Abstract
Resveratrol (3, 4', 5-trihydroxystilbene), a naturally-occurring phytoalexin readily available in the diet, is reported to possess both chemopreventive and chemotherapeutic activities in several cancers. However, despite the identification of numerous molecular targets, the underlying mechanisms involved in the anticancer activities of resveratrol are not completely understood. Resveratrol is postulated to function as a potential signaling pathway modulator and as such, is demonstrated to affect a multitude of signal transduction pathways associated with tumorigenesis and/or carcinogenesis; it is likely that this collective activity, rather than just a single effect, may play an important role in the anticancer properties of resveratrol. Since transcription factors control the expression of many genes, the elucidation of molecular targets of resveratrol involved in transcriptional regulation is necessary to better understand how this dietary phytochemical affects chemopreventive and chemotherapeutic processes. As a result, investigators have increasingly searched for and examined possible targets of resveratrol. In this review, we summarize the current knowledge on molecular targets, specifically transcription factors, that contribute to the observed anticancer effects of resveratrol related to: (1) inhibition of carcinogenic activation and induction of carcinogen detoxification, (2) induction of growth arrest and apoptosis, and (3) suppression of pro-inflammatory signaling pathways related to cancer progression.
Keywords: AhR, ATF3, Experimental, FOXO, Resveratrol
INTRODUCTION
Epidemiological and current laboratory studies suggest consumption of certain types of fruits and vegetables, containing phytochemicals, is associated with reduced cancer risk (1). Furthermore, it is postulated that dietary phytochemicals can function as chemopreventive and/or adjuvant chemotherapeutic agents. One such phytochemical is resveratrol (3, 4', 5-trihydroxystilbene), a naturally-occurring phytoalexin readily available in the diet and to which a plethora of health-promoting effects have been ascribed. Resveratrol, first identified as a bioactive compound in 1992, is found in several plants, particularly in the skin of red grapes (2). This compound has elicited much attention as a potential anticancer agent since its inhibitory effect on carcinogenic processes (initiation, promotion, and progression) was first reported in 1997 (3). Subsequently, numerous studies, using both in vitro and in vivo model systems, have illustrated resveratrol's capacity to modulate a multitude of signaling pathways associated with cellular growth and division, apoptosis, angiogenesis, invasion, and metastasis (4).
Despite substantial progress in understanding the molecular basis of resveratrol's anticancer activities, few clinical trials have been undertaken to confirm its use as a chemopreventive and/or adjuvant chemotherapeutic agent. Preclinical studies have demonstrated the inhibitory effects of resveratrol in different cancers (5) and its ability to act as an adjuvant to traditional chemotherapeutics (6-9). In this review, we summarize the current knowledge on molecular targets of resveratrol, specifically transcription factors, that contribute to observed anticancer effects of this dietary phytochemical; these transcription factors include the aryl hydrocarbon receptor (AhR), nuclear factor E2-related factor 2 (Nrf2), p53, forkhead box subgroup O (FoxO), nuclear factor-κB (NF-κB), and activating transcription factor 3 (ATF3).
ARYL HYDROCARBON RECEPTOR AND NUCLEAR FACTOR E2-RELATED FACTOR 2
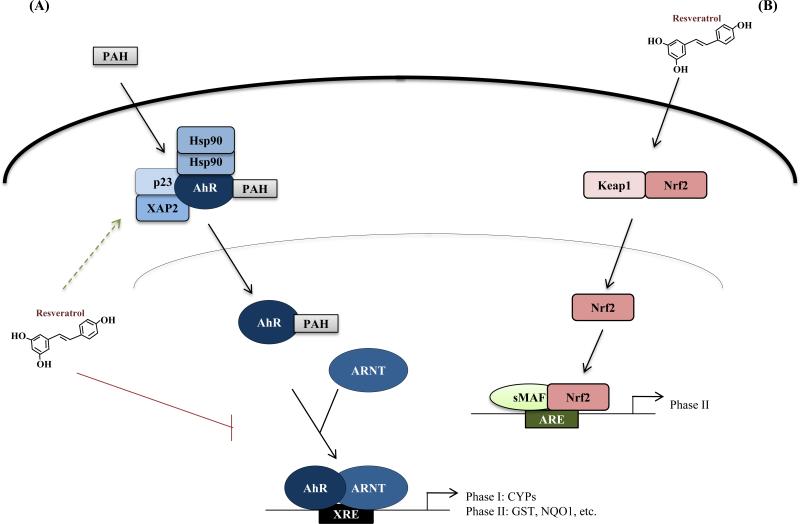
Oxidative metabolism by phase I enzymes, such as those belonging to the cytochrome P450 (CYP) family, results in the conversion of pro-carcinogens to reactive electrophilic intermediates, which are further metabolized by phase II enzymes via the conjugation of hydrophilic moieties. Resultant metabolites are detoxified and eliminated. However, inadequate detoxification by phase II enzymes potentiates genotoxicity of phase I products, thus initiating the carcinogenic process (10). Reports of modification of both phase I and phase II xenobiotic metabolizing enzymes by resveratrol suggest an explanation for the compound's chemopreventive effect (Fig. 1). In this regard, two primary molecular mechanisms have been identified: (1) inhibition of AhR-mediated activation of phase I enzymes and (2) induction of Nrf2-mediated activation of phase II enzymes.
Fig. 1. Effects of resveratrol on AhR/Nrf2 signaling pathways.
For a detailed description of AhR and Nrf2 signaling, refer to the text. Briefly, (A) PAH binds AhR (bound by the complex) and facilitates AhR translocation to the nucleus, where it forms a heterodimer with ARNT. The AhR/ARNT heterodimer then binds and trans-activates XRE-driven phase I/II enzyme promoters and initiates carcinogenesis (12). Resveratrol is demonstrated to inhibit AhR/ARNT recruitment to the promoter (red line; see text for references) and is speculated to displace PAH ligand binding and stabilize the cytosolic AhR complex (dotted green arrow; see text for reference). (B) Resveratrol-induced Nrf2 signaling confers protection against activated phase I enzymes (see text for references). Resveratrol promotes Nrf2 dissociation from Keap1 and nuclear translocation. In the nucleus, Nrf2 forms a heterodimer with small Maf proteins and trans-activates ARE-driven gene promoters (17).
Aryl hydrocarbon receptor (AhR)
Resveratrol is reported to alter phase I enzyme expression and activity in both an AhR-dependent and AhR-independent manner (11). In this review, we focus on resveratrol-mediated repression of AhR-induced phase I enzyme expression. The canonical AhR-dependent signaling pathway is thought to contribute to carcinogenic initiation by phase I enzyme-activated polycyclic aromatic hydrocarbons (PAH), and inhibition of AhR signaling by resveratrol is thought to suppress this initiative process. Under basal conditions, unbound AhR forms a multimeric complex in the cytosol. However, upon ligand binding (e.g. binding by PAHs), AhR translocates to the nucleus (shedding the multimeric complex), forms a heterodimer with the AhR nuclear translocator (ARNT), and binds to gene promoters containing xenobiotic response elements (XRE) resulting in the trans-activation of phase I enzymes (12).
Several reports demonstrate the inhibitory effects of resveratrol on AhR-mediated activation of phase I enzymes. For example, resveratrol was shown to impair TCDD (2, 3, 7, 8-tetrachlorodibenzo-pdioxin)-induced recruitment of AhR and ARNT to the CYP1A1/1B1 and CYP1A1/1A2 promoter in MCF-7 breast and HepG2 liver cancer cells, respectively, resulting in decreased expression (13). Resveratrol also effectively blocked TCDD-induced, AhR-dependent transcription in both an estrogen receptor (ER)-dependent and ER-independent manner (14, 15). Using ER-positive cancer cells, Perdew et al.(14) observed that at micro-molar concentrations, resveratrol reduced the AhR/ARNT complex at the CYP1A1 promoter, resulting in near-complete inhibition of CYP1A1 expression and metabolic activity; however, at nano-molar concentrations, resveratrol repressed AhR-mediated induction of CYP1A1 without interfering with AhR association with XREs after TCDD exposure. On the other hand, MacPherson and Matthews (15) showed resveratrol's competitive displacement of TCDD from AhR, indicating prevention of TCDD-induced CYP1A1/1B1 expression mediated by AhR/ARNT complex recruitment to the promoter in both ER-positive and ER-negative breast cancer cells. Furthermore, resveratrol was demonstrated to reverse TCDD-induced, AhR-mediated CYP1A1 and matrix metalloproteinase 9 expression in the gastric cancer cell line AGS (16). Thus, resveratrol plays a suppressive role in AhR-mediated activation of phase I enzymes, contributing to the anticancer activity of this dietary phytoalexin.
Nuclear factor E2-related factor 2 (Nrf2)
Induction of Nrf2 signaling by resveratrol is thought to confer protection against phase I enzyme-activated carcinogens and associated carcinogenicity via the trans-activation of antioxidant and phase II detoxifying enzymes. Under basal conditions, Kelch-like ECH-associated protein 1 (Keap1) sequesters Nrf2 in the cytoplasm, targeting the transcription factor for proteasomal degradation. However, when induced by electrophiles, reactive oxygen species, or dietary phytochemicals such as resveratrol, Nrf2 dissociates from Keap1 and translocates to the nucleus where it dimerizes with small Maf proteins and activates antioxidant response element (ARE)-driven gene promoters (17).
Bishayee et al. (18) demonstrated that attenuation of DENA (diethyl nitrosamine)-induced liver carcinogenesis by resveratrol was mediated by increased Nrf2 expression. Resveratrol induction of NAD(P)H quinone oxidoreductase (NQO1) expression in TCDD-treated MCF10F immortalized breast cells involved Nrf2, leading to the suppression of DNA adduct formation (19). NQO1 was also increased by resveratrol after 4-hydroxyestradiol and estradiol-3, 4-quinone treatment (20). Induction of Nrf2 signaling by resveratrol resulted in increased expression of NQO1, heme-oxygenase 1 (HO-1), and glutamate cysteine ligase catalytic subunit in cigarette smoke extract-treated bronchial epithelial cells (21). Kode et al. (22) observed restored glutathione levels in cigarette smoke extract-treated A549 lung alveolar epithelial cancer cells by resveratrol; this effect was mediated via Nrf2-induced glutamate cysteine ligase expression and activity through the inhibition of cigarette smoke extract-modified Nrf2 post-translation. Resveratrol protected primary hepatocytes exposed to oxidative stress via increased Nrf2-mediated NQO1, catalase, superoxide dismutase, glutathione reductase, glutathione peroxidase, and glutathione-S-transferase expression (23). Furthermore, resveratrol increased NQO1 expression and activity in the K562 leukemia cell line, which was associated with resveratrol-induced Nrf2/Keap1 complex disruption, Nrf2 nuclear translocation, and subsequent binding to ARE within the NQO1 promoter (24). These results indicate that Nrf2 is a key protein that controls resveratrol-induced anti-tumorigenesis in several cancers. In contrast, Kawai et al. (25) observed Nrf2 cytoplasmic accumulation and inhibition on Nrf2-dependent transcription by resveratrol, presumably mediated through induced SIRT1 deacetylase activity, in both K562 leukemia and HepG2 hepatocellular carcinoma cell lines. Thus, resveratrol could affect not only translocation but also accumulation of Nrf2.
P53 TUMOR SUPPRESSOR
The tumor suppressor protein p53 is a critical transcription factor involved in the regulation of cell proliferation and apoptosis; as such, it is a key mediator in the prevention of carcinogenesis (26). It has been shown that resveratrol can cause apoptosis through p53-dependent and p53-independent pathways (27). Herein, we highlight reported resveratrol-induced, p53-mediated anticancer mechanisms.
Several groups, including our laboratory, have implicated the activation of p53-dependent pathway(s) in the observed anti-proliferative effects of resveratrol. For example, Heiss et al. (28) demonstrated that chronic administration of resveratrol at a sub-apoptotic dose resulted in senescent-like growth arrest in different carcinoma cell lines. This effect was due to increased reactive oxygen species generation, ataxia telangiectasia mutated kinase (ATM) and p53 activation (via p38MAPK-mediated p53 phosphorylation at serine 15), induction of p21, and subsequent induction of senescence. Resveratrol is also reported to induce apoptosis via activation of both intrinsic (mitochondria-mediated) and extrinsic (death receptor-mediated) pathways (27). Resveratrol altered the Bax:Bcl2 ratio in the A431 epidermoid cancer cell line, leading to mitochondrial membrane depolarization and subsequent induction of caspase-dependent apoptosis, presumably mediated through p53 activation (29). Using MCF-7 breast carcinoma cells, Singh et al. (30) reported resveratrol-induced G0/G1 and S phase growth arrest associated with p53 phosphorylation at serine 15, increased expression of p53-regulated pro-apoptotic proteins (p21, Bax, and Fas), caspase 8/9 activation, and decreased Bcl2 expression; similar results were observed with resveratrol and cyclophosphamide co-treatment. Furthermore, Bishayee and Dhir (31) demonstrated reduced tumor incidence resulting from resveratrol-induced apoptosis associated with increased Bax:Bcl2 ratio (increased Bax and decreased Bcl2 expression) in a diethylnitrosamine-initiated, phenobarbital-promoted in vivo model of liver carcinogenesis. Resveratrol also increased p53-mediated expression of pro-apoptotic proteins (e.g. Bax, Bak, Bim, PUMA, Noxa, etc.) and the release of mitochondria proteins (e.g. cytochrome c, Smac/DIABLO, etc.) to the cytosol, thus triggering suppression of inhibitors of apoptosis proteins (e.g. Bcl2, Bcl-XL, survivin, XIAP, etc.) and caspase activation in several cancers (9, 32-34).
How resveratrol increases p53 expression is not clear; it is believed that resveratrol treatment causes DNA damage, which facilitates p53 activation. Tyagi et al. (35) observed resveratrol-induced cell cycle arrest was mediated through increased Cdc2 tyrosine 15 phosphorylation via the activation of the DNA damage pathway (i.e. ATM/ATR-Chk1/2-Cdc25 pathway). Resveratrol treatment also suppressed the metastasis-associated protein-1/nucleosome remodeling deacetylation complex, allowing for increased p53 acetylation and subsequent activation of pro-apoptotic genes (36). Moreover, our laboratory reported that resveratrol-induced expression of the pro-apoptotic protein non-steroidal anti-inflammatory drug-activated gene-1 is mediated by p53, resulting in induction of cell death (37).
FORKHEAD BOX, SUBGROUP O (FOXO) TRANSCRIPTION FACTORS
Proteins known as FoxO, divergent members of the Fox/winged-helix transcription factor superfamily, are recognized tumor suppressors that play a critical role in cell fate decisions (38). Composed of four members (FoxO1, 3, 4, and 6), FoxO transcription factors coordinate the expression of genes involved in diverse cellular processes, including cell cycle progression, apoptosis, and oxidative stress response. The function of FoxO proteins is regulated by post-translational modification(s), specifically phosphorylation and acetylation, which dictates their subcellular localization and transcriptional activity (39).
Abrogation of FoxO function occurs in numerous cancers due in large part to the constitutive activation of the phosphatidylinositol 3-kinase (PI3K)/Akt pathway, a key regulator of FoxO activity. PI3K/Akt-mediated phosphorylation results in the inactivation of FoxO transcription factors. FoxO phosphorylation by PI3K/Akt facilitates their interaction with 14-3-3 chaperone proteins and nuclear export; cytoplasmic sequestration inhibits FoxO-dependent transcription (38). FoxOs are also regulated by de/acetylation in response to oxidative stress. Several studies identify FoxOs as targets of the NAD-dependent class III histone/protein deacetylase sirtuin 1 (SIRT1). Under conditions of oxidative stress, SIRT1 forms a complex with and deacetylates FoxO transcription factors, resulting in the preferential activation of cell cycle arrest/stress resistance-related genes, thereby promoting cell survival (40-42). Furthermore, silencing of SIRT1 resulted in FoxO4-mediated apoptosis in epithelial-derived cancer cells (43). Thus, targeting these inhibitory pathways may prove advantageous in the prevention/treatment of cancers.
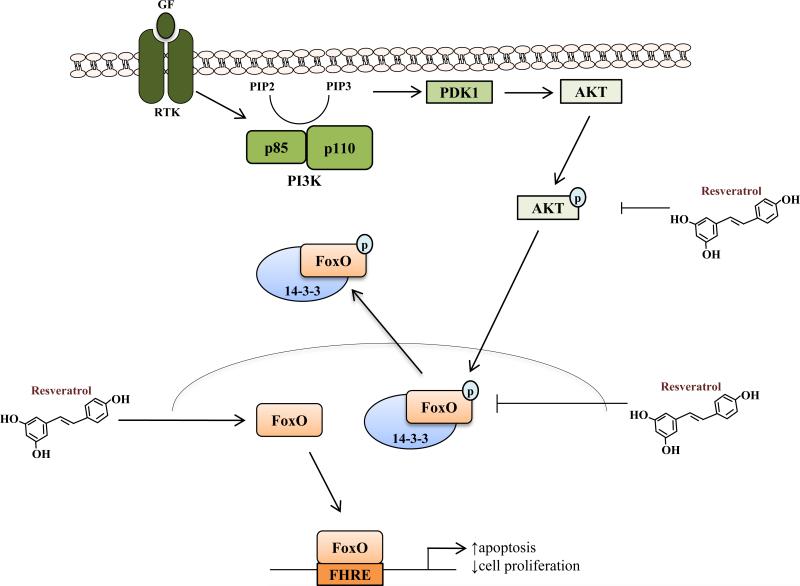
The activation of FoxO transcription factors is implicated in the observed anticancer activities of resveratrol. Using prostate cancer cells, Chen et al. (44) demonstrated resveratrol's ability to inhibit the phosphorylation of PI3K/Akt (i.e. PI3K/Akt inactivation) resulting in decreased FoxO phosphorylation. Resveratrol increased nuclear translocation, DNA binding affinity, and transcriptional activity of FoxOs. Furthermore, the anti-proliferative effect (Bim/TRAIL/DR4/DR5/p27KIP1 induction and cyclin D1 inhibition) of resveratrol on prostate cancer cells is FoxO-dependent. Similar results were also observed in vivo (45). Additionally, the anti-migratory/angiogenic effects of resveratrol observed in human umbilical vein epithelial cells were FoxO-dependent and predicated on PI3K/Akt pathway inhibition (46). A schematic representation of resveratrol-mediated FoxO regulation is shown in Fig. 2.
Fig. 2. Effects of resveratrol on the FoxO signaling pathway.
PI3K/Akt-mediated phosphorylation results in the inactivation of FoxO transcription factors. FoxO phosphorylation by PI3K/Akt facilitates FoxO interaction with 14-3-3 chaperone proteins and nuclear export; cytoplasmic sequestration inhibits FoxO-dependent transcription (38). Resveratrol blocks Akt activation and subsequent FoxO phosphorylation and nuclear export; on the other hand, resveratrol-induced FoxO expression facilitates trans-activation of anti-proliferative/pro-apoptotic forkhead response element (FHRE)-driven promoters (see text for references). GF, growth factor; RTK, receptor tyrosine kinase.
As described, SIRT1-induced FoxO deacetylation results in the preferential activation of cell survival-related genes. Being that resveratrol is considered a SIRT1 agonist, although currently controversial, one could speculate the activation of a FoxO-mediated pro-survival mechanism induced by resveratrol may occur through increased SIRT1 deacetylase activity. Indeed, this is demonstrated in cardiomyocytes; resveratrol, presumably through SIRT1, increased FoxO transcriptional activation of stress resistance-related genes and subsequent phosphorylation by Akt (47). However, to the best of our knowledge, the resveratrol/SIRT1/FoxO signaling axis has not been studied in cancer cells thus allowing one to infer such an effect is context dependent.
NUCLEAR FACTOR-κB (NF-κB)
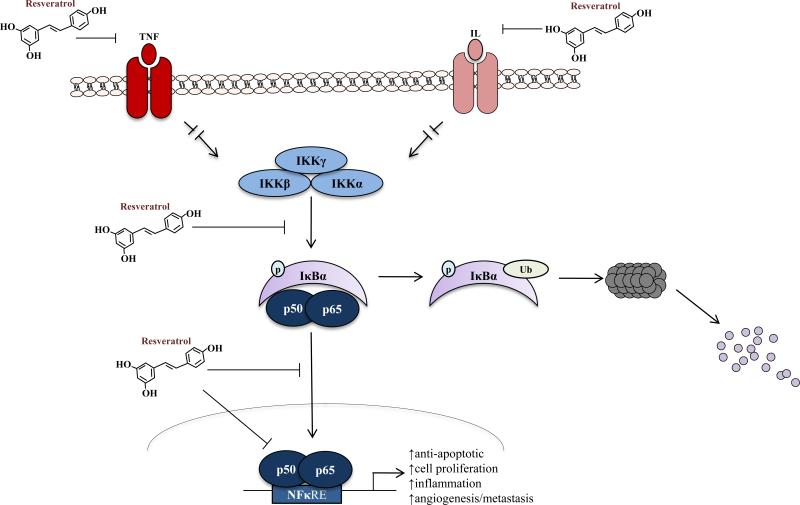
The link between inflammation and cancer is well established; these inflammatory processes contribute to the development and progression of carcinogenesis, including tumor growth, angiogenesis, invasion, and metastasis (48). A key mediator of inflammation-induced cellular transformation is the transcription factor NF-κB. Cytoplasmic sequestration by IκBα prevents NF-κB-dependent trans-activation. Upon activation, IKKβ phosphorylates IκBα, resulting in its degradation, and facilitates NF-κB nuclear translocation and subsequent activation of transcription (49).
It has been postulated that the anticancer effects of resveratrol are attributable to the inactivation of NF-κB-dependent signaling. Resveratrol inhibited IKKβ-mediated IκBα phosphorylation, resulting in increased IκBα expression, NF-κB cytoplasmic retention, and subsequent NF-κB inactivation (50, 51). Resveratrol also blocked interleukin-1β (IL-1β)-, tumor necrosis factor α (TNF-α)-, and HO-1-induced NF-κB activation (52-54). Resveratrol treatment of multiple myeloma cells resulted in cell cycle arrest and suppression of NF-κB-dependent signaling related to proliferation (cyclin D1), survival (Bcl2, Bcl-XL, XIAP, c-IAP, and survivin), angiogenesis (vascular endothelial growth factor), and metastasis (matrix metalloproteinase 9 and IL-6) (6, 55). Similar results were observed in vivo using a DENA-initiated liver carcinogenesis model (56) and Mia PaCa-2 orthotopic model of pancreatic cancer (8). Furthermore, resveratrol prevented PMA (phorbol 12-myristate 12-acetate)-induced HT1080 fibrosarcoma cell adhesion to endothelial cells via modulation of intercellular adhesion molecule-1 expression and NF-κB activity (57). Taken together, it is clear that resveratrol not only curbs expression of NF-κB, but also impedes the phosphorylation of IκBα thereby keeping the constitutive NF-κB subunit in an inactive state, resulting in suppression of the inflammatory and pro-tumorigenic changes associated with this pathway (Fig. 3).
Fig. 3. Effects of resveratrol on the NF-κB signaling pathway.
Activation by cytokine results in IKK phosphorylation of IκBα, resulting in IκBα degradation, and facilitation of NF-κB nuclear translocation and subsequent activation of transcription (49). Resveratrol is demonstrated it inhibit NF-κB signaling at all steps (see text for references).
ACTIVATING TRANSCRIPTION FACTOR 3 (ATF3)
Recently, our laboratory identified ATF3 as a novel molecular target of resveratrol in colorectal carcinoma cells (58). ATF3, a member of the ATF/CREB family of transcription factors, is characterized as a stress-inducible or adaptive response gene (59). Much controversy exists as to the role of ATF3 in tumorigenesis, and ATF3 is demonstrated to be a positive or negative modulator of tumor progression. However, several lines of evidence suggest that ATF3 may function as a tumor suppressor gene in colorectal carcinogenesis. Firstly, ATF3 expression is markedly reduced in cancer tissues when compared to normal adjacent tissue (60). Secondly, ATF3 over-expression is demonstrated to elicit a number of cellular responses, including inhibition of proliferation (61), induction of apoptosis (62-65), inhibition of invasion and associated genes (66-68), and retardation of tumor formation in vivo (63, 66). Finally, ATF3 is reported to mediate or enhance induction of apoptosis by compounds demonstrated to possess anti-tumor properties (64, 69-72). Thus, it is believed that ATF3 plays an anti-tumorigenic role in colorectal cancer.
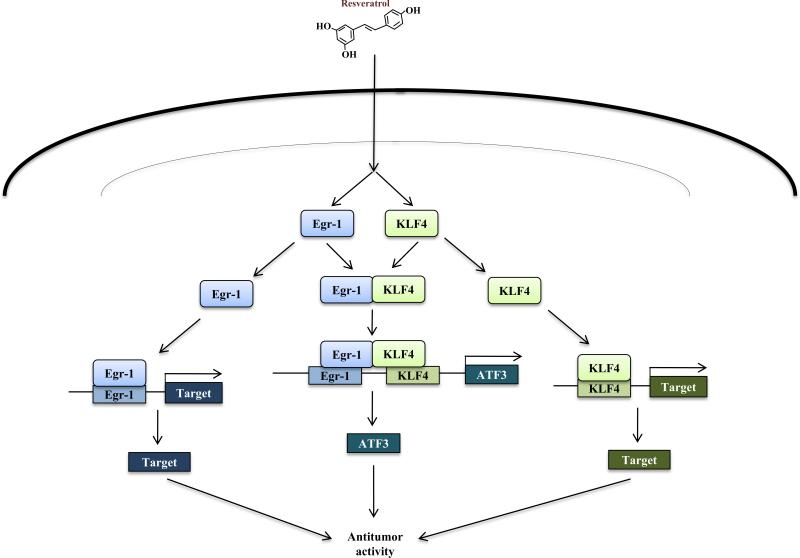
As stated, we identified ATF3 as a novel target of resveratrol in colorectal cancer cells and showed resveratrol involvement in the transcription factor's regulation. Specifically, resveratrol-induced ATF3 expression is mediated by the induction and interaction of C2H2-type zinc finger transcription factors early growth response-1 (Egr-1) and Krüppel-like factor 4 (KLF4). Egr-1 and KLF4 belong to a family of immediate early response transcription factor genes whose expression is transiently induced in response to various environmental stimuli (73, 74). Both Egr-1 and KLF4 are suggested to act as master regulatory proteins involved in cell fate decisions (75, 76); as such, these transcription factors coordinate the expression of genes associated with cell proliferation, differentiation, and apoptosis (74, 77, 78). Nonetheless, as with ATF3, much controversy exists as to the role of Egr-1 and KLF4 in cancer development, and their biological function appears largely context dependent. Several studies have demonstrated that Egr-1 and KLF4 expression facilitates tumor progression in vivo (79-81); however, there is ample evidence supporting a tumor suppressive role for both transcription factors (82-88). Because resveratrol can increase Egr-1 and KLF4 expression and transcription activity, one could speculate that observed anticancer properties of resveratrol may be facilitated through either an Egr-1-mediated or KLF4-mediated mechanism (Fig. 4). Furthermore, increased ATF3 expression by resveratrol facilitated induction of apoptosis, at least partially, by the dietary compound (58). However, continued validation of ATF3 as a target of resveratrol is necessary to determine if such phenomena are specific to colorectal cancer cells or can be observed in other cancer phenotypes, both in vitro and in vivo.
Fig. 4. Schematic diagram of ATF3-mediated resveratrol action in colorectal cancer.
Resveratrol increases the expression of both Egr-1 and KLF4, facilitating their interaction. The Egr-1/KLF4 complex binds to its response elements on the ATF3 promoter and mediates ATF3 trans-activation. Increased ATF3 expression results in increase of antitumor activity (58). Alternatively, resveratrol-increased Egr-1 or KLF4 expression results in trans-activation of antitumor-related target genes.
CONCLUDING REMARKS
The continued identification of dietary phytochemicals and their related derivatives with chemopreventive and/or chemotherapeutic activities offers an alternative and complementary approach to the prevention and treatment of cancers. Studies investigating the use of these compounds for cancer prevention or as adjuvants to traditional treatment have revealed several potential benefits: (1) suppression of tumorigenesis and carcinogenesis in vitro and in vivo, (2) sensitization of cancer cells to drug-induced growth inhibition, and (3) minimization of adverse effects associated with conventional therapies. Most importantly, dietary phytochemicals are demonstrated to target multiple signal transduction pathways involved in tumorigenesis and carcinogenesis, an important advantage due to the inherent heterogeneity of cancers. Thus, it is not surprising that the number of studies involving dietary phytochemicals, such as resveratrol, has dramatically increased in the past decade.
As discussed in previous sections, resveratrol is demonstrated to modulate the expression and/or activity of transcription factors involved in critical pathways of carcinogenesis, including carcinogen activation and detoxification, growth arrest and apoptosis, and pro-inflammatory-mediated signaling pathways (e.g. inflammation-promoted cell proliferation, angiogenesis, invasion, and metastasis). Resveratrol is demonstrated to participate in both pro-survival and pro-death cellular mechanisms, either by favoring the preservation of the functional status of cells and possibly elongating cellular life span or inducing death of those cells whose physiological conditions have become deranged. Resveratrol also affects the transcriptional machinery resulting in trans-activation of key regulatory proteins. Thus, resveratrol may function as a signaling pathway modulator and as such, is demonstrated to affect a multitude of signal transduction pathways associated with tumorigenesis and/or carcinogenesis through the alteration of key transcription factors. Yet, it is likely that the observed anticancer properties of resveratrol are due to the collective modification of numerous signaling pathways, rather than just a single effect. Because transcription factors control the expression of many genes, the elucidation of molecular targets of resveratrol involved in transcriptional regulation is necessary to better understand how this dietary phytochemical affects chemopreventive and chemotherapeutic processes. As a result, researchers continue to investigate the molecular and cellular effects of resveratrol in cancer in hopes of unraveling the mysteries of this fascinating and promising dietary molecule.
ACKNOWLEDGEMENT
We thank Misty Bailey for her critical reading of this manuscript. This work was supported by the American Cancer Society grant CNE-111611, NIH grant RO1CA108975, and The University of Tennessee Center of Excellence in Livestock Diseases and Human Health.
REFERENCES
- 1.Surh Y-J. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 2.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 3.Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 4.Kundu JK, Surh Y-J. Cancer chemopreventive and therapeutic potential of resveratrol: Mechanistic perspectives. Cancer Lett. 2008;269:243–261. doi: 10.1016/j.canlet.2008.03.057. [DOI] [PubMed] [Google Scholar]
- 5.Bishayee A. Cancer prevention and treatment with resveratrol: from rodent studies to clinical trials. Cancer Prev Res. 2009;2:409–418. doi: 10.1158/1940-6207.CAPR-08-0160. [DOI] [PubMed] [Google Scholar]
- 6.Bhardwaj A, Sethi G, Vadhan-Raj S, Bueso-Ramos C, Takada Y, et al. Resveratrol inhibits proliferation, induces apoptosis, and overcomes chemoresistance through down-regulation of STAT3 and nuclear factor-κB-regulated antiapoptotic and cell survival gene products in human multiple myeloma cells. Blood. 2007;109:2293–2302. doi: 10.1182/blood-2006-02-003988. [DOI] [PubMed] [Google Scholar]
- 7.Gatouillat G, Balasse E, Joseph-Pietras D, Morjani H, Madoulet C. Resveratrol induces cell-cycle disruption and apoptosis in chemoresistant B16 melanoma. J Cell Biochem. 2010;110:893–902. doi: 10.1002/jcb.22601. [DOI] [PubMed] [Google Scholar]
- 8.Harikumar KB, Kunnumakkara AB, Sethi G, Diagaradjane P, Anand P, et al. Resveratrol, a multitargeted agent, can enhance antitumor activity of gemcitabine in vitro and in orthotopic mouse model of human pancreatic cancer. Int J Cancer. 2010;127:257–268. doi: 10.1002/ijc.25041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shankar S, Chen Q, Siddiqui I, Sarva K, Srivastava R. Sensitization of TRAIL-resistant LNCaP cells by resveratrol (3, 4', 5 tri-hydroxystilbene): molecular mechanisms and therapeutic potential. J Mol Signal. 2007;2:7. doi: 10.1186/1750-2187-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Androutsopoulos V, Tsatsakis A, Spandidos D. Cytochrome P450 CYP1A1: wider roles in cancer progression and prevention. BMC Cancer. 2009;9:187. doi: 10.1186/1471-2407-9-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee J-E, Safe S. Involvement of a post-transcriptional mechanism in the inhibition of CYP1A1 expression by resveratrol in breast cancer cells. Biochem Pharmacol. 2001;62:1113–1124. doi: 10.1016/s0006-2952(01)00763-8. [DOI] [PubMed] [Google Scholar]
- 12.Dietrich C, Kaina B. The aryl hydrocarbon receptor (AhR) in the regulation of cell–cell contact and tumor growth. Carcinogenesis. 2010;31:1319–1328. doi: 10.1093/carcin/bgq028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beedanagari SR, Bebenek I, Bui P, Hankinson O. Resveratrol inhibits dioxin-induced expression of human CYP1A1 and CYP1B1 by inhibiting recruitment of the aryl hydrocarbon receptor complex and RNA Polymerase II to the regulatory regions of the corresponding genes. Toxicol Sci. 2009;110:61–67. doi: 10.1093/toxsci/kfp079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perdew GH, Hollingshead BD, DiNatale BC, Morales JL, Labrecque MP, et al. Estrogen receptor expression is required for low-dose resveratrol-mediated repression of aryl hydrocarbon receptor activity. J Pharmacol Exp Ther. 2010;335:273–283. doi: 10.1124/jpet.110.170654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacPherson L, Matthews J. Inhibition of aryl hydrocarbon receptor-dependent transcription by resveratrol or kaempferol is independent of estrogen receptor α expression in human breast cancer cells. Cancer Lett. 2010;299:119–129. doi: 10.1016/j.canlet.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peng T-L, Chen J, Mao W, Song X, Chen M-H. Aryl hydrocarbon receptor pathway activation enhances gastric cancer cell invasiveness likely through a c-Jun-dependent induction of matrix metalloproteinase-9. BMC Cell Biology. 2009;10:27. doi: 10.1186/1471-2121-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kensler TW, Wakabayashi N. Nrf2: friend or foe for chemoprevention? Carcinogenesis. 2010;31:90–99. doi: 10.1093/carcin/bgp231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bishayee A, Barnes KF, Bhatia D, Darvesh AS, Carroll RT. Resveratrol suppresses oxidative stress and inflammatory response in diethylnitrosamine-initiated rat hepatocarcinogenesis. Cancer Prev Res. 2010;3:753–763. doi: 10.1158/1940-6207.CAPR-09-0171. [DOI] [PubMed] [Google Scholar]
- 19.Lu F, Zahid M, Wang C, Saeed M, Cavalieri EL, et al. Resveratrol prevents estrogen-DNA adduct formation and neoplastic transformation in MCF-10F cells. Cancer Prev Res. 2008;1:135–145. doi: 10.1158/1940-6207.CAPR-08-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zahid M, Gaikwad NW, Ali MF, Lu F, Saeed M, et al. Prevention of estrogen-DNA adduct formation in MCF-10F cells by resveratrol. Free Radic Biol Med. 2008;45:136–145. doi: 10.1016/j.freeradbiomed.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang H, Shih A, Rinna A, Forman HJ. Exacerbation of tobacco smoke mediated apoptosis by resveratrol: An unexpected consequence of its antioxidant action. Int J Biochem Cell Biol. 2011;43:1059–1064. doi: 10.1016/j.biocel.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kode A, Rajendrasozhan S, Caito S, Yang S-R, Megson IL, et al. Resveratrol induces glutathione synthesis by activation of Nrf2 and protects against cigarette smoke-mediated oxidative stress in human lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2008;294:L478–488. doi: 10.1152/ajplung.00361.2007. [DOI] [PubMed] [Google Scholar]
- 23.Rubiolo JA, Mithieux G, Vega FV. Resveratrol protects primary rat hepatocytes against oxidative stress damage: Activation of the Nrf2 transcription factor and augmented activities of antioxidant enzymes. Eur J Pharmacol. 2008;591:66–72. doi: 10.1016/j.ejphar.2008.06.067. [DOI] [PubMed] [Google Scholar]
- 24.Hsieh T-c, Lu X, Wang Z, Wu JM. Induction of quinone reductase NQO1 by resveratrol in human K562 cells involves the antioxidant response element are and is accompanied by nuclear translocation of transcription factor Nrf2. Med Chem. 2006;2:275–285. doi: 10.2174/157340606776930709. [DOI] [PubMed] [Google Scholar]
- 25.Kawai Y, Garduño L, Theodore M, Yang J, Arinze IJ. Acetylation-deacetylation of the transcription factor Nrf2 (nuclear factor erythroid 2-related factor 2) regulates its transcriptional activity and nucleocytoplasmic localization. J Biol Chem. 2011;286:7629–7640. doi: 10.1074/jbc.M110.208173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farnebo M, Bykov VJN, Wiman KG. The p53 tumor suppressor: A master regulator of diverse cellular processes and therapeutic target in cancer. Biochem Biophys Res Commun. 2010;396:85–89. doi: 10.1016/j.bbrc.2010.02.152. [DOI] [PubMed] [Google Scholar]
- 27.Fan E, Jiang S, Zhang L, Bai Y. Molecular mechanism of apoptosis induction by resveratrol, a natural cancer chemopreventive agent. Int J Vitam Nutr Res. 2008;78:3–8. doi: 10.1024/0300-9831.78.1.3. [DOI] [PubMed] [Google Scholar]
- 28.Heiss EH, Schilder YDC, Dirsch VM. Chronic treatment with resveratrol induces redox stress- and ataxia telangiectasia-mutated (atm)-dependent senescence in p53-positive cancer cells. J Biol Chem. 2007;282:26759–26766. doi: 10.1074/jbc.M703229200. [DOI] [PubMed] [Google Scholar]
- 29.Madan E, Prasad S, Roy P, George J, Shukla Y. Regulation of apoptosis by resveratrol through JAK/STAT and mitochondria mediated pathway in human epidermoid carcinoma A431 cells. Biochem Biophys Res Commun. 2008;377:1232–1237. doi: 10.1016/j.bbrc.2008.10.158. [DOI] [PubMed] [Google Scholar]
- 30.Singh N, Nigam M, Ranjan V, Sharma R, Balapure AK, et al. Caspase mediated enhanced apoptotic action of cyclophosphamide-and resveratrol-treated MCF-7 cells. J Pharmacol Sci. 2009;109:472–485. doi: 10.1254/jphs.08173fp. [DOI] [PubMed] [Google Scholar]
- 31.Bishayee A, Dhir N. Resveratrol-mediated chemoprevention of diethylnitrosamine-initiated hepatocarcinogenesis: Inhibition of cell proliferation and induction of apoptosis. Chem Biol Interact. 2009;179:131–144. doi: 10.1016/j.cbi.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 32.Shankar S, Siddiqui I, Srivastava R. Molecular mechanisms of resveratrol (3,4,5-trihydroxy-trans-stilbene) and its interaction with TNF-related apoptosis inducing ligand (TRAIL) in androgen-insensitive prostate cancer cells. Mol Cell Biochem. 2007;304:273–285. doi: 10.1007/s11010-007-9510-x. [DOI] [PubMed] [Google Scholar]
- 33.Jiang H, Zhang L, Kuo J, Kuo K, Gautam SC, et al. Resveratrol-induced apoptotic death in human U251 glioma cells. Mol Cancer Ther. 2005;4:554–561. doi: 10.1158/1535-7163.MCT-04-0056. [DOI] [PubMed] [Google Scholar]
- 34.Jazirehi AR, Bonavida B. Resveratrol modifies the expression of apoptotic regulatory proteins and sensitizes non-Hodgkin's lymphoma and multiple myeloma cell lines to paclitaxel-induced apoptosis. Mol Cancer Ther. 2004;3:71–84. [PubMed] [Google Scholar]
- 35.Tyagi A, Singh RP, Agarwal C, Siriwardana S, Sclafani RA, et al. Resveratrol causes Cdc2-tyr15 phosphorylation via ATM/ATR–Chk1/2–Cdc25C pathway as a central mechanism for S phase arrest in human ovarian carcinoma Ovcar-3 cells. Carcinogenesis. 2005;26:1978–1987. doi: 10.1093/carcin/bgi165. [DOI] [PubMed] [Google Scholar]
- 36.Kai L, Samuel SK, Levenson AS. Resveratrol enhances p53 acetylation and apoptosis in prostate cancer by inhibiting MTA1/NuRD complex. Int J Cancer. 2010;126:1538–1548. doi: 10.1002/ijc.24928. [DOI] [PubMed] [Google Scholar]
- 37.Baek SJ, Wilson LC, Eling TE. Resveratrol enhances the expression of non-steroidal anti-inflammatory drug-activated gene (NAG-1) by increasing the expression of p53. Carcinogenesis. 2002;23:425–432. doi: 10.1093/carcin/23.3.425. [DOI] [PubMed] [Google Scholar]
- 38.Fu Z, Tindall DJ. FOXOs, cancer and regulation of apoptosis. Oncogene. 2008;27:2312–2319. doi: 10.1038/onc.2008.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang X, Tang N, Hadden TJ, Rishi AK. Akt, FoxO and regulation of apoptosis. Biochim Biophys Acta. doi: 10.1016/j.bbamcr.2011.03.010. In Press. [DOI] [PubMed] [Google Scholar]
- 40.Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 41.van der Horst A, Tertoolen LGJ, de Vries-Smits LMM, Frye RA, Medema RH, et al. FOXO4 is acetylated upon peroxide stress and deacetylated by the longevity protein hSir2SIRT1. J Biol Chem. 2004;279:28873–28879. doi: 10.1074/jbc.M401138200. [DOI] [PubMed] [Google Scholar]
- 42.Yang Y, Hou H, Haller EM, Nicosia SV, Bai W. Suppression of FOXO1 activity by FHL2 through SIRT1-mediated deacetylation. EMBO J. 2005;24:1021–1032. doi: 10.1038/sj.emboj.7600570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ford J, Jiang M, Milner J. Cancer-specific functions of SIRT1 enable human epithelial cancer cell growth and survival. Cancer Res. 2005;65:10457–10463. doi: 10.1158/0008-5472.CAN-05-1923. [DOI] [PubMed] [Google Scholar]
- 44.Chen Q, Ganapathy S, Singh KP, Shankar S, Srivastava RK. Resveratrol induces growth arrest and apoptosis through activation of FOXO transcription factors in prostate cancer cells. PLoS ONE. 2010;5:e15288. doi: 10.1371/journal.pone.0015288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ganapathy S, Chen Q, Singh KP, Shankar S, Srivastava RK. Resveratrol enhances antitumor activity of TRAIL in prostate cancer xenografts through activation of FOXO transcription factor. PLoS ONE. 2010;5:e15627. doi: 10.1371/journal.pone.0015627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Srivastava R, Unterman T, Shankar S. FOXO transcription factors and VEGF neutralizing antibody enhance antiangiogenic effects of resveratrol. Mol Cell Biochem. 2010;337:201–212. doi: 10.1007/s11010-009-0300-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ni YG, Wang N, Cao DJ, Sachan N, Morris DJ, et al. FoxO transcription factors activate Akt and attenuate insulin signaling in heart by inhibiting protein phosphatases. Proc Natl Acad Sci. 2007;104:20517–20522. doi: 10.1073/pnas.0610290104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu Y, Zhou B. Inflammation: a driving force speed cancer metastasis. Cell Cycle. 2009;8:3267–3273. doi: 10.4161/cc.8.20.9699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Muriel P. NF-κB in liver diseases: a target for drug therapy. J Appl Toxicol. 2009;29:91–100. doi: 10.1002/jat.1393. [DOI] [PubMed] [Google Scholar]
- 50.Roy P, Kalra N, Nigam N, George J, Ray RS, et al. Resveratrol enhances ultraviolet B-induced cell death through nuclear factor-κB pathway in human epidermoid carcinoma A431 cells. Biochem Biophys Res Commun. 2009;384:215–220. doi: 10.1016/j.bbrc.2009.04.100. [DOI] [PubMed] [Google Scholar]
- 51.Benitez DA, Hermoso MA, Pozo-Guisado E, Fernández-Salguero PM, Castellón EA. Regulation of cell survival by resveratrol involves inhibition of NFκB-regulated gene expression in prostate cancer cells. The Prostate. 2009;69:1045–1054. doi: 10.1002/pros.20953. [DOI] [PubMed] [Google Scholar]
- 52.Estrov Z, Shishodia S, Faderl S, Harris D, Van Q, et al. Resveratrol blocks interleukin-1{beta}-induced activation of the nuclear transcription factor NF-κB, inhibits proliferation, causes S-phase arrest, and induces apoptosis of acute myeloid leukemia cells. Blood. 2003;102:987–995. doi: 10.1182/blood-2002-11-3550. [DOI] [PubMed] [Google Scholar]
- 53.Yu H, Pan C, Zhao S, Wang Z, Zhang H, et al. Resveratrol inhibits tumor necrosis factor-α-mediated matrix metalloproteinase-9 expression and invasion of human hepatocellular carcinoma cells. Biomed Pharmacother. 2008;62:366–372. doi: 10.1016/j.biopha.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 54.Liu P-L, Tsai J-R, Charles AL, Hwang J-J, Chou S-H, et al. Resveratrol inhibits human lung adenocarcinoma cell metastasis by suppressing heme oxygenase 1-mediated nuclear factor-kappaB pathway and subsequently downregulating expression of matrix metalloproteinases. Mol Nutr Food Res. 2010;54:S196–S204. doi: 10.1002/mnfr.200900550. [DOI] [PubMed] [Google Scholar]
- 55.Sun C, Hu Y, Liu X, Wu T, Wang Y, et al. Resveratrol downregulates the constitutional activation of nuclear factor-κB in multiple myeloma cells, leading to suppression of proliferation and invasion, arrest of cell cycle, and induction of apoptosis. Cancer Genet Cytogenet. 2006;165:9–19. doi: 10.1016/j.cancergencyto.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 56.Bishayee A, Waghray A, Barnes K, Mbimba T, Bhatia D, et al. Suppression of the inflammatory cascade is implicated in resveratrol chemoprevention of experimental hepatocarcinogenesis. Pharm Res. 2010;27:1080–1091. doi: 10.1007/s11095-010-0144-4. [DOI] [PubMed] [Google Scholar]
- 57.Park J, Kim K, Kim M, Chang H, Baek M, et al. Resveratrol inhibits tumor cell adhesion to endothelial cells by blocking ICAM-1 expression. Anticancer Res. 2009;29:355–362. [PubMed] [Google Scholar]
- 58.Whitlock NC, Bahn JH, Lee S-H, Eling TE, Baek SJ. Resveratrol-induced apoptosis is mediated by early growth response-1, krüppel-like factor 4, and activating transcription factor 3. Cancer Prev Res. 2011;4:116–127. doi: 10.1158/1940-6207.CAPR-10-0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lu D, Chen J, Hai T. The regulation of ATF3 gene expression by mitogen-activated protein kinases. Biochem J. 2007;401:559–567. doi: 10.1042/BJ20061081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yan C, Boyd DD. ATF3 regulates the stability of p53: a link to cancer. Cell Cycle. 2006;5:926–929. doi: 10.4161/cc.5.9.2714. [DOI] [PubMed] [Google Scholar]
- 61.Fan F, Jin S, Amundson SA, Tong T, Fan W, et al. ATF3 induction following DNA damage is regulated by distinct signaling pathways and over-expression of ATF3 protein suppresses cell growth. Oncogene. 2002;21:7488–7495. doi: 10.1038/sj.onc.1205896. [DOI] [PubMed] [Google Scholar]
- 62.Huang X, Li X, Guo B. KLF6 induces apoptosis in prostate cancer cells through up-regulation of ATF3. J Biol Chem. 2008;283:29795–29801. doi: 10.1074/jbc.M802515200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lu D, Wolfgang CD, Hai T. Activating transcription factor 3, a stress-inducible gene, suppresses Ras-stimulated tumorigenesis. J Biol Chem. 2006;281:10473–10481. doi: 10.1074/jbc.M509278200. [DOI] [PubMed] [Google Scholar]
- 64.Yamaguchi K, Lee S-H, Kim J-S, Wimalasena J, Kitajima S, et al. Activating Transcription factor 3 and early growth response 1 are the novel targets of LY294002 in a phosphatidylinositol 3-kinase–independent pathway. Cancer Res. 2006;66:2376–2384. doi: 10.1158/0008-5472.CAN-05-1987. [DOI] [PubMed] [Google Scholar]
- 65.Turchi L, Aberdam E, Mazure N, Pouyssegur J, Deckert M, et al. Hif-2alpha mediates UV-induced apoptosis through a novel ATF3-dependent death pathway. Cell Death Differ. 2008;15:1472–1480. doi: 10.1038/cdd.2008.74. [DOI] [PubMed] [Google Scholar]
- 66.Bottone FG, Moon Y, Kim JS, Alston-Mills B, Ishibashi M, et al. The anti-invasive activity of cyclooxygenase inhibitors is regulated by the transcription factor ATF3 (activating transcription factor 3). Mol Cancer Ther. 2005;4:693–703. doi: 10.1158/1535-7163.MCT-04-0337. [DOI] [PubMed] [Google Scholar]
- 67.Stearns ME, Kim G, Garcia F, Wang M. Interleukin-10 induced activating transcription factor 3 transcriptional suppression of matrix metalloproteinase-2 gene expression in human prostate CPTX-1532 cells. Mol Cancer Res. 2004;2:403–416. [PubMed] [Google Scholar]
- 68.Yan C, Wang H, Boyd DD. ATF3 represses 72-kDa type IV collagenase (MMP-2) expression by antagonizing p53-dependent trans-activation of the collagenase promoter. J Biol Chem. 2002;277:10804–10812. doi: 10.1074/jbc.M112069200. [DOI] [PubMed] [Google Scholar]
- 69.Lee S-H, Kim J-S, Yamaguchi K, Eling TE, Baek SJ. Indole-3-carbinol and 3,3'-diindolylmethane induce expression of NAG-1 in a p53-independent manner. Biochem Biophys Res Commun. 2005;328:63–69. doi: 10.1016/j.bbrc.2004.12.138. [DOI] [PubMed] [Google Scholar]
- 70.Yan C, Jamaluddin MS, Aggarwal B, Myers J, Boyd DD. Gene expression profiling identifies activating transcription factor 3 as a novel contributor to the proapoptotic effect of curcumin. Mol Cancer Ther. 2005;4:233–241. [PubMed] [Google Scholar]
- 71.Mashima T, Udagawa S, Tsuruo T. Involvement of transcriptional repressor ATF3 in acceleration of caspase protease activation during DNA damaging agent-induced apoptosis. J Cell Physiol. 2001;188:352–358. doi: 10.1002/jcp.1130. [DOI] [PubMed] [Google Scholar]
- 72.Wang Q, Mora-Jensen H, Weniger MA, Perez-Galan P, Wolford C, et al. ERAD inhibitors integrate ER stress with an epigenetic mechanism to activate BH3-only protein NOXA in cancer cells. Proc Natl Acad Sci. 2009;106:2200–2205. doi: 10.1073/pnas.0807611106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thiel G, Cibelli G. Regulation of life and death by the zinc finger transcription factor Egr-1. J Cell Physiol. 2002;193:287–292. doi: 10.1002/jcp.10178. [DOI] [PubMed] [Google Scholar]
- 74.Zhang W, Geiman DE, Shields JM, Dang DT, Mahatan CS, et al. The gut-enriched Krüppel-like Factor (Krüppel-like Factor 4) mediates the transactivating effect of p53 on the p21WAF1/Cip1 promoter. J Biol Chem. 2000;275:18391–18398. doi: 10.1074/jbc.C000062200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yan S-F, Fujita T, Lu J, Okada K, Shan Zou Y, et al. Egr-1, a master switch coordinating upregulation of divergent gene families underlying ischemic stress. Nat Med. 2000;6:1355–1361. doi: 10.1038/82168. [DOI] [PubMed] [Google Scholar]
- 76.Zhou Q, Hong Y, Zhan Q, Shen Y, Liu Z. Role for Kruppel-like factor 4 in determining the outcome of p53 response to DNA damage. Cancer Res. 2009;69:8284–8292. doi: 10.1158/0008-5472.CAN-09-1345. [DOI] [PubMed] [Google Scholar]
- 77.Liu C, Rangnekar V, Adamson E, Mercola D. Suppression of growth and transformation and induction of apoptosis by EGR-1. Cancer Gene Ther. 1998;5:3–28. [PubMed] [Google Scholar]
- 78.Yoon HS, Chen X, Yang VW. Kruppel-like factor 4 mediates p53-dependent G1/S cell cycle arrest in response to DNA damage. J Biol Chem. 2003;278:2101–2105. doi: 10.1074/jbc.M211027200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Baron V, De Gregorio G, Krones-Herzig A, Virolle T, Calogero A, et al. Inhibition of Egr-1 expression reverses transformation of prostate cancer cells in vitro and in vivo. Oncogene. 2003;22:4194–4204. doi: 10.1038/sj.onc.1206560. [DOI] [PubMed] [Google Scholar]
- 80.Chen Y-J, Wu C-Y, Chang C-C, Ma C-J, Li M-C, et al. Nuclear Kruppel-like factor 4 expression is associated with human skim squamous cell carcinoma progression and metastasis. Cancer Biol Ther. 2008;7:777–782. doi: 10.4161/cbt.7.5.5768. [DOI] [PubMed] [Google Scholar]
- 81.Pandya AY, Talley LI, Frost AR, Fitzgerald TJ, Trivedi V, et al. Nuclear localization of KLF4 is associated with an aggressive phenotype in early-stage breast cancer. Clin Cancer Res. 2004;10:2709–2719. doi: 10.1158/1078-0432.ccr-03-0484. [DOI] [PubMed] [Google Scholar]
- 82.Lee S-H, Bahn JH, Choi CK, Whitlock NC, English AE, et al. ESE-1/EGR-1 pathway plays a role in tolfenamic acid-induced apoptosis in colorectal cancer cells. Mol Cancer Ther. 2008;7:3739–3750. doi: 10.1158/1535-7163.MCT-08-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Choi BH, Kim CG, Bae Y-S, Lim Y, Lee YH, et al. p21Waf1/Cip1 expression by curcumin in U-87MG human glioma cells: role of early growth response-1 expression. Cancer Res. 2008;68:1369–1377. doi: 10.1158/0008-5472.CAN-07-5222. [DOI] [PubMed] [Google Scholar]
- 84.Park SE, Lee SW, Hossain MA, Kim MY, Kim M-N, et al. A chenodeoxycholic derivative, HS-1200, induces apoptosis and cell cycle modulation via Egr-1 gene expression control on human hepatoma cells. Cancer Lett. 2008;270:77–86. doi: 10.1016/j.canlet.2008.04.038. [DOI] [PubMed] [Google Scholar]
- 85.Zagurovskaya M, Shareef MM, Das A, Reeves A, Gupta S, et al. EGR-1 forms a complex with YAP-1 and upregulates Bax expression in irradiated prostate carcinoma cells. Oncogene. 2009;28:1121–1131. doi: 10.1038/onc.2008.461. [DOI] [PubMed] [Google Scholar]
- 86.Ghaleb AM, McConnell BB, Nandan MO, Katz JP, Kaestner KH, et al. Haploinsufficiency of Kruppel-like factor 4 promotes adenomatous polyposis coli dependent intestinal tumorigenesis. Cancer Res. 2007;67:7147–7154. doi: 10.1158/0008-5472.CAN-07-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hu W, Hofstetter WL, Li H, Zhou Y, He Y, et al. Putative tumor-suppressive function of Krüppel-like factor 4 in primary lung carcinoma. Clin Cancer Res. 2009;15:5688–5695. doi: 10.1158/1078-0432.CCR-09-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wei D, Kanai M, Jia Z, Le X, Xie K. Kruppel-like factor 4 induces p27Kip1 expression in and suppresses the growth and metastasis of human pancreatic cancer cells. Cancer Res. 2008;68:4631–4639. doi: 10.1158/0008-5472.CAN-07-5953. [DOI] [PMC free article] [PubMed] [Google Scholar]