Abstract
The role of CD8+ T cells in the pathogenesis of asthma remains controversial, as both pro- and anti-inflammatory functions have been suggested. This study was designed to examine the endogenous CD8+ T cell response in a biphasic ovalbumin (OVA)–induced model of allergic airway disease (AAD) and its subsequent resolution with the development of local inhalational tolerance (LIT). We observed increases in OVA-specific CD8+ T cell numbers in the local lung compartments (bronchoalveolar lavage, lung tissue, hilar lymph node) at AAD and LIT; systemic compartments (spleen, inguinal lymph node) displayed no such increases in CD8+ T cell numbers. OVA-specific CD8+ T cells appeared to exhibit plasticity both phenotypically and functionally. They possessed pro-inflammatory characteristics at AAD, with high phenotypic expression of CD11a and increased functional expression of granzyme B and interferon-γ. In contrast, at LIT they showed increased phenotypic expression of the inhibitory marker NKG2A and functionally did not produce granzyme B or interferon-γ. In addition, in a discontinuous model the OVA-specific CD8+ T cells could be recalled on re-exposure to OVA, demonstrating memory. Finally, confocal microscopy results showed that OVA-specific CD8+ T cells at AAD are associated with B cell aggregates in lung tissue. These B cell aggregates resembled tertiary ectopic lymphoid tissue and may thus provide a local environment for the salient cellular interactions that contribute to the development of LIT.
Research over the last three decades has provided evidence that T helper 2 (Th2) CD4+ lymphocytes are a major contributor to the development of allergic airway disease (AAD) in animals and asthma in humans.1–3 These Th2 cells produce cytokines that promote many of the features associated with AAD, including B cell class switching and production of IgE (IL-4), lung eosinophilia (IL-5), and increased mucus levels (IL-13). Furthermore, in murine models of AAD, CD4+Foxp3+ T regulatory cells (Tregs) have been shown to play a role in resolution of the disease and the onset of local inhalational tolerance (LIT).4,5 Analogous to CD4+ Th1 and Th2 cells, CD8+ T cells are capable of forming cytokine-specific subsets termed Tc1 and Tc2 cells, respectively.6 In addition, CD8+ T cells can develop into cytotoxic T-lymphocytes (CTLs), which express granzyme, produce interferon (IFN)–γ, and are capable of antigen-specific killing through T-cell receptor recognition of the cognate antigen presented on MHC I.7 Although it is apparent that Th2 cells play a critical role in the development of AAD and Tregs in the resolution phase at LIT, the role of CD8+ T cells has not been fully elucidated.
Both pro- and anti-inflammatory roles have been demonstrated for CD8+ T cells in the development of AAD and asthma.8–14 Although CD8+ T cells have not been directly implicated with eosinophilia, it has been demonstrated that they contribute to airway hyperresponsiveness (AHR) in animals during AAD.11,13 Certain models have also been shown to elicit Tc2 CD8+ T cells, which produce the Th2 cytokines associated with asthma.12,14 Conversely, in other models, CD8+ T cells have been shown to suppress IgE production15 and to inhibit eosinophilic airway inflammation.14,16 Previously, in a biphasic ovalbumin (OVA)–induced murine model of AAD, in which resolution occurs with long-term continuous antigen challenge, our laboratory has demonstrated increased numbers of CD8+ cells in lung tissue and in the bronchoalveolar lavage (BAL) at AAD and LIT compared with that in sensitized animals, and increases in these cells in the draining lymph node at LIT.4 Other investigators have demonstrated that the magnitude of the antigen-specific CD8+ T cell responses generated during AAD is dependent on the dose of antigen used during sensitization and inversely correlated with the severity of disease.17 Together, these findings suggest a possible role for antigen-specific CD8+ cells in the development of AAD and LIT.
Using the biphasic OVA-induced model of AAD, we examined the distribution of endogenous OVA-specific CD8+ T cells (OVA-TET+) in the local lung compartments and systemic tissues via flow cytometry and confocal microscopy. The purpose of this study was to determine, using phenotypic and functional assessments, whether endogenous CD8+ T cells were comparable or exhibit plasticity at each stage (AAD and LIT) of the biphasic OVA-induced model.
Materials and Methods
Animals and OVA Exposure Protocol
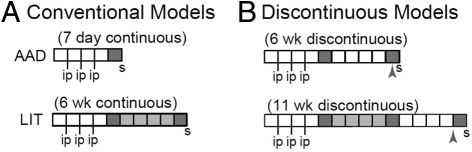
Specific pathogen free (SPF) female C57BL/6J mice (stock number 000664) were obtained from The Jackson Laboratory (Bar Harbor, ME). The results for testing of various viruses, bacteria, and mycoplasma organisms for shipment were provided by the Jackson Laboratory, confirming SPF status. The SPF status during housing at the University of Connecticut Health Center (UCHC) is verified with quarterly serological testing. The only pathogen that tested positive was the ubiquitous mouse norovirus (MNV). Mice were not tested for antibodies to cilia-associated respiratory (CAR) bacillus. Regarding possible bacillus contamination, we have not observed any abnormal lung pathology in control naive or OVA-sensitized mice. The mice were housed in IVC cages stored in a Thoren unit for ventilation in the animal facility at the University of Connecticut Health Center and were treated in accordance with all Institutional and Office of Laboratory Animal Welfare guidelines. As previously described4,18,19 and as shown in Figure 1, mice were immunized weekly with intraperitoneal injections of 25 μg OVA in 2 mg of alum for 3 consecutive weeks (sensitized). Starting 1 week after the last injection, mice were exposed to 1% aerosolized OVA in physiological saline, 1 hour per day (estimated inhaled daily dose of 30–40 μg/mouse), following one of four protocols classified as either conventional or discontinuous models (Figure 1). Conventional models (Figure 1A) were as follows: i) Allergic Airway Disease (AAD), in which sensitized mice were exposed to OVA aerosol 1 hour per day for 7 days (7-day continuous); and ii) resolution or Local Inhalational Tolerance (LIT), in which sensitized mice were exposed to OVA aerosol 1 hour per day for 7 days, followed by 1 hour per day, 5 days per week for 4 weeks, then 1 hour per day for 7 days (6-week continuous). Discontinuous models (Figure 1B) included the following: iii) 6-week discontinuous, whereby mice were sensitized and received OVA aerosol following the AAD protocol. This was followed by no aerosol exposure for 4 weeks, and then an additional 7 days of OVA aerosol for 1 hour per day. In the last model, iv) 11-week discontinuous, mice were sensitized and exposed to OVA following the LIT protocol, then no aerosol exposure for 4 weeks and then an additional 7 days OVA aerosol for 1 hour per day. AAD and LIT rested groups were used as controls for the discontinuous models, which, following AAD or LIT, received 4 to 5 weeks of no aerosol before sacrifice.
Figure 1.
Models of allergic airway disease. A: Conventional models of AAD and LIT with continuous aerosol of OVA post i.p. sensitization. B: Discontinuous (6-week and 11-week) models of AAD with staggered aerosol of OVA post i.p. sensitization. Dark gray and light gray areas represent weeks in which animals received 7 days or 5 days of 1% OVA aerosol, respectively. For each model group, the letter S indicates when animals were sacrificed. Arrowheads indicate rested (no aerosol) control groups for the discontinuous models. These groups were euthanized after receiving 4 to 5 weeks of rest subsequent to the initial aerosol protocol without receiving a second challenge.
Bronchoalveolar Lavage and Tissue Analysis
At sacrifice, bronchoalveolar lavage (BAL) fluid, hilar lymph node (HLN), lung tissue, inguinal lymph node (ILN), and the spleen from each animal was harvested and processed for the isolation and enumeration of leukocytes. It should be noted that our use of the term “HLN” more specifically refers to the tracheobronchial lymph node, whereas “ILN” refers to the subiliac lymph node.20 We use the more generic HLN and ILN to represent the region, as they are commonly used in many publications. For collection of BAL, lungs were lavaged in situ with five 1.0-mL aliquots of sterile saline. Lymph nodes and spleens were harvested and mechanically disrupted into a single-cell suspension. Lung tissues were minced and treated with collagenase solution (150 U/mL, Gibco, Grand Island, NY) for 1 hour at 37°C. The minced tissue was mechanically disrupted through a 40-μm nylon filter (BD Falcon, Bedford, MA). For the lung tissue and spleen, red blood cells were lysed with Tris ammonium chloride. For all tissue samples, total nucleated cell counts were obtained using a hemocytometer with nigrosin dye exclusion as a measure of viability.
Antibodies, Immunofluorescence Reagents, and Flow Cytometry
The following monoclonal antibodies were used for cellular surface staining: α-CD8α (53–6.7), α-CD3 (145-2c11), α-CD11a (2D7), α-CD3 (145-2c11), α-CD62L (MEL-14), and α-NKG2AB6 (16a11) and were purchased from eBioscience (San Diego, CA) or BioLegend (San Diego, CA). H-2Kb tetramers containing the OVA-derived peptide SIINFEKL were generated in the laboratory as described previously.21 After surface marker staining, cells were lysed and fixed using Cytofix/perm buffer (BD Pharmingen, San Jose, CA) as instructed by the manufacturer. Cells were then stained with α-granzyme B (GB11; eBioscience).
For intracellular cytokine staining, 1 × 106 cells from the BAL, lung or HLN were plated in a 96-well plate and treated with BD Golgi Plug (BD Pharmingen). Cells were stimulated with SIINFEKL peptide or left unstimulated for 5 hours. Cells were then stained for surface cell markers, treated with Cytofix/perm buffer (BD Pharmingen), followed by intracellular cytokine staining with α-IFN-γ(XMG1.2), α-IL-4(11B11), α-IL-5 (TRFK5), or α-IL-13 (ebio13A) (eBioscience). After staining, all samples were run on a Becton Dickinson LSR II and analyzed with FlowJo (Tree Star Inc., Ashland, OR) software. Standard gating strategies based on isotype controls began by gating on lymphocytes (side by forward scatter), which was followed by gating on CD8 T cells (CD3 by CD8). CD8 T cells were further gated by OVA-TET and CD11a for identification of OVA-TET+ CD8+ T cells. Since tissue single cell suspensions are prepared differently, a specific gating strategy was used for each.
Enrichment of Antigen-Specific CD8+ T Cells
Enrichment of OVA-TET+ CD8+ T cells from sensitized and naive mice were performed as previously described.22 Briefly, single cell suspensions from the spleen or pooled lymph nodes (axillary, mandibular, cervical, HLN, ILN, colic, jejunal, and caudal mesenteric) were prepared as described above, stained with both phosphatidylethanolamine- and allophycocyanin (APC)-labeled tetramers and α-CD8 antibody. They were then stained with α-phosphatidylethanolamine microbeads as per the instructions of the manufacturer (Miltenyi Biotec, Auburn, CA). The samples were then run on an AutoMACs (Miltenyi Biotec) magnetic column cell separator. After enrichment, cells were stained with α-CD11a, α-CD62L, α-CD4, α-CD19, α-IAb, and α-CD11b for 30 minutes at 4°C. Cells were then washed and fixed with 2% paraformaldehyde. The entire sample was then analyzed with an LSRII cytometer (Becton Dickinson Biosciences, San Jose, CA).
Light and Confocal Microscopy
After sacrifice, nonmanipulated lungs from each animal (n = 4/group) not subjected to BAL were removed, fixed with 10% buffered formalin, and processed in a standard manner for light microscopy. Lung tissue were transversely sliced/sectioned and stained with hematoxylin and eosin. All five lobes from each animal were examined under light microscopy.
Whole-mount tissue preparations were used to visualize the location of the endogenous CD8+ T cells in the lung tissue via confocal microscopy.23,24 Lung tissue from sensitized, AAD, or LIT mice were transversely sliced/sectioned into sections 300 to 500 μm thick. Tissues were washed and stained overnight at 4°C in round-bottomed, 48-well plates with Cy5-labeled CD8a (53–6.7, eBioscience), α-B220 with Alexa Fluor 488 (Invitrogen, Grand Island, NY), and APC-conjugated H-2Kb tetramer containing the SIINFEKL (OVA366–374) peptide (1 μg/mL) diluted in 2% normal goat serum and 2% FBS/PBS solution. The tissues were extensively washed and fixed in 2% paraformaldehyde for 2 hours at 4°C. The fixed tissues were washed again with PBS and incubated overnight at 4°C. The next day, the tissues were washed extensively and were incubated overnight at 4°C with rabbit α-APC antibody. The tissues were again washed extensively and incubated overnight at 4°C with Alexa Fluor 546 goat anti-rabbit IgG (Invitrogen). Stained tissues were washed extensively and then mounted on slides using Immu-Mount (Thermo Shandon, Pittsburgh, PA).
Images were collected using a Zeiss LSM-510-Meta confocal microscope mounted on an Axiovert 100M with automated xyz control equipped with an argon laser (emission at 458, 488, and 514 nm), and two HeNe lasers (emission wavelengths at 543 and 633 nm). Image analysis was performed using Imaris Suite (Bitplane, South Windsor, CT). Images were processed using the median filter algorithm.
Statistical Analysis
Normal distribution was determined via the Shapiro–Wilk test. Statistical comparisons between the means of multiple groups were made with analysis of variance followed by a Student's unpaired t-test. Comparisons between the mean of two groups were made via unpaired t-test. All statistical analysis was performed using JMP 5.1 statistical software (SAS Institute, Cary, NC). In all comparisons, P ≤ 0.05 was used to determine statistical significance.
Results
Endogenous OVA-TET+ CD8+ T Cells Increase in Local Lung Compartments (BAL, Lung, and HLN) but Not in Systemic Tissues (Spleen and ILN)
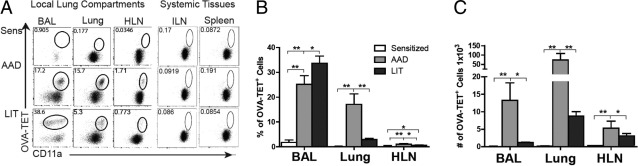
As shown in the representative dot plots (Figure 2A), OVA-TET+ CD8+ T cells were not identified in sensitized mice in any of the local lung compartments or the systemic tissues examined using our standard protocol for isolating cells from tissue. Tetramer enrichment was used to identify OVA-TET+ CD8+ T cells and demonstrated significantly increased numbers of OVA-TET+ CD8+ T cells in sensitized mice compared with naive animals (see Supplemental Figure S1A at http://ajp.amjpathol.org).22 In sensitized animals, increased numbers of these cells were identified in the spleen as compared with the pooled lymph nodes but there was no difference in the frequency of these cells between the two compartments (see Supplemental Figures S1, B and C at http://ajp.amjpathol.org). At AAD, significant increases in the percentage of OVA-TET+ CD8+ T cells were observed in the local lung compartments compared with sensitized mice: BAL 24% vs 1% (P < 0.01); lung 17% vs 0.1% (P < 0.01); HLN 1% vs 0.1% (P < 0.01) (Figure 2B). Unlike the HLN and lung, the percentage of BAL OVA-TET+ CD8+ T cells was found to be increased at LIT (31%) as compared with sensitized mice (P < 0.01) and AAD (P < 0.05; Figure 2B). Furthermore, at LIT, the percentages in the HLN (0.5%) and lung (3.5%) were significantly decreased as compared with AAD mice (HLN P < 0.05; lung P < 0.01) but remained elevated over those tissues from sensitized mice (P < 0.05 each, Figure 2B). In the systemic tissues, OVA-TET+ CD8+ T cells were not detected at any of the time points examined (Figure 2A).
Figure 2.
Identification of OVA-TET+ CD8+ T cells in local lung compartments and systemic tissues. Mice exposed to the conventional model protocol were sacrificed at sensitization, AAD, and LIT, and OVA-TET+ CD8+ T cells were isolated and counted from the various lung (BAL, lung tissue, HLN) and systemic tissue (ILN and spleen) compartments as described in the text. A: Representative dot plots from one mouse in each group are shown for CD8+ T cells gated on OVA-TET and CD11a; circles identify all OVA-TET+ CD8+ T cells in each tissue and time point. Mean values of OVA-TET+ CD8+ T cells for all mice in each group are shown as a percentage (B) and as total number (C) identified in the BAL, lung tissue, and HLN at each respective time point. OVA-TET+ CD8+ T cells were below detectable levels in the systemic tissues, and therefore the percentage and total numbers were not calculated. Data represent the mean ± SEM of six to eight mice per group, *P ≤ 0.05, **P ≤ 0.01.
In each of the local lung compartments, the total number of OVA-TET+ CD8+ T cells significantly increased in mice at AAD as compared with sensitized mice, and then decreased in mice at LIT. The BAL at AAD had 13 × 103 OVA-TET+ CD8+ T cells, as compared to 0.001 × 103 cells from sensitized mice (P < 0.01) and 1.2 × 103 cells from LIT mice (P < 0.05); the lung at AAD had 74 × 103 CD8+ T cells, as compared to 1.2 × 103 cells from sensitized mice (P < 0.01) and 8 × 103 cells from LIT mice (P < 0.01); the HLN had 6 × 103 OVA-TET+ CD8+ T cells from AAD mice, as compared to 0.3 × 103 cells from sensitized mice (P < 0.01) and 3 × 103 cells from LIT mice (P < 0.05; Figure 2C).
Light Histopathology and Confocal Microscopy Images of Lung Tissue from Sensitized, AAD, and LIT Mice Demonstrate Prominent Lymphoid Aggregates at AAD
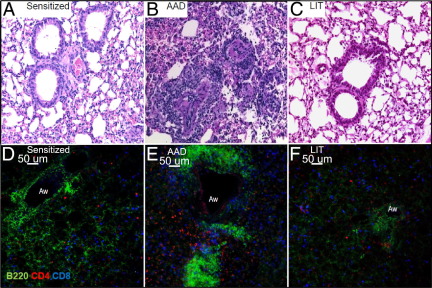
Lymphoid aggregates of B cells (B220+) were identified in lung tissue of mice at AAD (Figure 3, B and E) but were absent in lung tissue from sensitized mice (Figure 3, A and D). These aggregates were prominent in perivascular and peribronchial regions at AAD. No aggregates could be found after 1 day of OVA aerosol, and only small clusters were visualized after 3 days of OVA aerosol (see Supplemental Figure S2 at http://ajp.amjpathol.org). Larger aggregates were first observed at 5 days of OVA challenge and peaked at 7 days (AAD). After 14 days of OVA aerosol, these aggregates had begun to diminish (see Supplemental Figure S2 at http://ajp.amjpathol.org),23,24 and they remained at low density at LIT (Figure 3, C and F).
Figure 3.
Light histopathology and confocal microscopy images of lung tissue from sensitized, AAD, and LIT mice demonstrating prominent lymphoid aggregates at AAD. Mice at various stages of the model (sensitized, AAD, and LIT) were sacrificed and the lungs removed and prepared for standard light and confocal microscopy as described in the Materials and Methods. Briefly, for light microscopy, formalin-fixed lung tissue was processed in a standard manner and stained with hematoxylin and eosin. For confocal microscopy, freshly isolated lung tissue was sliced/sectioned and stained with specific antibodies overnight at 4°C before fixation with 2% paraformaldehyde. Light microscopy images (×20) from the lungs of sensitized (A) and LIT (C) mice appear normal, with no apparent infiltration of inflammatory cells. Prominent peribronchial and perivascular inflammation was not present in lungs of naive (not shown) or sensitized animals (A), but was apparent with aggregates of lymphoid-like cells in AAD mice (B). Confocal microscopy images (×20) confirmed the absence of B-cell aggregates in sensitized animals (D), but their presence at AAD (E). Corresponding with resolution of inflammation, these lymphoid aggregates were considerably diminished in lung tissue from LIT mice (F). Aw = airway. Images shown are from one representative mouse for each group and are consistent with images from a total of four mice per group.
OVA-TET+ CD8+ T Cells Identified via Confocal Microscopy in Lung Tissue of AAD Mice Are Associated with Peribronchial and Perivascular B Cell Aggregates
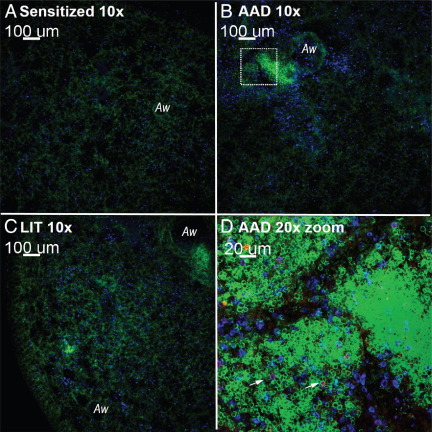
Few OVA-TET+ CD8+ T cells could be visualized in lung tissue of sensitized mice (Figure 4A), but these cells were readily identified at AAD (Figure 4, B and D) with fewer being seen at LIT (Figure 4C). The OVA-TET+ CD8+ T cells were found within the B-cell aggregates.
Figure 4.
Confocal microscopy images identify OVA-TET+ CD8+ T cells in B-cell aggregates at AAD. Mice at various stages of the model (sensitized, AAD, and LIT) were sacrificed, and confocal microscopy was performed as described in the Materials and Methods. In addition to the general T and B cell stains, the APC-conjugated H-2Kb tetramer containing the SIINFEKL OVA366–374 peptide stain was used to identify OVA-specific CD8+ T cells. Representative lung sections from sensitized (A), AAD (B), and LIT (C) mice were assessed at low magnification (×10). B cell (B220+) aggregates were visible at AAD (B and D) and diminished considerably in lung tissue from LIT mice (C). Digital zoom photograph (×20) (D) taken from the white square in B illustrates CD8+ T cells and OVA-TET+ CD8+ T cells associated with the B-cell aggregates. OVA-TET+ CD8+ T cells are indicated with white arrows. Green indicates B220+ cells; blue indicates CD8+ cells; and, with a dual stain overlay, magenta represents OVA-TET+ CD8+ T cells. Images shown are from one representative mouse for each group and are consistent with images from a total of four mice per group.
These B-cell aggregates were a common feature of AAD, were widespread throughout the lung, and demonstrated co-association with CD8+ T cells (Figure 4D; see also Supplemental Figure S3 at http://ajp.amjpathol.org).
Phenotypic Changes of Activation Markers Occur in OVA-TET+ CD8+ T Cells at AAD and LIT
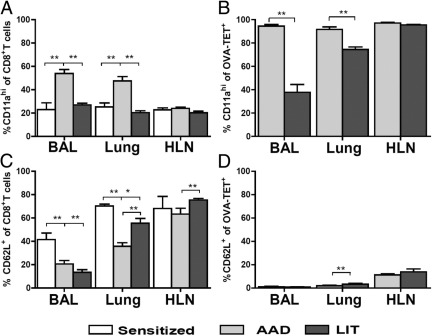
Expression of known T-cell activation markers CD11a and CD62L was examined on CD8+ T cells and OVA-TET+ CD8+ T cells in the local lung compartments of sensitized, AAD and LIT mice (Figure 5). OVA-TET+ CD8+ T cells were not identifiable in the local lung compartments of sensitized animals (data not shown). In the entire CD8+ T cell population, significant increases in the percentage of CD11ahi cells were observed in the BAL (53%) and lung (46%) at AAD as compared with sensitized animals (BAL 23%, P < 0.01; lung 25%, P < 0.01 each) and LIT animals (BAL 27%, P < 0.01; lung 20%, P < 0.01; Figure 5A). Moreover, within the antigen-specific CD8+ T cell subpopulation, CD11ahi OVA-TET+ CD8+ T cells at AAD were also increased in BAL (90%) and lung (88%) compared with LIT (BAL 39%, P < 0.01; lung 74%, P < 0.01; Figure 5B). Thus, the percentage of CD11alo OVA-TET+ CD8+ T cells increased at LIT in the BAL (61%) and lung (26%) compared with AAD (BAL 10% and lung 12%). No differences were observed in CD11ahi expression on CD8+ T cells or on OVA-TET+ CD8+ T cells in the HLN at the time points examined.
Figure 5.
Phenotypic changes on CD8+ T cells and OVA-TET CD8+ T cells were identified in the local lung compartments. Expression of CD11a and CD62L were examined on CD8+ T cells (A, C) and OVA-TET+ CD8+ T cells (B, D) in BAL, lung tissue, and HLN of sensitized, AAD, and LIT mice. OVA-TET+ CD8+ T cells were not identifiable in sensitized animals and therefore are not included in B or D. Data represent the mean ± SEM of five to eight mice per group. *P ≤ 0.05, **P ≤ 0.01.
The percentages of CD62L+ CD8+ T cells in sensitized BAL (41%) and lung (70%) were significantly increased compared with AAD (BAL 21%, P < 0.01; lung 34%, P < 0.01) and LIT (BAL 13%, P < 0.01; lung 59%, P < 0.05) (Figure 5C). The percentage of CD62L+ CD8+ T cells rebounded toward sensitized levels in lung at LIT compared with AAD (P < 0.01). There was a similar increase in CD62L+ CD8+ T cells in the HLN at LIT (75%) compared with AAD (58%, P < 0.01, Figure 5C). The percentage of CD62L+ OVA-TET+ CD8+ T cells observed in AAD BAL (2%) and HLN (12%) were equivalent with those at LIT (BAL 1%; HLN 12%). In the lung, there was a significant increase of these cells at LIT (4%) compared with AAD (2%, P < 0.01, Figure 5D).
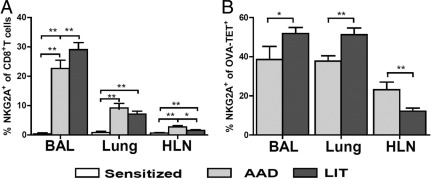
Increased Percentages of NKG2A-Positive OVA-TET+ CD8+ T Cells in Lung Compartments Are Observed at AAD and LIT
NKG2A has been shown to be a phenotypic marker of regulatory CD8 T cells, which interact with Qa-1b.25 Few NKG2A+ CD8+ T cells were found in BAL, lung, and HLN of sensitized mice (<1% each). The percentages of NKG2A+ CD8+ T cells significantly increased at AAD in all compartments (BAL 22%, P < 0.01; lung 8%, P < 0.01; HLN 2%, P < 0.01) and remained increased at LIT (BAL 28%, P < 0.01; lung 7% P < 0.01; HLN 1.5%, P < 0.01). Compared with AAD, these cells at LIT were significantly increased in BAL (P < 0.05), remained the same in lung, and were significantly decreased in HLN (P < 0.05, Figure 6A). NKG2A+ OVA-TET+ CD8+ T cells were significantly increased at LIT (BAL 52%; lung 51%) compared with AAD (BAL 39%, P < 0.01; lung 37%, P < 0.01). In the HLN, a decrease was observed in these cells at LIT (12%) compared with AAD (23%, P < 0.01, Figure 6B).
Figure 6.
Increased percentages of NKG2A-positive OVA-TET+ CD8+ T cells in lung compartments were observed at AAD and LIT. Expression of NKG2A was examined on CD8+ T cells (A) and OVA-TET+ CD8+ T cells (B) in BAL, lung tissue, and HLN of sensitized, AAD, and LIT mice. OVA-TET+ CD8+ T cells were not identifiable in sensitized animals and therefore are not included in B. Data represent the mean ± SEM of five to eight mice per group. *P ≤ 0.05, **P ≤ 0.01.
Functional Changes, Granzyme B, and IFN-γ Expression, Occur in CD8+ T Cells at AAD and LIT
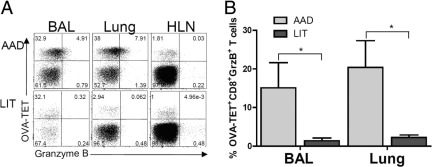
Granzyme B
As demonstrated in representative dot plots (Figure 7), OVA-TET+ CD8+ T cells from the BAL, lung, or HLN were analyzed for granzyme B expression. Granzyme B positive OVA-TET+ CD8+ T were observed in the BAL and in lung at AAD but not at LIT or in the HLN. There was little granzyme B detected in the CD8+ T cells that were OVA-TET negative (Figure 7A). At AAD, in the BAL and lung, 15% and 20% of OVA-TET+ CD8+ T cells were granzyme B+, which was significantly increased compared with LIT BAL 1% (P < 0.05) and lung 2% (P < 0.05; Figure 7B).
Figure 7.
The percentage of OVA-TET+ CD8+ T cells expressing granzyme B was increased in BAL and lung tissue at AAD. Mice at various stages of the model (AAD and LIT) were sacrificed, and OVA-TET+ CD8+ T cells were isolated from the BAL, lung tissue, and HLN. Intracellular granzyme B (GrzB) expression was determined via flow cytometry. A: Representative dot plots from one mouse in each group are shown for CD8+ T cells gated by OVA-TET and positive for GrzB, and demonstrate increased double-positive cells at AAD as compared with LIT. B: The mean number of GrzB postive OVA-TET+ CD8+ T cells was shown to be significantly elevated in the BAL and lung tissue at AAD as compared with LIT. HLN levels of GrzB were below detectable levels and are not represented graphically. Data represent the mean ± SEM of four mice per group. *P ≤ 0.05.
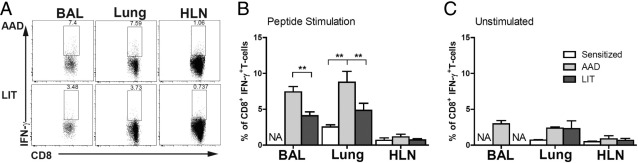
IFN-γ
As demonstrated in representative dot plots, the BAL and lung of AAD animals had an increased percentage of IFN-γ+ CD8+ T cells after OVA peptide stimulation, which was not observed in the HLN (Figure 8A). The percentage of IFN-γ+ CD8+ cells in lung (2.5%) was significantly increased in AAD lung (8%, P < 0.01) but not in LIT lung (4%) after peptide stimulation. A corresponding increase in the percentage of IFN-γ+ CD8+ cells was observed in the BAL at AAD (7%) compared with LIT (4%, P < 0.01) but was not observed in the (HLN <1%) at any time point. The number of IFN-γ+ CD8+ cells isolated from the BAL of sensitized mice decreased below the detectable level and are not represented (Figure 8B). No changes were observed in the percentage of IFN-γ+ CD8+ T cells in unstimulated cells from any of the lung compartments of sensitized, AAD, and LIT animals (Figure 8C). In addition, CD8+ T cells were examined for Tc2 cytokines IL-4, IL-5, and IL-13 in the lung compartments of sensitized, AAD, and LIT mice. Few (<1%) CD8+ T cells produced any of the Tc2 cytokines examined with or without peptide stimulation (data not shown).
Figure 8.
The percentage of CD8+ T cells expressing IFN-γ production in response to SIINFEKL peptide stimulation was increased in BAL and lung tissue at AAD. Mice at various stages of the conventional model (AAD and LIT) were sacrificed, and CD8+ T cells isolated from the BAL, lung tissue, and HLN. Intracellular IFN-γ expression was determined via flow cytometry. A: Representative dot plots from one mouse in each group are shown for cells gated on CD8+ T cells and IFN-γ and demonstrate increased double-positive cells in BAL and lung tissue at AAD as compared with LIT. The percentage of IFN-γ+ CD8+ T cells was shown to be significantly elevated in the BAL and lung tissue at AAD as compared with LIT after peptide stimulation (B), but no significant differences were seen after nonstimulated control experiments (C). CD8 T cells positve for IFN-γ from BAL of sensitized and LIT animals were below detectable levels and are not represented graphically. The data represent the mean ± SEM of four to eight mice per group. **P ≤ 0.01.
Increases in BAL Eosinophilia and OVA-TET+ CD8+ T Cells Are Observed in Discontinuous Models
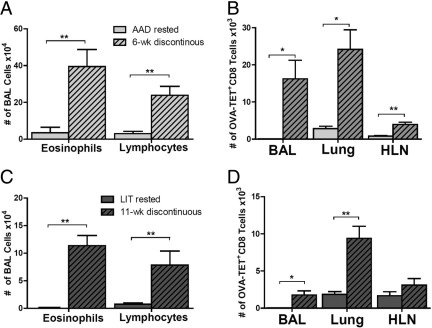
The number of BAL eosinophils and lymphocytes from 6-week discontinuously treated mice were significantly increased compared with AAD rested animals (Figure 9A). The total number of OVA-TET+ CD8+ T cells were also significantly increased in the lung compartments of 6-week discontinuously treated mice (BAL 13 × 103; lung 24 × 103; HLN 4 × 103) as compared with AAD rested (no aerosol) mice (BAL 0.1 × 103, P < 0.01; lung 3 × 103, P < 0.01; HLN 0.5 × 103, P < 0.01; Figure 9B). Like the 6-week discontinuously treated mice, significant increases were identified in the total number of eosinophils and lymphocytes of 11-week discontinuously treated mice compared with LIT rested animals (P < 0.01, Figure 9C). These significant increases were also reflected in the total number of OVA-TET+ CD8+ T cells in the 11-week discontinuously treated animals (BAL 1.8 × 103; lung 11 × 103; HLN 3 × 103) compared with LIT rested mice (no aerosol for 4 weeks) (BAL 0.1 × 103, P < 0.01; lung 2 × 103, P < 0.01; HLN 1.5 × 103, P < 0.05; Figure 9D). Similar increases were also seen in the percentage of BAL eosinophils and lymphocytes between the discontinuously treated and rested control groups (data not shown).
Figure 9.
Local lung OVA-TET+ CD8+ T cells were capable of re-expanding after re-exposure to inhaled OVA in the 6-week and 11-week discontinuous animal models. Mice in the discontinuous models with long periods of rest (no inhaled OVA) were sacrificed, and OVA-TET+ CD8+ T cells were isolated from the BAL, lung tissue, and HLN. In both the 6-week (A) and 11-week (C) discontinuous models, there was a significant increase in the total number of BAL eosinophils and lymphocytes as compared with their respective rested controls. In a similar manner, in both the 6-week (B) and 11-week (D) discontinuous models, there was a significant increase in the total number of OVA-TET+ CD8+ T cells in the BAL and lung tissue compartments as compared with their respective rested controls. Data represent the mean ± SEM of three to eight mice per group. *P ≤ 0.05, **P ≤ 0.01.
Discussion
In a biphasic OVA-induced model of AAD, an endogenous antigen-specific OVA-TET+ CD8+ T cell response developed in local lung compartments but not in systemic tissues. The location and distribution of an endogenous antigen-specific CD8+ T cell response has never been examined at AAD or LIT (Figure 3 and 4).21,22 We have demonstrated that systemic sensitization alone with OVA and alum significantly increased the number of OVA-TET+ CD8+ T cells in lymphoid tissues as compared with those found in naive mice by >10-fold. The OVA-TET+ CD8+ T cell response was further enhanced in specific lung compartments post inhalation of OVA with the development of AAD. Both the percentages and total numbers of OVA-TET+ CD8+ T cells were higher in lung tissue than in HLN at AAD and LIT, suggesting a local lung tissue rather than lymphatic site of action. These antigen-specific OVA-TET+ CD8+ T cells associated with B-cell aggregates in peribronchial and perivascular regions of the lung. Formation and size of the aggregates corresponded with the inflammatory stage of the disease, with multiple high-density aggregates seen at AAD and few low-density clusters observed at LIT. These transient aggregates resemble tertiary ectopic lymphoid tissue (TELT) or inducible bronchus associated lymph tissue (iBALT), which have been reported to play an important role in protective immunity against viral infections and to support memory CD8+ T cells in the absence of secondary lymph nodes.26,27 The aggregates appear more TELT–like in that they lack the organization and vasculature of a BALT structure, although no attempt at this time has been made to further characterize the aggregates. BALT–like structures are not uncommon in mice in response to pathogens but, to our knowledge, have not been implicated in asthma.
Changes in CD8+ T cell densities and localizations between AAD and LIT were accompanied by phenotypic and functional differences in the cells. The OVA-TET+ CD8+ T cells in the local lung compartments at AAD and LIT had an activated phenotype, with increased levels of CD11a (LFA–1) and decreased levels of CD62L (L–Selectin). The low expression of CD62L suggested that most of the OVA-TET+ CD8+ T cells were of an effector or effector–memory phenotype.28 Unlike other tissues and time points, CD11ahi and CD11alo OVA-TET+ CD8+ T cells were identified in the BAL at LIT. Previous studies using viral infections have demonstrated that the two populations represent either cells that have been in the BAL for a prolonged period of time (CD11alo) or recent immigrants into the BAL (CD11ahi).29 Analogously, our findings would suggest that AAD BAL OVA-TET+ CD8+ T cells consisted almost entirely of recently recruited cells (ie, 90% CD11ahi) whereas LIT BAL OVA-TET+ CD8+ T cells were a combination of long-lived CD11alo (61%) and recent immigrant CD11ahi (39%) cells maintained by constant recruitment. These cells may be playing a role in maintaining tolerance or participating in the inflammatory process but are not deleted by continued antigen exposure.
In addition, we demonstrated that the regulatory NK receptor, NKG2A, was minimally expressed on CD8+ T cells in sensitized animals but increased at AAD and increased further at LIT in BAL and lung. The majority of these NKG2A+ CD8+ T cells were antigen specific OVA-TET+ CD8+ T cells. NKG2A affords preferential survival to CD8+ cells expressing it and has been demonstrated to suppress CD8+ T cell function.30–34 Furthermore, it has been shown to be induced by transforming growth factor–β, which has been demonstrated to be an important driver of tolerance in this biphasic OVA model.35,36 NKG2A has been associated with CD8+ regulatory cells and recently linked to suppressing B cell responses via T follicular helper cells.37 The increased expression of NKG2A may afford regulatory potential to the OVA-TET CD8+ T cells via interactions with its ligand Qa-1b, or it may be inhibiting their pro-inflammatory effects and limiting lung damage. Its presence suggests a possible inhibitory role for OVA-TET CD8+ T cells on potential Qa-1b+ B-cells and/or Qa-1b+ CD4+ effector T cells within the TELT-like aggregates identified in the lung tissue.
Functionally, we demonstrated that expression of granzyme B, an important molecule for cytotoxic killing, was reserved to the antigen specific OVA-TET CD8+ T cells in the BAL and lung at AAD but not found in these cells at LIT or in the HLN. These observations correlate with clinical findings, which have identified increased levels of granzyme B in the BAL of asthma patients while being decreased in the serum.38,39 Similarly, we demonstrated that CD8+ T cells in the BAL and lung at AAD produced increased IFN-γ, a Tc1 cytokine. The presence of granzyme B positive CD8+ T cells and IFN-γ+ CD8+ T cells at AAD and their absence at LIT suggest a functional plasticity in the lung microenvironment. The increased expression of granzyme B and NKG2A by OVA-TET+ CD8+ T cells suggests that these cells are capable of direct killing, potentially of antigen-activated Qa-1b+ CD4+ effector T cells.25
Collectively, changes in phenotypic (CD11ahi, CD62L, and NKG2A) and functional (granzyme B and IFN-γ) markers illustrate plasticity in the OVA-TET+ CD8+ T cell response, which may contribute to the development of AAD and/or LIT. Conceivably, TELT-like structures seen at AAD may be needed to establish a local environment conducive for intercellular communication. This environment may contribute to optimal signaling exchange for switchover to a regulatory phenotype and may promote the establishment of LIT.
Uniquely, our discontinuous models (Figure 1B) allowed us to examine the ability of antigen-specific CD8+ T cells generated at AAD or LIT to respond to re-exposure of aerosolized antigen (Figure 9). As we have previously described,19 resting (ie, no aerosol exposure to OVA) either AAD or LIT animals and re-exposing them to OVA aerosol (6-week or 11-week discontinuous model), resulted in the return of AAD with increased BAL eosinophils. In both cases, the expansion of eosinophils in the BAL was accompanied by significant increases in the percentages and numbers of OVA-TET+ CD8+ T cells in the BAL and lung tissue, demonstrating that the OVA-TET+ CD8+ T cells generated at AAD or LIT were responsive to antigen re-challenge and were not permanently exhausted. Such a CD8+ memory response has been suggested as an ideal target for a vaccination strategy for asthma.40,41 Several vaccination studies have demonstrated the increased production of IFN-γ from CD8+ T cells and have suggested it as a mechanism of limiting asthma. Our data demonstrate that antigen-specific IFN-γ+ CD8+ T cells developed at AAD and may contribute to the development of LIT.
In summary, in our biphasic OVA murine asthma model, antigen-specific CD8+ T cells in the local lung compartments at AAD appeared as typical activated effector cells (CD11ahi,CD62L−, expressing granzyme B, and IFN-γ) whereas at LIT these CD8+ T cells appeared to be regulatory, with increased expression of NKG2A and no ability to express granzyme B or IFN-γ. The possible plasticity of these CD8+ T cells exists in a somewhat analogous manner as has been seen to occur in murine asthma models with a shift from CD4+Foxp3− T effector cells to CD4+Foxp3+ regulatory cells. An alternative to a plasticity paradigm is the recruitment of new subpopulations of suppressive/regulatory CD8+ T cells phenotypically expressing NKG2A. It is intriguing to consider that the formation of the TELT-like structures provides the appropriate local environment for either of these paradigms while preserving homeostatic systemic responsiveness. Structures such as iBALT have been shown to be present in the lungs of asthmatic individuals.42 What roles CD8+ T cells and these TELT-like aggregates may play in AAD and in the induction of tolerance at LIT remain unresolved; nonetheless, these results could provide important insight into this biphasic model and allergic reactions in general, calling for further examination in future studies.
Footnotes
Supported by NIH grants R21-AI079533 (C.M.S.), R01-AI043573 (L.L.), R01-AI43573 (R.S.T.), and T32-AI007080 (J.T.M.).
Supplemental material for this article can be found at http://ajp.amjapthol.org or at doi: 10.1016/j.ajpath.2012.01.043.
Supplementary data
Identification of OVA-TET+ CD8+ T cells in naive and sensitized mice using tetramer enrichment. Single-cell suspensions from the spleen and lymph nodes (axillary, mandibular, cervical, HLN, ILN, colic, jejunal, and caudal mesenteric) were obtained from naive and OVA-sensitized mice. Identification of OVA-TET+ CD8+ T cells was performed as described in Materials and Methods. Cells from the spleen and lymph nodes of naive and sensitized mice were pooled for comparison of the number of OVA-TET+ CD8+ T cells in the total body (A). There was a significant increase in the total number of OVA-TET+ CD8+ T cells in the sensitized mice as compared with the naive mice. There was no difference observed in the total number of OVA-TET+ CD8+ T cells identified in the spleen as compared with the pooled lymph nodes of sensitized mice (B). Furthermore, there was no difference observed in the frequency of OVA-TET+ CD8+ T cells to CD8+ T cells in either compartment of the sensitized mice (C). Data represent the mean ± SEM of five animals per group. **P ≤ 0.01.
Development and resolution of B220+ cell aggregates in the lung tissue. Whole-mount tissue preparations were used to visualize via confocal microscopy the location of the B cells and CD8+ T cells in the lung tissue.23,24 Lung tissue from mice at various stages of the AAD model (3, 5, 7, 14, and 42 days post inhalation of OVA) was transversely sliced into sections 300 to 500 μm thick. Tissues were washed and stained overnight at 4°C in round-bottomed, 48-well plates with α-B220 with Alexa Fluor 488 (Invitrogen) and Cy5-labeled CD8a (53-6.7, eBioscience). Aggregates of B220+ cells were not observed at day 1 (A) but could be identified after day 3 (B) and progressively increased in size after day 5 (C) and day 7 (D) of daily OVA aerosol. These aggregates then progressively diminished after 14 days (E) and 42 days (F) of daily OVA aerosols. Representative colors for confocal microscopy: green, B220+ cells; blue, CD8+ cells. Aw = airway; bv = blood vessel. Original magnification, ×20.
Visualization of OVA-TET+ CD8+ T cells in the lung tissue.
Whole-mount tissue preparations were used to visualize, via confocal microscopy, the location of OVA-TET+ CD8+ T cells in the lung tissue. Lung tissue from mice at various stages of the AAD model (sensitized, AAD, and LIT) were transversely sliced into sections 300 to 500 μm thick. Tissues were washed and stained overnight at 4°C in round-bottomed, 48-well plates with α-B220 with Alexa Fluor 488 (Invitrogen) and Cy5-labeled CD8a (53-6.7, eBioscience). Large numbers of OVA-TET+ CD8+ T cells could be identified in the lung tissue of AAD animals (A, B, and D). OVA-TET+ CD8+ T cells could be identified in the lung tissue of LIT animals (E); however, it was difficult to find these cells in sensitized lung tissue from sensitized mice (C). (D) Digital zoom photograph (×20) taken from the white square in B illustrates CD8+ T cells and OVA-TET+ CD8+ T cells associating with the B220+ cell aggregates. Examples of OVA-TET+ CD8+ T cells are highlighted with white arrows. Green indicates B220+ cells; blue indicates CD8+ cells; and, with a dual stain overlay, magenta represents OVA-TET+ CD8+ T cells. Aw = airway. Magnification: ×10 (A and B); ×20 (C, D, and E).
References
- 1.Mosmann T.R., Coffman R.L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 2.Ricci M., Rossi O., Bertoni M., Matucci A. The importance of Th2-like cells in the pathogenesis of airway allergic inflammation. Clin Exp Allergy. 1993;23:360–369. doi: 10.1111/j.1365-2222.1993.tb00340.x. [DOI] [PubMed] [Google Scholar]
- 3.Larche M., Robinson D.S., Kay A.B. The role of T lymphocytes in the pathogenesis of asthma. J Allergy Clin Immunol. 2003;111:450–463. doi: 10.1067/mai.2003.169. [DOI] [PubMed] [Google Scholar]
- 4.Carson W.F., 4th, Guernsey L.A., Singh A., Vella A.T., Schramm C.M., Thrall R.S. Accumulation of regulatory T cells in local draining lymph nodes of the lung correlates with spontaneous resolution of chronic asthma in a murine model. Int Arch Allergy Immunol. 2008;145:231–243. doi: 10.1159/000109292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Venuprasad K., Kong Y.C., Farrar M.A. Control of Th2-mediated inflammation by regulatory T cells. Am J Pathol. 2010;177:525–531. doi: 10.2353/ajpath.2010.090936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woodland D.L., Dutton R.W. Heterogeneity of CD4(+) and CD8(+) T cells. Curr Opin Immunol. 2003;15:336–342. doi: 10.1016/s0952-7915(03)00037-2. [DOI] [PubMed] [Google Scholar]
- 7.Lefrançois L., Obar J.J. Once a killer, always a killer: from cytotoxic T cell to memory cell. Immunol Rev. 2010;235:206–218. doi: 10.1111/j.0105-2896.2010.00895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Betts R.J., Kemeny D.M. CD8+ T cells in asthma: friend or foe. Pharmacol Ther. 2009;121:123–131. doi: 10.1016/j.pharmthera.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Hamelmann E., Oshiba A., Paluh J., Bradley K., Loader J., Potter T.A., Larsen G.L., Gelfand E.W. Requirement for CD8+ T cells in the development of airway hyperresponsiveness in a murine model of airway sensitization. J Exp Med. 1996;183:1719–1729. doi: 10.1084/jem.183.4.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leggat J.A., Gibbons D.L., Haque S.F., Smith A.L., Wells J.W., Choy K., Lloyd C.M., Hayday A.C., Noble A. Innate responsiveness of CD8 memory T-cell populations nonspecifically inhibits allergic sensitization. J Allergy Clin Immunol. 2008;122:1014–1021. doi: 10.1016/j.jaci.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyahara N., Swanson B.J., Takeda K., Taube C., Miyahara S., Kodama T., Dakhama A., Ott V.L., Gelfand E.W. Effector CD8+ T cells mediate inflammation and airway hyper-responsiveness. Nat Med. 2004;10:865–869. doi: 10.1038/nm1081. [DOI] [PubMed] [Google Scholar]
- 12.Stock P., Kallinich T., Akbari O., Quarcoo D., Gerhold K., Wahn U., Umetsu D.T., Hamelmann E. CD8(+) T cells regulate immune responses in a murine model of allergen-induced sensitization and airway inflammation. Eur J Immunol. 2004;34:1817–1827. doi: 10.1002/eji.200324623. [DOI] [PubMed] [Google Scholar]
- 13.Koya T., Miyahara N., Takeda K., Matsubara S., Matsuda H., Swasey C., Balhorn A., Dakhama A., Gelfand E.W. CD8+ T cell-mediated airway hyperresponsiveness and inflammation is dependent on CD4+IL-4+ T cells. J Immunol. 2007;179:2728–2796. doi: 10.4049/jimmunol.179.5.2787. [DOI] [PubMed] [Google Scholar]
- 14.Sawicka E., Noble A., Walker C., Kemeny D.M. Tc2 cells respond to soluble antigen in the respiratory tract and induce lung eosinophilia and bronchial hyperresponsiveness. Eur J Immunol. 2004;34:2599–2608. doi: 10.1002/eji.200425018. [DOI] [PubMed] [Google Scholar]
- 15.Sedgwick J.D., Holt P.G. Suppression of IgE responses in inbred rats by repeated respiratory tract exposure to antigen: responder phenotype influences isotype specificity of induced tolerance. Eur J Immunol. 1984;14:893–897. doi: 10.1002/eji.1830141006. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki M., Taha R., Ihaku D., Hamid Q., Martin J.G. CD8+ T cells modulate late allergic airway responses in Brown Norway rats. J Immunol. 1999;163:5574–5581. [PubMed] [Google Scholar]
- 17.Aguilar-Pimentel J.A., Alessandrini F., Huster K.M., Jakob T., Schulz H., Behrendt H., Ring J., de Angelis M.H., Busch D.H., Mempel M., Ollert M. Specific CD8 T cells in IgE-mediated allergy correlate with allergen dose and allergic phenotype. Am J Respir Crit Care Med. 2010;181:7–16. doi: 10.1164/rccm.200902-0190OC. [DOI] [PubMed] [Google Scholar]
- 18.Yiamouyiannis C.A., Schramm C.M., Puddington L., Stengel P., Baradaran-Hosseini E., Wolyniec W.W., Whitley H.E., Thrall R.S. Shifts in lung lymphocyte profiles correlate with the sequential development of acute allergic and chronic tolerant stages in a murine asthma model. Am J Pathol. 1999;154:1911–1921. doi: 10.1016/S0002-9440(10)65449-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schramm C.M., Puddington L., Wu C., Guernsey L., Gharaee-Kermani M., Phan S.H., Thrall R.S. Chronic inhaled ovalbumin exposure induces antigen-dependent but not antigen-specific inhalational tolerance in a murine model of allergic airway disease. Am J Pathol. 2004;164:295–304. doi: 10.1016/S0002-9440(10)63119-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van den Broeck W., Derore A., Simoens P. Anatomy and nomenclature of murine lymph nodes: descriptive study and nomenclatory standardization in BALB/cAnNCrl mice. J Immunol Methods. 2006;312:12–19. doi: 10.1016/j.jim.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 21.Altman J.D., Moss P.A., Goulder P.J., Barouch D.H., McHeyzer-Williams M.G., Bell J.I., McMichael A.J., Davis M.M. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. doi: 10.1126/science.274.5284.94. [DOI] [PubMed] [Google Scholar]
- 22.Obar J.J., Khanna K.M., Lefrancois L. Endogenous naive CD8+ T cell precursor frequency regulates primary and memory responses to infection. Immunity. 2008;28:859–869. doi: 10.1016/j.immuni.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khanna K.M., Bonneau R.H., Kinchington P.R., Hendricks R.L. Herpes simplex virus-specific memory CD8+ T cells are selectively activated and retained in latently infected sensory ganglia. Immunity. 2003;18:593–603. doi: 10.1016/s1074-7613(03)00112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khanna K.M., McNamara J.T., Lefrancois L. In situ imaging of the endogenous CD8 T cell response to infection. Science. 2007;318:116–120. doi: 10.1126/science.1146291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu L., Werneck M.B., Cantor H. The immunoregulatory effects of Qa-1. Immunol Rev. 2006;212:51–59. doi: 10.1111/j.0105-2896.2006.00418.x. [DOI] [PubMed] [Google Scholar]
- 26.Rangel-Moreno J., Moyron-Quiroz J.E., Hartson L., Kusser K., Randall T.D. Pulmonary expression of CXC chemokine ligand 13, CC chemokine ligand 19, and CC chemokine ligand 21 is essential for local immunity to influenza. Proc Natl Acad Sci USA. 2007;104:10577–10582. doi: 10.1073/pnas.0700591104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moyron-Quiroz J.E., Rangel-Moreno J., Hartson L., Kusser K., Tighe M.P., Klonowski K.D., Lefrancois L., Cauley L.S., Harmsen A.G., Lund F.E., Randall T.D. Persistence and responsiveness of immunologic memory in the absence of secondary lymphoid organs. Immunity. 2006;25:643–654. doi: 10.1016/j.immuni.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 28.Obar J.J., Lefrancois L. Memory CD8+ T cell differentiation. Ann NY Acad Sci. 2010;1183:251–266. doi: 10.1111/j.1749-6632.2009.05126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ely K.H., Cookenham T., Roberts A.D., Woodland D.L. Memory T cell populations in the lung airways are maintained by continual recruitment. J Immunol. 2006;176:537–543. doi: 10.4049/jimmunol.176.1.537. [DOI] [PubMed] [Google Scholar]
- 30.McMahon C.W., Zajac A.J., Jamieson A.M., Corral L., Hammer G.E., Ahmed R., Raulet D.H. Viral and bacterial infections induce expression of multiple NK cell receptors in responding CD8(+) T cells. J Immunol. 2002;169:1444–1452. doi: 10.4049/jimmunol.169.3.1444. [DOI] [PubMed] [Google Scholar]
- 31.Gunturi A., Berg R.E., Forman J. The role of CD94/NKG2 in innate and adaptive immunity. Immunol Res. 2004;30:3429–3432. doi: 10.1385/IR:30:1:029. [DOI] [PubMed] [Google Scholar]
- 32.Gunturi A., Berg R.E., Forman J. Preferential survival of CD8 T and NK cells expressing high levels of CD94. J Immunol. 2003;170:1737–1745. doi: 10.4049/jimmunol.170.4.1737. [DOI] [PubMed] [Google Scholar]
- 33.Zhou J., Matsuoka M., Cantor H., Homer R., Enelow R.I. Cutting edge: engagement of NKG2A on CD8+ effector T cells limits immunopathology in influenza pneumonia. J Immunol. 2008;180:25–29. doi: 10.4049/jimmunol.180.1.25. [DOI] [PubMed] [Google Scholar]
- 34.Gunturi A., Berg R.E., Crossley E., Murray S., Forman J. The role of TCR stimulation and TGF-beta in controlling the expression of CD94/NKG2A receptors on CD8 T cells. Eur J Immunol. 2005;35:766–775. doi: 10.1002/eji.200425735. [DOI] [PubMed] [Google Scholar]
- 35.Ostroukhova M., Seguin-Devaux C., Oriss T.B., Dixon-McCarthy B., Yang L., Ameredes B.T., Corcoran T.E., Ray A. Tolerance induced by inhaled antigen involves CD4(+) T cells expressing membrane-bound TGF-beta and FOXP3. J Clin Invest. 2004;114:28–38. doi: 10.1172/JCI20509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh A., Carson W.F., 4th, Secor E.R., Jr., Guernsey L.A., Flavell R.A., Clark R.B., Thrall R.S., Schramm C.M. Regulatory role of B cells in a murine model of allergic airway disease. J Immunol. 2008;180:7318–7326. doi: 10.4049/jimmunol.180.11.7318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim H.J., Verbinnen B., Tang X., Lu L., Cantor H. Inhibition of follicular T-helper cells by CD8(+) regulatory T cells is essential for self tolerance. Nature. 2010;467:328–332. doi: 10.1038/nature09370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bratke K., Bottcher B., Leeder K., Schmidt S., Kupper M., Virchow J.C., Jr., Luttmann W. Increase in granzyme B+ lymphocytes and soluble granzyme B in bronchoalveolar lavage of allergen challenged patients with atopic asthma. Clin Exp Immunol. 2004;136:542–548. doi: 10.1111/j.1365-2249.2004.02468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bratke K., Haupt F., Kuepper M., Bade B., Faehndrich S., Luttmann W., Virchow J.C., Jr Decrease of cytotoxic T cells in allergic asthma correlates with total serum immunglobulin E. Allergy. 2006;61:1351–1357. doi: 10.1111/j.1398-9995.2006.01192.x. [DOI] [PubMed] [Google Scholar]
- 40.Takeda K., Dow S.W., Miyahara N., Kodama T., Koya T., Taube C., Joetham A., Park J.W., Dakhama A., Kedl R.M., Gelfand E.W. Vaccine-induced CD8+ T cell-dependent suppression of airway hyperresponsiveness and inflammation. J Immunol. 2009;183:181–190. doi: 10.4049/jimmunol.0803967. [DOI] [PubMed] [Google Scholar]
- 41.Wells J.W., Choy K., Lloyd C.M., Noble A. Suppression of allergic airway inflammation and IgE responses by a class I restricted allergen peptide vaccine. Mucosal Immunol. 2009;2:54–62. doi: 10.1038/mi.2008.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elliot J.G., Jensen C.M., Mutavdzic S., Lamb J.P., Carroll N.G., James A.L. Aggregations of lymphoid cells in the airways of nonsmokers, smokers, and subjects with asthma. Am J Respir Crit Care Med. 2004;69:712–718. doi: 10.1164/rccm.200308-1167OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Identification of OVA-TET+ CD8+ T cells in naive and sensitized mice using tetramer enrichment. Single-cell suspensions from the spleen and lymph nodes (axillary, mandibular, cervical, HLN, ILN, colic, jejunal, and caudal mesenteric) were obtained from naive and OVA-sensitized mice. Identification of OVA-TET+ CD8+ T cells was performed as described in Materials and Methods. Cells from the spleen and lymph nodes of naive and sensitized mice were pooled for comparison of the number of OVA-TET+ CD8+ T cells in the total body (A). There was a significant increase in the total number of OVA-TET+ CD8+ T cells in the sensitized mice as compared with the naive mice. There was no difference observed in the total number of OVA-TET+ CD8+ T cells identified in the spleen as compared with the pooled lymph nodes of sensitized mice (B). Furthermore, there was no difference observed in the frequency of OVA-TET+ CD8+ T cells to CD8+ T cells in either compartment of the sensitized mice (C). Data represent the mean ± SEM of five animals per group. **P ≤ 0.01.
Development and resolution of B220+ cell aggregates in the lung tissue. Whole-mount tissue preparations were used to visualize via confocal microscopy the location of the B cells and CD8+ T cells in the lung tissue.23,24 Lung tissue from mice at various stages of the AAD model (3, 5, 7, 14, and 42 days post inhalation of OVA) was transversely sliced into sections 300 to 500 μm thick. Tissues were washed and stained overnight at 4°C in round-bottomed, 48-well plates with α-B220 with Alexa Fluor 488 (Invitrogen) and Cy5-labeled CD8a (53-6.7, eBioscience). Aggregates of B220+ cells were not observed at day 1 (A) but could be identified after day 3 (B) and progressively increased in size after day 5 (C) and day 7 (D) of daily OVA aerosol. These aggregates then progressively diminished after 14 days (E) and 42 days (F) of daily OVA aerosols. Representative colors for confocal microscopy: green, B220+ cells; blue, CD8+ cells. Aw = airway; bv = blood vessel. Original magnification, ×20.
Visualization of OVA-TET+ CD8+ T cells in the lung tissue.
Whole-mount tissue preparations were used to visualize, via confocal microscopy, the location of OVA-TET+ CD8+ T cells in the lung tissue. Lung tissue from mice at various stages of the AAD model (sensitized, AAD, and LIT) were transversely sliced into sections 300 to 500 μm thick. Tissues were washed and stained overnight at 4°C in round-bottomed, 48-well plates with α-B220 with Alexa Fluor 488 (Invitrogen) and Cy5-labeled CD8a (53-6.7, eBioscience). Large numbers of OVA-TET+ CD8+ T cells could be identified in the lung tissue of AAD animals (A, B, and D). OVA-TET+ CD8+ T cells could be identified in the lung tissue of LIT animals (E); however, it was difficult to find these cells in sensitized lung tissue from sensitized mice (C). (D) Digital zoom photograph (×20) taken from the white square in B illustrates CD8+ T cells and OVA-TET+ CD8+ T cells associating with the B220+ cell aggregates. Examples of OVA-TET+ CD8+ T cells are highlighted with white arrows. Green indicates B220+ cells; blue indicates CD8+ cells; and, with a dual stain overlay, magenta represents OVA-TET+ CD8+ T cells. Aw = airway. Magnification: ×10 (A and B); ×20 (C, D, and E).