Abstract
An aging population in the developing world has led to an increase in musculoskeletal diseases such as osteoporosis and bone metastases. Left untreated many bone diseases cause debilitating pain and in the case of cancer, death. Many potential drugs are effective in treating diseases but result in side effects preventing their efficacy in the clinic. Bone, however, provides an unique environment of inorganic solids, which can be exploited in order to effectively target drugs to diseased tissue. By integration of bone targeting moieties to drug-carrying water-soluble polymers, the payload to diseased area can be increased while side effects decreased. The realization of clinically relevant bone targeted polymer therapeutics depends on (1) understanding bone targeting moiety interactions, (2) development of controlled drug delivery systems, as well as (3) understanding drug interactions. The latter makes it possible to develop bone targeted synergistic drug delivery systems.
Keywords: Bone-targeting, Drug delivery, Cathepsin K, HPMA copolymer, PLGA copolymer, Hydroxyapatite, Poly(ethylene glycol) (PEG), Prostaglandin, Statins, PTH1-34, Osteoporosis, Bone metastasis, Rheumatoid arthritis, Bone fracture
1. Introduction
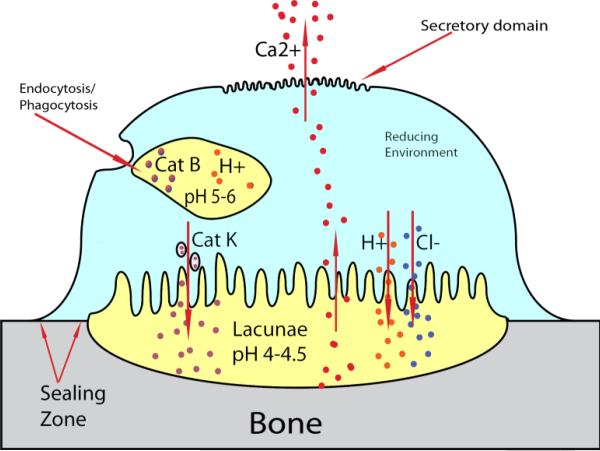
Bone is a complex organ responsible for structure and calcium storage as well as being a structure for hematopoiesis to occur. Adult bone is 50-70% mineral, 20-40% organic matrix, 5-10% water and 1-5% lipids. Hydroxyapatite (HAp) (Ca10(PO4)6(OH)2) is the main mineral component of bone. The crystallinity of HAp increases with time, however, the needle-like crystals never grow much more than 300 Å in length [1–3]. These crystals are continuously being broken down and replaced during bone turnover. Initially, monocytes receive several signals pushing them to differentiate into osteoclasts. Critical to this process, osteoblasts present receptor activator of nuclear factor κB ligand (RANKL) to the receptor activator of nuclear factor κB (RANK) surface receptor triggering the TNF receptor associated factor 6 (TRAF6) cascade in monocytes, ensuring osteoclast development [4,5]. Mature osteoclasts then perform the catabolic process on bone. The osteoclast creates a sealing zone (Fig. 1) over resorption sites (lacunae) and proceeds to release cathepsin K (Cat K) to degrade the organic matrix and HCl to dissolve the HAp. These mechanisms in combination with reactive oxygen species (ROS) produced by tartrate-resistant acid phosphatase (TRAP) degrade the bone preparatory for osteoblasts to lay down organic matrix called osteoid. The osteoid, primarily composed of type I collagen, is then calcified and becomes new bone [6,7].
Figure 1.
General osteoclast structure and function demonstrating unique environments, which can be utilized for site-specific release of drugs. Osteoclasts sequester portions of bone by sealing off areas called lacunae. The adjacent membrane to the bone ruffles and releases cathepsin K, and HCl, reducing the pH to 4-4.5 and dissolving the bone. Calcium from the bone is then transported to the secretory domain and released into the interstitial space. Although not specific to osteoclasts endosomes/lysosomes reduce pH to 5-6 and contain cathepsin B, two environmental specific attributes which can be used for the design of polymer-drug linkers.
When catabolism or anabolism in bone turnover is altered, the bone may become diseased. Osteoporosis, where catabolism effectively outweighs anabolism, predominately affects men and women over 50. Approximately 44 million people in the US suffer from low bone mass and 10 million people already have osteoporosis. An estimated 61 million will have the disease by 2020 [8]. Complications of this disease include debilitating vertebral and hip fractures cost between in $13.7 billion and $20.3 billion in 2005 [9]. Other imbalances in bone turnover include osteosarcoma and bone metastasis. Osteolytic and tumor creating cancers of the bone both result in significant pain for patients and morbidity. Osteoarthritis, osteomyelitis, infections, and fractures all increase the economic toll on health systems worldwide as well as decrease quality of life for millions of people each year.
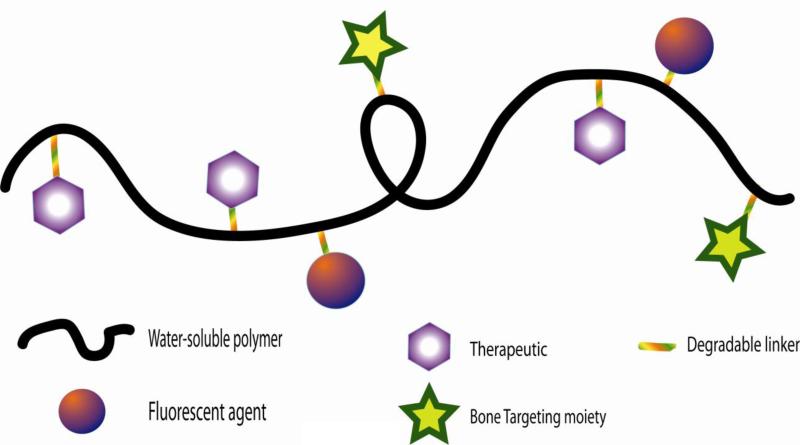
Nanomedicine plays an important role in recent advancements in bone therapeutics. Diseases that affect the bone may carry a range of receptor-specific targeting opportunities or they may carry none at all. However, by exploiting the mineral portion of the bone, bone diseases can be targeted using molecules such as bisphosphonate and acidic oligopeptides. Nanomedicines can be easily conjugated to several ionic targeting ligands, increasing binding avidity to bone. HAp crystal structures or HAp exposure are disease specific, and as such, modification of the targeting ligand itself or the density of the ligand may provide specific targeting opportunities. In addition to targeting, nanomedicines create an opportunity to image and synergistically combine multiple drugs to treat the diseased portion of the bone (Fig. 2).
Figure 2.
General structure of bone-targeted polymeric nanomedicines.
Aspects of this field have been reviewed previously [10-15]. In this review we discuss the selection of proper targeting agents, polymers, and drugs for application to specific bone-disease states. The analysis also includes methods of increasing local drug accumulation by modification of the molecular weight and drug-polymer release mechanisms. We will also elaborate on how various bone anabolic agents interact as well as how synergism of chemotherapeutics can be achieved by nanomedicine.
2. Targeting
Each bone disease state has similarities in that each causes local inflammation and/or results in the exposure of HAp to blood. These two characteristics can be utilized in order to deliver high drug loads specifically to diseased tissue. Inflamed tissue fills with exudate as various cytokines dilate vasculature. Macromolecular therapeutics then are able to travel into the tissue due to vascular pressure and be retained in the tissue due to lack of diffusivity back into the vasculature. This enhanced permeability and retention (EPR) has been well documented in several publications and is extensively discussed in several reviews [16-19]. Delivery of therapeutics to bone also depends on the extravasation through vessels in or near the bone [20]. Larger molecules, for example albumin and polyvinylpyrrolidone (Mw 35 kDa), can extravasate and have been found in bone after i.v. administration [21]. In fact, it has been long known that the vasculature in bone is fenestrated with pore sizes up to 80 nm [22], which exceeds the hydrodynamic size of most circulating nanomedicines. Exposure of HAp to blood carries its own targeting possibilities. Various tetracyclines, bisphosphonates, acidic oligopeptides, chelating compounds and salivary proteins have all been employed to target bone diseases. These compounds bind to the inorganic HAp part of bone and have specificity for a certain size of HAp crystal. Therefore, as the crystallinity of newly deposited bone in a tumor is different from the crystallinity of HAp exposed during a bone fracture, so targeting molecules can be selected for specific disease states.
2.1 Tetracycline
Tetracycline was introduced as an antibiotic in 1947 [23]. The antibiotic is derived from Streptomyces rimosus and inhibits aminoacyl-tRNA from entering into bacterial ribosomes, inhibiting protein elongation [24]. Tetracycline affects general bacterial metabolism and is prescribed for a wide range of gram-positive and gram-negative bacteria. Soon after its incorporation into medicine, however, tetracycline was found to bind strongly to bone. Its usage was discontinued in pediatric medicine as the high affinity to HAp caused children's teeth to stain yellow [25]. Furthermore, other early studies found that tetracycline may inhibit skeletal growth in children [26,27]. Its use as an antibiotic in adults and a targeting ligand in bone disease though, has not persisted.
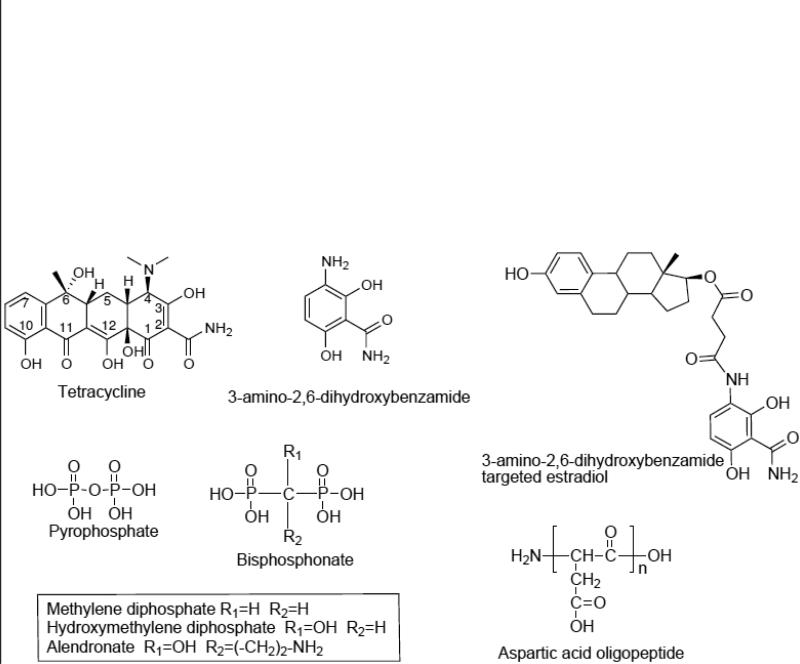
Early studies indicate that tetracycline must be in the correct orientation in order to bind HAp. Oxygens bound to C2, C10, and C12 are among the primary HAp binding atoms [28]. As such, modifications around the 5, 6, and 7 carbons can be made with minimal effects on binding and biological activity (Fig. 3). Other studies have shown that bone binding and antimicrobial activity may be retained even with a simplified tetracycline molecule. Neale et al. tried to reduce potential side effects caused by tetracycline's biological activity by minimalizing tetracycline structure so that it retains no biological activity yet is still able to bind to HAp. They found that 3-amino-2,6-dihydroxybenzamide retains 50% of the ability to bind to HAp when compared to native tetracycline (Fig. 3). To achieve bone anabolism, the new targeting ligand was bound to estradiol via a succinate linker. Following conjugation, the compound had a binding affinity of 105% over tetracycline alone. As estradiol alone did not bind to HAp the increased affinity may be attributed to the addition of a succinate linker [29].
Figure 3.
Structures of several bone-targeting molecules including tetracycline as well as minimalized tetracycline (3-amino-2,6-dihydroxybenzamide) and its conjugate with estradiol [29]. Also shown, are the structures of several bisphosphonates in comparison with pyrophosphate. Acidic oligopeptides such as aspartic acid (shown) or glutamic acid 4-10 amino acids long are also excellent bone-targeting molecules.
2.2 Bisphosphonates
Bisphosphonates (BPs) also bind strongly to HAp and retain much of the binding affinity after conjugation to other molecules or carriers. The first biological activity associated with BPs was noticed in 1968 [30]. Later, their effects on bone metabolism were discovered and they were prescribed to treat osteoporosis. BPs are derived from pyrophosphate and contain a carbon substitution for pyrophosphate's central oxygen. From the central carbon most BPs have been functionalized with a hydroxyl group and an R group by which they are classified (R groups containing nitrogen and those that do not) (Fig. 3). Non-nitrogen containing BPs incorporate into AMP creating a modified ATP, which cannot be hydrolyzed. Buildup of this ATP analog inside osteoclasts leads to apoptosis and reduced bone turnover. Nitrogen containing BPs (nBP) are much more potent. They inhibit farnesyl pyrophosphate synthase activity and by so doing disrupt the mevalonic acid pathway. Without the mevalonic acid pathway, protein prenylation is inhibited and osteoclasts lose their functionality or apoptose [31]. This inhibition may not completely arrest bone turnover as mevalonic acid pathway inhibitors increase bone morphogenic protein production as well [32-34]. Clinically, the activity of BPs has a profound effect on osteoporosis. Clinical data demonstrates that BPs yield a 47% reduction in vertebral fractures as well as wrist fractures, 50% in hip fractures, and a 19% overall drop in all non-vertebral fractures [35].
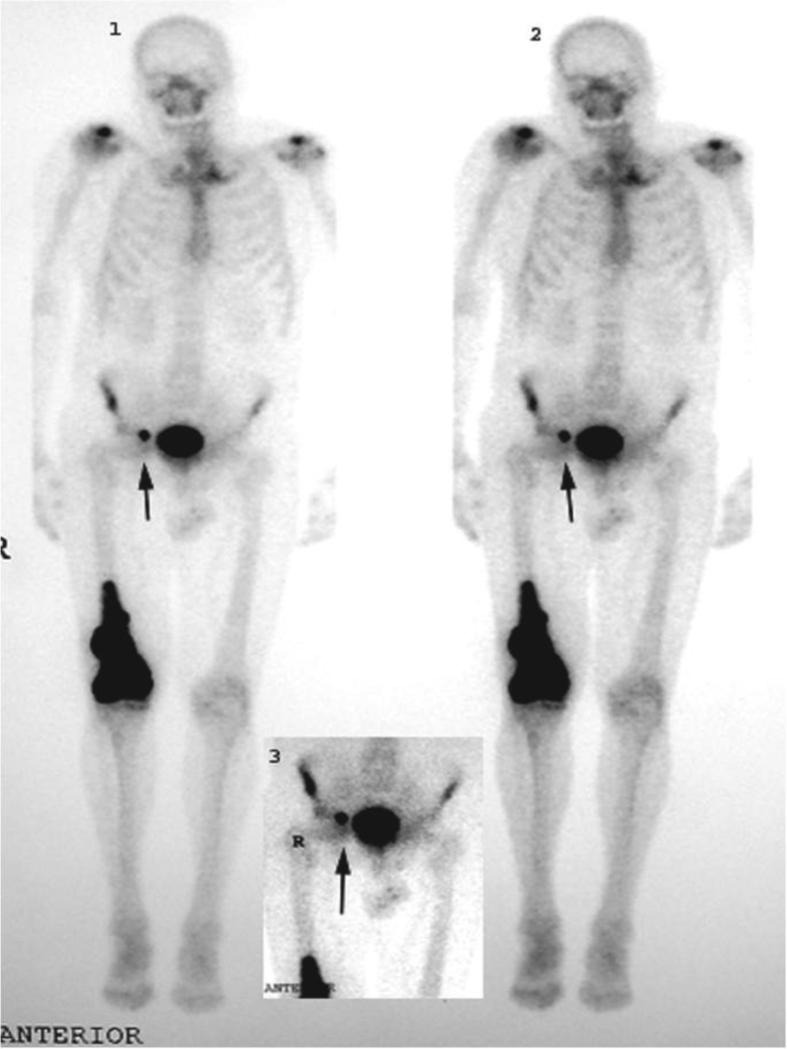
BP affinity to raw HAp is also clinically relevant in bone scintigraphy. Tc99 labeled methylene diphosphate (MDP) or hydroxy methylene diphosphate (HMDP) are used clinically for imaging bone abnormalities ranging from osteomyelitis to stress fractures. In many bone diseases bone is deposited (as in a tumor) or degraded (as in osteoporosis and osteoarthritis). Consequently, HAp crystals are exposed and can be targeted using bisphosphonates such as MDP (Fig. 4).
Figure 4.
MDP-Tc99 scan demonstrates the targeting specificity of bisphosphonates. Shown here is a primary osteogenic sarcoma from a 72-year-old man with Paget's disease. Dark arrows indicate a metastasis to inguinal lymph node. Reprinted with permission from reference [36].
The use of BPs as polymer/nanoparticle targeting moieties has several advantages. nBPs contain a primary amine and can therefore readily be conjugated to carboxylic acids. Secondly, as with tetracycline, if they are conjugated to nanomedicines via a degradable linker such as a pH sensitive hydrazone bond, they are pharmacologically active and may produce synergistic effects when coupled with appropriate drugs. Finally, the patent protection of many BPs will expire soon, which makes them an economic option for a targeting moiety.
BPs are one of the most studied bone targeting molecules and much research has been done determining their binding properties to HAp [37-39]. Hrubý et al. determined that the number of targeting moieties on a polymer did not dramatically affect the rate of adsorption. Rather, the amount of adsorbed polymer was affected by the number of targeting moieties as more ligands yielded higher binding avidity. The authors also concluded that there is a need for multiple targeting moieties to ensure that at least one is exposed [40]. Logically then, multiple targeting ligands become more critical as the nanomedicine size increases [41].
BP adsorption to HAp is a thermodynamic process primarily driven by entropy. Mukherjee et al. measured the thermodynamics of adsorption for several clinically used BPs as well as modified BPs in order to determine what aspects of the molecules are important for HAp binding. For instance, strongly basic side chain substituents, such as the nitrogen in alendronate, contribute dramatically to the binding in the free form. Furthermore, when measuring the difference between one Pi bond forming with HAp versus two Pi bonds forming (one phosphate versus both phosphates binding) they found that the hydroxyl group originating from the central carbon atom is necessary for both phosphates to bind [42]. By reducing a nanomedicine's thermodynamic strength of adsorption, either by manipulating the number of ligands or by modification of the ligand itself, one can target only the thermodynamically favored diseased area over the kinetically favored skeleton.
Numerous BP conjugates with natural or synthetic macromolecules demonstrate bone targeting in vivo [43-45]. Targeted osteoprotegerin (OPG) is one example. OPG is naturally produced by osteoblast's Wnt-β-catenin signaling pathway and is a potent RANKL inhibitor preventing osteoclastogenesis. With the intent to treat osteoarthritis, OPG was modified with a thiol-BP and administered intravenously (i.v.) to rats. Overall bone deposition of BP targeted OPG was twice that of free OPG. In an osteoarthritis rat model targeted OPG accumulated in the tibia 4x and femur 6x that of free OPG, demonstrating BP targeting abilities [46].
BPs exhibit anti-angiogenic properties by causing apoptosis, inhibiting migration, and reducing angiogenic sprouts of endothelial tissue making it a good antineoplastic agent but reducing capabilities to heal bones [47]. Moreover, the half-life of BPs such as alendronate in the bone is more than 10 years [48]. The inhibition of osteoclasts for extended periods of time can have serious detrimental effects on bone turnover as osteoclasts secrete insulin-like growth factors and BMPs to promote the maturation of osteons to osteoblasts. Jawbones have a high blood supply and high turnover resulting in BP accumulation. The combination of low bone turnover and lower blood flow causes osteonecrosis of the jaw (ONJ). ONJ is primarily associated, but not limited, to potent BPs used for chemotherapy, which completely inhibit osteoclast function [48,49]. Other toxicities include nephrotoxicity, hypocalcemia, and ocular dysfunction [50]. Therefore, BPs should not be used without considering some of the side effects.
2.3 Acidic oligopeptides (AO)
Bone sialoprotein is one of several naturally occurring proteins that exhibit strong affinity for HAp. Sialoprotein has several strings of acidic amino acids. Modeled after sialoprotein, glutamic acid and aspartic acid oligopeptides 4-10 amino acids (AA) long provide a more biocompatible option when adequate blood flow and bone turnover are needed [51]. Biocompatibility can be further increased as d peptides, which are not easily recognized by the body's immune system, can replace l peptides. Similarly to BPs, the HAp binding capabilities of these oligopeptides are retained after conjugation to a nanomedicine carrier via the peptide's alpha amino group [52].
AO's chain length can be modified to fit an application. Sekido et al. compared binding rates of Asp2-10 and Glu2-10 to HAp (Table 1). Increase in AA chain length resulted in enhanced binding rate (Bmax) up to hexapeptides; then the rate of binding plateaued and further increase in chain length was without effect. The dissociation constant (Kd) decreased with increasing numbers of AA throughout the experiment and no differences were found between d and l AA. This indicates that an AO over 6 AA long is ideal for optimal binding efficiencies [53]. Nanomedicines, however, are not limited to a single targeting moiety; rather multiple ligands may also decrease Kd. Ouyang et al. demonstrated the effect of multiple sets of AOs on binding rates. Dendrimers were synthesized with 1, 2 and 3 chains of Asp4-6 and a HAp binding assay was performed. The results indicated that two chains had nearly three times the binding of one alone but that the addition of a third chain resulted in poorer binding than two chains alone [52]. In the case of dendrimers, this means that steric hindrance may prevent binding of multiple AO chains. The extra chains then decrease the binding by increasing the size of the dendrimer itself or by reducing the ionic attraction of other dendrimers to the HAp by increasing the negative charge on the surface.
Table 1.
Maximal binding rate (Bmax) and dissociation constant (Kd) of acidic oligopeptides with increasing number of amino acids.
| Compound | Kd (μM) | Bmax (nmol/h/100μg HAp) | Bmax/Kd |
|---|---|---|---|
| Fmoc-(L-Asp)n | |||
| n=2 | >100 | n.d. | |
| 4 | 12.1 ± 1.3* | 0.34 ± 0.04 | 0.028 |
| 6 | 6.03 ± 0.84 | 1.57 ± 0.16 | 0.026 |
| 8 | 5.24 ± 0.55 | 1.61 ± 0.18 | 0.307 |
| 10 | 2.52 ± 0.23 | 1.66 ± 0.17 | 0.659 |
| Fmoc-(L-Glu)n | |||
| n=2 | >100 | n.d. | |
| 4 | 13.2 ± 1.1 | 0.42 ± 0.05 | 0.032 |
| 6 | 6.38 ± 0.71 | 159 ± 0.17 | 0.249 |
| 8 | 5.21 ± 0.61 | 161 ± 0.13 | 0.309 |
| 10 | 2.39 ± 0.25 | 1.66 ± 0.16 | 0.695 |
Standard errors are shown. Adapted from reference [53].
2.4 Estradiol analogs
Estrogen replacement therapy is being used for the treatment of osteoporosis related to low estrogen levels. Crooks and coworkers developed estradiol analogs that would localize in bone tissue but are lacking estrogenic properties. They designed bone-targeting compounds by attaching calcium chelators to an estradiol moiety through succinoyl [54] or carboxyethyl [55] linkers. To further improve the targeting potential they prepared a series of phosphate esters of the carboxyethyl linker-containing compound that possessed similar bone-targeting properties as tetracycline [56].
2.5 Specific applications
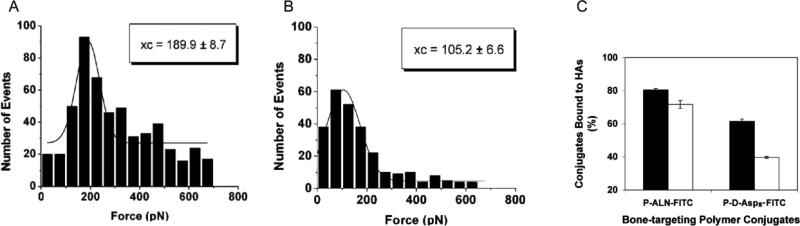
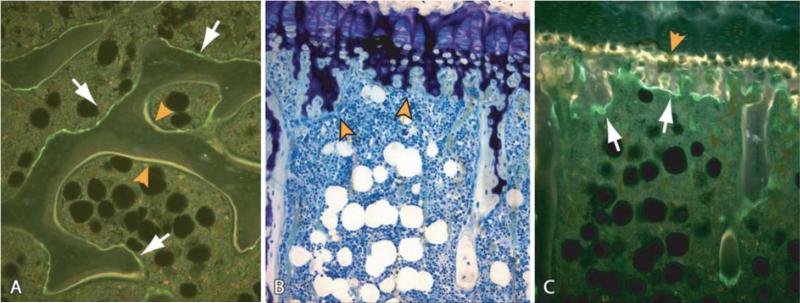
Reflecting their molecular structures, tetracycline, BPs, and AO do have some unique binding capabilities, which may affect the disease states for which they are most appropriate. The rate of binding of AOs to HAp is faster than that of BP [57]. One may hypothesize this to be a result of the larger binding area of AOs. Meanwhile, BPs have a greater binding strength, most likely due to greater specificity of BPs for HAp [57]. Wang et al. found that BP binds to all bone while Asp8 binds preferentially to higher crystalline HAp and thus to resorption surfaces [58]. In a follow-up study, AFM cantilever tips were modified with either Asp8 or alendronate (ALN). Interactions with HAp such as binding, adhesion events and rupture forces were measured (Fig. 5). ALN binding at zero distance was 190 pN as opposed to 105 pN with Asp8. Furthermore, ALN had higher rupture forces [3]. There were two sets of rupture forces for ALN, which lends itself to the bonding mechanism described by Mukherjee et al. [42]. Using two HA surfaces with differing crystallinity, Wang et al. confirmed that ALN is less affected by crystallinity and that Asp prefers higher crystalline HAp [3]. Bringing targeting via ionic interactions full circle, Miller et al. demonstrated using fluorescence microscopy that tetracycline deposits preferentially onto growing surfaces, or those with low crystallinity (Fig. 6) [59]. Application of these principles means that targeting osteoclasts should be done with AOs, which prefer absorbing surfaces, while targeting osteoblast should be done with tetracycline, which prefers growing surfaces of bone.
Figure 5.
Atomic force microscopy histograms demonstrating rupture forces of (A) alendronate and (B) d-Asp8 modified cantilever tips from a tooth enamel surface. (C) Binding ability of FITC labeled HPMA copolymer-ALN conjugate (P-ALN-FITC) and HPMA copolymer-d-Asp8 conjugate (P-d-Asp8-FITC) to hydroxyapatites with different crystallinity; black bars are high crystallinity and white bars exhibit low crystallinity. Adapted from reference [3].
Figure 6.
Initial uptake and localization of FITC labeled HPMA copolymer-Asp8 conjugate in bone compared with the uptake of tetracycline. (A) The conjugate preferentially incorporates in scalloped-appearing eroded surfaces in cancellous bone (white arrows); tetracycline (yellow label) is incorporated onto active bone mineralization surfaces. (B,C) Stained (B) and unstained (C) section of the same region of the proximal tibial growth plate and primary spongiosa. Tetracycline (yellow label) incorporated into the mineralizing zone of the growth plate (C, orange arrowhead) as expected, whereas HPMA copolymer-Asp8 conjugate (green label) localized in the resorption areas of the primary spongiosa (C, white arrows). Magnifications: A=150x; B,C=125x. Reprinted with permission from reference [59].
3. Polymer carriers
The conjugation of multiple targeting ligands to a polymeric backbone is an obvious advantage that polymeric drug delivery systems have over small molecule systems. In addition to targeting ligands, modification of polymer size has a profound effect on blood circulation time as well as on whole body biodistribution. Once in target tissue, polymeric drug delivery systems have several modes of drug release, which range from passive diffusion from a degrading nanosphere to enzymatic cleavage of drug-polymer linkers. These drug release mechanisms in combination with biodistribution play a critical role in ensuring the drug is delivered to the right area for the right duration of time.
3.1 High molecular weight vs. low molecular weight carriers
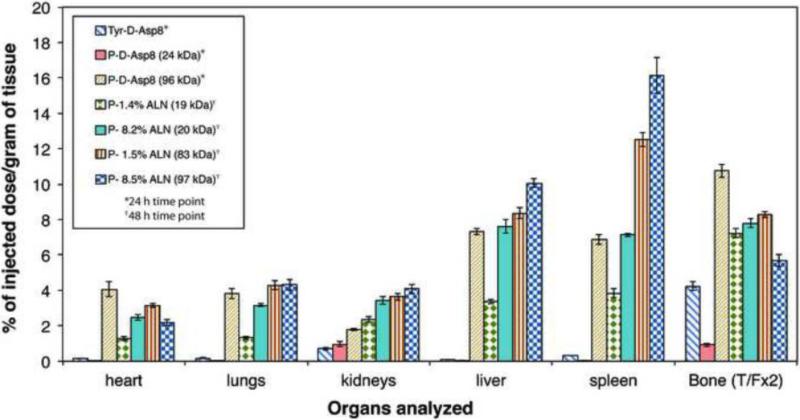
The molecular weight and ultimately the hydrodynamic radius of polymeric carriers have a profound effect on the efficacy of drug delivery. Higher molecular weight polymers have longer circulating half-lives and ultimately higher deposition in diseased sites. However, as the molecular weight increases, so does uptake in the reticuloendothelial system (RES). Therefore, careful selection of molecular weights with appropriate targeting ligand concentration (number per macromolecule) is essential to ensure biocompatibility and high deposition of drug in the affected area. Wang et al. compared 24 kDa, 46 kDa, and 96 kDa Asp8 containing HPMA copolymers in a biodistribution study. Each polymer contained 4.4, 5.6, and 9.6 Asp8 chains per macromolecule, respectively. Also included in the study was Tyr-d-Asp8 as a control. As hypothesized, the study demonstrated enhanced blood circulation time for high molecular weight conjugates with the highest bone accumulation being 96 kDa HPMA copolymer by several fold. However, the highest bone to RES accumulation ratio was the Asp8 targeting ligand by itself, followed by 24 kDa HPMA copolymer conjugates [58]. The same group performed a similar biodistribution study on ALN containing HPMA copolymers of two molecular weights - 20 kDa and 90 kDa. In addition to molecular weight, the concentration of ALN (0%, 1.5%, and 8.5%) was also varied (Fig. 7). All conjugates showed an initially high uptake in the heart, lungs, and kidneys, which reduced over time. Similarly to [58] the low molecular weight, 20 kDa conjugate cleared the body first as expected and each targeted molecule increased bone accumulation over time. The high molecular weight (90 kDa) conjugate yielded a higher RES uptake. Similarly, higher ALN content also yielded higher RES uptake. This indicates that the identification of ideal targeting ligand concentrations needs to take into account the polymer's overall charge (and therefore RES uptake), number of targeting ligands per macromolecule (related to charge), and molecular weight. In the case above, the drug with the highest bone to RES ratio was the 20 kDa, 1.5% ALN polymer [60]. In conditions where metabolism of a drug by the RES is a concern, moderate molecular weights with a low percentage of targeting moieties are beneficial. However, uptake by the RES generally means phagocytosis by mononuclear phagocytes. Phagosomes eventually fuse with lysosomes, creating an acidic environment with several proteolytic enzymes. Therefore, delivery of proteins and other molecules, which will be degraded by lysosomal enzymes, may not create biocompatibility issues following RES uptake. In these situations, a high molecular weight and a high targeting moiety content may be the best option.
Figure 7.
Biodistribution in BALB/c mice of 125I-labeled HPMA copolymer-Asp8 conjugates (P-Asp8) 24 h and 125I-labeled HPMA copolymer-ALN conjugates (P-ALN) 48 h after i.v. administration. The impact of molecular weight and ALN content was evaluated. Adapted from references [58,60].
3.2 Degradable linkers
As critical as the biodistribution of a drug, the proper release rate and mechanism will determine the efficacy of a drug delivery system. Modification of the linker, which connects polymer with the drug, can create release profiles that deliver high amounts of drug immediately upon arrival or deliver small amounts over time, extending the effective dose time of the drug. Furthermore, by utilizing bone specific enzymes, disease site-specific pro-drugs can be created.
The bone itself has several enzymes, which can be used to release drugs. Among these enzymes are matrix metalloproteinases (MMP) 1, 2, 3, 4, 7, 8, 9, 12, 13, and 14 and Cat K. MMPs are expressed by osteoclasts, osteoblasts and are overexpressed in many bone metastases [61-71]. Most MMPs function by cleaving peptide bonds holding collagen and other glycoproteins together. MMPs assist osteoclasts’ function by degrading collagen within the bone at resorption lacunae while bone metastases use MMPs to degrade the basement membrane and spread through out the body. Using MMP-sensitive linkers may provide site-specific delivery of drugs to bone; however, bone delivery using MMPs is limited by the fact that MMPs are inhibited by tetracycline and derivatives such as doxycycline [72]. Cat K on the other hand is expressed at resorption lacunae and is not sensitive to tetracycline derivatives (Fig. 1). Due to expression of Cat K outside the cell, Cat K specific linkers are an excellent choice for signaling compounds such as prostaglandins and bone morphogenic protein-2 (BMP-2), whereas hydrazone bonds, disulfide bonds and cathepsin B (Cat B)-sensitive linkers are better options for drugs such as chemotherapeutics, which rely on internalization by the cells prior to release.
Many classic degradable drug-polymer linkers rely on receptor-mediated endocytosis of cells or phagocytosis by osteoclasts. Following endocytosis, the endosomal compartment acidifies and ultimately fuses with lysosomes into an acidic organelle (pH ~5). Acid-sensitive linkers are also effective in the low pH present in the interstitial space of tumors (pH 5-6) and the resorption lacuna of osteoclasts (pH 4-4.5) [73]. Hydrazone bonds are acid-sensitive linkers and are able to rapidly release an unaltered drug at pH 5.5 but remain intact at physiological pH 7.4. Hrubý et al. conjugated doxorubicin to an HPMA copolymer using a hydrazone linker and measured release kinetics of the HAp adsorbed polymer as well as the polymer in solution at pH 5 and 7.4. Release kinetics at pH 5 was logarithmic, with over 95% of the copolymer conjugate in solution releasing the DOX within 10 h. Release kinetics of the HAp bound polymer was slower as after 10 h approximately 55% of the DOX was released and the remaining DOX was nearly all released over the first 50 h. Meanwhile, at pH 7.4 the drug linker was relatively stable, only releasing 20% of drug after 50 h [40].
There are other release systems that rely on the endosomal environment. As the lysosome and endosome fuse, polymers within the endosome are exposed to proteases such as Cat B. Hrubý et al. also studied the release kinetics of a Cat B sensitive tetrapeptide GFLG (glycine-phenylalanine-leucine-glycine) [74]. Release kinetics were nearly linear and much slower than the hydrazone bond discussed earlier. When the polymer was in solution, after 50h less than 45% of the drug was released. Steric hindrance, preventing Cat B from accessing the tetrapeptide linker, reduced the DOX cleavage rate in HAp bound polymer to half the rate in solution, as only 22% was released after 50 h incubation [40].
Elongated spacers, which separate the enzymatically cleaved bond from the drug by a self-eliminating group, were designed by several groups [75-79]. The predominant example of an electronic cascade spacer is the 1,6-elimination spacer [77]. It contains, e.g., a bifunctional p-aminobenzyl alcohol group that is linked to an enzymatically cleavable group through an amine moiety. After enzymatic cleavage, the strong electron-donating amine group of the 1,6-elimination spacer is unmasked and immediately initiates an electronic cascade that leads to cleavage of the benzyl-carbamate bond and release of carbamic acid. The unstable carbamic acid rapidly releases carbon dioxide and yields the unmodified drug [78,79]. Use of such spacers was shown in bone-targeted delivery of PGE1 [59] and anticancer drugs to bone [80-82].
Once a polymer exits the endosome, it enters the cytosol. The cytosol has a reducing environment that is able to reduce disulfide linkers. Evidence also suggests that this reductive environment may start as early as the endosome during receptor-mediated endocytosis [83]. Kurtoglu et al. used disulfide linkers for dendrimer drug delivery and described the release at pH 5 and pH 7.4. Using either cysteine or glutathione at pH 7.4, release occurred within 20 min, while at pH 5, complete drug release occurred over 6 h or more. Furthermore, the amount of drug released was dramatically affected by glutathione and cysteine concentrations as one cysteine can release only one drug [84].
3.2.1 Example of cathepsin K sensitive spacer design
The design of an oligopeptide sequence susceptible to cleavage by Cat K is demonstrated here. The aim was to design a conjugate that would deliver prostaglandin E1 (PGE1) to bone with subsequent PGE1 release by osteoclast-secreted Cat K in the resorption lacuna (for macromolecular entry into osteoclasts see [85-87]).
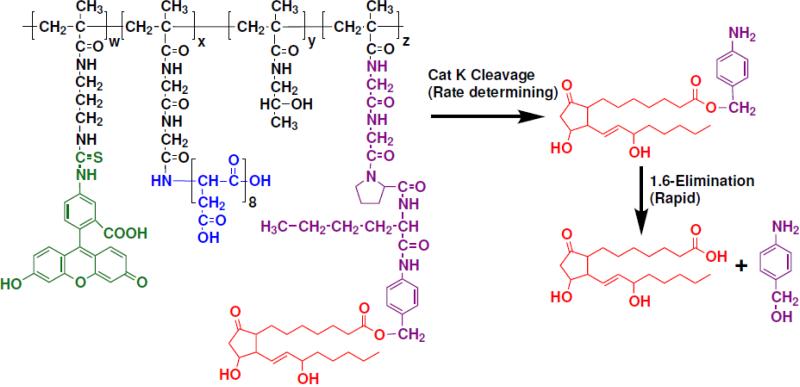
The Cat K sensitive spacer was designed based on known interactions of peptide substrates with the active site of the enzyme [88]. The specificities of S1–S4 subsites (subsite nomenclature from Schechter and Berger [89]) of the Cat K active site have been screened with positional scanning libraries [90]. Their most striking result was the unique preference for proline in P2 position; therefore, it was selected. The favored amino acid residues in the P1 position were lysine and arginine. However, to ensure that the spacer is stable in the circulation [91], basic amino acid residues have been avoided and norleucine, the most active neutral amino acid residue, was chosen for position P1. It was shown that Gly-Pro in positions P3 and P2 were favorable for cleavage [90]. Consequently, glycine was seleceted for position P3. It had been observed previously that expanding the length of the oligopeptide spacer in side-chains of HPMA copolymers from three to four amino acid residues resulted in decreased steric hindrance upon the formation of the enzyme-substrate complex for chymotrypsin [92] and Cat B [74]. Thus, glycine was introduced into the position P4.
Chemically, the C end of Gly-Gly-Pro-Nle spacer cannot be directly connected to PGE1 because the latter lacks a NH2 group. To overcome this problem, a 1,6-elimination spacer based on 4-aminobenzyl alcohol (AB-OH) was inserted. The latter was used to link the peptide to the C-1 COOH group of PGE1. Upon enzymatic cleavage of the peptide spacer, the strong electronic-donating amine group of the 1,6-elimination spacer is unmasked [75,76] and initiates an electronic cascade that leads to the cleavage of the ester bond and immediate release of unmodified PGE1 (Fig. 8). Finally, a methacryloyl group was attached at the N-terminus of the construct; the resulting macromonomer, MA-Gly-Gly-Pro-Nle-AB-PGE1, may be copolymerized with HPMA to produce new PGE1-containing macromolecular therapeutics. The release of PGE1 from HPMA + MA-Gly-Gly-Pro-Nle-AB-PGE1 copolymer indicated that a high rate of cleavage occurred in osteoclast cultures followed by osteoblast cultures. Polymer incubation with non-skeletal and precursor cells resulted in minimal release of PGE1, demonstrating the specificity of this linker [93]. The specificity of the linker was also validated in vivo [59,80,81].
Figure 8.
Scheme of release of unmodified prostaglandin E1 (PGE1) from HPMA copolymer-Asp8-PGE1 conjugate. Rate controlling cathepsin K cleavage is followed by fast 1,6 elimination.
3.3 Different polymers studied
Each polymer exhibits unique characteristics based on chemical and structural properties. We have classified polymers by their structural properties whether they form linear hydrophilic polymers or spherical micelle and nanoparticle structures.
3.3.1 Linear polymers
3.3.1.1 Cationic polymers
Polyethyleneimine (PEI) and poly(l-lysine) (PLL) are frequently-used cationic polymers in drug and gene delivery systems. Uludağ and coworkers incorporated a BP, 2-(3-mercaptopropylsulfanyl)-ethyl-1,1-bisphosphonic acid, to both polymers using heterobifunctional reagents. In vitro (HAp) and in vivo (unprocessed bone matrix from femurs/tibiae) mineral affinity of BP containing polymers was evaluated and compared with unmodified polymers. Interestingly, they found that in vitro binding to HAp was similar or lower after conjugation to BP and that both PEI and PLL were able to bind nearly 100% to HAp in many conditions on their own. This is not surprising, as positively charged molecules should adsorb to HAp. In vivo results on a rat model also showed no significant difference in bone affinity of modified and unmodified polymers [94]. In addition, PEI and other cationic polymers are associated with toxicity in vivo [95,96].
Recently, modified PEI was used for the development of bone-seeking radiopharmaceuticals. To this end, PEI was functionalized with methylene phosphonate groups thereby incorporating targeting and metal chelating ability in the polymer itself. In vitro tests demonstrated that fractionated N,N’,N’-trimethylenephosphonate-poly(ethyleneimine) (PEI-MP) was able to carry radioactive tin ions to an HAp surface and then release them upon contact. By doing so, PEI-MP may work well as an imaging agent and as a possible radiotherapy delivery agent for metastatic bone cancer [97,98]. As such, prevention of any biocompatibility issues may be avoided by integrating PEI-MP into a block-copolymer of a more biocompatible polymer.
3.3.1.2 Poly[N-(2-hydroxypropyl)methacrylamide]
HPMA is one of the more studied polymer therapeutics to bone. PolyHPMA's biodistribution and bone targeting abilities have been well documented and are discussed throughout this paper [58-60,80,81]. Other advantages of HPMA copolymers include a low toxicity profile and the ability to control the molecular weight and therefore biodistribution by RAFT polymerization [99-101]. This process also permits the incorporation of methacryloylated drug derivatives into the polymer by copolymerization [99,100], eliminating the chance of having unreacted (residual) groups remaining on the polymer backbone after post-polymerization modification. HPMA copolymers have also been used in the design of micelles [102,103] and dendrimers [104].
3.3.1.3 Other polymer carriers
Korzhikov et al. synthesized aldehyde-containing copolymers based on 2-deoxy-2-methacrylamido-d-glucose (MAG). Two methods were used for the introduction of aldehyde groups into the polysaccharide chains: i) copolymerization of MAG with N-vinylpyrrolidone and a comonomer that can be easily converted to an aldehyde, such as diethylacetal acrolein; and ii) the periodate oxidation of the saccharide units. The water-soluble polymers adsorbed on HAp. The ultimate aim is to use these polymers for construction of composite scaffolds for tissue regeneration [105].
Wang and coworkers synthesized a polyrotaxane delivery system targeted by ALN. They conjugated ALN to α-cyclodextrin and threaded it onto PEG (2000 mol. wt.) producing a pseudopolyrotaxane. Then, employing copper(I)-catalyzed Huisgen 1,3-dipolar cycloaddition, the pseudopolyrotaxane was copolymerized with bulky monomers containing imaging and/or therapeutic agents. The osteotropicity of the constructs was confirmed in vivo [106].
3.3.2 Vesicular polymer carriers
3.3.2.1 Micelles and liposomes
Micelles and liposomes are able to hold a great deal of drug when compared to linear polymers due to their three-dimensional shape. Although in vivo stability may be an issue for many polymeric spherical micelles and liposomes, these delivery systems are able to release their contents over long periods of time [107,108]. Finally, as alluded to before, this class of drug carriers may be able to protect drugs they carry from proteasomal degradation as well as protect the body from non-targeted effects of the drugs.
Critical to their success, bone targeted liposomes are able to retain their targeting ability. Hengst et al. designed a 1,2-diacyl-Sn-glycero-3-phosphocholine (EPC) liposome containing a BP targeting agent anchored by cholesterol. Each of the four conjugates tested had a low polydispersity of ≤0.1. Mole percents of the targeting ligand, cholesteryl-trisoxyethylene-bisphosphonic acid (CHOL-TOE-BP), were 0%, 3%, 14%, and 25% with zeta potentials of -18 ± 2, -28± 1, -34± 2, and -40± 2, respectively. The determination of the binding of each liposome revealed that 50% HAp binding was achieved using the 3 mol% CHOL-TOE-BP and binding progressed nearly linearly until 100% binding was achieved using the 25 mol% CHOL-TOE-BP liposome. This data suggests the bone targeting potential of this liposome [109].
Uludağ and coworkers incorporated BP-derivatized liposomes into collagen/hydroxyapatite scaffolds and decreased the rate of model drug release from the constructs. This provides a sustained drug release approach for bone regeneration [107].
The liposomal drug formulations for the treatment of rheumatoid arthritis have been recently reviewed [110].
3.3.2.2 Poly(lactic-co-glycolic acid)
(PLGA) nanospheres are a well-studied drug delivery modality that has been applied to bone targeting. Choi et al. designed PEG-PLGA block copolymers intermixed with ALN functionalized PLGA to create surface modified nanoparticles. The monomethoxy PEG formed a hydrophilic layer on the surface of nanoparticles with the potential to decrease RES accumulation, and ALN was used as the targeting moiety. The study of the relationship between structure and adsorption on HAp revealed decreasing adsorption correlated with a decreasing concentration on ALN at the surface; the increase in PEG mol. wt. (550, 750, and 2000) resulted in decreased adsorption apparently due to shielding of the ALN moieties by longer PEG chains [41]. As PLGA degradation yields naturally occurring products it also has a low toxicity profile. Cenni et al. was able to synthesize ALN functionalized nanospheres with a polydispersity index of 0.348 ± 0.020 nm and test their toxicity. Each in vitro test (hemolysis, leukocyte proliferation, platelet number, coagulation, and complement consumption) showed acceptable blood compatibility and an absence of cytotoxicity [111]. In follow-up studies, the authors loaded ALN functionalized PLGA nanoparticles with doxorubicin (DOX) and evaluated their properties in an orthotopic mouse model of breast cancer bone metastases. Both free DOX and DOX-loaded nanoparticles reduced the occurrence of metastases in mice. Loaded and drug-free nanoparticles decreased the number of osteoclasts at the tumor site; the authors hypothesized that this may be the result of ALN activity [112].
Other advantages of PLGA include well-studied drug release kinetics. By modification of lactic glycolic acid ratios, PLGA nanospheres can release their contents over a wide time scale [113-115]. More in vivo tests such as bone targeting and whole body biodistribution are needed, however, in order to truly determine the efficacy of these targeted polymeric delivery systems.
3.3.2.3 Other vesicular structures
Ozcan et al. used nanoprecipitation to produce poly(γ-benzyl-l-glutamate) (PLGB) nanoparticles (< 80 nm; low polydispersity), which were functionalized with PEG-ALN for bone targeting. ALN presence did increase bone targeting dramatically in vivo. By labeling the PBLG-PEG-ALN nanospheres with FITC, Ozcan et al. was able to demonstrate preferential accumulation of targeted drug in bone [116].
Wang et al. evaluated bovine serum albumin (BSA) nanoparticles surface-coated with PEI-PEG-thiolBP and used them for the delivery of bone morphogenetic protein-2 (BMP-2). They found that the nanoparticles were not effective in bone targeting after i.v. administration. However, they were useful in localized delivery of BMP-2 in bone repair and regeneration [117].
3.4 Dendrimers, dendrons, dendronized polymers
Dendrimers are symmetrical vesicular carriers, whereas dendrons are tree-like fragments of dendrimers that can be attached to water-soluble polymers [118]. Thus, the “dendron-like” systems possess complex structures characterized by high density of functional groups and/or targeting moieties. Ideally (without structural defects), dendrimers are monodisperse, spherical compounds. Their size depends on the generation, i.e., the number of successive synthetic steps used. Numerous attempts have been made to exploit dendrimers in drug delivery including targeting to bone. Ouyang et al. synthesized naproxene containing polyamide and poly-ether-amide dendrimers and decorated them with 2 or 3 Asp(4-6) peptides and demonstrated affinity for HAp [52]. In the following study the same group synthesized the “next generation” of naproxene containing dendritic compounds with four or eight Asp(4-6) fragments. They did not observe a difference in HAp binding of constructs containing low or high Asp(4-6) content and the total HAp binding only ranged from 63% to 75% [119]. Clementi et al. synthesized PEG-based dendrimers from heterobifunctional PEG; they were decorated with H2N-PEG-β-Glu-(β-Glu)2-(COOH)4, exposing four carboxylic groups for the attachment of ALN and/or paclitaxel. This construct was able to achieve nearly 100% HAp binding and nearly 80% HAp binding following conjugation to paclitaxel (PTX). These conjugates were designed for the treatment of bone neoplasms and used a pH-sensitive linker for rapid release of the drug. In vitro studies demonstrated that drug release was highly effective resulting in conjugated PTX having a similar IC50 as PTX alone, apparently due to fast drug release at physiological pH [120].
3.5 Vesicular vs. linear polymer carriers
The decision to use a spherical or linear polymer depends on the application. Both types can be designed so that they release drugs rapidly. Longer periods of time (such as a day to a week) may be achieved using linear polymers conjugated to drugs via enzyme degradable linkers. For periods over a week, spherical polymers may be designed to release drug as the polymer degrades. The question then arises as to which yields better targeting and how it affects the treatment of disease. This may be answered by looking at zeta potentials. Excess surface charge is associated with high RES uptake [121]. This means that for a specific polymer at a specific hydrodynamic radius, the addition of surface charge, in this case as a targeting moiety, could increase RES uptake. As such, linear polymers may have an advantage. Linear polymers are able to bend and conform to a surface thereby reducing the number of targeting moieties needed to achieve bone specificity. Spherical polymers on the other hand have a three-dimensional shape, which reduces the amount of surface that can bind to bone thereby increasing the need for several targeting moieties and increasing the zeta potential. Though this theory needs to be tested, one may consider that if RES uptake does not contribute to a decrease in biocompatibility (such as with drugs that can be degraded by RES lysosomes) then a spherical polymer as well as a linear polymer would work. However, if RES uptake results in toxicity, as with nondegradable chemotherapeutics, then one may consider the use of a linear polymer over a spherical one.
4. Therapeutics
Life expectancy in the developed world continues to increase at a constant rate [122]. As such, age-related health complications also increase and the need for therapeutics to treat bone degenerative diseases and cancers expands. Critical to solving problems associated with bone disease is the selection of proper therapeutics. Bone disease causes a wide range of problems: too little bone growth, cancer and overgrowth, inflammation, and infection. In this paper, focus will be on bone anabolic agents (for osteoporosis) as well as cytotoxic agents (for cancer). Other topics such as inflammation [123] and siRNA delivery to bone [124] are discussed in other reviews within this volume.
4.1 Osteoporosis and other low bone mineral density (BMD) related diseases
Osteoporosis is among the most common of the degenerative diseases. It is estimated that 1 out of 5 women over the age of 50 have osteoporosis and that approximately half of the women over 50 will fracture their hip, wrist or vertebra [125]. BPs, although helpful for some people, have several side effects as described previously (section 2.3). Furthermore, a need exists for alternatives to Cat K and RANKL inhibitors [126-130]. Ultimately, these drugs yield inadequate bone turnover and therefore poor bone quality. The 1-34 fragment of recombinant parathyroid hormone (PTH), FDA approved in late 2002, is an effective bone anabolic agent. When compared to oral 10 mg/day ALN, the administration of s.c. injections 40 μg/day PTH yielded greater bone density [131]. However, there is a concern that lifelong injections may cause osteosarcomas to develop, and consequently, the drug may only be prescribed for 2 years during one's lifetime [132]. Other bone anabolic agents have also been investigated for treatment of osteoporosis but ultimately have significant drawbacks preventing their use. Prostaglandins are in clinical use for several medical conditions. In bone, they exhibit strong anabolic effects; however, the short half-life and serious cardiovascular side effects of free prostaglandins have prevented their use in osteoporosis. BMPs have been clinically applied to fuse vertebrae. BMPs have low systemic side effects, which may be in part due to their short blood half-life, which prohibits effective systemic administration. Targeting bone anabolic agents and/or extending their circulation half-life may open the door for new PTH alternatives.
4.2 Anabolic agents
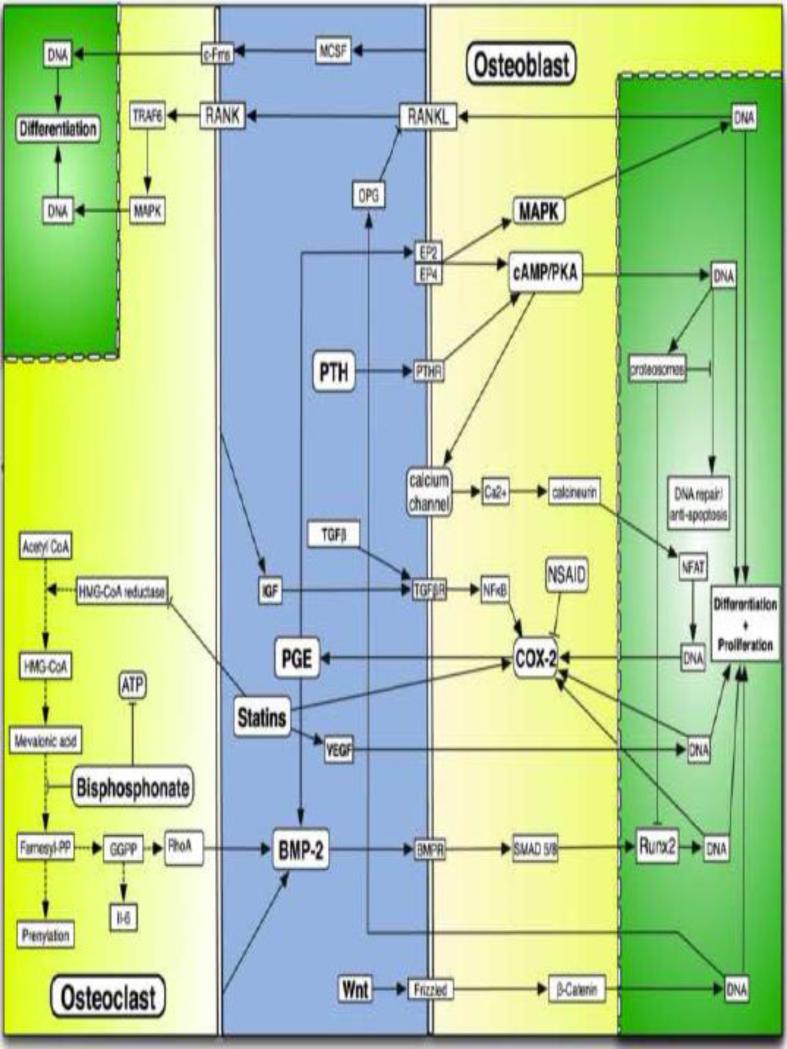
Several potential bone anabolic agents exist. We will discuss their interactions in the hopes of elucidating possible synergistic mechanisms, which may be employed to create super-drugs (Fig. 9).
Figure 9.
General pathways affecting bone anabolism and cross talk between osteoclasts and osteoblasts. Most notably, PGE levels affect BMP-2 levels and vice versa, however, each contributes to bone anabolism by independent signaling pathways. Statins upregulate both BMP-2 and PGE through independent pathways. PGE and PTH1-34 upregulate cAMP however, stimulation of EP2 or EP4 by PGE will also trigger MAPK cascades. Not shown, PTH1-34 affects calcium levels in the body by regulating resorption in the kidneys and intestine. Wnt plays a critical role in bone turnover by production of osteoprotegerin and therefore inhibition of RANKL-RANK interactions. Also of note, COX-2 represents basic components PGE production rather than upregulation of COX-2 enzyme.
4.2.1 Prostaglandins
Prostaglandins (PGEs), especially the E series (such as PGE1 and PGE2), have been studied extensively for their bone anabolic properties. Their importance in bone turnover is demonstrated by the lack of bone repair when patients are administered NSAIDs [133-137]. Systemically administered prostaglandins, however, cause diarrhea, lethargy, and flushing and thus are too toxic for non-local or non-targeted dosing [138]. Locally administered to bone, PGE affects osteoblasts by binding to four receptor subtypes (EP1-4) of PGEs. EP2 and EP4 are the primary receptors associated with bone turnover; EP2 is normally associated with bone anabolism and EP4 generally affects catabolism by upregulating RANKL through the MAPK pathway [139-143]. Both EP2 and EP4 have traditionally been associated with a dramatic increase in cAMP levels; however, these two receptors also contribute to the p38 MAPK and ERK MAPK signaling pathways, respectively [144]. There is also evidence that prostaglandins upregulate BMP-2 production [32]; however, another study contends that it is not through EP2 or EP4 [144].
Several studies have attempted to treat osteoporosis by either modification of PGE2, making it specific for EP2 or EP4 receptors, or by conjugation to targeting moieties [59,138,145,146]. Miller et al. has attached PGE1 via a Cat K sensitive spacer, Gly-Gly-Pro-Nle, to an HPMA copolymer (Mw 37.2 kDa), P-Asp8-FITC-PGE1. Ovariectomized female Sprague-Dawley rats were administered a single i.v. bolus of either 10 mg P-FITC, P-Asp8-FITC or P-Asp8-FITC-PGE1 (0.61 mg of PGE1 equivalent), followed by a tetracycline label administration at the end of the study on day 25. Measuring the distance between the FITC-labeled polymer and the tetracycline label on the bone surface, they were able to determine the surface-referent bone formation rates (μm2 μm-1 day-1). Bone deposition rates attributed to P-FITC, P-Asp8-FITC or P-Asp8-FITC-PGE1 were 0.0362 ± 0.0062, 0.0541 ± 0.0108, and 0.1142 ± 0.0077, respectively, demonstrating the potential anabolic effects of targeted PGE1 against osteoporosis [59].
4.2.2 Statins/bisphosphonates
Statins and BPs affect bone turnover by interacting with the mevalonic acid pathway, and down-regulating protein prenylation. It is believed that down-regulating protein prenylation, down-regulates RhoA and by so doing upregulates eNOS. The presence of eNOS increases synthesis of BMP-2 and therefore increases bone anabolism [147,148]. Furthermore, statins and BPs may also directly upregulate the PGE pathway, as COX-2 inhibitors dramatically reduce statin induced bone anabolism by up to 77% [149]. Statins also induce VEGF, which contributes to osteoblast differentiation [150,151]. Statins, having been administered systemically for lowering cholesterol, also affect on bone growth. Clinical evaluation of systemic delivery has shown no decrease in fractures, minimal increase in BMD and no increase in bone turnover markers in post-menopausal women [152,153]; however, local administration in animals has shown significant bone turnover [33,154-158]. Systemic delivery of bone-targeted statins may increase local bone concentrations enough for higher BMD and bone turnover.
4.2.3 TGFβ superfamily
BMP-2 is a member of the TGFβ superfamily. It is produced by osteoclasts and the signal differentiation of osteoblasts through the SMAD5/8 pathway. SMAD5/8 combines with SMAD1 and translocates to the nucleus where Cbfa1/Runx2 is upregulated. CBFA1/Runx2 is responsible for promotion of several osteoblast differentiation genes as well as the upregulation of COX2 [159]. Although overlap can be seen in the two pathways, BMP-2 operates seperately from PGE. Research suggests that BMP-2 has a synergistic effect with PGE [160]. BMP-2 is clinically applied locally for spinal, oro-maxillary, and trauma surgeries [161-169]. Also part of the TGFβ superfamily, TGF-β1 and IGF-I are produced by osteoclasts [15] and affect transcription via several SMAD proteins. TGF-β1 and IGF-I activate NFκB leading to increased levels of COX-2 and PGE2 production [170,171]. TGF-β1 is generally considered to inhibit osteoclasts, however, as with many growth hormones, their action is dose dependent [172-174].
4.2.4 Fragment 1-34 of parathyroid hormone (PTH)
PTH1-34 (PTH contains 84 AA) is prescribed for treatment of osteoporosis. Systemically, PTH1-34 affects calcium resorption in the kidneys and intestine [175]. Within bone, PTH 1-34 affects osteoblasts and leads to increased cAMP/PKC production and differentiation even when COX-2 is inhibited [176]. Increased cAMP levels also open up calcium channels leading to increased calcium levels in the cell. Consequently, the calcineurin pathway is triggered, which activates NFAT and yields increases in COX-2 expression [177]. Elevated levels of PTH1-34 reduce apoptosis by increasing PKC levels there by upregulating FOXO3a DNA repair mechanisms [178]. Overexpression of cAMP/PKC leads to deleterious proteasomal (smurf1) degradation of CBFA1/Runx2, and therefore, regulation of BMP-2 effects [179-181]. Furthermore, proteosomal degradation will negate the anti-apoptotic effects of FOXO3a [182]. Therefore, intermittent administration of PTH1-34 leads to anabolism, while chronic administration leads to catabolism [183].
4.3 Delivery of Proteins
Numerous attempts have been made to modify protein-based therapeutics with bone-seeking moieties to enhance their localization at skeletal sites [11,12,184]. Bansal et al. synthesized clustered BP-based targeting moieties composed of four bisphosphonic acid moieties linked through a benzene ring and conjugated them to bovine serum albumin (BSA) as a model protein. In vitro binding in both saline and 60% serum solutions resulted in less than 60% bound protein. However, in vivo results indicated a 4.7 fold increased accumulation in rat tibiae over controls (BSA and BSA targeted with a thiol-BP moiety). Thus the clustered BP possessed a considerably increased bone-seeking capacity in vivo when compared to HS-BP [185]. Interestingly, the accumulation of thiolBP modified osteoprotegerin in osteoarthritic rats undergoing active bone remodeling increased more than 4x over that of control unmodified OPG 24 h after i.v. administration [186].
Proteins such as BMP-2 have a circulation half-life of only about 1 min [187]. For successful systemic administration they may have to be modified by semitelechelic (ST) water-soluble polymers. It is well known that covalent attachment of PEG [188] or ST polyHPMA [189] may extend the intravascular half-life of proteins and/or vesicular drug carriers.
4.4 Bone metastases
Paget's 1889 “seed and soil” theory of bone metastases remains the generally accepted theory regarding metastasis locating to bone. This theory simply reasons that as metastatic cells (the seeds) enter into the bloodstream they will be carried everywhere, but they will only attach and grow where the conditions are right (the soil) [190]. Examples of this include bone marrow expression of CXCL12 [191,192], whose complementary CXCR4 is found on several prostate and breast cancers [193-195]. Once in the bone, bone anabolism and catabolism causes patients severe bone pain and increased mortality [196]. Treatment of these metastases often involves small molecule therapeutics, which target various osteoclast mechanisms. Several small molecule osteoclast targeted therapeutics have been studied: vacuolar H+-ATPase inhibitors, Reveromycin A, Methyl Gerfelin, C-Src Inhibitors, aVb3 integrin inhibitors, Cat K inhibitors) [197] and BPs [50]. Although these do reduce bone pain and some degree of metastatic growth, their effects may be increased as other synergistic drugs are added to reduce growth and tumor progression.
As bone metastases progress, HAp is exposed, providing a target for BP attachment [198]. The Satchi-Fainaro group conducted several experiments combining the anti-angiogenic properties of ALN with other chemotherapies. Paclitaxel (PTX) has anti-angiogenic properties in low doses. Miller et al. used ALN targeted PTX containing HPMA copolymers to reduce migration and proliferation of human umbilical vein endothelial cells (HUVEC). Cat B is a protease expressed by both proliferating endothelial and prostate cancer cells. Miller et al. incorporated a Cat B sensitive linker (GFLG) for both PTX and ALN. In vitro data suggest that the HPMA copolymer inhibited migration and proliferation of both human prostate adenocarcinoma (PC3) and HUVEC cells [199].
In a follow up study Miller et al. demonstrated the in vivo tumor inhibition and safety profiles on Balb/c mice. The PTX, ALN, HPMA copolymer showed no significant WBC toxicity while free PTX significantly reduced WBC counts. Intratibial injections of mCherry-labeled 4T1 cells were used to mimic breast cancer metastasis to the bone. Following i.v. administration of either PTX, ALN HPMA copolymer or free PTX+ ALN in combination, the polymer conjugate inhibited tumor growth by 60%, while free PTX + ALN only inhibited 37% as compared to controls [200].
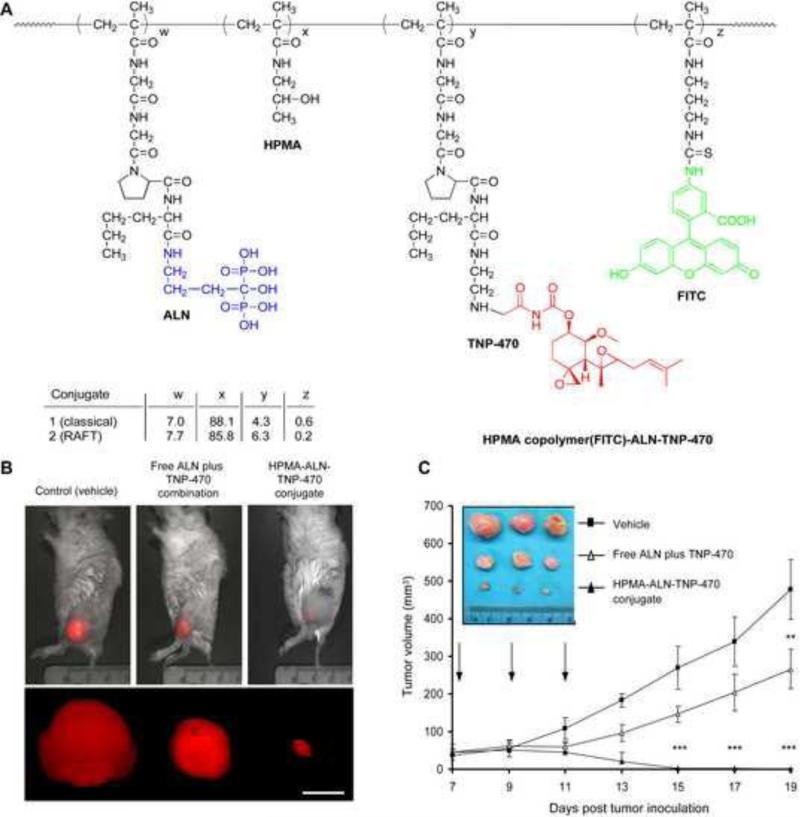
Segal et al. conducted a similar set of experiments using TNP-470 bound to an HPMA copolymer conjugate targeted with ALN. TNP-470 demonstrated high efficacy in clinical trials but too many side effects prevented clinical applications [201]. Segal et al. sought to reduce TNP-470 side effects by conjugation and targeting. Both ALN and TNP-470 were conjugated to HPMA using Cat K sensitive linkers (Gly-Gly-Pro-Nle). In vivo studies demonstrated (Fig. 10) that not only do ALN and TNP-470 have synergism, but revealed that the HPMA copolymer decreased osteosarcoma growth by 96% compared to the control, as opposed to 45% with free ALN in combo with TNP-470 [80]. Similar to the PTX, ALN, HPMA copolymer, TNP-470, ALN HPMA copolymer's toxicity is low, as opposed to ALN + TNP-470, which caused in vivo weight loss, neurological dysfunction, and low WBC counts [81].
Figure 10.
Treatment of mCherry-labeled MG-63-Ras osteosarcoma tumor-bearing mice with free ALN and TNP-470, HPMA copolymer-ALN-TNP-470 conjugate, or vehicle alone. (A) Structure of the HPMA copolymer-ALN-TNP-470 conjugate. (B) Intravital non-invasive fluorescence imaging of the tumor. (C) Tumor volume of mCherry-labeled MG-63-Ras tumor-bearing mice treated with free ALN and TNP-470 (open triangles), HPMA copolymer-ALN-TNP-470 conjugate (closed triangles), or vehicle alone (closed squares) [80].
4.5 Radiation therapy
Targeted delivery of radioisotopes provides opportunities to open up the field of theranostics. Several groups have effectively reduced tumor size and alleviated bone pain by radiolabeling BPs [202,203]. PEI-MP bound tin is one of the few studies which uses polymer attributes in radiation therapy to bone. Jansen et al. demonstrated effective HAp binding of Sn2+ and Sn4+ chelated PEI-MP. In order to demonstrate the EPR effect in combination with HAp adsorption, however, an in vivo study is still necessary [97,98]. Although more tests are needed in order to determine the efficacy of delivering radiolabeled polymers to bone metastases, one must still be curious about the possibilities. Although radioactive polymers may need to be kept small as to reduce circulation time, one may still see greater tumor accumulation due to the EPR effect. Furthermore, the dose can be increased with polymer therapeutics as several chelating moieties can be attached to one molecule. Finally, a single polymer may be able to destroy cancer stem cells by radiosensitizing a tumor using a compound such as perifosin and then killing the stem cells using radiation [204].
5 Conclusions
Targeted polymer therapeutics continue to find niches in the medical world. Much of this research has focused on the EPR effect and delivery of chemotherapeutics and siRNA to solid tumors. Although this is excellent research, the characteristics of bone supply a fertile ground for future clinically relevant targeted-polymer drug research. Many applications favor macromolecular drug delivery over small molecule drugs. For example, one must consider that achieving the highest binding constant possible may not suit one's needs. Having an extremely low dissociation constant means that the polymer will most likely bind kinetically to the first bone that it comes in contact with. Modification of mole percent targeting ligands, or the ligand itself may reduce the Kd, thereby favoring thermodynamic adsorption over kinetic adsorption. By doing so, one may reduce promiscuity of the targeting ligand by limiting adsorption to the diseased area, such as a tumor. This method of targeting lends itself to targeted polymer therapeutics rather than their small molecule counterparts. Furthermore, many bone diseases are chronic conditions. Osteoporosis and osteoarthritis sufferers would benefit from extended controlled drug release mechanisms, which suit macromolecular drug delivery systems. As an underutilized application for targeted polymer drug delivery, bone targeted polymer therapeutics have yet to reach their full potential.
5.2 Gaps in current research
Many novel bone-drug applications have yet to be explored, but first it is important to better develop disease-specific targeting and discover specific biochemical pathways involved in disease states. More accurate targeting to specific diseases involves finding out what bone conditions are present in each disease and then tailoring targeting mechanisms to those conditions. In this paper we discussed several mechanisms by which modification of targeting mechanisms will lead to drug accumulation on varied HAp crystalline states. Currently, most papers stop prior to in vivo data, and pre-in vivo binding studies use a standard HAp. Appropriate pre-in vivo testing should include binding assays using HAp crystals sized for specific disease conditions. Information also lacking with regards to many bone diseases is a deep understanding of the pathology and biochemistry which governs each disease. For example, this paper discussed some of the basic principles of bone anabolism affecting diseases such as osteoporosis. Some crosstalk between signaling pathways such as BMP-2 and PGEs were discussed, but great gaps in our knowledge remain. Studies such as Lee et al., where basic research on bone anabolic pathways are elucidated can be performed side-by-side with applied research on discovering novel delivery approaches and are critical for progression of the field [115]. The realization of synergistic drugs delivered to bone depends on this basic research.
5.3 Future of the field
With attention to proper targeting techniques, the applications in bone-targeted therapeutics have several functions it can fulfill. Relatively few novel developments of bone targeted antineoplastic agents delivered by polymers have been explored. Bone metastases are manifestations of severe cancers representing a largely unsolved problem in oncology. As such, small improvements may go a long way in the clinic. As previously discussed, locally applied radiation therapy delivered with a sensitization drug, or two chemotherapeutics acting on different pathways are a good place to begin in solving this problem.
Combination therapies can also be applied to osteoporosis. It is clear that combining drugs such as BMP-2 and PGE2 is worth exploring but dosing regimens may also yield some dogma-changing discoveries. Development of bone anabolic agents for the treatment of osteoporosis has always been a tricky balancing act. As seen with PTH1-34, overstimulation of an anabolic biological pathway over time can result in osteosarcoma. Reducing the chance of osteosarcoma may be accomplished by the development of several targeted bone anabolic agents that affect different pathways and applying them over different lengths of time, never exacerbating one particular pathway.
Lessons learned from targeting osteoporosis may then be applied to developing anabolic agents to aid healing following orthopedic surgeries and reduce long-term bone resorption around prosthetic stems. Furthermore, treatment of deep bone infections may be reduced by a pre- and post- surgery targeted antibiotic rather than antibiotic-loaded bone cements, which some argue have toxicity issues.
Bone diseases continue to increase in prevalence with aging populations. Successful advancements in bone targeted polymer therapeutics will yield clinically relevant drugs that will reduce pain and increase quality of life for millions of people.
Acknowledgment
The research was supported in part by NIH grant GM069847.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shea JE, Miller SC. Skeletal function and structure: Implications for tissue-targeted therapeutics. Adv. Drug Delivery Rev. 2005;57:945–957. doi: 10.1016/j.addr.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 2.Posner AS, Betts F. Synthetic amorphous calcium phosphate and its relation to bone mineral structure. Acc. Chem. Res. 1975;8:273–281. [Google Scholar]
- 3.Wang D, Miller SC, Shlyakhtenko LS, Portillo AM, Liu X-M, Papangkorn K, Kopečková P, Lyubchenko Y, Higuchi WI, Kopeček J. Osteotropic peptide that differentiates functional domains of the skeleton. Bioconjugate Chem. 2007;18:1375–1378. doi: 10.1021/bc7002132. [DOI] [PubMed] [Google Scholar]
- 4.Lacey D, Timms E, Tan HL, Kelley M, Dunstan C, Burgess T, Elliott R, Colombero A, Elliott G, Scully S. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 5.Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc. Natl. Acad. Sci. USA. 1998;95:3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Väänänen H, Zhao H, Mulari M, Halleen JM. The cell biology of osteoclast function. J. Cell Sci. 2000;113:377–381. doi: 10.1242/jcs.113.3.377. [DOI] [PubMed] [Google Scholar]
- 7.Harada S. Control of osteoblast function and regulation of bone mass. Nature. 2003;423:349–355. doi: 10.1038/nature01660. [DOI] [PubMed] [Google Scholar]
- 8.Bartl R, Frisch B, Bartl C. Osteoporosis: Diagnosis, Prevention, Therapy. Springer; 2009. [Google Scholar]
- 9.Dempster DW. Osteoporosis and the burden of osteoporosis-related fractures. Am. J. Manag. Care. 2011;17:S164–S169. [PubMed] [Google Scholar]
- 10.Wang D, Miller SC, Kopečková P, Kopeček J. Bone-targeting macromolecular therapeutics. Adv. Drug Delivery Rev. 2005;57:1049–1076. doi: 10.1016/j.addr.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 11.Gittens SA, Bansal G, Zernicke RF, Uludağ H. Designing proteins for bone targeting. Adv. Drug Delivery Rev. 2005;57:1011–1036. doi: 10.1016/j.addr.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 12.Uludağ H, Yang J. Targeting systemically administered proteins to bone by bisphosphonate conjugation. Biotechnol. Prog. 2002;18:604–611. doi: 10.1021/bp0200447. [DOI] [PubMed] [Google Scholar]
- 13.Segal E, Satchi-Fainaro R. Design and development of polymer conjugates as antiangiogenic agents. Adv. Drug Delivery Rev. 2009;61:1159–1176. doi: 10.1016/j.addr.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Blackwell KA, Raisz LG, Pilbeam CC. Prostaglandins in bone: Bad cop, good cop? Trends Endocrinol. Metab. 2010;21:294–301. doi: 10.1016/j.tem.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsuo K, Irie N. Osteoclast-osteoblast communication. Arch. Biochem. Biophys. 2008;473:201–209. doi: 10.1016/j.abb.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 16.Maeda H, Bharate GY, Daruwalla J. Polymeric drugs for efficient tumor-targeted drug delivery based on EPR-effect. Eur. J. Pharm. Biopharm. 2009;71:409–419. doi: 10.1016/j.ejpb.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 17.Fang J, Nakamura H, Maeda H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv. Drug Delivery Rev. 2010;63:136–151. doi: 10.1016/j.addr.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 18.Maeda H. Tumor-selective delivery of macromolecular drugs via the EPR effect: Background and future prospects. Bioconjugate Chem. 2010;21:797–802. doi: 10.1021/bc100070g. [DOI] [PubMed] [Google Scholar]
- 19.Torchilin V. Tumor delivery of macromolecular drugs based on the EPR effect. Adv. Drug Delivery Rev. 2010;63:131–135. doi: 10.1016/j.addr.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 20.Silverthorn DU, Ober WC, Garrison CW, Silverthorn AC, Johnson BR. Human Physiology: An Integrated Approach. Pearson/Benjamin Cummings; San Francisco, CA: 2004. [Google Scholar]
- 21.Owen M, Howlett CR, Triffitt JT. Movement of 125I albumin and 125I polyvinylpyrrolidone through bone tissue fluid. Calcif. Tissue Res. 1977;23:103–112. doi: 10.1007/BF02012773. [DOI] [PubMed] [Google Scholar]
- 22.Howlett CR, Dickson M, Sheridan AK. The fine structure of the proximal growth plate of the avian tibia: vascular supply. J. Anat. 1984;139(Pt 1):115–132. [PMC free article] [PubMed] [Google Scholar]
- 23.Griffin MO, Ceballos G, Villarreal FJ. Tetracycline compounds with non-antimicrobial organ protective properties: Possible mechanisms of action. Pharmacol. Res. 2011;63:102–107. doi: 10.1016/j.phrs.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Semenkov Yu.P., Makarov EM, Makhno VI, Kirillov SV. Kinetic aspects of tetracycline action on the acceptor (A) site of Escherichia coli ribosomes. FEBS Lett. 1982;144:125–129. doi: 10.1016/0014-5793(82)80584-x. [DOI] [PubMed] [Google Scholar]
- 25.Madison JF. Tetracycline pigmentation of teeth. Arch. Dermatol. 1963;88:58–59. doi: 10.1001/archderm.1963.01590190064008. [DOI] [PubMed] [Google Scholar]
- 26.Lojodice G, Vento R, Cinque NA, Gilli G. Effect on dental and skeletal development of administration of tetracycline in the infant. Minerva Pediatr. 1965;17:1358–1365. [PubMed] [Google Scholar]
- 27.Bevelander G, Nakahara H, Rolle GK. Inhibition of skeletal formation in the chick embryo following administration of tetracycline. Nature. 1959;184(Suppl. 10):728–729. doi: 10.1038/184728b0. [DOI] [PubMed] [Google Scholar]
- 28.Perrin DD. Binding of tetracyclines to bone. Nature. 1965;208:787–788. doi: 10.1038/208787a0. [DOI] [PubMed] [Google Scholar]
- 29.Neale JR, Richter NB, Merten KE, Grant Taylor K, Singh S, Waite LC, Emery NK, Smith NB, Cai J, Pierce WM., Jr. Bone selective effect of an estradiol conjugate with a novel tetracycline-derived bone-targeting agent. Bioorg. Med. Chem. Lett. 2009;19:680–683. doi: 10.1016/j.bmcl.2008.12.051. [DOI] [PubMed] [Google Scholar]
- 30.Fleisch H, Russell RG, Bisaz S, Casey PA, Mühlbauer RC. The influence of pyrophosphate analogues (diphosphonates) on the precipitation and dissolution. Calcif. Tissue Res. 1968;(Suppl.):10–10a. doi: 10.1007/BF02065192. [DOI] [PubMed] [Google Scholar]
- 31.Kavanagh KL, Guo K, Dunford JE, Wu X, Knapp S, Ebetino FH, Rogers MJ, Russel RGG, Oppermann U. The molecular mechanism of nitrogen-containing bisphosphonates as antiosteoporosis drugs. Proc. Natl. Acad. Sci. USA. 2006;103:7829–34. doi: 10.1073/pnas.0601643103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bradley J, Cleverly D, Burns A, Helm N, Schmid M, Marx D, Cullen DM, Reinhardt RA. Cyclooxygenase-2 inhibitor reduces simvastatin-induced bone morphogenetic protein-2 and bone formation in vivo. J. Periodontal Res. 2007;42:267–273. doi: 10.1111/j.1600-0765.2006.00943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ayukawa Y, Yasukawa E, Moriyama Y, Ogino Y, Wada H, Atsuta I, Koyano K. Local application of statin promotes bone repair through the suppression of osteoclasts and the enhancement of osteoblasts at bone-healing sites in rats, Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology. Endodontology. 2009;107:336–342. doi: 10.1016/j.tripleo.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 34.Ohnaka K, Shimoda S, Nawata H, Shimokawa H, Kaibuchi K, Iwamoto Y, Takayanagi R. Pitavastatin enhanced BMP-2 and osteocalcin expression by inhibition of Rho-associated kinase in human osteoblasts. Biochem. Biophys. Res. Commun. 2001;287:337–342. doi: 10.1006/bbrc.2001.5597. [DOI] [PubMed] [Google Scholar]
- 35.Black DM, Cummings SR. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet. 1996;348:1535–1541. doi: 10.1016/s0140-6736(96)07088-2. [DOI] [PubMed] [Google Scholar]
- 36.Arkader A, Morris CD. Lymphatic spread of pagetic osteogenic sarcoma detected by bone scan. Cancer Imaging. 2008;8:131–134. doi: 10.1102/1470-7330.2008.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lawson MA, Xia Z, Barnett BL, Triffitt JT, Phipps RJ, Dunford JE, Locklin RM, Ebetino FH, Russell RG. Differences between bisphosphonates in binding affinities for hydroxyapatite. J. of Biomedical Materials Res. Part B: Applied Biomaterials. 2010;92B:149–155. doi: 10.1002/jbm.b.31500. [DOI] [PubMed] [Google Scholar]
- 38.Jahnke W, Henry C. An in vitro Assay to Measure Targeted Drug Delivery to Bone Mineral. Chem. MedChem. 2010;5:770–776. doi: 10.1002/cmdc.201000016. [DOI] [PubMed] [Google Scholar]
- 39.Wen D, Qing L, Harrison G, Golub E, Akintoye S. Anatomic site variability in rat skeletal uptake and desorption of fluorescently labeled bisphosphonate. Oral Diseases. 2010;4:427–432. doi: 10.1111/j.1601-0825.2010.01772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hrubý M, Etrych T, Kučka J, Forsterová M, Ulbrich K. Hydroxybisphosphonate-containing polymeric drug-delivery systems designed for targeting into bone tissue. J. Appl. Polymer Sci. 2006;101:3192–3201. [Google Scholar]
- 41.Choi S-W, Kim J-H. Design of surface-modified poly(D,L-lactide-co-glycolide) nanoparticles for targeted drug delivery to bone. J. Control. Release. 2007;122:24–30. doi: 10.1016/j.jconrel.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 42.Mukherjee S. Thermodynamics of bisphosphonates binding to human bone: a two-site model. J. Am. Chem. Soc. 2009;131:8374–8375. doi: 10.1021/ja902895p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ross RD, Roeder RK. Binding affinity of surface functionalized gold nanoparticles to hydroxyapatite. Journal of Biomedical Materials Research Part A. 2011;99A:58–66. doi: 10.1002/jbm.a.33165. [DOI] [PubMed] [Google Scholar]
- 44.Franc G, Turrin CO, Cavero E, Costes JP, Duhayon C, Caminade AM, Majoral JP. gem-Bisphosphonate-Ended Group Dendrimers: Design and Gadolinium Complexing Properties. European Journal of Organic Chemistry. 2009;2009:4290–4299. [Google Scholar]
- 45.Shao XH, Xu JQ, Jiao YP, Zhou CR. Synthesis and Characterization of an Alendronate-Chitosan Conjugate. Applied Mechanics and Materials. 2012;140:53–57. [Google Scholar]
- 46.Doschak MR, Kucharski CM, Wright JE, Zernicke RF, Uludağ H. Improved bone delivery of osteoprotegerin by bisphosphonate conjugation in a rat model of osteoarthritis. Mol. Pharmaceutics. 2009;6:634–640. doi: 10.1021/mp8002368. [DOI] [PubMed] [Google Scholar]
- 47.Ziebart T, Pabst A, Klein MO, Kämmerer P, Gauss L, Brüllmann D, Al-Nawas B, Walter C. Bisphosphonates: restrictions for vasculogenesis and angiogenesis: inhibition of cell function of endothelial progenitor cells and mature endothelial cells in vitro. Clin. Oral Invest. 2009;15:105–111. doi: 10.1007/s00784-009-0365-2. [DOI] [PubMed] [Google Scholar]
- 48.Marx RE, Sawatari Y, Fortin M, Broumand V. Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: risk factors, recognition, prevention, and treatment. J. Oral Maxillofacial Surg. 2005;63:1567–1575. doi: 10.1016/j.joms.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 49.Hoff AO, Toth BB, Altundag K, Johnson MM, Warneke CL, Hu M, Nooka A, Sayegh G, Desrouleaux V, Cui J, Adamus A, Gagel RF, Hortobagyi GN. Frequency and risk factors associated with osteonecrosis of the jaw in cancer patients treated with intravenous bisphosphonates. J. Bone Miner. Res. 2008;23:826–836. doi: 10.1359/JBMR.080205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prommer EE. Toxicity of bisphosphonates. J. Palliative Med. 2009;12:1061–1065. doi: 10.1089/jpm.2009.9936. [DOI] [PubMed] [Google Scholar]
- 51.Ishizaki J. Selective drug delivery to bone using acidic oligopeptides. J. Bone Mineral Metabolism. 2009;27:1–8. doi: 10.1007/s00774-008-0004-z. [DOI] [PubMed] [Google Scholar]
- 52.Ouyang L, Huang W, He G, Guo L. Bone targeting prodrugs based on peptide dendrimers, Synthesis and hydroxyapatite binding in vitro. Letters Org. Chem. 2009;6:272–277. [Google Scholar]
- 53.Sekido T, Sakura N, Higashi Y, Miya K, Nitta Y, Nomura M, Sawanishi H, Morito K, Masamune Y, Kasugai S, Yokogawa K, Miyamoto K. Novel drug delivery system to bone using acidic oligopeptide: pharmacokinetic characteristics and pharmacological potential. J. Drug Target. 2001;9:111–121. doi: 10.3109/10611860108997922. [DOI] [PubMed] [Google Scholar]
- 54.Neale JR, Richter NB, Merten KE, Taylor KG, Singh S, Waite LC, Emery NK, Smith B, Cai J, Pierce WM., Jr. Bone selective effect of an estradiol conjugate wih a novel tetracycline-derived bone-targeting agent. Bioorg. Med. Chem. Lett. 2009;19:680–683. doi: 10.1016/j.bmcl.2008.12.051. [DOI] [PubMed] [Google Scholar]
- 55.Nasim S, Vartak A, Pierce WM, Jr., Taylor KG, Crooks PA. Improved and scalable synthetic route to the synthon 17-(2-carboxyethyl)-1,3,5 (10)-estratriene: An important itermediate in the synthesis of bone-targeting estrogens. Synth. Commun. 2010;40:772–781. [Google Scholar]
- 56.Nasim S, Vartak AP, Pierce WM, Jr., Taylor KG, Smith N, Crooks PA. 3-O-Phosphate ester conjugates of 17-β-O-{1-[2-carboxy-(2-hydroxy-4-methoxy-3-carboxamido) anilido]ethyl}1,3,5(10)-estratriene as novel bone-targeting agents. Bioorg. Med. Chem. Lett. 2010;20:7450–7453. doi: 10.1016/j.bmcl.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 57.Murphy MB. Synthesis and in vitro hydroxyapatite binding of peptides conjugated to calcium-binding moieties. Biomacromolecules. 2007;8:2237–2243. doi: 10.1021/bm070121s. [DOI] [PubMed] [Google Scholar]
- 58.Wang D, Sima M, Mosley RL, Davda JP, Tietze N, Miller SC, Gwilt PR, Kopečková P, Kopeček J. Pharmacokinetic and biodistribution studies of a bone-targeting drug delivery system based on N-(2-hydroxypropyl)methacrylamide copolymers. Mol. Pharmaceutics. 2006;3:717–725. doi: 10.1021/mp0600539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miller S, Pan H, Wang D, Bowman B, Kopečková P, Kopeček J. Feasibility of using a bone-targeted, macromolecular delivery system coupled with prostaglandin E1 to promote bone formation in aged, estrogen-deficient rats. Pharmaceutical Res. 2008;25:2889–2895. doi: 10.1007/s11095-008-9706-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pan H, Sima M, Kopečková P, Wu K, Gao S, Liu J, Wang D, Miller SC, Kopeček J. Biodistribution and pharmacokinetic studies of bone-targeting N-(2-hydroxypropyl)methacrylamide copolymer-alendronate conjugates. Mol. Pharmaceutics. 2008;5:548–558. doi: 10.1021/mp800003u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Noh EM, Kim JS, Hur H, Park BH, Song EK, Han MK, Kwon KB, Yoo WH, Shim IK, Lee SJ. Cordycepin inhibits IL-1β-induced MMP-1 and MMP-3 expression in rheumatoid arthritis synovial fibroblasts. Rheumatology. 2009;48:45–48. doi: 10.1093/rheumatology/ken417. [DOI] [PubMed] [Google Scholar]
- 62.Hu F, Wang C, Guo S, Sun W, Mi D, Gao Y, Zhang J, Zhu T, Yang S. δEF1 promotes osteolytic metastasis of MDA-MB-231 breast cancer cells by regulating MMP-1 expression. Biochim. Biophys.Acta-Gene Reg.Mech. 2011;1809:200–210. doi: 10.1016/j.bbagrm.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 63.Nyman JS, Lynch CC, Perrien DS, Thiolloy S, O'Quinn EC, Patil CA, Pharr GM, Mahadevan-Jansen A, Mundy GR. Differential effects between the loss of MMP-2 and MMP-9 on structural and tissue-level properties of bone. J. Bone Mineral Res. 2011;26:1252–1260. doi: 10.1002/jbmr.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Takaishi H, Kimura T, Dalal S, Okada Y, D'Armiento J. Joint diseases and matrix metalloproteinases: a role for MMP-13. Curr. Pharmaceutical Biotech. 2008;9:47–54. doi: 10.2174/138920108783497659. [DOI] [PubMed] [Google Scholar]
- 65.Franco GCN, Kajiya M, Nakanishi T, Ohta K, Rosalen PL, Groppo FC, Ernst CWO, Boyesen JL, Bartlett JD, Strashenko P. Inhibition of matrix metalloproteinase-9 activity by doxycycline ameliorates RANK ligand-induced osteoclast differentiation in vitro and in vivo. Exp. Cell Res. 2011;317:1454–1464. doi: 10.1016/j.yexcr.2011.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mosig RA, Dowling O, DiFeo A, Ramirez MCM, Parker IC, Abe E, Diouri J, Aqeel AA, Wylie JD, Oblander SA. Loss of MMP-2 disrupts skeletal and craniofacial development and results in decreased bone mineralization, joint erosion and defects in osteoblast and osteoclast growth. Human Mol. Genetics. 2007;16:1113–1123. doi: 10.1093/hmg/ddm060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lynch CC, Hikosaka A, Acuff HB, Martin MD, Kawai N, Singh RK, Vargo-Gogola TC, Begtrup JL, Fingleton B. MMP-7 promotes prostate cancer-induced osteolysis via the solubilization of RANKL. Cancer Cell. 2005;7:485–496. doi: 10.1016/j.ccr.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 68.Mun SH, Kim HS, Kim JW, Ko NY, Kim DK, Lee BY, Kim B, Won HS, Shin HS, Han JW. Oral administration of curcumin suppresses production of matrix metalloproteinase (MMP)-1 and MMP-3 to ameliorate collagen-induced arthritis: Inhibition of the PKCδ/JNK/c-Jun Pathway. J. Pharmacol. Sci. 2009;111:13–21. doi: 10.1254/jphs.09134fp. [DOI] [PubMed] [Google Scholar]
- 69.Luo XH, Guo LJ, Shan PF, Xie H, Wu XP, Zhang H, Cao XZ, Yuan LQ, Liao EY. Relationship of circulating MMP–2, MMP–1, and TIMP–1 levels with bone biochemical markers and bone mineral density in postmenopausal Chinese women. Osteoporosis Int. 2006;17:521–526. doi: 10.1007/s00198-005-0017-6. [DOI] [PubMed] [Google Scholar]
- 70.Gwack C, Kim SS, Park SB, Son WS, Kim YD, Jun ES, Park MH. The expression of MMP-1,-8, and-13 mRNA in the periodontal ligament of rats during tooth movement with cortical punching. Korean J. Orthodontics. 2008;38:187–201. [Google Scholar]
- 71.Lynch CC, Matrisian LM. Matrix metalloproteinases as key regulators of tumor–bone interaction. Cancer Degradome. 2008:541–566. [Google Scholar]
- 72.Smith GN, Mickler EA, Hasty KA, Brandt KD. Specificity of inhibition of matrix metalloproteinase activity by doxycycline: relationship to structure of the enzyme. Arthritis Rheum. 1999;42:1140–1146. doi: 10.1002/1529-0131(199906)42:6<1140::AID-ANR10>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 73.Georges S, Ruiz Velasco C, Trichet V, Fortun Y, Heymann D, Padrines M. Proteases and bone remodeling. Cytokine Growth Factor Rev. 2009;20:29–41. doi: 10.1016/j.cytogfr.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 74.Rejmanová P, Pohl J, Baudyš M, Kostka V, Kopeček J. Polymers containing enzymatically degradable bonds. 8. Degradation of oligopeptide sequences in N-(2-hydroxypropyl)methacrylamide copolymers by bovine spleen cathepsin B. Makromol. Chem. 1983;184:2009–2020. [Google Scholar]
- 75.Gao S, Lu ZR, Petri B, Kopečková P, Kopeček J. Colon-specific 9-aminocamptothecin-HPMA copolymer conjugates containing a 1,6-elimination spacer. J. Control. Release. 2006;110:323–331. doi: 10.1016/j.jconrel.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 76.Greenwald RB, Pendri A, Conover CD, Zhao H, Choe YH, Martinez A, Shum K, Guan S. Drug delivery systems employing 1,4- or 1,6-elimination: poly(ethylene glycol) prodrugs of amine-containing compounds. J. Med. Chem. 1999;42:3657–3667. doi: 10.1021/jm990166e. [DOI] [PubMed] [Google Scholar]
- 77.Carl PL, Chakravarty PK, Katzenellenbogen JA. A novel connector linkage applicable in prodrug design. J. Med. Chem. 1981;24:479–480. doi: 10.1021/jm00137a001. [DOI] [PubMed] [Google Scholar]
- 78.Toki BE, Cerveny CG, Wahl AF, Senter PD. Protease-mediated fragmentation of p-amidobenzyl ethers: A new strategy for the activation of anticancer prodrugs. J. Org. Chem. 2002;67:1866–1872. doi: 10.1021/jo016187+. [DOI] [PubMed] [Google Scholar]
- 79.de Groot FM, Loos WJ, Koekkoek R, van Berkom LW, Busscher GF, Seelen AE, Albrecht C, de Bruijn P, Scheeren HW. Elongated multiple electronic cascade and cyclization spacer systems in activatible anticancer prodrugs for enhanced drug release. J. Org. Chem. 2001;66:8815–8830. doi: 10.1021/jo0158884. [DOI] [PubMed] [Google Scholar]
- 80.Segal E, Pan H, Ofek P, Udagawa T, Kopečková P, Kopeček J, Satchi-Fainaro R. Targeting angiogenesis-dependent calcified neoplasms using combined polymer therapeutics. PLoS One. 2009;4:e5233. doi: 10.1371/journal.pone.0005233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Segal E, Pan H, Benayoun L, Kopečková P, Shaked Y, Kopeček J, Satchi-Fainaro R. Enhanced anti-tumor activity and safety profile of targeted nano-scaled HPMA copolymer-alendronate-TNP-470 conjugate in the treatment of bone malignances. Biomaterials. 2011;32:4450–4463. doi: 10.1016/j.biomaterials.2011.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Miller K, Erez R, Segal E, Shabat D, Satchi-Fainaro R. Targeting bone metastases with a bispecific anticancer and antiangiogenic polymer-alendronate-taxane conjugates. Ang. Chem. Int. Ed. 2009;48:2949–2954. doi: 10.1002/anie.200805133. [DOI] [PubMed] [Google Scholar]
- 83.Yang J, Chen H, Vlahov IR, Cheng J-X, Low PS. Evaluation of disulfide reduction during receptor-mediated endocytosis by using FRET imaging. Proc. Natl. Acad. Sci. USA. 2006;103:13872–13877. doi: 10.1073/pnas.0601455103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kurtoglu YE, Navath RS, Wang B, Kannan S, Romero R, Kannan RM. Poly(amidoamine) dendrimer-drug conjugates with disulfide linkages for intracellular drug delivery. Biomaterials. 2009;30:2112–2121. doi: 10.1016/j.biomaterials.2008.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nesbitt SA, Horton MA. Trafficking of matrix collagens through bone-resorbing osteoclasts. Science. 1997;276:266–273. doi: 10.1126/science.276.5310.266. [DOI] [PubMed] [Google Scholar]
- 86.Palokangas H, Mulari M, Väänänen HK. Endocytic pathway from basal plasma membrane to the ruffled border membrane in bone-resorbing osteoclasts. J. Cell Sci. 1997;110:1767–1780. doi: 10.1242/jcs.110.15.1767. [DOI] [PubMed] [Google Scholar]
- 87.Mulari MTK, Zhao HB, Lakkakorpi PT, Väänänen HK. Osteoclast ruffled border has distinct subdomains for secretion and degraded matrix uptake. Traffic. 2003;4:113–125. doi: 10.1034/j.1600-0854.2003.40206.x. (2003) [DOI] [PubMed] [Google Scholar]
- 88.Pan H, Kopečková P, Wang D, Yang J, Miller S, Kopeček J. Water-soluble HPMA copolymer—prostaglandin E1 conjugates containing a cathepsin K sensitive spacer. J. Drug Targeting. 2006;14:425–435. doi: 10.1080/10611860600834219. [DOI] [PubMed] [Google Scholar]
- 89.Schechter I, Berger A. On the size of active site in proteases. I. Papain. Biochem. Biophys. Res. Commun. 1967;27:157–162. doi: 10.1016/s0006-291x(67)80055-x. [DOI] [PubMed] [Google Scholar]
- 90.Lecaille F, Choe Y, Brandt W, Li Z, Craik CS, Brömme D. Selective inhibition of the collagenolytic activity of human cathepsin K by altering its S2 subsite specificity. Biochemistry. 2002;41:8447–8454. doi: 10.1021/bi025638x. [DOI] [PubMed] [Google Scholar]
- 91.Rejmanová P, Kopeček J, Duncan R, Lloyd JB. Stability in rat plasma and serum of lysosomally degradable oligopeptide sequences in N-(2-hydroxypropyl)methacrylamide copolymers. Biomaterials. 1985;6:45–48. doi: 10.1016/0142-9612(85)90037-7. [DOI] [PubMed] [Google Scholar]
- 92.Kopeček J, Rejmanová P, Chytrý V. Polymers containing enzymatically degradable bonds. I. Chymotrypsin catalyzed hydrolysis of p-nitroanilides of phenylalanine and tyrosine attached to side-chains of copolymers of N-(2-hydroxypropyl)methacrylamide. Makromol. Chem. 1981;182:799–809. [Google Scholar]
- 93.Pan H, Liu J, Dong Y, Sima M, Kopečková P, Brandi ML, Kopeček J. Release of prostaglandin E1 from N-(2-Hydroxypropyl)methacrylamide copolymer conjugates by bone cells. Macromol. Biosci. 2008;8:599–605. doi: 10.1002/mabi.200700338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang S, Wright JEI, Ozber N, Uludağ H. The interaction of cationic polymers and their bisphosphonate derivatives with hydroxyapatite. Macromol. Biosci. 2007;7:656–670. doi: 10.1002/mabi.200600286. [DOI] [PubMed] [Google Scholar]
- 95.Wen Y, Pan S, Luo X, Zhang X, Zhang W, Feng M. A biodegradable low molecular weight polyethylenimine derivative as low toxicity and efficient gene vector. Bioconjugate Chem. 2009;20:322–332. doi: 10.1021/bc800428y. [DOI] [PubMed] [Google Scholar]
- 96.Aravindan L, Bicknell KA, Brooks G, Khutoryanskiy VV, Williams AC. Effect of acyl chain length on transfection efficiency and toxicity of polyethylenimine. Int. J. Pharmaceutics. 2009;378:201–210. doi: 10.1016/j.ijpharm.2009.05.052. [DOI] [PubMed] [Google Scholar]
- 97.Jansen DR, Rijn Zeevaart J, Denkova A, Kolar ZI, Krijger GC. Hydroxyapatite chemisorption of N,N′,N′-trimethylenephosphonate−poly(ethyleneimine) (PEI−MP) combined with Sn2+ or Sn4+ Langmuir. 2009;25:2790–2796. doi: 10.1021/la802485g. [DOI] [PubMed] [Google Scholar]
- 98.Jansen DR, Krijger GC, Wagener J, Senwedi RM, Gabanamotse K, Kgadiete M, Kolar ZI, Zeevaart JR. Blood plasma model predictions for the proposed bone-seeking radiopharmaceutical [117mSn]Sn(IV)-N,N’,N’-trimethylenephosphonate-poly(ethyleneimine) J. Inorganic Biochem. 2009;103:1265–1272. doi: 10.1016/j.jinorgbio.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 99.Yang J, Luo K, Pan H, Kopečková P, Kopeček J. Synthesis of biodegradable multiblock copolymers by click coupling of RAFT-generated heterotelechelic polyHPMA conjugates. Reactive Functional Polym. 2011;71:294–302. doi: 10.1016/j.reactfunctpolym.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pan H, Yang J, Kopečková P, Kopeček J. Backbone degradable multiblock HPMA copolymer conjugates via RAFT polymerization and thiol-ene coupling reaction. Biomacromolecules. 2011;12:247–252. doi: 10.1021/bm101254e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Luo K, Yang J, Kopečková P, Kopeček J. Biodegradable multiblock N-(2-hydroxypropyl)methacrylamide copolymers via reversible addition-fragmentation chain transfer polymerization and click chemistry. Macromolecules. 2011;44:2481–2488. doi: 10.1021/ma102574e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Talelli M, Rijcken CJF, van Nostrum CF, Storm G, Hennink WE. Micelles based on HPMA copolymers. Adv. Drug Delivery Rev. 2010;62:231–239. doi: 10.1016/j.addr.2009.11.029. [DOI] [PubMed] [Google Scholar]
- 103.Krimmer S, Pan H, Liu J, Yang J, Kopeček J. Synthesis and characterization of poly(ε-caprolactone)-block-poly[N-(2-hydroxypropyl)methacrylamide] micelles for drug delivery. Macromol. Biosci. 2011;11:1041–1051. doi: 10.1002/mabi.201100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang D, Kopečková JP, Minko T, Nanayakkara V, Kopeček J. Synthesis of starlike N-(2-hydroxypropyl)methacrylamide copolymers: potential drug carriers. Biomacromolecules. 2000;1:313–319. doi: 10.1021/bm0000236. [DOI] [PubMed] [Google Scholar]
- 105.Korzhikov VA, Diederichs S, Nazarova OV, Vlakh EG, Kasper C, Panarin EV, Tennikova TB. Water-soluble aldehyde-bearing polymers of 2-deoxy-2-methacrylamido-D-glucose for bone tissue engineering. J. Appl. Polym. Sci. 2008;108:2386–2397. [Google Scholar]
- 106.Hein CD, Liu XM, Chen F, Cullen DM, Wang D. The synthesis of a multiblock ostotropic polyrotaxane by copper(I)-catalyzed Huisgen 1,3-dipolar cycloaddition. Macromol. Biosci. 2010;10:1544–1556. doi: 10.1002/mabi.201000205. [DOI] [PubMed] [Google Scholar]
- 107.Wang G, Babadagli ME, Uludağ H. Bisphosphonate-derivatized liposomes to control drug release from collagen/hydroxyapatite scaffolds. Mol. Pharmaceutics. 2011;8:1025–1034. doi: 10.1021/mp200028w. [DOI] [PubMed] [Google Scholar]
- 108.Li LY, He WD, Li J, Zhang BY, Pan TT, Sun XL, Ding ZL. Shell-cross-linked micelles from PNIPAM-b-(PLL)2Y-shaped miktoarm star copolymer as drug carriers. Biomacromolecules. 2010;11:1882–1890. doi: 10.1021/bm1004383. [DOI] [PubMed] [Google Scholar]
- 109.Hengst V, Oussoren C, Kissel T, Storm G. Bone targeting potential of bisphosphonate-targeted liposomes: Preparation, characterization and hydroxyapatite binding in vitro. Int. J. Pharmaceutics. 2007;331:224–227. doi: 10.1016/j.ijpharm.2006.11.024. [DOI] [PubMed] [Google Scholar]
- 110.van den Hoven JM, Van Tomme SR, Metselaar JM, Nuijen B, Beijnen JH, Storm G. Liposomal drug formulations in the treatment of rheumatoid arthritis. Mol. Pharmaceutics. 2011;8:1002–1005. doi: 10.1021/mp2000742. [DOI] [PubMed] [Google Scholar]
- 111.Cenni E, Granchi D, Avnet S, Fotia C, Salerno M, Micieli D, Sarpietro MG, Pignatello R, Castelli F, Baldini N. Biocompatibility of poly(D,L-lactide-co-glycolide) nanoparticles conjugated with alendronate. Biomaterials. 2008;29:1400–1411. doi: 10.1016/j.biomaterials.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 112.Salerno M, Cenni E, Fotia C, Avnet S, Granchi D, Castelli F, Micieli D, Pignatello R, Capulli M, Rucci N, Angelucci A, Del Fattore A, Teti A, Zini N, Giunti A, Baldini N. Bone-targeted doxorubicin-loaded nanoparticles as a tool for the treatment of skeletal metastases. Curr. Cancer Drug Targets. 2010;10:649–659. doi: 10.2174/156800910793605767. [DOI] [PubMed] [Google Scholar]
- 113.Wischke C, Zhang Y, Mittal S, Schwendeman SP. Development of PLGA-based injectable delivery systems for hydrophobic fenretinide. Pharmaceutical Res. 2010;27:2063–2074. doi: 10.1007/s11095-010-0202-y. [DOI] [PubMed] [Google Scholar]
- 114.Faisant N, Siepmann J, Benoit JP. PLGA-based microparticles: elucidation of mechanisms and a new, simple mathematical model quantifying drug release. Eur. J. Pharmaceutical Sci. 2002;15:355–366. doi: 10.1016/s0928-0987(02)00023-4. [DOI] [PubMed] [Google Scholar]
- 115.Mundargi RC, Babu VR, Rangaswamy V, Patel P, Aminabhavi TM. Nano/micro technologies for delivering macromolecular therapeutics using poly(D,L-lactide-co-glycolide) and its derivatives. J. Control. Release. 2008;125:193–209. doi: 10.1016/j.jconrel.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 116.Ozcan I, Bouchemal K, Segura-Sánchez F, Ozer O, Güneri T, Ponchel G. Synthesis and characterization of surface-modified PBLG nanoparticles for bone targeting: In vitro and in vivo evaluations. J Pharmaceutical Sci. 2011;100:4877–87. doi: 10.1002/jps.22678. [DOI] [PubMed] [Google Scholar]
- 117.Wang G, Kucharski C, Lin X, Uludağ H. Bisphosphonate-coated BSA nanoparticles lack bone targeting after systemic administration. J. Drug Targeting. 2010;18:611–626. doi: 10.3109/10611861003622560. [DOI] [PubMed] [Google Scholar]
- 118.Bai S, Thomas C, Rawat A, Ahsan F. Recent progress in dendrimer-based nanocarriers. Crit. Rev. Ther. Drug Carrier Syst. 2006;23:437–495. doi: 10.1615/critrevtherdrugcarriersyst.v23.i6.10. [DOI] [PubMed] [Google Scholar]
- 119.Ouyang L, Pan J, Zhang Y, Guo L. Synthesis of second-and third-generation Asp oligopeptide conjugated dendrimers for bone-targeting drug delivery. Synth. Commun. 2009;39:4039–4052. [Google Scholar]
- 120.Clementi C, Miller K, Mero A, Satchi-Fainaro R, Pasut G. Dendritic poly(ethylene glycol) bearing paclitaxel and alendronate for targeting bone neoplasms. Mol. Pharmaceutics. 2011;8:1063–1072. doi: 10.1021/mp2001445. [DOI] [PubMed] [Google Scholar]
- 121.Xiao K, Li Y, Luo J, Lee JS, Xiao W, Gonik AM, Agarwai RG, Lam KS. The effect of surface charge on in vivo biodistribution of PEG-oligocholic acid based micellar nanoparticles. Biomaterials. 2011;32:3435–3446. doi: 10.1016/j.biomaterials.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Christensen K, Doblhammer G, Rau R, Vaupel JW. Ageing populations: the challenges ahead. Lancet. 2009;374:1196–1208. doi: 10.1016/S0140-6736(09)61460-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yuan F, Quan LD, Cui L, Goldring S, Wang D. Macromolecular prodrug delivery for arthritis. Adv. Drug Delivery Rev. doi: 10.1016/j.addr.2012.03.006. this volume. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Grainger G. Targeting RNA-based therapeutics to osteoporosis. Adv. Drug Delivery Rev. this volume. [Google Scholar]
- 125. [September 2011];Osteoporosis information. available at, http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0001400.
- 126.Thompson SK, Halbert SM, Bossard MJ, Tomaszek TA, Levy MA, Zhao B, Smith WW, Abdel-Meguid SS, Janson CA, D'Alessio KJ, McQueeney MS, Amegadzie BY, Hanning CR, DesJarlais RL, Briand J, Sarkar SK, Huddleston MJ, Ijames CF, Carr SA, Garnes KT, Shu A, Heys RJ, Bradbeer J, Zembryki D, Lee-Rykaczeweski L, James IE, Lark MW, Drake FH, Gowen M, Cleason JG, Verber DF. Design of potent and selective human cathepsin K inhibitors that span the active site. Proc. Natl. Acad. Sci. USA. 1997;94:14249–14254. doi: 10.1073/pnas.94.26.14249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang D, Li W, Pechar M, Kopečková P, Brömme D, Kopeček J. Cathepsin K inhibitor–polymer conjugates: potential drugs for the treatment of osteoporosis and rheumatoid arthritis. Int. J. Pharmaceutics. 2004;277:73–79. doi: 10.1016/j.ijpharm.2003.03.003. [DOI] [PubMed] [Google Scholar]
- 128.Wang D, Pechar M, Li W, Kopečková P, Brömme D, Kopeček J. Inhibition of cathepsin K with lysosomotropic macromolecular inhibitors. Biochemistry. 2002;41:8849–8859. doi: 10.1021/bi0257080. [DOI] [PubMed] [Google Scholar]
- 129.Thompson SK, Smith WW, Zhao B, Halbert SM, Tomaszek TA, Tew DG, Levy MA, Janson CA, DAlessio KJ, McQueney MS, Kurdyla J, Jones CS, DesJarlais RL, Abdel-Meguid SS, Veber DF. Structure-based design of cathepsin K inhibitors containing a benzyloxy-substituted benzoyl peptidomimetic. J. Med. Chem. 1998;41:3923–3927. doi: 10.1021/jm980474x. [DOI] [PubMed] [Google Scholar]
- 130.Tanaka S. Role of RANKL in physiological and pathological bone resorption and therapeutics targeting the RANKL–RANK signaling system. Immunol. Rev. 2005;208:30–49. doi: 10.1111/j.0105-2896.2005.00327.x. [DOI] [PubMed] [Google Scholar]
- 131.Body JJ, Gaich GA, Scheele WH, Kulkarni PM, Miller PD, Peretz A, Dore RK, Correa-Rotter R, Papaioannou A, Cumming DC. A randomized double-blind trial to compare the efficacy of teriparatide [recombinant human parathyroid hormone (1–34)] with alendronate in postmenopausal women with osteoporosis. J. Clin. Endocrinol. Metabolism. 2002;87:4528–4535. doi: 10.1210/jc.2002-020334. [DOI] [PubMed] [Google Scholar]
- 132.Okazaki R. Osteosarcoma in rats receiving long-term PTH injection. Clin Calcium. 2003;13:42–44. [PubMed] [Google Scholar]
- 133.Chikazu D, Fujikawa Y, Fujihara H, Suenaga H, Saijo H, Ohkubo K, Ohkubo T, Ogasawara T, Mori Y, Iino M, Takato T. Cyclooxygenase-2 activity is important in craniofacial fracture repair. Int. J. Oral Maxillofac. Surg. 2011;40:322–326. doi: 10.1016/j.ijom.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 134.Gerstenfeld LC, Einhorn TA. COX inhibitors and their effects on bone healing. Exp. Opin. Drug Safety. 2004;3:131–136. doi: 10.1517/eods.3.2.131.27335. [DOI] [PubMed] [Google Scholar]
- 135.Vuolteenaho K, Moilanen T, Moilanen E. Non-steroidal anti-inflammatory drugs, cyclooxygenase-2 and the bone healing process. Basic Clin. Pharmacol. Toxicol. 2008;102:10–14. doi: 10.1111/j.1742-7843.2007.00149.x. [DOI] [PubMed] [Google Scholar]
- 136.Glassman SD, Rose SM, Dimar JR, Puno RM, Campbell MJ, Johnson JR. The effect of postoperative nonsteroidal anti-inflammatory drug administration on spinal fusion. Spine. 1998;23:834–838. doi: 10.1097/00007632-199804010-00020. [DOI] [PubMed] [Google Scholar]
- 137.Giannoudis P, MacDonald D, Matthews S, Smith R, Furlong A, De Boer P. Nonunion of the femoral diaphysis: the influence of reaming and non-steroidal anti-inflammatory drugs. J. Bone Joint Surg. 2000;82:655–658. doi: 10.1302/0301-620x.82b5.9899. [DOI] [PubMed] [Google Scholar]
- 138.Li M, Ke HZ, Qi H, Healy DR, Li Y, Crawford DT, Paralkar VM, Owen TA, Cameron KO, Lefker BA. A novel, non-prostanoid EP2 receptor-selective prostaglandin E2 agonist stimulates local bone formation and enhances fracture healing. J. Bone Mineral Res. 2003;18:2033–2042. doi: 10.1359/jbmr.2003.18.11.2033. [DOI] [PubMed] [Google Scholar]
- 139.Hirata M, Harada S, Matsumoto C, Takita M, Miyaura C, Inada M. Role of prostaglandin E in receptor activator of nuclear factor-κB ligand (RANKL) expression in osteoblasts induced by cell adhesion to bone marrow B-lymphocytes. J. Health Sci. 2009;55:832–837. [Google Scholar]
- 140.Tsutsumi R, Xie C, Wei X, Zhang M, Zhang X, Flick LM, Schwarz EM, O'Keefe RJ. PGE2 signaling through the EP4 receptor on fibroblasts upregulates RANKL and stimulates osteolysis. J. Bone Mineral Res. 2009;24:1753–1762. doi: 10.1359/JBMR.090412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Park JY, Bae M, Cheon HG, Kim SS, Hong JM, Kim TH, Choi JY, Kim SH, Lim J, Choi CH. A novel PPAR [gamma] agonist, KR62776, suppresses RANKL-induced osteoclast differentiation and activity by inhibiting MAP kinase pathways. Biochem. Biophys. Res. Commun. 2009;378:645–649. doi: 10.1016/j.bbrc.2008.11.115. [DOI] [PubMed] [Google Scholar]
- 142.Idris A, Mrak E, Greig I, Guidobono F, Ralston SH, van't Hof R. ABD56 causes osteoclast apoptosis by inhibiting the NFκB and ERK pathways. Biochem. Biophys. Res. Commun. 2008;371:94–98. doi: 10.1016/j.bbrc.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 143.Tsai HY, Lin HY, Fong YC, Wu JB, Chen YF, Tsuzuki M, Tang CH. Paeonol inhibits RANKL-induced osteoclastogenesis by inhibiting ERK, p38 and NF-κB pathway. Eur. J. Pharmacol. 2008;588:124–133. doi: 10.1016/j.ejphar.2008.04.024. [DOI] [PubMed] [Google Scholar]
- 144.Minamizaki T, Yoshiko Y, Kozai K, Aubin JE, Maeda N. EP2 and EP4 receptors differentially mediate MAPK pathways underlying anabolic actions of prostaglandin E2 on bone formation in rat calvaria cell cultures. Bone. 2009;44:1177–1185. doi: 10.1016/j.bone.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 145.Gil L, Han Y, Opas EE, Rodan GA, Ruel R, Seedor JG, Tyler PC, Young RN. Prostaglandin E2-bisphosphonate conjugates: potential agents for treatment of osteoporosis. Bioorg. Med. Chem. 1999;7:901–919. doi: 10.1016/s0968-0896(99)00045-0. [DOI] [PubMed] [Google Scholar]
- 146.Kamolratanakul P, Hayata T, Ezura Y, Kawamata A, Hayashi C, Yamamoto Y, Hemmi H, Nagao M, Hanyu R, Natomi T, Nakamoto T, Amagasa T, Akyoshi K, Noda M. Nanogel-based scaffold delivery of prostaglandin E2 receptor (EP4) specific agonist in combination with low dosage of growth factor heals critical size bone defect. Arthritis Rheumatism. 2010;10:1–11. doi: 10.1002/art.30151. [DOI] [PubMed] [Google Scholar]
- 147.Fromigue O, Hay E, Modrowski D, Bouvet S, Jacquel A, Auberger P, Marie PJ. RhoA GTPase inactivation by statins induces osteosarcoma cell apoptosis by inhibiting p42/p44-MAPKs-Bcl-2 signaling independently of BMP-2 and cell differentiation. Cell Death Different. 2006;13:1845–1856. doi: 10.1038/sj.cdd.4401873. [DOI] [PubMed] [Google Scholar]
- 148.Kanazawa I, Yamaguchi T, Yano S, Yamauchi M, Sugimoto T. Activation of AMP kinase and inhibition of Rho kinase induce the mineralization of osteoblastic MC3T3-E1 cells through endothelial NOS and BMP-2 expression. Am. J. Physiol. Endocrinol. Metabolism. 2009;296:E139–E146. doi: 10.1152/ajpendo.90677.2008. [DOI] [PubMed] [Google Scholar]
- 149.Lee Y, Liu X, Nawshad A, Marx DB, Wang D, Reinhardt RA. Role of prostaglandin pathway and alendronate-based carriers to enhance statin-induced bone. Mol. Pharmaceutics. 2011;8:1035–1042. doi: 10.1021/mp200045p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Midy V, Plouët J. Vasculotropin/vascular endothelial growth factor induces differentiation in cultured osteoblasts. Biochem Biophys Res Commun. 1994;199:380–386. doi: 10.1006/bbrc.1994.1240. [DOI] [PubMed] [Google Scholar]
- 151.Wong RWK, Rabie ABM. Early healing pattern of statin-induced osteogenesis. Brit. J. Oral Maxillofacial Sur. 2005;43:46–50. doi: 10.1016/j.bjoms.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 152.Uzzan B, Cohen R, Nicolas P, Cucherat M, Perret GY. Effects of statins on bone mineral density: A meta-analysis of clinical studies. Bone. 2007;40:1581–1587. doi: 10.1016/j.bone.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 153.Yue J. Statins and bone health in postmenopausal women: a systematic review of randomized controlled trials. Menopause. 2010;17:1071–1079. doi: 10.1097/gme.0b013e3181d3e036. [DOI] [PubMed] [Google Scholar]
- 154.Moriyama Y, Ayukawa Y, Ogino Y, Atsuta I, Todo M, Takao Y, Koyano K. Local application of fluvastatin improves peri-implant bone quantity and mechanical properties: A rodent study. Acta Biomater. 2010;6:1610–1618. doi: 10.1016/j.actbio.2009.10.045. [DOI] [PubMed] [Google Scholar]
- 155.Stein D, Lee Y, Schmid MJ, Killpack B, Genrich MA, Narayana N, Marx DB, Cullen DM, Reinhardt RA. Local simvastatin effects on mandibular bone growth and inflammation. J. Periodontology. 2005;76:1861–1870. doi: 10.1902/jop.2005.76.11.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pauly S, Luttosch F, Morawski M, Haas N, Schmidmaier G, Wildemann B. Simvastatin locally applied from a biodegradable coating of osteosynthetic implants improves fracture healing comparable to BMP-2 application. Bone. 2009;45:505–511. doi: 10.1016/j.bone.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 157.Masuzaki T, Ayukawa Y, Moriyama Y, Jinno Y, Atsuta I, Ogino Y, Koyano K. The effect of a single remote injection of statin-impregnated poly (lactic-co-glycolic acid) microspheres on osteogenesis around titanium implants in rat tibia. Biomaterials. 2010;31:3327–3334. doi: 10.1016/j.biomaterials.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 158.Lee Y, Schmid MJ, Marx DB, Beatty MW, Cullen DM, Collins ME, Reinhardt RA. The effect of local simvastatin delivery strategies on mandibular bone formation in vivo. Biomaterials. 2008;29:1940–1949. doi: 10.1016/j.biomaterials.2007.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chikazu D, Li X, Kawaguchi H, Sakuma Y, Voznesensky OS, Adams DJ, Xu M, Hoshio K, Katavic V, Hershman HR, Raisz LG, Pilbeam CC. Bone morphogenetic protein 2 induces cyclo-oxygenase 2 in osteoblasts via a Cbfal binding site: role in effects of bone morphogenetic protein 2 in vitro and in vivo. J. Bone Mineral Res. 2002;17:1430–1440. doi: 10.1359/jbmr.2002.17.8.1430. [DOI] [PubMed] [Google Scholar]
- 160.Blackwell KA, Hortschansky P, Sanovic S, Choudhary S, Raisz LG, Pilbeam CC. Bone morphogenetic protein 2 enhances PGE2 stimulated osteoclast formation in murine bone marrow cultures. Prostaglandins Other Lipid Mediat. 2009;90:76–80. doi: 10.1016/j.prostaglandins.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mummaneni PV, Pan J, Haid RW, Rodts GE. Contribution of recombinant human bone morphogenetic protein-2 to the rapid creation of interbody fusion when used in transforaminal lumbar interbody fusion: a preliminary report. J. Neurosurg. Spine. 2004;1:19–23. doi: 10.3171/spi.2004.1.1.0019. [DOI] [PubMed] [Google Scholar]
- 162.Schwender JD, Holly LT, Rouben DP, Foley KT. Minimally invasive transforaminal lumbar interbody fusion (TLIF): technical feasibility and initial results. J Spinal Disord. Tech. 2005;18(Suppl):S1–6. doi: 10.1097/01.bsd.0000132291.50455.d0. [DOI] [PubMed] [Google Scholar]
- 163.Baskin DS, Ryan P, Sonntag V, Westmark R, Widmayer MA. A prospective, randomized, controlled cervical fusion study using recombinant human bone morphogenetic protein-2 with the CORNERSTONE-SR allograft ring and the ATLANTIS anterior cervical plate. Spine. 2003;28:1219–1224. doi: 10.1097/01.BRS.0000065486.22141.CA. discussion 1225. [DOI] [PubMed] [Google Scholar]
- 164.Riedel GE, Valentin-Opran A. Clinical evaluation of rhBMP-2/ACS in orthopedic trauma: a progress report. Orthopedics. 1999;22:663–665. [PubMed] [Google Scholar]
- 165.Govender S, Csimma C, Genant HK, Valentin-Opran A. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J. Bone Joint Surg. Am. 2002;84-A:2123–2134. doi: 10.2106/00004623-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 166.Boyne PJ, Marx RE, Nevins M, Triplett G, Lazaro E, Lilly LC, Alder M. A feasibility study evaluating rhBMP-2/absorbable collagen sponge for maxillary sinus floor augmentation. Int. J. Periodontics Restorative Dent. 1997;17:11–25. [PubMed] [Google Scholar]
- 167.Haid RW, Jr, Branch CL, Jr, Alexander JT, Burkus JK. Posterior lumbar interbody fusion using recombinant human bone morphogenetic protein type 2 with cylindrical interbody cages. Spine J. 2004;4:527–538. doi: 10.1016/j.spinee.2004.03.025. discussion 538-539. [DOI] [PubMed] [Google Scholar]
- 168.Katayama Y, Matsuyama Y, Yoshihara H, Sakai Y, Nakamura H, Imagama S, Ito Z, Wakao N, Kamiya M, Yukawa Y. Clinical and radiographic outcomes of posterolateral lumbar spine fusion in humans using recombinant human bone morphogenetic protein-2: An average five-year follow-up study. Int. Orthopaedics. 2009;33:1061–1067. doi: 10.1007/s00264-008-0600-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Triplett RG, Nevins M, Marx RE, Spagnoli DB, Oates TW, Moy PK, Boyne PJ. Pivotal, randomized, parallel evaluation of recombinant human bone morphogenetic protein-2/absorbable collagen sponge and autogenous bone graft for maxillary sinus floor augmentation. J. Oral Maxillofacial Surg. 2009;67:1947–1960. doi: 10.1016/j.joms.2009.04.085. [DOI] [PubMed] [Google Scholar]
- 170.Shen W. Interaction between macrophages, TGF- β1, and the COX- 2 pathway during the inflammatory phase of skeletal muscle healing after injury. J. Cell Phys. 2008;214:405–412. doi: 10.1002/jcp.21212. [DOI] [PubMed] [Google Scholar]
- 171.Niu Z, Wang L, Hu X, Wang H, Ouyang J, Huang W, Yu L, Qui X. Promotion effect of nuclear factor kappa B p65 on early fracture healing of rat radius by elevating prostaglandins E2 production and regulating inhibitor of DNA binding 2 protein expression. C. J. Repar and Recon Surg. 2011;25:569–574. [PubMed] [Google Scholar]
- 172.Yasui T, Kadono Y, Nakamura M, Oshima Y, Matsumoto T, Masuda H, Hirose J, Omata Y, Yasuda H, Imamura T, Nakamura K, Tanaka S. Regulation of RANKL-induced osteoclastogenesis by TGF-β through molecular interaction between Smad3 and Traf6. J. Bone Mineral Res. 2011;26:1447–1456. doi: 10.1002/jbmr.357. [DOI] [PubMed] [Google Scholar]
- 173.Gingery A, Bradley EW, Pederson L, Ruan M, Horwood NJ, Oursler MJ. TGFβ coordinately activates TAK1/MEK/AKT/NFkB and SMAD pathways to promote osteoclast survival. Exp. Cell Res. 2008;314:2725–2738. doi: 10.1016/j.yexcr.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Houde N, Chamoux E, Bisson M, Roux S. Transforming growth factor-beta1 (TGF-β1) induces human osteoclast apoptosis by up-regulating Bim. J. Biol. Chem. 2009;284:23397–23404. doi: 10.1074/jbc.M109.019372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Peterson MC, Riggs MM. A physiologically based mathematical model of integrated calcium homeostasis and bone remodeling. Bone. 2010;46:49–63. doi: 10.1016/j.bone.2009.08.053. [DOI] [PubMed] [Google Scholar]
- 176.Choudhary S, Huang H, Raisz L, Pilbeam C. Anabolic effects of PTH in cyclooxygenase-2 knockout osteoblasts in vitro. Biochem. Biophys. Res. Commun. 2008;372:536–541. doi: 10.1016/j.bbrc.2008.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Huang H, Chikazu D, Voznesensky OS, Herschman HR, Kream BE, Drissi H, Pilbeam CC. Parathyroid hormone induction of cyclooxygenase-2 in murine osteoblasts: role of the calcium-calcineurin-NFAT pathway. J. Bone Mineral Res. 2010;25:819–829. doi: 10.1359/jbmr.091019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Schnoke M, Midura SB, Midura RJ. Parathyroid hormone suppresses osteoblast apoptosis by augmenting DNA repair. Bone. 2009;45:590–602. doi: 10.1016/j.bone.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Tintut Y, Parhami F, Le V, Karsenty G, Demer LL. Inhibition of osteoblast-specific transcription factor Cbfa1 by the cAMP pathway in osteoblastic cells. J. Biol. Chem. 1999;274:28875–28879. doi: 10.1074/jbc.274.41.28875. [DOI] [PubMed] [Google Scholar]
- 180.Zhao M, Qiao M, Oyajobi BO, Mundy GR, Chen D. E3 ubiquitin ligase Smurf1 mediates core-binding factor α1/Runx2 degradation and plays a specific role in osteoblast differentiation. J. Biol. Chem. 2003;278:27939–27944. doi: 10.1074/jbc.M304132200. [DOI] [PubMed] [Google Scholar]
- 181.Murray EJB, Bentley GV, Grisanti MS, Murray SS. The ubiquitin-proteasome system and cellular proliferation and regulation in osteoblastic cells. Exp. Cell Res. 1998;242:460–469. doi: 10.1006/excr.1998.4090. [DOI] [PubMed] [Google Scholar]
- 182.Bellido T, Ali AA, Plotkin LI, Fu Q, Gubrij I, Roberson PK, Weinstein RS, O'Brien CA, Manolagas SC, Jilka RL. Proteasomal degradation of Runx2 shortens parathyroid hormone-induced anti-apoptotic signaling in osteoblasts. J. Biol. Chem. 2003;278:50259–50272. doi: 10.1074/jbc.M307444200. [DOI] [PubMed] [Google Scholar]
- 183.Jilka RL. Molecular and cellular mechanisms of the anabolic effect of intermittent PTH. Bone. 2007;40:1434–1446. doi: 10.1016/j.bone.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zhang S, Gangal G, Uludağ H. “Magic bullets” for bone diseases: progress in rational design of bone-seeking medicinal agents. Chem. Soc. Rev. 2007;36:507–531. doi: 10.1039/b512310k. [DOI] [PubMed] [Google Scholar]
- 185.Bansal G, Wright JE, Kucharski C, Uludağ H. A dendritic tetra(bisphosphonic acid) for improved targeting of proteins to bone. Angew. Chem. Int. Ed. 2005;44:3710–3714. doi: 10.1002/anie.200500350. [DOI] [PubMed] [Google Scholar]
- 186.Doschak MR, Kucharski CM, Wright JEI, Zernicke RF, Uludağ H. Improved bone delivery of osteoprotegerin by bisphosphonate conjugation in a rat model of osteoarthritis. Mol. Pharmaceutics. 2009;6:634–640. doi: 10.1021/mp8002368. [DOI] [PubMed] [Google Scholar]
- 187.Vaibhav B, Nilesh P, Vikram S, Anshul C. Bone morphogenic protein and its application in trauma cases: a current concept update. Injury. 2007;38:1227–1235. doi: 10.1016/j.injury.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 188.Harris JM, Chess RB. Effect of pegylation on pharmaceuticals. Nature Drug Disc. Rev. 2003;2:214–221. doi: 10.1038/nrd1033. [DOI] [PubMed] [Google Scholar]
- 189.Kamei S, Kopeček J. Prolonged blood circulation in rats of nanospheres surface-modified with semitelechelic poly[N-(2-hydroxypropyl)methacrylamide] Pharmaceutical Res. 1995;12:663–668. doi: 10.1023/a:1016247206531. [DOI] [PubMed] [Google Scholar]
- 190.Clines GA, Guise TA. Hypercalcaemia of malignancy and basic research on mechanisms responsible for osteolytic and osteoblastic metastasis to bone. Endocrine-Related Cancer. 2005;12:549–583. doi: 10.1677/erc.1.00543. [DOI] [PubMed] [Google Scholar]
- 191.Schajnovitz A, Itkin T, D'Uva G, Kalinkovich A, Golan K, Ludin A, Cohen D, Shulman Z, Avigdor A, Nagler A. CXCL12 secretion by bone marrow stromal cells is dependent on cell contact and mediated by connexin-43 and connexin-45 gap junctions. Nature Immunol. 2011;12:391–398. doi: 10.1038/ni.2017. [DOI] [PubMed] [Google Scholar]
- 192.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 193.Akashi T, Koizumi K, Tsuneyama K, Saiki I, Takano Y, Fuse H. Chemokine receptor CXCR4 expression and prognosis in patients with metastatic prostate cancer. Cancer Sci. 2008;99:539–542. doi: 10.1111/j.1349-7006.2007.00712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Chinni SR, Yamamoto H, Dong Z, Sabbota A, Bonfil RD, Cher ML. CXCL12/CXCR4 transactivates HER2 in lipid rafts of prostate cancer cells and promotes growth of metastatic deposits in bone. Mol. Cancer Res. 2008;6:446–457. doi: 10.1158/1541-7786.MCR-07-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Liang Z, Yoon Y, Votaw J, Goodman MM, Williams L, Shim H. Silencing of CXCR4 blocks breast cancer metastasis. Cancer Res. 2005;65:967–971. [PMC free article] [PubMed] [Google Scholar]
- 196.Burdick MJ, Sartor O. Bone-targeted therapy in metastatic prostate cancer: osteoclast inhibitors and bone-seeking radiopharmaceuticals. Drug Disc. Today: Ther. Strat. 2010;7:23–29. [Google Scholar]
- 197.Kawatani M. Osteoclast- targeting small molecules for the treatment of neoplastic bone metastases. Cancer Sci. 2009;100:1999–2005. doi: 10.1111/j.1349-7006.2009.01294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Saad F, Lipton A. SRC kinase inhibition: targeting bone metastases and tumor growth in prostate and breast cancer. Cancer Treat. Rev. 2010;36:177–184. doi: 10.1016/j.ctrv.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 199.Miller K, Erez R, Segal E, Shabat D, Satchi-Fainaro R. Targeting bone metastases with a bispecific anticancer and antiangiogenic polymer-alendronate-taxane conjugate. Angew. Chem. Int. Ed. 2009;48:2949–2954. doi: 10.1002/anie.200805133. [DOI] [PubMed] [Google Scholar]
- 200.Miller K, Eldar-Boock A, Polyak D, Segal E, Benayoun L, Shaked Y, Satchi-Fainaro R. Antiangiogenic antitumor activity of HPMA copolymer-paclitaxel-alendronate conjugate on breast cancer bone metastasis mouse model. Mol. Pharmaceutics. 2011;8:1052–1062. doi: 10.1021/mp200083n. [DOI] [PubMed] [Google Scholar]
- 201.Bhargava P, Marshall JL, Rizvi N, Dahut W, Yoe J, Figuera M, Phipps K, Ong VS, Kato A, Hawkins MJ. A Phase I and pharmacokinetic study of TNP-470 administered weekly to patients with advanced cancer. Clin. Cancer Res. 1999;5:1989–1995. [PubMed] [Google Scholar]
- 202.Larsen RH, Murud KM, Akabani G, Hoff P, Bruland ØS, Zalutsky MR. 211At- and 131I-Labeled bisphosphonates with high in vivo stability and bone accumulation. J. Nucl. Med. 1999;40:1197–1203. [PubMed] [Google Scholar]
- 203.Ogawa K, Kawashima H, Shiba K, Washiyama K, Yoshimoto M, Kiyono Y, Ueda M, Mori H, Saji H. Development of [90Y]DOTA-conjugated bisphosphonate for treatment of painful bone metastases. Nucl. Med. Biol. 2009;36:129–135. doi: 10.1016/j.nucmedbio.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 204.Hambardzumyan D, Becher OJ, Rosenblum MK, Pandolfi PP, Manova-Todorova K, Holland EC. PI3K pathway regulates survival of cancer stem cells residing in the perivascular niche following radiation in medulloblastoma in vivo. Genes Devel. 2008;22:436–448. doi: 10.1101/gad.1627008. [DOI] [PMC free article] [PubMed] [Google Scholar]