgE >14 kUa/L without known tolerance to peanut. In addition, this sample only included subjects whose peanut allergy had not resolved during the observation period.
Statistical Methods
All analyses were performed using STATA/SE 11. For the NHANES data, logistic regression was performed to model the odds of history of food allergy according to season of birth. Based on exploratory analyses of the NHANES data, season of birth was defined as fall (September, October or November) or non-Fall. Ethnicity, categorized as Caucasian or non-Caucasian (including Hispanic, African-American and other), was assessed as a potential modifier of the association between season and food allergy. Analyses were done using the replicate weights provided with the dataset to minimize technical difficulties with small strata.
In order to assess whether the distribution of birthdays among those diagnosed with food allergy at the Johns Hopkins Pediatric Clinic deviated from expected, we estimated the expected distribution of birth months in the general population by collating data on the months of birth of all infants born in Maryland from 1990 to 2005. This time frame covered >95% of the births among our cohort (total range of clinic birth years 1979–2006). We then made the assumption that the clinic cohort was drawn from this larger cohort of Maryland births, and that all babies born in this cohort would have been at risk for presenting to the clinic, had they developed food allergy. Thus, the outcome of this analysis was the odds of being diagnosed with food allergy in this clinic according to season of birth. Because clinical data was not available for the general Maryland population, in order to determine if eczema diagnosis was related to season of birth among our participants, history of eczema was modeled as the outcome among our population of food allergic children, with season of birth as the predictor using logistic regression. A p-value of ≤0.05 was considered significant.
Results
Baseline Characteristics
NHANES III (Table I)
Table I.
Baseline Characteristics: NHANES III
| No history of food reaction (5578) | History of food reaction (284) | P value for comparison | |
|---|---|---|---|
| Median Age, (range) | 8.3 (8.2–8.5) | 9.7 (9.1–10.3) | 0.001 |
| Gender (% female) | 48.9% | 44.6% | 0.45 |
| Race/ethnicity (%white) | 63.8% | 68.8% | 0.25 |
| Income | 2.25 | 2.21 | 0.83 |
5862 children had non-missing data for all covariates and were included in the analysis. Table 1 shows their demographic characteristics. An estimated 7% of the population reported a history of a reaction to a food.
JHPAC (Table II)
Table II.
Baseline characteristics for JH Pediatric Allergy Clinic
| Number of subjects | 1514 |
| Median Year of Birth, range | 2000 (1979–2005) |
| Gender (% female) | 29% |
| History of Eczema | 70% |
| History of Asthma | 52% |
| History of Allergic rhinitis | 51% |
| Represented in peanut database | 51% |
| Represented in milk database | 56% |
| Represented in egg database | 26%% |
| Represented in all three databases | 5% |
1514 children were diagnosed with milk, egg and/or peanut allergy. Table 2 shows their demographic characteristics. The dates of birth of these children were compared to 1,114,701 births in Maryland between 1990 and 2005.
Being born in the fall increases the odds of food allergy
NHANES III (Table III, Fig 1)
Table III.
Relationship between fall birth and food allergy, stratified by ethnicity, among subjects in NHANES III.
| NHANES III | |||||
|---|---|---|---|---|---|
| OR (95% CI) for history of food reaction, p value | |||||
| Unadjusted | Adjusted for race, age, income and sex | Among non-Caucasians | Among Caucasians | Interaction p value | |
| Comparing fall to non-fall birth | 1.94 (1.34– 2.82), 0.001 | 1.91 (1.31–2.77), 0.001 | 1.19 (0.77– 1.86), 0.43 | 2.34 (1.43– 3.82), 0.001 | 0.04 |
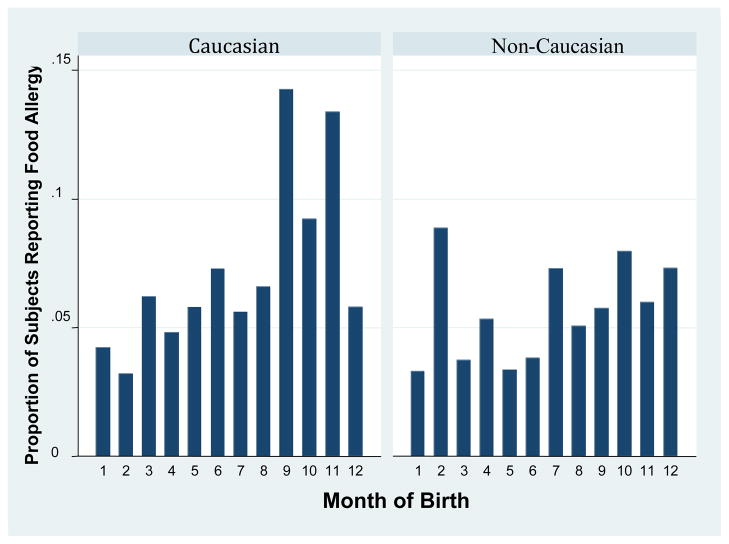
Figure 1.
Proportion of children in the NHANES dataset born in each month reporting food allergy, by month of birth and ethnic status.
In adjusted analyses, the odds of a history of food reaction was 1.91 times higher (95% for OR: 1.31–2.77) among those born in the fall. Neither history of doctor diagnosed asthma (OR 0.98, 95% CI 0.79–1.22, p=0.86) nor allergic rhinitis (OR 1.12, 95% CI 0.87–1.45, p=0.37) were associated with fall birth, although each disease had its own distinct seasonal trend. Asthma was less common among those born in the spring (OR 0.69, 95% CI 0.55–0.86, p=0.002), while allergic rhinitis tended to be more common among those born in the spring (OR 1.31, 95% CI 0.90–1.92, p=0.16), although this was not statistically significant.
JHPAC (Table IV, Fig 2)
Table IV.
Relationship between fall birth and food allergy, stratified by eczema history, among subjects in NHANES III.
| JH Allergy Clinic | ||||
|---|---|---|---|---|
| OR (95% CI) for diagnosis of milk, peanut or egg allergy at JH clinic, p value | ||||
| Overall | Among those without Eczema | Among those with Eczema | Interaction p value | |
| Comparing fall to non-fall birth | 1.31 (1.18–1.47), p<0.001 | 1.00 (0.80–1.23), p=0.97 | 1.47 (1.29–1.67), p<0.001 | 0.002 |
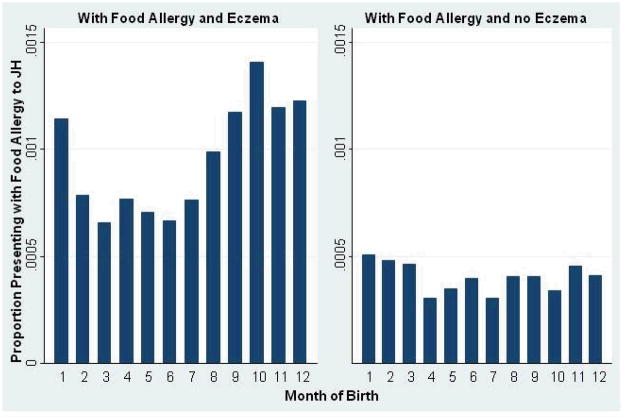
Figure 2.
Proportion of Maryland births from 1990–2005 presenting to the JH allergy clinic with food allergy, by eczema status.
The odds of being diagnosed with food allergy by our clinic were 1.32 times higher (95% CI for OR: 1.18–1.47) among those born in the fall.
This relationship is modified by ethnicity
NHANES III (Table III, Fig 1)
As is seen in table 3, ethnicity modified the relationship between season of birth and food allergy. Fall birth was only a risk factor for food allergy among Caucasians (OR 2.34, 95% CI 1.43–3.82, compared to OR 1.19, 95% CI 0.77–1.86 among non-Caucasians, p=0.04 for interaction). As described above, there was insufficient ethnic diversity in the Johns Hopkins Clinic to examine ethnicity in this group.
This relationship is only found among those with eczema
JHPAC (Table IV, Fig 2)
As is seen in table 3, fall birth was only a risk factor for food allergy among those with a history of eczema (OR 1.47, 95% CI 1.29–1.67, compared to OR 1.00, 95% CI 0.80–1.23 among those without eczema, p=0.002 for interaction). There was no interaction between a history of either asthma or allergic rhinitis and fall birth on food allergy risk (p=0.62, and p=0.98, respectively). Eczema data was not available for NHANES III.
Discussion
In this study, we examined relationships between season of birth and food allergy in two large populations; a nationally representative sample of more than 5000 children and a large population of food allergic patients from a referral clinic. We found a strong relationship between birth in the fall and odds of food allergy in both populations; those born in the fall were 30–90% more likely to develop food allergy than those born in other seasons. In addition, we found from the NHANES database that this association was restricted to Caucasians and that the association between food allergy and season was only present among those with a history of eczema in the clinic population where the patients’ atopic phenotype was more fully characterized.
Several hypotheses for the association between season and food allergy and other atopic diseases have been proposed, including pre- and post- natal allergen exposure (15, 16, 24–29), viral infection (30) and relative vitamin D deficiency (6, 17). Because the critical time period for development of most allergic diseases is not known, and because season is only a proxy for the underlying causal factor, the mechanism by which season of birth is associated with allergy is difficult to determine with certainty. In addition, it is unlikely that there is a single seasonal factor which underlies increased risk of all allergic diseases, given that the seasonal pattern and biology vary among the diseases. For asthma, elegant analyses have demonstrated that an increased risk of disease is conferred upon children born 4 months prior to the peak winter virus season, and that the timing of this risk varies with yearly variations in virus peak (30). For allergic rhinitis, increased prevalence has been reported in those born in during the pollen season among several populations (15, 16, 24–29), a finding attributed to the effects of early exposure to pollen allergens. For eczema, data are more sparse, with several studies showing no seasonal relationship (27, 31, 32), but one from Japan showing increased rates among those born in the fall (33). For food allergy, previous studies reported increased risk with fall/winter birth (15, 17, 19). Here, fall birth conferred a higher risk of food allergy than winter birth in both populations, although the difference was only significant in the NHANES population (data not shown). Because children born in the fall (and to a lesser extent, winter) potentially have less UV exposure and lower vitamin D in early life, and because vitamin D has widespread immune effects, vitamin D has been cited most commonly as the mechanism of seasonal variation in food allergy (8, 17–19).
The role of relative vitamin D deficiency in general in the development of allergic diseases is controversial. Epidemiologic studies have associated vitamin D deficiency with a wide range of allergic diseases, including asthma (34, 35) and food allergy/sensitization (7, 36), although some have suggested that vitamin D may actually increase the risk of allergic diseases (37–42). Recently the Institute of Medicine (United States) stated that there is insufficient evidence to recommend vitamin D supplementation for any non-bone related disease (43), and the role of vitamin D remains hotly contested.
Here we explored the hypothesis that vitamin D may be involved in the relationship between fall birth and food allergy by investigating how this relationship interacts with ethnicity. Because approximately 5–10 times more sun exposure is required to produce the same amount of vitamin D in African-Americans and other dark skinned minorities compared to Caucasians (44), seasonal variations in vitamin D may be less pronounced in darker skinned populations (45, 46). Thus, finding that fall birth was a risk factor for food allergy only in those with potentially high seasonal variation in infancy (i.e., Caucasians) is consistent with the theory that vitamin D is a mediator of the seasonal distribution of food allergy. However, other explanations, such as an increased genetic susceptibility among Caucasians for other reasons to the seasonal factor and the joint influence of other environmental differences between these populations, cannot be excluded.
We then examined whether the relationship varied according to the presence of eczema. Our finding that fall birth was a risk factor only among those with a history of eczema suggests a hypothesis that sun exposure in early life may protect against skin inflammation, thereby protecting against food allergy during a critical period in development. This theory is biologically plausible; the first 3–6 months of life are thought to be critical for future development of food allergy, with eczema that manifests during this period, but not others, associated with later food allergy (47). Although the hypothesis that food sensitization occurs through the skin remains controversial (48), evidence for this hypothesis has been mounting, including animal models of skin sensitization (49–55), human epidemiologic studies (56, 57), and, finally, new evidence that primary genetic defects in skin barrier are associated not only with eczema (58) but also with risk of peanut allergy (59). If impaired skin barrier leads to food allergy, then UV light and vitamin D are likely to modify this path. UV light has been shown to decrease the ease of both IgE and hapten-mediated sensitization through the skin in animal models (60–64), and cutaneous exposure to ovalbumin after UV exposure results in the generation of antigen specific T regulatory cells and the induction of systemic tolerance (65). Vitamin D, whether applied locally, or given systemically, also improves skin inflammation in both mouse and human models (66–69). Finally, our findings that decreased risk of food allergy associated with non-fall birth is only found in those who had impaired skin barrier at some point in childhood (i.e. had a history eczema) points to the pathway we propose. However, there are other pathways that could also be plausible, including alterations in the gut microbiome by season (8) that could potentially modify the relationship between eczema and food allergy.
There are several limitations to our analyses. First, the definition of food allergy may be imprecise in both of our populations. The NHANES III data did not contain laboratory tests to confirm the history of food allergy; NHANES 2005–6 did have IgE, but no clinical data, for food allergy, and did not have freely available season of birth data. In general, food allergy is over-reported (70), although the question used here to determine history of food allergy may have more specificity than other surveys and the overall prevalence of food allergy using this definition is only slightly higher than other estimates from other NHANES surveys that instead used food-specific IgE to estimate rates (2). In our clinic population, diagnosis was made by a combination of history and confirmatory testing, but not all subjects received a food challenge. Thus it is possible that some subjects were misclassified in both populations. However, we would expect that any misclassification of food allergy would be non-differential by season of birth, leading to a bias to the null in our analyses. Other limitations include that there was insufficient ethnic variability in the clinic population to confirm the interaction with Caucasian race/ethnicity and season that we found in NHANES. Similarly, this NHANES dataset had no data about history of eczema or other rashes, so we could not confirm our findings from the clinic population about eczema. Finally, for eczema, our data could be consistent with either the theory that season affects the risk of eczema, thus indirectly affecting the risk of food allergy, or that it directly affects the relationship between eczema and food allergy. Other studies with more detailed information about both eczema and food allergy in early life will be needed to answer this question.
This study represents the first time that season of birth has been tied to food allergy in a nationally representative sample of the US. In addition, we found that the seasonal association with food allergy was only seen among Caucasians and children with eczema. Based on these findings we propose a theory that fall birth increases the risk of food allergy by leading to decreased vitamin D during a critical time for development of food allergy, and further hypothesize that decreased vitamin D may increase the risk of food allergy by increasing the risk for skin barrier impairment during that critical time. More research during the early life period to test these hypotheses will be needed to determine the true mechanisms of the association of fall birth with food allergy.
Acknowledgments
Sources of Support: This research was made possible in part by NIH grant numbers 5T32-AI07007, and 1KL2RR025006-01 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp. The Eudowood foundation also supported this work.
Footnotes
Conflicts of Interest: None of the authors have relevant conflicts of interest.
Author Contributions: CAK designed the study, analyzed the data, wrote the manuscript and revised it. ECM contributed to the design of the study, analysis and interpretation of data, and revision. JHS, DLN and JS contributed to the design of the study, collected the data, and critically reviewed the manuscript. RDP contributed to the design, analysis and interpretation of the data and critically reviewed the manuscript. RAW contributed to the design, collection and interpretation of the data and critically reviewed the manuscript.
Contributor Information
Corinne A. Keet, Division of Allergy and Immunology, Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, MD.
Elizabeth C. Matsui, Division of Allergy and Immunology, Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, MD.
Jessica H. Savage, Division of Allergy and Clinical Immunology, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, MD
Dara L. Neuman-Sunshine, Division of Allergy and Clinical Immunology, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, MD
Justin Skripak, ENT and Allergy Associates, Hoboken, NJ.
Roger D. Peng, Department of Biostatistics, Johns Hopkins Bloomberg School of Public Health.
Robert A. Wood, Division of Allergy and Clinical Immunology, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, MD.
References
- 1.Gupta RS, Springston EE, Warrier MR, Smith B, Kumar R, Pongracic J, et al. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics. 2011;128(1):e9–17. doi: 10.1542/peds.2011-0204. [DOI] [PubMed] [Google Scholar]
- 2.Liu AH, Jaramillo R, Sicherer SH, Wood RA, Bock SA, Burks AW, et al. National prevalence and risk factors for food allergy and relationship to asthma: results from the National Health and Nutrition Examination Survey 2005–2006. J Allergy Clin Immunol. 2010;126(4):798–806. e13. doi: 10.1016/j.jaci.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyce JA, Assa’a A, Burks AW, Jones SM, Sampson HA, Wood RA, et al. Guidelines for the diagnosis and management of food allergy in the United States: summary of the NIAID-Sponsored Expert Panel Report. Nutrition. 2011;27(2):253–67. doi: 10.1016/j.nut.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Okada H, Kuhn C, Feillet H, Bach JF. The ‘hygiene hypothesis’ for autoimmune and allergic diseases: an update. Clin Exp Immunol. 2010;160(1):1–9. doi: 10.1111/j.1365-2249.2010.04139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ly NP, Litonjua A, Gold DR, Celedon JC. Gut microbiota, probiotics, and vitamin D: interrelated exposures influencing allergy, asthma, and obesity? J Allergy Clin Immunol. 2011;127(5):1087–94. doi: 10.1016/j.jaci.2011.02.015. quiz 95–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mullins RJ, Camargo CA. Latitude, Sunlight, Vitamin D, and Childhood Food Allergy/Anaphylaxis. Curr Allergy Asthma Rep. 2011 doi: 10.1007/s11882-011-0230-7. [DOI] [PubMed] [Google Scholar]
- 7.Sharief S, Jariwala S, Kumar J, Muntner P, Melamed ML. Vitamin D levels and food and environmental allergies in the United States: results from the National Health and Nutrition Examination Survey 2005–2006. J Allergy Clin Immunol. 2011;127(5):1195–202. doi: 10.1016/j.jaci.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vassallo MF, Camargo CA., Jr Potential mechanisms for the hypothesized link between sunshine, vitamin D, and food allergy in children. J Allergy Clin Immunol. 2010;126(2):217–22. doi: 10.1016/j.jaci.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 9.Koplin JJ, Osborne NJ, Wake M, Martin PE, Gurrin LC, Robinson MN, et al. Can early introduction of egg prevent egg allergy in infants? A population-based study. J Allergy Clin Immunol. 2010;126(4):807–13. doi: 10.1016/j.jaci.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 10.Hesselmar B, Saalman R, Rudin A, Adlerberth I, Wold A. Early fish introduction is associated with less eczema, but not sensitization, in infants. Acta Paediatr. 2010;99(12):1861–7. doi: 10.1111/j.1651-2227.2010.01939.x. [DOI] [PubMed] [Google Scholar]
- 11.Virtanen SM, Kaila M, Pekkanen J, Kenward MG, Uusitalo U, Pietinen P, et al. Early introduction of oats associated with decreased risk of persistent asthma and early introduction of fish with decreased risk of allergic rhinitis. Br J Nutr. 2010;103(2):266–73. doi: 10.1017/S0007114509991541. [DOI] [PubMed] [Google Scholar]
- 12.Wennergren G. What if it is the other way around? Early introduction of peanut and fish seems to be better than avoidance. Acta Paediatr. 2009;98(7):1085–7. doi: 10.1111/j.1651-2227.2009.01342.x. [DOI] [PubMed] [Google Scholar]
- 13.Greer FR, Sicherer SH, Burks AW. Effects of early nutritional interventions on the development of atopic disease in infants and children: the role of maternal dietary restriction, breastfeeding, timing of introduction of complementary foods, and hydrolyzed formulas. Pediatrics. 2008;121(1):183–91. doi: 10.1542/peds.2007-3022. [DOI] [PubMed] [Google Scholar]
- 14.Tarini BA, Carroll AE, Sox CM, Christakis DA. Systematic review of the relationship between early introduction of solid foods to infants and the development of allergic disease. Arch Pediatr Adolesc Med. 2006;160(5):502–7. doi: 10.1001/archpedi.160.5.502. [DOI] [PubMed] [Google Scholar]
- 15.Aalberse RC, Nieuwenhuys EJ, Hey M, Stapel SO. ‘Horoscope effect’ not only for seasonal but also for non-seasonal allergens. Clin Exp Allergy. 1992;22(11):1003–6. doi: 10.1111/j.1365-2222.1992.tb03028.x. [DOI] [PubMed] [Google Scholar]
- 16.Pyrhonen K, Laara E, Hiltunen L, Kaila M, Hugg T, Nayha S. Season of the first trimester of pregnancy predicts sensitisation to food allergens in childhood: a population-based cohort study from Finland. J Epidemiol Community Health. 2010 doi: 10.1136/jech.2009.105411. [DOI] [PubMed] [Google Scholar]
- 17.Vassallo MF, Banerji A, Rudders SA, Clark S, Camargo CA., Jr Season of birth and food-induced anaphylaxis in Boston. Allergy. 2010;65(11):1492–3. doi: 10.1111/j.1398-9995.2010.02384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vassallo MF, Banerji A, Rudders SA, Clark S, Mullins RJ, Camargo CA., Jr Season of birth and food allergy in children. Ann Allergy Asthma Immunol. 2010;104(4):307–13. doi: 10.1016/j.anai.2010.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mullins RJ, Clark S, Katelaris C, Smith V, Solley G, Camargo CA., Jr Season of birth and childhood food allergy in Australia. Pediatr Allergy Immunol. 2011;22(6):583–9. doi: 10.1111/j.1399-3038.2011.01151.x. [DOI] [PubMed] [Google Scholar]
- 20.Savage JH, Matsui EC, Skripak JM, Wood RA. The natural history of egg allergy. J Allergy Clin Immunol. 2007;120(6):1413–7. doi: 10.1016/j.jaci.2007.09.040. [DOI] [PubMed] [Google Scholar]
- 21.Skripak JM, Matsui EC, Mudd K, Wood RA. The natural history of IgE-mediated cow’s milk allergy. J Allergy Clin Immunol. 2007;120(5):1172–7. doi: 10.1016/j.jaci.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 22.Neuman-Sunshine DLEJA, Keet CA, Matsui EC, Peng RD, Lenehan PJ, Wood RA. The natural history of persistent peanut allergy. Ann Allergy. 2012 doi: 10.1016/j.anai.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention. National Center for Health Statistics. VitalStats. 2011 [updated 2011; cited]; Available from: http://www.cdc.gov/nchs/vitalstats.htm.
- 24.Graf N, Johansen P, Schindler C, Wuthrich B, Ackermann-Liebrich U, Gassner M, et al. Analysis of the relationship between pollinosis and date of birth in Switzerland. Int Arch Allergy Immunol. 2007;143(4):269–75. doi: 10.1159/000100572. [DOI] [PubMed] [Google Scholar]
- 25.Guerra S, Sherrill DL, Cottini M, Michetti G, Allegra L. On the association between date of birth and pollen sensitization: is age an effect modifier? Allergy Asthma Proc. 2002;23(5):303–10. [PubMed] [Google Scholar]
- 26.Kemp AS. Relationship between the time of birth and the development of immediate hypersensitivity to grass-pollen antigens. Med J Aust. 1979;1(7):263–4. doi: 10.5694/j.1326-5377.1979.tb112072.x. [DOI] [PubMed] [Google Scholar]
- 27.Saitoh Y, Dake Y, Shimazu S, Sakoda T, Sogo H, Fujiki Y, et al. Month of birth, atopic disease, and atopic sensitization. J Investig Allergol Clin Immunol. 2001;11(3):183–7. [PubMed] [Google Scholar]
- 28.Sibbald B, Rink E. Birth month variation in atopic and non-atopic rhinitis. Clin Exp Allergy. 1990;20(3):285–8. doi: 10.1111/j.1365-2222.1990.tb02685.x. [DOI] [PubMed] [Google Scholar]
- 29.Carosso A, Ruffino C, Bugiani M. The effect of birth season on pollenosis. Ann Allergy. 1986;56(4):300–3. [PubMed] [Google Scholar]
- 30.Wu P, Dupont WD, Griffin MR, Carroll KN, Mitchel EF, Gebretsadik T, et al. Evidence of a causal role of winter virus infection during infancy in early childhood asthma. Am J Respir Crit Care Med. 2008;178(11):1123–9. doi: 10.1164/rccm.200804-579OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arshad SH, Stevens M, Hide DW. The effect of genetic and environmental factors on the prevalence of allergic disorders at the age of two years. Clin Exp Allergy. 1993;23(6):504–11. doi: 10.1111/j.1365-2222.1993.tb03238.x. [DOI] [PubMed] [Google Scholar]
- 32.Schafer T, Przybilla B, Ring J, Kunz B, Greif A, Uberla K. Manifestation of atopy is not related to patient’s month of birth. Allergy. 1993;48(4):291–4. doi: 10.1111/j.1398-9995.1993.tb00731.x. [DOI] [PubMed] [Google Scholar]
- 33.Kuzume K, Kusu M. Before-birth climatologic data may play a role in the development of allergies in infants. Pediatr Allergy Immunol. 2007;18(4):281–7. doi: 10.1111/j.1399-3038.2006.00526.x. [DOI] [PubMed] [Google Scholar]
- 34.Keet CA, McCormack MC, Peng RD, Matsui EC. Age- and atopy-dependent effects of vitamin D on wheeze and asthma. J Allergy Clin Immunol. 2011;128(2):414–16. e5. doi: 10.1016/j.jaci.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Devereux G, Litonjua AA, Turner SW, Craig LC, McNeill G, Martindale S, et al. Maternal vitamin D intake during pregnancy and early childhood wheezing. Am J Clin Nutr. 2007;85(3):853–9. doi: 10.1093/ajcn/85.3.853. [DOI] [PubMed] [Google Scholar]
- 36.Camargo CA, Jr, Clark S, Kaplan MS, Lieberman P, Wood RA. Regional differences in EpiPen prescriptions in the United States: the potential role of vitamin D. J Allergy Clin Immunol. 2007;120(1):131–6. doi: 10.1016/j.jaci.2007.03.049. [DOI] [PubMed] [Google Scholar]
- 37.Hypponen E, Sovio U, Wjst M, Patel S, Pekkanen J, Hartikainen AL, et al. Infant vitamin d supplementation and allergic conditions in adulthood: northern Finland birth cohort 1966. Ann N Y Acad Sci. 2004;1037:84–95. doi: 10.1196/annals.1337.013. [DOI] [PubMed] [Google Scholar]
- 38.Back O, Blomquist HK, Hernell O, Stenberg B. Does vitamin D intake during infancy promote the development of atopic allergy? Acta Derm Venereol. 2009;89(1):28–32. doi: 10.2340/00015555-0541. [DOI] [PubMed] [Google Scholar]
- 39.Kull I, Bergstrom A, Melen E, Lilja G, van Hage M, Pershagen G, et al. Early-life supplementation of vitamins A and D, in water-soluble form or in peanut oil, and allergic diseases during childhood. J Allergy Clin Immunol. 2006;118(6):1299–304. doi: 10.1016/j.jaci.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 40.Wjst M. The vitamin D slant on allergy. Pediatr Allergy Immunol. 2006;17(7):477–83. doi: 10.1111/j.1399-3038.2006.00456.x. [DOI] [PubMed] [Google Scholar]
- 41.Wjst M. Allergy risk of vitamin D supplements has been described in various settings. J Allergy Clin Immunol. 2008;121(4):1065–6. doi: 10.1016/j.jaci.2008.01.020. author reply 6. [DOI] [PubMed] [Google Scholar]
- 42.Wjst M. Introduction of oral vitamin D supplementation and the rise of the allergy pandemic. Allergy Asthma Clin Immunol. 2009;5(1):8. doi: 10.1186/1710-1492-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53–8. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosen CJ. Clinical practice. Vitamin D insufficiency. N Engl J Med. 2011;364(3):248–54. doi: 10.1056/NEJMcp1009570. [DOI] [PubMed] [Google Scholar]
- 45.Bodnar LM, Simhan HN, Powers RW, Frank MP, Cooperstein E, Roberts JM. High prevalence of vitamin D insufficiency in black and white pregnant women residing in the northern United States and their neonates. J Nutr. 2007;137(2):447–52. doi: 10.1093/jn/137.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harris SS, Dawson-Hughes B. Seasonal changes in plasma 25-hydroxyvitamin D concentrations of young American black and white women. Am J Clin Nutr. 1998;67(6):1232–6. doi: 10.1093/ajcn/67.6.1232. [DOI] [PubMed] [Google Scholar]
- 47.Hill DJ, Hosking CS, de Benedictis FM, Oranje AP, Diepgen TL, Bauchau V. Confirmation of the association between high levels of immunoglobulin E food sensitization and eczema in infancy: an international study. Clin Exp Allergy. 2008;38(1):161–8. doi: 10.1111/j.1365-2222.2007.02861.x. [DOI] [PubMed] [Google Scholar]
- 48.Du Toit G, Lack G. Can food allergy be prevented? The current evidence. Pediatr Clin North Am. 2011;58(2):481–509. xii. doi: 10.1016/j.pcl.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 49.Dunkin D, Berin MC, Mayer L. Allergic sensitization can be induced via multiple physiologic routes in an adjuvant-dependent manner. J Allergy Clin Immunol. 2011 doi: 10.1016/j.jaci.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Naito S, Maeyama J, Mizukami T, Takahashi M, Hamaguchi I, Yamaguchi K. Transcutaneous immunization by merely prolonging the duration of antigen presence on the skin of mice induces a potent antigen-specific antibody response even in the absence of an adjuvant. Vaccine. 2007;25(52):8762–70. doi: 10.1016/j.vaccine.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 51.Oyoshi MK, Larson RP, Ziegler SF, Geha RS. Mechanical injury polarizes skin dendritic cells to elicit a T(H)2 response by inducing cutaneous thymic stromal lymphopoietin expression. J Allergy Clin Immunol. 2010;126(5):976–84. 84, e1–5. doi: 10.1016/j.jaci.2010.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spergel JM, Mizoguchi E, Brewer JP, Martin TR, Bhan AK, Geha RS. Epicutaneous sensitization with protein antigen induces localized allergic dermatitis and hyperresponsiveness to methacholine after single exposure to aerosolized antigen in mice. J Clin Invest. 1998;101(8):1614–22. doi: 10.1172/JCI1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Strid J, Hourihane J, Kimber I, Callard R, Strobel S. Disruption of the stratum corneum allows potent epicutaneous immunization with protein antigens resulting in a dominant systemic Th2 response. Eur J Immunol. 2004;34(8):2100–9. doi: 10.1002/eji.200425196. [DOI] [PubMed] [Google Scholar]
- 54.Kondo H, Ichikawa Y, Imokawa G. Percutaneous sensitization with allergens through barrier-disrupted skin elicits a Th2-dominant cytokine response. Eur J Immunol. 1998;28(3):769–79. doi: 10.1002/(SICI)1521-4141(199803)28:03<769::AID-IMMU769>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 55.Strid J, Hourihane J, Kimber I, Callard R, Strobel S. Epicutaneous exposure to peanut protein prevents oral tolerance and enhances allergic sensitization. Clin Exp Allergy. 2005;35(6):757–66. doi: 10.1111/j.1365-2222.2005.02260.x. [DOI] [PubMed] [Google Scholar]
- 56.Lack G, Fox D, Northstone K, Golding J. Factors associated with the development of peanut allergy in childhood. N Engl J Med. 2003;348(11):977–85. doi: 10.1056/NEJMoa013536. [DOI] [PubMed] [Google Scholar]
- 57.Fox AT, Sasieni P, du Toit G, Syed H, Lack G. Household peanut consumption as a risk factor for the development of peanut allergy. J Allergy Clin Immunol. 2009;123(2):417–23. doi: 10.1016/j.jaci.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 58.Baurecht H, Irvine AD, Novak N, Illig T, Buhler B, Ring J, et al. Toward a major risk factor for atopic eczema: meta-analysis of filaggrin polymorphism data. J Allergy Clin Immunol. 2007;120(6):1406–12. doi: 10.1016/j.jaci.2007.08.067. [DOI] [PubMed] [Google Scholar]
- 59.Brown SJ, Asai Y, Cordell HJ, Campbell LE, Zhao Y, Liao H, et al. Loss-of-function variants in the filaggrin gene are a significant risk factor for peanut allergy. J Allergy Clin Immunol. 2011;127(3):661–7. doi: 10.1016/j.jaci.2011.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Krutmann J. Phototherapy for atopic dermatitis. Clin Exp Dermatol. 2000;25(7):552–8. doi: 10.1046/j.1365-2230.2000.00700.x. [DOI] [PubMed] [Google Scholar]
- 61.Jungersted JM, Hogh JK, Hellgren LI, Jemec GB, Agner T. The impact of ultraviolet therapy on stratum corneum ceramides and barrier function. Photodermatol Photoimmunol Photomed. 2011;27(6):331–3. doi: 10.1111/j.1600-0781.2011.00618.x. [DOI] [PubMed] [Google Scholar]
- 62.Patrizi A, Savoia F, Giacomini F, Tabanelli M, Gurioli C. The effect of summer holidays and sun exposure on atopic dermatitis. G Ital Dermatol Venereol. 2009;144(4):463–6. [PubMed] [Google Scholar]
- 63.Toews GB, Bergstresser PR, Streilein JW. Epidermal Langerhans cell density determines whether contact hypersensitivity or unresponsiveness follows skin painting with DNFB. J Immunol. 1980;124(1):445–53. [PubMed] [Google Scholar]
- 64.Gorman S, McGlade JP, Lambert MJ, Strickland DH, Thomas JA, Hart PH. UV exposure and protection against allergic airways disease. Photochem Photobiol Sci. 2010;9(4):571–7. doi: 10.1039/b9pp00136k. [DOI] [PubMed] [Google Scholar]
- 65.Ghoreishi M, Dutz JP. Tolerance induction by transcutaneous immunization through ultraviolet-irradiated skin is transferable through CD4+CD25+ T regulatory cells and is dependent on host-derived IL-10. J Immunol. 2006;176(4):2635–44. doi: 10.4049/jimmunol.176.4.2635. [DOI] [PubMed] [Google Scholar]
- 66.Hart PH, Gorman S, Finlay-Jones JJ. Modulation of the immune system by UV radiation: more than just the effects of vitamin D? Nat Rev Immunol. 2011;11(9):584–96. doi: 10.1038/nri3045. [DOI] [PubMed] [Google Scholar]
- 67.Hartmann B, Riedel R, Jorss K, Loddenkemper C, Steinmeyer A, Zugel U, et al. Vitamin D Receptor Activation Improves Allergen-Triggered Eczema in Mice. J Invest Dermatol. 2011 doi: 10.1038/jid.2011.296. [DOI] [PubMed] [Google Scholar]
- 68.Sidbury R, Sullivan AF, Thadhani RI, Camargo CA., Jr Randomized controlled trial of vitamin D supplementation for winter-related atopic dermatitis in Boston: a pilot study. Br J Dermatol. 2008;159(1):245–7. doi: 10.1111/j.1365-2133.2008.08601.x. [DOI] [PubMed] [Google Scholar]
- 69.Javanbakht MH, Keshavarz SA, Djalali M, Siassi F, Eshraghian MR, Firooz A, et al. Randomized controlled trial using vitamins E and D supplementation in atopic dermatitis. J Dermatolog Treat. 2011;22(3):144–50. doi: 10.3109/09546630903578566. [DOI] [PubMed] [Google Scholar]
- 70.Food allergy: a practice parameter. Ann Allergy Asthma Immunol. 2006;96(3 Suppl 2):S1–68. [PubMed] [Google Scholar]