Abstract
Excessive extracellular matrix production by fibroblasts in response to tissue injury contributes to fibrotic diseases, such as idiopathic pulmonary fibrosis (IPF). Epithelial-mesenchymal transition, involving transition of alveolar epithelial cells (AECs) to pulmonary fibroblasts, appears to be an important contributory process to lung fibrosis. Although aberrant expression of microRNAs (miRs) is involved in a variety of pathophysiologic processes, the role of miRs in fibrotic lung diseases is less well understood. In the present study, we found that miR-200a, miR-200b, and miR-200c are significantly down-regulated in the lungs of mice with experimental lung fibrosis. Levels of miR-200a and miR-200c were reduced in the lungs of patients with IPF. miR-200 had greater expression in AECs than in lung fibroblasts, and AECs from mice with experimental pulmonary fibrosis had diminished expression of miR-200. We found that the miR-200 family members inhibit transforming growth factor-β1–induced epithelial-mesenchymal transition of AECs. miR-200 family members can reverse the fibrogenic activity of pulmonary fibroblasts from mice with experimental pulmonary fibrosis and from patients with IPF. Indeed, the introduction of miR-200c diminishes experimental pulmonary fibrosis in mice. Thus, the miR-200 family members participate importantly in fibrotic lung diseases and suggest that restoring miR-200 expression in the lungs may represent a novel therapeutic approach in treating pulmonary fibrotic diseases.
Fibroblast activation with generation of provisional extracellular matrix (ECM) is a primary tissue response to injury.1 Successful wound repair relies on a balance of ECM synthesis and resolution, as well as re-epithelization of damaged epithelial surfaces.1,2 Abnormal tissue repair is often associated with excessive ECM production that ultimately leads to fibrosis, including idiopathic pulmonary fibrosis (IPF).1,3 ECM-producing lung fibroblasts arise from several sources, including the following: i) resident pulmonary fibroblasts, ii) circulating fibrocytes that then infiltrate into the lung, and iii) alveolar epithelial cells (AECs) through a process termed epithelial-mesenchymal transition (EMT).4,5
EMT is a biological process that allows an epithelial cell to undergo multiple biochemical changes, resulting in mesenchymal cell features, including enhanced migratory capacity, production of ECM components, and loss of epithelial cell characteristics.6,7 EMT has been an essential step during implantation of the fertilized ovum, embryogenesis, and organ development.6,7 However, it also appears to be an important source of fibroblasts during repair of tissue injury associated with pathological fibrotic processes.6,7
Transforming growth factor (TGF)-β1 is a central mediator of lung fibrosis and can induce EMT of AECs both in vitro and in vivo.8–15 The mechanism by which TGF-β1 induces EMT involves recruitment of several transcriptional repressors, including SNAI1, SNAI2, Twist1, ZEB1, and ZEB2, to the promoters of genes required to preserve epithelial cell phenotypes.9 Furthermore, TGF-β1 induces differentiation of pulmonary fibroblasts into myofibroblasts that are characterized by expression of smooth muscle actin (SMA)-α and elevated synthesis of ECM proteins.16,17
MicroRNAs (miRs) are noncoding small RNAs, 22 nucleotides in length, that bind to the 3′ UTR of target genes and, thereby, repress translation and/or induce degradation of target gene mRNAs.18 miRs have regulated numerous molecular and cellular processes.18 Aberrant expression of miRs is associated with initiation and progression of pathological processes, including diabetes, cancer, and cardiovascular disease.19–22 However, the role of miRs in TGF-β1–induced EMT of AECs and in lung fibrosis is not well characterized.23 An improved understanding of the roles that specific miRs play in the pathogenesis of lung fibrosis is likely to suggest important new directions for the treatment of IPF and other interstitial lung diseases.
In the present study, we demonstrate that three miR-200 family members, including miR-200a, miR-200b, and miR-200c, are significantly down-regulated in the lungs of mice with bleomycin-induced lung fibrosis. The levels of miR-200a and miR-200c were decreased in the lungs of patients with IPF. We found that the miR-200 family members inhibit TGF-β1–induced EMT of AECs and can reverse the fibrogenic activity of pulmonary fibroblasts from mice with experimental pulmonary fibrosis and from patients with IPF. Furthermore, we found that introduction of miR-200c diminishes experimental pulmonary fibrosis in mice. Overall, these data suggest that miR-200 family members play an important role in the pathogenesis of lung fibrosis and suggest that restoring miR-200 expression in the lungs may represent a novel therapeutic approach for the treatment of pulmonary fibrotic diseases.
Materials and Methods
Experimental Pulmonary Fibrosis Model
The experimental pulmonary fibrosis model was established, as previously described.24 The animal protocol was approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee. For miR-200 treatment, control mimics (1.5 mg/kg body weight in 40 μL of saline), control mimics plus bleomycin (1.5 mg/kg mimics plus 1 U/kg bleomycin in 40 μL of saline), miR-200c mimics (1.5 mg/kg in 40 μL of saline), or miR-200c mimics plus bleomycin (1.5 mg/kg mimics plus 1 U/kg bleomycin in 40 μL of saline) were instilled intratracheally through oropharyngeal cavities. At 14 days after the treatment, the severity of lung fibrosis was evaluated.
Reagents
Human recombinant TGF-β1 was obtained from Peprotech (Rocky Hill, NJ). miR mimics and inhibitors were from Ambion (Grand Island, NY).
miR Array Analysis
miR array analysis was performed as previously described.24 Briefly, total RNAs were isolated from mouse lungs harvested at days 0, 7, and 14 after bleomycin instillation (three mice per group) with the miRNAeasy Mini Kit (Qiagen, Valencia, CA). The miR array was performed using the miRCURY LNA microRNA Array (Exiqon, Woburn, MA). The miR profiling data were deposited into Array Express (accession number E-MEXP-2749) and are free to public access.
Isolation and Culture of Primary Mouse AECs and Pulmonary Fibroblasts
Primary AECs were isolated from saline- and bleomycin-treated mice, as previously described, with modifications.25 In brief, lungs were lavaged through the right ventricle with 10 mL of sterile PBS, and tissues were minced and digested with 0.1% collagenase, 0.005% trypsin, and 0.04% DNase. The suspension was filtered through 40-μm nylon meshes and centrifuged at 200 × g for 10 minutes. The pellet was resuspended in modified Eagle's media, and negative selection for lymphocytes/macrophages was performed by incubation on CD16/32- and CD45-coated Petri dishes for 30 minutes at 37°C. Negative selection for fibroblasts was performed by adherence for 45 minutes on cell culture dishes.
The adherent lung fibroblasts from the previously described procedures were cultured in modified Eagle's media containing 10% fetal bovine serum (FBS). The fibroblasts at passage 2 were trypsinized, and the same numbers of cells were plated for experiments. AECs or lung fibroblasts from each mouse were used as an independent line. Four to five mice were used for each condition in the study.
Isolation and Culture of Primary Rat AECs
Isolation and culture of primary rat AECs were performed essentially as previously described.26 Before being treated with TGF-β1, the cells were starved in media containing 0.5% FBS for 24 hours.
Cell Lines
The human primary pulmonary fibroblast line, MRC-5, and the rat ATII cell line, RLE-6TN, were obtained from American Type Culture Collection (Manassas, VA) and cultured according to the manufacturer's instructions.
Human Lung Tissue
IPF and histologically normal lung tissue samples were obtained from the NIH Lung Tissue Research Consortium and the University of Alabama at Birmingham Tissue Procurement and Cell Culture Core. The protocol was approved by the Institutional Review Board at the University of Alabama at Birmingham.
Real-Time PCR
The assay was performed as previously described.24,27 TaqMan probes for miR-200a, miR-200b, miR-200c, RNU48, snoRNA, and sno135 were obtained from Applied Biosystems (Carlsbad, CA). The expression of SMA-α, fibronectin (Fn), collagen 1A1, E-cadherin, GATA3, fibroblast-specific protein (FSP) 1, zona occludens-1 (ZO-1), ZEB1, and ZEB2 was determined using the SYBR Green Master Mix kit (Roche, Indianapolis, IN). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control. Primer sequences were as follows: human Fn, 5′-GTGTTGGGAATGGTCGTGGGGAATG-3′ (sense) and 5′-CCAATGCCACGGCCATAGCAGTAGC-3′ (antisense); mouse Fn, 5′-TCTGGGAAATGGAAAAGGGGAATGG-3′ (sense) and 5′-CACTGAAGCAGGTTTCCTCGGTTGT-3′ (antisense); mouse SMA-α, 5′-GACGCTGAAGTATCCGATAGAACACG-3′ (sense) and 5′-CACCATCTCCAGAGTCCAGCACAAT-3′ (antisense); mouse collagen 1A1, 5′-GGAGGGCGAGTGCTGTGCTTT-3′ (sense) and 5′-GGGACCAGGAGGACCAGGAAGT-3′ (antisense); rat ZEB1, 5′-TTTGTCTCCCAGTCAGCCACCTTTA-3′ (sense) and 5′-GGAATCTGTCCAGCTTGCATCTTTT-3′ (antisense); rat ZEB2, 5′-GCAGCACTTAGGTGTAGGGTTAGAAGC-3′ (sense) and 5′-GACCGACGGCTGGAATACTAGGAGA-3′ (antisense); rat GAPDH, 5′-ATGCTGGTGCTGAGTATGTCGTGGAG-3′ (sense) and 5′-TGAGGGAGTTGTCATATTTCTCGTGGTTC-3′ (antisense); mouse E-cadherin, 5′-GTGTGCTCACCTCTGGGCTGGAC-3′ (sense) and 5′-GAGTGTTGGGGGCATCATCATCG-3′ (antisense); mouse GAPDH, 5′-CGACTTCAACAGCAACTCCCACTCTTCC-3′ (sense) and 5′-TGGGTGGTCCAGGGTTTCTTACTCCTT-3′ (antisense); human GAPDH, 5′-GCTGGCGCTGAGTACGTCGTGGAGT-3′ (sense) and 5′-CACAGTCTTCTGGGTGGCAGTGATGG-3′ (antisense); mouse ZO-1, 5′-TCTGGCATCATTCGCCTTCATACA-3′ (sense) and 5′-CGCATAATTAAGACGATCAACCGC-3′ (antisense); mouse FSP1, 5′-TCCACAAATACTCAGGCAAAGAGGG-3′ (sense) and 5′-TGTTGCTGTCCAAGTTGCTCATCAC-3′ (antisense); and rat GATA3, 5′-CCATTACCACCTATCCGCCCTAT-3′ (sense) and 5′-GCAGTTCACACACTCCCTGCCTT-3′ (antisense).
Western Blot Analysis
Western blot analysis was performed as previously described.28 Mouse anti-Fn antibody and rabbit anti-GAPDH antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse anti-SMA-α was from Sigma-Aldrich (St. Louis, MO). Mouse anti-vimentin antibody was from Abcam (Cambridge, MA). Mouse anti-E-cadherin and mouse anti-N-cadherin antibodies were from BD Bioscience (Sparks, MA).
Immunofluorescence Assay
Immunofluorescence assays were performed as previously described.28 Confocal microscopy was also performed.
Immunohistochemical Data
Immunohistochemistry (IHC) assays were performed as previously described.24 Briefly, sections of mouse lungs (10 μm thick) were prepared, deparaffinized with xylene, and then rehydrated in water. Antigen retrieval was performed in a pressure cooker in Tris-EDTA solution, pH 9.0. After incubation in Tris-buffered saline with Tween for 10 minutes and 3% H2O2 for 10 minutes, the sections were blocked with avidin-biotin blocker and affinity-purified goat anti-mouse IgG [heavy chain (H) and light chain (L)]. The sections were then incubated with mouse anti-α-SMA (Sigma) overnight at 4°C, secondary antibody for 45 minutes, avidin–horseradish peroxidase for 45 minutes, and diaminobenzidine (DAB) chromogen, sequentially. Finally, the sections were counterstained with hematoxylin.
Collagen Content Determination
The right lungs from mice were collected and homogenized in 5 mL of 0.5 mol/L acetic acid in PBS containing 0.6% pepsin. The extracts were rotated at 4°C overnight and cleared by centrifugation at 17,200 × g for 15 minutes. Collagen content was measured using the Sircol Collagen Assay kit (Biocolor Ltd, Carrickfergus, UK), according to the manufacturer's instructions. Collagen content is presented as micrograms of acid-soluble collagen per right lung.
Statistical Analysis
One-way analysis of variance, followed by the Holm-Sidak or Tukey-Kramer test, was performed for multiple group comparisons. The Student's t-test was used for comparison between two groups. P < 0.05 was considered significant.
Results
miR-200 Is Down-Regulated in the Lungs of Mice with Experimental Pulmonary Fibrosis
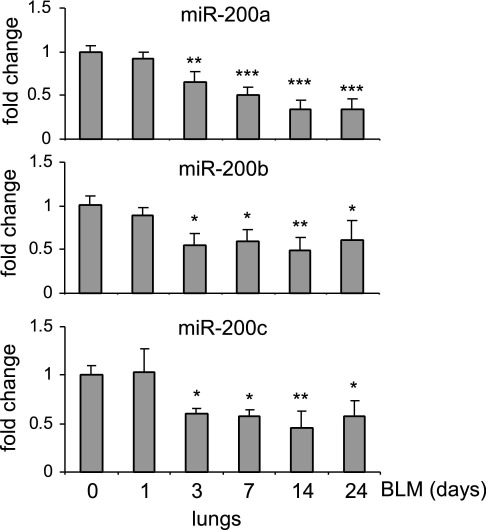
To examine alterations of miR in pulmonary fibrosis, we performed miR array analyses on RNA samples isolated from lungs of mice that were given intratracheal bleomycin, a well-studied pulmonary fibrosis model.29,30 We found that several miRs demonstrate altered expression in fibrotic mouse lungs.24 We found that miR-21, an miR with enhanced expression in fibrotic mouse lungs, regulates pulmonary fibrogenesis in response to bleomycin-induced lung injury.24 In our effort to characterize miRs with reduced expression in fibrotic mouse lungs, we defined the role of miR-200 family members in the regulation of pulmonary fibrosis. To confirm the alterations of miR-200 expression in fibrotic lungs, we performed real-time PCR analysis and found that the expression of miR-200 family members, including miR-200a, miR-200b, and miR-200c, is time dependently decreased in murine lungs after intratracheal injection of bleomycin (Figure 1). These data suggest that miR-200 may play a role in the regulation of lung fibrosis.
Figure 1.
miR-200 is down-regulated in the lungs of mice with experimental pulmonary fibrosis. Mice were injected intratracheally with saline or bleomycin (BLM; 1 U/kg in 50 μL of saline) and sacrificed at days 0, 1, 3, 4, 7, 14, or 24 after injection. Total RNA from the lungs was isolated, and the levels of miR-200a, miR-200b, and miR-200c were determined by real-time PCR assays. A small nucleolar RNA, snoRNA 135, was used as an internal control (n = 3 mice in each group). Data are given as mean ± SD. *P < 0.05, **P < 0.01, and ***P < 0.001 versus day 0.
miR-200 Demonstrates Greater Expression in AECs Than in Lung Fibroblasts and Is Down-Regulated in AECs Isolated from Fibrotic Mouse Lungs
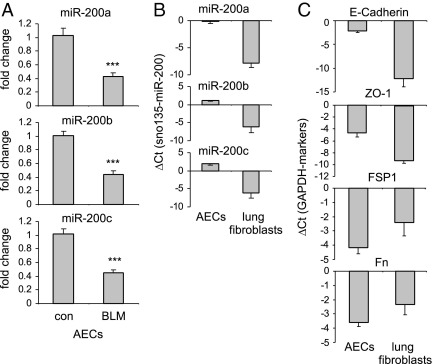
The AECs and lung fibroblasts are the most relevant cell populations involved in pulmonary fibrosis. To determine the levels of miR-200 in these two cell populations, we isolated AECs and lung fibroblasts from control untreated mice and from mice after bleomycin administration. We found that the expression of miR-200 is down-regulated in the AECs isolated from mice with experimental lung fibrosis (Figure 2A). Furthermore, we found that miR-200 is expressed in the AECs at remarkably higher levels than in the lung fibroblasts (Figure 2B). These data suggest that the diminished levels of miR-200 in the fibrotic lungs are primarily derived from the decrease in the expression of miR-200 in AECs. The AECs demonstrated much greater expression of E-cadherin and ZO-1 (two epithelial cell markers), whereas they demonstrated decreased amounts of Fn and FSP1 (two fibroblast markers), than did lung fibroblasts. These data indicate the purity of the studied cell populations (Figure 2C). Because AECs undergoing EMT are a major source of lung fibroblasts that produce excessive ECM in fibrotic lungs, the diminished expression of miR-200 in AECs suggests that miR-200 may participate in the regulation of EMT involving AECs during lung fibrosis.
Figure 2.
miR-200 demonstrates greater expression in AECs than in lung fibroblasts and is down-regulated in AECs isolated from fibrotic mouse lungs. Mice were injected intratracheally with saline or bleomycin (BLM; 1 U/kg in 50 μL of saline) and sacrificed 14 days after the injection. AECs and pulmonary fibroblasts were isolated and cultured as described in Materials and Methods, and RNA from these cells was isolated. A: The levels of miR-200a, miR-200b, and miR-200c in the cells were determined by real-time PCR. con indicates control. B: The levels of miR-200a, miR-200b, and miR-200c in the AECs and pulmonary fibroblasts were determined by real-time PCR and are shown as ΔCT relative to those of a small nucleolar RNA, snoRNA 135, in the same cells. C: The levels of E-cadherin, ZO-1, FSP1, and Fn in the AECs and pulmonary fibroblasts were determined by real-time PCR and are shown as ΔCTrelative to those of GAPDH in the same cells (n = 5 mice per group). Data are given as mean ± SD. ***P < 0.001 versus the con group.
miR-200 Is Down-Regulated in the Lungs of Patients with IPF
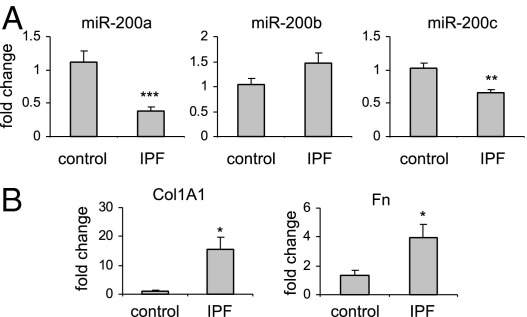
To determine whether the expression of miR-200 family members is altered in the lungs of patients with IPF, we measured the levels of miR-200 in control normal human lung tissue and in the lungs of patients with IPF. As shown in Figure 3A, the levels of miR-200a and miR-200c were significantly decreased in the lungs of patients with IPF compared with those in the healthy controls, although miR-200b demonstrated a slight increase in the lungs of patients with IPF. The expression of two ECM proteins, collagen 1A1 and Fn, was increased in the lungs of patients with IPF (Figure 3B), consistent with previous studies.25,31
Figure 3.
miR-200 is down-regulated in the lungs of patients with IPF. A: Total RNA was isolated from histologically normal human lungs and from the lungs of patients with IPF. The levels of miR-200a, miR-200b, and miR-200c in the lungs were determined by real-time PCR. A small nucleolar RNA, RNU48, was used as an internal control (n = 8 for healthy controls, and n = 18 for IPF lungs). Data are given as mean ± SEM. B: The expression of collagen 1A1 (Col1A1) and Fn was determined in the same RNA samples. *P < 0.05, **P < 0.01, and ***P < 0.001 versus the control group.
miR-200 Is Down-Regulated in TGF-β1–Treated AECs
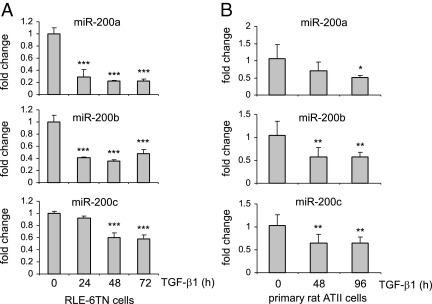
TGF-β1 is one of the major mediators of pulmonary fibrosis and induces EMT of AECs in vitro.8–15 To investigate whether miR-200 has a role in TGF-β1–induced EMT transition, we first sought to determine whether TGF-β1 regulates miR-200 expression in AECs. In these experiments, the rat alveolar type II cell line, RLE-6TN, was treated with TGF-β1 for 0 to 3 days. As shown in Figure 4A, the expression of miR-200a, miR-200b, and miR-200c was significantly decreased after TGF-β1 treatment. These findings suggest that miR-200 may play a role in TGF-β1–induced EMT of AECs.
Figure 4.
miR-200 is down-regulated in TGF-β1–treated AECs. A: RLE-6TN cells were starved in media containing 0.5% FBS for 24 hours and then treated with 10 ng/mL TGF-β1 for 0, 24, 48, or 72 hours. RNA was isolated, and the levels of miR-200a, miR-200b, and miR-200c in the cells were determined by real-time PCR. Experiments were performed in triplicate. Data are given as mean ± SD. ***P < 0.001 versus time 0. B: Rat primary ATII cells were isolated and cultured as described in Materials and Methods. The cells were treated with 10 ng/mL TGF-β1 for 0, 48, or 96 hours. RNA was isolated, and the levels of miR-200a, miR-200b, and miR-200c in the cells were determined by real-time PCR. Each condition contained six replicates. Data are given as mean ± SD. *P < 0.05, **P < 0.01 versus time 0.
To exclude the possibility that the diminished expression of miR-200 after TGF-β1 treatment only occurs in immortalized AEC lines, we treated rat primary alveolar type II cells with TGF-β1. As shown in Figure 4B, TGF-β1 treatment markedly diminished miR-200 expression in the primary rat alveolar type II cells.
miR-200 Promotes EMT in AECs
The EMT process is characterized by the loss of epithelial cell phenotypes, such as diminished expression of E-cadherin and ZO-1, and the acquisition of mesenchymal cell phenotypes, such as enhanced expression of ECM proteins, vimentin, SMA-α, and N-cadherin.6,7 To study the role of miR-200 in the EMT of AECs during pulmonary fibrosis, we first determined the expression of E-cadherin in the lungs of bleomycin- treated mice. As shown in Supplemental Figure S1A (available at http://ajp.amjpathol.org), the expression of E-cadherin in the lungs of mice with bleomycin-induced pulmonary fibrosis is markedly decreased compared with that in normal control lungs. More specifically, we found that the expression of E-cadherin is significantly decreased in AECs isolated from mice with bleomycin-induced lung fibrosis (see Supplemental Figure S1B at http://ajp.amjpathol.org). In addition, the expression of Fn was significantly increased in AECs isolated from mice with experimental lung fibrosis (see Supplemental Figure S1B at http://ajp.amjpathol.org). These data confirm previous findings8–15 that EMT occurs during the development of lung fibrosis.
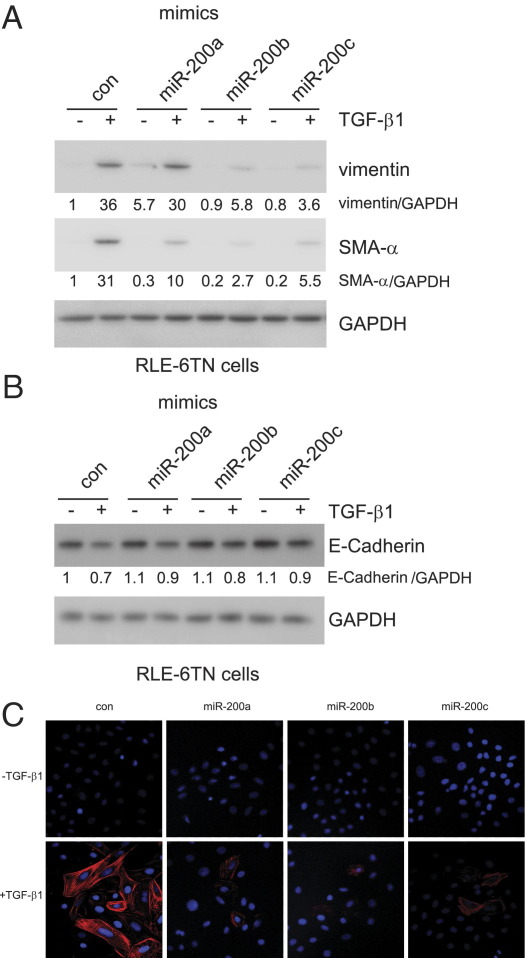
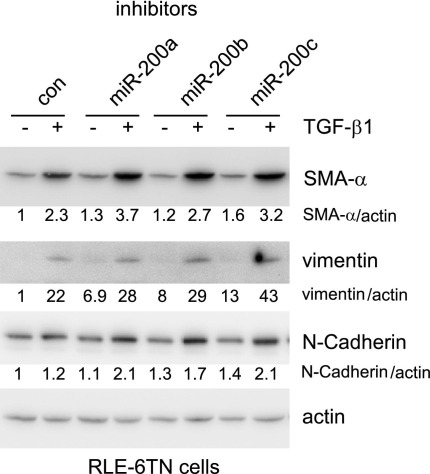
To determine the role of miR-200 in modulating EMT, we transfected RLE-6TN cells with control miR mimics or mimics for miR-200a, miR-200b, or miR-200c and then treated the cells with TGF-β1. As shown in Figure 5, A and C, overexpression of miR-200, particularly miR-200b and miR-200c, attenuated TGF-β1–induced expression of the mesenchymal cell markers, including SMA-α and vimentin, and TGF-β1–induced mesenchymal cell morphological characteristics. Furthermore, we found that miR-200 enhanced E-cadherin expression and attenuated TGF-β1–repressed E-cadherin expression (Figure 5B). Inhibition of miR-200 enhanced TGF-β1–induced expression of mesenchymal markers, including SMA-α, vimentin, and N-cadherin in rat AECs (Figure 6), although such effects were less striking, as suggested by those found with miR-200 mimics. This may be because of relatively low basal levels of miR-200s in the rat cell line. Together, these data suggest that miR-200 has an inhibitory role in TGF-β1–induced EMT in AECs.
Figure 5.
Overexpression of miR-200 diminishes EMT in AECs. A and B: RLE-6TN cells were transfected with 40 nmol/L control (con) miR mimics or mimics for miR-200a, miR-200b, or miR-200c. Three days after the transfection, the cells were starved in medium containing 0.5% FBS for 24 hours and then treated with 5 ng/mL TGF-β1 for 48 hours. The cells were collected, and cell extracts were prepared. The levels of vimentin, SMA-α, E-cadherin, and the loading con, GAPDH, were determined by using Western blot analysis. C: RLE-6TN cells, plated on coverslips, were transfected and treated as in A and B. The cells were then fixed and permeabilized. Immunofluorescence assays were performed with anti-SMA-α antibody. Nuclei were stained with DAPI. Original magnification, ×40.
Figure 6.
Inhibition of miR-200 enhances EMT in AECs. RLE-6TN cells were transfected with 40 nmol/L control (con) miR inhibitors or inhibitors to miR-200a, miR-200b, or miR-200c. Three days after the transfection, the cells were starved in media containing 0.5% FBS for 24 hours and then treated with 5 ng/mL TGF-β1 for 48 hours. The cells were collected, and cell extracts were prepared. The levels of vimentin, SMA-α, N-cadherin, and the loading control, actin, were determined by using Western blot analysis.
miR-200 Regulates the Expression of GATA3, ZEB1, and ZEB2
miRs regulate various cellular processes by down-regulating the expression of their target genes. As shown in Supplemental Figure S2 (available at http://ajp.amjpathol.org), transfection of miR-200 mimics significantly decreased the mRNA levels of GATA3, ZEB1, and ZEB2, transcriptional factors that have regulated EMT,32,33 in rat AECs. These data suggest that the ability of miR-200 to diminish expression of GATA3, ZEB1, and ZEB2 may be a mechanism by which miR-200 inhibits EMT in AECs.
miR-200 Diminishes the Fibrogenic Activity of TGF-β1 in Lung Fibroblasts
Lung fibroblasts function as effectors in pulmonary fibrosis by producing excessive collagen and Fn.4 The finding that miR-200 inhibits EMT in AECs suggests that restoration of expression of miR-200 family members in the lungs may have potential therapeutic utility in the treatment of pulmonary fibrosis. However, although we have shown that miR-200 is primarily expressed in AECs, miR-200 mimics may be taken up by both AECs and lung fibroblasts if they are introduced intratracheally or systemically.
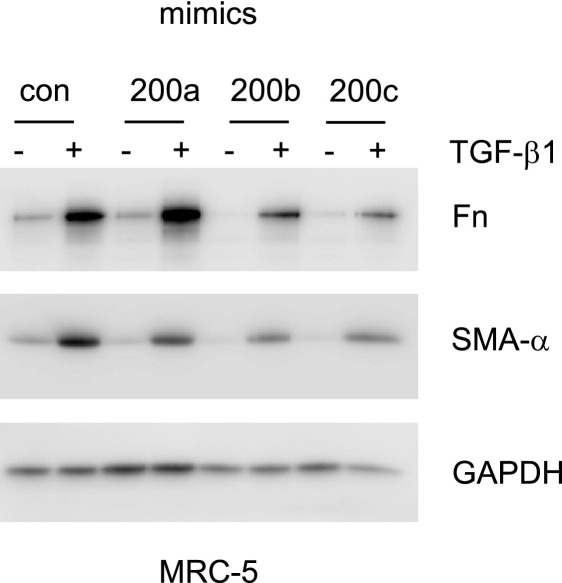
To determine whether miR-200 has a role in regulating the fibrogenic activity of lung fibroblasts, we transfected primary human lung fibroblasts with control or miR-200 mimics. As shown in Figure 7, overexpression of miR-200, particularly miR-200b and miR-200c, markedly attenuated TGF-β1–induced expression of Fn and SMA-α. Given that TGF-β1 is a central mediator of lung fibrosis, these data suggest that therapies able to increase miR-200 expression in the lungs may be able to diminish TGF-β1–mediated fibrogenesis and may attenuate lung fibrosis.
Figure 7.
miR-200 diminishes the fibrogenic activity of TGF-β1 in lung fibroblasts. Primary human lung fibroblasts were transfected with 40 nmol/L control (con) miR mimics or mimics for miR-200a, miR-200b, or miR-200c. Three days after transfection, the cells were starved in medium containing 0.1% FBS for 24 hours and then treated with 2 ng/mL TGF-β1 for 48 hours. The cells were collected, and cell extracts were prepared. The levels of Fn, SMA-α, and the loading control, GAPDH, were determined by using Western blot analysis.
miR-200 Attenuates the Fibrogenic Activity of Lung Fibroblasts from Mice with Experimental Pulmonary Fibrosis
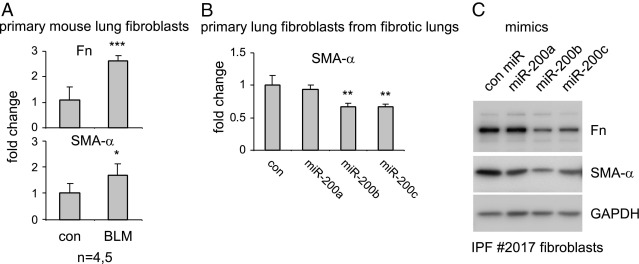
Lung fibroblasts can differentiate into myofibroblasts that are characterized by enhanced expression of SMA-α and elevated fibrogenic activity.4 To determine whether miR-200 participates in the regulation of this process, we isolated lung fibroblasts from control mice or those with experimental pulmonary fibrosis. As shown in Figure 8A, lung fibroblasts from mice with experimental pulmonary fibrosis demonstrated significantly enhanced expression of Fn and SMA-α compared with those from the lungs of untreated mice. These data are consistent with previous findings and suggest differentiation of fibroblasts into myofibroblasts in fibrotic lungs.25,34,35 Next, we transfected control miR or miR-200 mimics into lung fibroblasts from mice with experimental pulmonary fibrosis. We found that overexpression of miR-200, particularly miR-200b and miR-200c, significantly diminished the expression of SMA-α (Figure 8B), suggesting that miR-200 can reverse the differentiation of lung fibroblasts into myofibroblasts.
Figure 8.
miR-200 attenuates the fibrogenic activity of lung fibroblasts from mice with experimental pulmonary fibrosis and from a patient with IPF. A: Primary lung fibroblasts from mice that were intratracheally injected with saline or bleomycin (BLM) were isolated and, after culture, RNA from these cells was purified. The expression of Fn and SMA-α was determined by real-time PCR assays. GAPDH was used as an internal control (con). *P < 0.05, ***P < 0.001 versus the con group. B: One fibroblast line from a mouse with experimental pulmonary fibrosis was transfected with 40 nmol/L con miR mimics or mimics for miR-200a, miR-200b, or miR-200c. Three days after the transfection, the cells were collected and RNA was isolated. The expression of SMA-α was determined by real-time PCR. Experiments were repeated with a second fibroblast line from fibrotic mouse lungs and showed the same results. **P < 0.001 versus the con group. C: IPF lung fibroblasts were transfected with 40 nmol/L con miR mimics or mimics for miR-200a, miR-200b, or miR-200c. Three days after the transfection, the cells were collected and cell extracts were prepared. The levels of Fn, SMA-α, and the loading con, GAPDH, were determined by using Western blot analysis.
miR-200 Attenuates the Fibrogenic Activity of Human IPF Lung Fibroblasts
To further define the role of miR-200 in regulating the fibrogenic activity of lung fibroblasts, we transfected control miR and miR-200 mimics into lung fibroblasts isolated from a patient with IPF and found that miR-200, particularly miR-200b and miR-200c, diminished the expression of SMA-α and Fn in these cells (Figure 8C). Taken together, our data suggest that introduction of miR-200 into lungs diminishes EMT in AECs, thereby reducing the numbers of ECM-producing lung fibroblasts, and also attenuates the fibrogenic activity of lung fibroblasts and reverses the differentiation of lung fibroblasts into myofibroblasts.
Introduction of miR-200c into Lungs Diminishes Experimental Pulmonary Fibrosis in Mice
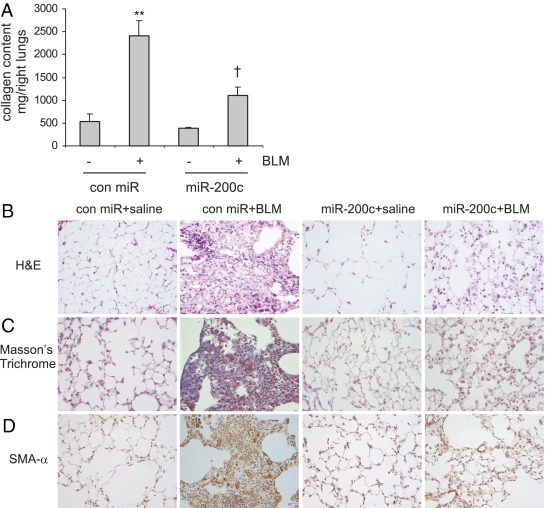
We previously showed that miR-200, particularly miR-200b and miR-200c, decreases EMT in AECs and fibrogenesis in IPF lung fibroblasts. We next determined if introduction of miR-200 into lungs diminishes bleomycin-induced lung fibrosis. As shown in Figure 9A, there was significantly increased collagen deposition in the lungs of bleomycin-instilled mice that received control mimics. However, the enhanced collagen deposition was remarkably attenuated in the lungs of bleomycin-instilled mice that received miR-200c mimics. These data suggest that miR-200c prevents lung fibrosis. H&E staining also demonstrated dramatic attenuation of bleomycin-induced lung fibrosis in mice that received miR-200c probes; this finding was confirmed by Masson's trichrome staining, which highlights collagen deposition (Figure 9, B and C). Likewise, the introduction of miR-200c probes prevented the accumulation of myofibroblasts in bleomycin-treated lungs, as demonstrated by IHC staining with anti- SMA-α antibody (Figure 9D).
Figure 9.
Introduction of miR-200 into lungs diminishes bleomycin (BLM)–induced pulmonary fibrosis. A: Control (con) mimics, con mimics plus bleomycin, miR-200c mimics, or miR-200c mimics plus BLM were instilled intratracheally through oropharyngeal cavities. At 14 days after the treatment, collagen contents in the right lungs were determined by Sircol assays (n = 3, 9, 3, and 9, respectively). Data are given as mean±SEM. **P < 0.01 versus con miR-; †P < 0.01 versus con miR+. B–D: The experiments were performed as in A. Lung tissue sections were prepared, and H&E staining (B), Masson's trichrome staining (C), and IHC staining for SMA-α (D) were performed. Original magnification, ×40.
Discussion
miRs regulate numerous physiological and pathological processes. However, the role of miRs in the pathogenesis of lung fibrosis has not been well characterized.19–22 In our previous study,24 we found that the expression of miR-21 is enhanced in the lungs of mice with bleomycin-induced lung fibrosis and in the lungs of patients with IPF. Blocking miR-21 attenuated experimental lung fibrosis.24 In another study, Pandit et al23 demonstrated that let-7d is down-regulated in the lungs of patients with IPF. They further provided evidence showing that let-7d is a negative regulator of pulmonary fibrosis.23 These previous studies suggest that miRs play important roles in the initiation and progression of lung fibrosis.
In the present study, we defined the role in pulmonary fibrosis of members of the miR-200 family that were identified by miR array assays as showing decreased expression in fibrotic mouse lungs. Three of the miR-200 family members, specifically miR-200a, miR-200b, and miR-200c, were all down-regulated in the lungs of bleomycin-treated mice. Previous studies33,36,37 had shown that miR-200 promotes EMT in cancer cells and is involved in cancer metastasis, in which EMT plays an important role.
We found that miR-200 demonstrated much greater expression in AECs than in lung fibroblasts. These data suggest that miR-200 is involved in the phenotypic abnormalities of AECs that accompany pulmonary fibrosis but may not play a significant role in the production of collagen and other ECM proteins by lung fibroblasts. The important contribution of EMT of AECs to lung fibrosis has been well documented.8–15 In the present study, we found that the epithelial cell marker, E-cadherin, is significantly down-regulated, whereas Fn is significantly up-regulated in AECs isolated from mice with bleomycin-induced lung fibrosis. Our data, together with previous studies, are consistent with the presence of EMT during lung fibrosis and suggest that down-regulation of miR-200 in AECs may contribute to EMT, thereby enhancing pulmonary fibroblast accumulation and lung fibrosis.
TGF-β1 plays a central role in the pathogenesis of lung fibrosis by enhancing the fibrogenic activity of lung fibroblasts and inducing the differentiation of lung fibroblasts into myofibroblasts.38–42 Furthermore, TGF-β1 is a potent inducer of EMT of AECs both in vivo and in vitro.8–15 In the present study, we found that TGF-β1 markedly down-regulated miR-200 expression in primary rat ATII cells and in the rat ATII cell line, RLE-6TN. These data suggest that the miR-200 family may be a negative regulator of TGF-β1–induced EMT of AECs. Indeed, we found that overexpression of miR-200, particularly miR-200b and miR-200c, diminishes TGF-β1–induced EMT of AECs, as demonstrated by attenuation of TGF-β1–induced expression of mesenchymal markers and preservation of the expression of epithelial cell markers in AECs transfected with miR-200. Conversely, we found that inhibition of miR-200 enhances TGF-β1–induced EMT of AECs. Taken together, these data suggest that miR-200 participates in the pathogenesis of lung fibrosis by regulating TGF-β1–induced EMT in AECs.
We found that miR-200 is down-regulated in the lungs of patients with IPF. These data are significant because miR-200 may be a target in developing novel therapeutics for the treatment of pulmonary fibrosis. A recent study43 found increased expression of miR-200 in the lungs of patients with interstitial lung disease, although the authors suggested a negative regulation of pulmonary fibrosis by miR-200. The discrepancy could be explained by diverse disease stages and diagnoses of the selected patient populations. The mechanism by which miR-200 is down-regulated in the lungs of patients with IPF is unknown but may be a response to the enhanced activity of TGF-β1 in this setting. Although miR-200a, miR-200b, and miR-200c are all down-regulated in the lungs of mice with experimental pulmonary fibrosis, we found decreased expression of miR-200a and miR-200c, but not miR-200b, in IPF lungs. Given that the miR-200a and miR-200b genes are located in the same genomic locus and controlled by the same promoter, these data suggest that the expression of miR-200b in IPF lungs is regulated at the transcriptional level and by post-transcriptional mechanisms that may be associated with other disease conditions, such as exacerbation.
Recent studies25,34,35 have shown that pulmonary fibroblasts from the lungs of patients with IPF and from the lungs of mice with experimental pulmonary fibrosis possess increased fibrogenic and proliferative properties compared with those from normal lungs, even in the absence of extracellular stimuli. These data suggest that fibroblasts in fibrotic lungs have acquired an intrinsic transition to a pathological phenotype that enables these cells to produce excessive ECM proteins or to become fibrogenic myofibroblasts, even without a profibrogenic microenvironment, although the mechanism by which these phenomena occur is unclear. Consistent with those studies, we found that pulmonary fibroblasts from mice with experimental lung fibrosis demonstrate significantly enhanced expression of Fn and SMA-α. However, although miR-200 has minimal expression in lung fibroblasts, the transfection of fibroblasts from the fibrotic mouse lungs with miR-200 resulted in significantly down-regulated expression of SMA-α. More important, transfection with miR-200 of IPF fibroblasts attenuated expression of SMA-α and Fn. These data suggest that miR-200 may be able to reverse the intrinsically acquired pathological phenotypes of fibroblasts in the lungs of patients with IPF. This result also implies that the introduction of miR-200 into fibrotic lungs may blunt EMT among AECs, thereby reducing the accumulation of ECM-producing lung fibroblasts, and may reverse the fibrogenic phenotypes of pulmonary fibroblasts in fibrotic lungs. Therefore, miR-200 may produce benefit through a dual mechanism in treating lung fibrosis. Indeed, we found that introduction of miR-200c diminishes bleomycin-induced pulmonary fibrosis in mice, suggesting that restoring miR-200c may be a novel approach for treating lung fibrosis.
Although the present data suggest that miR-200 can attenuate and perhaps even reverse the fibrogenic activity of active pulmonary fibroblasts in fibrotic lungs, it is possible that the mechanism for this effect of miR-200 is distinct from the participation of miR-200 in modulating EMT. Given that miRs usually have multiple effects through regulating the expression of more than one target gene, the identification of miR-200 targets may shed light on how fibroblasts acquire autonomous fibrogenic phenotypes in fibrotic lungs. These questions are being investigated in our laboratory.
The administration of miR-200c diminishes bleomycin-induced pulmonary fibrosis; however, miR mimics may be short-lived molecules. Given the progressive nature of human pulmonary fibrosis, miR molecules with therapeutic potency require chemical modifications that have optimal combinations of being able to enhance the stability and preserve the activity of miR in vivo.
Footnotes
Supported by grants from the NIH (5U01ES015676 to S.M.; HL076206 to E.A.; HL105473 and HL097218 to G.L.) and an award from the American Heart Association (10SDG4210009 to G.L.).
Author contributions: G.L. conceived and designed the study; V.J.T., E.A., and G.L. analyzed the data; S.Y., S.B., A.d.F., and G.L. performed the experiments; Y.Y.S., Q.D., S.M., and V.J.T. provided important materials; and E.A. and G.L. wrote the manuscript.
CME Disclosure: None of the authors disclosed any relevant financial relationships.
Supplemental material for this article can be found at http://ajp.amjpathol.org or at doi: 10.1016/j.ajpath.2011.10.005.
Supplementary data
AECs undergo EMT in lung fibrosis. Mice were injected intratracheally with saline or bleomycin (BLM; 1 U/kg in 50 μL of saline) and sacrificed 14 days after the injection. A: Lung extracts from these mice were prepared, and the levels of E-cadherin and actin in the lungs were determined by using Western blot analysis. B: AECs from these mice were isolated and cultured as described in Materials and Methods, and RNA from the cells was isolated. The expression of E-cadherin and Fn in the AECs was determined by real-time PCR assays (n = 5 mice per group). Data are given as mean ± SD. ***P < 0.001 versus the control (con) group.
miR-200 regulates GATA3, ZEB1, and ZEB2 in AECs. A–C: RLE-6TN cells were transfected with 40 nmol/L of control (con) miR mimics or mimics for miR-200a, miR-200b, or miR200c. At 3 days after the transfection, the cells were starved in medium containing 0.5% FBS for 24 hours and then treated with 5 ng/mL TGF-β1 for 48 hours. The cells were collected, and RNA was isolated. The expression of GATA3 (A), ZEB1 (B), ZEB2 (C), and GAPDH was determined by real-time PCR. The experiments were performed in triplicate. Data are given as mean ± SD. *P < 0.05, **P < 0.05, and ***P < 0.001 versus the con group.
References
- 1.Tomasek J.J., Gabbiani G., Hinz B., Chaponnier C., Brown R.A. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 2.Wynn T.A. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest. 2007;117:524–529. doi: 10.1172/JCI31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thannickal V.J., Toews G.B., White E.S., Lynch J.P., 3rd, Martinez F.J. Mechanisms of pulmonary fibrosis. Annu Rev Med. 2004;55:395–417. doi: 10.1146/annurev.med.55.091902.103810. [DOI] [PubMed] [Google Scholar]
- 4.Hardie W.D., Glasser S.W., Hagood J.S. Emerging concepts in the pathogenesis of lung fibrosis. Am J Pathol. 2009;175:3–16. doi: 10.2353/ajpath.2009.081170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chapman H.A. Epithelial-mesenchymal interactions in pulmonary fibrosis. Annu Rev Physiol. 2011;73:413–435. doi: 10.1146/annurev-physiol-012110-142225. [DOI] [PubMed] [Google Scholar]
- 6.Kalluri R., Weinberg R.A. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thiery J.P., Acloque H., Huang R.Y., Nieto M.A. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 8.Yao H.W., Xie Q.M., Chen J.Q., Deng Y.M., Tang H.F. TGF-beta1 induces alveolar epithelial to mesenchymal transition in vitro. Life Sci. 2004;76:29–37. doi: 10.1016/j.lfs.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 9.Xu J., Lamouille S., Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009;19:156–172. doi: 10.1038/cr.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willis B.C., Borok Z. TGF-beta-induced EMT: mechanisms and implications for fibrotic lung disease. Am J Physiol Lung Cell Mol Physiol. 2007;293:L525–L534. doi: 10.1152/ajplung.00163.2007. [DOI] [PubMed] [Google Scholar]
- 11.Willis B.C., Liebler J.M., Luby-Phelps K., Nicholson A.G., Crandall E.D., du Bois R.M., Borok Z. Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-beta1: potential role in idiopathic pulmonary fibrosis. Am J Pathol. 2005;166:1321–1332. doi: 10.1016/s0002-9440(10)62351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kasai H., Allen J.T., Mason R.M., Kamimura T., Zhang Z. TGF-beta1 induces human alveolar epithelial to mesenchymal cell transition (EMT) Respir Res. 2005;6:56. doi: 10.1186/1465-9921-6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim K.K., Kugler M.C., Wolters P.J., Robillard L., Galvez M.G., Brumwell A.N., Sheppard D., Chapman H.A. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci U S A. 2006;103:13180–13185. doi: 10.1073/pnas.0605669103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu Z., Yang L., Cai L., Zhang M., Cheng X., Yang X., Xu J. Detection of epithelial to mesenchymal transition in airways of a bleomycin induced pulmonary fibrosis model derived from an alpha-smooth muscle actin-Cre transgenic mouse. Respir Res. 2007;8:1. doi: 10.1186/1465-9921-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Degryse A.L., Tanjore H., Xu X.C., Polosukhin V.V., Jones B.R., Boomershine C.S., Ortiz C., Sherrill T.P., McMahon F.B., Gleaves L.A., Blackwell T.S., Lawson W.E. TGFβ signaling in lung epithelium regulates bleomycin-induced alveolar injury and fibroblast recruitment. Am J Physiol Lung Cell Mol Physiol. 2011;300:L887–L897. doi: 10.1152/ajplung.00397.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cutroneo K.R., White S.L., Phan S.H., Ehrlich H.P. Therapies for bleomycin induced lung fibrosis through regulation of TGF-beta1 induced collagen gene expression. J Cell Physiol. 2007;211:585–589. doi: 10.1002/jcp.20972. [DOI] [PubMed] [Google Scholar]
- 17.Lee C.G., Cho S., Homer R.J., Elias J.A. Genetic control of transforming growth factor-beta1-induced emphysema and fibrosis in the murine lung. Proc Am Thorac Soc. 2006;3:476–477. doi: 10.1513/pats.200603-040MS. [DOI] [PubMed] [Google Scholar]
- 18.Stefani G., Slack F.J. Small non-coding RNAs in animal development. Nat Rev Mol Cell Biol. 2008;9:219–230. doi: 10.1038/nrm2347. [DOI] [PubMed] [Google Scholar]
- 19.Croce C.M. Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet. 2009;10:704–714. doi: 10.1038/nrg2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Latronico M.V., Condorelli G. MicroRNAs and cardiac pathology. Nat Rev Cardiol. 2009;6:419–429. doi: 10.1038/nrcardio.2009.56. [DOI] [PubMed] [Google Scholar]
- 21.Pandey A.K., Agarwal P., Kaur K., Datta M. MicroRNAs in diabetes: tiny players in big disease. Cell Physiol Biochem. 2009;23:221–232. doi: 10.1159/000218169. [DOI] [PubMed] [Google Scholar]
- 22.Thum T., Gross C., Fiedler J., Fischer T., Kissler S., Bussen M., Galuppo P., Just S., Rottbauer W., Frantz S., Castoldi M., Soutschek J., Koteliansky V., Rosenwald A., Basson M.A., Licht J.D., Pena J.T., Rouhanifard S.H., Muckenthaler M.U., Tuschl T., Martin G.R., Bauersachs J., Engelhardt S. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008;456:980–984. doi: 10.1038/nature07511. [DOI] [PubMed] [Google Scholar]
- 23.Pandit K.V., Corcoran D., Yousef H., Yarlagadda M., Tzouvelekis A., Gibson K.F., Konishi K., Yousem S.A., Singh M., Handley D., Richards T., Selman M., Watkins S.C., Pardo A., Ben-Yehudah A., Bouros D., Eickelberg O., Ray P., Benos P.V., Kaminski N. Inhibition and role of let-7d in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2010;182:220–229. doi: 10.1164/rccm.200911-1698OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu G., Friggeri A., Yang Y., Milosevic J., Ding Q., Thannickal V.J., Kaminski N., Abraham E. miR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J Exp Med. 2010;207:1589–1597. doi: 10.1084/jem.20100035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Konigshoff M., Kramer M., Balsara N., Wilhelm J., Amarie O.V., Jahn A., Rose F., Fink L., Seeger W., Schaefer L., Gunther A., Eickelberg O. WNT1-inducible signaling protein-1 mediates pulmonary fibrosis in mice and is upregulated in humans with idiopathic pulmonary fibrosis. J Clin Invest. 2009;119:772–787. doi: 10.1172/JCI33950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen L., Bosworth C.A., Pico T., Collawn J.F., Varga K., Gao Z., Clancy J.P., Fortenberry J.A., Lancaster J.r., Jr, Matalon S. DETANO and nitrated lipids increase chloride secretion across lung airway cells. Am J Respir Cell Mol Biol. 2008;39:150–162. doi: 10.1165/rcmb.2008-0005OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu G., Friggeri A., Yang Y., Park Y.J., Tsuruta Y., Abraham E. miR-147, a microRNA that is induced upon Toll-like receptor stimulation, regulates murine macrophage inflammatory responses. Proc Natl Acad Sci U S A. 2009;106:15819–15824. doi: 10.1073/pnas.0901216106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu G., Park Y.J., Abraham E. Interleukin-1 receptor-associated kinase (IRAK)-1-mediated NF-kappaB activation requires cytosolic and nuclear activity. FASEB J. 2008;22:2285–2296. doi: 10.1096/fj.07-101816. [DOI] [PubMed] [Google Scholar]
- 29.Chua F., Gauldie J., Laurent G.J. Pulmonary fibrosis: searching for model answers. Am J Respir Cell Mol Biol. 2005;33:9–13. doi: 10.1165/rcmb.2005-0062TR. [DOI] [PubMed] [Google Scholar]
- 30.Moore B.B., Hogaboam C.M. Murine models of pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2008;294:L152–L160. doi: 10.1152/ajplung.00313.2007. [DOI] [PubMed] [Google Scholar]
- 31.Wang X.M., Zhang Y., Kim H.P., Zhou Z., Feghali-Bostwick C.A., Liu F., Ifedigbo E., Xu X., Oury T.D., Kaminski N., Choi A.M. Caveolin-1: a critical regulator of lung fibrosis in idiopathic pulmonary fibrosis. J Exp Med. 2006;203:2895–2906. doi: 10.1084/jem.20061536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yan W., Cao Q.J., Arenas R.B., Bentley B., Shao R. GATA3 inhibits breast cancer metastasis through the reversal of epithelial-mesenchymal transition. J Biol Chem. 2010;285:14042–14051. doi: 10.1074/jbc.M110.105262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gregory P.A., Bert A.G., Paterson E.L., Barry S.C., Tsykin A., Farshid G., Vadas M.A., Khew-Goodall Y., Goodall G.J. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 34.Ramos C., Montano M., Garcia-Alvarez J., Ruiz V., Uhal B.D., Selman M., Pardo A. Fibroblasts from idiopathic pulmonary fibrosis and normal lungs differ in growth rate, apoptosis, and tissue inhibitor of metalloproteinases expression. Am J Respir Cell Mol Biol. 2001;24:591–598. doi: 10.1165/ajrcmb.24.5.4333. [DOI] [PubMed] [Google Scholar]
- 35.Xia H., Diebold D., Nho R., Perlman D., Kleidon J., Kahm J., Avdulov S., Peterson M., Nerva J., Bitterman P., Henke C. Pathological integrin signaling enhances proliferation of primary lung fibroblasts from patients with idiopathic pulmonary fibrosis. J Exp Med. 2008;205:1659–1672. doi: 10.1084/jem.20080001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang Y., Ahn Y.H., Gibbons D.L., Zang Y., Lin W., Thilaganathan N., Alvarez C.A., Moreira D.C., Creighton C.J., Gregory P.A., Goodall G.J., Kurie J.M. The Notch ligand Jagged2 promotes lung adenocarcinoma metastasis through a miR-200-dependent pathway in mice. J Clin Invest. 2011;121:1373–1385. doi: 10.1172/JCI42579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park S.M., Gaur A.B., Lengyel E., Peter M.E. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 2008;22:894–907. doi: 10.1101/gad.1640608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kang H.R., Cho S.J., Lee C.G., Homer R.J., Elias J.A. Transforming growth factor (TGF)-beta1 stimulates pulmonary fibrosis and inflammation via a Bax-dependent, bid-activated pathway that involves matrix metalloproteinase-12. J Biol Chem. 2007;282:7723–7732. doi: 10.1074/jbc.M610764200. [DOI] [PubMed] [Google Scholar]
- 39.Finlay G.A., Thannickal V.J., Fanburg B.L., Paulson K.E. Transforming growth factor-beta 1-induced activation of the ERK pathway/activator protein-1 in human lung fibroblasts requires the autocrine induction of basic fibroblast growth factor. J Biol Chem. 2000;275:27650–27656. doi: 10.1074/jbc.M000893200. [DOI] [PubMed] [Google Scholar]
- 40.Sheppard D. Transforming growth factor beta: a central modulator of pulmonary and airway inflammation and fibrosis. Proc Am Thorac Soc. 2006;3:413–417. doi: 10.1513/pats.200601-008AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gharaee-Kermani M., Hu B., Phan S.H., Gyetko M.R. Recent advances in molecular targets and treatment of idiopathic pulmonary fibrosis: focus on TGFbeta signaling and the myofibroblast. Curr Med Chem. 2009;16:1400–1417. doi: 10.2174/092986709787846497. [DOI] [PubMed] [Google Scholar]
- 42.Thannickal V.J., Lee D.Y., White E.S., Cui Z., Larios J.M., Chacon R., Horowitz J.C., Day R.M., Thomas P.E. Myofibroblast differentiation by transforming growth factor-beta1 is dependent on cell adhesion and integrin signaling via focal adhesion kinase. J Biol Chem. 2003;278:12384–12389. doi: 10.1074/jbc.M208544200. [DOI] [PubMed] [Google Scholar]
- 43.Cho J.H., Gelinas R., Wang K., Etheridge A., Piper M.G., Batte K., Dakhallah D., Price J., Bornman D., Zhang S., Marsh C., Galas D. Systems biology of interstitial lung diseases: integration of mRNA and microRNA expression changes. BMC Med Genomics. 2011;4:8. doi: 10.1186/1755-8794-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
AECs undergo EMT in lung fibrosis. Mice were injected intratracheally with saline or bleomycin (BLM; 1 U/kg in 50 μL of saline) and sacrificed 14 days after the injection. A: Lung extracts from these mice were prepared, and the levels of E-cadherin and actin in the lungs were determined by using Western blot analysis. B: AECs from these mice were isolated and cultured as described in Materials and Methods, and RNA from the cells was isolated. The expression of E-cadherin and Fn in the AECs was determined by real-time PCR assays (n = 5 mice per group). Data are given as mean ± SD. ***P < 0.001 versus the control (con) group.
miR-200 regulates GATA3, ZEB1, and ZEB2 in AECs. A–C: RLE-6TN cells were transfected with 40 nmol/L of control (con) miR mimics or mimics for miR-200a, miR-200b, or miR200c. At 3 days after the transfection, the cells were starved in medium containing 0.5% FBS for 24 hours and then treated with 5 ng/mL TGF-β1 for 48 hours. The cells were collected, and RNA was isolated. The expression of GATA3 (A), ZEB1 (B), ZEB2 (C), and GAPDH was determined by real-time PCR. The experiments were performed in triplicate. Data are given as mean ± SD. *P < 0.05, **P < 0.05, and ***P < 0.001 versus the con group.