Abstract
Cellular mechanisms of carotid intima-media thickening (IMT) are largely unknown. The receptor tyrosine kinase Axl is essential for function of both bone marrow (BM) and non-BM cells. We studied the mechanisms by which Axl expression in BM-derived cells (compared with non-BM-derived cells) mediates carotid IMT. Partial ligation of the left carotid artery resulted in a similar carotid blood flow reduction in Axl chimeras. Neither irradiation nor bone marrow transplantation had any effect on the 40% difference in carotid IMT between Axl genotypes. Axl-dependent survival is very important for intimal leukocytes; however, Axl expression in BM cells contributes to <30% of carotid IMT. Axl in non-BM cells has a greater effect on carotid remodeling. Expression of Axl in non-BM cells is crucial for the up-regulation of several key proinflammatory signals (eg, IL-1) in the carotid. We found that Axl is involved in immune activation of cultured smooth muscle cells and in immune heterogeneity of medial cells (measured by major histocompatibility complex class II) after carotid injury. Finally, a lack of Axl in non-BM cells increased collagen Iα expression, which may play a critical role in carotid remodeling. Our data suggest that Axl contributes to carotid remodeling not only by inhibition of apoptosis but also via regulation of immune heterogeneity of vascular cells, cytokine/chemokine expression, and extracellular matrix remodeling.
Risks of heart disease and stroke are associated with increase in carotid intima-media thickening (IMT) in humans.1 However, the cellular mechanisms of carotid IMT are largely unknown. Although it is well accepted that non-bone marrow (non-BM) cells play a central role in intima formation,2 recent studies in atherosclerotic mice showed that intimal cells are derived from multiple origins and are immunologically heterogeneous.3 Immune heterogeneity of endothelial, smooth muscle, and immune cells was documented by expression of major histocompatibility complex class II (MHC II) in the carotid neointima after balloon injury in rats.4
The TAM (Tyro3, Axl, and Mertk) family of receptor tyrosine kinases controls various cell functions.5 Two ligands activate TAM receptors: growth arrest-specific protein 6 (Gas6) and protein S.6 TAM receptors control survival and phagocytosis, as well as production of proinflammatory cytokines in innate immune cells.7 Recent data suggest that Gas6/TAM-dependent immune responses involve interactions among multiple cell types, including vascular and immune cells, in cancer development.8,9 The Gas6/Axl pathway is involved in pathogenesis of vascular diseases.10–14 In particular, Axl contributes to carotid IMT in response to low blood flow, and Axl inhibited vascular apoptosis and affected vascular inflammation during carotid artery remodeling.11 Our goal in the present study was to investigate the role of Axl expressed in BM-derived and non-BM cells in carotid IMT in response to low blood flow.
Materials and Methods
Animals
Male Axl knockout (Axl−/−) mice were obtained from our colony. B6.SJL-PtprcaPep3b/BoyJ mice purchased from the Jackson Laboratory (Bar Harbor, ME) were bred in house. After confirming that the B6.SJL-PtprcaPep3b/BoyJ (CD45.1+) mice expressed the Axl gene [with Axl wild-type (Axl+/+) littermates from our colony taken as the standard; see Supplemental Figure S1 at http://ajp.amjpathol.org], we used these B6.SJL-PtprcaPep3b/BoyJ mice as Axl+/+ (CD45.1+) for the present study. DNA isolation from tails and genotyping were performed as described previously.11 All experiments were approved by the University of Rochester Animal Care Committee and were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and with American Heart Association guidelines (8th edition).
Primary Vascular Cell Culture
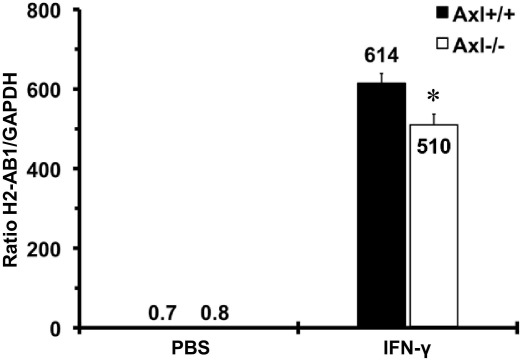
We established primary mouse aortic smooth muscle cell (MASMC) lines from Axl mice, as described previously.15 There were no differences in cell growth between MASMCs harvested from Axl+/+ and Axl−/− mice (data not shown). Mouse interferon-γ (IFN-γ; 1000 U/mL; 24 hours) was used to stimulate major histocompatibility complex class II (MHC II) expression (measured by relative expression of the mouse H2-Ab1 gene) in Axl MASMCs at passage 4, as described previously for human vascular smooth muscle cells (VSMCs).16 Control MASMCs were treated with PBS for 24 hours. mRNAs isolated from MASMCs and quantitative RT-PCR were assayed for mouse Axl, H2-Ab1, and GAPDH as described previously.17
Bone Marrow Transplantation
For detection of BM cells, we used a naturally occurring allelic variant of transmembrane CD45 glycoprotein in BM transplantation (BMT). The more common CD45.2 allele is carried by C57BL/6 mice, which is the background strain for our Axl−/− colony (CD45.2+). The CD45.1 allele is carried by B6.SJL-PtprcaPep3b/BoyJ mice (also on a C57BL/6 background). We used 5- to 7-week-old male mice for BMT. Briefly, BM cells were harvested from tibias and femurs of age-matched Axl−/− or Axl+/+ donor mice. Before BMT, recipient mice were irradiated (9.0 Gy) to ablate the host BM, using an RS2000 irradiator (Rad Source Technologies, Suwanee, GA). Recipient mice were injected with 6 × 106 donor BM cells via tail vein.
Flow Cytometry
Blood collection was done by the submandibular bleeding method without anesthesia. The ACK erythrocyte lysing buffer (Invitrogen, Carlsbad, CA) was used to lyse red blood cells. Flow cytometry analyses were performed using a six-color FACSCanto II system (BD Biosciences, San Jose, CA) and FlowJo software version 7.6.3 (Tree Star, Ashland, OR). The engraftment of the recipient BM was confirmed by analyses of the peripheral blood stained with a cocktail of CD45.1-fluorescein isothiocyanate and CD45.2-phycoerythrin antibodies (1:500; eBioscience, San Diego, CA) 6 weeks after injections.18
Carotid Artery Ligation
Upon successful BM repopulation, Axl chimeras were anesthetized with injection of ketamine (130 mg/kg i.p.) and xylazine (8.8 mg/kg i.p.) in saline (10 mL/kg) and maintained at 37°C on a heating pad. Reduction of blood flow in the left common carotid artery was achieved by ligation of external and internal branches as described previously.19 We administered flunixin (Banamine; 2.5 mg/kg, i.p.) preoperatively, and then again 24 hours later if needed to alleviate discomfort. The Axl chimeras were allowed to recover and were housed individually under specific-pathogen-free conditions with 12/12 hours light/dark cycle for 2 weeks after the surgery. An ultrasonic transit-time volume flowmeter (Transonic Systems, Ithaca, NY) was used to measure common carotid blood flow, as described previously.19
Morphometry and Immunohistochemistry
For euthanasia, all animals were anesthetized with injection of ketamine (130 mg/kg i.p.) and xylazine (8.8 mg/kg i.p.). Tissues were perfusion fixed with 10% paraformaldehyde in sodium phosphate buffer (pH 7.0), as described previously.19 Tissue processing, staining with H&E (Dako, Carpinteria, CA), and morphometry analyses (MCID image software version 6.0; Imaging Research-GE Healthcare Niagara, St. Catherine's, ON, Canada) of the 10 sections every 200 μm from carotid bifurcation were performed as described previously.19 Variations in carotid intima+media (or media) and adventitia areas were greater over a length of 2 mm in the ligated left carotid artery (LCA), compared with the contralateral right carotid artery (RCA) among Axl chimeras (see Supplemental Figures S2, B and C, and S3, B and C, at http://ajp.amjpathol.org). The compartment volumes were calculated based on lumen, intima, media, adventitia, and the external elastic lamina areas over the 1600-μm length of the carotid artery.19
Carotid cross-sections taken ∼1 mm proximal from carotid bifurcation from Axl chimeras were double-stained with biotinylated CD45.1 (1:100) and CD45.2 (1:100) antibodies (BD Pharmingen, San Diego, CA) with methyl green (Dako) counterstain. MHC II (OX6) antibody or collagen Iα (Col I) were counterstained with hematoxylin, as described previously.4,20 A high temperature (120°C) was used for antigen retrieval with Decloaker solution (pH 6.0; Biocare Medical, Concord, CA) for 25 minutes for CD45.1/CD45.2. Primary antibodies were subsequently incubated at 37°C for 60 minutes, followed by incubation with polymer MM-HRP (CD45.1+) and polymer MM-AP (CD45.2+) for 20 minutes each (MM kits; Biocare Medical). The peroxidase-binding sites (CD45.1+, MHC II+, and Col I+) were verified with 3,3′-diaminobenzidine (Dako). The alkaline-phosphatase-binding sites (CD45.2+) were validated by the Fast Red method (Vulcan Fast Red; Biocare Medical). For DNA fragmentation of apoptotic cells we used a Chemicon ApopTag peroxidase in situ apoptosis detection kit (Millipore Bioscience Research Reagents, Temecula, CA) with methyl green counterstain.11 Vascular calcification was determined by staining carotids with Alizarin Red S with fast green counterstain. Images of immunoassayed carotid sections were captured at various magnifications (×20 to ×60) with a SPOT Insight camera (Spot Imaging Systems-Diagnostic Instruments, Sterling Heights, MI) mounted on an Olympus BX41 microscope. We uniformly adjusted size and contrast of the images to meet journal guidelines (Adobe Photoshop CS3 version 10.0.1). Analyses of relative expression of the antigens in three to five mice (two to three sections/mouse) were performed using ImagePro software version 6.2.21
Quantitative RT-PCR
In a separate set of Axl chimeras (n = 3 per group of 4), carotids were harvested 2 weeks after the surgery and immediately frozen in liquid nitrogen as described previously.17 Vascular RNA was isolated with a Qiagen (Valencia, CA) RNeasy micro kit, and amplified cDNA was isolated with a NuGEN (San Carlos, CA) Ovation RNA amplification system V2.17 We assayed cDNA samples using an SABiosciences mouse inflammatory cytokines and receptors PCR Array (PAMM-011; Qiagen, Valencia, CA). Expression of the three housekeeping genes [β-glucuronidase (Gusb), hypoxanthine guanine phosphoribosyl transferase 1 (Hprt), and β-actin (Actb)] was the same across Axl mice. Therefore we chose these genes for normalization performed with an Excel-based template file from SABiosciences (Qiagen). We performed bioinformatics analyses of transregulation of transcription factors based on gene expression profiles from carotids across Axl chimeras, using the curated InnateDB resource (http://www.innatedb.ca).
Statistical Analysis
Data are expressed as means ± SEM. We used JMP5.1.2 software (SAS Institute, Cary, NC) for statistical tests, except for multiple quantitative RT-PCRs. One-way analysis of variance with repeated measurements was used to evaluate differences among the morphometry data of carotids from Axl chimeras. Relative expression of immunoreactive markers was analyzed by nonparametric Wilcoxon test across Axl chimeras. Quantitative RT-PCR gene expression differences between two groups were analyzed by the Student's t-test. Analyses of the PCR arrays were done in three steps, using an Excel-based template from SABiosciences (Qiagen). First, the template automatically performed all CT-based fold-change calculations from the uploaded raw threshold cycle data. Second, it executed pairwise comparison between two groups of experimental replicates and defined fold-change and statistical significance thresholds. Finally, the obtained gene expression results were presented as dots on volcano plots with positive and negative cutoffs and P = 0.05 indicated. The level of P < 0.05 was considered significant.
Results
Bone Marrow Transplantation between Axl Genotypes
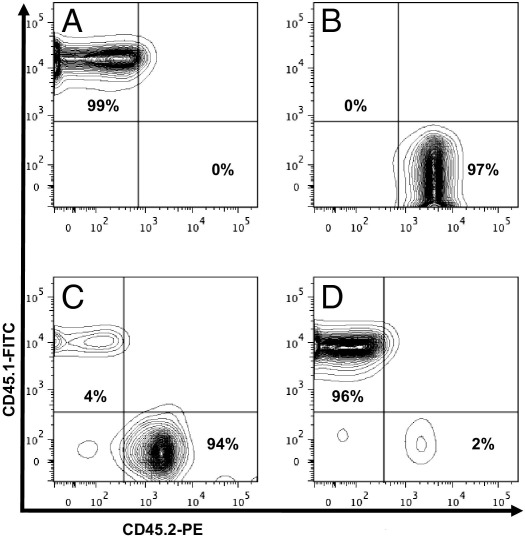
To directly address the role for Axl-derived BM cells on vascular remodeling, we created Axl chimeras by BMT. Representative flow cytometry charts show a repopulation of the BM in four groups of Axl chimeras 6 weeks after BMT (Figure 1). As expected, control chimeras [Axl+/+ → Axl+/+ (CD45.1+) versus Axl−/− → Axl−/− (CD45.2+)] exhibited nearly 100% replacement of the donor BM (Figure 1, A and B). We successfully generated Axl chimeras with replaced peripheral blood cells of the opposite genotype (Figure 1, C versus A and D versus B). We concluded that engraftment in Axl chimeras is appropriate to study the relative contribution of BM and non-BM cells to vascular remodeling.
Figure 1.
Flow cytometry analyses of peripheral blood from Axl chimeras. A–D: Representative CD45.1/CD45.2 double staining of peripheral blood from chimeras Axl+/+ → Axl+/+ (A) Axl−/− → Axl−/− (B), Axl−/− → Axl+/+ (C), and Axl+/+ → Axl−/− (D). CD45.1+ cells are Axl+/+; CD45.2+ cells are Axl−/−. Percentages in the upper-left and lower-right quadrants indicate extent of engraftment 6 weeks after BMT in Axl chimeras. FITC, fluorescein isothiocyanate; PE, phycoerythrin.
Carotid Remodeling in Axl Chimeras
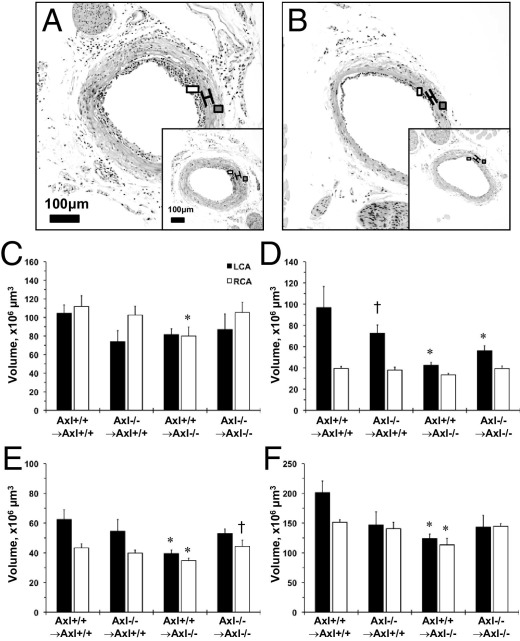
The ligation procedure resulted in very similar carotid blood flow reduction in the common LCA across Axl chimeras (Table 1). The Axl+/+ → Axl+/+ chimeras had significantly thicker LCA, compared with Axl−/− → Axl−/− chimeras (Figure 2, A and B). These findings are in agreement with our original observations in Axl littermates without BMT.11 The RCA media volume was the same across Axl chimeras, but the LCA intima+media volume was lower in Axl−/−, compared with Axl+/+ mice (Figure 2D). Relative changes in intima+media between chimeras of Axl genotypes were similar to those in Axl littermates.11 Expression of Axl in BM cells did not increase LCA intima+media volume in Axl+/+ → Axl−/− chimeras, compared with Axl+/+ → Axl+/+ (Figure 2D). However, Axl depletion in BM-derived cells in Axl−/− → Axl+/+ chimeras resulted in a 25% reduction in intima+media, compared with the Axl+/+ → Axl+/+ group (Figure 2D). The only Axl−/− non-BM chimeras exhibited small reduction of RCA lumen and adventitia of both carotid volumes (Figure 2, C and E). The size of the ligated carotid (measured by external elastic lamina volume) was significantly smaller in chimeras lacking Axl in BM, non-BM, or both cell lineages, compared with Axl+/+ → Axl+/+ mice (Figure 2F). Taken together, Axl deficiency in non-BM-derived cells had a much greater effect on flow-induced carotid remodeling than in BM-derived cells.
Table 1.
Blood Flow in the Common Carotid Artery in Axl Chimera Mice after Ligation
| Chimera⁎ | Blood flow (mL/min) |
|
|---|---|---|
| LCA | RCA | |
| Axl+/+ → Axl+/+ | 0.07 ± 0.03† | 0.98 ± 0.07 |
| Axl−/− → Axl−/− | 0.05 ± 0.02† | 1.05 ± 0.10 |
| Axl+/+ → Axl−/− | 0.06 ± 0.01† | 1.04 ± 0.05 |
| Axl−/− → Axl+/+ | 0.07 ± 0.02† | 1.03 ± 0.06 |
LCA, left carotid artery; RCA, right carotid artery.
n = 3 mice/chimera.
P < 0.05 versus RCA blood flow (Student's t-test).
Figure 2.
Flow-induced carotid remodeling in Axl chimeras. A and B: Photomicrographs of the left carotid artery (LCA) cross-sections from Axl chimeras 2 weeks after ligation: Axl−/− → Axl+/+ (inset:Axl+/+ → Axl+/+) (A) and Axl+/+ → Axl−/− (inset:Axl−/− → Axl−/−) (B). Brackets span the area between internal and external elastic lamina. Open boxes indicate intima formation; gray boxes indicate adventitia. Original magnification, 20×. Scale bar = 100 μm. C–F: Vessel component volumes: lumen (C), intima+media (D), adventitia (E), and external elastic lamina (F). Axl chimeras are shown on the x axis. Axl+/+ → Axl+/+, n = 5; Axl−/− → Axl−/−, n = 5; Axl−/− → Axl+/+, n = 7; Axl+/+ → Axl−/−, n = 7. Data are expressed as means ± SEM. *P < 0.05 versus Axl+/+ → Axl+/+; †P < 0.05 versus Axl+/+ → Axl−/−.
Carotid Leukocytes in Axl Chimeras
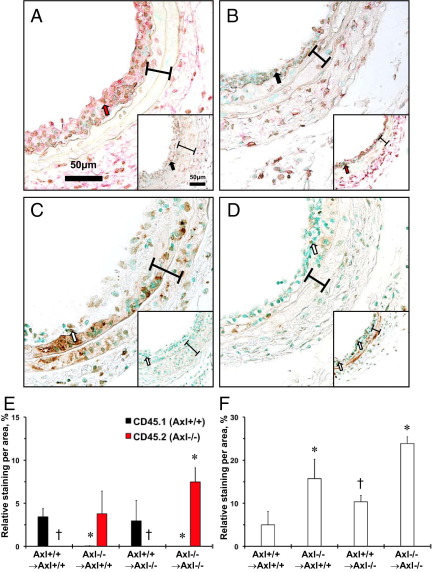
We were able to distinguish BM-derived cells in the carotid arteries after ligation across Axl chimeras using immunohistochemistry (Figure 3). The majority of the CD45+ cells were localized to the intima and adventitia (Figure 3, A and B). We recapitulated previous observations of higher levels of CD45.2+ cells in the intima from Axl−/− mice, compared with CD45.1+ cells in Axl+/+ mice (Figure 3, A and B). Less than 0.05% of host CD45.1+ cells were detected in the intima of chimeras with Axl−/− in BM cells (Figure 3E). However, there were no detectable CD45.2+ (Axl−/−) cells in the intima of carotids from Axl+/+ → Axl−/− chimeras (Figure 3E). Thus, the Axl genotype of intimal leukocytes reflected CD45 expression in peripheral blood from Axl chimeras (Figure 1).
Figure 3.
Immunohistochemical evaluation of intimal leukocytes and apoptosis in carotids from Axl chimeras. A and B: Representative CD45.1/CD45.2 double staining of carotids: Axl−/− → Axl+/+ (inset:Axl+/+ → Axl+/+) (A) and Axl+/+ → Axl−/− (inset:Axl−/− → Axl−/−) (B). CD45.1+ (Axl+/+) cells staining dark brown (black arrows); CD45.2+ (Axl−/−) cells stain red (red arrows). C and D: Representative Apoptag staining of carotids: Axl−/− → Axl+/+ (inset:Axl+/+ → Axl+/+) (C) and Axl+/+ → Axl−/− (inset:Axl−/− → Axl−/−) (D). Apoptotic cells stain dark brown (open arrows). Brackets span the area between internal and external elastic laminae. Original magnification, ×60. Scale bar = 50 μm. E: Relative intimal leukocyte staining for CD45.1+, and CD45.2+ cells. Axl+/+ → Axl+/+, n = 5; Axl−/− → Axl−/−, n = 4; Axl−/− → Axl+/+, n = 4; Axl+/+ → Axl−/−, n = 4. F: Relative intimal apoptosis staining Axl+/+ → Axl+/+, n = 3; Axl−/− → Axl−/−, n = 3; Axl−/− → Axl+/+, n = 3; Axl+/+ → Axl−/−, n = 3. Data are expressed as means ± SEM. *P < 0.05 versus Axl+/+ → Axl+/+; †P < 0.05 versus Axl−/− → Axl−/−.
Carotid Apoptosis in Axl Chimeras
More apoptotic cells were observed in the intima than in the media in ligated carotids from Axl−/− → Axl−/− mice (Figure 3, C and D). Intimal apoptosis was five times greater in Axl−/− chimeras, compared with Axl+/+ chimeras (Figure 3F). Axl expression in BM-derived cells significantly reduced apoptosis in the intima, compared with Axl−/− → Axl−/− mice (Figure 3, D and F). Conversely, deletion of Axl in BM cells increased apoptosis in the intima, compared with the Axl+/+ → Axl+/+ group (Figure 3, D and F). The Gas6/Axl pathway has been shown to increase survival and protected smooth muscle cells from calcium deposition in vitro.22 However, we found no evidence of calcification in the remodeled carotids from Axl chimeras (see Supplemental Figure S4 at http://ajp.amjpathol.org). Taken together, our data suggest that Axl-dependent pathways are crucial for survival of intimal leukocytes, but have a modest effect on carotid IMT (Figure 2).
Cytokine and Chemokine Expression in Carotids from Axl Chimeras
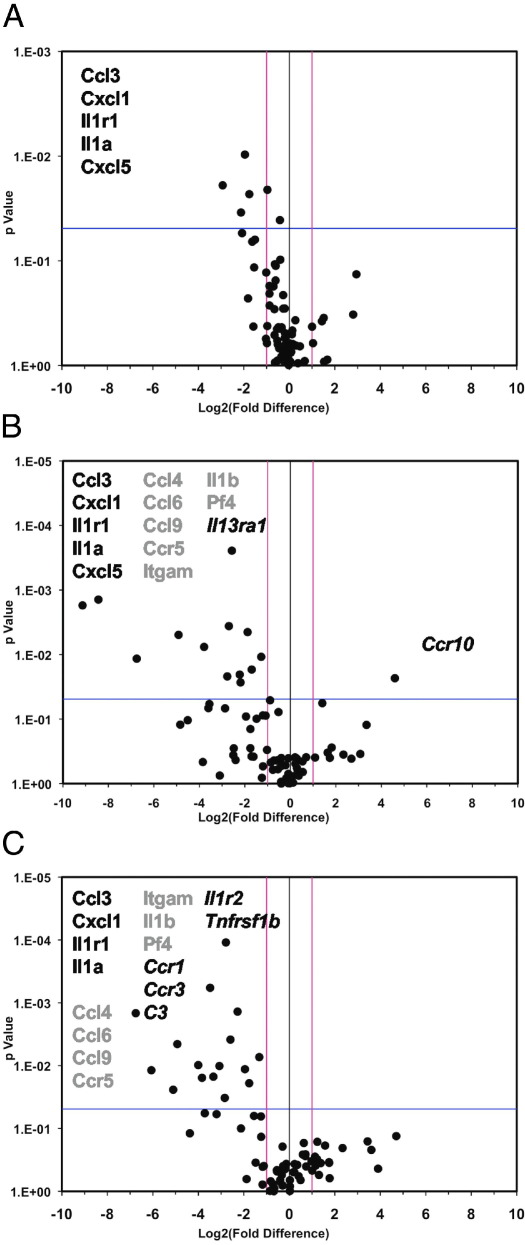
We evaluated expression of cytokines/chemokines and their receptors, to gain insight into mechanisms by which Axl in non-BM cells determines carotid IMT (Supplemental Tables S1–S3 at http://ajp.amjpathol.org). Five genes were significantly down-regulated in the ligated carotids with Axl depletion in both lineages (Figure 4A). In particular, reduced expression of interleukin-1α (Il-1α), interleukin-1 receptor type 1 (Il1r), C-C motif chemokine 3 (Ccl3), and the C-X-C motif chemokines 1 and 5 (Cxcl1 and Cxcl5) was evident in carotids from Axl−/− mice. Even though carotid IMT was slightly reduced in chimeras with deleted Axl in BM cells, all five genes were expressed at lower levels (Figure 4B). However, one chemokine receptor (Ccr10) was up-regulated and eight more genes were down-regulated in chimeras with Axl−/− in BM cells. Depletion of Axl in non-BM cells resulted in even more genes being down-regulated (Figure 4C). In chimeras that lack Axl in BM or non-BM cells, expression of seven common genes (Ccl4, Ccl6, Ccl9, Ccr5, Itgam, Il1b, and Pf4) was decreased in ligated carotids (Figure 4, B and C). These findings suggest that Axl expression equally affects IL-1, chemokine, and integrin α-M (Itgam) signals by cells of both lineages during carotid remodeling. Importantly, deficiency of Axl in non-BM cells further decreased expression of five genes (Ccr1, Ccr3, C3, Il1r2, and Tnfrsf1b), compared with Axl+/+ mice (Figure 4C). We found a very complex network of transcription factors that transregulate target genes in our experiments (see Supplemental Figure S5 at http://ajp.amjpathol.org). However, the most common transcription factors (linked to two to five target genes, including Foxa1, Aire, Sp1, and Elk1) were very similar in chimeras with different levels of expression of Axl. In contrast, a significant reduction of the complement component 3 (C3) gene may suggest a reduced antigen-presenting cell (APC) activity in chimeras with Axl−/− in non-BM cells (Figure 4C). It was documented23 that C3 knockout (C3−/−) mice exhibited reduced stimulatory activity of APC and MHC II expression. Thus, we conclude that Axl expression is critical for immune activation of non-BM cells and by production of proinflammatory cytokines and chemokines during carotid remodeling.
Figure 4.
Cytokine and chemokine gene profiling in carotids from Axl chimeras. A–C: Effects of Axl deletion in both BM and non-BM lineages (A), in BM cells (B), and in non-BM cells (C). Individual genes are represented as dots on the volcano plots, in which log2-transformed fold changes in gene expression are plotted against P values. Vertical lines indicate zero and both positive and negative cutoffs; the horizontal line indicates the significance level (P = 0.05). Genes listed to the left of the zero line (negative values) are down-regulated; those to the right (positive values) are up-regulated. Groups of genes indicated by type style (black, gray, or italic) are as discussed in the text. Expression data were obtained from three carotids per group.
Axl-Dependent Immune Modulation of Vascular Cells
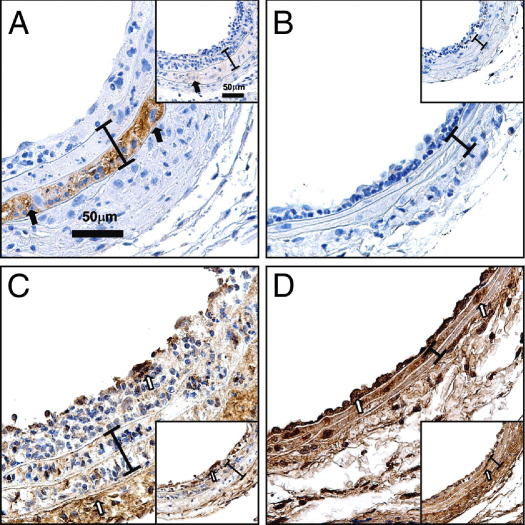
We found that MASMCs harvested from Axl+/+ and Axl−/− mice grew at the same rate, whereas levels of Axl were diminished in Axl−/− cultured cells (data not shown). Expression of MHC II was similar between unstimulated MASMCs from both Axl genotypes (Figure 5). Intensity of MHC II was dramatically increased in outer medial layer in chimeras that express Axl in non-BM cells only (Axl−/− → Axl+/+; Figure 6A). However, a dramatic up-regulation of MHC II was significantly attenuated in Axl−/−, compared with Axl+/+ MASMCs after IFN-γ treatment (Figure 5). Moreover, ligation slightly increased MHC II in the media of Axl+/+ → Axl+/+, but not in Axl−/− → Axl−/− chimeras (Figure 6B). Increase in MHC II has been correlated with reduction of Col I expression in smooth muscle cells.24 Similarly, Col I was significantly increased in carotids from chimeras with deletion of Axl in non-BM cells and coincided with the absence of MHC II immunoreactivity (Figure 6, C and D). Increased expression of Col I in the intima-media (Figure 6, C and D) predicted lower values of carotid IMT (Figure 2D). In addition, adventitial deposition of Col I (Figure 6, C and D) correlated with reduced external elastic lamina in Axl chimeras (Figure 2F). In summary, we found that Axl expression in non-BM cells controls MHC II, which determines the extent of the carotid intima-media thickening via regulation of immune heterogeneity and alteration of extracellular matrix.
Figure 5.
Immune modulation of vascular cells by Axl cultured MASMCs. Relative expression of the MHC II gene H2-Ab1 in Axl MASMCs in vitro. Wild-type (Axl+/+) or knockout (Axl−/−) cells were treated with PBS or IFN-γ. Axl+/+, n = 3; Axl−/−, n = 3. Data are expressed as means ± SEM. *P < 0.05 versus Axl+/+.
Figure 6.
Immune modulation and collagen expression in carotids from Axl chimeras. A and B: Representative MHC II staining: Axl−/− → Axl+/+ (inset:Axl+/+ → Axl+/+) (A) and Axl+/+ → Axl−/− (inset:Axl−/− → Axl−/−) (B). C and D: Representative Col I staining: Axl−/− → Axl+/+ (inset:Axl+/+ → Axl+/+) (C) and Axl+/+ → Axl−/− (inset:Axl−/− → Axl−/−) (D). MHC II+ cells (dark brown) are indicated by black arrows and Col I+ cells (dark brown) are indicated by open arrows. A bracket spans the area between internal and external elastic lamina. Original magnification, ×60. Scale bar = 50 μm.
Discussion
The present findings provide new insights into mechanisms by which Axl contributes to vascular remodeling (Figure 7). First, Axl-dependent survival is very important for intimal leukocytes. However, Axl-dependent signals in BM-derived cells may explain <30% of carotid IMT. Second, Axl in non-BM cells has a much greater effect on carotid remodeling. Third, we found that Axl regulates immune heterogeneity of medial cells (measured by MHC II), which directs carotid IMT; we show, for the first time, that lack of Axl reduced activation of MHC II in cultured smooth muscle cells in response to IFN-γ. Fourth, depletion of Axl in non-BM cells caused a greater down-regulation of several key cytokine/chemokine signals (eg, IL-1) via a complex network of transcription factors in carotids. Finally, absence of Axl in non-BM cells increased expression of Col I and reduced carotid IMT. Taken together, our findings suggest that Axl contributes to carotid remodeling via regulation of immune heterogeneity of vascular cells, cytokine/chemokine expression, and extracellular matrix remodeling, in addition to the known function of Axl in survival (Figure 7).
Figure 7.
Mechanisms by which Axl regulates carotid intima-media thickening, with known effects of Axl on survival during carotid intima-media thickening (blue) and new mechanisms of Axl-dependent immune activation of non-BM cells, cytokine/chemokine expression, and inhibition of Col I expression in the remodeled carotids (orange). Synthetic VSMCs are indicated in purple; contractile VSMCs are indicated in red. BM, bone marrow; Col I, collagen Iα; EEL, external elastic lamina; IEL, internal elastic lamina; MHC II, major histocompatibility complex class II; VSMC, vascular smooth muscle cell.
Our data from Axl chimeras are in agreement with a major role for non-BM cells on vascular remodeling (Figure 2D). Most current efforts are focused on the origin of the intimal cells.2 However, recent findings in experimental atherosclerosis suggest that intimal smooth muscle cells are derived from multiple origins and exhibit immune heterogeneity.3 Notably, some subsets of endothelial cells, immune cells, and VSMCs expressed high levels of MHC II in the neointima in later phases after balloon injury in rats.4 We found significant compensation of MHC II in the outer layer of carotid media in chimeras with Axl expressed in non-BM cells only (Figure 6A). This unique position of MHC II+ cells in the remodeled arterial wall may allow interactions with progenitor cells from multiple origins. This may also suggest that immune activation of non-BM cells is crucial for later phases in response to injury, as recently proposed.25 Likewise, Axl was up-regulated during later phases of neointimal proliferation in a rat balloon injury model.10
The most plausible mechanism for Axl in non-BM cells is triggering of the PI3K/Akt pathway.26 Activation of the PI3K/Akt pathway (by depletion of an inhibitor, PTEN) resulted in increased production of the chemokine Cxcl1 in murine VSMCs and enhanced intima formation.27 We also showed that Axl is responsible for up-regulation of Cxcl1 in response to low flow (Figure 4). A critical role for Axl in immune activation of VSMCs (by antigen presentation) is supported by significant reduction of C3 in carotids from chimeras with Axl−/− in non-BM cells (Figure 4C). A reduction of APC activity and MHC II was documented in C3−/− mice.23 IFN-γ is a powerful stimulator of MHC II in human VSMCs.16 However, the molecular mechanisms of MHC II up-regulation are incompletely understood. Activation of the PI3K/Akt/mTOR pathway has been implicated in IFN-γ signal transduction.28 In addition, immune heterogeneity of resident vascular cell (MHC II positivity) might be related to down-regulation of Col I expression.24 Specifically, immune activation of smooth muscle cells up-regulated expression of major histocompatibility class II transactivator (CIITA) that increased MHC II gene expression and reduced Col I expression in vitro. However, we found no effect of Axl on CIITA in MASMCs (data not shown). Changes in extracellular matrix are very important for cell function (eg, migration) during vascular remodeling. Collagen deposition preceded inward remodeling after balloon injury, possibly via stabilization of the vessel wall.29 Our findings in chimeras suggest that lack of Axl in non-BM cells shifted the balance toward more Col I over MHC II expression, which reduced carotid remodeling (Figure 6). Future studies are required for dissection of the molecular mechanisms of MHC II activation by Axl in smooth muscle cells.
We found that Axl-dependent pathways are most crucial for survival of the BM cells in the intima (Figure 3). Notably, apoptotic cells were positive in the intimal and the inner layer of the media of carotids from chimeras with Axl+/+ in non-BM cells, whereas the outer medial layer was MHC II+ (Figures 4C and 6A). Increases in apoptosis of VSMCs (by induction of human diphtheria toxin receptor) dramatically enhanced atherosclerosis by intimal inflammation, medial degradation, and calcification in proatherogenic (Apoe−/−) mice.30,31 Experimental data in two mouse models of atherosclerosis (ApoE−/− and Ldlr−/−) suggest that a clearance of apoptotic cells relies on Mertk.12,13 Lack of Mertk in BM and non-BM cells accelerated atherosclerotic lesions, because of excessive necrosis. However, subsets of innate immune cells can use different TAM receptors for the clearance of apoptotic cells.32 In contrast to Mertk, loss of Gas6 had no effect on atherosclerotic plaque size, but dramatically changed composition of the plaque in Apoe−/− mice.14 Similarly, we found that only deletion of Axl in both lineages increased intimal leukocytes (Figure 3E). A relatively small human genetic study attempted to evaluate association of the GAS6 and TAM polymorphisms with carotid atherosclerosis.33 The authors reported a very weak relationship between carotid atherosclerosis and GAS6 or AXL variants, which was contradictory to their previous reports on associations between GAS6 and stroke.34–36 Future experiments will be required to determine roles for AXL in atherosclerotic plaque composition and size in human and animal models.
TAM receptors regulate survival and phagocytosis of innate immune cells.37 In addition, triple-TAM knockout mice exhibited lymphoproliferative abnormalities, because of increased expression of proinflammatory cytokines by activated macrophages. However, this autoimmunity phenotype could be due to Mertk deficiency in leukocytes.38 Gas6/TAM-mediated immune responses require interactions between multiple cell types, including vascular and immune cells.8 These cell-to-cell interactions are responsible for neovascularization and tumorigenesis in breast cancer and are controlled by Axl.39 Recent studies in the CT26 model of colon cancer9 showed that this type of tumor growth progresses via production of the Axl ligand, Gas6, by infiltrating leukocytes. Our results in flow-induced carotid remodeling further emphasize a key role for Axl-dependent pathways for interactions between multiple cell lineages via production of proinflammatory cytokines and chemokines. The IL-1 receptor type I knockout (Il1r1−/−) mice reduced intima formation in response to complete ligation.40 We can speculate that expression of Axl in both lineages is required for immune activation, even though the extent of reduction in blood flow was different in experiments on Il1r1−/− (−100%) mice,40 compared with our studies (−90%) in Axl chimeras. Recent microarray analyses discovered number of mechanosensitive genes in a similar mouse model of flow-dependent carotid remodeling.41 Authors found significant over-representation of the immune pathways with up-regulation of three genes (Ccl4, Ccr5, and Spp1) that are controlled by Axl during carotid IMT (Figure 4). Notably, integrin β subunits (Itgb2, Itgb4, and Itgb7) are highly regulated in the carotid intima early after flow reduction.41 We found that deletion of Axl in either lineage significantly reduced expression of Itgam in the carotid artery (Figure 4, B and C). Intima proliferation and leukocyte recruitment to the carotid were attenuated in Mac-1 (Itgam) knockout mice after mechanical injury.42 Therefore, increases in intimal leukocytes in Axl−/− → Axl−/− chimeras may be explained, in part, through regulation of Itgam.
There are three potential limitations of the present study. First, a background mouse strain (C57BL/6) of Axl chimeras exhibits significant but relatively small carotid IMT, compared with other inbred strains.43 Despite this disadvantage of the background, we were able to show that Axl expressed in non-BM cells was responsible for carotid IMT (Figure 2). A second limitation is related to the Axl-mediated transcriptional regulation of cytokine/chemokine production in cell lineages. At this point, we were unable to identify specific transcription factor or factors regulated by Axl. Finally, activation of Axl has been reported in endothelial cells that are critical for survival.44,45 It is possible that Axl-dependent signals in endothelial or other resident cell types are important for carotid IMT. Nonetheless, based on in vitro experiments using MASMCs and MHC II expression patterns in the ligated carotids from Axl chimeras, it is likely that smooth muscle cells determine IMT (Figures 5 and 6).
In the present study, we identified a new mechanism by which residential wall cells orchestrate carotid remodeling by activation of Axl (Figure 7). In particular, for the first time we show that Axl regulates immune heterogeneity of non-BM cells and extracellular matrix reorganization in the remodeled artery. We also identified several key cytokine and chemokine pathways, which are dependent on Axl during carotid IMT. Finally, Axl-dependent signaling protects intimal leukocytes against apoptosis. However, these prosurvival effects of Axl in BM-derived cells contribute to <30% of carotid IMT. Our findings provide new insights into immune modifications of vascular resident cells, by which Axl controls vascular remodeling.
Acknowledgments
We thank Michelle Zanche and Dr. Stephen Welle (Functional Genomics Core) and Mitchele Au and Dr. Tim Bushnell (Flow Cytometry Core) for assistance with gene expression and flow cytometry assays.
Footnotes
Supported by grants from the American Heart Association (SDG 0430267N), NYSTEM (C023056), and the NIH (R01 HL105623) to V.A.K.
Supplemental material for this article can be found at http://ajp.amjpathol.org or at doi: 10.1016/j.ajpath.2012.01.036.
Supplementary data
The Axl gene is expressed in B6.SJL-PtprcaPep3b/BoyJ mice. Ethidium bromide-stained PCR products after gel electrophoresis are shown for two CD45.1+ B6.SJL-PtprcaPep3b/BoyJ mice and for CD45.2+Axl+/+ and Axl−/− littermates from our colony.
The carotid component areas plotted against length of left carotid artery (LCA) cross-sections from Axl chimeras. A: LCA lumen. B: LCA intima+media. C: LCA adventitia. Gray highlighting marks the area (along 1600 μm of the carotid length) used to calculate the carotid compartment volume. Data are expressed as means + SEM and n indicates the number of animals.
The carotid component areas plotted against length of right carotid artery (RCA) cross-sections from Axl chimeras. A: RCA lumen. B: RCA intima+media. C: RCA adventitia. Gray highlighting marks the area (along 1600 μm of the carotid length) used to calculate the carotid compartment volume. Data are expressed as means + SEM and n indicates the number of animals.
Vascular calcification in the ligated carotids from Axl chimeras: Axl+/+ → Axl+/+ (A), Axl−/− → Axl−/− (B), Axl−/− → Axl+/+ (C), and Axl+/+ → Axl−/− (D). Brackets span the area between internal and external elastic lamina. Open boxes indicate intima formation; gray boxes indicate adventitia. E: Calcified bones in mouse embryos at e16 stain red (positive). Original magnification, 20×. Scale bar = 100 μm.
Transcription factor analyses based on cytokine and chemokine gene profiling in carotids from Axl chimeras. A–C: Effects of Axl deletion in both lineages (A), in BM cells (B), and in non-BM cells (C). Differentially expressed genes are shown below the line (in Transregulation) and transcription factors are shown above the line (in Nucleus). Construction of the network of interactions between transcription factors and differentially expressed genes is indicated by lines.
References
- 1.O'Leary D.H., Polak J.F., Kronmal R.A., Manolio T.A., Burke G.L., Wolfson S.K., Jr Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults: Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340:14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 2.Daniel J.M., Bielenberg W., Stieger P., Weinert S., Tillmanns H., Sedding D.G. Time-course analysis on the differentiation of bone marrow-derived progenitor cells into smooth muscle cells during neointima formation. Arterioscler Thromb Vasc Biol. 2010;30:1890–1896. doi: 10.1161/ATVBAHA.110.209692. [DOI] [PubMed] [Google Scholar]
- 3.Yu H., Stoneman V., Clarke M., Figg N., Xin H.B., Kotlikoff M., Littlewood T., Bennett M. Bone marrow-derived smooth muscle-like cells are infrequent in advanced primary atherosclerotic plaques but promote atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31:1291–1299. doi: 10.1161/ATVBAHA.110.218578. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka H., Sukhova G.K., Swanson S.J., Clinton S.K., Ganz P., Cybulsky M.I., Libby P. Sustained activation of vascular cells and leukocytes in the rabbit aorta after balloon injury. Circulation. 1993;88:1788–1803. doi: 10.1161/01.cir.88.4.1788. [DOI] [PubMed] [Google Scholar]
- 5.O'Bryan J.P., Frye R.A., Cogswell P.C., Neubauer A., Kitch B., Prokop C., Espinosa R., 3rd, Le Beau M.M., Earp H.S., Liu E.T. Axl, a transforming gene isolated from primary human myeloid leukemia cells, encodes a novel receptor tyrosine kinase. Mol Cell Biol. 1991;11:5016–5031. doi: 10.1128/mcb.11.10.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stitt T.N., Conn G., Gore M., Lai C., Bruno J., Radziejewski C., Mattsson K., Fisher J., Gies D.R., Jones P.F., Masiakowski P., Ryan T.E., Tobkes N.J., Chen D.H., DiStefano P.S., Long G.L., Basilico C., Goldfarb M.P., Lemke G., Glass D.J., Yancopoulos G.D. The anticoagulation factor protein S and its relative, Gas6, are ligands for the Tyro 3/Axl family of receptor tyrosine kinases. Cell. 1995;80:661–670. doi: 10.1016/0092-8674(95)90520-0. [DOI] [PubMed] [Google Scholar]
- 7.Rothlin C.V., Lemke G. TAM receptor signaling and autoimmune disease. Curr Opin Immunol. 2010;22:740–746. doi: 10.1016/j.coi.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tjwa M., Bellido-Martin L., Lin Y., Lutgens E., Plaisance S., Bono F., Delesque-Touchard N., Hervé C., Moura R., Billiau A.D., Aparicio C., Levi M., Daemen M., Dewerchin M., Lupu F., Arnout J., Herbert J.M., Waer M., García de Frutos P., Dahlbäck B., Carmeliet P., Hoylaerts M.F., Moons L. Gas6 promotes inflammation by enhancing interactions between endothelial cells, platelets, and leukocytes. Blood. 2008;111:4096–4105. doi: 10.1182/blood-2007-05-089565. [DOI] [PubMed] [Google Scholar]
- 9.Loges S., Schmidt T., Tjwa M., van Geyte K., Lievens D., Lutgens E., Vanhoutte D., Borgel D., Plaisance S., Hoylaerts M., Luttun A., Dewerchin M., Jonckx B., Carmeliet P. Malignant cells fuel tumor growth by educating infiltrating leukocytes to produce the mitogen Gas6. Blood. 2010;115:2264–2273. doi: 10.1182/blood-2009-06-228684. [DOI] [PubMed] [Google Scholar]
- 10.Melaragno M.G., Wuthrich D.A., Poppa V., Gill D., Lindner V., Berk B.C., Corson M.A. Increased expression of Axl tyrosine kinase after vascular injury and regulation by G protein-coupled receptor agonists in rats. Circ Res. 1998;83:697–704. doi: 10.1161/01.res.83.7.697. [DOI] [PubMed] [Google Scholar]
- 11.Korshunov V.A., Mohan A.M., Georger M.A., Berk B.C. Axl, a receptor tyrosine kinase, mediates flow-induced vascular remodeling. Circ Res. 2006;98:1446–1452. doi: 10.1161/01.RES.0000223322.16149.9a. [DOI] [PubMed] [Google Scholar]
- 12.Thorp E., Cui D., Schrijvers D.M., Kuriakose G., Tabas I. Mertk receptor mutation reduces efferocytosis efficiency and promotes apoptotic cell accumulation and plaque necrosis in atherosclerotic lesions of apoe−/− mice. Arterioscler Thromb Vasc Biol. 2008;28:1421–1428. doi: 10.1161/ATVBAHA.108.167197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ait-Oufella H., Pouresmail V., Simon T., Blanc-Brude O., Kinugawa K., Merval R., Offenstadt G., Lesèche G., Cohen P.L., Tedgui A., Mallat Z. Defective mer receptor tyrosine kinase signaling in bone marrow cells promotes apoptotic cell accumulation and accelerates atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28:1429–1431. doi: 10.1161/ATVBAHA.108.169078. [DOI] [PubMed] [Google Scholar]
- 14.Lutgens E., Tjwa M., Garcia de Frutos P., Wijnands E., Beckers L., Dahlbäck B., Daemen M.J., Carmeliet P., Moons L. Genetic loss of Gas6 induces plaque stability in experimental atherosclerosis. J Pathol. 2008;216:55–63. doi: 10.1002/path.2381. [DOI] [PubMed] [Google Scholar]
- 15.Satoh K., Matoba T., Suzuki J., O'Dell M.R., Nigro P., Cui Z., Mohan A., Pan S., Li L., Jin Z.G., Yan C., Abe J., Berk B.C. Cyclophilin A mediates vascular remodeling by promoting inflammation and vascular smooth muscle cell proliferation. Circulation. 2008;117:3088–3098. doi: 10.1161/CIRCULATIONAHA.107.756106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Warner S.J., Friedman G.B., Libby P. Regulation of major histocompatibility gene expression in human vascular smooth muscle cells. Arteriosclerosis. 1989;9:279–288. doi: 10.1161/01.atv.9.3.279. [DOI] [PubMed] [Google Scholar]
- 17.Korshunov V.A., Nikonenko T.A., Tkachuk V.A., Brooks A., Berk B.C. Interleukin-18 and macrophage migration inhibitory factor are associated with increased carotid intima-media thickening. Arterioscler Thromb Vasc Biol. 2006;26:295–300. doi: 10.1161/01.ATV.0000196544.73761.82. [DOI] [PubMed] [Google Scholar]
- 18.Lessner S.M., Prado H.L., Waller E.K., Galis Z.S. Atherosclerotic lesions grow through recruitment and proliferation of circulating monocytes in a murine model. Am J Pathol. 2002;160:2145–2155. doi: 10.1016/S0002-9440(10)61163-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korshunov V.A., Berk B.C. Flow-induced vascular remodeling in the mouse: a model for carotid intima-media thickening. Arterioscler Thromb Vasc Biol. 2003;23:2185–2191. doi: 10.1161/01.ATV.0000103120.06092.14. [DOI] [PubMed] [Google Scholar]
- 20.Chiang H.Y., Korshunov V.A., Serour A., Shi F., Sottile J. Fibronectin is an important regulator of flow-induced vascular remodeling. Arterioscler Thromb Vasc Biol. 2009;29:1074–1079. doi: 10.1161/ATVBAHA.108.181081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smolock E.M., Korshunov V.A. Pharmacological inhibition of Axl affects smooth muscle cell functions under oxidative stress. Vascul Pharmacol. 2010;53:185–192. doi: 10.1016/j.vph.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Collett G.D., Sage A.P., Kirton J.P., Alexander M.Y., Gilmore A.P., Canfield A.E. Axl/phosphatidylinositol 3-kinase signaling inhibits mineral deposition by vascular smooth muscle cells. Circ Res. 2007;100:502–509. doi: 10.1161/01.RES.0000258854.03388.02. [DOI] [PubMed] [Google Scholar]
- 23.Zhou W., Patel H., Li K., Peng Q., Villiers M.B., Sacks S.H. Macrophages from C3-deficient mice have impaired potency to stimulate alloreactive T cells. Blood. 2006;107:2461–2469. doi: 10.1182/blood-2005-08-3144. [DOI] [PubMed] [Google Scholar]
- 24.Buttice G., Miller J., Wang L., Smith B.D. Interferon-gamma induces major histocompatibility class II transactivator (CIITA), which mediates collagen repression and major histocompatibility class II activation by human aortic smooth muscle cells. Circ Res. 2006;98:472–479. doi: 10.1161/01.RES.0000204725.46332.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoglund V.J., Dong X.R., Majesky M.W. Neointima formation: a local affair. Arterioscler Thromb Vasc Biol. 2010;30:1877–1879. doi: 10.1161/ATVBAHA.110.211433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melaragno M.G., Cavet M.E., Yan C., Tai L.K., Jin Z.G., Haendeler J., Berk B.C. Gas6 inhibits apoptosis in vascular smooth muscle: role of Axl kinase and Akt. J Mol Cell Cardiol. 2004;37:881–887. doi: 10.1016/j.yjmcc.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 27.Furgeson S.B., Simpson P.A., Park I., Vanputten V., Horita H., Kontos C.D., Nemenoff R.A., Weiser-Evans M.C. Inactivation of the tumour suppressor, PTEN, in smooth muscle promotes a pro-inflammatory phenotype and enhances neointima formation. Cardiovasc Res. 2010;86:274–282. doi: 10.1093/cvr/cvp425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gough D.J., Levy D.E., Johnstone R.W., Clarke C.J. IFNgamma signaling—does it mean JAK-STAT? Cytokine Growth Factor Rev. 2008;19:383–394. doi: 10.1016/j.cytogfr.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt M.R., Maeng M., Kristiansen S.B., Andersen H.R., Falk E. The natural history of collagen and alpha-actin expression after coronary angioplasty. Cardiovasc Pathol. 2004;13:260–267. doi: 10.1016/j.carpath.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 30.Clarke M.C., Figg N., Maguire J.J., Davenport A.P., Goddard M., Littlewood T.D., Bennett M.R. Apoptosis of vascular smooth muscle cells induces features of plaque vulnerability in atherosclerosis. Nat Med. 2006;12:1075–1080. doi: 10.1038/nm1459. [DOI] [PubMed] [Google Scholar]
- 31.Clarke M.C., Littlewood T.D., Figg N., Maguire J.J., Davenport A.P., Goddard M., Bennett M.R. Chronic apoptosis of vascular smooth muscle cells accelerates atherosclerosis and promotes calcification and medial degeneration. Circ Res. 2008;102:1529–1538. doi: 10.1161/CIRCRESAHA.108.175976. [DOI] [PubMed] [Google Scholar]
- 32.Seitz H.M., Camenisch T.D., Lemke G., Earp H.S., Matsushima G.K. Macrophages and dendritic cells use different Axl/Mertk/Tyro3 receptors in clearance of apoptotic cells. J Immunol. 2007;178:5635–5642. doi: 10.4049/jimmunol.178.9.5635. [DOI] [PubMed] [Google Scholar]
- 33.Hurtado B., Abasolo N., Muñoz X., García N., Benavente Y., Rubio F., García de Frutos P., Krupinski J., Sala N. Association study between polymorphism in GAS6-TAM genes and carotid atherosclerosis. Thromb Haemost. 2010;104:592–598. doi: 10.1160/TH09-11-0787. [DOI] [PubMed] [Google Scholar]
- 34.Muñoz X., Sumoy L., Ramírez-Lorca R., Villar J., de Frutos P.G., Sala N. Human vitamin K-dependent GAS6: gene structure, allelic variation, and association with stroke. Hum Mutat. 2004;23:506–512. doi: 10.1002/humu.20025. [DOI] [PubMed] [Google Scholar]
- 35.Muñoz X., Obach V., Hurtado B., de Frutos P.G., Chamorro A., Sala N. Association of specific haplotypes of GAS6 gene with stroke. Thromb Haemost. 2007;98:406–412. [PubMed] [Google Scholar]
- 36.Jiang L., Liu C.Y., Yang Q.F., Wang P., Zhang W. Plasma level of growth arrest-specific 6 (GAS6) protein and genetic variations in the GAS6 gene in patients with acute coronary syndrome. Am J Clin Pathol. 2009;131:738–743. doi: 10.1309/AJCP3CX3AUVRBHCF. [DOI] [PubMed] [Google Scholar]
- 37.Lu Q., Lemke G. Homeostatic regulation of the immune system by receptor tyrosine kinases of the Tyro 3 family. Science. 2001;293:306–311. doi: 10.1126/science.1061663. [DOI] [PubMed] [Google Scholar]
- 38.Williams J.C., Wagner N.J., Earp H.S., Vilen B.J., Matsushima G.K. Increased hematopoietic cells in the mertk−/− mouse peritoneal cavity: a result of augmented migration. J Immunol. 2010;184:6637–6648. doi: 10.4049/jimmunol.0902784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holland S.J., Powell M.J., Franci C., Chan E.W., Friera A.M., Atchison R.E., McLaughlin J., Swift S.E., Pali E.S., Yam G., Wong S., Lasaga J., Shen M.R., Yu S., Xu W., Hitoshi Y., Bogenberger J., Nör J.E., Payan D.G., Lorens J.B. Multiple roles for the receptor tyrosine kinase axl in tumor formation. Cancer Res. 2005;65:9294–9303. doi: 10.1158/0008-5472.CAN-05-0993. [DOI] [PubMed] [Google Scholar]
- 40.Rectenwald J.E., Moldawer L.L., Huber T.S., Seeger J.M., Ozaki C.K. Direct evidence for cytokine involvement in neointimal hyperplasia. Circulation. 2000;102:1697–1702. doi: 10.1161/01.cir.102.14.1697. [DOI] [PubMed] [Google Scholar]
- 41.Ni C.W., Qiu H., Rezvan A., Kwon K., Nam D., Son D.J., Visvader J.E., Jo H. Discovery of novel mechanosensitive genes in vivo using mouse carotid artery endothelium exposed to disturbed flow. Blood. 2010;116:e66–e73. doi: 10.1182/blood-2010-04-278192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simon D.I., Dhen Z., Seifert P., Edelman E.R., Ballantyne C.M., Rogers C. Decreased neointimal formation in Mac-1(−/−) mice reveals a role for inflammation in vascular repair after angioplasty. J Clin Invest. 2000;105:293–300. doi: 10.1172/JCI7811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Korshunov V.A., Berk B.C. Strain-dependent vascular remodeling: the “Glagov phenomenon” is genetically determined. Circulation. 2004;110:220–226. doi: 10.1161/01.CIR.0000134958.88379.2E. [DOI] [PubMed] [Google Scholar]
- 44.Healy A.M., Schwartz J.J., Zhu X., Herrick B.E., Varnum B., Farber H.W. Gas 6 promotes Axl-mediated survival in pulmonary endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2001;280:L1273–L1281. doi: 10.1152/ajplung.2001.280.6.L1273. [DOI] [PubMed] [Google Scholar]
- 45.D'Arcangelo D., Ambrosino V., Giannuzzo M., Gaetano C., Capogrossi M.C. Axl receptor activation mediates laminar shear stress anti-apoptotic effects in human endothelial cells. Cardiovasc Res. 2006;71:754–763. doi: 10.1016/j.cardiores.2006.06.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The Axl gene is expressed in B6.SJL-PtprcaPep3b/BoyJ mice. Ethidium bromide-stained PCR products after gel electrophoresis are shown for two CD45.1+ B6.SJL-PtprcaPep3b/BoyJ mice and for CD45.2+Axl+/+ and Axl−/− littermates from our colony.
The carotid component areas plotted against length of left carotid artery (LCA) cross-sections from Axl chimeras. A: LCA lumen. B: LCA intima+media. C: LCA adventitia. Gray highlighting marks the area (along 1600 μm of the carotid length) used to calculate the carotid compartment volume. Data are expressed as means + SEM and n indicates the number of animals.
The carotid component areas plotted against length of right carotid artery (RCA) cross-sections from Axl chimeras. A: RCA lumen. B: RCA intima+media. C: RCA adventitia. Gray highlighting marks the area (along 1600 μm of the carotid length) used to calculate the carotid compartment volume. Data are expressed as means + SEM and n indicates the number of animals.
Vascular calcification in the ligated carotids from Axl chimeras: Axl+/+ → Axl+/+ (A), Axl−/− → Axl−/− (B), Axl−/− → Axl+/+ (C), and Axl+/+ → Axl−/− (D). Brackets span the area between internal and external elastic lamina. Open boxes indicate intima formation; gray boxes indicate adventitia. E: Calcified bones in mouse embryos at e16 stain red (positive). Original magnification, 20×. Scale bar = 100 μm.
Transcription factor analyses based on cytokine and chemokine gene profiling in carotids from Axl chimeras. A–C: Effects of Axl deletion in both lineages (A), in BM cells (B), and in non-BM cells (C). Differentially expressed genes are shown below the line (in Transregulation) and transcription factors are shown above the line (in Nucleus). Construction of the network of interactions between transcription factors and differentially expressed genes is indicated by lines.