Abstract
We demonstrated previously that urine contains low-molecular-weight (LMW) (<300 bp), circulation-derived DNA that can be used to detect cancer-specific mutations if a tumor is present. The goal of this study was to develop an assay to detect the colorectal cancer (CRC)–associated, circulation-derived, epigenetic DNA marker hypermethylated vimentin gene (mVIM) in the urine of patients with CRC. An artificial 18-nucleotide DNA sequence was tagged at the 5′ end of the primers of the first PCR cycle to increase the amplicon size, which was then integrated into the primers of the second PCR cycle. A quantitative MethyLight PCR-based assay targeting a 39-nucleotide template was developed and used to quantify mVIM in CRC tissues and matched urine samples. mVIM was detected in 75% of LMW urine DNA samples from patients with CRC (n = 20) and in 10% of urine samples of control subjects with no known neoplasia (n = 20); 12 of 17 LMW urine DNA samples (71%) but only 2 of 17 high-molecular-weight urine DNA samples (12%) from patients with mVIM-positive tissues contained detectable mVIM, suggesting that the mVIM detected in LMW urine DNA is derived from the circulation. The detection of mVIM in urine was significantly associated with CRC compared with controls (P < 0.0001, by Fisher's exact test). A potential urine test for CRC screening using epigenetic markers is discussed.
Colorectal cancer (CRC) remains the second leading cause of cancer deaths in the United States (>49,380 projected deaths in 2011)1 despite the availability of sensitive screening tests, such as colonoscopy. The inconvenience of the test and the risks involved contribute to the low adherence rate (40%) in US adults. The noninvasive fecal occult blood test is also available, but its sensitivity is low (∼30%). Fecal DNA tests for CRC screening have been extensively studied and gave encouraging results (up to 90% sensitivity) with open-labeled study participants.2–6 However, the sensitivity to detect CRC fell to ≤52% in large multicenter validation studies in an average-risk population7,8 and as reviewed by Bonanno et al9 and Levin et al.10 This result could be due to the massive contamination from bacterial DNA in stool.11,12 Thus, early detection of colon cancer by currently available screening methods remains a major challenge.
Circulating DNA has been studied for decades for cancer detection, including detection of CRC.12–22 However, it has not proved to be sufficiently sensitive to detect tumor-associated DNA alterations in the circulation. We and others have shown that urine contains DNA from the circulation15,23–29 and that circulation-derived DNA in urine is fragmented into segments of <300 bp [low-molecular-weight (LMW) urine DNA] that can be used to detect cancer-derived genetic mutations if the tumor is present. We have also shown that preferentially isolating LMW urine DNA from total urine DNA to use as the substrate enhanced the sensitivity and specificity of the test for detecting tumor-derived circulating K-ras–mutated DNA marker.30 Thus, we suggest that LMW DNA in urine could be used as a substrate for detecting circulation-derived DNA markers. However, because tumor-derived circulating DNA in urine is fragmented,24,31 several researchers, including us, have suggested that an assay targeting a small template size is required to have sufficient sensitivity to detect the DNA of interest.12,32–36
Aberrant hypermethylation of tumor suppressor genes occurs early and throughout the process of colorectal carcinogenesis.5,37–43 These DNA alterations could be used as biomarkers for cancer detection and disease management.37,43,44 Among the epigenetic DNA markers, the aberrant hypermethylated vimentin gene (mVIM) has shown great promise in fecal DNA tests for CRC screening8,37–39,45–47 and was detected in the serum of patients with CRC.20 Detection of methylated DNA markers has been challenging when the source of substrate DNA was fragmented or in low quantity, such as DNA isolated from formalin-fixed, paraffin-embedded sections, because the process of bisulfite (BS) conversion further fragments the DNA,48,49 and it was suggested that approximately 99.9% of the DNA was lost in this process.48 To detect circulation-derived mVIM in the urine of patients with CRC in this study, we developed an assay targeting a 39-nucleotide (nt) segment of the hypermethylated region in the vimentin gene that was associated with CRC. Using LMW urine DNA as the substrate and the short amplicon assay that we developed, we demonstrated that the circulation-derived, CRC-associated methylated DNA marker mVIM can be detected in the urine of patients with CRC. The potential of this assay for developing a urine test to detect CRC is discussed.
Materials and Methods
Study Participants and Specimens
Participants were recruited from the Great Lakes–New England Clinical Epidemiology and Validation Center (Ann Arbor, MI) under institutional review board approval. Patients with cancer were enrolled from surgical or oncologic services before treatment, and controls with “no known neoplasia” were enrolled from endoscopy suites, where they had undergone colonoscopies that yielded negative results. All the participants gave informed consent. The clinical profiles of patients with CRC and no-known-neoplasia controls are summarized in Table 1.
Table 1.
Clinical Profiles of Patients with CRC and No-Known-Neoplasia Controls
| ID | CRC |
No known neoplasia⁎ |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Urine sample ID | Tissue sample ID | TNM | Stage | Age (years) | Sex | Urine sample ID | Age (years) | Sex | |
| A | U20 | T15 | 2/0/? | I | 63 | M | N1 | 75 | F |
| B | U19 | T13 | 2/1/? | IIIa | 69 | M | N2 | 63 | F |
| C | U12 | T14 | 3/1/? | 58 | M | N3 | 71 | F | |
| D | U11 | T17 | 3/1/X | IIIb | 79 | F | N4 | 78 | M |
| E | U1 | T5 | 3/1/1 | IV | 54 | M | N5 | 72 | M |
| F | U5 | T12 | 3/0/X | IIa | 75 | F | N6 | 79 | M |
| G | U18 | T18 | 1/0/0 | I | 80 | M | N7 | 79 | F |
| H | U3 | T8 | 4/0/1 | IV | 45 | M | N8 | 85 | M |
| I | U14 | T20 | 3/2/X | IIIc | 69 | M | N9 | 82 | F |
| J | U16 | T10 | 2/0/X | Ic | 69 | M | N10 | 68 | F |
| K | U8 | T9 | 3/0/X | IIa | 36 | M | N11 | 66 | M |
| L | U10 | T2 | 3/2/1 | IV | 64 | M | N12 | 69 | F |
| M | U9 | T16 | 3/0/X | IIa | 65 | F | N13 | 77 | F |
| N | U15 | T4 | 4/0/0 | IIb | 32 | M | N14 | 65 | F |
| O | U17 | T6 | 4/2/1 | IV | 59 | F | N15 | 67 | F |
| P | U13 | T19 | 3/0/X | IIa | 74 | M | N16 | 82 | M |
| Q | U7 | T11 | 1/0/X | I | 46 | F | N17 | 69 | F |
| R | U4 | T3 | 3/0/0 | IIa | 46 | M | N18 | 88 | M |
| S | U6 | T7 | 3/2/0 | IIIc | 67 | F | N19 | 62 | M |
| T | U2 | T1 | 3/0/X | IIa | 59 | M | N20 | 79 | F |
| Age, mean (years) (P = 0.00048†) | 60.45 | 73.8 | |||||||
| Sex, F/M (No.) (P = 0.11‡) | 6/14 | 12/8 | |||||||
F, female; M, male; TNM, tumor node metastasis.
Controls with no known neoplasia were enrolled from endoscopy suites, where they had undergone colonoscopies that yielded negative results.
By Student's t-test.
By Fisher's exact test.
Urine Collection, DNA Isolation, and Fractionation of LMW and High-Molecular-Weight DNA
A total of 0.5 mol/L EDTA, pH 8.0, was added to freshly collected urine to a final concentration of 10 mmol/L EDTA to inhibit possible nuclease activity; the mixture was stored at −70°C. To isolate total urine DNA, the frozen urine sample was thawed at room temperature and then placed immediately in ice before the DNA was isolated. Total urine DNA was isolated from the thawed urine within an hour as described previously.24 The LMW urine DNA and high-molecular-weight (HMW) urine DNA fractions were obtained using carboxylated magnetic beads (Agencourt Bioscience Corp., Beverly, MA) and a binding method developed previously by our laboratory (Philadelphia, PA).30 DNA from paraffin-embedded tissue sections was isolated using the MasterPure DNA kit (Epicentre Biotechnologies, Madison, WI) per the manufacturer's instructions.
BS Treatment
BS conversion of DNA was performed using EpiTect BS conversion kits (Qiagen Inc, Valencia, CA) according to the manufacturer's specifications. To evaluate the efficiency of the BS conversion, BS-converted DNA was subjected to PCR amplification using BS-specific primer sets, as described previously.14 The PCR product was analyzed and visualized by gel containing ethidium bromide, excised, and purified by the Qiagen gel extraction kit (Qiagen Inc.); it was then sent to NapCore (Children's Hospital of Philadelphia, Philadelphia, PA) for DNA sequencing. The DNA sequencing data were compared with the sequence generated by the Methyl Primer Express software (Applied Biosystems, Foster City, CA) using ClustalW software (European Bioinformatics Institute, Cambridge, UK). The efficiency of the BS conversion was determined as the percentage of the number of non-CpG cytosine molecules that became thymidine compared with the percentage of the total number of non-CpG cytosine molecules in the region. Only samples that were converted with an efficiency of ≥95% were analyzed.
DNA Quantification by Real-Time PCR
DNA was quantified by a real-time PCR amplifying globin DNA as previously described.50 To quantify the BS-converted DNA, we developed a real-time PCR assay, BS-actin, targeting the BS-converted actin gene sequences. The primers, listed in Table 2, were designed within the regions that did not have any CpG sites in the gene so that the status of CpG methylation would not affect the primer binding. The BS-actin PCR was performed using the LightCycler 480 real-time PCR system (Roche Biochemical, Mannheim, Germany) and the LightCycler 480 SYBR Green I master kit (Roche Biochemical). The reaction contained 1× SYBR Green master mix, 1.0 μmol/L primers, and the BS-converted DNA template. The PCR was performed under the following conditions: 95°C for 10 minutes to activate the Taq polymerase, then 95°C for 10 seconds, 55°C for 20 seconds, 72°C for 10 seconds for 45 cycles, followed by determination of the melting curve at 95°C for 5 seconds, 65°C for 1 minute, and 97°C for continuous hold. Cooling occurred at 40°C for 30 seconds. The linearity and sensitivity of the assay were determined by performing the assay using a 10-fold dilution of the BS-converted human universal methylated DNA control (Zymo Research, Irvine, CA) (see Supplemental Figure S1 at http://jmd.amjpathol.org).
Table 2.
Oligonucleotides Used in the Study
| Assay (Genbank) | Oligonucleotide sequences⁎ | Annealing temperature (°C) |
|---|---|---|
| BS-actin (NT_007819) | Forward: 5′-GATGTATGAAGGTTTTTGG-3′ | 55 |
| Reverse: 5′-CTAACTACCTCCACCCACTC-3′ | ||
| MSP2945 (AL133415) | MSP29F: 5′-TCGTTTCGAGGTTTTCGCGTTAGAGAC-3′ | 68 |
| MSP29R: 5′-CGACTAAAACTCGACCGACTCGCGA-3′ | ||
| VIM29R_LNA 2-step MethyLight | 1F: 5′-GCTCTTCGTGGTGTGGTGCGGTTCGGGTATCGC-3′ | First PCR: 62 |
| 1R: 5′-GCTCTTCGTGGTGTGGTGCTCCGACTAAAACTCGACC-3′ | ||
| 2F: 5′-GTGTGGTGCGGTTC-3′ | Second PCR: 60 | |
| 2R: 5′-GTGTGGTGCTCCGAC-3′ | ||
| TaqMan probe: FAM-ATCGCGAGTCGGTCGAGTT-BHQ1 |
The artificial DNA sequences are underlined, and LNAs are in bold.
Development of PCR Assays for the Aberrant Methylation of the Vimentin Gene
To develop the MethyLight assay for mVIM, target sequences from the promoter and first exon regions of the vimentin gene were obtained from GenBank (AL133415) using PubMed software (National Center for Biotechnology Information, Bethesda, MD). CpG analysis was performed using Methyl Primer Express software (Applied Biosystems). Primers and probes for a two-step nested MethyLight assay targeting template sizes of 39 nt were designed (Table 2). The first PCR was performed in a thermocycler (Eppendorf, Hamburg, Germany) using the first PCR primer set (0.1 μmol/L), dNTP (20 μmol/L), and HotStar Taq (Qiagen Inc.) under the following conditions: 95°C for 15 minutes to activate the HotStar Taq polymerase, then 95°C for 30 seconds, 62°C for 30 seconds, 72°C for 20 seconds for 30 cycles, and 72°C for 5 minutes. The second PCR was performed using the LightCycler 2.0 PCR instrument with the second PCR primers (0.5 μmol/L each) and TaqMan probe (0.15 μmol/L), 1 μL of first PCR product (1:20 dilution) with Roche LightCycler TaqMan 5x master mix under the following conditions: 95°C for 10 minutes and 40 cycles at 95°C for 10 seconds, 60°C for 10 seconds, and 72°C for 10 seconds; cooling occurred at 40°C for 30 seconds.
To determine the linearity and sensitivity of the assay, reconstituted standard samples ranging from 5 to 500 copies of a series of BS-converted universal methylated human DNA standard (Zymo Research), with a negative control, 100 copies of BS-treated human unmethylated DNA standard (Zymo Research) were used to assess assay sensitivity. A previously established methylation-specific PCR assay for the mVIM, the MSP29 assay,37 was used as a control assay to evaluate the methylation status of the vimentin gene in CRC tissue.
Results
Development of a MethyLight Assay to Detect the mVim Gene in Fragmented Short DNA Templates
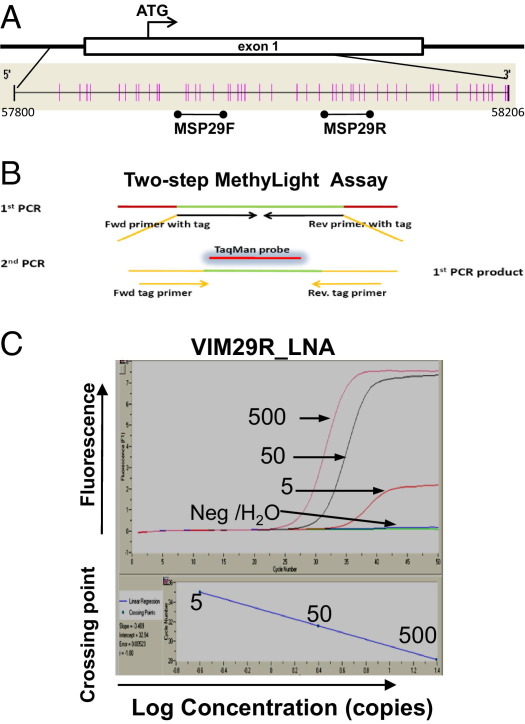
It has been suggested that PCR assays that target template sequences of ≤50 nt are necessary to obtain a sensitivity >50% to detect DNA of interest in urine derived from the circulation.34,35 To develop an assay for detecting mVIM in circulation-derived urine DNA, we first identified a target region, the MSP29R region (Figure 1A), for assay development. The MSP29R region was chosen because of the results of a comprehensive study by Chen et al45 that analyzed the promoter and first exon regions of the vimentin gene. The results indicated that the region studied using the methylation-specific PCR assay MSP29 had the highest specificity and sensitivity as a biomarker for CRC; the specificity of using the MSP29 assay for detecting mVIM in CRC was confirmed by other investigators.39,46,47 Because the primer sequences determine the specificity of the PCR assay, the reverse primer region of MSP29 (designated MSP29R; Figure 1) was chosen for assay design.
Figure 1.
Development of a two-step MethyLight PCR assay for the mVIM gene. A: Schematic location of the CpG sites generated by the Methyl Primer Express software in the promoter and the first exon regions of the vimentin gene (GenBank AL133415) and the locations of the forward and reverse primer regions of the MSP29 methylation-specific PCR assay37 are indicated. Each vertical line represents a CpG site. B: Schematic diagrams of the two-step nested quantitative MethyLight assay. The green line represents the target sequence. For the two-step PCR assay, the forward and reverse primers of the first PCR cycle include the targeted sequence and an artificial sequence (orange). The second PCR cycle has a forward and a reverse primer (with mostly artificial sequences shown in orange) and a TaqMan probe. C: Amplification curves of the mVIM MethyLight assay VIM29R_LNA. The amplification curves and the linear standard were generated from a reconstituted standard as described in Materials and Methods. The numbers of copies of positive control DNA, negative control DNA (Neg), and H2O are indicated.
Two strategies were applied to shorten the target size and enhance assay sensitivity, as illustrated in Figure 1, A and B; the primer sequences are listed in Table 2. First, an artificial 18-nt DNA sequence, 5′-GCTCTTCGTGGTGTGGTG-3′, was tagged at the 5′ end of the first PCR primers to increase the amplicon size, and then it was used as part of the primer sequences for the second PCR (Table 2). Second, two locked nucleic acids (LNAs) were incorporated into the reverse primer of the first PCR assay at two CpG sites to allow for more sensitive priming of the assay. Accordingly, a two-step MethyLight assay targeting a template of 39 nt, designated VIM29R_LNA, produced a specific PCR product from the positive control templates (100 copies of BS-actin–quantified, BS-converted WiDr DNA) and no product from the negative control templates (500 copies of BS-actin–quantified, BS-converted HepG2 DNA) (data not shown). The amplification curve and linearity of the VIM29R_LNA assay are shown in Figure 1C. This assay can detect ≥5 copies of mVIM per reaction quantitatively in the presence of 500 copies of BS-converted HepG2 DNA (negative control).
Detection of mVIM in Colorectal Tumor and Matched Urine Samples in a Blinded Study
We evaluated whether the developed assay, VIM29R_LNA, could detect mVIM in the urine of patients with CRC. A blinded study was performed using matching tissue and urine DNA samples from patients with CRC. The 20 CRC tissue samples and 20 urine samples from patients with CRC were provided with barcodes; the researchers did not know which tissue sample matched which urine sample. Total urine DNA was isolated, fractionated into LMW and HMW DNA, subjected to BS conversion, and quantified by the BS-actin real-time PCR assay, as described in Materials and Methods. We first assessed the variability of the VIM29R_LNA assay in clinical urine samples. Each urine DNA sample was tested in triplicate. DNA, equivalent to 1 mL of urine, was subjected to each assay. Since no detectable amplification signal was generated with 500 copies of BS-converted HepG2 DNA (negative control), we valued any positive data generated even if it represented <5 copies, the lowest standard that was used in the assay. The mean ± SEM values for mVIM were calculated for each sample (Table 3). The percentage of the SE for all the triplicate data sets except two (U2 and U8) was less than 10%, showing high reproducibility. Encouragingly, 75% (15 of 20) of the LMW urine DNA from patients with CRC contained detectable mVIM. In contrast, only two HMW urine DNA fractions, U2 and U11, contained detectable amounts of mVIM (Table 4). As expected, the LMW urine DNA fractions of U2 and U11 were positive for mVIM.
Table 3.
Variability of the VIM29R_LNA Assay for Detecting mVIM in Urine DNA
| Sample ID | Methylated VIM29R (copies/mL of urine) |
|||
|---|---|---|---|---|
| Experiment 1 | Experiment 2 | Experiment 3 | Mean ± SEM | |
| U1 | 47 | 43.75 | 34 | 38.88 ± 6.89 |
| U2 | 348.95 | 965.5 | 220.6 | 511.68 ± 398.22 |
| U3 | 3.85 | 2.8 | 4.6 | 3.75 ± 0.90 |
| U4 | 27.95 | 29.5 | 29.4 | 28.95 ± 0.87 |
| U5 | 6.5 | 7.7 | 6.35 | 6.85 ± 0.74 |
| U6 | 13.7 | 9.25 | 12.45 | 11.8 ± 2.30 |
| U7 | ND | ND | ND | NA |
| U8 | 291 | 450 | 419.15 | 386.7 ± 84.3 |
| U9 | ND | ND | ND | NA |
| U10 | 400.45 | 406.15 | 394.45 | 400.4 ± 5.85 |
| U11 | 3.7 | ND | 2.75 | 3.23 ± 0.67 |
| U12 | 93.6 | 91.7 | 82.9 | 89.4 ± 5.71 |
| U13 | ND | ND | ND | NA |
| U14 | 2.65 | 1.7 | ND | 2.18 ± 0.67 |
| U15 | ND | ND | ND | NA |
| U16 | ND | 6 | 3.9 | 4.95 ± 1.48 |
| U17 | ND | ND | ND | NA |
| U18 | 58.2 | 74.3 | 60.5 | 64.3 ± 8.7 |
| U19 | 46.4 | 40.2 | 41.4 | 42.67 ± 3.29 |
| U20 | 4.1 | 4.75 | 4.2 | 4.35 ± 0.35 |
NA, not applicable; ND, not detectable.
Table 4.
Detection of mVIM in LMW Urine DNA Isolated from the Urine of Patients with CRC and No-Known-Neoplasia Controls by the VIM29R_LNA Assay
| CRC |
No known neoplasia |
|||||||
|---|---|---|---|---|---|---|---|---|
| Sample ID | LMW (copies/mL)⁎ |
HMW (copies/mL)⁎ |
Sample ID | LMW (copies/mL)⁎ |
||||
| VIM29R_LNA | MSP29 | BS-actin | VIM29R_LNA | BS-actin | VIM29R_LNA | BS-actin | ||
| U1 | 38.88 | ND | 267.3 | ND | 36.3 | N1 | ND | 83.6 |
| U2 | 511.7 | ND | 149.5 | 4.6 | 5.8 | N2 | 60 | 294 |
| U3 | 3.75 | ND | 174.5 | ND | 210 | N3 | ND | 38.8 |
| U4 | 28.95 | ND | 256.3 | ND | 33.9 | N4 | ND | 1148 |
| U5 | 6.85 | ND | 673.8 | ND | 979 | N5 | 9.4 | 80.4 |
| U6 | 11.8 | ND | 59.7 | ND | 19.8 | N6 | ND | 43.6 |
| U7 | ND | ND | 239.3 | ND | 190 | N7 | ND | 33.2 |
| U8 | 386.7 | ND | 628.2 | ND | 234 | N8 | ND | 123.6 |
| U9 | ND | ND | 477.9 | ND | 150 | N9 | ND | 32.8 |
| U10 | 400.35 | ND | 79.1 | ND | 15 | N10 | ND | 97.2 |
| U11 | 3.23 | ND | 51.8 | 4.5 | 10.3 | N11 | ND | 237.6 |
| U12 | 89.4 | ND | 222.2 | ND | 31 | N12 | ND | 784 |
| U13 | ND | ND | 57.4 | ND | 42.6 | N13 | ND | 20.4 |
| U14 | 2.18 | ND | 97.3 | ND | 117.2 | N14 | ND | 82.8 |
| U15 | ND | ND | 68.6 | ND | 101.3 | N15 | ND | 148.8 |
| U16 | 4.95 | ND | 264.4 | ND | 149.7 | N16 | ND | 46.4 |
| U17 | ND | ND | 173.7 | ND | 817 | N17 | ND | 35.6 |
| U18 | 64.3 | ND | 16.4 | ND | 11.2 | N18 | ND | 26.6 |
| U19 | 42.8 | ND | 135.8 | ND | 258 | N19 | ND | 16.4 |
| U20 | 4.35 | ND | 926.7 | ND | 152 | N20 | ND | 30 |
ND, not detectable.
The quantity of DNA presented is the average of two or three independent assays.
It was of interest to see whether a previously established mVIM assay, MSP29, which amplified a 216-nt segment of template,45 could detect mVIM in LMW urine DNA fractions from patients with CRC. None of the LMW urine DNA fractions were positive for mVIM by the MSP29 assay. As controls, 20 LMW urine DNA fractions from the no-known-neoplasia group were also subjected to the VIM29_LNA assay. Two of 20 LMW urine DNA fractions had a low amount of mVIM (Table 4). As a control for the amount of input DNA templates in mVIM-negative DNA samples, the amount of BS-converted β-actin templates in each DNA sample determined by the BS-actin PCR assay was also listed. Thus, the detection of mVIM in urine is significantly associated with CRC [15 of 20; 95% confidence interval (CI), 10.55–17.83[ compared with the no-known-neoplasia controls (2 of 20; 95% CI, 0.31–6.264) (P < 0.0001 by Fisher's exact test).
Next, a blinded study was performed by determining the amount of mVIM in CRC tissue and urine DNA. We subjected 5 ng of CRC tissue DNA to the VIM29R_LNA assay in triplicate; the averages are given in Table 5. mVIM was detectable in 85% (17 of 20) of the CRC tissue DNA samples, although some of the samples contained a low level of mVIM. After all the samples were tested, the urine and tissue ID numbers were unblinded and matched as listed in Table 5. Although this was a pilot study to determine whether the VIM29R_LNA assay could detect circulation-derived mVIM, it was of interest to see whether there was any correlation between the mVIM-positive CRC tissue and matched urine DNA samples and whether the amount of mVIM detected in urine could be correlated with the stage of the cancer. We, therefore, generated a two-way contingency table (Table 6) and found no significant association between the detection of mVIM in urine and in matched CRC tissue (P = 0.5395, by Fisher's exact test). To compare the amount of mVIM detected in urine with the stages of CRC, we divided the CRC samples in two groups (stages I and II and stages III and IV) and calculated the average amount of mVIM detected in the urine samples of each group. The average amount of mVIM was 91.73 copies/mL of urine for stages I and II and 63.2 copies/mL of urine for stages III and IV. The P value was 0.700 by Student's t-test, suggesting that the amount of mVIM detected in urine is not correlated with the stage of CRC.
Table 5.
Detection of mVIM in Tissue and Corresponding Urine DNA Samples
| Patient ID | Matched samples, after unblinding | Methylated VIM29R copies⁎ |
|
|---|---|---|---|
| Tissue | Urine | ||
| A | T15/U20 | 0.9 | 4.4 |
| B | T13/U19 | 1.8 | 42.8 |
| C | T14/U12 | 2 | 89.4 |
| D | T17/U11 | 2.1 | 3.3 |
| E | T5/U1 | 5 | 41.6 |
| F | T12/U5 | 33.5 | 6.9 |
| G | T18/U18 | 145 | 64.4 |
| H | T8/U3 | 235 | 3.5 |
| I | T20/U14 | 430 | 2.2 |
| J | T10/U16 | 990 | 5.9 |
| K | T9/U8 | 1975 | 386.7 |
| L | T2/U10 | 3150 | 400.4 |
| M | T16/U9 | 19 | ND |
| N | T4/U15 | 24.5 | ND |
| O | T6/U17 | 60 | ND |
| P | T19/U13 | 460 | ND |
| Q | T11/U7 | 4305 | ND |
| R | T3/U4 | ND | 29.0 |
| S | T7/U6 | ND | 11.8 |
| T | T1/U2 | ND | 511.7 |
| Incidence [No./total No. (%)] | 17/20 (85) | 15/20 (75) | |
ND, not detectable.
Values are given as means (n = 3).
Table 6.
Two-Way Contingency Table of Detection of mVIM in Tissue versus Urine
| Tissue mVIM | Urine mVIM |
Total | |
|---|---|---|---|
| + | − | ||
| + | 12 | 5 | 17 |
| − | 3 | 0 | 3 |
| Total | 15 | 5 | 20 |
Table entries are number of participants; Fisher's exact t-test, P = 0.5395.
Discussion
We detected, for the first time, a CRC-associated epigenetic hypermethylation DNA marker in the urine of patients with CRC using a two-step MethyLight PCR assay targeting a 39-nt sequence of mVIM that was developed in this study. In addition to genetic mutation markers, epigenetic methylated DNA markers could potentially be included in a urine test for the detection of CRC.
This project emphasizes the importance of having an assay that can detect small templates if the substrates are short fragments. The VIM29R_LNA assay, which targets the 39-nt template, detected mVIM in 75% of LMW urine DNA fractions, whereas the MSP29 assay, which also detected 5 copies of mVIM per reaction (data not shown), targeting the 216-nt template, did not detect mVIM in any of the LMW urine DNA fractions. This difference cannot be attributed to the difference in the region assayed because the VIM29R_LNA assay targets the reverse primer of MSP29; 80% of the matched CRC tissues were positive for mVIM, which is similar to the incidence of mVIM in CRC in previous studies using the MSP29 assay.45
Although we detected mVIM in 75% of the LMW urine DNA samples (n = 12) from patients with CRC, only two of those corresponding HMW urine DNA fractions contained detectable amounts of mVIM. As suggested from previous studies,24,30 the HMW urine DNA was derived mostly from sloughed off cell debris from the urinary tract. The circulation-derived DNA was found primarily in the LMW urine DNA fraction. The present data further confirm that urine contains DNA from the circulation and that circulation-derived urine DNA is mostly in the LMW urine DNA fraction. Moreover, we demonstrated that the mVIM detected in the urine of patients with CRC was derived from the circulation, probably from CRC tissue. Ten percent of urine samples from the no-known-neoplasia controls were positive for mVIM in urine. This finding is not surprising because the methylation of mVIM was found in approximately 10% to 15% of normal colon tissue samples.39,45,46 It is also possible that the 10% of mVIM in urine samples was from tissue other than colon tissue.
LNA molecules have been successfully used by us50–52 and others53–59 to increase PCR assay sensitivity. By incorporating LNAs into the reverse primer of the first PCR reaction, we successfully increased 10-fold the sensitivity of the two-step PCR assay for detecting mVIM (data not shown). In the process of assay development, we also tested the forward and reverse primers that contained LNAs at the CpG sites for the first PCR reaction. When both primers were LNA-containing oligos, the specificity of the assay decreased with nonspecific priming during the PCR (data not shown). Although we used various combinations of LNA and regular DNA-only primers, the only combination that demonstrated a 10-fold increase in sensitivity and generated only one expected PCR product was that of the regular DNA-only forward primer and the LNA-containing reverse primer in the first PCR reaction. This observation could be due to the nature of the LNA, which has a high binding affinity, thus increasing the opportunity for intermolecular interaction.
The detection of mVIM in CRC tissues (Table 4) is consistent with the results of previous studies in which mVIM was found in 70% to 80% of CRC tissue samples.39,45–47 In this pilot study, with a sample size of 20, we also detected a similar proportion (75%) of urine samples containing mVIM from patients with CRC. Although the detection of mVIM in urine is significantly associated with CRC compared with no known neoplasia (P < 0.0001 by Fisher's exact test), the amount of mVIM detected in urine was not correlated with the stages of CRC (P = 0.700 by Student's t-test) when comparing the average of mVIM in stages I and II (91.73 copies/mL of urine) versus stages III and IV (63.2 copies/mL of urine). The two-way contingency table analysis (Table 6) suggests that the concordance between detection of mVIM in urine and in its matched CRC tissue is not significant (P = 0.5395), probably because the sample size is too small.
In patients R, S, and T, mVIM was not detected in CRC tissue samples but was detected in the corresponding urine samples (Table 5). This difference may be due to the fact that the DNA extracted from the tissue samples had no mVIM, whereas the DNA from other sites in the body had mVIM that was detected in the urine assay. However, some tissue samples had large amounts of mVIM, such as T11 and T19, whereas the corresponding urine samples had none. One explanation could be that the rate of apoptosis of these particular tumors was limited; thus, the quantity of apoptotic-derived tumor DNA was not sufficient to be detected in patients' urine. Alternatively, plasma DNA could be used for the detection of mVIM. Interestingly, a previous study36 suggested that the concentration of mutated K-ras DNA in plasma is similar to that in urine of patients with CRC. Urine can be collected in a larger volume with less contamination; thus, more DNA could be used, which would result in a higher frequency of detection of mutated K-ras DNA from patients with mutated K-ras CRC. Thus, we do not think that using plasma DNA would increase the detection of circulating mVIM in patients with CRC compared with using LMW urine DNA.
In summary, the detection of circulation-derived, hypermethylated CRC-associated DNA markers in the urine of patients with CRC provides a promising approach for developing a urine test for CRC screening that includes methylated DNA markers. A larger sample size to determine the performance of mVIM alone as a urine marker or combined with other CRC genetic markers in urine to detect CRC or adenoma is the next step toward the development of a urine screening test for the early detection of CRC. The success of this study could deliver a urine-based screening test for colon cancer and, ultimately, for other cancers with known genetic and epigenetic alterations that could be used for screening, detecting the tumor early, monitoring recurrence, and managing the disease.
Acknowledgments
We thank Xiao-He Wang for technical assistance and Pamela Fried for editorial help.
Footnotes
Supported by NIH grant R01 CA125642 (Y.-H.S.), Department of Defense grant CA093176 (Y.-H.S.), a Prevent Cancer Foundation Postdoctoral Fellowship Award (S.J.), an Early Detection Research Network grant from the National Cancer Institute (T.M.B.), and an appropriation from the Commonwealth of Pennsylvania (T.M.B.).
Supplemental material for this article can be found at http://jmd.amjpathol.org or at doi: 10.1016/j.jmoldx.2011.12.003.
Contributor Information
Surbhi Jain, Email: surbhi.jain@drexelmed.edu.
Ying-Hsiu Su, Email: ying-hsiu.su@drexelmed.edu.
Supplementary data
Amplification curve and linearity of the BS-actin real-time PCR assay. The amplification curve (top) and the linear standard (bottom) were generated using a 10-fold dilution the bisulfite-treated human universal methylated DNA control (Zymo Research, Irvine, CA). The amount of DNA (copy) for each curve is indicated.
References
- 1.Siegel R., Ward E., Brawley O., Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Dong S.M., Traverso G., Johnson C., Geng L., Favis R., Boynton K., Hibi K., Goodman S.N., D'Allessio M., Paty P., Hamilton S.R., Sidransky D., Barany F., Levin B., Shuber A., Kinzler K.W., Vogelstein B., Jen J. Detecting colorectal cancer in stool with the use of multiple genetic targets. J Natl Cancer Inst. 2001;93:858–865. doi: 10.1093/jnci/93.11.858. [DOI] [PubMed] [Google Scholar]
- 3.Osborn N.K., Ahlquist D.A. Stool screening for colorectal cancer: molecular approaches. Gastroenterology. 2005;128:192–206. doi: 10.1053/j.gastro.2004.10.041. [DOI] [PubMed] [Google Scholar]
- 4.Syngal S., Stoffel E., Chung D., Willett C., Schoetz D., Schroy P., Jagadeesh D., Morel K., Ross M. Detection of stool DNA mutations before and after treatment of colorectal neoplasia. Cancer. 2006;106:277–283. doi: 10.1002/cncr.21558. [DOI] [PubMed] [Google Scholar]
- 5.Ahlquist D.A., Skoletsky J.E., Boynton K., Harrington J.J., Mahoney D.W., Pierceall W.E., Thibodeau S.N., Shuber A.P. Colorectal cancer screening by detection of altered human DNA in stool: feasibility of a multitarget assay panel. Gastroenterology. 2000;119:1219–1227. doi: 10.1053/gast.2000.19580. [DOI] [PubMed] [Google Scholar]
- 6.Traverso G., Shuber A., Levin B., Johnson C., Olsson L., Schoetz D.J., Jr., Hamilton S.R., Boynton K., Kinzler K.W., Vogelstein B. Detection of APC mutations in fecal DNA from patients with colorectal tumors. N Engl J Med. 2002;346:311–320. doi: 10.1056/NEJMoa012294. [DOI] [PubMed] [Google Scholar]
- 7.Imperiale T.F., Ransohoff D.F., Itzkowitz S.H., Turnbull B.A., Ross M.E., Colorectal Cancer Study Group Fecal DNA versus fecal occult blood for colorectal-cancer screening in an average-risk population. N Engl J Med. 2004;351:2704–2714. doi: 10.1056/NEJMoa033403. [DOI] [PubMed] [Google Scholar]
- 8.Ahlquist D.A., Sargent D.J., Loprinzi C.L., Levin T.R., Rex D.K., Ahnen D.J., Knigge K., Lance M.P., Burgart L.J., Hamilton S.R., Allison J.E., Lawson M.J., Devens M.E., Harrington J.J., Hillman S.L. Stool DNA and occult blood testing for screen detection of colorectal neoplasia. Ann Intern Med. 2008;149:441–450. doi: 10.7326/0003-4819-149-7-200810070-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonanno E., Rulli F., Galatà G., Pucci S., Sesti F., Farinon A.M., Spagnoli L.G. Stool test for colorectal cancer screening: what is going on. Surg Oncol. 2007;16(Suppl 1):43–45. doi: 10.1016/j.suronc.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 10.Levin B., Lieberman D.A., McFarland B., Andrews K.S., Brooks D., Bond J., Dash C., Giardiello F.M., Glick S., Johnson D., Johnson C.D., Levin T.R., Pickhardt P.J., Rex D.K., Smith R.A., Thorson A., Winawer S.J., American Cancer Society Colorectal Cancer Advisory Group; US Multi-Society Task Force; American College of Radiology Colon Cancer Committee Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134:1570–1595. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Klaassen C.H.W., Jeunink M.A.F., Prinsen C.F.M., Ruers T.J.M., Tan A.C.I.T.L., Strobbe L.J.A., Thunnissen F.B.J.M. Quantification of human DNA in feces as a diagnostic test for the presence of colorectal cancer. Clin Chem. 2003;49:1185–1187. doi: 10.1373/49.7.1185. [DOI] [PubMed] [Google Scholar]
- 12.Diehl F., Schmidt K., Durkee K.H., Moore K.J., Goodman S.N., Shuber A.P., Kinzler K.W., Vogelstein B. Analysis of mutations in DNA isolated from plasma and stool of colorectal cancer patients. Gastroenterology. 2008;135:489–498. doi: 10.1053/j.gastro.2008.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anker P. Quantitative aspects of plasma/serum DNA in cancer patients. Ann N Y Acad Sci. 2000;906:5–7. doi: 10.1111/j.1749-6632.2000.tb06580.x. [DOI] [PubMed] [Google Scholar]
- 14.Anker P., Mulcahy H., Chen X.Q., Stroun M. Detection of circulating tumour DNA in the blood (plasma/serum) of cancer patients. Cancer Metastasis Rev. 1999;18:65–73. doi: 10.1023/a:1006260319913. [DOI] [PubMed] [Google Scholar]
- 15.Diehl F., Schmidt K., Choti M.A., Romans K., Goodman S., Li M., Thornton K., Agrawal N., Sokoll L., Szabo S.A., Kinzler K.W., Vogelstein B., Diaz L.A., Jr Circulating mutant DNA to assess tumor dynamics. Nat Med. 2008;14:985–990. doi: 10.1038/nm.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gautschi O., Bigosch C., Huegli B., Jermann M., Marx A., ChassÉ E., Ratschiller D., Weder W., Joerger M., Betticher D.C., Stahel R.A., Ziegler A. Circulating deoxyribonucleic acid as prognostic marker in non-small-cell lung cancer patients undergoing chemotherapy. J Clin Oncol. 2004;22:4157–4164. doi: 10.1200/JCO.2004.11.123. [DOI] [PubMed] [Google Scholar]
- 17.Herman J.G. Circulating methylated DNA. Ann N Y Acad Sci. 2004;1022:33–39. doi: 10.1196/annals.1318.006. [DOI] [PubMed] [Google Scholar]
- 18.Mulcahy H.E., Lyautey J., Lederrey C., Chen X.Q., Lefort F., Vasioukhin V., Anker P., Alstead E.M., Farthing M.J., Stroun M. Plasma DNA k-ras mutations in patients with gastrointestinal malignancies. Ann N Y Acad Sci. 2000;906:25–28. doi: 10.1111/j.1749-6632.2000.tb06585.x. [DOI] [PubMed] [Google Scholar]
- 19.Ryan B.M., Lefort F., McManus R., Daly J., Keeling P.W.N., Weir D.G., Kelleher D. A prospective study of circulating mutant kras2 in the serum of patients with colorectal neoplasia: strong prognostic indicator in postoperative follow up. Gut. 2003;52:101–108. doi: 10.1136/gut.52.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shirahata A., Sakuraba K., Goto T., Saito M., Ishibashi K., Kigawa G., Nemoto H., Hibi K. Detection of vimentin (vim) methylation in the serum of colorectal cancer patients. Anticancer Res. 2010;30:5015–5018. [PubMed] [Google Scholar]
- 21.Grutzmann R., Molnar B., Pilarsky C., Habermann J.K., Schlag P.M., Saeger H.D., Miehlke S., Stolz T., Model F., Roblick U.J., Bruch H.P., Koch R., Liebenberg V., Devos T., Song X., Day R.H., Sledziewski A.Z., Lofton-Day C. Sensitive detection of colorectal cancer in peripheral blood by septin 9 DNA methylation assay. PLoS One. 2008;3:e3759. doi: 10.1371/journal.pone.0003759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lofton-Day C., Model F., DeVos T., Tetzner R., Distler J., Schuster M., Song X., Lesche R., Liebenberg V., Ebert M., Molnar B., Grützmann R., Pilarsky C., Sledziewski A. DNA methylation biomarkers for blood-based colorectal cancer screening. Clin Chem. 2008;54:414–423. doi: 10.1373/clinchem.2007.095992. [DOI] [PubMed] [Google Scholar]
- 23.Lichtenstein A.V., Melkonyan H.S., Tomei D., Umansky S.R. Circulating nucleic acids and apoptosis. Ann N Y Acad Sci. 2001;945:239–249. doi: 10.1111/j.1749-6632.2001.tb03892.x. [DOI] [PubMed] [Google Scholar]
- 24.Su Y.H., Wang M., Brenner D.E., Ng A., Melkonyan H., Umansky S., Syngal S., Block T.M. Human urine contains small, 150 to 250 nucleotide-sized, soluble DNA derived from the circulation and may be useful in the detection of colorectal cancer. J Mol Diagn. 2004;6:101–107. doi: 10.1016/S1525-1578(10)60497-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan A.K.C., Chiu R.W.K., Lo Y.M.D. Cell-free nucleic acids in plasma, serum and urine: a new tool in molecular diagnosis. Ann Clin Biochem. 2003;40:122–130. doi: 10.1258/000456303763046030. [DOI] [PubMed] [Google Scholar]
- 26.Pathak A.K., Bhutani M., Kumar S., Mohan A., Guleria R. Circulating cell-free DNA in plasma/serum of lung cancer patients as a potential screening and prognostic tool. Clin Chem. 2006;52:1833–1842. doi: 10.1373/clinchem.2005.062893. [DOI] [PubMed] [Google Scholar]
- 27.Anker P., Lyautey J., Lederrey C., Stroun M. Circulating nucleic acids in plasma or serum. Clin Chim Acta. 2001;313:143–146. doi: 10.1016/s0009-8981(01)00666-0. [DOI] [PubMed] [Google Scholar]
- 28.Jahr S., Hentze H., Englisch S., Hardt D., Fackelmayer F.O., Hesch R., Knippers R. DNA fragments in the blood plasma of cancer patients: quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res. 2001;61:1659–1665. [PubMed] [Google Scholar]
- 29.Stroun M., Maurice P., Vasioukhin V., Lyautey J., Lederrey C., Lefort F., Rossier A., Chen X.Q., Anker P. The origin and mechanism of circulating DNA. Ann N Y Acad Sci. 2000;906:161–168. doi: 10.1111/j.1749-6632.2000.tb06608.x. [DOI] [PubMed] [Google Scholar]
- 30.Su Y.-H., Song J., Wang Z., Wang X., Wang M., Brenner D.E., Block T.M. Removal of high molecular weight DNA by carboxylated magnetic beads enhances the detection of mutated k-ras DNA in urine. Ann N Y Acad Sci. 2008;1137:82–91. doi: 10.1196/annals.1448.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang M., Block T.M., Steel L., Brenner D.E., Su Y.H. Preferential isolation of fragmented DNA enhances the detection of circulating mutated k-ras DNA. Clin Chem. 2004;50:211–213. doi: 10.1373/clinchem.2003.026914. [DOI] [PubMed] [Google Scholar]
- 32.Chan K.C.A., Leung S.F., Yeung S.W., Chan A.T.C., Lo Y.M.D. Quantitative analysis of the transrenal excretion of circulating EBV DNA in nasopharyngeal carcinoma patients. Clin Cancer Res. 2008;14:4809–4813. doi: 10.1158/1078-0432.CCR-08-1112. [DOI] [PubMed] [Google Scholar]
- 33.Melkonyan H.S., Feaver W.J., Meyer E., Scheinker V., Shekhtman E.M., Xin Z., Umansky S.R. Transrenal nucleic acids: from proof of principle to clinical tests; problems and solutions. Ann N Y Acad Sci. 2008;1137:73–81. doi: 10.1196/annals.1448.015. [DOI] [PubMed] [Google Scholar]
- 34.Shekhtman E.M., Anne K., Melkonyan H.S., Robbins D.J., Warsof S.L., Umansky S.R. Optimization of transrenal DNA analysis: detection of fetal DNA in maternal urine. Clin Chem. 2009;55:723–729. doi: 10.1373/clinchem.2008.113050. [DOI] [PubMed] [Google Scholar]
- 35.Sikora A., Zimmermann G., Rusterholz C., Birri D., Kolla V., Lapaire O., Hoesli I., Kiefer V., Jackson L., Hahn S. Detection of increased amounts of cell-free fetal DNA with short PCR amplicons. Clin Chem. 2010;56:136–138. doi: 10.1373/clinchem.2009.132951. [DOI] [PubMed] [Google Scholar]
- 36.Su Y.-H., Wang M., Norton P.A., Brenner D.E., Block T.M. Detection of mutated k-ras DNA in urine, plasma and serum from patients with colorectal carcinoma or adenomatous polyps. Ann N Y Acad Sci. 2008;1137:197–201. doi: 10.1196/annals.1448.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahlquist D.A. Molecular detection of colorectal neoplasia. Gastroenterology. 2010;138:2127–2139. doi: 10.1053/j.gastro.2010.01.055. [DOI] [PubMed] [Google Scholar]
- 38.Zou H., Harrington J.J., Shire A.M., Rego R.L., Wang L., Campbell M.E., Oberg A.L., Ahlquist D.A. Highly methylated genes in colorectal neoplasia: implications for screening. Cancer Epidemiol Biomarkers Prev. 2007;16:2686–2696. doi: 10.1158/1055-9965.EPI-07-0518. [DOI] [PubMed] [Google Scholar]
- 39.Itzkowitz S.H., Jandorf L., Brand R., Rabeneck L., Schroy Iii P.C., Sontag S., Johnson D., Skoletsky J., Durkee K., Markowitz S., Shuber A. Improved fecal DNA test for colorectal cancer screening. Clin Gastroenterol Hepatol. 2007;5:111–117. doi: 10.1016/j.cgh.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 40.Kimura N., Nagasaka T., Murakami J., Sasamoto H., Murakami M., Tanaka N., Matsubara N. Methylation profiles of genes utilizing newly developed CpG island methylation microarray on colorectal cancer patients. Nucleic Acids Res. 2005;33:e46. doi: 10.1093/nar/gni046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grady W.M., Carethers J.M. Genomic and epigenetic instability in colorectal cancer pathogenesis. Gastroenterology. 2008;135:1079–1099. doi: 10.1053/j.gastro.2008.07.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iacobuzio-Donahue C.A. Epigenetic changes in cancer. Annu Rev Pathol. 2009;4:229–249. doi: 10.1146/annurev.pathol.3.121806.151442. [DOI] [PubMed] [Google Scholar]
- 43.Kim M., Lee J., Sidransky D. DNA methylation markers in colorectal cancer. Cancer Metastasis Rev. 2010;29:181–206. doi: 10.1007/s10555-010-9207-6. [DOI] [PubMed] [Google Scholar]
- 44.Markowitz S.D., Bertagnolli M.M. Molecular basis of colorectal cancer. N Engl J Med. 2009;361:2449–2460. doi: 10.1056/NEJMra0804588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen W.D., Han Z.J., Skoletsky J., Olson J., Sah J., Myeroff L., Platzer P., Lu S., Dawson D., Willis J., Pretlow T.P., Lutterbaugh J., Kasturi L., Willson J.K.V., Rao J.S., Shuber A., Markowitz S.D. Detection in fecal DNA of colon cancer-specific methylation of the nonexpressed vimentin gene. J Natl Cancer Inst. 2005;97:1124–1132. doi: 10.1093/jnci/dji204. [DOI] [PubMed] [Google Scholar]
- 46.Zou H., Harrington J., Rego R.L., Ahlquist D.A. A novel method to capture methylated human DNA from stool: implications for colorectal cancer screening. Clin Chem. 2007;53:1646–1651. doi: 10.1373/clinchem.2007.086223. [DOI] [PubMed] [Google Scholar]
- 47.Zou H., Taylor W.R., Harrington J.J., Hussain F.T.N., Cao X., Loprinzi C.L., Levine T.R., Rex D.K., Ahnen D., Knigge K.L., Lance P., Jiang X., Smith D.I., Ahlquist D.A. High detection rates of colorectal neoplasia by stool DNA testing with a novel digital melt curve assay. Gastroenterology. 2009;136:459–470. doi: 10.1053/j.gastro.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 48.Tanaka K., Okamoto A. Degradation of DNA by bisulfite treatment. Bioorg Med Chem Lett. 2007;17:1912–1915. doi: 10.1016/j.bmcl.2007.01.040. [DOI] [PubMed] [Google Scholar]
- 49.Raizis A.M., Schmitt F., Jost J.P. A bisulfite method of 5-methylcytosine mapping that minimizes template degradation. Anal Biochem. 1995;226:161–166. doi: 10.1006/abio.1995.1204. [DOI] [PubMed] [Google Scholar]
- 50.Lin S.Y., Dhillon V., Jain S., Chang T.T., Hu C.T., Lin Y.J., Chen S.H., Chang K.C., Song W., Yu L., Block T.M., Su Y.H. A locked nucleic acid clamp-mediated PCR assay for detection of a p53 codon 249 hotspot mutation in urine. J Mol Diagn. 2011;13:474–484. doi: 10.1016/j.jmoldx.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ren X.D., Lin S.Y., Wang X., Zhou T., Block T.M., Su Y.H. Rapid and sensitive detection of hepatitis b virus 1762T/1764A double mutation from hepatocellular carcinomas using LNA-mediated PCR clamping and hybridization probes. J Virol Methods. 2009;158:24–29. doi: 10.1016/j.jviromet.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Su Y.H., Wang M., Block T.M., Landt O., Botezatu I., Serdyuk O., Lichtenstein A., Melkonyan H., Tomei L.D., Umansky S. Transrenal DNA as a diagnostic tool: important technical notes. Ann N Y Acad Sci. 2004;1022:81–89. doi: 10.1196/annals.1318.014. [DOI] [PubMed] [Google Scholar]
- 53.Ballantyne K.N., van Oorschot R.A.H., Mitchell R.J. Locked nucleic acids in PCR primers increase sensitivity and performance. Genomics. 2008;91:301–305. doi: 10.1016/j.ygeno.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 54.Gustafson K.S. Locked nucleic acids can enhance the analytical performance of quantitative methylation-specific polymerase chain reaction. J Mol Diagn. 2008;10:33–42. doi: 10.2353/jmoldx.2008.070076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Latorra D., Arar K., Hurley J.M. Design considerations and effects of LNA in PCR primers. Mol Cell Probes. 2003;17:253–259. doi: 10.1016/s0890-8508(03)00062-8. [DOI] [PubMed] [Google Scholar]
- 56.Maertens O., Legius E., Speleman F., Messiaen L., Vandesompele J. Real-time quantitative allele discrimination assay using 3′ locked nucleic acid primers for detection of low-percentage mosaic mutations. Anal Biochem. 2006;359:144–146. doi: 10.1016/j.ab.2006.07.039. [DOI] [PubMed] [Google Scholar]
- 57.Collado M., Landt O., Barragan E., Lass U., Cervera J., Sanz M.A., Bolufer P. Locked nucleic acid-enhanced detection of 1100delc*chek2 germ-line mutation in Spanish patients with hematologic malignancies. Clin Chem. 2004;50:2201–2204. doi: 10.1373/clinchem.2004.038331. [DOI] [PubMed] [Google Scholar]
- 58.Oldenburg R.P., Liu M.S., Kolodney M.S. Selective amplification of rare mutations using locked nucleic acid oligonucleotides that competitively inhibit primer binding to wild-type DNA. J Invest Dermatol. 2009;128:398–402. doi: 10.1038/sj.jid.5700920. [DOI] [PubMed] [Google Scholar]
- 59.Ugozzoli L.A., Latorra D., Pucket R., Arar K., Hamby K. Real-time genotyping with oligonucleotide probes containing locked nucleic acids. Anal Biochem. 2004;324:143–152. doi: 10.1016/j.ab.2003.09.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Amplification curve and linearity of the BS-actin real-time PCR assay. The amplification curve (top) and the linear standard (bottom) were generated using a 10-fold dilution the bisulfite-treated human universal methylated DNA control (Zymo Research, Irvine, CA). The amount of DNA (copy) for each curve is indicated.