Abstract
The myocardial extracellular matrix (ECM), an interwoven meshwork of proteins, glycoproteins, proteoglycans, and glycosaminoglycans that is dominated by polymeric fibrils of type I collagen, serves as the mechanical scaffold on which myocytes are arrayed for coordinated and synergistic force transduction. Following ischemic injury, cardiac ECM remodeling is initiated via localized proteolysis, the bulk of which has been assigned to matrix metalloproteinase (MMP) family members. Nevertheless, the key effector(s) of myocardial type I collagenolysis both in vitro and in vivo have remained unidentified. In this study, using cardiac explants from mice deficient in each of the major type I collagenolytic MMPs, including MMP-13, MMP-8, MMP-2, MMP-9, or MT1-MMP, we identify the membrane-anchored MMP, MT1-MMP, as the dominant collagenase that is operative within myocardial tissues in vitro. Extending these observations to an in vivo setting, mice heterozygous for an MT1-MMP–null allele display a distinct survival advantage and retain myocardial function relative to wild-type littermates in an experimental model of myocardial infarction, effects associated with preservation of the myocardial type I collagen network as a consequence of the decreased collagenolytic potential of cardiac fibroblasts. This study identifies MT1-MMP as a key MMP responsible for effecting postinfarction cardiac ECM remodeling and cardiac dysfunction.
The cardiac extracellular matrix (ECM) is an interwoven meshwork of proteins, glycoproteins, proteoglycans, and glycosaminoglycans that provide the structural scaffold on which myocytes, cardiac fibroblasts, and endothelial cells are arrayed to form functional heart tissue.1–4 Dominated by fibrils of triple helical type I collagen, the cardiac ECM serves to maintain myocyte geometrical integrity for synergistic force transduction while also serving as a reservoir of latent bioactive signaling molecules that regulate cardiac function.1–4 In the setting of myocardial infarction (MI), ischemic tissue injury generates profound changes in the architecture and composition of the cardiac ECM: hypoxic cell death of myocytes and oxidative damage following reperfusion induces an influx of inflammatory leukocytes that collaborate with stromal cells to degrade the native structural components of the ventricular wall, resulting in ventricular thinning and dilation.5–10 Following clearance of necrotic tissue, neomatrix is deposited, with the wound resolving as an inelastic scar.6,7,11 Loss of functional cardiac tissue manifests either acutely, if uncompensated, as cardiogenic shock or chronically as congestive heart failure, resulting in significant morbidity and mortality.2,5–8,12 As such, recent studies have focused on identifying the key effectors of post-MI cardiac ECM remodeling in an effort to develop therapeutic interventions that might better preserve tissue architecture and function.11,13–15
The matrix metalloproteinases (MMPs), a family of more than 20 zinc-dependent endopeptidases that collectively can degrade all ECM macromolecules, are molecular effectors of post-MI tissue remodeling, likely via the catabolism of type I collagen within the cardiac interstitium.12,15,16 Consistent with this hypothesis, multiple studies have demonstrated increased MMP expression and activation in the post-MI state.15,17–20 Further, a functional role for MMPs in post-MI cardiac remodeling is supported by studies wherein MMP inhibition preserves cardiac function in animal models.13,15,21–24 Complementing these studies, genetically engineered mice carrying disrupted genes encoding a subset of MMPs or endogenous MMP inhibitors display significant alterations in post-MI cardiac remodeling or survival.25–34 Although these studies support a model wherein excessive MMP-mediated cardiac ECM remodeling contributes adversely to post-MI systolic dysfunction, the key MMPs responsible for type I collagenolysis in the infarcted heart remain controversial.
Until recently, the dissolution of type I collagen-rich networks in murine tissues has largely been attributed to the secreted type I collagenolytic MMPs, MMP-13, MMP-8, MMP-2, or MMP-9.16,35 However, membrane type-1 MMP (MT1-MMP) is a cell-surfaced anchored MMP that also functions as a highly effective, pericellular type I collagenase.35–38 Studies using mice deficient for this MMP have identified required roles for the enzyme in diverse developmental settings,39–42 but the role played by MT1-MMP within the ischemic myocardium remains largely unexplored. Herein, using explanted myocardial tissues isolated from MMP-13−/−, MMP-8−/−, MMP-2−/−, MMP-9−/−, or MT1-MMP−/− mice, we demonstrate that MT1-MMP is the major type I collagenase operative within the ischemic myocardium. Furthermore, mice heterozygous for a null allele of MT1-MMP display functional advantages post-MI due to preserved cardiac function that occurs in tandem with the attenuated remodeling of the cardiac ECM. These results identify MT1-MMP as a key effector of the post-MI ECM remodeling that accompanies cardiac dysfunction.
Materials and Methods
Mice
All ex vivo and in vivo studies were performed with MMP2−/− C57BL/6 mice,43 MMP8−/− C57BL/6/129 mice,44 MMP9−/− 129SvEv mice,45 MMP13−/− C57BL/6 mice,46 or MT1-MMP−/− or MT1-MMP+/− Swiss Black mice,39 all backcrossed >6 generations. Age-matched C57BL/6 animals were used as controls for MMP-2−/−, MMP-8−/−, and MMP-13−/− mice, and 129SvEv animals were used as controls for MMP-9−/− mice. For studies of MT1-MMP−/− or MT1-MMP+/− mice, paired analyses were performed with wild-type (WT) littermates.
Tissue Explants and Cell Isolation
Whole-heart tissue explants were isolated from mice perfused with saline, minced into 1-mm3 fragments, and placed in 10-mL centrifuge tubes with Dulbecco's modified Eagle's medium (Gibco BRL, Carlsbad, CA) supplemented with 10% fetal bovine serum and incubated under 5% CO2 at 37°C.47 In selected experiments, tissue samples were cultured in the presence of the synthetic MMP inhibitors 5 μmol/L BB-94 (Tocris, Minneapolis, MN) or 25 μmol/L GM6001 (Calbiochem, Merck, Darmstadt, Germany). After 3 and 5 days in culture, supernatant and heart tissue samples were collected for analysis. Collagen degradation products were quantified by hydroxyproline release into the conditioned medium after an ethanol precipitation step (70% v/v) as described.37,48 Sirius Red staining was performed as described,49 whereas denatured type I collagen degradation products were detected in situ with a monoclonal antibody directed against hydrolyzed type I collagen.40,50
Cardiac fibroblasts were isolated from the left ventricle of 10- to 12-week-old MT1-MMP+/+, MT1-MMP+/−, or MT1-MMP−/− mice (passage 2 to 4) and were cultured in Dulbecco's modified Eagle's medium supplemented with 10% heat-inactivated fetal calf serum (Atlanta Biologicals, Lawrenceville, GA), 100 units/mL penicillin, 100 μg/mL streptomycin, 0.25 μg/mL Fungizone, and 2 mmol/L l-glutamine (Gibco).
Animal Models
Female mice, ranging in age from 10 weeks to 12 weeks, and in weight from 20 g to 30 g, underwent coronary artery ligation for the induction of MI. Surgical procedures have been described in detail elsewhere.51 Briefly, mice were sedated with intraperitoneal sodium pentobarbital (45 mg/kg), intubated orally, and ventilated via a pressure-controlled ventilator (Harvard Apparatus, Holliston, MA) with 1% isoflurane in 100% oxygen at a peak inspiratory pressure of 15 cm H2O and a respiratory rate of 60 breaths per minute. With the aid of a dissecting microscope, the heart was exposed via a left thoracotomy, and the proximal portion of the left coronary artery was ligated 1 to 2 mm from the left atrium with a 7-0 silk suture. Pallor, regional hypokinesia, and enlargement of the left ventricle confirmed the presence of an infarction. The chest was filled with warm sterile saline to evacuate air, and the incision was closed in layers using 5-0 silk suture. The animals were then extubated and allowed to recover from surgery under a heating lamp for 1 hour. Antibiotic prophylaxis was not given, but no apparent infection developed in any animal during the course of the study or at the time of autopsy. Bone marrow transplantation of 8- to 10-week-old MT1-MMP+/− mice with MT1-MMP+/+ or MT1-MMP+/− marrow was performed as described.52 Animals were maintained under standard housing conditions following experimental MI with careful monitoring for health status. Animals were euthanized at 3, 14, or 28 days post-MI for analysis of cardiac disease as described below. Post-MI mortality was monitored throughout this period. All animal protocols were approved by the University of Michigan Committee on Use and Care of Animals. Mice were housed in the American Association for Accreditation of Laboratory Animal Care–approved facility of the University of Michigan.
Echocardiography
Echocardiographic studies were performed under light anesthesia with spontaneous respiration using inhaled isoflurane, l-chloro-2,2,2-trifluoroethyl difluoromethyl ether (Avertin; Sigma-Aldrich, St. Louis, MO). An ultrasonographer experienced in rodent imaging performed the echocardiographic studies, using commercially available equipment (Sonos Model 5500; Hewlett-Packard Medical Products Group, Andover, MA) and a 15- to 60-MHz transducer (Hewlett-Packard). The heart was imaged with two-dimensional–guided M-mode echocardiography in the parasternal long-axis view. From this view, an M-mode cursor was positioned perpendicular to the interventricular septum and posterior wall of the left ventricle at the level of the papillary muscles, and a sweep speed of 100 mm/s was used. Diastolic and systolic left ventricular (LV) wall thickness and left ventricular end-diastolic and end-systolic chamber dimensions were measured. Doppler was used for analysis of mitral valve inflow patterns [early (E) and late (A) filling waves]. Doppler tissue imaging was used to measure the early (Ea) and late (Aa) diastolic tissue velocities of the mitral annulus in the apical four-chamber view. For each measurement, three consecutive cardiac cycles were measured and averaged. All measurements were done from leading edge to leading edge according to the American Society of Echocardiography guidelines.
Histopathology
After in vivo echocardiographic and hemodynamic studies, the hearts were excised and dissected. From the mid-left ventricle transverse sections, 5-μm sections were cut and stained with hematoxylin and eosin for determination of myocyte cross-sectional area.
Myocardial leukocyte infiltration was quantified in hematoxylin-and-eosin–stained sections by determination of nuclear density (nuclei/mm2). In each animal, five independent high-power fields were analyzed. To further determine the number of polymorphonuclear leukocytes and macrophages, immunohistochemical analysis using specific antibodies against mouse CD45 and F4/80 (BD Biosciences PharMingen, San Jose, CA), respectively, was performed.
Myocardium collagen volume fraction was determined by quantitative morphometry of Sirius Red–stained sections (Sigma, St. Louis, MO)49 on a subset of six mice (three MT1-MMP+/− mice and three MT1-MMP+/+ mice) with infarcts. The relative abundance of thin (immature) versus thick (mature) interstitial collagen fibers was estimated by spectral analysis of collagen fiber color emission (green/yellow versus red/orange, respectively) under polarized microscopy as described previously.53–56 Tissue collagen content and collagen degradation fragments were determined as described above for cardiac explants. Apoptosis was assessed by TUNEL assay according to the manufacturer's protocol (Roche, Basel, Switzerland).57
Fluorescent and light microscopy images were captured by a microscope (DMLB; Leica Microsystems, Wetzlar, Germany) equipped with a SPOT RT camera (Spot Imaging Solutions, Diagnostic Instruments, Sterling Heights, MI) after calibration using an objective micrometer.
In Situ Hybridization
Probes specific for mouse MT1-MMP or murine Col1a1 (for type I collagen) were developed as previously described.17 After vector linearization, sense and antisense digoxigenin (Dig)-labeled probes were generated using the Dig RNA labeling mix (Roche) and SP6 or T7 RNA polymerase, respectively. Five-micrometer sections were deparaffinized, rehydrated, and treated with proteinase K (5 μg/mL) for 10 minutes at room temperature and then fixed in paraformaldehyde. Slides were incubated in acetylation solution [triethanolamine (pH 8), HCl, acetic anhydride] for 10 minutes to reduce nonspecific, charge-based hybridization. Hybridization with 20 ng of probe was performed overnight at 65°C in a humidity chamber. Slides were then sequentially washed with 5× standard saline citrate at room temperature for 5 minutes, 1× standard saline citrate/50% formamide at 60°C for 30 minutes, and TNE [10 mmol/L Tris (pH 7.5), 500 mmol/L NaCl, 1 mmol/L EDTA (pH 8)] for 10 minutes at 37°C. Sections were treated with RNase A (20 μg/mL in TNE) for 10 minutes and then washed again in 2× standard saline citrate followed by 0.2× standard saline citrate at 60°C. Dig detection was performed with the anti-Dig-AP antibody (Roche) and developed with the BM purple substrate (Roche) for 5 days with one change of the substrate solution as per manufacturer's directions.58
RT-PCR Analysis
RNA was isolated from the mouse heart tissue using TRIzol reagent (Life Technologies, Grand Island, NY). RT-PCR amplification using specific oligonucleotide primers for MMP-2, MMP-8, MMP-9, MMP-13, and MT1-MMP was performed as described.37 The identities of the PCR products were confirmed by sequence analysis.
Tissue Zymography
Myocardial MMP-2 and MMP-9 activity were determined in the left ventricle from 12 week-old mice at baseline and postinfarction by gelatin zymography.31,37,59 The left ventricular myocardial samples were homogenized (∼30-second bursts) in 1 mL of an ice-cold extraction buffer containing cacodylic acid (10 mmol/L), NaCl (0.15 mol/L), ZnCl2 (20 mmol/L), NaN3 (1.5 mmol/L), and 0.01% Triton X-100 (pH 5.0). The homogenate was then centrifuged (4°C, 10 minutes, 10,000 × g), and the supernatant was decanted and saved on ice. The pH levels of the samples were adjusted to 7.5 with Tris (1 mol/L). The final protein concentration of the myocardial extracts was determined using a standardized colorimetric assay. The myocardial extracts were then loaded onto electrophoretic gels (SDS-PAGE) containing 1 mg/mL gelatin under nonreducing conditions. Five micrograms of protein was loaded onto the gels using a 3:1 sample buffer [10% SDS, 4% sucrose, 0.25 mol/L Tris Cl, and 0.1% bromophenol blue (pH 6.8)]. The gels were run at 15 mA through the stacking phase (4%) and at 20 mA for the separating phase (10%), whereas the running buffer temperature was maintained at 4°C. After SDS-PAGE, the gels were washed twice in 2.5% Triton X-100 for 30 minutes each, rinsed in water, and then incubated for 24 hours in a substrate buffer at 37°C [50 mmol/L Tris HCl, 5 mmol/L, CaCl2, and 0.02% NaN3 (pH 7.5)]. After incubation, the gels were stained with Coomassie Brilliant Blue R-250.
Subjacent Collagen Degradation Assays
Type I collagen gel films (100 μg/cm2) were labeled with tetramethylrhodamine isocyanate (TRITC; Molecular Probes, Carlsbad, CA)37 and isolated cardiac fibroblasts (5 × 104) were cultured atop the collagen films for 7 days in Dulbecco's modified Eagle's medium/10% fetal bovine serum and PDGF-BB (10 ng/ml) at 37°C. The samples were fixed, polymerized actin was visualized with Alexa Fluor-488 phalloidin (Molecular Probes), and fluorescent images were captured by a laser scanning confocal microscope equipped with acquisition software (Service Pack 2) to visualize the formation of degradative zones.37
Alternatively, type I collagen degradation was visualized by a collagen film assay wherein 24-well plates were coated with collagen gel (100 μg/well) and 5 × 104 fibroblasts in 35 μL of medium were seeded in the center of each well and allowed to adhere for 8 hours.35,60 After washing, 0.5 mL of serum-free medium was added to each well with PDGF-BB (10 ng/mL). After 5 days, cells were removed by trypsin/EDTA or detergent lysis, and the remaining collagen film was stained with Coomassie Brilliant Blue.
Statistical Analysis
All data are expressed as mean ± SEM. A survival analysis was performed by the Kaplan-Meier method, and between-group difference in survival was tested by the log-rank test. Between-group comparisons of the means were performed by one-way analysis of variance followed by paired Student's t-test.
Results
MT1-MMP Is Required for Interstitial Collagen Degradation in Ischemic Myocardial Explants
To assess the collagenolytic potential of myocardium-derived MMPs ex vivo, explants of WT adult mouse hearts were cultured under no-flow conditions designed to simulate an ischemic environment.47 Over the course of the 5-day culture period, the collagen volume fraction decreased as assessed by i) Sirius Red staining; ii) the accumulation of degraded type I collagen as monitored by the immunohistochemical detection of hydrolyzed collagen fragments in the explant tissue; and iii) the release of small Mr, hydroxyproline-containing peptides into the extracellular media (Figure 1, A and B). To determine the role of MMPs in the collagenolytic activity detected under these conditions, explants were cultured in the presence of the broad-spectrum MMP inhibitors, BB-94 or GM6001.37 Either agent preserved the collagen network of the myocardial interstitium, attenuated the appearance of type I collagen cleavage products within the interstitium, and inhibited the appearance of soluble collagen degradation products (Figure 1, A and B).
Figure 1.
Ex vivo model assessing role of MT1-MMP in collagen degradation using ischemic myocardial explants. A: Sirius Red staining of interstitial collagen (red) and denatured collagen staining (green) in adult WT heart tissue at baseline and following 5 days incubation in the absence or presence of BB-94. Scale bars: 20 μm. Quantification of collagen volume fraction (%) and degraded collagen (arbitrary units, AU) as shown (mean ± SEM). *P < 0.05. B: Collagen degradation as monitored by the release of hydroxyproline-containing fragments from explants of adult WT mouse heart tissue after 3 days or 5 days incubation in the absence or presence of the MMP inhibitors, BB-94 or GM6001 (mean ± SEM). C: RT-PCR analysis of MMP-2, MMP-8, MMP-9, MMP-13, and MT1-MMP expression in heart explants of WT tissue at baseline and after a 5-day culture period. Results are representative of three independent experiments performed. D: Hydroxyproline solubilization was determined after a 5-day culture period of cardiac explants isolated from adult WT, MMP-2−/−, MMP-8−/−, MMP-9−/−, MMP-13−/−, MT1-MMP−/−, or MT1-MMP+/− mice (mean ± SEM). E: Gelatin zymography of heart tissue explants from MT1-MMP+/− or MT1-MMP+/+ explants at baseline versus after a 5-day culture period. The pro- and processed forms of MMP-9 and MMP-2 are indicated.
To define the relative roles of individual MMPs in myocardial collagenolysis, the induction of transcripts encoding each of the known murine type I collagenases (ie, MMP-13, MMP-8, MMP-2, MMP-9, and MT1-MMP37) was examined in myocardial explants over a 5-day period. mRNAs encoding MMP-13, MMP-9, and MT1-MMP were induced over the 5-day culture period, whereas MMP-2 levels remained stable (Figure 1C). MMP-8 could not be detected under these conditions (Figure 1C). To identify the MMP(s) participating in collagenolysis, myocardial explants were isolated from mice with targeted disruption of the genes encoding MMP-2, MMP-8, MMP-9, MMP-13, or MT1-MMP and cultured under no-flow conditions for 5 days. The total collagen content of the explants harvested from WT or each of the MMP-deficient mice was identical at baseline as assessed by hydroxyproline analysis (data not shown). Although deficiency of any of the four secreted collagenases (ie, MMP-13, MMP-8, MMP-2, or MMP-9) failed to significantly blunt ischemia-induced collagenolysis, the collagenolytic activity of MT1-MMP−/− cardiac explants was attenuated to levels comparable to those observed when WT tissues were cultured in the presence of the synthetic MMP inhibitors (Figure 1, B and D). As MT1-MMP–null mice display profound defects in multiple organ systems that might indirectly affect myocardial responses to noxious stimuli,39,41,42 the collagenolytic activity of MT1-MMP heterozygous explants were examined. MT1-MMP+/− mice are phenotypically indistinguishable from WT mice,49,61 but heterozygous explants displayed an intermediate level of hydroxyproline release that was significantly decreased relative to WT controls (Figure 1D). Although MT1-MMP can initiate the processing of the MMP-2 and MMP-9 zymogens to their active forms,62,63 levels of processed MMP-2 and MMP-9 were unaffected by decreasing the MT1-MMP gene dosage (Figure 1E).
MT1-MMP Is Engaged in Ischemic Myocardium in Vivo and Regulates Acute Post-MI Survival
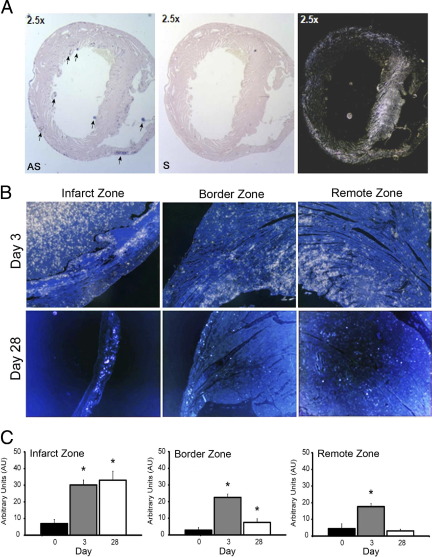
As MT1-MMP served as the key effector of myocardial collagenolysis in ex vivo explants, expression of the mRNA encoding this protease was monitored during early (ie, 3 days) and late (28 days) post-MI cardiac remodeling in vivo. MT1-MMP expression was barely detected by in situ hybridization of normal hearts (ie, noninfarcted, control mice; data not shown), but a dramatic increase in MT1-MMP mRNA expression was detected both within the zone of infarction as well as in remote myocardial tissues at 3 days postinfarction (Figure 2, A and B). Expression of MT1-MMP mRNA was sustained in the infarct zone throughout the late remodeling phase, with MT1-MMP expression observed in the thinned left ventricular wall at 28 days postinfarction (Figure 2, B and C), correlating with persistent MT1-MMP–dependent (as well as other MT-MMP family member–dependent) activity as assessed by proteolytic processing of MMP-2.36
Figure 2.
Expression kinetics and localization of MT1-MMP in the post-MI myocardium. A:In situ hybridization of cardiac cross sections 3 days post-MI using MT1-MMP antisense (AS) or sense (S) probes. Arrows indicate scattered foci of MT1-MMP expression. Images obtained with the sense probe were highlighted in darkfield micrographs (right). B: Representative micrographs of in situ hybridization for MT1-MMP expression in cross sections of WT hearts using darkfield images (MT1-MMP antisense probe appears as white/silver foci) at baseline and 3 or 28 days post-MI in the infarct, border, and remote zones, respectively. Scale bars: 100 μm. C: Relative quantification of MT1-MMP in the infarct zone and border zone or remote viable myocardium in WT heart tissue at baseline, 3, or 28 days postinfarction (mean ± SEM). *P < 0.05.
To assess the functional role of MT1-MMP expression on post-MI remodeling and cardiac function in vivo, adult MT1-MMP+/− mice were used in place of the null animals because MT1-MMP−/− mice display early morbidity and mortality that preclude further testing.39,42 Consistent with the normal phenotypic appearance of MT1-MMP+/− mice, no significant differences in baseline cardiac function and relevant co-parameters including heart and body weight, hemodynamics, echocardiographic parameters or electrocardiographic intervals were detected between heterozygous mice and littermate controls (Table 1). Further, within 24 hour of coronary artery ligation, acute mortality was comparable between the WT and MT1-MMP+/− mice (data not shown). Infarction was confirmed by wall-motion abnormality on echocardiography, changes on electrocardiography, and distal coronary vessel blanching following ligation (data not shown). Subsequent long-term post-MI overall survival, however, was increased over twofold at 4 weeks in the MT1-MMP+/− population (78%) relative to MT1-MMP+/+ mice (36%; Figure 3A). At 4 weeks post-MI, marked differences in the expression of MMP-2, MMP-8, MMP-13, or MMP-9 were not detected between MT1-MMP+/+ and MT1-MMP+/− mice (Figure 3B). Furthermore, levels of latent and processed MMP-2 or MMP-9 in MT1-MMP+/− myocardial tissue were comparable to those detected in WT tissue (Figure 3C). Death in both groups of mice was attributed most frequently to acute heart failure or ventricular arrhythmia, whereas ventricular rupture occurred only rarely (data not shown).
Table 1.
Baseline Comparison of MT1-MMP+/− and MT1-MMP+/+ Mice
| MT1-MMP+/+ | MT1-MMP+/− | |
|---|---|---|
| Baseline | ||
| BW, g | 25.7 ± 0.4 | 26.2 ± 1.1 |
| HW/BW ratio, mg/g | 6.52 ± 0.10 | 6.43 ± 0.13 |
| Systolic blood pressure, mm Hg | 131 ± 5 | 129 ± 9 |
| HR, beats/minute | 529 ± 72 | 520 ± 36 |
| Heart rate variability, beats/minute | 11.1 ± 3.0 | 9.8 ± 3.6 |
| PR, ms | 25.5 ± 2.8 | 24.5 ± 1.6 |
| QRS, ms | 10.3 ± 0.9 | 9.6 ± 0.7 |
| QTc, ms | 45.9 ± 3.2 | 44.6 ± 1.9 |
| LV mass, mg | 35.9 ± 11.8 | 39.1 ± 9.8 |
| LV mass/BW, mg/g | 3.60 ± 0.09 | 3.62 ± 0.93 |
| LV ejection fraction, % | 79 ± 4 | 81 ± 3 |
| Fractional shortening, % | 41 ± 4 | 43 ± 4 |
| Left ventricular internal diameter, mm | 2.57 ± 0.40 | 2.65 ± 0.41 |
| Interventricular septal thickness, mm | 0.60 ± 0.10 | 0.63 ± 0.10 |
| Relative wall thickness, mm/mm | 0.42 ± 0.03 | 0.43 ± 0.13 |
| E/A ratio | 1.9 ± 0.3 | 1.8 ± 0.4 |
| Cardiac output, L/minute | 5.9 ± 2.4 | 6.9 ± 3.1 |
| Cardiac index, mL/minute/g | 584 ± 74 | 591 ± 33 |
Comparisons were made using echocardiography, electrocardiography, and noninvasive sphygmomanometry techniques on mice at 12 weeks of age.
BW, body weight; HR, heart rate; HW, heart weight; LV, left ventricular; QTc, heart rate–correct QT interval.
Figure 3.
Enhanced postinfarction survival in MT1-MMP+/− mice. A: Survival of MT1-MMP+/+ and MT1-MMP+/− mice after coronary artery ligation. Lifespan was estimated by the Kaplan-Meier method. Percentages of surviving WT (MT1-MMP+/+; n = 11) and MT1-MMP heterozygote (MT1-MMP+/−; n = 10) are shown at 0, 7, 14, 21, and 28 days post-MI. *P < 0.05. B: RT-PCR analysis of MMP-2, MMP-8, MMP-9, MMP-13, and MT1-MMP expression in MT1-MMP+/+ and MT1-MMP+/− infarcted tissue at 28 days post-MI. C: Gelatin zymography of infarcted heart tissue isolated from MT1-MMP+/+ and MT1-MMP+/− mice 28 days post-MI. The pro- and active forms of MMP-9 and MMP-2 are indicated. Results are representative of three independent experiments performed.
Postinfarct Cardiac Structure/Function Preservation in MT1-MMP+/− Mice
Three days post-MI, MT1-MMP+/+, and MT1-MMP+/− hearts displayed similar areas at risk as assessed by histology and echocardiography, indicating that comparable areas of injury arise following coronary ligation (Figure 4A). However, progressive changes subsequently ensued that resulted in a significant reduction in the final infarct size in the MT1-MMP+/− mice at 28 days (Figure 4B). In addition, MT1-MMP+/− hearts exhibited a small, but statistically significant, decrease in left ventricular dilatation, as reflected by a higher ventricular wall thinning ratio (ie, ratio of infarcted wall thickness to septal thickness) (Figure 4B). Moreover, compensatory post-MI myocardial hypertrophic responses were correspondingly blunted in the MT1-MMP+/− mice as assessed by decreases in myocyte cross-sectional area as well as LV mass and LV diameter in noninfarcted regions of the heart (Figure 4B).
Figure 4.
Preservation of cardiac structure and function in MT1-MMP heterozygous mice. A: Micrographs of H&E-stained cross sections of whole hearts recovered from adult MT1-MMP+/+ and MT1-MMP+/− mice at baseline, 3, and 28 days post-MI. Scale bar = 1 mm. B: Quantitative assessment of left ventricular remodeling using established measures of infarct size, septal wall thickness (wall thinning ratio), myocyte hypertrophy, LV internal diameter (at diastole and at systole), relative LV wall thickness at systole, and LV mass at baseline, 14, or 28 days post-MI. (mean ± SEM). Structural assessment of the left ventricular internal diameter and relative wall thickness were determined using two-dimensional echocardiography on MT1-MMP+/+ and MT1-MMP+/− mice at baseline and 14 or 28 days post-MI. Myocyte hypertrophy (area) was quantified using established measures in histological sections at baseline and 28 days post-MI (mean ± SEM). *P < 0.05 compared to baseline within genotypes; †P < 0.05 compared to WT heart at the respective time points. C: Measurement of ejection fractions and fractional shortening using standard echocardiography are shown in MT1-MMP+/+ and MT1-MMP+/− mice at baseline and 14 versus 28 days post-MI (mean ± SEM). *P < 0.05 compared to baseline within genotypes; †P < 0.05 compared to WT heart at the respective time points.
Consistent with the improved structural status of the post-infarct myocardium in MT1-MMP+/− mice, cardiac functional parameters (ie, fractional shortening and ejection fraction) were improved significantly in these mice 28 days postligation (Figure 4C). Furthermore, whereas MT1-MMP+/+ mice displayed reduced cardiac function as early as 3 days post-MI, MT1-MMP+/− mice did not display comparable decremental changes in function until 7 to 14 days (Figure 4C, Supplemental Figures S1 and S2 at http://ajp.amjpathol.org, and data not shown). Diastolic parameters of isovolumic relaxation time and E/A ratio showed a trend toward less dysfunction in the MT1-MMP+/− mice, but the degree of change was not statistically significant (see Supplemental Figure S1 at http://ajp.amjpathol.org). Covariable parameters, including resting heart rates and systemic vascular resistance, were also followed in both treatment groups, but no significant differences between the MT1-MMP+/+ and MT1-MMP+/− mice were detected (see Supplemental Figure S2 at http://ajp.amjpathol.org). Taken together, these findings demonstrate that MT1-MMP deficiency imparts a protective effect on the adverse LV remodeling that follows myocardial infarction by reducing both the extension of infarct size and the degree of LV dilation.
MT1-MMP Gene Dose Regulates Functional Remodeling of the Cardiac Stroma in a Myocardium-Intrinsic Fashion
Following MI, MT1-MMP–dependent remodeling of the cardiac ECM is potentially effected by resident cardiomyoctyes and cardiac stromal cells (ie, fibroblasts and endothelial cells) working in collaboration with infiltrating inflammatory leukocyte populations and other bone marrow–derived cell populations.9 To begin characterizing the tissue of origin of cells responsible for MT1-MMP–dependent cardiac remodeling, inflammatory cell influx was assessed in MT1-MMP+/+ and MT1-MMP+/− hearts following coronary ligation. At 3 days post-MI, inflammatory cell influx into the necrotic myocardium and border zone region consisted mainly of polymorphonuclear leukocytes (CD45+) and mononuclear cells/macrophages (F4/80+) (Figure 5A). Although slightly lower numbers of neutrophils were observed within the infarcted region of MT1-MMP+/− mice, these differences were not significant (Figure 5A). By contrast, significantly reduced numbers of macrophages (as well as total leukocyte numbers) localized to the infarcted region of MT1-MMP+/− mice (Figure 5A). Despite the decreased macrophage content of the MT1-MMP+/− infarcted myocardium, levels of circulating monocytes as well as neutrophils were increased in MT1-MMP+/− mice relative to MT1-MMP+/+ mice at 3 days post-MI (0.62 ± 0.28 × 103 vs. 0.27 ± 0.19 × 103 cells/μL and 1.21 ± 0.07 × 103 vs. 0.39 ± 0.04 × 103 cells/μL, respectively).
Figure 5.
Myocardium-intrinsic role of MT1-MMP in regulating post-MI cardiac function. A: Micrographs (top) of H&E sections of the infarct zone and the peri-infarct, viable myocardium from adult MT1-MMP+/+ and MT1-MMP+/− mice at 3 days post-MI. Scale bar = 100 μm. Arrows indicate examples of infiltrating leukocytes within the interstitial space. Neutrophil, macrophage, and leukocyte infiltration in the infarct and remote LV regions was quantified (bottom) using CD45- and F4/80-staining and morphological analysis, respectively (mean ± SEM). *P < 0.05. B: PCR analysis of HPRT (minicassette marker designating disrupted allele encoding MT1-MMP) and WT MT1-MMP locus in samples of bone marrow, peripheral blood cells and splenic T-cells in mice 12 weeks after receiving bone marrow transplantation showing complete engraftment of donor marrow. C: Measurement of systolic cardiac function parameter ejection fraction and structural cardiac assessment of the left ventricle internal diameter in Group A [WT to heterozygous (HT) transplant, n = 4] versus Group B (HT to HT transplant, n = 4) mice at baseline, 14 days, and 28 days post-MI (mean ± SEM). *P < 0.05. D: Representative micrograph of PECAM-1 (red) stained sections in the border zone of MT1-MMP+/+ and MT1-MMP+/− mice at 3 days post-MI. Number of microvessels per square millimeter were quantified (mean ± SEM). *P < 0.05. E:MT1-MMP+/+ or MT1-MMP+/− myocardium was stained for TUNEL 3 days post-MI. Numbers of TUNEL-positive cells per square millimeter were tabulated (mean ± SEM).
Macrophage-derived MT1-MMP has been implicated in pathologic interstitial ECM remodeling,64,65 raising the possibility that the enhanced post-MI survival observed in MT1-MMP+/− mice arises as a result of altered macrophage function. As such, congenic WT marrow was stably transplanted into irradiated MT1-MMP+/− mice (Figure 5B). Leukocyte blood counts performed 12 weeks after transplantation in MT1-MMP+/− mice were similar to those in WT mice, as was the degree of initial inflammatory cell infiltration during the immediate post-MI period (data not shown). However, the post-MI LV functional status of MT1-MMP+/− mice transplanted with MT1-MMP+/+ bone marrow was similar, if not identical, to that observed in MT1-MMP+/− mice transplanted with MT1-MMP+/− bone marrow (Figure 5C). Hence, the preserved cardiac function observed post-MI in MT1-MMP+/− mice is mediated by cells intrinsic to the myocardium itself, with little or no effect of the MT1-MMP gene dose expressed within bone marrow–derived cell populations.
Given a dominant role for myocardial-derived MT1-MMP in regulating post-MI cardiac structure and function, the features of cells intrinsic to the myocardium during post-MI tissue remodeling were next assessed. As endothelial cells mobilize MT1-MMP during angiogenesis, a critical aspect of tissue remodeling,40 microvessel density was assessed in the border zone adjacent to the infarct zone at 3 and 28 days post-MI in MT1-MMP+/+ and MT1-MMP+/− hearts. As assessed by platelet endothelial cell adhesion molecule 1 (PECAM-1) staining,40 vessel numbers were not significantly different between genotypes (Figure 5D). Moreover, significant differences in the number of apoptotic myocytes and fibroblasts were not detected post-MI between the two genotypes (Figure 5E).
Post-MI Collagen Catabolism Is Attenuated in MT1-MMP–Deficient Mice, Resulting in Preserved Interstitial Collagen Matrix
Given MT1-MMP's role as a pericellular collagenase and its regulation of collagen metabolism in the myocardium ex vivo, collagen metabolism was examined in WT and MT1-MMP+/− hearts following coronary artery ligation. At 3 days post-MI, type I collagen degradation products were readily detected both within the infarct zone and border region surrounding necrotic cardiomyocytes in WT mice, whereas decreased levels of type I collagen degradation were detected in the infarct and border zone (but not remote) regions of the MT1-MMP+/− mice (Figure 6A). To determine whether the decrease in collagenolysis associated with MT1-MMP heterozygosity altered steady-state collagen levels in the postinfarct heart, the fibrillar collagen content was determined within both the infarct border and remote regions in MT1-MMP+/− and MT1-MMP+/+ hearts. As assessed by Sirius Red staining under circularly polarized light, thicker interstitial collagen fibers (constituting type I and/or type III collagen-containing fibrils) were identified as the dominant fibrillar collagen in the post-MI myocardium as compared to thinner collagen fibers at the 28-day time point (Figure 6B).53–56,66 Relative to WT mice, however, MT1-MMP+/− animals retained a relatively higher content of the thicker collagen fibers in post-MI tissue with an equal content of thinner collagen fibrils, resulting in a net increase in the ratio of thick-to-thin fibers (Figure 6B). Consistent with these findings, the collagen content of the infarct zone as determined by total hydroxyproline content was increased significantly by ∼20% in MT1-MMP+/− mice (Figure 6B). Although MT1-MMP activity can potentially impact type I collagen expression by modulating the local transforming growth factor (TGF)-β1 concentration,67–70 type I collagen transcript levels were indistinguishable in the infarct border and remote zones of MT1-MMP+/+ and MT1-MMP+/− hearts as assessed by in situ hybridization (Figure 6C). Taken together, these results suggest that myocardial MT1-MMP regulates post-MI ventricular remodeling by effecting turnover of the type I collagen ECM.
Figure 6.
MT1-MMP regulates LV remodeling post-MI through catabolism of type I collagen fibrils in the myocardial interstitium. A: Micrographs of myocardial tissue in the infarct zone, border zone, or remote viable myocardium of adult MT1-MMP+/+ and MT1-MMP+/− mice following immunostaining with an antibody specific for denatured type I collagen (brown). Tissue was isolated 3 days post-MI. Scale bar = 10 μm. Quantification of type I collagen degradation activity at baseline, 3 days, or 28 days post-MI for MT1-MMP+/+ and MT1-MMP+/− mice in the three myocardial zones (AU, arbitrary units; mean ± SEM). *P < 0.05 compared to baseline within genotypes; †P < 0.05 compared to WT heart at the respective time points. B: Sirius Red staining of interstitial collagens using light microscopy and circularly polarized light (insets) in the infarct, border zone, and remote viable myocardium from adult MT1-MMP+/+ and MT1-MMP+/− mice 28 days post-MI. Scale bar = 10 μm. Quantitative assessment of collagen volume content using digital analysis of Sirius Red–stained sections imaged with circularly polarized light; total collagen content in tissue by hydroxyproline analysis; and relative thick and thin collagen fibril content using hue analysis of Sirius Red–stained sections in the MI zone and remote myocardium in MT1-MMP+/+ and MT1-MMP+/− mice at 28-day postinfarction (mean ± SEM). *P < 0.05 compared to baseline within genotypes; †P < 0.05 compared to WT heart. C: Micrographs of in situ hybridization in the infarct zone, border zone, and remote viable myocardium of adult MT1-MMP+/+ and MT1-MMP+/− mice hearts using standard light microscopy with darkfield imaging of the type I collagen mRNA antisense probe 3 days post-MI. Scale bar = 10 μm. Quantification of type I collagen probe signal at baseline, 3 days, or 28 days post-MI for MT1-MMP+/+ and MT1-MMP+/− mice in the three myocardial zones (mean ± SEM). *P < 0.05.
Regulation of Collagen Degradation by MT1-MMP in Cardiac Fibroblasts
Current evidence suggests that cardiac fibroblasts and myofibroblasts play a central role in remodeling of the cardiac interstitial matrix.71–73 Hence, cardiac fibroblasts were isolated from MT1-MMP+/+, MT1-MMP+/−, and MT1-MMP−/− myocardial tissues and their relative type I collagenolytic activity assessed in vitro. As determined by RNA microarray analysis, these cells express vimentin and α-smooth muscle actin at comparable levels, with no desmin expression detected (data not shown). First, a suspension of cardiac fibroblasts was allowed to adhere in the center of a three-dimensional sheet of type I collagen fibrils. After culturing the fibroblasts in the presence of platelet-derived growth factor BB (PDGF-BB) for 5 days, the cells were lysed and the residual collagen film stained with Coomassie Blue. As shown in Figure 7A, MT1-MMP+/− fibroblasts degraded qualitatively less subjacent type I collagen than WT fibroblasts, whereas MT1-MMP−/− fibroblasts did not display any detectable type I collagenolytic activity. Next, to quantitatively assess MT1-MMP–dependent type I collagenolysis, PDGF-BB–stimulated fibroblasts were cultured atop a bed of Alexa Fluor-594–labeled type I collagen and collagen degradation monitored by confocal laser microscopy. WT cells degraded the subjacent type I collagen film (visualized as dark “holes”) of variable two-dimensional areas via a process that was abolished in MT1-MMP−/− fibroblasts (Figure 7, B and C). Although MT1-MMP+/− cells retained significant collagenolytic activity relative to MT1-MMP–null cells, the heterozygous cardiac fibroblasts generated significantly fewer degradative zones with smaller average two-dimensional area, reflecting less efficient type I collagenolysis (Figure 7, B and C). Thus, in a fashion consistent with the enhanced preservation of the type I collagen network observed post-MI in MT1-MMP+/− mice, these results define an essential role for MT1-MMP in regulating cardiac fibroblast–mediated type I collagenolysis.
Figure 7.
MT1-MMP–dependent collagen degradation in isolated cardiac fibroblasts. A: Collagenolytic activity of PDGF-BB–stimulated (50 ng/mL) MT1-MMP+/+, MT1-MMP+/−, or MT1-MMP–null cardiac fibroblasts seeded atop type I collagen gels. Zones of collagenolytic activity are visualized after Coomassie Blue staining following a 5 days culture period. Hydroxyproline release was quantified after a 5-day culture period (n = 3; mean ± SEM) *P < 0.05. B: Isolated cardiac fibroblasts from WT, MT1-MMP heterozygote, and MT1-MMP–deficient cardiac fibroblasts were cultured atop TRITC-labeled type I collagen gel films (100 μg/cm2) for 7 days in the presence of PDGF-BB (50 ng/mL) and visualized with Alexa Fluor-488 phalloidin with fluorescent images captured by laser scanning confocal microscopy. Scale bar = 10 μm. C: Quantification of the number and size of degradative zones per high-powered field formed in vitro by cardiac fibroblasts isolated from MT1-MMP+/+, MT1-MMP+/−, or MT1-MMP−/− mice as described in (B). The number and size of degradative zones was determined in five randomly selected fields.
Discussion
Following MI, the collagenous fabric of the myocardium undergoes extensive remodeling, both acutely and chronically, as wound healing programs are initiated to remove damaged tissues and viable portions of the injured heart mount adaptive hypertrophic processes in an effort to maintain mechanical function.6,7,74,75 As a consequence of these matrix remodeling events, the alterations that occur in collagen synthesis, degradation, and organization in the post-MI state ultimately precipitate cardiac dysfunction.2,5–7 Despite the wealth of evidence that collagenolytic MMPs are expressed at increased levels in the diseased myocardium,15,76–78 and that cardiac function can worsen or improve in tandem with respective increases or decreases in MMP activity,13,23–26,28,68,79,80 the identity of the collagenolytic MMP(s) responsible for these effects has remained the subject of speculation. From a developmental perspective, secreted collagenases have long been posited to play dominant roles in regulating type I collagen turnover in the in vivo setting.16,35,37 More recently, this proposition has undergone revision as studies of MMP-8−/−, MMP-13−/−, MMP-2−/−, or MMP-9−/− mice have demonstrated that each of the respective knockout animals are viable and fertile, with only subtle defects in ECM remodeling.35,44–46,81 By contrast, mounting evidence indicates that MT1-MMP plays a central role in type I collagen turnover—a finding consistent with the fact that the null nice display multiple defects in tissue remodeling in vivo and die within weeks of birth.39,41,42 Interestingly, however, the structural/functional impact of MT1-MMP targeting on cardiac remodeling in the post-MI heart has remained largely unstudied.
To probe for collagenolytic systems activated within infarcted tissues, we first focused our efforts on culturing cardiac explants recovered from WT mice under no-flow conditions designed to recapitulate an ischemic environment.47 In response to this in vitro stress, explants up-regulated expression of MMP-9, MMP-13, and MT1-MMP—a pattern similar to that observed in vivo.18,76–78,82,83 Although MMP-8 mRNA was not detected under these conditions, this result is consistent with the fact that in vivo, MMP-8 expression is confined largely to infiltrating neutrophil populations.78 Further confirming the relevance of the model, and coincident with the expression of collagenolytic proteases, type I collagen degradation products accumulated within the explanted tissues in a process that could be blocked by MMP inhibitors. When this experimental model was extended to myocardial explants of MMP-targeted mice, only MT1-MMP deficiency ablated the collagenolytic activity of the cardiac explants to a degree similar to that observed with the pan-specific, synthetic MMP inhibitors. Our inability to implicate secreted MMPs in the collagenolytic phenotype activated in tissue explants is consistent with findings demonstrating that MMP-8, MMP-13, MMP-2, or MMP-9 display only limited collagen-degradative activity in the antiprotease-rich, plasma- or serum- replete environments that characterize the in vivo setting.35,36,84 These observations do not allow for the conclusion that secreted collagenases are uniformly inoperative in all in vivo scenarios. Rather, these enzymes are only able to circumvent the antiprotease screen under conditions in which either the local concentration of active proteases exceeds that of protease inhibitors or antiprotease function is dissipated as a consequence of oxidative or proteolytic inactivation.35,84 By contrast, MT1-MMP, which is expressed at the cell surface in a membrane-tethered, active form, retains full collagenolytic activity at the cell–matrix interface even in the presence of high concentrations of antiproteases.35,36,38
Given the morbid status of MT1-MMP–null mice, in vivo studies designed to interrogate the role of MT1-MMP in the post-MI state were limited to the use of mice heterozygous for an MT1-MMP–null allele. Although MT1-MMP+/− mice are phenotypically normal, recent studies have demonstrated that these animals enjoy a protected status when challenged by pathological injuries ranging from intimal hyperplasia to high-fat diet–induced obesity.49,61 With regard to cardiovascular function, we now find that MT1-MMP+/− mice also display a significant post-MI survival benefit while better preserving myocardial structure and function. Reductions in post-MI LV dilation and dysfunction along with increased cardiac performance in MT1-MMP+/− mice each correlated closely with increases in local type I collagen content as a consequence of decreased interstitial collagenolysis. These findings do, however, contrast with those reported recently by Spinale and colleagues68–70 wherein MT1-MMP has been proposed to regulate post-MI fibrotic responses within the myocardium by controlling TGF-β1 signaling. In these authors most recent work, MT1-MMP+/− mice were likewise noted to display a post-MI protected status, but this correlated with a decrease—rather than the increase observed in our report—in type I collagen content within the infarct zone as assessed by Sirius Red staining.70 As opposed to the studies presented herein, although collagen volume fractions were determined by morphometry, neither local type I collagen degradation nor total collagen hydroxyproline content was quantified, and the bulk of the effect observed regarding the decreased collagen content of MT1-MMP+/− infarct zones appears to be attributed to the lower collagen content of the heterozygote hearts before infarction—an effect that we were unable to verify.70 Furthermore, although Zavadzkas et al70 postulated that the decreased type I collagen content observed in the infarct zone of MT1-MMP+/− mice arose as a consequence of a local decrease in MT1-MMP–dependent TGF-β activation, a number of caveats apply. First, the assumed decrease in TGF-β activation is based on the ability of myocardial extracts to hydrolyze a synthetic peptide whose sequence includes a previously identified MT1-MMP cleavage site in the TGF-β latency-binding protein, LTBP-1.70 The in situ cleavage of LTBP-1 in MT1-MMP+/+ or MT1-MMP+/− mice was not actually monitored in their studies.70 Second, this issue notwithstanding, although others have demonstrated previously that MT1-MMP can release latent TGF-β1 from ECM-bound sites as a consequence of hydrolyzing TGF-β latent-binding proteins,85 the ability of MT1-MMP to participate in the activation of the solubilized, but still latent, TGF-β1 remains unclear. Third, though data are presented in support of decreased TGF-β signaling in the postinfarct MT1-MMP+/− myocardium (as assessed by Smad3 phosphorylation), total Smad3 levels were inexplicably reduced in the cardiac tissues harvested from the heterozygote mice.70 Because decreased levels of Smad3 are frequently observed as a consequence of increased TGF-β signaling,86 the almost complete absence of Smad3 in post-MI tissues harvested from MT1-MMP+/− mice may indicate that other mechanisms are operative here. Finally, type I collagen mRNA levels (which one would predict to be decreased as a consequence of diminished TGF-β signaling11) were not monitored.70 As noted, we were unable to detect changes in local type I collagen expression post-MI in MT1-MMP+/+ versus MT1-MMP+/− mice, suggesting that the observed increased collagen in the infarct zone of MT1-MMP+/− mice 28 days post-MI likely results from attenuated proteolysis as a consequence of 50% reduction in the MT1-MMP gene dose. Indeed, preliminary analyses of MT1-MMP+/+ and MT1-MMP−/− gene expression in cardiac fibroblasts cultured in three-dimensional type I collagen hydrogels have failed to detect differences in TGF-β–related signaling pathways or the expression of downstream TGF-β targets (G. Koenig, F.S., and S.J.W., unpublished data). Conclusions similar to our own were also reached in a recent study by Kandalam et al27 wherein postinfarct remodeling of fibrillar collagen was monitored in mice harboring an inactivating mutation in TIMP2, the major inhibitor of MT1-MMP in vivo. In these studies, TIMP2 deficiency resulted in an increase in MT1-MMP activity within the infarct zone, whereas local fibrillar collagen content was decreased.27 Even so, given the range of potential MT1-MMP targets,87 it will likely prove difficult, if not impossible, to identify a single target for the protease in the infarcted myocardium. Based on the observation that transgenic mice harboring knockin type I collagen mutations (r/r collagen) that render the molecule resistant to collagenolytic attack are not protected from pathological post-MI interstitial remodeling, Lindsey et al88 concluded that pathological activity attributed to cardiac MMPs arises as a function of a substrate repertoire that lies outside of type I collagen per se. However, this conclusion can only be cautiously embraced as we have found that r/r collagen is readily degraded by mouse fibroblasts in vitro as well as in vivo (F.S. and S.J.W., unpublished data). Nevertheless, as type I collagen plays an unchallenged role in defining cardiac structure and function,1,2,4–7 it seems likely that the collagenous matrix remains a dominant MT1-MMP–targeted substrate.
Independent of the identification of the critical MT1-MMP substrates in the damaged myocardium, caution must also be exercised in attributing all changes in ECM remodeling to the ability of MT1-MMP to function as a direct-acting, proteolytic effector. In this regard, MT1-MMP–dependent effects are frequently attributed to its ability to directly or indirectly catalyze the processing of MMP-2, MMP-13, or MMP-9 zymogens to their active forms.62,63,89,90 Previous studies have demonstrated that MMP-2−/− as well as MMP-9−/− mice are also protected—to varying degrees and via distinct mechanisms—from postinfarct cardiac dysfunction.30–32 Recently, it was reported that an MMP-2–null status afforded transgenic mice with a survival advantage following infarct induction by modulating macrophage accumulation and the clearance of necrotic debris from damaged tissues.32 Though long-term improvements in cardiac function were not detected in this study, other reports suggest that left ventricular dysfunction is blunted in MMP-2−/− mice, but without changes in collagen content.31 MMP-9−/− mice may also show improvements in cardiac function following coronary artery ligation, but in these animals, type I collagen content decreased in the knockout cohort, whereas other investigators have documented increases in post-MI angiogenic responses.30,91 Clearly, these studies indicate that secreted MMPs also impact the post-MI remodeling program, but that the underlying mechanisms are complex and unlikely to reflect direct changes in type I collagen degradation or remodeling. Rather, these protective effects are more likely linked to the ability of MMP-2 and MMP-9 to modulate growth factor release, the release of bioactive ECM fragments or the local activation/inactivation of chemokines and growth factors.16,92 Nevertheless, despite potential roles for MMP-2 and MMP-9 in postinfarct remodeling, we noted that cardiac tissues recovered from MT1-MMP+/− mice express levels of processed MMP-2 and MMP-9 similar to those observed in WT littermates. As such, we posit that the protective effects exerted by the MT1-MMP heterozygous state manifest themselves as a direct consequence of MT1-MMP activity alone.
The multiplicity of cell types capable of generating MT1-MMP in vivo complicates efforts to assign a particular cell type as the primary effector of collagenolytic activity in the postinfarct myocardium. Cardiomyocytes, endothelial cells, and cardiac fibroblasts are all able to generate MT1-MMP.40,78,93,94 Furthermore, inflammatory cell populations, including monocytes and macrophages as well as bone marrow–derived cells (eg, bone marrow stromal/stem cells, fibroblasts, etc) that are recruited to sites of cardiac damage likely express MT1-MMP as well.64,65 In our studies, however, the replacement of the heterozygous bone marrow with transplanted WT marrow did not alter the cardiac-protective effects exerted by the MT1-MMP+/− status. Within the myocardium itself, cardiac fibroblasts seem the most likely cell type responsible for type I collagenolytic activity as these cells are the dominant effectors of type I collagen synthesis, deposition, and remodeling.71,72 Indeed, our studies of isolated cardiac fibroblasts demonstrated an absolute requirement for MT1-MMP in type I collagenolytic activity, and confirmed the predicted blunting of the collagen-degradative potential of MT1-MMP+/− cells. Although endothelial cell–derived MT1-MMP plays a critical role in angiogenesis,40,42,93 the vascularization of the infarcted myocardium was unaffected in MT1-MMP+/− mice relative to the control population. Likewise, cardiomyocytes have also been reported to express MT1-MMP,78 but its function in these cells remains to be established. In this regard, it is important to stress that cardiomyocytes are ensheathed by a basement membrane that would limit MT1-MMP access to the surrounding interstitial matrix.2 Though transgenic mice that specifically overexpress MT1-MMP in cardiomyocytes develop a number of cardiac abnormalities and display aberrant post-MI remodeling,68–70 the requirement for endogenous cardiomyocyte-derived MT1-MMP in post-MI remodeling remains to be determined. In preliminary studies, we have generated transgenic mice wherein MT1-MMP expression in cardiomyocytes has been deleted specifically, and analyses of these mice are currently underway.
A key role for MT1-MMP in precipitating acute myocardial damage as well as pathological left ventricular remodeling in the post-MI heart, coupled with the protective effect exerted by the MT1-MMP heterozygous state, raises the question of the utility of targeting the protease for therapeutic intervention. Indeed, the continued expression of MT1-MMP throughout the entire 28-day post-MI period monitored in our study supports a model wherein continued type I collagen remodeling exacerbates left ventricular dysfunction. This conclusion is consistent with prior studies using pan-specific MMP inhibitors to reduce post-MI left ventricular remodeling and improve cardiac function.21–24 However, despite complementary reports describing increased levels of MT1-MMP following MI, these results contrast with the lack of benefit of an MMP inhibitor given post-MI in human trials.95 Nevertheless, interpretation of these results are complicated by the fact that the dosing of MMP inhibitors used in these studies appear to fall below those required to inhibit MT1-MMP activity.24,96 Furthermore, as the mammalian MMP family contains over 20 members whose wide-ranging activities can both promote and inhibit myriad cell functions,16,97 the use of pan-specific MMP inhibitors further obfuscates attempts to gauge in vivo impact. Given these caveats, many investigators in the field have concluded that targeting specific MMPs, such as MT1-MMP, in the in vivo setting may not be possible. Interestingly, this hurdle may have been negotiated with the more recent generation of humanized monoclonal antibodies that specifically inhibit MT1-MMP catalytic activity in vitro as well as in vivo.98 The availability of this new class of MT1-MMP–specific inhibitors could provide investigators with new tools to gauge the clinical utility of targeted MT1-MMP inhibition in conditions associated with maladaptive myocardial remodeling.
Footnotes
Supported by NIH grants 5R01CA88308 (S.J.W.), 5R01CA71699 (S.J.W.), and R01HL09338 (S.M.D.), as well as an NIH/National Heart, Lung, and Blood Institute T-32 Postdoctoral Fellowship (G.C.K.).
G.C.K. and R.G.R. contributed equally to this work.
Supplemental material for this article can be found at http://ajp.ampathol.org or at doi: 10.1016/j.ajpath.2012.01.022.
Current address of G.C.K., Henry Ford Hospital and Wayne State University, Detroit, Michigan; of R.G.R., Children's Hospital Boston, Boston, Massachusetts; of J.J.A., Washington University School of Medicine, St. Louis, Missouri; and of K.R.C, University Hospitals, Cleveland, Ohio.
Supplementary data
Diastolic parameters post-myocardial infarction (MI). Isovolumetric relaxation time (A) and E/A ratio (B) in MT1-MMP+/− mice and wild-type littermates (mean ± SEM).
Covariable parameters post-myocardial infarction (MI). Heart rate (A) and systemic vascular resistance (SVR) (B) in MT1-MMP+/− mice and wild-type littermates (mean ± SEM).
References
- 1.Borg T.K., Caulfield J.B. The collagen matrix of the heart. Fed Proc. 1981;40:2037–2041. [PubMed] [Google Scholar]
- 2.Weber K.T. Cardiac interstitium in health and disease: the fibrillar collagen network. J Am Coll Cardiol. 1989;13:1637–1652. doi: 10.1016/0735-1097(89)90360-4. [DOI] [PubMed] [Google Scholar]
- 3.Berk B.C., Fujiwara K., Lehoux S. ECM remodeling in hypertensive heart disease. J Clin Invest. 2007;117:568–575. doi: 10.1172/JCI31044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fomovsky G.M., Thomopoulos S., Holmes J.W. Contribution of extracellular matrix to the mechanical properties of the heart. J Mol Cell Cardiol. 2010;48:490–496. doi: 10.1016/j.yjmcc.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weber K.T., Sun Y., Tyagi S.C., Cleutjens J.P. Collagen network of the myocardium: function, structural remodeling and regulatory mechanisms. J Mol Cell Cardiol. 1994;26:279–292. doi: 10.1006/jmcc.1994.1036. [DOI] [PubMed] [Google Scholar]
- 6.Zamilpa R., Lindsey M.L. Extracellular matrix turnover and signaling during cardiac remodeling following MI: causes and consequences. J Mol Cell Cardiol. 2010;48:558–563. doi: 10.1016/j.yjmcc.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gallagher G.L., Jackson C.J., Hunyor S.N. Myocardial extracellular matrix remodeling in ischemic heart failure. Front Biosci. 2007;12:1410–1419. doi: 10.2741/2157. [DOI] [PubMed] [Google Scholar]
- 8.Pfeffer M.A., Braunwald E. Ventricular remodeling after myocardial infarction: Experimental observations and clinical implications. Circulation. 1990;81:1161–1172. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- 9.Frangogiannis N.G., Smith C.W., Entman M.L. The inflammatory response in myocardial infarction. Cardiovasc Res. 2002;53:31–47. doi: 10.1016/s0008-6363(01)00434-5. [DOI] [PubMed] [Google Scholar]
- 10.Dobaczewski M., Gonzalez-Quesada C., Frangogiannis N.G. The extracellular matrix as a modulator of the inflammatory and reparative response following myocardial infarction. J Mol Cell Cardiol. 2010;48:504–511. doi: 10.1016/j.yjmcc.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jugdutt B.I. Ventricular remodeling after infarction and the extracellular collagen matrix: when is enough enough? Circulation. 2003;108:1395–1403. doi: 10.1161/01.CIR.0000085658.98621.49. [DOI] [PubMed] [Google Scholar]
- 12.Vilahur G., Juan-Babot O., Pena E., Onate B., Casani L., Badimon L. Molecular and cellular mechanisms involved in cardiac remodeling after acute myocardial infarction. J Mol Cell Cardiol. 2011;50:522–533. doi: 10.1016/j.yjmcc.2010.12.021. [DOI] [PubMed] [Google Scholar]
- 13.Mukherjee R., Brinsa T.A., Dowdy K.B., Scott A.A., Baskin J.M., Deschamps A.M., Lowry A.S., Escobar G.P., Lucas D.G., Yarbrough W.M., Zile M.R., Spinale F.G. Myocardial infarct expansion and matrix metalloproteinase inhibition. Circulation. 2003;107:618–625. doi: 10.1161/01.cir.0000046449.36178.00. [DOI] [PubMed] [Google Scholar]
- 14.Apple K.A., Yarbrough W.M., Mukherjee R., Deschamps A.M., Escobar P.G., Mingoia J.T., Sample J.A., Hendrick J.W., Dowdy K.B., McLean J.E., Stroud R.E., O'Neill T.P., Spinale F.G. Selective targeting of matrix metalloproteinase inhibition in post-infarction myocardial remodeling. J Cardiovasc Pharmacol. 2006;47:228–235. doi: 10.1097/01.fjc.0000200989.23987.b8. [DOI] [PubMed] [Google Scholar]
- 15.Spinale F.G. Myocardial matrix remodeling and the matrix metalloproteinases: influence on cardiac form and function. Physiol Rev. 2007;87:1285–1342. doi: 10.1152/physrev.00012.2007. [DOI] [PubMed] [Google Scholar]
- 16.Page-McCaw A., Ewald A.J., Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson E.M., Spinale F.G. Myocardial remodelling and matrix metalloproteinases in heart failure: turmoil within the interstitium. Ann Med. 2001;33:623–634. doi: 10.3109/07853890109002108. [DOI] [PubMed] [Google Scholar]
- 18.Deschamps A.M., Yarbrough W.M., Squires C.E., Allen R.A., McClister D.M., Dowdy K.B., McLean J.E., Mingoia J.T., Sample J.A., Mukherjee R., Spinale F.G. Trafficking of the membrane type-1 matrix metalloproteinase in ischemia and reperfusion: relation to interstitial membrane type-1 matrix metalloproteinase activity. Circulation. 2005;111:1166–1174. doi: 10.1161/01.CIR.0000157149.71297.3A. [DOI] [PubMed] [Google Scholar]
- 19.Su H., Spinale F.G., Dobrucki L.W., Song J., Hua J., Sweterlitsch S., Dione D.P., Cavaliere P., Chow C., Bourke B.N., Hu X.Y., Azure M., Yalamanchili P., Liu R., Cheesman E.H., Robinson S., Edwards D.S., Sinusas A.J. Noninvasive targeted imaging of matrix metalloproteinase activation in a murine model of postinfarction remodeling. Circulation. 2005;112:3157–3167. doi: 10.1161/CIRCULATIONAHA.105.583021. [DOI] [PubMed] [Google Scholar]
- 20.Webb C.S., Bonnema D.D., Ahmed S.H., Leonardi A.H., McClure C.D., Clark L.L., Stroud R.E., Corn W.C., Finklea L., Zile M.R., Spinale F.G. Specific temporal profile of matrix metalloproteinase release occurs in patients after myocardial infarction: relation to left ventricular remodeling. Circulation. 2006;114:1020–1027. doi: 10.1161/CIRCULATIONAHA.105.600353. [DOI] [PubMed] [Google Scholar]
- 21.Rohde L.E., Ducharme A., Arroyo L.H., Aikawa M., Sukhova G.H., Lopez-Anaya A., McClure K.F., Mitchell P.G., Libby P., Lee R.T. Matrix metalloproteinase inhibition attenuates early left ventricular enlargement after experimental myocardial infarction in mice. Circulation. 1999;99:3063–3070. doi: 10.1161/01.cir.99.23.3063. [DOI] [PubMed] [Google Scholar]
- 22.Lindsey M.L., Gannon J., Aikawa M., Schoen F.J., Rabkin E., Lopresti-Morrow L., Crawford J., Black S., Libby P., Mitchell P.G., Lee R.T. Selective matrix metalloproteinase inhibition reduces left ventricular remodeling but does not inhibit angiogenesis after myocardial infarction. Circulation. 2002;105:753–758. doi: 10.1161/hc0602.103674. [DOI] [PubMed] [Google Scholar]
- 23.Villarreal F.J., Griffin M., Omens J., Dillmann W., Nguyen J., Covell J. Early short-term treatment with doxycycline modulates postinfarction left ventricular remodeling. Circulation. 2003;108:1487–1492. doi: 10.1161/01.CIR.0000089090.05757.34. [DOI] [PubMed] [Google Scholar]
- 24.Yarbrough W.M., Mukherjee R., Escobar G.P., Mingoia J.T., Sample J.A., Hendrick J.W., Dowdy K.B., McLean J.E., Lowry A.S., O'Neill T.P., Spinale F.G. Selective targeting and timing of matrix metalloproteinase inhibition in post-myocardial infarction remodeling. Circulation. 2003;108:1753–1759. doi: 10.1161/01.CIR.0000091087.78630.79. [DOI] [PubMed] [Google Scholar]
- 25.Creemers E.E., Davis J.N., Parkhurst A.M., Leenders P., Dowdy K.B., Hapke E., Hauet A.M., Escobar P.G., Cleutjens J.P., Smits J.F., Daemen M.J., Zile M.R., Spinale F.G. Deficiency of TIMP-1 exacerbates LV remodeling after myocardial infarction in mice. Am J Physiol Heart Circ Physiol. 2003;284:H364–H371. doi: 10.1152/ajpheart.00511.2002. [DOI] [PubMed] [Google Scholar]
- 26.Tian H., Cimini M., Fedak P.W., Altamentova S., Fazel S., Huang M.L., Weisel R.D., Li R.K. TIMP-3 deficiency accelerates cardiac remodeling after myocardial infarction. J Mol Cell Cardiol. 2007;43:733–743. doi: 10.1016/j.yjmcc.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Kandalam V., Basu R., Abraham T., Wang X., Soloway P.D., Jaworski D.M., Oudit G.Y., Kassiri Z. TIMP2 deficiency accelerates adverse post-myocardial infarction remodeling because of enhanced MT1-MMP activity despite lack of MMP2 activation. Circ Res. 2010;106:796–808. doi: 10.1161/CIRCRESAHA.109.209189. [DOI] [PubMed] [Google Scholar]
- 28.Koskivirta I., Kassiri Z., Rahkonen O., Kiviranta R., Oudit G.Y., McKee T.D., Kyto V., Saraste A., Jokinen E., Liu P.P., Vuorio E., Khokha R. Mice with tissue inhibitor of metalloproteinases 4 (Timp4) deletion succumb to induced myocardial infarction but not to cardiac pressure overload. J Biol Chem. 2010;285:24487–24493. doi: 10.1074/jbc.M110.136820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ikonomidis J.S., Hendrick J.W., Parkhurst A.M., Herron A.R., Escobar P.G., Dowdy K.B., Stroud R.E., Hapke E., Zile M.R., Spinale F.G. Accelerated LV remodeling after myocardial infarction in TIMP-1-deficient mice: effects of exogenous MMP inhibition. Am J Physiol Heart Circ Physiol. 2005;288:H149–H158. doi: 10.1152/ajpheart.00370.2004. [DOI] [PubMed] [Google Scholar]
- 30.Ducharme A., Frantz S., Aikawa M., Rabkin E., Lindsey M., Rohde L.E., Schoen F.J., Kelly R.A., Werb Z., Libby P., Lee R.T. Targeted deletion of matrix metalloproteinase-9 attenuates left ventricular enlargement and collagen accumulation after experimental myocardial infarction. J Clin Invest. 2000;106:55–62. doi: 10.1172/JCI8768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hayashidani S., Tsutsui H., Ikeuchi M., Shiomi T., Matsusaka H., Kubota T., Imanaka-Yoshida K., Itoh T., Takeshita A. Targeted deletion of MMP-2 attenuates early LV rupture and late remodeling after experimental myocardial infarction. Am J Physiol Heart Circ Physiol. 2003;285:H1229–H1235. doi: 10.1152/ajpheart.00207.2003. [DOI] [PubMed] [Google Scholar]
- 32.Matsumura S., Iwanaga S., Mochizuki S., Okamoto H., Ogawa S., Okada Y. Targeted deletion or pharmacological inhibition of MMP-2 prevents cardiac rupture after myocardial infarction in mice. J Clin Invest. 2005;115:599–609. doi: 10.1172/JCI22304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heymans S., Luttun A., Nuyens D., Theilmeier G., Creemers E., Moons L., Dyspersin G.D., Cleutjens J.P., Shipley M., Angellilo A., Levi M., Nube O., Baker A., Keshet E., Lupu F., Herbert J.M., Smits J.F., Shapiro S.D., Baes M., Borgers M., Collen D., Daemen M.J., Carmeliet P. Inhibition of plasminogen activators or matrix metalloproteinases prevents cardiac rupture but impairs therapeutic angiogenesis and causes cardiac failure. Nat Med. 1999;5:1135–1142. doi: 10.1038/13459. [DOI] [PubMed] [Google Scholar]
- 34.Kim H.E., Dalal S.S., Young E., Legato M.J., Weisfeldt M.L., D'Armiento J. Disruption of the myocardial extracellular matrix leads to cardiac dysfunction. J Clin Invest. 2000;106:857–866. doi: 10.1172/JCI8040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sabeh F., Li X.Y., Saunders T.L., Rowe R.G., Weiss S.J. Secreted versus membrane-anchored collagenases: relative roles in fibroblast-dependent collagenolysis and invasion. J Biol Chem. 2009;284:23001–23011. doi: 10.1074/jbc.M109.002808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hotary K., Allen E., Punturieri A., Yana I., Weiss S.J. Regulation of cell invasion and morphogenesis in a three-dimensional type I collagen matrix by membrane-type matrix metalloproteinases 1, 2, and 3. J Cell Biol. 2000;149:1309–1323. doi: 10.1083/jcb.149.6.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sabeh F., Ota I., Holmbeck K., Birkedal-Hansen H., Soloway P., Balbin M., Lopez-Otin C., Shapiro S., Inada M., Krane S., Allen E., Chung D., Weiss S.J. Tumor cell traffic through the extracellular matrix is controlled by the membrane-anchored collagenase MT1-MMP. J Cell Biol. 2004;167:769–781. doi: 10.1083/jcb.200408028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li X.Y., Ota I., Yana I., Sabeh F., Weiss S.J. Molecular dissection of the structural machinery underlying the tissue-invasive activity of membrane type-1 matrix metalloproteinase. Mol Biol Cell. 2008;19:3221–3233. doi: 10.1091/mbc.E08-01-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holmbeck K., Bianco P., Caterina J., Yamada S., Kromer M., Kuznetsov S.A., Mankani M., Robey P.G., Poole A.R., Pidoux I., Ward J.M., Birkedal-Hansen H. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 1999;99:81–92. doi: 10.1016/s0092-8674(00)80064-1. [DOI] [PubMed] [Google Scholar]
- 40.Chun T.H., Sabeh F., Ota I., Murphy H., McDonagh K.T., Holmbeck K., Birkedal-Hansen H., Allen E.D., Weiss S.J. MT1-MMP-dependent neovessel formation within the confines of the three-dimensional extracellular matrix. J Cell Biol. 2004;167:757–767. doi: 10.1083/jcb.200405001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chun T.H., Hotary K.B., Sabeh F., Saltiel A.R., Allen E.D., Weiss S.J. A pericellular collagenase directs the 3-dimensional development of white adipose tissue. Cell. 2006;125:577–591. doi: 10.1016/j.cell.2006.02.050. [DOI] [PubMed] [Google Scholar]
- 42.Zhou Z., Apte S.S., Soininen R., Cao R., Baaklini G.Y., Rauser R.W., Wang J., Cao Y., Tryggvason K. Impaired endochondral ossification and angiogenesis in mice deficient in membrane-type matrix metalloproteinase I. Proc Natl Acad Sci U S A. 2000;97:4052–4057. doi: 10.1073/pnas.060037197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuzuya M., Kanda S., Sasaki T., Tamaya-Mori N., Cheng X.W., Itoh T., Itohara S., Iguchi A. Deficiency of gelatinase a suppresses smooth muscle cell invasion and development of experimental intimal hyperplasia. Circulation. 2003;108:1375–1381. doi: 10.1161/01.CIR.0000086463.15540.3C. [DOI] [PubMed] [Google Scholar]
- 44.Balbin M., Fueyo A., Tester A.M., Pendas A.M., Pitiot A.S., Astudillo A., Overall C.M., Shapiro S.D., Lopez-Otin C. Loss of collagenase-2 confers increased skin tumor susceptibility to male mice. Nat Genet. 2003;35:252–257. doi: 10.1038/ng1249. [DOI] [PubMed] [Google Scholar]
- 45.Liu Z., Shipley J.M., Vu T.H., Zhou X., Diaz L.A., Werb Z., Senior R.M. Gelatinase B-deficient mice are resistant to experimental bullous pemphigoid. J Exp Med. 1998;188:475–482. doi: 10.1084/jem.188.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Inada M., Wang Y., Byrne M.H., Rahman M.U., Miyaura C., Lopez-Otin C., Krane S.M. Critical roles for collagenase-3 (Mmp13) in development of growth plate cartilage and in endochondral ossification. Proc Natl Acad Sci U S A. 2004;101:17192–17197. doi: 10.1073/pnas.0407788101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang J.G., Ghosh S., Ockleford C.D., Galinanes M. Characterization of an in vitro model for the study of the short and prolonged effects of myocardial ischaemia and reperfusion in man. Clin Sci (Lond) 2000;99:443–453. [PubMed] [Google Scholar]
- 48.Creemers L.B., Jansen D.C., van Veen-Reurings A., van den Bos T., Everts V. Microassay for the assessment of low levels of hydroxyproline. Biotechniques. 1997;22:656–658. doi: 10.2144/97224bm19. [DOI] [PubMed] [Google Scholar]
- 49.Filippov S., Koenig G.C., Chun T.H., Hotary K.B., Ota I., Bugge T.H., Roberts J.D., Fay W.P., Birkedal-Hansen H., Holmbeck K., Sabeh F., Allen E.D., Weiss S.J. MT1-matrix metalloproteinase directs arterial wall invasion and neointima formation by vascular smooth muscle cells. J Exp Med. 2005;202:663–671. doi: 10.1084/jem.20050607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu J., Rodriguez D., Petitclerc E., Kim J.J., Hangai M., Moon Y.S., Davis G.E., Brooks P.C. Proteolytic exposure of a cryptic site within collagen type IV is required for angiogenesis and tumor growth in vivo. J Cell Biol. 2001;154:1069–1079. doi: 10.1083/jcb.200103111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Day S.M., Westfall M.V., Fomicheva E.V., Hoyer K., Yasuda S., La Cross N.C., D'Alecy L.G., Ingwall J.S., Metzger J.M. Histidine button engineered into cardiac troponin I protects the ischemic and failing heart. Nat Med. 2006;12:181–189. doi: 10.1038/nm1346. [DOI] [PubMed] [Google Scholar]
- 52.Day S.M., Reeve J.L., Pedersen B., Farris D.M., Myers D.D., Im M., Wakefield T.W., Mackman N., Fay W.P. Macrovascular thrombosis is driven by tissue factor derived primarily from the blood vessel wall. Blood. 2005;105:192–198. doi: 10.1182/blood-2004-06-2225. [DOI] [PubMed] [Google Scholar]
- 53.Namba T., Tsutsui H., Tagawa H., Takahashi M., Saito K., Kozai T., Usui M., Imanaka-Yoshida K., Imaizumi T., Takeshita A. Regulation of fibrillar collagen gene expression and protein accumulation in volume-overloaded cardiac hypertrophy. Circulation. 1997;95:2448–2454. doi: 10.1161/01.cir.95.10.2448. [DOI] [PubMed] [Google Scholar]
- 54.Nicoletti A., Heudes D., Hinglais N., Appay M.D., Philippe M., Sassy-Prigent C., Bariety J., Michel J.B. Left ventricular fibrosis in renovascular hypertensive rats: Effect of losartan and spironolactone. Hypertension. 1995;26:101–111. doi: 10.1161/01.hyp.26.1.101. [DOI] [PubMed] [Google Scholar]
- 55.Bradshaw A.D., Baicu C.F., Rentz T.J., Van Laer A.O., Bonnema D.D., Zile M.R. Age-dependent alterations in fibrillar collagen content and myocardial diastolic function: role of SPARC in post-synthetic procollagen processing. Am J Physiol Heart Circ Physiol. 2010;298:H614–H622. doi: 10.1152/ajpheart.00474.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yano T., Miura T., Whittaker P., Miki T., Sakamoto J., Nakamura Y., Ichikawa Y., Ikeda Y., Kobayashi H., Ohori K., Shimamoto K. Macrophage colony-stimulating factor treatment after myocardial infarction attenuates left ventricular dysfunction by accelerating infarct repair. J Am Coll Cardiol. 2006;47:626–634. doi: 10.1016/j.jacc.2005.09.037. [DOI] [PubMed] [Google Scholar]
- 57.Hotary K.B., Allen E.D., Brooks P.C., Datta N.S., Long M.W., Weiss S.J. Membrane type I matrix metalloproteinase usurps tumor growth control imposed by the three-dimensional extracellular matrix. Cell. 2003;114:33–45. doi: 10.1016/s0092-8674(03)00513-0. [DOI] [PubMed] [Google Scholar]
- 58.Atkinson J.J., Toennies H.M., Holmbeck K., Senior R.M. Membrane type 1 matrix metalloproteinase is necessary for distal airway epithelial repair and keratinocyte growth factor receptor expression after acute injury. Am J Physiol Lung Cell Mol Physiol. 2007;293:L600–L610. doi: 10.1152/ajplung.00028.2007. [DOI] [PubMed] [Google Scholar]
- 59.Hotary K.B., Yana I., Sabeh F., Li X.Y., Holmbeck K., Birkedal-Hansen H., Allen E.D., Hiraoka N., Weiss S.J. Matrix metalloproteinases (MMPs) regulate fibrin-invasive activity via MT1-MMP-dependent and -independent processes. J Exp Med. 2002;195:295–308. doi: 10.1084/jem.20010815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Netzel-Arnett S., Mitola D.J., Yamada S.S., Chrysovergis K., Holmbeck K., Birkedal-Hansen H., Bugge T.H. Collagen dissolution by keratinocytes requires cell surface plasminogen activation and matrix metalloproteinase activity. J Biol Chem. 2002;277:45154–45161. doi: 10.1074/jbc.M206354200. [DOI] [PubMed] [Google Scholar]
- 61.Chun T.H., Inoue M., Morisaki H., Yamanaka I., Miyamoto Y., Okamura T., Sato-Kusubata K., Weiss S.J. Genetic link between obesity and MMP14-dependent adipogenic collagen turnover. Diabetes. 2010;59:2484–2494. doi: 10.2337/db10-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sato H., Takino T., Kinoshita T., Imai K., Okada Y., Stetler Stevenson W.G., Seiki M. Cell surface binding and activation of gelatinase A induced by expression of membrane-type-1-matrix metalloproteinase (MT1-MMP) FEBS Lett. 1996;385:238–240. doi: 10.1016/0014-5793(96)00389-4. [DOI] [PubMed] [Google Scholar]
- 63.Toth M., Chvyrkova I., Bernardo M.M., Hernandez-Barrantes S., Fridman R. Pro-MMP-9 activation by the MT1-MMP/MMP-2 axis and MMP-3: role of TIMP-2 and plasma membranes. Biochem Biophys Res Commun. 2003;308:386–395. doi: 10.1016/s0006-291x(03)01405-0. [DOI] [PubMed] [Google Scholar]
- 64.Xiong W., Knispel R., MacTaggart J., Greiner T.C., Weiss S.J., Baxter B.T. Membrane-type 1 matrix metalloproteinase regulates macrophage-dependent elastolytic activity and aneurysm formation in vivo. J Biol Chem. 2009;284:1765–1771. doi: 10.1074/jbc.M806239200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schneider F., Sukhova G.K., Aikawa M., Canner J., Gerdes N., Tang S.M., Shi G.P., Apte S.S., Libby P. Matrix-metalloproteinase-14 deficiency in bone-marrow-derived cells promotes collagen accumulation in mouse atherosclerotic plaques. Circulation. 2008;117:931–939. doi: 10.1161/CIRCULATIONAHA.107.707448. [DOI] [PubMed] [Google Scholar]
- 66.Whittaker P., Boughner D.R., Kloner R.A. Analysis of healing after myocardial infarction using polarized light microscopy. Am J Pathol. 1989;134:879–893. [PMC free article] [PubMed] [Google Scholar]
- 67.Sounni N.E., Dehne K., van Kempen L., Egeblad M., Affara N.I., Cuevas I., Wiesen J., Junankar S., Korets L., Lee J., Shen J., Morrison C.J., Overall C.M., Krane S.M., Werb Z., Boudreau N., Coussens L.M. Stromal regulation of vessel stability by MMP14 and TGFbeta. Dis Model Mech. 2010;3:317–332. doi: 10.1242/dmm.003863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Spinale F.G., Escobar G.P., Mukherjee R., Zavadzkas J.A., Saunders S.M., Jeffords L.B., Leone A.M., Beck C., Bouges S., Stroud R.E. Cardiac-restricted overexpression of membrane type-1 matrix metalloproteinase in mice: effects on myocardial remodeling with aging. Circ Heart Fail. 2009;2:351–360. doi: 10.1161/CIRCHEARTFAILURE.108.844845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Spinale F.G., Mukherjee R., Zavadzkas J.A., Koval C.N., Bouges S., Stroud R.E., Dobrucki L.W., Sinusas A.J. Cardiac restricted overexpression of membrane type-1 matrix metalloproteinase causes adverse myocardial remodeling following myocardial infarction. J Biol Chem. 2010;285:30316–30327. doi: 10.1074/jbc.M110.158196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zavadzkas J.A., Mukherjee R., Rivers W.T., Patel R.K., Meyer E.C., Black L.E., McKinney R.A., Oelsen J.M., Stroud R.E., Spinale F.G. Direct regulation of membrane type-1 matrix metalloproteinase following myocardial infarction causes changes in survival, cardiac function, and remodeling. Am J Physiol Heart Circ Physiol. 2011;301:H1656–H1666. doi: 10.1152/ajpheart.00141.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Souders C.A., Bowers S.L., Baudino T.A. Cardiac fibroblast: the renaissance cell. Circ Res. 2009;105:1164–1176. doi: 10.1161/CIRCRESAHA.109.209809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Camelliti P., Borg T.K., Kohl P. Structural and functional characterisation of cardiac fibroblasts. Cardiovasc Res. 2005;65:40–51. doi: 10.1016/j.cardiores.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 73.Zhou L., Aon M.A., Liu T., O'Rourke B. Dynamic modulation of Ca2+ sparks by mitochondrial oscillations in isolated guinea pig cardiomyocytes under oxidative stress. J Mol Cell Cardiol. 2011;51:632–639. doi: 10.1016/j.yjmcc.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van den Borne S.W., van de Schans V.A., Strzelecka A.E., Vervoort-Peters H.T., Lijnen P.M., Cleutjens J.P., Smits J.F., Daemen M.J., Janssen B.J., Blankesteijn W.M. Mouse strain determines the outcome of wound healing after myocardial infarction. Cardiovasc Res. 2009;84:273–282. doi: 10.1093/cvr/cvp207. [DOI] [PubMed] [Google Scholar]
- 75.Sutton M.G., Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101:2981–2988. doi: 10.1161/01.cir.101.25.2981. [DOI] [PubMed] [Google Scholar]
- 76.Cleutjens J.P., Kandala J.C., Guarda E., Guntaka R.V., Weber K.T. Regulation of collagen degradation in the rat myocardium after infarction. J Mol Cell Cardiol. 1995;27:1281–1292. doi: 10.1016/s0022-2828(05)82390-9. [DOI] [PubMed] [Google Scholar]
- 77.Chen J., Tung C.H., Allport J.R., Chen S., Weissleder R., Huang P.L. Near-infrared fluorescent imaging of matrix metalloproteinase activity after myocardial infarction. Circulation. 2005;111:1800–1805. doi: 10.1161/01.CIR.0000160936.91849.9F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vanhoutte D., Schellings M., Pinto Y., Heymans S. Relevance of matrix metalloproteinases and their inhibitors after myocardial infarction: a temporal and spatial window. Cardiovasc Res. 2006;69:604–613. doi: 10.1016/j.cardiores.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 79.Cox M.J., Hawkins U.A., Hoit B.D., Tyagi S.C. Attenuation of oxidative stress and remodeling by cardiac inhibitor of metalloproteinase protein transfer. Circulation. 2004;109:2123–2128. doi: 10.1161/01.CIR.0000127429.53391.78. [DOI] [PubMed] [Google Scholar]
- 80.King M.K., Coker M.L., Goldberg A., McElmurray J.H., 3rd, Gunasinghe H.R., Mukherjee R., Zile M.R., O'Neill T.P., Spinale F.G. Selective matrix metalloproteinase inhibition with developing heart failure: effects on left ventricular function and structure. Circ Res. 2003;92:177–185. doi: 10.1161/01.res.0000052312.41419.55. [DOI] [PubMed] [Google Scholar]
- 81.Itoh T., Tanioka M., Yoshida H., Yoshioka T., Nishimoto H., Itohara S. Reduced angiogenesis and tumor progression in gelatinase A-deficient mice. Cancer Res. 1998;58:1048–1051. [PubMed] [Google Scholar]
- 82.Deschamps A.M., Zavadzkas J., Murphy R.L., Koval C.N., McLean J.E., Jeffords L., Saunders S.M., Sheats N.J., Stroud R.E., Spinale F.G. Interruption of endothelin signaling modifies membrane type 1 matrix metalloproteinase activity during ischemia and reperfusion. Am J Physiol Heart Circ Physiol. 2008;294:H875–H883. doi: 10.1152/ajpheart.00918.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wilson E.M., Moainie S.L., Baskin J.M., Lowry A.S., Deschamps A.M., Mukherjee R., Guy T.S., St John-Sutton M.G., Gorman J.H., 3rd, Edmunds L.H., Jr., Gorman R.C., Spinale F.G. Region- and type-specific induction of matrix metalloproteinases in post-myocardial infarction remodeling. Circulation. 2003;107:2857–2863. doi: 10.1161/01.CIR.0000068375.40887.FA. [DOI] [PubMed] [Google Scholar]
- 84.Weiss S.J. Tissue destruction by neutrophils. N Engl J Med. 1989;320:365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- 85.Tatti O., Vehviläinen P., Lehti K., Keski-Oja J. MT1-MMP releases latent TGF-beta1 from endothelial cell extracellular matrix via proteolytic processing of LTBP-1. Exp Cell Res. 2008;314:2501–2514. doi: 10.1016/j.yexcr.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 86.Poncelet A.C., Schnaper H.W., Tan R., Liu Y., Runyan C.E. Cell phenotype-specific down-regulation of Smad3 involves decreased gene activation as well as protein degradation. J Biol Chem. 2007;282:15534–15540. doi: 10.1074/jbc.M701991200. [DOI] [PubMed] [Google Scholar]
- 87.Butler G.S., Dean R.A., Tam E.M., Overall C.M. Pharmacoproteomics of a metalloproteinase hydroxamate inhibitor in breast cancer cells: dynamics of membrane type 1 matrix metalloproteinase-mediated membrane protein shedding. Mol Cell Biol. 2008;28:4896–4914. doi: 10.1128/MCB.01775-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lindsey M.L., Yoshioka J., MacGillivray C., Muangman S., Gannon J., Verghese A., Aikawa M., Libby P., Krane S.M., Lee R.T. Effect of a cleavage-resistant collagen mutation on left ventricular remodeling. Circ Res. 2003;93:238–245. doi: 10.1161/01.RES.0000085580.45279.60. [DOI] [PubMed] [Google Scholar]
- 89.Itoh Y., Seiki M. MT1-MMP: a potent modifier of pericellular microenvironment. J Cell Physiol. 2006;206:1–8. doi: 10.1002/jcp.20431. [DOI] [PubMed] [Google Scholar]
- 90.Sato H., Takino T. Coordinate action of membrane-type matrix metalloproteinase-1 (MT1-MMP) and MMP-2 enhances pericellular proteolysis and invasion. Cancer Sci. 2010;101:843–847. doi: 10.1111/j.1349-7006.2010.01498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lindsey M.L., Escobar G.P., Dobrucki L.W., Goshorn D.K., Bouges S., Mingoia J.T., McClister D.M., Jr., Su H., Gannon J., MacGillivray C., Lee R.T., Sinusas A.J., Spinale F.G. Matrix metalloproteinase-9 gene deletion facilitates angiogenesis after myocardial infarction. Am J Physiol Heart Circ Physiol. 2006;290:H232–H239. doi: 10.1152/ajpheart.00457.2005. [DOI] [PubMed] [Google Scholar]
- 92.Rowe R.G., Weiss S.J. Navigating ECM barriers at the invasive front: the cancer cell-stroma interface. Annu Rev Cell Dev Biol. 2009;25:567–595. doi: 10.1146/annurev.cellbio.24.110707.175315. [DOI] [PubMed] [Google Scholar]
- 93.Hiraoka N., Allen E., Apel I.J., Gyetko M.R., Weiss S.J. Matrix metalloproteinases regulate neovascularization by acting as pericellular fibrinolysins. Cell. 1998;95:365–377. doi: 10.1016/s0092-8674(00)81768-7. [DOI] [PubMed] [Google Scholar]
- 94.Stawowy P., Margeta C., Kallisch H., Seidah N.G., Chretien M., Fleck E., Graf K. Regulation of matrix metalloproteinase MT1-MMP/MMP-2 in cardiac fibroblasts by TGF-beta1 involves furin-convertase. Cardiovasc Res. 2004;63:87–97. doi: 10.1016/j.cardiores.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 95.Hudson M.P., Armstrong P.W., Ruzyllo W., Brum J., Cusmano L., Krzeski P., Lyon R., Quinones M., Theroux P., Sydlowski D., Kim H.E., Garcia M.J., Jaber W.A., Weaver W.D. Effects of selective matrix metalloproteinase inhibitor (PG-116800) to prevent ventricular remodeling after myocardial infarction: results of the PREMIER (Prevention of Myocardial Infarction Early Remodeling) trial. J Am Coll Cardiol. 2006;48:15–20. doi: 10.1016/j.jacc.2006.02.055. [DOI] [PubMed] [Google Scholar]
- 96.Spinale F.G., Wilbur N.M. Matrix metalloproteinase therapy in heart failure. Curr Treat Options Cardiovasc Med. 2009;11:339–346. doi: 10.1007/s11936-009-0034-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Overall C.M., Kleifeld O. Tumour microenvironment—opinion: validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy. Nat Rev Cancer. 2006;6:227–239. doi: 10.1038/nrc1821. [DOI] [PubMed] [Google Scholar]
- 98.Devy L., Huang L., Naa L., Yanamandra N., Pieters H., Frans N., Chang E., Tao Q., Vanhove M., Lejeune A., van Gool R., Sexton D.J., Kuang G., Rank D., Hogan S., Pazmany C., Ma Y.L., Schoonbroodt S., Nixon A.E., Ladner R.C., Hoet R., Henderikx P., Tenhoor C., Rabbani S.A., Valentino M.L., Wood C.R., Dransfield D.T. Selective inhibition of matrix metalloproteinase-14 blocks tumor growth, invasion, and angiogenesis. Cancer Res. 2009;69:1517–1526. doi: 10.1158/0008-5472.CAN-08-3255. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Diastolic parameters post-myocardial infarction (MI). Isovolumetric relaxation time (A) and E/A ratio (B) in MT1-MMP+/− mice and wild-type littermates (mean ± SEM).
Covariable parameters post-myocardial infarction (MI). Heart rate (A) and systemic vascular resistance (SVR) (B) in MT1-MMP+/− mice and wild-type littermates (mean ± SEM).