Abstract
This study investigated the efficacy of a combination gene therapy to repress IL-1 and RANKL for the treatment of particulate debris-induced aseptic loosening, and tried to explore the molecular mechanism the exogenous gene modifications on osteoclastogenesis. RAW cells activated by titanium particles were transduced with DFG-IL-1Ra and AAV-OPG individually or in combination for 4 weeks. Pro-inflammatory cytokines in culture media were determined by ELISA, and gene expressions of RANK, IL-1β, c-Fos, TRAF6, JNK1, and CPK were examined using real-time PCR. An established knee-implant-failure mouse model was employed to evaluate the efficacy of the in vivo double-gene therapy. The surgical implantation of a titanium alloy pin into the proximal tibia was followed by monthly challenge with titanium debris. Peri-implant gene transfers of IL-1Ra and OPG (respectively or in combination) were given three weeks after surgery. The combination of OPG and IL-1Ra gene transfer exhibited strong synergetic effects in blockage of inflammation and osteoclastogenesis at 8-weeks after gene modification. The combination therapy reversed peri-implant bone resorption and restored implant stability when compared with either single gene transduction. Real-time PCR data indicated that the action of IL-1Ra gene therapy may be mediated via the JNK1 pathway, while the reduction of osteoclastogenesis by OPG gene modification may be regulated by c-Fos expression. In addition, both gene modifications resulted in significantly diminishment of TRAF6 expression.
Keywords: periprosthetic osteolysis, osteoclastogenesis, synergetic effects
Introduction
Aseptic loosening due to periprosthetic osteolysis remains an important cause of the long-term failure of total joint arthroplasty, accounting for approximately 75% of the failure cases1. With increased life spans and numbers of joint prostheses in younger, more active patients, osteolytic aseptic loosening has become the dominant reason for revision surgery. Understanding the precise mechanism of AL and developing potential therapeutic means to halt the loosening process is therefore critical and urgent.
It has been reported that wear particles from the bearing surface play a critical role in periprosthetic osteolysis2. Wear particles exert their biological activity via macrophages, foreign body giant cells, and fibroblasts within the periprosthetic membrane. Activated cells provoke the periprosthetic inflammation by means of proinflammatory mediators, including IL-1β, TNF-α, IL-6 and PGE2, which in turn contribute to the osteoclast differentiation and survival through the receptor activator of nuclear factor NF-kappa B ligand (RANKL) and its receptor RANK pathway3–6. Extensive research efforts have focused on osteoprotegerin (OPG), a native soluble protein that competes for the binding of RANKL to its signal-transmitting receptor RANK. Animal experiments have established the pivotal role of RANKL/RANK pathway as the central regulator of osteoclastogenesis7, and demonstrated that the introduction of exogenous OPG to alter the RANKL:OPG ratio may negatively regulate the osteoclastogenesis and bone remodeling4–6. Our previous studies have suggested that OPG effectively blocked orthopaedic biomaterial particle-induced bone resorption in animal models7, 8.
One of the most common pathological findings at the site of osteolytic prosthetic loosening is a layer of periprosthetic tissue with numerous macrophages and osteoclasts9. Neale and Athanasou detected strong IL-1, IL-2 and TNF receptors present on macrophages and osteoclasts associated with revision arthroplasty, suggesting that these cytokines play an important role in periprosthetic bone resorption10. IL-1, TNF or IL-6 may directly activate osteoclast activity and promote the production and activation of other potent mediators of bone loss11, 12. We hypothesize that debris-associated local inflammation and bone resorption are associated, yet separated, pathological processes. Therefore therapies targeting inflammation and osteolysis might have synergetic effects in controlling wear debris associated aseptic joint prosthesis loosening. The current study extends our previous findings7, 8, 13 and aims to evaluate the efficacy and potential molecular mechanisms of a combination gene modification against both IL-1 and RANKL on wear debris-induced aseptic implant loosening in mice.
Results
The anti-inflammation effects of the combination gene therapy
The pre-osteoclastic mouse RAW 264.7 cells (2×104) were activated in vitro in the presence of titanium alloy particles (1×106/ml), followed by virus-mediated gene transduction of interleukin-1 receptor antagonist (DFG-IL-1Ra-neo) and osteoprotegerin (rAAV-GFP-OPG), individually or in combination. The combination group received one-half dosage of each individual viral vector (0.4 ×104 pfu) to ensure transduction equivalence. RAW cells transduced with 107 particles/ml of AAV-LacZ were used as non-therapeutic control. Enzyme-linked immunosorbent assay (ELISA) for IL-1 in the culture media indicated significant inhibition of IL-1 release in IL-1Ra treated group and the combination group at the first week following gene modification in comparison to LacZ controls, and the inhibition effect persisted through the 3-week culture (Figure 1a). During the later time period, all the gene therapy groups resulted in the diminished IL-1 expressions, including OPG gene modification (with less efficiency). At the transcriptional level, real-time PCR on the RAW cell preparations at 4 weeks of culture post treatment revealed a significant decrease of IL-1β mRNA expression in all the therapeutic treatment groups, with best effect in combination group (Figure 1b, p<0.05).
Figure 1.
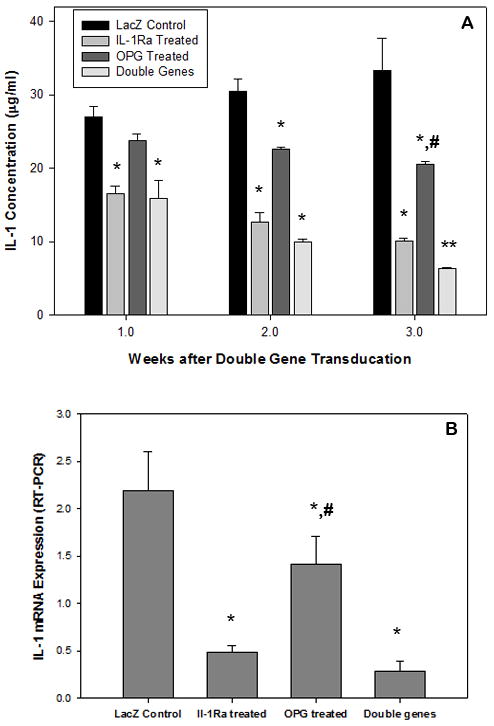
(a) ELISA was performed to determine IL-1β protein release in the culture media following gene modifications; while (b) summarizes the mRNA expression of IL-1 from the cells receiving treatment. (*p<0.05 and **p<0.01 to LacZ controls; #p<0.05 to double gene modification group)
Effect of combination therapy group on osteoclast differentiation
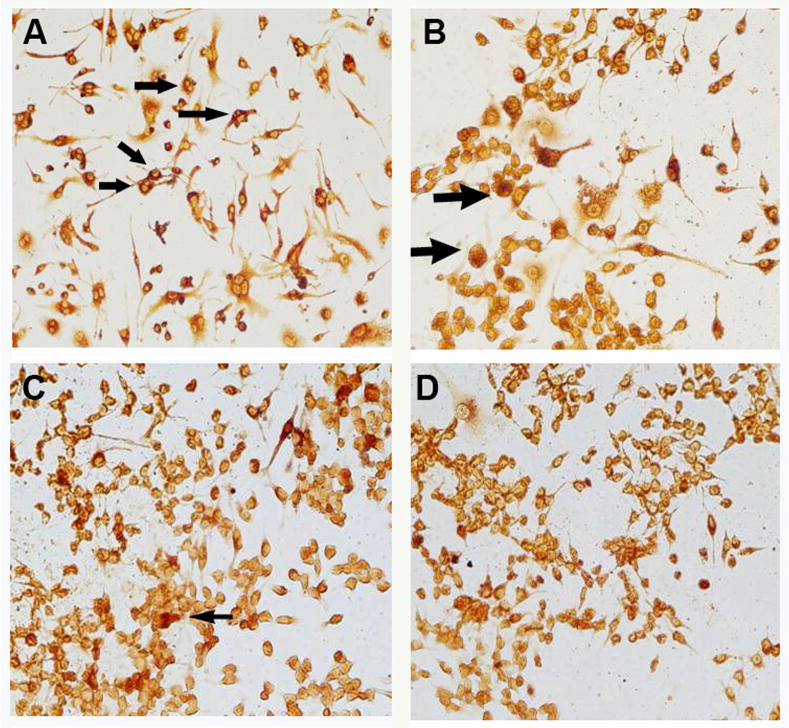
RAW cells were harvested at 4 weeks after gene modifications. Tartrate-resistant acid phosphatase (TRAP) staining was performed to quantify mature osteoclasts (Figure 2a–d). Significantly fewer TRAP-positive cells were present following OPG and OPG+IL-1Ra gene modifications, while IL-1Ra modification alone suggested less effectiveness (Figure 2e). Although there were relatively fewer multinucleated cells existed in this type of cell line, acquirement of TRAP+ characteristics suggests an indication of mature osteoclastic cells. Real-time PCR data further indicated that the double gene modification resulted in the most marked reduction of RANK mRNA expressions within all treatment groups, revealed synergetic inhibition influence over the individual exogenous IL-1Ra or OPG gene transduction (Figure 2f, p<0.01).
Figure 2.
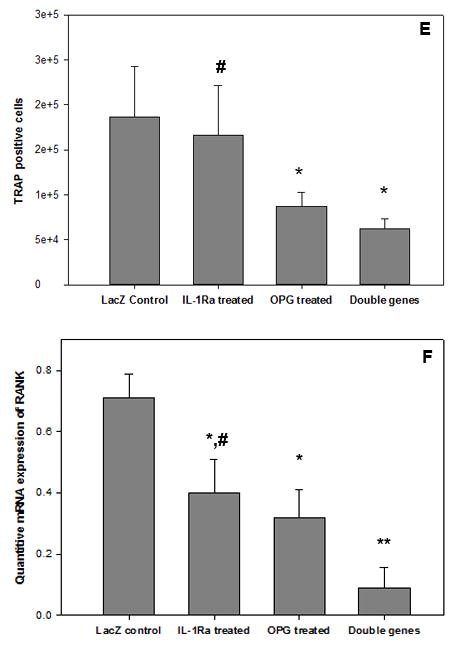
TRAP staining was performed to identify the mature osteoclast following gene modification: (a) LacZ, (b) IL-1Ra, (c) OPG, and (d) IL-1Ra + OPG; while (e) summaries the quantitative analysis of TRAP+ cells by ImagePro® software among groups (*p<0.05 to LacZ control; #p<0.05 to double gene therapy). Panel (f) exhibits the real-time PCR result of the RANK mRNA expressions (*p<0.05 and **p<0.01 to LacZ control; #p<0.05 to double gene group).
In vivo combination gene therapeutic effects on bone remodeling
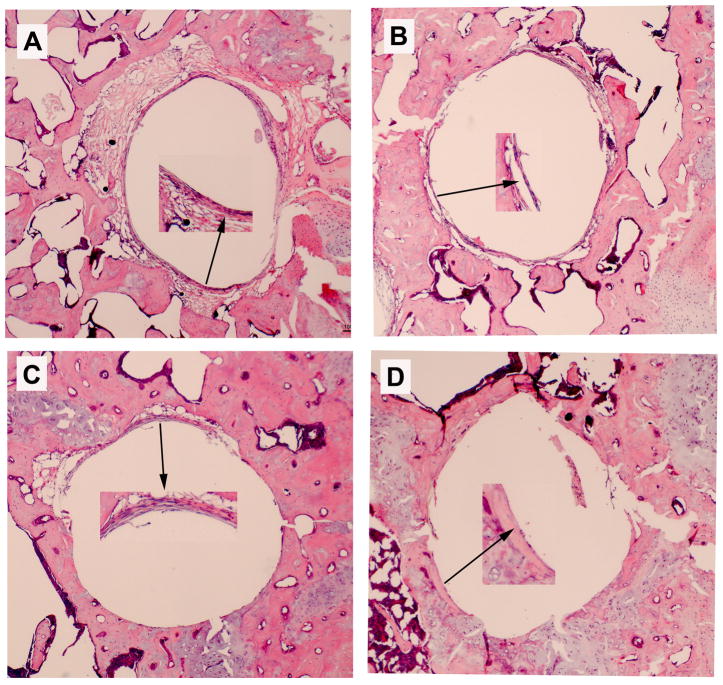
The mouse knee pin-implantation-failure model14 was used to evaluate the in vivo therapeutic efficacy of the gene modification therapies. Three weeks after titanium pin implantation into the proximal tibia and peri-implant titanium particles challenge, media containing DFG-IL-1Ra and AAV-GFP-OPG (individually or in combination) were injected intra-articularly in the implanted knee joint. Mice with AAV-LacZ viral vector injection were included as controls. Histological assessment of the proximal tibiae harvested 8 weeks following gene modifications exhibited ubiquitous peri-implant pseudo-membranes in LacZ gene treated group, while dramatically thinner or disappearing peri-implant soft tissue was exhibited in most of the harvested specimens following therapeutic gene modification(s) (Figure 3). Quantitative measurement of the pseudo-membrane thickness using a computerized image analysis system confirmed that all therapeutic gene therapies, especially OPG and the double gene modification groups significantly reversed the peri-implant pseudo-membrane formation and bone resorption compared to LacZ controls (Figure 3e, p<0.05)
Figure 3.
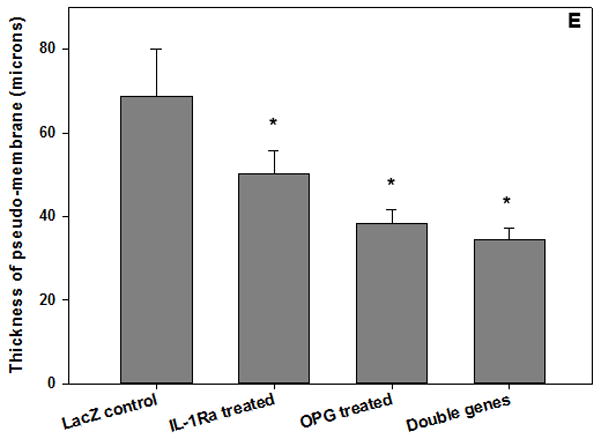
Histological appearance of the peri-implant tibiae (H&E stained) and the measurement of the thickness of the pseudo-membrane. (a) LacZ-control; (b) IL-1Ra treated; (c) OPG gene modified; (d) double gene transduced (all 40x magnification except the Inserts 200x); and (e) quantification of the pseudo-membrane thickness at 2 months following gene modifications (*p<0.05).
Micro-CT assessment of bone mineral density changes and osteolysis
A Scanco in vivo μCT system was used to quantify peri-implant bone volume and bone mineral density changes following gene modification treatments. Panels (a) through (d) of Figure 4 illustrate representative micro-computed tomography (μCT) 3-D reconstruction images of pin-implanted tibiae at 8-week post gene modifications. Focal bone osteolysis at the interface was remarkably diminished following the double gene therapy (Figure 4d). Quantification of the bone volume/total volume (BV/TV) and the bone mineral density (BMD) of the peri-implant bone specimens was also compared and summarized on Figure 4e. Therapeutic modifications significantly protected against the bone fraction volume and BMD loss in comparison with the LacZ controls (Figure 4d, p<0.05).
Figure 4.
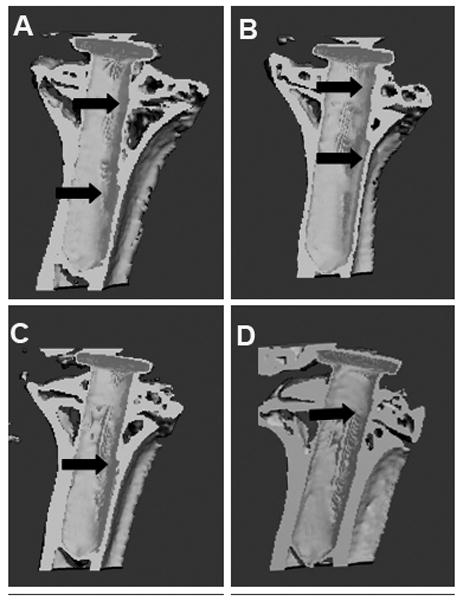
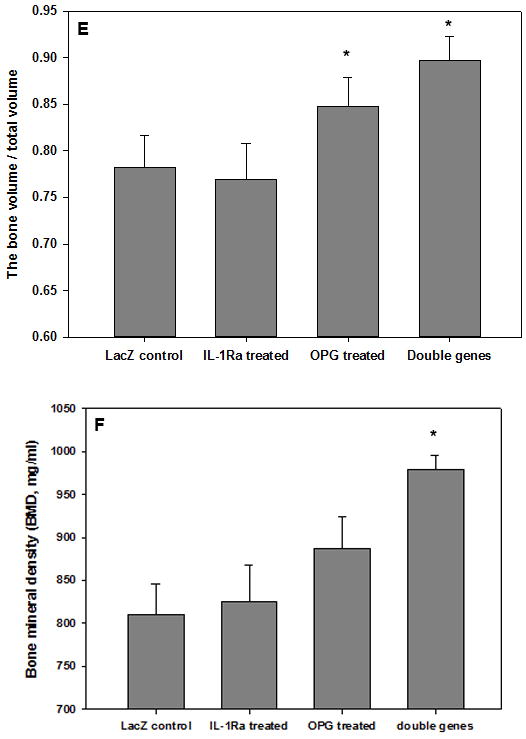
The analysis of bone remodeling. Representative μCT 3D construction images of the mouse prosthetic tibiae after 8 weeks of (a) LacZ-control; (b) IL-1Ra treated; (c) OPG gene modified; (d) double gene modifications. Arrows point out the focal bone erosions. The plot (e) summarizes the bone volume over total volume calculations by the μCT software among groups (*p<0.05); whereas (f) illustrates the quantitative analysis of bone mineral density changes following treatments (*P<0.05).
Biomechanical testing of the knee-implant tibia after combination gene therapy
Since peri-implant osteolysis weakens the stability of the pin implant, a pin pull-out test was performed to examine the implant’s biomechanical stability after eight weeks of gene modification treatments, With a customer-made fixture on a BOSE actuator (Model 3220-AT, Bose ElectroForce System, Eden Prairie, MN), the force curve to dissociate the pin from the surrounding bone was recorded and analyzed (Figure 5, insert). Although there were variations among the individual specimen’s pullout force in OPG gene modification group, all the pins in double gene therapy group exhibited well-fixed mechanical stability that required significantly more force to dissociate the pin implants compared to the LacZ control group (Figure 5, p<0.05). However, the pullout test on the IL-1Ra transduction group did not reach statistical significant difference from the LacZ controls (p=0.21).
Figure 5.
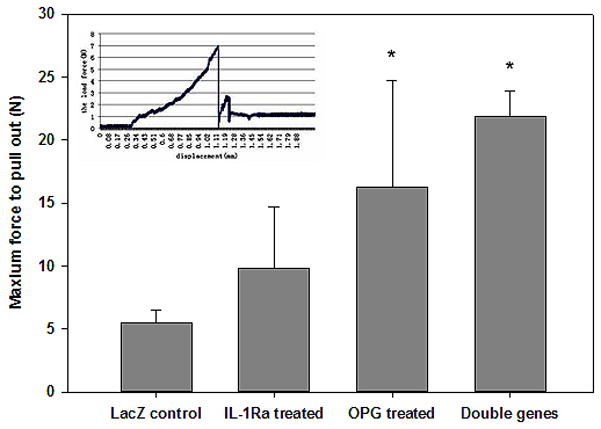
Quantitative analysis of the pin pull-out forces to dissociate the titanium pins from the mouse tibiae among groups for the implant stabilities (*p<0.05). The insert illustrates an example pulling curve.
The molecular explorationof the exogenous gene modifications on osteoclastogenesis
Real-time PCR was performed to examine specific gene expression profiles on cells following the therapeutic gene modifications. Using gene expression data from the LacZ group as the baseline, c-Fos was diminished 54%, 67%, and 88% in IL-1Ra, OPG, and double-gene groups respectively (Figure 6); while TRAF6 was inhibited 25%, 31% and 44% respectively (Figure 6). JNK1 was inhibited in the combination group and IL-1Ra group to 20%, and 16% of levels in the LacZ control, whereas no significant difference was reached in OPG group (Figure 6). Assessment of osteoclast marker cathepsin K (CPK) indicated that the combination gene therapy reduced its expression to more than 50% compared to LacZ controls, more efficient than other two single gene modification groups (Figure 6).
Figure 6.
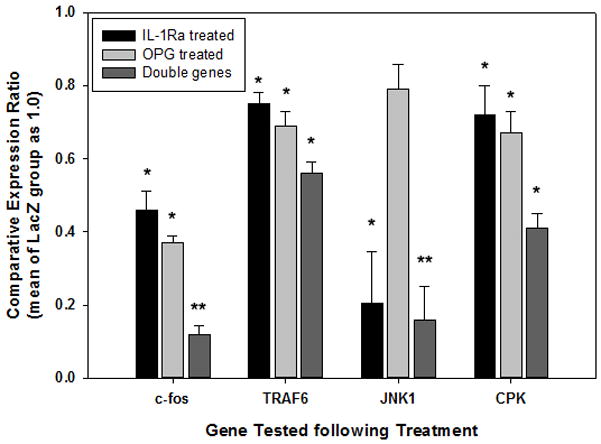
Real time PCR to examine some signal transduction gene expression profile of the cells following exogenous gene modifications. All data was normalized to the expression data from LacZ treated cells (*p<0.05, **p<0.01).
Discussion
Wear-debris associated aseptic loosening is the major long-term complication of total joint replacement. Studies suggested that the implant wear particles from the implant bearing surface attract inflammatory cellular infiltration and initiate the formation of the periprosthetic tissue, which often results in the elevated local expression of pro-inflammatory cytokines including IL-1, TNF, IL-6, prostaglandin E2, matrix metalloproteinases, and other factors15, 16. Those cytokines in turn promote more inflammatory cell activation and osteoclastogenesis, leading to periprosthetic bone resorption and aseptic loosening7. In addition, recent research efforts have identified signaling crosstalk between immune and skeletal system in osteoimmunology17. As early as 1990, in vitro experiments had suggested that the maturation of osteoclasts required the presence of bone marrow stromal cells or their osteoblast progeny18. Since the identification of two essential molecules response for osteoclast differentiation (RANKL and M-CSF) and the naturally existing decoy RANKL receptor (OPG), the imbalance of RANK, RANKL and OPG expressions has been theorized as the major cause of bone remodeling disorders including osteolytic aseptic prosthetic loosening8, 19. We have hypothesized that debris-associated local inflammation and bone resorption are associated yet separated pathological processes, thus therapies targeting inflammation and osteolysis may have synergetic effects in controlling wear debris associated aseptic joint prosthetic loosening. The current study was designed to test our hypothesis to evaluate a double gene modification targeting both IL-1 and OPG on titanium particle-induced knee pin-implant loosening. We have previously shown the protective effects of the retrovirus mediated IL-1 receptor antagonist (IL-1Ra) gene modification to wear debris induced local inflammation and osteolysis on small animal models19–21. Previous data from our group and others also suggested the protective and therapeutic influence of directly blockade of RANK/RANKL interactions using osteoprotegerin (OPG) or RANK:Fc for the wear-debris associated osteolysis in vivo7, 13, 22–24. In vitro data in this study confirmed the previous findings that IL-1Ra and OPG gene modification effectively blocked local inflammation and osteoclastogenesis, respectively. More importantly, this study suggested that combination of OPG and IL-Ra gene transfers with equivalent viral vector load exhibited a strong synergistic therapeutic effect in the blocking of proinflammatory cytokine expression and osteoclast maturation, with a significantly better efficacy over either single gene transduction. The data also supported a close relationship between the immune and skeletal systems in osteoimmunology25.
The knee-implantation-failure mouse model14 was also indicative of the potential long term therapeutic influence of the combination gene therapy. Peri-implant pseudo-membrane typically develops at the bone-pin interface within 2 weeks of the particulate debris challenge14. In the current study, therapeutic or control viral vectors were transduced in vivo at three weeks post particle challenge to evaluate and compare the therapeutic influence. The double gene modified mice exhibited a significant decrease in the thickness of pseudo-membrane and a significant increase in the bone fraction volume and bone density, while single gene modification exhibited a slightly less effectiveness. In addition, the double gene therapy reached the maximum implant stability confirmed by the biomechanical pullout test.
Real-time PCR illustrated signal transduction modifications following gene therapy. Takayanagi summarized the molecular pathways that RANKL/RANK associated in the osteoclastogenesis25. Although the role of the calcium-NFAT pathway25, 26 is currently controversial, the RANKL-RANK-c-Fos and RANKL-RANK-TRAF6 pathways have been widely accepted as relevant to osteoclastogenesis27–30. In this study, significantly decreased expression of TRAF6, c-Fos and JNK1 genes was observed in the cells receiving combination therapy of IL-1Ra and OPG. Although IL-1 is also thought playing important role through TRAF6 similar as RANKL to interact with TAK1 and promote osteoclastogenesis31, 32, the data presented here appears that combined blockage of both IL-1 and RANKL dramatically enhanced osteoclast depletion and osteolysis, suggesting some synergetic influence. Real-time PCR data further suggested that over-expression of IL-1Ra gene may alter JNK1 expression, while RANKL/RANK inhibition through elevated OPG expression directly blocked the initiation of the osteoclastogenesis. Besides the anti-osteoclastogenesis, the gene modifications also exhibited significantly ameliorated inflammation. It is interesting to reveal again that OPG over-expression significantly reduced the thickness of peri-pin implant pseudo-membranes and resulted in reduced IL-1 expression. A recent investigation complemented our findings 33, showing that both OPG-Fc and RANK-Fc reduced inflammatory cell counts and cytokine expression in a murine bacterial periodontitis model. Further investigations are on the way in our laboratory to explore the detailed molecular pathways regarding to this issue.
One of the potential arguments of the study may be that two types of the viral vectors were used to mediate the exogenous gene transduction. As a proof-of-concept study to test our hypothesis, we used these currently available vectors in our laboratoryfor the therapeutic gene modifications. Preliminary studies in our lab have suggested that retroviral vector behaves slower in reach the maximum expression of the transgene but the production sustains longer (unpublished data). We introduced DFG-IL-1Ra three days earlier than the AAV-OPG transduction, trying to increase IL-1Ra production first yet introduce the maximum production of the both therapeutic transgenes at the same time. Current investigation on the virus-mediated transgene kinetic equilibrium will be reported sequentially.
Overall, the combination gene therapy targeted towards anti-inflammation and anti-bone resorption may reduce the individual exogenous gene introduction and provide strong therapeutic influences in controlling wear debris-associated periprosthetic inflammation and osteolysis. The synergetic effect of double gene therapy may due to inhibition of c-Fos and JNK1 pathways in osteoclastogenesis, but further studies are warranted to explore the long-term safety issues of multiple gene modifications and proper dosages of exogenous genes.
Materials and methods
Cell culture and in vitro gene transfer
The RAW 264.7 cell line was purchased from the American Type Culture Collection. The titanium alloy particles (Ti-6Al-4V) used in the experiments was kindly provided by Zimmer, Inc. (Warsaw, IN) and the size of the particles (2.3 ± 0.168μm) was similar with the wear debris got from periprosthetic tissue of patients revised for aseptic loosening7. The recombinant adeno-associated virus transfer vector coding for OPG (pAAV-CMV-OPG-IRES-EGFP) was generated via multiple sub-cloning steps as detailed previously7, and packaged in the Gene Core Facility, University of North Carolina (UNC) at Chapel Hill, NC, who prepared the purified rAAV-OPG-IRES-EGFP using type 2 rAAV. The rAAV-LacZ control vectors were also obtained from the Gene Core Facility, UNC. Retroviral vectors encoding human IL-1Ra (DFG-IRAP-neo), viral β-galactosidase (MFG-LacZ) were constructed in and kindly provided by the laboratories of Dr Robbins in Pittsburgh, PA. RAW cells at 2 × 104/ml were cultured in the presence of Titanium particles (1×106/ml) for 72 hours before dividing into 5 groups: Group 1 was transduced with DFG-IL-1Ra (retroviral vectors code for interleukin-1 receptor antagonist gene) by adding 800 μl of 1×107 pfu viral medium; Group 2 cells were transduced by AAV-GFP-OPG (Adeno-associated viral vectors encoding osteoprotegerin) at 107 particles/ml; RAW cells in Group 3 were co-transduced sequentially with a half dose of AAV-OPG (0.4 × 104 particles/ml) and a half dose of DFG-IL1Ra (0.4 × 104 pfu); while the Group 4 RAW cells were transduced with 107 particles/ml of AAV-LacZ as controls. Group 5 was control cells without viral infections. To achieve the highest concentration of the double gene expressions in the combination therapy group, the infection of AAV-OPG was introduced 3 days later after the retrovirus-IL-1Ra transduction. The culture media were changed every two days, pooled weekly and stored in −20°C for ELISA examination. All the cells were harvested periodically during the 4-week cultivation.
Enzyme-linked immunosorbent assay (ELISA)
Release of proinflammatory cytokines IL-1β and TNFα in the cell culture media following gene transduction were assessed using ELISA kits (R&D systems, Minneapolis, MN) described previously21. The optical density was determined by an ELISA reader (Molecular Devices, Menlo Park, CA) at 405 nm wavelength, and levels of cytokines were determined by regression analysis against a standard curve.
Tartrate-resistant acid phosphatase (TRAP) stain for osteoclasts
The gene modified RAW 264.7 cells were harvested for TRAP (EC3.1.3.2) staining using a commercial kit (Sigma). The cells in 200μl suspension (1 × 106cells/ml) were cytospun onto a histological slide followed by fixation for 30s in buffered acetone in cold. The slide was incubated at 37°C for 1 h in 0.1 Macetate buffer (pH5.2), containing 0.5mM naphthol AS-BI phosphoric acid, 2.2mM FastGarnet GBC, and 10 mM sodium tartrate. The reaction was stopped by washing in several changes of distilled water. The presence of dark purple staining granules in the cytoplasm was considered as the specific criterion for identifying TRAP positive cells.
Real-time PCR for gene expression profile
Real-time reverse transcriptase polymerase chain reaction (RT-PCR) was performed to assess the efficacy of anti-inflammation and anti-bone resorption after gene modifications. Total RNA from RAW cells after gene modifications was extracted in TRIzol reagent (Invitrogen). cDNA was obtained by reverse transcription from 0.5 μg of total RNA in 40 μl of a reaction mixture containing 1x PCR buffer, 500 mM each of nucleotide triphosphates (dNTP), 0.5 U/ml of ribonuclease inhibitor, 2.5 mM of random hexamers, 5.5 mM of MgCl2, and 1.25 U/ml of reverse transcriptase (Perkin Elmer, CT). The reaction mixture was incubated in a DNA Thermal Cycler (Perkin Elmer, CT) at 25°C for 10 min, 48°C for 5 min followed by 95°C for 5 min. The expression of pro-inflammatory cytokines IL-1β, RANK, c-Fos,TRAF6, JNK1, CPK, and CTR was determined using the ABI Prism 7700 sequence detector (PE-Applied Biosystems, Foster City, California) as described previously20. All the mouse primers were designed using Primer3 (v.0.4.0 http//frodo.wi.mit.edu/primer3) and listed in Table I. With the Ct value of GAPDH as an internal control, the comparative quantification of gene expressions of the samples from OPG, IL-1Ra, and the double gene modified groups was calculated against the data from the LacZ control group according to the formula given in the manufacturer’s manual (PE Applied Biosystems).
Table 1.
The primer pairs for real-time PCR
| Genes | Left primer sequence | Right primer sequence |
|---|---|---|
| IL-1β | GGGCCTCAAAGGAAAGAATC | TACCAGTTGGGGAACTCTGC |
| RANK | GGGTGGGGCGCAGACTTCAC | ATGCCAGCAGCCTGCACCAG |
| c-Fos | CTCCCGTGGTCACCTGTACT | TTGCCTTCTCTGACTGCTCA |
| TRAF6 | GCAGTCGTTTCCTGCCGTGGTTT | GCACTGGGCTTCTCAATGCGGA |
| JNK1 | AGAAACTGTTCCCCGATGTG | TGATGTATGGGTGCTGGAGA |
| CPK | CGTGCAGCAGAACGGAGGCA | TAGCTGCCTTTGCCGTGGCG |
Establishment of the mouse pin-implantation model and in vivo gene transfer(s)
The animal procedures were approved by the Institutional Animal Care and Use Committees (IACUC, Wichita State University). BALB/c mice (Jackson Labs, Bar Harbor, ME) aged 10–12 weeks were quarantined in our local animal facility for 2 weeks prior to experimentation. Titanium alloy pins of 0.8 mm diameter and 5 mm long with a flat large top of 1.4 mm diameter were specially manufactured by Stryker Orthopaedic, Inc. (Mahwah, NJ). The mouse model was established as described previously14. Briefly, the tibial plateau of the mouse right knee was surgically exposed under strict sterile procedure, and a proximal 6 mm of the tibial intra-medullary canal through the center of the tibial plateau was reamed with a 0.8 mm dental drill. A titanium-alloy pin was then press-fit into the canal in a manner that the surface of pin head became flush with the cartilaginous surface of the tibial plateau and did not interfere with the motion of the knee. Following the surgery the x-ray was taken to confirm the correct position of the pin implantation. To mimic the prosthetic wear, 10 μl of a titanium-alloy particle suspension (4×104 particles of Ti–6Al–4V) was pipetted into the tibia canal before insertion of pin implant during surgery, and 20ul of Ti particles were intra-articularly injected into the prosthetic joint every 4 weeks following surgery. Three-week following the establishment of knee-implant-failure murine model, the mice (n=24) were randomly divided into four groups: 50μl and 25μl of the DFG-IL-1Ra at the titer of 107 pfu were injected into the prosthetic joint in the IL-1Ra group and the combination gene therapeutic group, respectively. Three days after the IL-1Ra gene transfer, 50 μl or 25 μl of the AAV-OPG at the titer of 1 × 107 particle/ml was injected into the prosthetic joint of the mice in the OPG group or the combination group, respectively. 50μl of AAC-LacZ (107 particle/ml) was injected into the prosthetic joint in the LacZ group as control. Mice were sacrificed at 8 weeks after gene transduction for biomechanical and histological tests. Previous experiments in our laboratory have confirmed that retroviral MFG-LacZ behaved similarly as AAV-LacZ in transgene expression with an exception of delayed peak expression, so the MFG-LacZ control was eliminated from this study.
Biomechanical test of pin implantation in tibia
The limb with tibia pin-implantation was surgically harvested at sacrifice. All soft tissue around the prosthetic joint was carefully removed to expose the implanted pin surface and proximal tibia. Approximately 10 mm of the distal tibia was cemented into a custom-designed jig with dental cement (Garreco; Herber Springs, AR). Proper vertical alignment of the tibia titanium implant to the loading axis of the BOSE ElectroForce® testing system was achieved with a customer-designed fixture, and the actuator positions and loading data during the pulling test were recorded.
Micro-computerized tomography (microCT) assessment
All mice were scanned using a Scanco VivaCT40 MicroCT scanner (Scanco Medical, Basserdorf, Switzerland) within a week of surgery to confirm the successful placement of the pin. A final scan was performed 8 weeks later at sacrifice for bone density measurements. The scans were performed with an isotropic voxel size of 30 μm, an energy level of 70 kVp, a current of 114 μA, and an integration time of 200 ms. Reconstruction and analysis were carried out using the manufacturer’s supplied software. A region of interest (ROI) was defined around the pin, and bone mineral densities (BMD) and bone and total volume (BV and TV) of the tibia were calculated.
Histological analyses
Formalin-fixed prosthetic joints were decalcified in a solution containing 22% formic acid and 10% sodium citrate before paraffin embedding. The sections were stained with hematoxylin and eosin to examine new bone formation or bone erosion around the prosthetic pin, and to evaluate debris-associated inflammation, including periprosthetic tissue formation and cellular infiltration. A computerized image analysis system with the Image-Pro Plus software package (Media Cybernetics, Silver Spring, MD) was used to quantify the histological data, as detailed previously8.
Statistical analysis
Statistical analysis among groups was performed by one-way ANOVA test; with the Schafer formula for post hoc multiple comparisons (SPSS v12, Chicago, IL). A p-value of less than 0.05 was considered as significant difference. Data are expressed as Mean ± Standard Errors of the Mean.
Acknowledgments
The authors wish to thank Ms. Zhengs Song and Ms. Tala Sandid for their excellent technical assistance. This study was supported in part by the National Institutes Health (5R03AR054929 S-YY) and Scholarship Funding from Kansas Bioscience Authority (S-YY and PHW). Hao Wang was supported by a Fellowship for Oversea Study from Graduate School of Shandong University, China.
Footnotes
Disclosure:The authors declare no conflict of interest.
References
- 1.Keener JD, Callaghan JJ, Goetz DD, Pederson DR, Sullivan PM, Johnston RC. Twenty-five-year results after Charnley total hip arthroplasty in patients less than fifty years old: a concise follow-up of a previous report. J Bone Joint Surg Am. 2003;85-A(6):1066–72. doi: 10.2106/00004623-200306000-00013. [DOI] [PubMed] [Google Scholar]
- 2.Maloney WJ, Smith RL. Periprosthetic osteolysis in total hip arthroplasty: the role of particulate wear debris. Instr Course Lect. 1996;45:171–82. [PubMed] [Google Scholar]
- 3.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423(6937):337–42. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 4.Collin-Osdoby P, Rothe L, Anderson F, Nelson M, Maloney W, Osdoby P. Receptor activator of NF-kappa B and osteoprotegerin expression by human microvascular endothelial cells, regulation by inflammatory cytokines, and role in human osteoclastogenesis. J Biol Chem. 2001;276(23):20659–72. doi: 10.1074/jbc.M010153200. [DOI] [PubMed] [Google Scholar]
- 5.Haynes DR, Crotti TN, Potter AE, Loric M, Atkins GJ, Howie DW, et al. The osteoclastogenic molecules RANKL and RANK are associated with periprosthetic osteolysis. J Bone Joint Surg Br. 2001;83(6):902–11. doi: 10.1302/0301-620x.83b6.10905. [DOI] [PubMed] [Google Scholar]
- 6.Leibbrandt A, Penninger JM. RANK(L) as a key target for controlling bone loss. Adv Exp Med Biol. 2009;647:130–45. doi: 10.1007/978-0-387-89520-8_9. [DOI] [PubMed] [Google Scholar]
- 7.Zhang T, Yu H, Gong W, Zhang L, Jia T, Wooley PH, et al. The effect of osteoprotegerin gene modification on wear debris-induced osteolysis in a murine model of knee prosthesis failure. Biomaterials. 2009;30(30):6102–8. doi: 10.1016/j.biomaterials.2009.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang SY, Mayton L, Wu B, Goater JJ, Schwarz EM, Wooley PH. Adeno-associated virus-mediated osteoprotegerin gene transfer protects against particulate polyethylene-induced osteolysis in a murine model. Arthritis Rheum. 2002;46(9):2514–23. doi: 10.1002/art.10527. [DOI] [PubMed] [Google Scholar]
- 9.Zhang K, Jia TH, McQueen D, Gong WM, Markel DC, Wooley PH, et al. Circulating blood monocytes traffic to and participate in the periprosthetic tissue inflammation. Inflamm Res. 2009;58(12):837–44. doi: 10.1007/s00011-009-0051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neale SD, Athanasou NA. Cytokine receptor profile of arthroplasty macrophages, foreign body giant cells and mature osteoclasts. Acta Orthop Scand. 1999;70(5):452–8. doi: 10.3109/17453679909000980. [DOI] [PubMed] [Google Scholar]
- 11.Amstutz HC, Campbell P, Kossovsky N, Clarke IC. Mechanism and clinical significance of wear debris-induced osteolysis. Clin Orthop Relat Res. 1992;(276):7–18. [PubMed] [Google Scholar]
- 12.Gowen M, Wood DD, Ihrie EJ, McGuire MK, Russell RG. An interleukin 1 like factor stimulates bone resorption in vitro. Nature. 1983;306(5941):378–80. doi: 10.1038/306378a0. [DOI] [PubMed] [Google Scholar]
- 13.Zhang L, Jia TH, Chong AC, Bai L, Yu H, Gong W, et al. Cell-based osteoprotegerin therapy for debris-induced aseptic prosthetic loosening on a murine model. Gene Ther. 2010;17(10):1262–9. doi: 10.1038/gt.2010.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang SY, Yu H, Gong W, Wu B, Mayton L, Costello R, et al. Murine model of prosthesis failure for the long-term study of aseptic loosening. J Orthop Res. 2007;25 (5):603–11. doi: 10.1002/jor.20342. [DOI] [PubMed] [Google Scholar]
- 15.Chiba J, Rubash HE, Kim KJ, Iwaki Y. The characterization of cytokines in the interface tissue obtained from failed cementless total hip arthroplasty with and without femoral osteolysis. Clin Orthop Relat Res. 1994;(300):304–12. [PubMed] [Google Scholar]
- 16.Jacobs JJ, Roebuck KA, Archibeck M, Hallab NJ, Glant TT. Osteolysis: basic science. Clin Orthop Relat Res. 2001;(393):71–7. doi: 10.1097/00003086-200112000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Takayanagi H. Inflammatory bone destruction and osteoimmunology. J Periodontal Res. 2005;40(4):287–93. doi: 10.1111/j.1600-0765.2005.00814.x. [DOI] [PubMed] [Google Scholar]
- 18.Udagawa N, Takahashi N, Akatsu T, Tanaka H, Sasaki T, Nishihara T, et al. Origin of osteoclasts: mature monocytes and macrophages are capable of differentiating into osteoclasts under a suitable microenvironment prepared by bone marrow-derived stromal cells. Proc Natl Acad Sci U S A. 1990;87(18):7260–4. doi: 10.1073/pnas.87.18.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim N, Takami M, Rho J, Josien R, Choi Y. A novel member of the leukocyte receptor complex regulates osteoclast differentiation. J Exp Med. 2002;195(2):201–9. doi: 10.1084/jem.20011681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang S, Wu B, Mayton L, Evans CH, Robbins PD, Wooley PH. IL-1Ra and vIL-10 gene transfer using retroviral vectors ameliorates particle-associated inflammation in the murine air pouch model. Inflamm Res. 2002;51(7):342–50. doi: 10.1007/pl00000313. [DOI] [PubMed] [Google Scholar]
- 21.Yang SY, Wu B, Mayton L, Mukherjee P, Robbins PD, Evans CH, et al. Protective effects of IL-1Ra or vIL-10 gene transfer on a murine model of wear debris-induced osteolysis. Gene Ther. 2004;11(5):483–91. doi: 10.1038/sj.gt.3302192. [DOI] [PubMed] [Google Scholar]
- 22.Childs LM, Paschalis EP, Xing L, Dougall WC, Anderson D, Boskey AL, et al. In vivo RANK signaling blockade using the receptor activator of NF-kappaB:Fc effectively prevents and ameliorates wear debris-induced osteolysis via osteoclast depletion without inhibiting osteogenesis. J Bone Miner Res. 2002;17(2):192–9. doi: 10.1359/jbmr.2002.17.2.192. [DOI] [PubMed] [Google Scholar]
- 23.Ulrich-Vinther M, Carmody EE, Goater JJ, KSb, O’Keefe RJ, Schwarz EM. Recombinant adeno-associated virus-mediated osteoprotegerin gene therapy inhibits wear debris-induced osteolysis. J Bone Joint Surg Am. 2002;84-A(8):1405–12. doi: 10.2106/00004623-200208000-00016. [DOI] [PubMed] [Google Scholar]
- 24.Tsutsumi R, Hock C, Bechtold CD, Proulx ST, Bukata SV, Ito H, et al. Differential effects of biologic versus bisphosphonate inhibition of wear debris-induced osteolysis assessed by longitudinal micro-CT. J Orthop Res. 2008;26(10):1340–6. doi: 10.1002/jor.20620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takayanagi H. Mechanistic insight into osteoclast differentiation in osteoimmunology. J Mol Med (Berl) 2005;83(3):170–9. doi: 10.1007/s00109-004-0612-6. [DOI] [PubMed] [Google Scholar]
- 26.Troen BR. The regulation of cathepsin K gene expression. Ann N Y Acad Sci. 2006;1068:165–72. doi: 10.1196/annals.1346.018. [DOI] [PubMed] [Google Scholar]
- 27.Franzoso G, Carlson L, Xing L, Poljak L, Shores EW, Brown KD, et al. Requirement for NF-kappaB in osteoclast and B-cell development. Genes Dev. 1997;11(24):3482–96. doi: 10.1101/gad.11.24.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grigoriadis AE, Wang ZQ, Cecchini MG, Hofstetter W, Felix R, Fleisch HA, et al. c-Fos: a key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science. 1994;266(5184):443–8. doi: 10.1126/science.7939685. [DOI] [PubMed] [Google Scholar]
- 29.Lomaga MA, Yeh WC, Sarosi I, Duncan GS, Furlonger C, Ho A, et al. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev. 1999;13(8):1015–24. doi: 10.1101/gad.13.8.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naito A, Azuma S, Tanaka S, Miyazaki T, Takaki S, Takatsu K, et al. Severe osteopetrosis, defective interleukin-1 signalling and lymph node organogenesis in TRAF6-deficient mice. Genes Cells. 1999;4(6):353–62. doi: 10.1046/j.1365-2443.1999.00265.x. [DOI] [PubMed] [Google Scholar]
- 31.Gallo J, Raska M, Mrazek F, Petrek M. Bone remodeling, particle disease and individual susceptibility to periprosthetic osteolysis. Physiol Res. 2008;57(3):339–49. doi: 10.33549/physiolres.931140. [DOI] [PubMed] [Google Scholar]
- 32.Armstrong AP, Tometsko ME, Glaccum M, Sutherland CL, Cosman D, Dougall WC. A RANK/TRAF6-dependent signal transduction pathway is essential for osteoclast cytoskeletal organization and resorptive function. J Biol Chem. 2002;277(46):44347–56. doi: 10.1074/jbc.M202009200. [DOI] [PubMed] [Google Scholar]
- 33.Yuan H, Gupte R, Zelkha S, Amar S. Receptor activator of nuclear factor kappa B ligand antagonists inhibit tissue inflammation and bone loss in experimental periodontitis. J Clin Periodontol. 2011;38(11):1029–36. doi: 10.1111/j.1600-051X.2011.01780.x. [DOI] [PubMed] [Google Scholar]