Abstract
OBJECTIVE
A major impediment toward the development of novel treatment strategies for fibromyalgia (FM) is the lack of an objective marker which tracks with spontaneous clinical pain report. Resting state intrinsic brain connectivity in FM has demonstrated increased insular connectivity to the default mode network (DMN), a network whose activity is increased during rest. Moreover increased insular connectivity to the DMN was associated with increased spontaneous pain levels. However as these analyses were cross-sectional in nature, they provided no insight to dynamic changes in connectivity and their relationship with variation in clinical pain report.
METHODS
17 FM patients underwent resting state fMRI at baseline and following 4 weeks of a non-pharmacological intervention to diminish pain. Intrinsic DMN connectivity was evaluated using probabilistic independent component analysis. A paired analysis evaluated longitudinal changes in intrinsic DMN connectivity and a multiple linear regression investigated correlations between longitudinal changes in clinical pain and changes in intrinsic DMN connectivity. Changes in clinical pain were assessed with the Short Form of the McGill Pain Questionnaire (SF-MPQ).
RESULTS
Clinical pain was reduced following therapy (SF-MPQ sensory scale: p<0.02). Intrinsic DMN connectivity to the insula was reduced, and this reduction was correlated with reductions in pain (corrected p<0.05).
CONCLUSIONS
Our findings suggest that intrinsic brain connectivity can be used as a candidate objective marker that tracks intra-subject with changes in spontaneous chronic pain in FM. We propose that intrinsic connectivity measures could potentially be used either in research or clinical settings as a complementary, more objective outcome.
Keywords: resting state fMRI, fcMRI, default mode network, fibromyalgia, acupuncture
Fibromyalgia (FM) is a functional chronic pain syndrome characterized by diffuse hyperalgesia and spontaneous widespread pain [1]. Multiple studies have described functional, structural and neurochemical brain changes in these patients [2]. Since most of these studies have been cross-sectional in nature they do not address whether these changes in brain structure, function, and neurochemistry are a cause of the pain, a consequence, or simply factors associated with chronic pain. Moreover most of the fMRI studies have assessed changes in response to pain stimuli and as such only provide information about hyperalgesia and not ongoing spontaneous pain. Recently we reported that a functional magnetic resonance imaging (fMRI) technique that assesses resting or intrinsic brain connectivity provides neurobiological correlates of spontaneous clinical pain report in FM [3]. FM patients displayed greater connectivity between the insula and the default mode network (DMN; see below) and the degree of connectivity was directly associated with the intensity of ongoing spontaneous pain. However this analysis was cross-sectional in nature and did not assess whether this marker could be a potential brain imaging marker reflecting dynamic longitudinal changes in pain in FM patients undergoing therapy.
The field of FM and chronic pain in general is in great need of both effective analgesic therapies, as well as better understanding of the pathophysiology underlying chronic pain syndromes. Indeed these two factors are likely related: a better understating of the mechanistic pathways underlying specific pathology in sub-groups of individual patients could then be used to optimize and enhance therapy for specific individuals with FM. Subjective self-reports of pain symptoms most likely lack the specificity needed to phenotype specific pathologic factors in individuals patients; however, as FM is increasingly characterized as a disorder in central nervous system functioning [1], a non-invasive neuroimaging marker could greatly improve our ability to characterize and track pain symptoms in FM patients in longitudinal trials.
Evaluation of intrinsic brain connectivity is a relatively recent approach in fMRI neuroimaging. Intrinsic connectivity assesses spontaneous neural and metabolic activity that occurs in a resting basal state since imaging is performed while subjects rest quietly in the scanner. We and others have proposed that this resting connectivity is related to the spontaneous chronic pain that these patients experience. It may reflect synaptic neurotransmission as inter-regional correlations in the fMRI signal follow known structural monosynaptic and polysynaptic pathways [4]. Thus, connectivity likely reflects neurophysiologically meaningful activity, and occurs within distinct networks of the brain. One such constellation is the Default Mode Network (DMN), an aggregate of brain structures which have been associated with self-referential cognition and autobiographical memory [5]. In addition to FM [3], altered intrinsic DMN connectivity has been detected in other chronic pain conditions [6]. We found that the DMN demonstrated greater connectivity to the insula in FM patients compared to healthy adults, and, perhaps even more importantly, increasing DMN/insula connectivity was associated with increasing levels of spontaneous pain [3]. These results suggested that intrinsic connectivity could serve as a brain biomarker for chronic pain - a hypothesis that would be bolstered if longitudinal changes in intrinsic DMN connectivity were associated with changes in the clinical pain state.
Our current study was designed to evaluate longitudinal changes in intrinsic brain connectivity in FM patients treated with a non-pharmacological intervention known to modulate pain levels in this patient population [7]. Subjects were scanned before and after therapy and were evaluated for intrinsic connectivity. We hypothesized that DMN-insula connectivity, would be reduced following therapy and would be correlated with diminished pain.
PATIENTS and METHODS
Subjects
As part of an ongoing study investigating non-pharmacologic treatment in FM, 17 female patients (age: 46.4±15.5 years, μ±σ) were evaluated with MRI during two scanning sessions spaced 29.8±4.0 days (μ±σ). Scanning sessions occurred before (4.0±2.4 days, μ±σ) and after (4.4±3.8 days) 9 treatments of either acupuncture or sham acupuncture using a protocol shown to decrease pain levels in FM patients [7, 8]. Analyses did not consider the treatment assignment, as we were not interested in any differences between treatment with acupuncture and sham acupuncture, but rather how changes in intrinsic brain connectivity are related to changes in pain. All subjects gave written informed consent, and protocols were approved by the University of Michigan Institutional Review Board.
Participant inclusion and exclusion criteria have been reported previously [3]. Briefly, FM patients: 1) met the 1990 American College of Rheumatology criteria for the diagnosis of FM for at least 1 year; 2) had continued presence of pain more than 50% of days; and 3) were willing to maintain their existing and limit the introduction of any new medications or treatment modalities for control of FM symptoms during the study.
Spontaneous Clinical Pain
Prior to scanning, subjects were asked to rate the intensity of their fibromyalgia pain using the Short Form of the McGill Pain Questionnaire (SF-MPQ) [9]. The sensory and affective subscales were contrast between baseline and post-therapy time points (Students’ T-test, significant at alpha = 0.05).
Intrinsic Connectivity fMRI Data Acquisition
Six minutes of resting state fMRI data were collected as the first functional scan run in the session. We used a spiral in-out gradient echo T2*-weighted BOLD pulse sequence (TR/TE=2000/30 ms, 180 volumes, 43 AC-PC aligned slices, voxel size=3.13 × 3.13 × 4.0 mm) running on a 3T Signa EXCITE scanner (GE, Milwaukee, USA) equipped with a 8-channel head coil. Subjects were instructed to close their eyes and to rest comfortably during the functional scan without moving or falling asleep. Structural data were also collected using a SPGR pulse sequence (TR/TE/TI = 14/5.5/300 ms, flip angle = 20 degrees, 124 contiguous axial slices, voxel size=1×1×1.5 mm).
Physiological data were collected simultaneously to fMRI data, as cardio-respiratory fluctuations are known to influence fMRI intrinsic connectivity estimation within several brain networks. Cardiac data were acquired using an infrared pulse oximeter (GE, Milwaukee, USA) attached to the right middle finger. Respiratory volume data were acquired using an MR-compatible belt (GE, Milwaukee, USA) placed around the subject’s ribcage.
Intrinsic Connectivity fMRI Data Analysis
Data analysis was performed using FSL (FMRIB’s Software Library, www.fmrib.ox.ac.uk/fsl). Data were corrected for motion artifact (FSL-MCFLIRT) and for cardio-respiratory artifacts using the RETROICOR algorithm. Brain extraction was performed on functional data (FSL-BET). Data were smoothed using a Gaussian kernel of FWHM 6 mm; and high-pass temporally filtered (f = 0.008Hz).
The within-subject resting fMRI data analysis was performed using the previously validated dual-regression ICA through Multivariate Exploratory Linear Optimized Decomposition into Independent Components (MELODIC, FSL). Importantly, this approach has been reported to have moderate to high test-retest reliability both short term (within-session) and long term (several weeks) in healthy adults [10]. We used the same approach in our previous cross-sectional study, investigating the relationship between pain and intrinsic brain connectivity in FM patients [3]. Dual-regression ICA allows for voxel-wise comparisons of resting state functional connectivity by first temporally concatenating resting fMRI data from all subjects, followed by back-reconstructing the group ICNs for individual subjects, which are then used for within- and between-subject group and difference maps. We limited the number of independent components (ICs) to 25 and used our previous goodness-of-fit methods [3, 11] to identify different ICNs. Our analysis was focused on the DMN network, as resting connectivity within this network was found to be altered in FM patients compared to healthy adults [3], while acupuncture has been shown to modulate resting DMN connectivity [11]. As in our previous analyses, the GLM included multiple temporal regressors of no interest including timeseries from white matter and ventricular regions, and cardiac and respiratory variability defined by convolving the heart rate timeseries and respiratory variations with appropriate cardiac and respiratory transfer functions. This was done to limit any residual shared variance with non-neuronal (e.g. cardio-respiratory physiological) processes. No global signal regression was used.
Mixed effects group analyses contrast intrinsic DMN connectivity at baseline versus post-therapy. To more closely link changes in resting DMN connectivity with analgesic response, a multiple linear regression was performed using the change in DMN connectivity and change in spontaneous pain rating (McGill sensory/affective sub-scores). This pain score was adjusted for age by including this variable in the model, as age is known to influence ICN connectivity. Results were threshold using cluster correction for multiple comparisons (corrected p-value=0.05).
RESULTS
Resting fMRI data were collected from all 17 female subjects, before and after therapy (inter-scan interval was 29.8±4.0 days, μ±σ). All subjects tolerated the intervention and there were no drop-outs.
Patients reported significant diminishment of clinical pain after therapy on the McGill-sensory subscore (Baseline=10.71±1.93, μ±SEM; Post-Therapy=6.06±0.85, p=0.02) and a trend for diminished pain on the McGill-affective subscore (Baseline=2.24±0.47; Post-Therapy=1.35±0.31, p=0.09; see Figure 1).
Figure 1.
Clinical pain levels were assessed just prior to fMRI scanning. There was a significant reduction in pain on the McGill Sensory subscale (p<0.05) and a trend (p=0.09) for diminished pain on the McGill affective subscale following therapy. n.b. * = p<0.05, + = 0.05 < p < 0.1
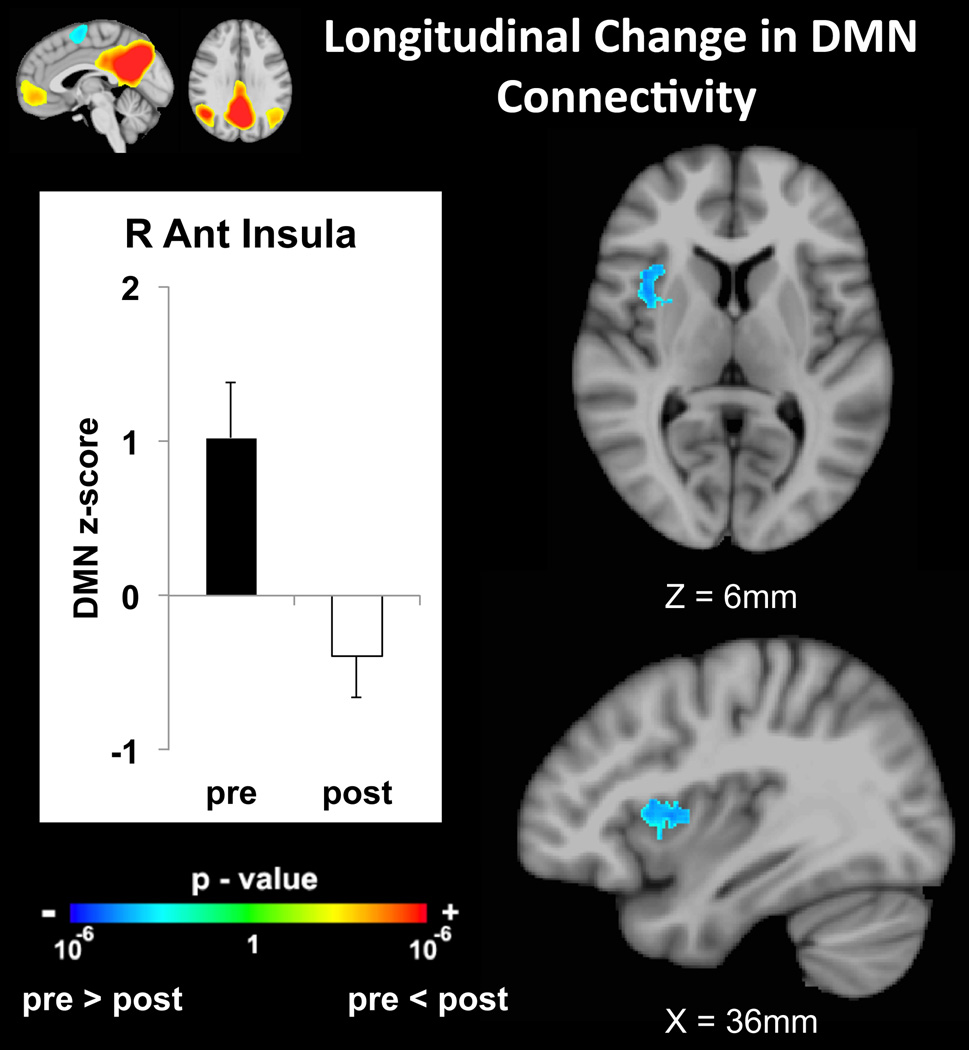
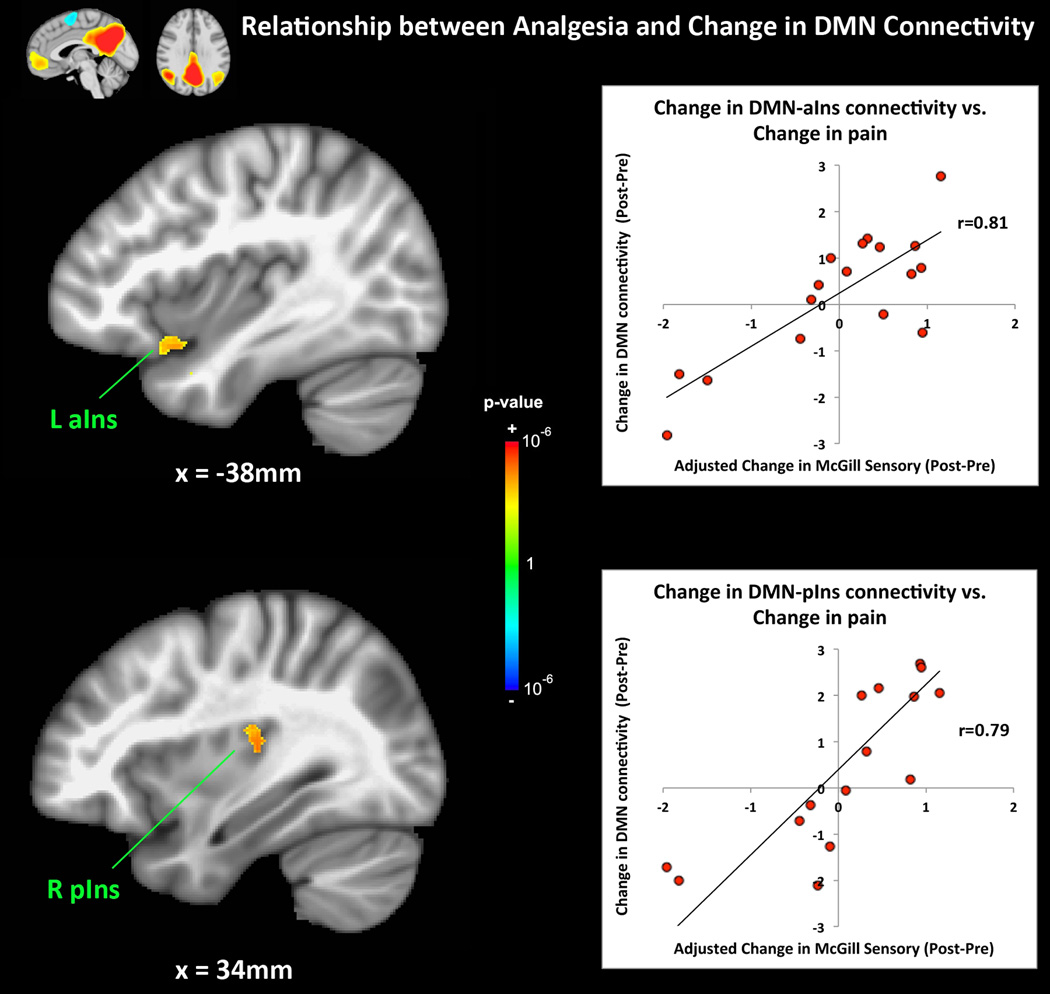
Following therapy, reduced intrinsic brain connectivity between the DMN and right anterior/middle insula was detected (z=−3.63, MNI(x,y,z)=39,7,5mm, Figure 2). Reduced connectivity was also noted between the DMN and right putamen post treatment (z=−3.57, MNI(x,y,z)=26,1,1mm). Interestingly diminished self-reporting of spontaneous clinical pain correlated positively with reduced connectivity between the DMN and left anterior insula (z=3.21, MNI(x,y,z)=−38,12,−18mm, Figure 3), as well as the left amygdala (z=3.22, MNI(x,y,z)=−24,1,−21mm). A sub-threshold cluster, which survived thresholding at uncorrected p<0.005, minimum cluster size 400mm2, was also found in the right posterior insula (z=3.74, MNI(x,y,z)=32,−24,16mm).
Figure 2.
DMN connectivity to the anterior insula, which was positive at baseline, was significantly reduced following therapy.
Figure 3.
A multiple linear regression analysis demonstrated that the change in age-adjusted clinical pain at the time of the scan was correlated (r=0.81) with reduced intrinsic DMN connectivity to the left anterior insula. A sub-threshold cluster was also found in the right posterior insula (r=0.79). n.b. aIns=anterior insula, pIns=posterior insula
DISCUSSION
Our previous studies linked increased intrinsic DMN-insula connectivity to the spontaneous pain state in FM patients [3]. This current study demonstrates that this connectivity is decreased following successful longitudinal therapy. Furthermore, diminished pain correlated positively with reduced intrinsic connectivity between the DMN and anterior insula. These results preliminarily support the use of intrinsic brain connectivity as a candidate objective marker for the subjective experience of clinical pain in FM. Moreover, we have demonstrated the utility of using intrinsic connectivity as a surrogate objective outcome for interventional trials in FM, especially in early “proof of concept” trials.
Previous studies have noted insular involvement in the multidimensional (sensory, affective, cognitive) pain state. The insula is one of the most commonly activated brain regions in neuroimaging studies of acute experimental pain [12]. However, the insula does not just process pain signals, and has been associated with multiple aversive or otherwise salient experiential states, both interoceptive [13] and exteroceptive [14]. As DMN processing has been attributed to self-referential cognitive processing, we could speculate that increased DMN-insula connectivity in FM may reflect a state of hyper-awareness to pain, which has been incorporated into the patient’s sense of self. Longitudinal reduction in DMN-insula connectivity may then follow, accompany, or even play a causal role in the reduction in pain experienced during analgesic intervention. At this time we cannot differentiate between these hypotheses.
The use of an insula-associated neuroimaging marker to track fibromyalgia pain has been explored in previous studies. For instance, we have shown that insular glutamate levels as assessed with proton magnetic resonance spectroscopy (H-MRS) were elevated in FM and were found to correlate positively with changes in clinical pain [8, 15]. Our current study adds to our understanding of insular involvement in clinical pain report and shows again that longitudinal changes in pain in FM are related to longitudinal changes in insular physiology.
We used acupuncture as the non-pharmacological therapy in this study. This intervention was used instead of a pharmacological intervention as (1) there would be less risk in modulating neurovascular coupling, and (2) acupuncture (both real and sham) are known to reduce pain levels in FM patients [7]. Our previous studies have also demonstrated that acupuncture increases intrinsic DMN connectivity to pain modulatory and affective brain areas such as the anterior cingulate cortex, periaqueductal gray, and amygdala [11]. This effect was short term (immediately following acupuncture stimulation), whereas our current study found that more sustained therapy reduced DMN connectivity to the insula. Although it is unknown at this time, the longitudinal changes found over multiple weeks in this trial may in fact be related to the more immediate stimulus-related change we observed previously. Future studies are needed to clarify this hypothesis.
In conclusion, our findings suggest that intrinsic brain connectivity can be used as a candidate objective marker sensitive enough to track pain levels in FM. Intrinsic connectivity can be used as a complementary, objective outcome in longitudinal clinical trials for different therapies in this patient population. Future studies should incorporate this marker into other clinical trials, thereby gaining a better understanding of the mechanistic pathways underlying various interventions.
Acknowledgements
We would like to thank the National Center for Complementary & Alternative Medicine, NIH for funding support: R01-AT004714 (Napadow), P01-AT002048 (Rosen), P01-AT006663 (Rosen), R01-AT005280 (Gollub/Kaptchuk), K01-AT01111 (Harris). Dr. Harris was also supported by Department of Army grant DAMD-Award Number W81XWH-07-2-0050 and a Dana Foundation Award in Brain and Immuno-imaging. The content is solely the responsibility of the authors and does not necessarily represent the official views of our funding agencies.
References
- 1.Clauw D, Williams D. Fibromyalgia. In: Mayer E, Bushnell M, editors. Functional Pain Syndromes: Presentation and Pathophysiology. Seattle: IASP Press; 2009. p. 580. [Google Scholar]
- 2.Nebel MB, Gracely RH. Neuroimaging of fibromyalgia. Rheumatic diseases clinics of North America. 2009;35(2):313–327. doi: 10.1016/j.rdc.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Napadow V, LaCount L, Park K, As-Sanie S, Clauw DJ, Harris RE. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheum. 2010;62(8):2545–2555. doi: 10.1002/art.27497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krienen FM, Buckner RL. Segregated fronto-cerebellar circuits revealed by intrinsic functional connectivity. Cereb Cortex. 2009;19(10):2485–2497. doi: 10.1093/cercor/bhp135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 6.Malinen S, Vartiainen N, Hlushchuk Y, Koskinen M, Ramkumar P, Forss N, Kalso E, Hari R. Aberrant temporal and spatial brain activity during rest in patients with chronic pain. Proc Natl Acad Sci U S A. 2010;107(14):6493–6497. doi: 10.1073/pnas.1001504107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harris RE, Tian X, Williams DA, Tian TX, Cupps TR, Petzke F, Groner KH, Biswas P, Gracely RH, Clauw DJ. Treatment of fibromyalgia with formula acupuncture: investigation of needle placement, needle stimulation, and treatment frequency. J Altern Complement Med. 2005;11(4):663–671. doi: 10.1089/acm.2005.11.663. [DOI] [PubMed] [Google Scholar]
- 8.Harris RE, Sundgren PC, Pang Y, Hsu M, Petrou M, Kim SH, McLean SA, Gracely RH, Clauw DJ. Dynamic levels of glutamate within the insula are associated with improvements in multiple pain domains in fibromyalgia. Arthritis Rheum. 2008;58(3):903–907. doi: 10.1002/art.23223. [DOI] [PubMed] [Google Scholar]
- 9.Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30(2):191–197. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- 10.Zuo XN, Kelly C, Adelstein JS, Klein DF, Castellanos FX, Milham MP. Reliable intrinsic connectivity networks: Test-retest evaluation using ICA and dual regression approach. Neuroimage. 2009 doi: 10.1016/j.neuroimage.2009.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dhond RP, Yeh C, Park K, Kettner N, Napadow V. Acupuncture modulates resting state connectivity in default and sensorimotor brain networks. Pain. 2008;136(3):407–418. doi: 10.1016/j.pain.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9(4):463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3(8):655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 14.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris RE, Sundgren PC, Craig AD, Kirshenbaum E, Sen A, Napadow V, Clauw DJ. Elevated insular glutamate in fibromyalgia is associated with experimental pain. Arthritis Rheum. 2009;60(10):3146–3152. doi: 10.1002/art.24849. [DOI] [PMC free article] [PubMed] [Google Scholar]