Abstract
The balance between alternatively activated macrophages (AAMs)/M2 cells and classically activated macrophages (M1 cells) is largely dependent on the effects of IL-4 and interferon (IFN)-γ, respectively. Although AAM/M2 cells can suppress inflammation and repair damaged tissue, M1 cells produce an array of pro-inflammatory molecules. Macrophage effector functions are critical for host protection against many infectious diseases, but it remains unknown whether lethal immunopathological characteristics, caused by Schistosoma mansoni infection in IL-4 receptor α–deficient mice (IL-4Rα−/−), results from the absence of M2 cells or increased numbers of M1 cells. In this study, we generated mice that completely lack IL-4Rα signaling in the context of a macrophage-specific loss of IFN-γ responsiveness (MIIG × IL-4Rα−/−). Contrary to what we expected, acute schistosomiasis resulted in greater liver injury and mortality in MIIG × IL-4Rα−/− mice compared with IL-4Rα−/− mice. Greater tissue injury in MIIG × IL-4Rα−/− mice was likely because of a lack of indoleamine 2,3 dioxygenase (IDO), a critical regulator of immunosuppression. Indeed, MIIG × IL-4Rα−/− failed to up-regulate IDO expression, and IL-4Rα−/− mice treated with an IDO antagonist underwent greater liver damage and mortality compared with mock-treated IL-4Rα−/− mice. Thus, we propose that, in the absence of AAM/M2 cells, IFN-γ–induced M1 cells suppress tissue-damaging inflammation during acute schistosomiasis through an IDO-dependent mechanism.
Worm ova released from adult-stage Schistosoma parasites cause granulomatous inflammation within any organ where the eggs become lodged.1 The hepatic and intestinal inflammation caused by Schistosoma mansoni eggs can result in substantial organ injury within the host, particularly in the absence of a counterinflammatory host response. For example, acute schistosomiasis is lethal in IL-4–deficient mice because of excessive liver injury and intestinal hemorrhage.2 IL-4 limits excessive tissue injury caused by worm ova through mechanisms that are largely dependent on CD4+ T-helper (TH) 2 cells and alternatively activated macrophages (AAMs)/M2 cells.3–5 During acute schistosomiasis, mice that specifically lack IL-4 receptor α (IL-4Rα)–expressing macrophages or mice that lack AAM/M2-associated genes develop excessive intestinal immunopathological characteristics that are driven by type 1 cytokine production.3 Furthermore, AAM/M2 cells have suppressed IL-12/23p40 release, which, in turn, suppresses the infection-induced expansion of TH1 and TH17 cells that drives mortality during murine schistosomiasis.6–8
It is unclear whether host survival during schistosomiasis is dependent on a relative increase of AAM/M2 cells or a decrease of M1 cells at the nidus of inflammation caused by the worm ovum. Compared with AAM/M2 cells, interferon (IFN)-γ–dependent M1 cells produce high amounts of pro-inflammatory mediators, such as inducible nitric oxide synthetase-2, tumor necrosis factor, and IL-12/23p40, that serve essential roles in host defense against microbial pathogens.9 However, these pro-inflammatory molecules can also cause significant collateral tissue damage within the host.9,10 Specifically, IFN-γ promotes severe hepatosplenic schistosomiasis,11 and increased nitric oxide synthetase-2 and IL-12/23p40 production contributes to severe immunopathological characteristics in S. mansoni–infected mice lacking IL-4 or arginase I.2,8,12 Because the increased production of type 1–associated inflammation often occurs in the absence of a strong type 2 response, it has been particularly difficult to discern the host-protective effects of AAM/M2 cells versus the potentially deleterious role(s) of M1 cells in the S. mansoni model system.
To address the roles of AAM/M2 and M1 cells in vivo, we bred mice that lack IL-4/IL-13 responsiveness in all cells (IL-4Rα−/−) with mice that lack macrophage-specific IFN-γ responsiveness (MIIG).13 This resulted in the generation of novel mice that were unable to generate AAM/M2 or M1 cells (MIIG × IL-4Rα−/−). Unexpectedly, acute schistosomiasis in these animals was more severe compared with IL-4Rα−/− mice, as determined by increased weight loss and mortality. This phenotype was not attributed to differences in cytokine production. Instead, MIIG × IL-4Rα−/− mice failed to up-regulate indoleamine 2,3 dioxygenase (IDO), an IFN-γ–induced gene that is highly immunosuppressive.14 Indeed, treatment of S. mansoni–infected IL-4Rα−/− mice with the IDO antagonist 1-methyltryptophan (1-MT) caused significantly worse cachexia and multiorgan injury compared with mock-treated IL-4Rα−/− mice. These data suggest that both M1 and AAM/M2 cells have protective roles during schistosomiasis and that the protective effects of M1 cells are likely to be IDO dependent.
Materials and Methods
Mice and the S. mansoni Model
The MIIG-transgenic mice that use the CD68-IVS1 promoter to drive an IFN-γ–dominant negative receptor have been previously described.13 These mice were bred with IL-4Rα−/− mice on a C57BL/6 background and were provided by Frank Bromabacher at the University of Cape Town, Cape Town, South Africa. Biomphalaria glabrata snails harboring S. mansoni cercariae were provided by the National Institute of Allergy and Infectious Diseases Schistosomiasis Resource Center at the Biomedical Research Institute (Rockville, MD), through National Institute of Allergy and Infectious Diseases contract N0-AI-30026. All infections were performed at Cincinnati Children's Hospital Medical Center, and procedures were approved by the Institutional Animal Care and Use Committee. Mice were anesthetized and percutaneously infected with 50 to 70 S. mansoni cercariae, as previously described.15
Serum Analysis
Aspartate transaminase (AST) serum levels were measured by a commercially available assay from Teco Diagnostics (Anaheim, CA). The serum lipopolysaccharide (LPS) concentration was measured using a commercially available limulus amebocyte lysate kit (BioWhittaker, Lancaster, MA), as previously described.8
ELISA
Single-cell suspensions were prepared from mesenteric lymph nodes at 7 weeks after infection and stimulated with S. mansoni–soluble egg antigen (SEA) at 10 μg/mL or left untreated. Supernatants were harvested 72 hours later, and IFN-γ, IL-5, IL-17A, and IL-12/23p40 levels were quantified by enzyme-linked immunosorbent assay (ELISA) (eBioscience, San Diego, CA). Nitrite levels in supernatant were determined using a Griess reaction kit (Promega, Madison, WI).
Real-Time PCR
Tissues were homogenized in TRIzol (Molecular Research Center, Cincinnati, OH) and RNA isolated using standard techniques. RNA was DNase I treated and cDNA prepared using SuperScript II Reverse Transcriptase (Invitrogen, Carlsbad, CA). A real-time PCR was performed on a Gene Amp 7500 instrument (PE Biosystems, Foster City, CA) with the SYBR Green detection reagent (Clonetech, Mountain View, CA). The CT values for genes evaluated were determined and expressed using the 1/ΔCT method. Primer sequences used were as follows: vimentin, 5′-GTGCGCCAGCAGTATGAAAG-3′ (forward) and 5′-GCATCGTTGTTCCGGGTTGG-3′ (reverse); IDO-1, 5′-GGATGCGTGACTTTGTGGAC-3′ (forward) and 5′-TTCTTTGCCAGCCTCGTGT-3′ (reverse); IDO-2, 5′-CTGGTGCTGACAAACTGGA-3′ (forward) and 5′-CTGACTGTGTTGCCGAATG-3′ (reverse); IL-10, 5′-AAGGGTTACTTGGGTTGCCA-3′ (forward) and 5′-TCACTCTTCACCTGCTCCAC-3′ (reverse); and transforming growth factor (TGF)-β1, 5′-CCGCAACAACGCCATCTATG-3′ (forward) and 5′-AGCCCTGTATTCCGTCTCCT-3′ (reverse).
1-MT Treatment
1-MT (Sigma-Aldrich, St. Louis, MO) was administered in the drinking water of mice at 2 mg/mL with the addition of sweetener (NutraSweet, Chicago, IL).16 Mock treatment was drinking water with sweetener. Treated drinking water was changed every 4 to 5 days during treatment.
Histological Features and Granuloma Measurement
Liver samples were formalin fixed for 24 hours, processed, embedded in paraffin, cut into sections (5 μm thick), and provided on coded slides by the Cincinnati Children's Hospital Medical Center morphological features core facility. H&E staining was used to visualize individual liver granuloma areas on coded slides. Granuloma areas were measured on coded slides; only granulomas that possessed a central egg were evaluated. Quantitation of area was performed using an SPOT Diagnostics imaging system and Simple PCI C-Imaging systems software version 4.0 (Cranberry Township, PA) for granuloma area measurement.
Statistical Analysis
To determine statistical significance between two groups, a one-tailed Student's t-test was used. For multiple groups, statistical significance was determined by analysis of variance for multiple groups, followed by Bonferroni's post hoc test. All tests were performed using Prism Graph Pad software version 5.0 (La Jolla, CA).
Results
Generation of Transgenic Mice that Specifically Lack IL-4/IL-13 Signaling and IFN-γ Responsiveness in Macrophages
To dissect the biological roles of M1 cells versus AAM/M2 cells in vivo, we generated a novel mouse line from an intercross between IL-4Rα−/− mice (a strain that lacks IL-4 and IL-13 responsiveness on all cells) and MIIG-transgenic mice (a strain that expresses a dominant-negative IFN-γ receptor specifically in macrophages).13 These mice (designated MIIG × IL-4Rα−/−) possess macrophages that are incapable of signaling in response to IFN-γ, IL-4, or IL-13.
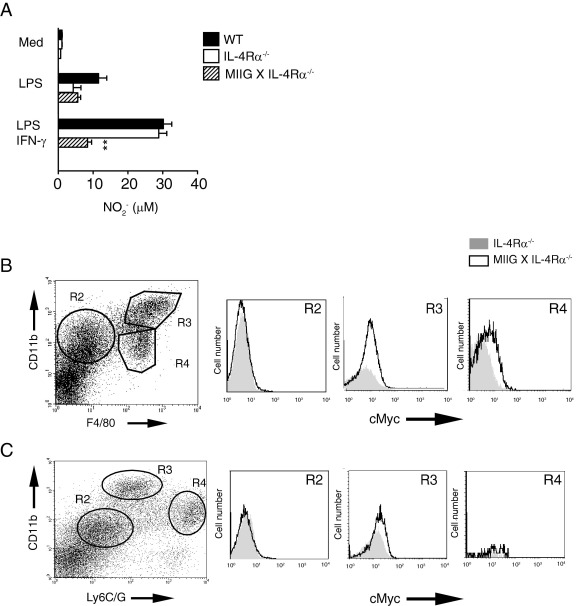
To ensure functional nonresponsiveness, bone marrow–derived macrophages were generated from wild-type (WT), IL-4Rα−/−, and MIIG × IL-4Rα−/− strains and evaluated for nitrite production after exposure to bacterial endotoxin (LPS) or a combination of LPS and recombinant murine IFN-γ. As expected, bone marrow–derived macrophages from MIIG × IL-4Rα−/− mice did not produce more nitrite after recombinant murine IFN-γ treatment compared with levels induced by LPS treatment alone, whereas LPS-induced nitrite production was equivalent among all three groups (Figure 1A). Flow cytometric analysis of thioglycollate-elicited peritoneal exudate cells from WT, IL-4Rα−/−, and MIIG × IL-4Rα−/− mice confirmed that CD68-driven transgene expression was primarily associated with CD11b+ F4/80+ double-positive cells, and not CD11b+ Ly6G/C+ cell populations (Figure 1, B and C). Thus, MIIG × IL-4Rα−/− mice are incapable of IFN-γ–driven M1 cell activation and IL-4/IL-13–driven AAM/M2 cell activation.
Figure 1.
Generation of mice with a macrophage-specific loss of IFN-γ responsiveness and a global loss of IL-4/IL-13 responsiveness. A: Nitrite production from WT, IL-4Rα−/−, or MIIG × IL-4Rα−/− bone marrow–derived macrophages (BMDMs) that were either left untreated (Med) or stimulated with Escherichia coli LPS, 100 ng/mL, or a combination of LPS and IFN-γ, 20 ng/mL, for 24 hours. Data show the mean ± SE of triplicate wells. The experiment was performed three times. **P < 0.001. Flow cytometric analysis of thioglycollate-elicited peritoneal exudate cells from IL-4Rα−/− or MIIG × IL-4Rα−/− mice cell surface expression of Myc (alias c-myc) on CD11b+ and F4/80+ cell populations (B) and CD11b+ and/or Ly6C/G+ cell populations (C). This is representative of three independent mice analyzed per group.
Acute Schistosomiasis in MIIG × IL-4Rα−/− Mice Is Characterized by Severe Weight Loss, Mortality, and Increased Liver Immunopathological Characteristics
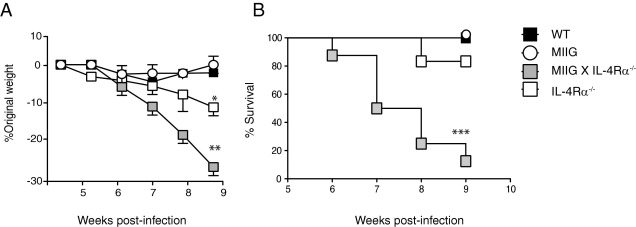
The absence of AAM/M2 cells during acute murine schistosomiasis results in exacerbated tissue injury, cachexia, and mortality.17 To determine whether M1 cells contribute to this severe disease phenotype, WT, MIIG, IL-4Rα−/−, and MIIG × IL-4Rα−/− mice were percutaneously infected with 50 to 60 S. mansoni cercariae and monitored for changes in weight or survival. Although no obvious differences in morbidity or mortality were observed between WT and MIIG mice, both IL-4Rα−/− and MIIG × IL-4Rα−/− strains lost a significant amount of weight from 5 to 8 weeks after infection (Figure 2A). Unexpectedly, cachexia was more severe in MIIG × IL-4Rα−/− mice compared with all other strains, and these animals experienced 90% mortality by 9 weeks after infection (Figure 2B).
Figure 2.
MIIG × IL-4Rα−/− mice develop accelerated weight loss and increased mortality relative to mice that lack IL-4/IL-13 responsiveness on all cells. A: Percentage weight change in WT, MIIG, IL-4Rα−/−, or MIIG × IL-4Rα−/− mice after infection of mice with 50 to 60 S. mansoni cercariae. Data show the mean ± SE (n = 6 to 8 mice per group). The experiment was performed four times. B: Percentage survival from a representative experiment described in A. *P < 0.05, **P < 0.01, and ***P < 0.001 as determined by one-way analysis of variance.
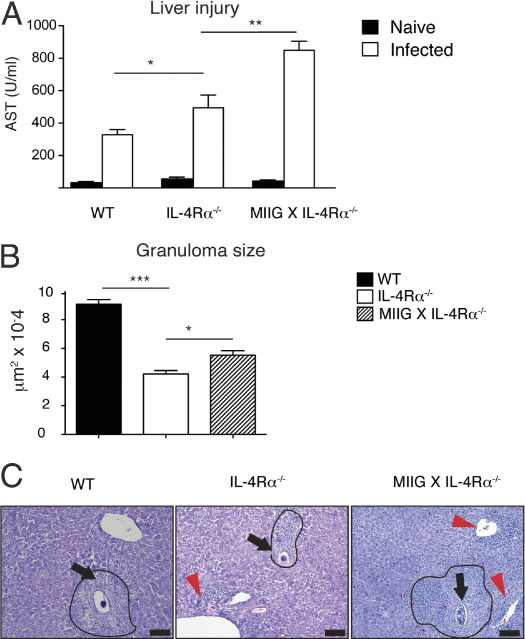
The S. mansoni eggs continuously accumulate within the liver during infection. Serum levels of AST and liver granuloma sizes were determined to evaluate whether weight loss was associated with an increase in hepatic tissue injury and immunopathological characteristics, respectively.15,17 As previously demonstrated, S. mansoni–infected IL-4Rα−/− mice produced higher levels of AST than WT mice.17 However, infected MIIG × IL-4Rα−/− mice produced even higher AST levels than IL-4Rα−/− mice (Figure 3A). A comparison of liver granuloma size among infected genotypes at 7.5 weeks after infection revealed that MIIG × IL-4Rα−/− granulomas were significantly larger than those in IL-4Rα−/− mice, although both were smaller than WT granulomas (Figure 3B). The exacerbated liver immunopathological characteristics in MIIG × IL-4Rα−/− mice were associated with greater perivascular inflammation within hepatic tissue of infected MIIG × IL-4Rα−/− mice compared with WT or IL-4Rα−/− strains (Figure 3C). We also noted that more severe peritonitis developed in MIIG × IL-4Rα−/− mice compared with WT or IL-4Rα−/− strains at 7.5 weeks after infection (data not shown). These data suggest that a combined lack of M1 and M2 cells within MIIG × IL-4Rα−/− mice resulted in greater liver inflammation during S. mansoni infection.
Figure 3.
MIIG × IL-4Rα−/− mice show greater liver injury and granulomatous pathological characteristics compared with IL-4Rα−/− mice during acute schistosomiasis. The S. mansoni–infected WT, IL-4Rα−/−, and MIIG × IL-4Rα−/− mice were evaluated at 7.5 weeks after infection for serum levels of AST (A) and liver granuloma cross-sectional area (B). Data show the mean ± SE (n = 300 granulomas per group). C: Representative images of liver granulomas after H&E staining of infected liver tissue at 7.5 weeks after infection. Photomicrographs show representative liver sections from S. mansoni–inoculated mice at 7.5 weeks after infection. Scale bar = 100 μm. Arrows, parasite ova; arrowheads, areas of perivascular inflammation. *P < 0.05, **P < 0.01, and ***P < 0.001.
Lack of IL-4 and IFN-γ Responsiveness in Macrophages Does Not Alter the Production of TH1, TH17, or Treg-Associated Cytokine Production
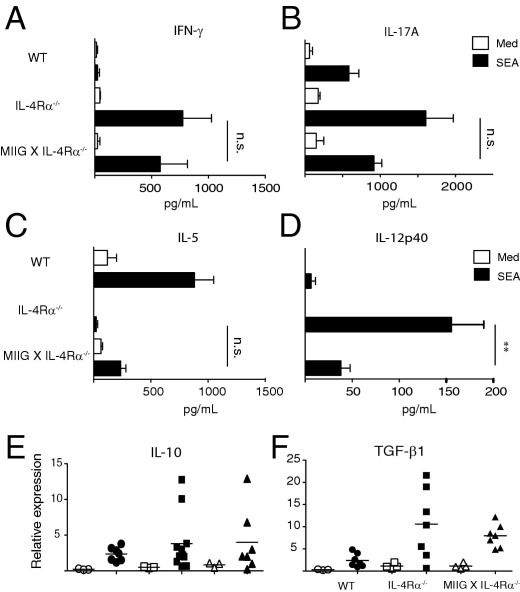
Host survival during schistosomiasis is dependent on the balanced production of pro- and anti-inflammatory cytokines.18 Thus, we asked whether severe disease in MIIG × IL-4Rα−/− mice was associated with dysregulated cytokine production. Mesenteric lymph node cells from WT, IL-4Rα−/−, and MIIG × IL-4Rα−/− mice were isolated at 7 weeks after infection and analyzed for the production of IFN-γ, IL-17A, IL-5, and IL-12p40 after restimulation with SEA. The mesenteric lymph nodes from both IL-4Rα−/− and MIIG × IL-4Rα−/− mice released significantly more IFN-γ and IL-17A and significantly less IL-5 than WT MLNs after SEA stimulation (Figure 4, A–C). However, there were only moderate differences between the cytokine profiles of IL-4Rα−/− and MIIG × IL-4Rα−/− mice. SEA-induced IL-12p40 release was significantly lower from MIIG × IL-4Rα−/− MLNs compared with IL-4Rα−/− mice, which is consistent with evidence that IFN-γ promotes IL-12 responses (Figure 4, A–C).19
Figure 4.
The S. mansoni–infected MIIG × IL-4Rα−/− mice and IL-4Rα−/− mice generate a similar cytokine response within lymphoid and hepatic tissues during the acute infection phase. Supernatant levels of IFN-γ (A), IL-17A (B), IL-5 (C), and IL-12p40 (D), produced from mesenteric lymph node cells of S. mansoni–infected mice at 7 weeks after infection, that were either left untreated (Med) or stimulated with 10 μg/mL SEA, as determined by ELISA. Data show the mean ± SE of triplicate wells (n = 4 mice per group). The experiment was performed three times. Hepatic mRNA expression levels of IL-10 (E) and TGF-β1 (F) were quantified in WT (circles), IL-4Rα−/− (squares), and MIIG × IL-4Rα−/− (triangles) mice at 7 weeks after infection. Open symbols, naïve; closed symbols, infected. The experiment was performed three times. **P < 0.01 as determined by one-way analysis of variance.
Redundant functions of IL-10 and TGF-β suppress hepatic immunopathological characteristics and promote host survival during murine schistosomiasis.15 To determine whether MIIG × IL-4Rα−/− mice failed to express these cytokines, hepatic mRNA expression levels of IL-10 and TGF-β1 were quantified in WT, IL-4Rα−/−, and MIIG × IL-4Rα−/− mice at 7 weeks after infection. Both IL-4Rα−/− and MIIG × IL-4Rα−/− mice expressed significantly higher levels of these cytokine transcripts than infected WT mice or any of the uninfected mouse strains (Figure 4, D–F). However, MIIG × IL-4Rα−/− mice did not generate markedly different cytokine responses compared with the IL-4Rα−/− strain, inasmuch as the production of TH1, TH17, TH2, and Treg-associated cytokines were similarly produced.
MIIG × IL-4Rα–Deficient Mice Have a Specific Defect in IDO Production that Is Required for Protection against Excessive Tissue Injury during Acute Schistosomiasis
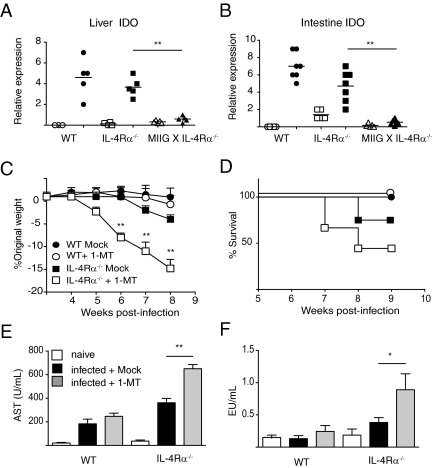
Given that IDO is an IFN-γ–inducible enzyme that mediates immunosuppression through tryptophan catabolism,20,21 we next asked whether MIIG × IL-4Rα−/− mice were uniquely defective in the expression of this gene. IDO-1 mRNA levels were threefold to fourfold increased within the liver and intestine of WT and IL-4Rα−/− strains versus naïve controls (Figure 5, A and B). However, MIIG × IL-4Rα−/− mice failed to increase IDO-1 mRNA expression versus baseline levels in either of these affected organs (Figure 5, A and B). Measurement of IDO-2 mRNA levels did not show increased expression during S. mansoni infection in any of the three strains analyzed (data not shown).
Figure 5.
The S. mansoni–infected MIIG × IL-4Rα−/− mice fail to up-regulate IDO, which protects IL-4Rα−/− mice against lethal multiorgan inflammation during acute schistosomiasis. Liver (A) and intestinal (B) mRNA expression levels for IDO at 7.5 weeks after infection. Data show the mean ± SE (n = 4 to 6 mice per group). Open symbols, naïve; closed symbols, infected. The experiment was performed twice. C: Percentage weight change in WT and IL-4Rα−/− mice treated with an IDO antagonist (1-MT) or left untreated. D: Percentage survival from the experiment described in C. Data show the mean ± SE (n = 6 to 8 mice per group). The experiment was repeated twice. AST (E) and endotoxin (F) levels in the serum samples of naïve and S. mansoni–infected WT and IL-4Rα−/− mice treated as described in C. Data show the mean ± SE (n = 6 to 8 mice per group). The experiment was performed twice. *P < 0.05 and **P < 0.01 as determined by one-way analysis of variance.
To determine whether IDO expression was functionally important, S. mansoni–infected mice were administered the specific IDO antagonist 1-MT in drinking water.16 The administration of 1-MT to S. mansoni–infected WT and IL-4Rα−/− mice from 4 weeks after infection had moderate effects on the former but severely affected the latter. Indeed, 1-MT–treated IL-4Rα−/− mice experienced rapid weight loss (Figure 5C) and 50% mortality by 8 weeks after infection (Figure 5D), which was a similar phenotype to MIIG × IL-4Rα−/− mice. The 1-MT treatment in IL-4Rα−/−–deficient mice also led to a marked increase in the AST (Figure 5E) and endotoxin (Figure 5F) levels in serum, the latter of which indicated a loss of intestinal mucosal barrier function.8,17 Conversely, 1-MT treatment did not alter the AST or LPS levels within infected WT mice (Figure 5, E and F). Overall, these data support a hypothesis that the effects of IFN-γ on macrophages are beneficial for S. mansoni–infected hosts that lack IL-4Rα expression.
Discussion
Macrophages are critical regulators of inflammation and immunopathology during murine schistosomiasis. Although IL-4/IL-13–dependent differentiation of AAM/M2 cells prevents TH1-associated lethality in this model, it remained unclear whether the mechanism required the suppression of M1 cell differentiation. In this study, we compared mice that lacked AAM/M2 cells with animals deficient in both AAM/M2 and M1 cells for differences in host susceptibility after acute infection with S. mansoni. Evidence shows that when the host is unable to generate AAM/M2 cells, IFN-γ–dependent M1 cell development actually suppresses hepatic inflammation and protects against lethal disease. This host-protective effect of M1 cells is most likely due to IDO, an IFN-γ–inducible enzyme known for its highly immunosuppressive effects. Thus, contrary to our initial hypothesis, our data suggest that both M1 and AAM/M2 cells serve as regulators of inflammation and immunosuppression during murine schistosomiasis.
Survival during murine schistosomiasis is dependent on the balance between pro- and anti-inflammatory cytokine production.15,22 Although mice unable to mount an IL-4–driven AAM/M2 response develop severe liver and intestinal injury within 7 to 8 weeks after infection, the overproduction of type 2 cytokines leads to excessive fibrosis and mortality beyond 20 weeks.11,23 Furthermore, the lack of sufficient IL-10 and TGF-β causes a predominant IFN-γ and IL-17 response that results in rapid death of infected hosts shortly after the onset of worm ova production.15 Given that the effects of IFN-γ on macrophages have profound effects on cytokine production, it was somewhat unexpected that antigen-induced IFN-γ and IL-17A production was not significantly different between IL-4Rα−/− and MIIG × IL-4Rα −/− mice. However, the increased production of these cytokines in these strains, compared with WT, is consistent with prior reports that evidence that AAM/M2-specific genes suppress IFN-γ and IL-17A during acute schistosomiasis. Interestingly, IL-4Rα−/− and MIIG × IL-4Rα−/− expressed higher levels of IL-10 and TGF-β, compared with WT, which made it unlikely that defective production of these anti-inflammatory cytokines could explain why MIIG × IL-4Rα−/− mice experienced greater disease susceptibility compared with WT and IL-4Rα−/− mice.
In most immunocompetent mouse strains, a fibrotic granulomatous reaction develops around each worm ova; however, granulomas in IL-4Rα−/− mice are significantly smaller. AAM/M2 cells are not required for liver granuloma development or fibrosis, yet the lack of AAM/M2 results in significantly greater hepatocellular injury.17 Although we expected that an increase of M1-associated inflammation was the culprit of exacerbated liver damage in mice lacking AAM/M2 cells, surprisingly, MIIG × IL-4Rα−/− mice experienced greater hepatic injury than IL-4Rα−/− mice. Furthermore, the liver granulomas in MIIG × IL-4Rα−/− mice were larger compared with IL-4Rα−/− mice. Notably, there was greater perivascular inflammation in MIIG × IL-4Rα−/− mice that did not appear to localize around worm ova, a finding that was much less evident in IL-4Rα−/− and nearly absent in WT liver tissue. Given that hepatocellular injury was greater in S. mansoni–infected MIIG × IL-4Rα−/− mice compared with the other strains, these data implied the loss of important counterregulatory mechanisms that were engaged in both WT and IL-4Rα−/− strains.
Demonstration that MIIG × IL-4Rα−/− mice fail to up-regulate IDO expression within liver and intestine provided critical insight to understanding how IFN-γ–dependent effects on macrophages promoted host protection. The 5′ flanking region of the IDO gene contains IFN-stimulated response elements and IFN-γ–activated site sequences, which drives IDO expression within hematopoietic and nonhematopoietic cell lineages.24,25 IDO is the rate-limiting enzyme for the catabolism of tryptophan, which is an essential amino acid required for cell proliferation. IDO production from myeloid antigen-presenting cells causes cell cycle arrest in lymphocytes.21,26 IDO expression within macrophages and dendritic cells is a particularly important mechanism that limits inflammation within the gastrointestinal tract and the tumor cell microenvironment.27 Lack of IDO production from MIIG × IL-4Rα−/− mice during acute schistosomiasis suggested that defective IDO production from macrophages resulted in the greater accumulation of inflammatory cells and excessive collateral damage to parasitized organs. Consistent with this hypothesis, IDO neutralization in IL-4Rα−/− mice significantly increased damage to the liver and intestine compared with mock-treated IL-4Rα−/− mice.
WT C57BL/6 mice expressed IDO within the liver and intestine at levels equivalent to IL-4Rα−/− mice, which was somewhat unexpected because of the low levels of IFN-γ production after infection. Although 1-MT treatment in WT mice did not significantly change disease outcome, the levels of IFN-γ produced from MLN cultures were higher compared with mock-treated WT mice (data not shown). However, 1-MT treatment of infected IL-4Rα−/− mice caused a dramatic increase in morbidity and mortality. Overall, these data suggest that, although IDO production in WT mice is important in limiting the production of type 1 cytokines, this function is not critical for host survival. However, in the absence of a strong TH2 response that promotes AAM/M2 cell activation, IFN-γ–mediated IDO from macrophages provides a critical secondary mechanism that limits overexuberant inflammation and immunopathology.28 This scenario bears potential relevance to schistosomiasis in humans because certain IFN-γ polymorphisms are associated with reduced periportal fibrosis and reduced liver immunopathological characteristics.29
In conclusion, during murine schistosomiasis, the absence of IL-4/IL-13–dependent AAM/M2 cells, combined with the additional loss of IFN-γ–driven M1 cells, results in significantly worse disease than the sole loss of AAM/M2 cells alone. The underlying mechanism is likely IFN-γ–dependent IDO production from macrophages, which limits the intensity of inflammation directed against worm ova and, therefore, protects the infected host against excess collateral damage to parasitized organs.
Footnotes
Supported by grants R01HL091769 (M.B.J.) and R01 GM 083204 and R01 AI 095289 (D.R.H.) from the NIH.
Current address of D.R.H., University of San Francisco, San Francisco, California.
References
- 1.Gryseels B., Polman K., Clerinx J., Kestens L. Human schistosomiasis. Lancet. 2006;368:1106–1118. doi: 10.1016/S0140-6736(06)69440-3. [DOI] [PubMed] [Google Scholar]
- 2.Brunet L.R., Finkelman F.D., Cheever A.W., Kopf M.A., Pearce E.J. IL-4 protects against TNF-alpha-mediated cachexia and death during acute schistosomiasis. J Immunol. 1997;159:777–785. [PubMed] [Google Scholar]
- 3.Nair M.G., Du Y., Perrigoue J.G., Zaph C., Taylor J.J., Goldschmidt M., Swain G.P., Yancopoulos G.D., Valenzuela D.M., Murphy A., Karow M., Stevens S., Pearce E.J., Artis D. Alternatively activated macrophage-derived RELM-{alpha} is a negative regulator of type 2 inflammation in the lung. J Exp Med. 2009;206:937–952. doi: 10.1084/jem.20082048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phillips S.M., Linette G.P., Doughty B.L., Byram J.E., Von Lichtenberg F. In vivo T cell depletion regulates resistance and morbidity in murine schistosomiasis. J Immunol. 1987;139:919–926. [PubMed] [Google Scholar]
- 5.Fallon P.G., Richardson E.J., McKenzie G.J., McKenzie A.N. Schistosome infection of transgenic mice defines distinct and contrasting pathogenic roles for IL-4 and IL-13: IL-13 is a profibrotic agent. J Immunol. 2000;164:2585–2591. doi: 10.4049/jimmunol.164.5.2585. [DOI] [PubMed] [Google Scholar]
- 6.Rutitzky L.I., Hernandez H.J., Yim Y.S., Ricklan D.E., Finger E., Mohan C., Peter I., Wakeland E.K., Stadecker M.J. Enhanced egg-induced immunopathology correlates with high IFN-gamma in murine schistosomiasis: identification of two epistatic genetic intervals. J Immunol. 2005;174:435–440. doi: 10.4049/jimmunol.174.1.435. [DOI] [PubMed] [Google Scholar]
- 7.Rutitzky L.I., Lopes da Rosa J.R., Stadecker M.J. Severe CD4 T cell-mediated immunopathology in murine schistosomiasis is dependent on IL-12p40 and correlates with high levels of IL-17. J Immunol. 2005;175:3920–3926. doi: 10.4049/jimmunol.175.6.3920. [DOI] [PubMed] [Google Scholar]
- 8.Herbert D.R., Orekov T., Roloson A., Ilies M., Perkins C., O'Brien W., Cederbaum S., Christianson D.W., Zimmermann N., Rothenberg M.E., Finkelman F.D. Arginase I suppresses IL-12/IL-23p40-driven intestinal inflammation during acute schistosomiasis. J Immunol. 2010;184:6438–6446. doi: 10.4049/jimmunol.0902009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mosser D.M., Edwards J.P. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kreider T., Anthony R.M., Urban J.F., Jr, Gause W.C. Alternatively activated macrophages in helminth infections. Curr Opin Immunol. 2007;19:448–453. doi: 10.1016/j.coi.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rutitzky L.I., Hernandez H.J., Stadecker M.J. Th1-polarizing immunization with egg antigens correlates with severe exacerbation of immunopathology and death in schistosome infection. Proc Natl Acad Sci U S A. 2001;98:13243–13248. doi: 10.1073/pnas.231258498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.La Flamme A.C., Patton E.A., Bauman B., Pearce E.J. IL-4 plays a crucial role in regulating oxidative damage in the liver during schistosomiasis. J Immunol. 2001;166:1903–1911. doi: 10.4049/jimmunol.166.3.1903. [DOI] [PubMed] [Google Scholar]
- 13.Lykens J.E., Terrell C.E., Zoller E.E., Divanovic S., Trompette A., Karp C.L., Aliberti J., Flick M.J., Jordan M.B. Mice with a selective impairment of IFN-gamma signaling in macrophage lineage cells demonstrate the critical role of IFN-gamma-activated macrophages for the control of protozoan parasitic infections in vivo. J Immunol. 2010;184:877–885. doi: 10.4049/jimmunol.0902346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim Y.H., Choi B.K., Kang W.J., Kim K.H., Kang S.W., Mellor A.L., Munn D.H., Kwon B.S. IFN-gamma-indoleamine-2,3 dioxygenase acts as a major suppressive factor in 4-1BB-mediated immune suppression in vivo. J Leukoc Biol. 2009;85:817–825. doi: 10.1189/jlb.0408246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herbert D.R., Orekov T., Perkins C., Finkelman F.D. IL-10 and TGF-beta redundantly protect against severe liver injury and mortality during acute schistosomiasis. J Immunol. 2008;181:7214–7220. doi: 10.4049/jimmunol.181.10.7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hou D.Y., Muller A.J., Sharma M.D., DuHadaway J., Banerjee T., Johnson M., Mellor A.L., Prendergast G.C., Munn D.H. Inhibition of indoleamine 2,3-dioxygenase in dendritic cells by stereoisomers of 1-methyl-tryptophan correlates with antitumor responses. Cancer Res. 2007;67:792–801. doi: 10.1158/0008-5472.CAN-06-2925. [DOI] [PubMed] [Google Scholar]
- 17.Herbert D.R., Holscher C., Mohrs M., Arendse B., Schwegmann A., Radwanska M., Leeto M., Kirsch R., Hall P., Mossmann H., Claussen B., Forster I., Brombacher F. Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity. 2004;20:623–635. doi: 10.1016/s1074-7613(04)00107-4. [DOI] [PubMed] [Google Scholar]
- 18.Hoffmann K.F., Cheever A.W., Wynn T.A. IL-10 and the dangers of immune polarization: excessive type 1 and type 2 cytokine responses induce distinct forms of lethal immunopathology in murine schistosomiasis. J Immunol. 2000;164:6406–6416. doi: 10.4049/jimmunol.164.12.6406. [DOI] [PubMed] [Google Scholar]
- 19.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 20.Mellor A.L., Munn D.H. Tryptophan catabolism and T-cell tolerance: immunosuppression by starvation? Immunol Today. 1999;20:469–473. doi: 10.1016/s0167-5699(99)01520-0. [DOI] [PubMed] [Google Scholar]
- 21.Munn D.H., Shafizadeh E., Attwood J.T., Bondarev I., Pashine A., Mellor A.L. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. 1999;189:1363–1372. doi: 10.1084/jem.189.9.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wynn T.A., Morawetz R., Scharton-Kersten T., Hieny S., Morse H.C., 3rd, Kuhn R., Muller W., Cheever A.W., Sher A. Analysis of granuloma formation in double cytokine-deficient mice reveals a central role for IL-10 in polarizing both T helper cell 1- and T helper cell 2-type cytokine responses in vivo. J Immunol. 1997;159:5014–5023. [PubMed] [Google Scholar]
- 23.Rutitzky L.I., Stadecker M.J. CD4 T cells producing pro-inflammatory interleukin-17 mediate high pathology in schistosomiasis. Mem Inst Oswaldo Cruz. 2006;101(Suppl 1):327–330. doi: 10.1590/s0074-02762006000900052. [DOI] [PubMed] [Google Scholar]
- 24.Hassanain H.H., Chon S.Y., Gupta S.L. Differential regulation of human indoleamine 2,3-dioxygenase gene expression by interferons-gamma and -alpha: analysis of the regulatory region of the gene and identification of an interferon-gamma-inducible DNA-binding factor. J Biol Chem. 1993;268:5077–5084. [PubMed] [Google Scholar]
- 25.Kadoya A., Tone S., Maeda H., Minatogawa Y., Kido R. Gene structure of human indoleamine 2,3-dioxygenase. Biochem Biophys Res Commun. 1992;189:530–536. doi: 10.1016/0006-291x(92)91590-m. [DOI] [PubMed] [Google Scholar]
- 26.Mellor A.L., Munn D.H. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 27.Munn D.H., Mellor A.L. IDO and tolerance to tumors. Trends Mol Med. 2004;10:15–18. doi: 10.1016/j.molmed.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 28.Pearce E.J., M Kane C., Sun J., J Taylor J., McKee A.S., Cervi L. Th2 response polarization during infection with the helminth parasite Schistosoma mansoni. Immunol Rev. 2004;201:117–126. doi: 10.1111/j.0105-2896.2004.00187.x. [DOI] [PubMed] [Google Scholar]
- 29.Chevillard C., Moukoko C.E., Elwali N.E., Bream J.H., Kouriba B., Argiro L., Rahoud S., Mergani A., Henri S., Gaudart J., Mohamed-Ali Q., Young H.A., Dessein A.J. IFN-gamma polymorphisms (IFN-gamma +2109 and IFN-gamma +3810) are associated with severe hepatic fibrosis in human hepatic schistosomiasis (Schistosoma mansoni) J Immunol. 2003;171:5596–5601. doi: 10.4049/jimmunol.171.10.5596. [DOI] [PubMed] [Google Scholar]