Abstract
IFN-γ−/− NOD.H-2h4 mice develop an autoimmune disease characterized by hyperplasia and proliferation of thyroid epithelial cells (TEC H/P). Proliferating TECs produce TGF-β, and IFN-γ inhibits TEC H/P. In the present study, cultured TECs were used to directly determine the mechanisms by which these cytokines act on TECs to result in proliferation or inhibition of proliferation. With TECs from IFN-γ−/− NOD.H-2h4 mice or mice expressing the dominant negative TGF-β type II receptor on TECs, TGF-β was shown to promote TEC proliferation and IFN-γ was shown to inhibit TEC proliferation in vitro. TGF-β may promote TEC proliferation by down-regulating antiproliferative molecules p21 and p27, whereas IFN-γ may inhibit proliferation by up-regulating antiproliferative molecules p18 and p21 and down-regulating the pro-proliferative molecule cyclin D. Inhibition of AKT abolished the effect of TGF-β on p21 and p27, resulting in similar proliferation of TGF-β-treated and control TECs. Increased expression of proliferating cell nuclear antigen (PCNA), TGF-β, and p-AKT and decreased expression of p21 and p27 by proliferating TECs correlated with the proliferative state of TEC H/P. Taken together, the results suggest that TGF-β promotes TEC proliferation by down-regulating p21 and p27 via the AKT pathway in IFN-γ−/− NOD.H-2h4 mice, which may have significant implications for development of effective therapeutic strategies targeting the TGF-β and AKT pathways for treatment of hyperplasia and/or neoplasia.
TGF-β is a multifunctional cytokine with diverse biological effects on many cellular processes, including cell proliferation.1–3 TGF-β exerts its functions through a cell surface receptor complex composed of type I (TGFBR1) and type II (TGFBR2) serine/threonine kinase receptors. The Smad pathway is a well-studied pathway used for TGF-β signaling, but TGF-β also uses other intracellular signaling pathways to regulate various cellular functions, including proliferation,1,4 which at least in part explains its functional versatility. TGF-β can promote cell proliferation in many tumor cells,2,3,5,6 but it also has antiproliferative effects on some cells, including epithelial cells.7,8 Considerable progress has been made toward understanding the signaling networks and downstream pathways after the binding of TGF-β with its receptors. Increasing evidence suggests that co-operation between Smad and non-Smad signaling pathways determines the final outcome of the cellular response to TGF-β.1,4 The noncanonical, non-Smad pathways are activated directly by ligand-occupied receptors to reinforce, attenuate, or otherwise modulate downstream cellular responses. The AKT pathway is one non-Smad pathway4 that has been shown to be crucial for a number of cellular responses to growth factors, including cell proliferation.9 It has been suggested that the signal transduced by TGF-β binding to its receptors depends on the cell type and the surrounding hormone/growth factor context.10,11
IFN-γ is another multifunctional cytokine that plays an important role in many autoimmune diseases, including thyroiditis. IFN-γ is the prototypic Th1 cytokine produced by CD4+ Th1 cells, CD8+ T cells, and natural killer cells.12,13 IFN-γ and TGF-β reciprocally regulate each other,14,15 and so regulate cell proliferation.
The eukaryotic cell cycle is tightly regulated to ensure that replication and division take place in a controlled manner.16–19 The balance between pro- and antiproliferative molecules plays an important role in cell proliferation. Cyclin D, cyclin E, and cyclin-dependent kinases are important pro-proliferative molecules. Down-regulation of cyclin D can delay or inhibit entry to the S phase of the cell cycle, and overexpression of cyclin D can shorten the G1 phase.16–19 Cyclin E is active in the late G1 phase and is maximal at the G1-to-S transition. p21, p27, p18, and p53, as well as p15, p16, p19, and p57, are important antiproliferative molecules that function to inhibit cyclin-dependent kinases and thus have antiproliferative effects.16–19
Autoimmune thyroid diseases are often associated with abnormal proliferation (hyperplasia) of thyroid epithelial cells (TECs). Thyroid hyperplasia is very common and can be associated with development of goiter, thyroid adenoma, and carcinoma.20 We previously showed that IFN-γ−/− NOD.H-2h4 mice develop an autoimmune disease characterized by severe TEC hyperplasia and proliferation (TEC H/P) and development of fibrosis.21 TEC proliferation is promoted by TGF-β, which is produced by the proliferating TECs.21 IFN-γ produced by T cells inhibits TEC proliferation in this model, presumably by acting directly on TECs.22 These in vivo studies provide indirect evidence suggesting that TGF-β acts directly on TECs to promote their proliferation, whereas IFN-γ suppresses TEC proliferation by interfering with the effects of TGF-β.21,22 The present study was conducted to extend these in vivo studies by using an in vitro system in which the interactions of cytokines with TECs could be controlled to directly define the mechanisms by which IFN-γ and TGF-β interact with TECs to inhibit or promote their proliferation.
Materials and Methods
Mice
NOD.H-2h4 mice express H-2Kk, I-Ak, and Dd on the NOD background.23 NOD.H-2h4 wild-type, IFN-γ−/−, IFN-γR−/−, and IFN-γ−/−-SCID mice were generated in our animal facility as described previously.22 To generate transgenic mice expressing the dominant negative TGF-β type II receptor (dnTβRII) on TECs, we used a plasmid containing the recombinant dnTβRII construct tagged with FLAG (kindly provided by Dr. H. Moses, Vanderbilt University). This construct was combined with the rat thyroglobulin (TG) promoter (with the assistance of Dr. J. Qiu, University of Kansas Medical Center), amplified in Escherichia coli, and digested using SalI and XbaI (Invitrogen, Carlsbad, CA). The 1.652K-bp recombined construct was microinjected into fertilized oocytes from NOD.H-2h4 female mice at the Transgenic Core Facility of the University of Missouri, Columbia. Transgenic founders were screened by PCR of tail DNA using the following primers: sense, 5′-GGAGCCAGGGCTGGGCATAAAA-3′; antisense, 5′-GACTCACCCTGAAGTTCTCAGGATCC-3′. A transgenic female founder was crossed with a NOD.H-2h4 IFN-γ−/− male, and Tg+ offspring were further crossed to obtain Tg+ IFN-γ−/− mice. Tg+ mice and Tg− IFN-γ−/− littermates were used for all experiments. Mice were bred and maintained in accordance with University of Missouri institutional guidelines for animal care.
Western Blot
Expression of TβRII and FLAG was quantified by Western blot analysis with rabbit anti-TβRII (Santa Cruz Biotechnology, Santa Cruz, CA) and anti-FLAG (Abcam, Cambridge, MA) by adding 30 μg of protein to a 15% SDS-PAGE gel as described previously.24 Expression of p21 and p27 was quantified using rabbit anti-p21 and anti-p27 antibodies (Santa Cruz Biotechnology) by adding 30 μg of protein to a 15% SDS-PAGE gel.24 For normalization of signals, the membranes were stripped and reprobed with 1/2000 rabbit anti-actin primary antibody (Santa Cruz Biotechnology) and 1/3000 HRP-conjugated anti-rabbit IgG as secondary antibody, as described previously.24
Real-Time PCR
Individual thyroid lobes were homogenized in TRIzol reagent (Invitrogen). RNA was extracted and 1 μg RNA was reverse transcribed as described previously.25,26 TβRII mRNA was quantified by real-time PCR using an ABI 7000 sequence detection system (Applied Biosystems, Foster City, CA). Amplification was performed for 40 cycles in a total volume of 30 μL; products were detected using SYBR Green (Thermo Scientific; ABgene, Epsom, UK). The relative expression levels of triplicate samples were determined by normalizing expression of each target to HPRT. Expression level of each normalized sample is given as relative expression units. Real-time PCR primers for TβRII were as follows: sense, 5′-AGCATCACGGCCATCTGTG-3′; antisense, 5′-TGGCAAACCGTCTCCAGAGT-3′. The size of the amplified product for TβRII is 166 bp.
Immunohistochemistry
IHC staining for TβRII, FLAG, PCNA, p-AKT, TGF-β, p21, and p27 was performed as described previously.27 Thyroid sections were deparaffinized in xylene, rehydrated through sequential ethanol, and rinsed in PBS. Sections were incubated with rabbit anti-TβRII, anti-FLAG, anti-PCNA, anti-p-AKT, anti-p21, and anti-p27 (Santa Cruz Biotechnology) and chicken anti-TGF-β antibodies (R&D Systems, Minneapolis, MN) for 60 minutes at room temperature. After incubation with the corresponding secondary biotinylated antibody (Jackson ImmunoResearch, West Grove, PA), immunoreactivity was demonstrated using the avidin-biotin complex immunoperoxidase system (Vector Laboratories, Burlingame, CA) and developed using Vector NovaRED as the chromogen. Slides were counterstained with hematoxylin. As a negative control, primary antibody was replaced with an equal amount of normal rabbit or chicken IgG; these controls were always negative.
Primary Culture of TECs from IFN-γ−/− NOD.H-2h4 Mice
Mouse primary TEC cultures were generated as described previously.28 In brief, thyroid lobes from groups of six 8- to 10-week-old naïve IFN-γ−/− NOD.H-2h4 mice or from dnTβRII Tg+ littermates were aseptically dissected, disrupted, and digested for 1 hour at 37°C in digestion medium consisting of 112 units/mL of type I collagenase and 1.2 units/mL of dispase II dissolved in Eagle's minimal essential medium. After centrifugation, pellets were resuspended in 15 mL of TEC culture medium, seeded in eight-well chamber slides (0.2 to 0.3 mL/well; Nalge Nunc International, Naperville, IL), and cultured at 37°C. When cultures reached 70% to 80% confluence, usually after 2 to 3 weeks, cultured TECs were treated for 3 days with different concentrations of IFN-γ (2 to 20 ng/mL; eBioscience, San Diego, CA), acid-activated TGF-β (0.1 to 2 ng/mL; PeproTech, Rocky Hill, NJ), or medium alone. IFN-γ and TGF-β concentrations and the duration of cytokine treatment were based on our previous studies28 and those of others.11
Determination of Proliferation of Cultured TECs
Proliferation of cultured TECs was determined by proliferation marker PCNA staining by IHC as described previously.21 NovaRED (Vector Laboratories) was used for color development, and slides were counterstained with hematoxylin. To quantify the number of proliferating TECs, all cells in five to six randomly selected high-power fields (×400 magnification) were manually counted using MetaMorph version 6.3r6 image analysis software (Molecular Devices Analytical Technologies, Sunnyvale, CA) as described previously.29 PCNA+ cells were expressed as a percentage of total cells. In some experiments, proliferation was determined using a Quick Cell proliferation assay kit (BioVision, Mountain View, CA) according to the manufacturer's instructions.30
TUNEL Staining
Apoptosis of cultured TECs was determined by TUNEL assay using an ApopTag kit (Millipore; Chemicon International, Temecula, CA) as described previously.28 TECs treated with resveratrol were used as a positive control for apoptosis.
RT-PCR
Cultured TECs were harvested, washed with PBS, centrifuged, and homogenized in TRIzol reagent (Invitrogen). RNA was extracted and 1 μg RNA was reverse transcribed as described previously.25,27 β-Actin was used as a housekeeping gene to verify that the same amount of RNA was amplified. Primer sequences were as follows: PCNA sense, 5′-GGTTGGTAGTTGTCGGTGTA-3′ and antisense, 5′-CAGGCTCATTCATCTCTATCG-3′; p21 sense, 5′-AGCCTGAAGACTGTGATGGG-3′ and antisense, 5′-AAAGTTCCACCGTTCTCGG-3′; p27 sense, 5′-AAGCACTGCCGGGATATGGA-3′ and antisense, 5′-AACCCAGCCTGATTGTCTGAC-3′; p18 sense, 5′-AGATTAACCATCCCAGTCCT-3′ and antisense, 5′-CTGAATGGGTGGATTAGGTA-3′; p53 sense, 5′-ACTGCATGGACGATCTGTTG-3′ and antisense, 5′-GCCATAGTTGCCCTGCTAAG-3′; and cyclin D sense, 5′-TCTACACTGACAACTCTATCCG-3′ and antisense, 5′-TAGCAGGAGAGGAAGTTGTTGG-3′.
Treatment of Cultured TECs with AKT Inhibitor
Cultured TECs, 70% to 80% confluent, were treated with an AKT inhibitor (AKT1/2 kinase inhibitor, 30 μmol/L; Sigma-Aldrich, St. Louis, MO) for 3 days with TGF-β (2 ng/mL) or medium alone. Cells were analyzed by IHC for PCNA and by RT-PCR for mRNA expression.
Induction of TEC H/P in Vivo
Splenocytes from IFN-γ−/− mice with severe TEC H/P and fibrosis were pooled and cultured for 72 hours in complete RPMI 1640 medium as described previously.31 Splenocytes (3 × 106) were transferred intravenously into NOD.H-2h4 IFN-γ−/− SCID mice. Mice were given 0.05% NaI water, and thyroid histology was assessed 28 days and either 35 or 60 days later.
Evaluation of TEC H/P Severity
Thyroids were removed, and one thyroid lobe was fixed in formalin, sectioned, and stained with H&E as described previously.31,32 All slides were scored by two individuals (H.B.-M. and Y.F. or S.Y.), one of whom had no knowledge of the experimental groups (Y.F. or S.Y.). Thyroid histopathology was scored for the extent of thyroid follicular cell hyperplasia/proliferation, using a scale of 0 to 5+, as described previously.31,32 Briefly, a score of 0 indicates a normal thyroid, and 0+ indicates mild follicular changes and/or a few inflammatory cells infiltrating the thyroids. A 1+ score indicates hyperplastic changes sufficient to cause replacement of several follicles. A 2+ score indicates hyperplastic changes causing replacement or destruction of up to 1/4 of the gland, 3+ indicates that 1/4 to 1/2 of the gland is destroyed by hyperplastic changes, and 4+ indicates that greater than 1/2 of the gland is destroyed. Thyroids given a score of 5+ had few or no remaining normal follicles and extensive collagen deposition (fibrosis). The severe lesions in IFN-γ−/− mice (graded 4+ to 5+, based on the percentage of normal thyroid follicles remaining) had widespread clusters of proliferating TECs and histiocytes with some lymphocyte infiltration. The areas of proliferating TECs were usually surrounded by collagen. All thyroids with mild or severe hyperplasia had infiltrating lymphocytes, but lymphocyte infiltration was much less than in thyroids of wild-type mice with spontaneous autoimmune thyroiditis.
Statistical Analysis
All experiments were repeated two or three times. Statistical analysis of data were performed using an unpaired two-tailed Student's t-test or the Mann-Whitney rank-sum test. A P value of <0.05 was considered significant.
Results
Generation of dnTβRII Transgenic Mice and Expression of dnTβRII and FLAG on TECs
Our previous studies indicated that overexpression of TGF-β on TECs promotes development of TEC H/P in vivo.21 To directly test the hypothesis that TGF-β promotes TEC proliferation in vitro and to determine possible mechanisms, dnTβRII transgenic mice were generated as described under Materials and Methods (see Supplemental Figure S1A at http://ajp.amjpathol.org). Western blot, real-time PCR, and IHC analyses showed that TβRII was constitutively expressed on TECs in Tg− littermates and that TβRII was significantly overexpressed on TECs in Tg+ mice (see Supplemental Figure S1, B–D, at http://ajp.amjpathol.org). Transgenic TβRII (detected by anti-FLAG) was expressed in TECs (see Supplemental Figure S1, B and D, at http://ajp.amjpathol.org), but not in other tissues (including spleen, liver, salivary gland, and cervical lymph nodes; data not shown). Western blot analysis and real-time PCR indicated that the dnTβRII levels in naïve Tg+ mice were five- to sixfold higher, compared with expression of the endogenous receptor in Tg− littermates (see Supplemental Figure S1, B and C, at http://ajp.amjpathol.org).
TGF-β Promotes Proliferation of Cultured TECs from IFN-γ−/− Tg− Mice but Not dnTβRII Tg+ Mice
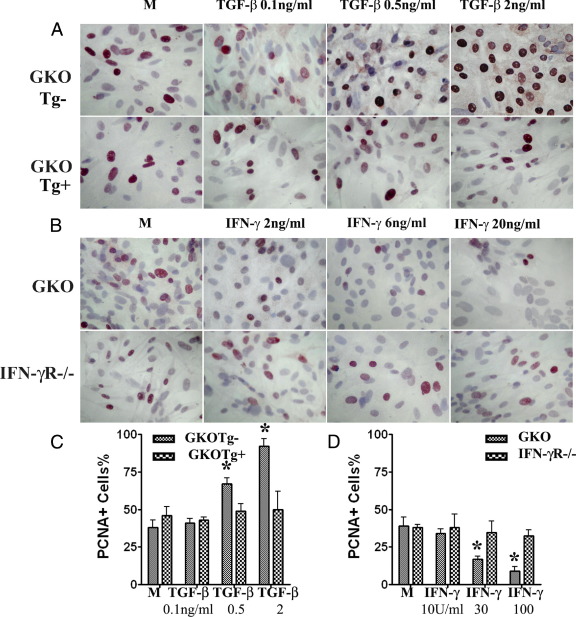
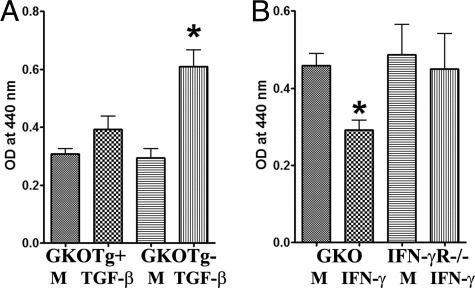
To directly determine whether TGF-β promotes proliferation of TECs in vitro, primary cultures of TECs from dnTβRII Tg+ IFN-γ−/− NOD.H-2h4 mice and their Tg− littermates were established, and various concentrations of TGF-β were added for 72 hours. Assessment of proliferation by PCNA staining (Figure 1, A and C) indicated that TGF-β promotes proliferation of TECs from nontransgenic IFN-γ−/− mice in vitro in a dose-dependent manner, but has no effect on TECs of dnTβRII Tg+ mice. Similar results were also obtained with a cell proliferation assay (Figure 2A) and by mRNA analysis for PCNA (Figure 3A). These results indicate that TGF-β directly promotes proliferation of cultured TECs by binding with its receptor.
Figure 1.
Effect of TGF-β and IFN-γ on TEC proliferation evaluated by IHC. Shown are IHC staining results for PCNA of cultured TECs from IFN-γ−/− (GKO) Tg− and Tg+ (dnTβRII) mice cultured with the indicated amounts of TGF-β (A) and results for PCNA of cultured TECs from GKO and IFN-γR−/− mice cultured with the indicated amounts of IFN-γ (B). Original magnification, ×400. C and D: PCNA+ cells (red) in five to six randomly selected high-power fields of three wells/group were counted using MetaMorph software. Summarized results are shown; 0.5 or 2 ng/mL TGF-β induced significant proliferation of TECs from Tg− mice but not from Tg+ mice (*P < 0.05), and 6 or 20 ng/mL IFN-γ inhibited proliferation of TECs from IFN-γ−/− mice (P < 0.05) but had no effect on TECs of IFN-γR−/− mice. The activity of IFN-γ is 5 U/ng.
Figure 2.
Effect of TGF-β and IFN-γ on TEC proliferation determined using a proliferation kit. A: TGF-β promotes proliferation of cultured TECs from GKO Tg− mice but not from GKO Tg+ mice. B: IFN-γ inhibits proliferation of cultured TECs from GKO but not from IFN-γR−/− mice. Results are expressed as the mean optical density (OD) ± SEM of TECs from four to five wells/group and are representative of three independent experiments. *P < 0.05 versus medium (M) alone.
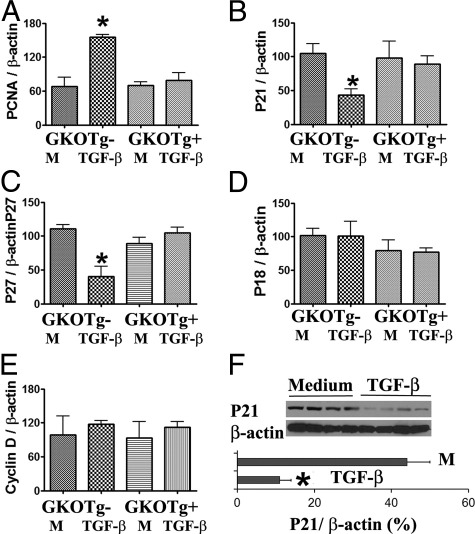
Figure 3.
Effect of TGF-β on pro- and antiproliferative molecules in cultured TECs. mRNA and proteins were extracted as described under Materials and Methods. A–E: RT-PCR. F: Western blot. Results are expressed as the mean ratio of PCNA, pro- and antiproliferative molecule densitometric units/β-actin ± SEM (×100) and are representative of two to three independent experiments. *P < 0.05 versus medium (M) alone.
IFN-γ Inhibits Proliferation of Cultured TECs from IFN-γ−/− but Not IFN-γR−/− NOD.H-2h4 Mice
Our previous studies showed that TEC H/P develops only in mice that lack IFN-γ or in mice whose TECs cannot respond to IFN-γ (IFN-γR−/− mice).22 IFN-γ-sufficient NOD.H-2h4 mice do not develop TEC H/P, and wild-type splenocytes able to produce IFN-γ suppress TEC H/P in IFN-γ−/− but not in IFN-γR−/− NOD.H-2h4 mice.22 This suggests that IFN-γ can directly suppress TEC proliferation in vitro. To address this possibility, primary cultures of TECs from naïve IFN-γ−/− and IFN-γR −/− NOD.H-2h4 mice were established, and various concentrations of IFN-γ were added for 3 days. Assessment of proliferation by PCNA staining indicated that IFN-γ inhibits proliferation of TECs from IFN-γ−/− NOD.H-2h4 mice in a dose-dependent manner in vitro, but has no effect on TECs from IFN-γR−/− NOD.H-2h4 mice (Figure 1, A and C). Similar results were obtained with a cell proliferation assay for TEC proliferation (Figure 2B) and by mRNA analysis for PCNA (Figure 4A). Consistent with our earlier in vivo results,21 these in vitro results directly demonstrate that TGF-β promotes and IFN-γ inhibits proliferation of cultured TECs.
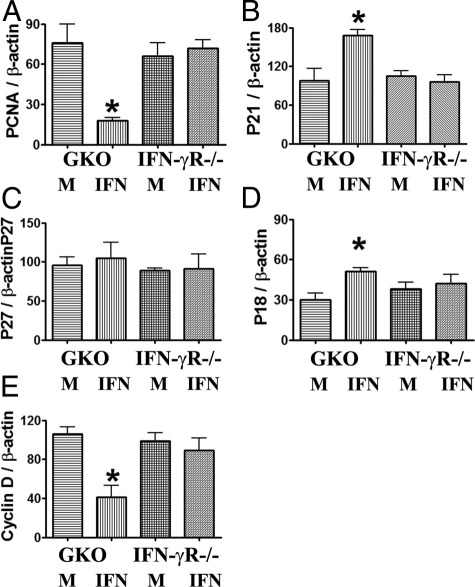
Figure 4.
IFN-γ inhibits proliferation of cultured TECs by modulating pro- and antiproliferative molecules. RT-PCR results are expressed as the mean ratio of PCNA, pro- and antiproliferative molecule densitometric units/β-actin ± SEM (×100), and are representative of three independent experiments. *P < 0.05 versus medium (M) alone.
TGF-β Promotes Proliferation of Cultured TECs by Modulating Antiproliferative Molecules
The balance between pro- and antiproliferative molecules plays an important role in cell proliferation.16–19 Cyclin D and cyclin E are important pro-proliferative molecules, and p21, p27, p18, and p53 are important antiproliferative molecules.16–19 To determine whether pro- and antiproliferative molecules are involved in TGF-β-induced proliferation of TECs, mRNA expression of pro- and antiproliferative molecules in cultured TECs from dnTβRII Tg+ mice and their Tg− littermates in the presence or absence of TGF-β was determined by RT-PCR. Consistent with the IHC and cell proliferation assay findings for TGF-β and IFN-γ (Figures 1 and 2), mRNA expression of PCNA was increased in TECs from nontransgenic mice after culture with TGF-β, but TGF-β had no effect on PCNA expression in TECs from dnTβRII Tg+ mice (Figure 3A). Of particular interest, mRNA expression of the antiproliferative molecules p21 and p27 was significantly lower in TECs from Tg− mice when TGF-β was added to the culture, but expression of these molecules by TECs of dnTβRII Tg+ mice was unaffected by addition of TGF-β (Figure 3, B and C). mRNA expression of other antiproliferative molecules such as p18 (Figure 3D) or p53 (data not shown), as well as mRNA expression of the pro-proliferative molecules cyclin D (Figure 3E) and cyclin E (data not shown), was unaffected by addition of TGF-β to TECs of Tg+ or Tg− mice. Western blot analysis further confirmed that p21 levels in TECs from Tg− mice were decreased in the presence of TGF-β (Figure 3F). Given that expression of all of these markers was unaffected in TECs from dnTβRII Tg+ mice, the results suggest that down-regulation of the antiproliferative molecules p21 and p27 is associated with TGF-β-induced proliferation of TECs.
IFN-γ Inhibits Proliferation of Cultured TECs by Modulating Pro- and Antiproliferative Molecules
To determine whether pro- and antiproliferative molecules are involved in IFN-γ-inhibited proliferation of TECs, mRNA expression of major pro- and antiproliferative molecules in cultured TECs from IFN-γ−/− and IFN-γR−/− NOD.H-2h4 mice was determined by RT-PCR. Consistent with the IHC and cell proliferation assay findings for TGF-β and IFN-γ (Figures 1 and 2), PCNA mRNA was significantly lower in TECs from IFN-γ−/− NOD.H-2h4 mice in the presence of IFN-γ. Expression of mRNA of the antiproliferative molecules p21 and p18 was significantly higher and that of the pro-proliferative molecule cyclin D was significantly lower in TECs from IFN-γ−/− NOD.H-2h4 mice in the presence of IFN-γ (Figure 4). Expression of the antiproliferative molecules p27 (Figure 4) and p53 (data not shown) or the pro-proliferative molecule cyclin E (data not shown) was unaffected by IFN-γ, and expression of all markers was unaffected in IFN-γR−/− TECs unable to respond to IFN-γ. These results indicate that up-regulation of the antiproliferative molecules p21 and p18 and down-regulation of the pro-proliferative molecule cyclin D are associated with IFN-γ-mediated inhibition of TEC proliferation.
TGF-β and IFN-γ Have Little Effect on TEC Apoptosis
Changes in apoptosis could contribute to the TGF-β-induced or IFN-γ-inhibited proliferation of TECs. To address the role of apoptosis in TEC proliferation, 70% to 80% confluent cultured TECs from dnTβRII Tg+ mice and their Tg− littermates were treated with or without TGF-β (2 ng/mL) and TECs from IFN-γ−/− NOD.H-2h4 mice were treated with IFN-γ (20 ng/mL) for 3 days. Apoptosis was detected by TUNEL staining. Few or no TUNEL-positive cells were detected in TECs cultured in the presence or absence of cytokines (data not shown), suggesting that apoptosis is not involved in the process of TGF-β-induced or IFN-γ-inhibited proliferation of TECs.
TGF-β-Induced Proliferation of TECs Is Associated with Increased p-AKT
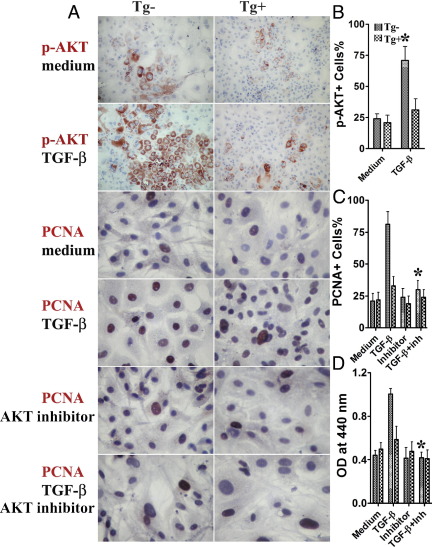
TGF-β makes use of several intracellular signaling pathways, in addition to the Smad pathway, to regulate cellular functions.1,4 The AKT pathway has been shown to be important for cell proliferation and other responses to growth factors,9 so it was of interest to determine whether the AKT pathway is involved in TGF-β-induced proliferation of TECs. To address this question, primary cultures of TECs from dnTβRII Tg+ IFN-γ−/− mice and their Tg− littermates were established, and TGF-β was added for 3 days. TGF-β induced p-AKT expression in TECs of Tg− mice, but not in TECs of dnTβRII Tg+ mice (Figure 5, A and B). Western blot analysis further confirmed that p-AKT was increased in TECs from Tg− mice in the presence of TGF-β (Figure 6A). These results suggest that TGF-β-induced proliferation of TECs is associated with increased p-AKT.
Figure 5.
TGF-β-induced proliferation of TECs is associated with increased p-AKT, and AKT inhibitor inhibits TGF-β-induced proliferation of TECs. Primary cultures of TECs from dnTβRII Tg+ IFN-γ−/− NOD.H-2h4 mice and their Tg− littermates were established, and TGF-β (2 ng/mL) was added for 3 days. A: IHC results for p-AKT and PCNA. Primary cultures of TECs from dnTβRII Tg+ mice and their Tg− littermates were established, and TGF-β (2 ng/mL) or medium with or without AKT inhibitor (30 μmol/L) was added for 3 days. Original magnification: ×100 (p-AKT); ×400 (PCNA). B and C: p-AKT+ or PCNA+ cells (red) in five to six randomly selected high power fields of three wells/group were counted using MetaMorph software. Summarized results are shown. *P < 0.05 versus medium alone (B). *P < 0.05 versus TGF-β alone (C). D: A cell proliferation kit was used to evaluate the effect of AKT inhibitor on TGF-β-induced proliferation of TECs. Results are expressed as the mean OD ± SEM of TECs from four to five wells/group. *P < 0.05 versus TGF-β alone. Results are representative of two or three independent experiments.
Figure 6.
TGF-β-induced proliferation of TECs is associated with increased p-AKT and AKT inhibitor reverses the ability of TGF-β to down-regulate antiproliferative molecules A: Western blot analysis. Results are expressed as the mean ratio of p-AKT densitometric units/β-actin ± SEM (×100). *P < 0.05 versus medium alone. B–D: Primary cultures of TECs from dnTβRII Tg+ mice and their Tg− littermates were established. TGF-β (2 ng/mL) with or without AKT inhibitor (30 μmol/L) was added for 3 days. mRNA expression of p21, p27, and PCNA in cultured TECs from dnTβRII Tg+ mice and their Tg− littermates was determined by RT-PCR. Results are expressed as the mean ratio of PCNA, pro- and antiproliferative molecule densitometric units/β-actin ± SEM (×100), and are representative of three independent experiments. Results are representative of two independent experiments. *P < 0.05 versus TGF-β alone.
AKT Inhibitor Inhibits TGF-β-Induced Proliferation of TECs
To further confirm the involvement of the AKT pathway in TGF-β-induced proliferation of TECs, an AKT inhibitor was used to attempt to block TGF-β-induced proliferation of TECs. Primary cultures of TECs from dnTβRII Tg+ mice and their Tg− littermates were established, and TGF-β or medium with or without AKT inhibitor was added for 3 days. AKT inhibitor significantly inhibited TGF-β-induced proliferation of TECs from Tg− mice, but had little effect on proliferation of TECs from dnTβRII Tg+ mice (Figure 5, A and C). Similar results were also obtained with a cell proliferation assay (Figure 5D) and by mRNA analysis for PCNA (Figure 6B). These results strongly indicate that TGF-β-induced proliferation of TECs is through the AKT pathway.
AKT Inhibitor Reverses the Effects of TGF-β on Antiproliferative Molecules
Because AKT inhibitor inhibits TGF-β-induced proliferation of TECs (Figure 5) and TGF-β-induced proliferation of TECs is associated with down-regulation of the antiproliferative molecules p21 and p27 (Figure 3), it is important to determine whether down-regulation of the antiproliferative molecules p21 and p27 is abrogated by the AKT inhibitor. To address this question, TGF-β with or without AKT inhibitor was added to primary cultures of TECs for 3 days, and mRNA expression of p21, p27 and PCNA was determined by RT-PCR. Consistent with the results described above (Figure 5), PCNA mRNA in TECs was significantly lower when both TGF-β and AKT inhibitor were added to the culture than when TGF-β alone was added (Figure 6B). Of particular interest, p21 and p27 mRNA was significantly higher in TECs cultured with TGF-β and AKT inhibitor, compared with TECs cultured with TGF-β alone (Figure 6, C and D). These results indicate that AKT inhibition reverses the ability of TGF-β to down-regulate p21 and p27. Taken together, the results suggest that TGF-β promotes proliferation of TECs by down-regulation of p21 and p27 via the AKT pathway.
Increased Proliferation of TECs Correlates with Increased Expression of TGF-β and p-AKT and Decreased Expression of p21 and p27 in TECs in Vivo
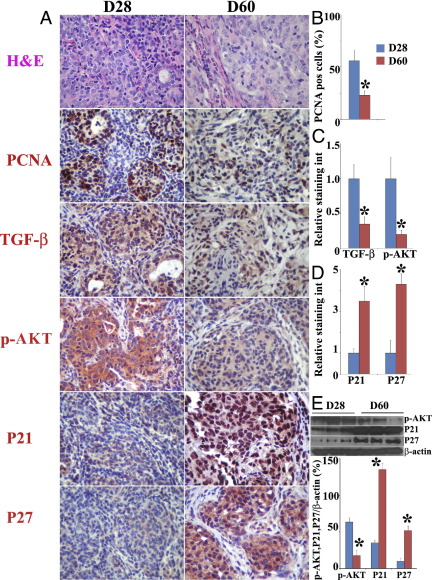
To determine whether our in vitro findings suggesting that TGF-β promotes proliferation of TECs by down-regulation of p21 and p27 via the AKT pathway correlate with expression of these molecules in vivo, we used a well-established murine model of TEC hyperplasia. IFN-γ−/− NOD.H-2h4 mice develop severe TEC H/P and fibrosis, whereas IFN-γ−/− SCID mice do not develop TEC H/P.31,32 Splenocytes from IFN-γ−/− mice with severe TEC H/P transfer severe TEC H/P to SCID recipients.31,32 At 28 days after cell transfer (Figure 7A), most recipients had severe TEC H/P (severity score, 4+ to 5+) with infiltration of thyroids by T cells, macrophages, and eosinophils, extensive proliferation of TECs, and some fibrosis. By day 60 (Figure 7A), thyroids were larger and there was more fibrosis and fewer infiltrating T cells, macrophages, and eosinophils. There were also fewer proliferating PCNA+ TECs, and proliferating TECs were surrounded by collagen, resulting in severe nodular hyperplasia (5+ TEC H/P).
Figure 7.
Increased proliferation of TECs correlates with increased expression of TGF-β and p-AKT and decreased expression of P21 and P27 by TECs in vivo. Splenocytes from IFN-γ−/− donors with 5+ TEC H/P severity scores were cultured with mouse thyroglobulin and were transferred to SCID recipient mice. A: Results of H&E staining and IHC staining for PCNA, TGF-β, p-AKT, p21, and p27 in thyroids at 28 and 60 days after cell transfer. Original magnification, ×400. B–D: PCNA+ cells (red) or relative staining intensity in five to six randomly selected high-power fields of three slides/group were counted or analyzed using MetaMorph software. Summarized results are shown. *P < 0.05 versus day 28. E: Protein expression levels of p-AKT, p21, and p27 evaluated by Western blot. *P < 0.05 versus day 28. Results shown are representative of two or three independent experiments.
The similarity in TEC H/P severity scores but differences in proliferating status of TECs at day 28 versus day 60 provided a good opportunity to determine whether the proliferation status of TECs correlates with expression of TGF-β, p-AKT, p21, and p27 in TECs in vivo. At day 28, there were many PCNA+ TECs (Figure 7, A and B), and they had strong staining for TGF-β and p-AKT (Figure 7, A and C), whereas the staining intensity for the antiproliferative molecules p21 and p27 was weaker (Figure 7, A and B). In contrast, at day 60, although the TEC H/P severity scores were similar to those at day 28, there were fewer PCNA+ TECs, the staining intensity for TGF-β and p-AKT in TECs was weaker, and the staining intensity for p21 and p27 was very strong (Figure 7, A–D). p21 and p27 were located both in the nucleus and the cytoplasm in TECs. The higher expression level of p-AKT and the lower expression levels of p21 and p27 at day 28, compared with those at day 60, were also confirmed by Western blot analysis (Figure 7E). Thus, increased proliferation of TECs correlates with increased expression of TGF-β and p-AKT and decreased expression of p21 and p27 in TECs in vivo.
Discussion
Regulation of thyroid growth and function is achieved by the balance between pro- and antiproliferative molecules.11,33,34 The present findings demonstrate that TGF-β promotes and IFN-γ inhibits TEC proliferation in a dose-dependent manner in vitro. The findings suggest that TGF-β may promote TEC proliferation by down-regulating antiproliferative molecules p21 and p27, whereas IFN-γ may inhibit proliferation by up-regulating antiproliferative molecules p18 and p21 and down-regulating the pro-proliferative molecule cyclin D. AKT inhibition abolished the effect of TGF-β on p21 and p27, resulting in similar proliferation between TECs treated with or without TGF-β. Furthermore, increased expression of PCNA, TGF-β, and p-AKT and decreased expression of p21 and p27 by proliferating TECs correlated with the proliferative state of TECs in vivo. The results suggest that TGF-β promotes TEC proliferation in IFN-γ−/− NOD.H-2h4 mice by down-regulation of p21 and p27 via the AKT pathway.
The present study is unique in that, to our knowledge, it is the first to demonstrate the pro-proliferative role of TGF-β on IFN-γ−/− murine TECs. These results are consistent with studies showing that TGF-β can promote proliferation of mesenchymal cells and fibroblasts35,36 and with studies showing that TGF-β can promote proliferation of goiter or thyroid tumor cells in vitro.37–39 TGF-β can also inhibit the growth of both rat and human TECs11,40,41 through the Smad2/3 pathway. These apparently contradictory findings may be explained, at least in part, by differences in species and/or the concentration of TGF-β. In recent years, several studies have demonstrated that there are multiple TGF-β signaling pathways, including both Smad and non-Smad pathways. Which pathway is predominant after the binding of TGF-β to its receptors is determined by many factors, including the cellular localization, phosphorylation state, and expression levels of the postreceptor signaling elements.1,42–46
The pro-proliferative role of TGF-β was directly demonstrated by using transgenic mice expressing the dnTβRII on their TECs. TECs from mice unable to respond to TGF-β did not proliferate in the presence of TGF-β, whereas TGF-β consistently promoted proliferation of cultured TECs from Tg− mice. On the other hand, proliferation of TECs was significantly inhibited after addition of IFN-γ (Figures 2 and 3), whereas IFN-γ had no effect on the proliferation of TECs from IFN-γR−/− mice (Figures 2 and 3). Thus, TGF-β and IFN-γ have contrasting roles in TEC proliferation. This is consistent with studies in vivo showing that TGF-β and IFN-γ reciprocally regulate each other.15,16,21 Our previous studies have shown that NOD.H-2h4 mice develop spontaneous autoimmune thyroiditis characterized by lymphocyte infiltration of the thyroid. IFN-γ−/−NOD.H-2h4 mice do not develop spontaneous autoimmune thyroiditis, but develop severe TEC H/P with production of TGF-β by proliferating TECs. This suggests that the pro-proliferative effect of TGF-β is enhanced when IFN-γ is absent. The contrasting roles of TGF-β and IFN-γ in TEC proliferation in vitro demonstrated in the present study thus provide direct support for our hypothesis.
TGF-β makes use of many intracellular signaling pathways in addition to Smads to regulate cellular functions, including proliferation.1–4 The AKT pathway is one of the most important non-Smad pathways considered to promote cell proliferation.47,48 Mechanistically, this has been linked to the ability of AKT to inhibit expression of the cyclin-dependent kinase inhibitor p27, resulting in cell cycle progression.49,50 In the present study, TGF-β-induced proliferation of TECs was associated with increased p-AKT and decreased p21 and p27 in cultured TECs. AKT inhibitor reverses the down-regulation effect of TGF-β on p21 and p27, abolishing TGF-β-induced proliferation of TECs. Thus, TGF-β may promote TEC proliferation by down-regulation of p21 and p27 via the AKT pathway. These findings are consistent with studies using other tissues.49,50 This hypothesis is also supported by in vivo results (Figure 7). In our TEC H/P transfer model, increased proliferation of TECs 28 days after cell transfer correlated with increased expression of TGF-β and p-AKT and decreased expression of p21 and p27 by TECs in vivo. (We are currently designing further in vivo studies to investigate whether AKT inhibitor can prevent or treat TEC H/P in this cell transfer model.) Notably, p21 and p27 were detected both in the nucleus and the cytoplasm in TECs (Figure 7). Further studies are needed to address the significance of cytoplasmic p21 and p27 in our vivo TEC H/P transfer model. Although the present study demonstrated the involvement of the AKT pathway in TEC proliferation induced by TGF-β, other pathways or mechanisms might also contribute to the pro-proliferative effect of TGF-β on TECs. Given that thyroid proliferation and hyperplasia constitute one of the pathological features in human thyroid diseases such as Graves' disease and thyroid adenoma and carcinoma,20 and given that TGF-β has been detected in these lesions,38,39,51 the present findings might, at least in part, provide an explanation for the mechanisms by which hyperplasia develops in these diseases.
In our animal model, anti-TGF-β decreased TEC H/P severity in SCID recipients of IFN-γ−/− splenocytes,31 and transgenic overexpression of TGF-β on TECs promoted development of TEC H/P,52 suggesting a critical role for TGF-β in TEC H/P. TNF-α also promotes TEC proliferation in vitro and anti-TNF-α also decreases TEC H/P severity in SCID recipients of IFN-γ−/− splenocytes.31 The balance between pro- and antiproliferative cytokines that use different signaling pathways and/or crosstalk with each other can influence the balance between pro- and antiproliferative molecules to direct the development of TEC H/P.
In summary, TGF-β promotes proliferation of thyroid epithelial cells in IFN-γ−/− mice, at least in part, by down-regulation of p21 and p27 via the AKT pathway. Our results highlight a critical role of the AKT pathway in TEC proliferation and hyperplasia. These findings could contribute to development of effective therapeutic strategies targeting both the TGF-β and AKT pathways for treatment of hyperplasia and abnormal cell proliferation.
Acknowledgments
We thank the staff of the Transgenic Core Facility at the University of Missouri for generating the transgenic founders and Edward Downey for excellent technical assistance. We also thank Dr. Harold Moses (Vanderbilt University) for providing the transgene construct and Dr. Jianming Qiu (University of Kansas Medical Center) for assistance in combining the transgene construct with the rat thyroglobulin promoter.
Footnotes
Supported by NIH grant R01-AI074857 (H.B.-M.) and by the Arthritis Foundation Eastern Missouri chapter.
Supplemental material for this article can be found at http://ajp.amjpathol.org or at doi: 10.1016/j.ajpath.2011.10.009.
Current address of S.Y.: Department of Biological Sciences, Arkansas Biosciences Institute, Arkansas State University, Jonesboro, AR 72467.
Contributor Information
Yujiang Fang, Email: fangy@health.missouri.edu.
Helen Braley-Mullen, Email: mullenh@health.missouri.edu.
Supplementary data
Generation of dnTβRII transgenic mice and expression of TβRII and FLAG on TECs. A: Map of transgene construct. B: Protein expression analysis by Western blot analysis in naïve wild-type and Tg+ thyroids. C: RNA expression by real-time PCR in naïve wild-type and Tg+ thyroids. Results are expressed as the mean relative ratio to actin ± SEM and are representative of two independent experiments. D: Representative IHC staining for TβRII and transgenic-specific FLAG.
References
- 1.Derynck R., Zhang Y.E. Smad-dependent and Smad-independent pathways in TGF-β family signaling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 2.Massagué J. TGFbeta in cancer. Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roberts A.B., Wakefield L.M. The two faces of transforming growth factor beta in carcinogenesis. Proc Natl Acad Sci USA. 2003;100:8621–8623. doi: 10.1073/pnas.1633291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y.E. Non-Smad pathways in TGF-beta signaling. Cell Res. 2009;19:128–139. doi: 10.1038/cr.2008.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang B., Vu M., Booker T., Santner S.J., Miller F.R., Anver M.R., Wakefield L.M. TGF-beta switches from tumor suppressor to prometastatic factor in a model of breast cancer progression. J Clin Invest. 2003;112:1116–1124. doi: 10.1172/JCI18899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu S.L., Reh D., Li A.G., Woods J., Corless C.L., Kulesz-Martin M., Wang X.J. Overexpression of transforming growth factor beta1 in head and neck epithelia results in inflammation, angiogenesis, and epithelial hyperproliferation. Cancer Res. 2004;64:4405–4410. doi: 10.1158/0008-5472.CAN-04-1032. [DOI] [PubMed] [Google Scholar]
- 7.Coppa A., Mincione G., Lazzereschi D., Ranieri A., Turco A., Lucignano B., Scarpa S., Ragano-Caracciolo M., Colletta G. Restored expression of TGF-beta type II receptor in K-ras-transformed thyroid cells: TGF-beta resistant, reverses their malignant phenotype. J Cell Physiol. 1997;172:200–208. doi: 10.1002/(SICI)1097-4652(199708)172:2<200::AID-JCP7>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 8.Bravo S.B., Pampin S., Cameselle-Teijeiro J., Carneiro C., Dominguez F., Barreiro F., Alvarez C.V. TGF-beta induced apoptosis in human thyrocytes is mediated by p27kip1 reduction and is overridden in neoplastic thyrocytes by NF-kappaB activation. Oncogene. 2003;22:7819–7830. doi: 10.1038/sj.onc.1207029. [DOI] [PubMed] [Google Scholar]
- 9.Remy I., Montmarquette A., Michnick S.W. PKB/Akt modulates TGF-beta signalling through a direct interaction with Smad3. Nat Cell Biol. 2004;6:358–365. doi: 10.1038/ncb1113. [DOI] [PubMed] [Google Scholar]
- 10.Massagué J., Wotton D. Transcriptional control by the TGF-beta/Smad signaling system. EMBO J. 2000;19:1745–1754. doi: 10.1093/emboj/19.8.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nicolussi A., D'Inzeo S., Santulli M., Colletta G., Coppa A. TGF-beta control of rat thyroid follicular cells differentiation. Mol Cell Endocrinol. 2003;207:1–11. doi: 10.1016/s0303-7207(03)00238-7. [DOI] [PubMed] [Google Scholar]
- 12.Boehm U., Klamp T., Groot M., Howard J. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 13.Fang Y., Yu S., Braley-Mullen H. Contrasting roles of IFN-gamma in murine models of autoimmune thyroid disease. Thyroid. 2007;17:989–994. doi: 10.1089/thy.2007.0261. [DOI] [PubMed] [Google Scholar]
- 14.Ishida Y., Kondo T., Takayasu T., Iwakura Y., Mukaida N. The essential involvement of crosstalk between IFN-gamma and TGF-beta in the skin wound-healing process. J Immunol. 2004;172:1848–1855. doi: 10.4049/jimmunol.172.3.1848. [DOI] [PubMed] [Google Scholar]
- 15.Ulloa L., Doody J., Massagué J. Inhibition of transforming growth factor-beta/SMAD signaling by interferon-gamma/STAT pathway. Nature. 1999;387:710–713. doi: 10.1038/17826. [DOI] [PubMed] [Google Scholar]
- 16.Johnson D.G., Walker C.L. Cyclins and cell cycle checkpoints. Annu Rev Pharmacol Toxicol. 1999;39:295–312. doi: 10.1146/annurev.pharmtox.39.1.295. [DOI] [PubMed] [Google Scholar]
- 17.Sherr C.J. G1 phase progression: cycling on cue. Cell. 1994;79:551–555. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- 18.Ohtsubo M., Theodoras A.M., Schumacher J., Roberts J.M., Pagano M. Human cyclin E, a nuclear protein essential for the G1-to-S phase transition. Mol Cell Biol. 1995;15:2612–2624. doi: 10.1128/mcb.15.5.2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sherr C.J. D-type cyclins. Trends Biochem Sci. 1995;20:187–190. doi: 10.1016/s0968-0004(00)89005-2. [DOI] [PubMed] [Google Scholar]
- 20.Kimura T., Van Keymeulen A., Golstein J., Fusco A., Dumont J.E., Roger P.P. Regulation of thyroid cell proliferation by TSH and other factors: a critical evaluation of in vitro models. Endocr Rev. 2001;22:631–656. doi: 10.1210/edrv.22.5.0444. [DOI] [PubMed] [Google Scholar]
- 21.Yu S., Sharp G.C., Braley-Mullen H. TGF-beta promotes thyroid epithelial cell hyperplasia and fibrosis in IFN-gamma-deficient NOD.H-2h4 mice. J Immunol. 2008;181:2238–2245. doi: 10.4049/jimmunol.181.3.2238. [DOI] [PubMed] [Google Scholar]
- 22.Yu S., Sharp G.C., Braley-Mullen H. Thyrocytes responding to IFN-gamma are essential for development of lymphocytic spontaneous autoimmune thyroiditis and for inhibition of thyrocyte hyperplasia. J Immunol. 2006;176:1259–1265. doi: 10.4049/jimmunol.176.2.1259. [DOI] [PubMed] [Google Scholar]
- 23.Podolin P.L., Pressey A., DeLarato N.H., Fischer P.A., Peterson L.B., Wicker L.S. I-E+ nonobese diabetic mice develop insulitis and diabetes. J Exp Med. 1993;178:793–803. doi: 10.1084/jem.178.3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fang Y., Wei Y., DeMarco V., Chen K., Sharp G.C., Braley-Mullen H. Murine FLIP transgene expressed on thyroid epithelial cells promotes resolution of granulomatous experimental autoimmune thyroiditis. Am J Pathol. 2007;170:875–887. doi: 10.2353/ajpath.2007.060816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fang Y., DeMarco V.G., Sharp G.C., Braley-Mullen H. Expression of transgenic FLIP on thyroid epithelial cells inhibits induction and promotes resolution of granulomatous experimental autoimmune thyroiditis in CBA/J mice. Endocrinology. 2007;148:5734–5745. doi: 10.1210/en.2007-0939. [DOI] [PubMed] [Google Scholar]
- 26.Fang Y., Sharp G.C., Braley-Mullen H. Interleukin-10 promotes resolution of granulomatous experimental autoimmune thyroiditis. Am J Pathol. 2008;172:1591–1602. doi: 10.2353/ajpath.2008.071067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fang Y., Sharp G.C., Yagita H., Braley-Mullen H. A critical role for TRAIL in resolution of granulomatous experimental autoimmune thyroiditis. J Pathol. 2008;216:505–513. doi: 10.1002/path.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fang Y., Braley-Mullen H. Cultured murine thyroid epithelial cells expressing transgenic Fas-associated death domain-like interleukin-1beta converting enzyme inhibitory protein are protected from Fas-mediated apoptosis. Endocrinology. 2008;149:3321–3329. doi: 10.1210/en.2008-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fang Y., Yu S., Ellis J.S., Sharav T., Braley-Mullen H. Comparison of sensitivity of Th1, Th2 and Th17 cells to Fas-mediated apoptosis. J Leuk Biol. 2010;87:1019–1028. doi: 10.1189/jlb.0509352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berridge M.V., Tan A.S., McCoy K.D., Wang R. The biochemical and cellular basis of cell proliferation assays that use tetrazolium salts. Boehringer Mannheim Biochemica. 1996;1996(4):12–20. [Google Scholar]
- 31.Yu S., Fang Y., Sharav T., Sharp G.C., Braley-Mullen H. CD8+ T cells induce thyroid epithelial cell hyperplasia and fibrosis. J Immunol. 2011;186:2655–2662. doi: 10.4049/jimmunol.1002884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu S., Sharp G.C., Braley-Mullen H. Thyroid epithelial cell hyperplasia in IFN-gamma-deficient NOD.H-2h4 mice. Clin Immunol. 2006;118:92–100. doi: 10.1016/j.clim.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 33.Franzén A., Piek E., Westermark B., ten Dijke P., Heldin N.E. Expression of transforming growth factor-beta1, activin A, and their receptors in thyroid follicle cells: negative regulation of thyrocyte growth and function. Endocrinology. 1999;140:4300–4310. doi: 10.1210/endo.140.9.6961. [DOI] [PubMed] [Google Scholar]
- 34.Gärtner R. Growth factors in thyroid cells. Curr Top Pathol. 1997;91:65–81. [PubMed] [Google Scholar]
- 35.Alexandrow M.G., Kawabata M., Aakre M., Moses H.L. Overexpression of the c-Myc oncoprotein blocks the growth-inhibitory response but is required for the mitogenic effects of transforming growth factor beta 1. Proc Natl Acad Sci USA. 1995;92:3239–3243. doi: 10.1073/pnas.92.8.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Machwate M., Jullienne A., Moukhtar M., Lomri A., Marie P.J. c-fos protooncogene is involved in the mitogenic effect of transforming growth factor-beta in osteoblastic cells. Mol Endocrinol. 1995;9:187–198. doi: 10.1210/mend.9.2.7776969. [DOI] [PubMed] [Google Scholar]
- 37.Logan A., Smith C., Becks G.P., Gonzalez A.M., Phillips I.D., Hill D.J. Enhanced expression of transforming growth factor-beta 1 during thyroid hyperplasia in rats. J Endocrinol. 1994;141:45–47. doi: 10.1677/joe.0.1410045. [DOI] [PubMed] [Google Scholar]
- 38.Morosini P., Taccaliti A., Di Loreto C., Arnaldi G., Faloia E., Giacchetti G., Mantero F. Transforming growth factor-beta1 is more expressed in thyroid follicular adenoma than in normal tissue. J Endocrinol Invest. 1994;17:335–340. doi: 10.1007/BF03348995. [DOI] [PubMed] [Google Scholar]
- 39.Cerutti J.M., Ebina K.N., Matsuo S.E., Martins L., Maciel R.M., Kimura E.T. Expression of Smad4 and Smad7 in human thyroid follicular carcinoma cell lines. J Endocrinol Invest. 2003;26:516–521. doi: 10.1007/BF03345213. [DOI] [PubMed] [Google Scholar]
- 40.Cirafici A.M., Pepe S., Mincione G., Esposito D., Colletta G. TGF beta inhibits rat thyroid cell proliferation without alterations in the expression of TSH-induced cell cycle-related genes. Biophys Res Commun. 1992;187:225–233. doi: 10.1016/s0006-291x(05)81482-2. [DOI] [PubMed] [Google Scholar]
- 41.Taton M., Lamy F., Roger P.P., Dumont J.E. General inhibition by transforming factor-beta1 of thyrotropin and cAMP responses in human thyroid cells in primary culture. Mol Cell Endocrinol. 1993;95:13–21. doi: 10.1016/0303-7207(93)90024-e. [DOI] [PubMed] [Google Scholar]
- 42.Itoh S., ten Dijke P. Negative regulation of TGF-beta receptor/Smad signal transduction. Curr Opin Cell Biol. 2007;19:176–184. doi: 10.1016/j.ceb.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 43.Pardali K., Kowanetz M., Heldin C.H., Moustakas A. Smad pathway-specific transcriptional regulation of the cell cycle inhibitor p21(WAF1/Cip1) J Cell Physiol. 2005;204:260–272. doi: 10.1002/jcp.20304. [DOI] [PubMed] [Google Scholar]
- 44.Bhowmick N.A., Ghiassi M., Bakin A., Aakre M., Lundquist C.A., Engel M.E., Arteaga C.L., Moses H.L. Transforming growth factor-beta1 mediates epithelial to mesenchymal transdifferentiation through a RhoA-dependent mechanism. Mol Biol Cell. 2001;12:27–36. doi: 10.1091/mbc.12.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Siegel P., Massagué J. Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nat Rev Cancer. 2003;3:807–821. doi: 10.1038/nrc1208. [DOI] [PubMed] [Google Scholar]
- 46.Rojas A., Padidam M., Cress D., Grady W.M. TGF-beta receptor levels regulate the specificity of signaling pathways activation and biological effect of TGF-beta. Biochim Biophys Acta. 2009;1793:1165–1173. doi: 10.1016/j.bbamcr.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liang J., Slingerland J.M. Multiple roles of the PI3K/PKB (Akt) pathway in cell cycle progression. Cell Cycle. 2003;2:339–345. [PubMed] [Google Scholar]
- 48.Testa J.R., Bellacosa A. AKT plays a central role in tumorigenesis. Proc Natl Acad Sci USA. 2001;98:10983–10985. doi: 10.1073/pnas.211430998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liang J., Zubovitz J., Petrocelli T., Kotchetkov R., Connor M.K., Han K., Lee J.H., Ciarallo S., Catzavelos C., Beniston R., Franssen E., Slingerland J.M. PKB/Akt phosphorylates p27, impairs nuclear import of p27 and opposes p27-mediated G1 arrest. Nat Med. 2002;8:1153–1160. doi: 10.1038/nm761. [DOI] [PubMed] [Google Scholar]
- 50.Narita Y., Nagane M., Mishima K., Huang H.J., Furnari F.B., Cavenee W.K. Mutant epidermal growth factor receptor signaling down-regulates p27 through activation of the phosphatidylinositol 3-kinase/Akt pathway in glioblastomas. Cancer Res. 2002;62:6764–6769. [PubMed] [Google Scholar]
- 51.Grubeck-Loebenstein B., Buchan G., Sadeghi R., Kissonerghis M., Londel M., Turner M., Pirich K., Roka R., Niederle B., Kassal H. Transforming growth factor beta regulates thyroid growth: Role in the pathogenesis of nontoxic goiter. J Clin Invest. 1989;83:764–769. doi: 10.1172/JCI113955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu S., Fang Y., Sharp G.C., Braley-Mullen H. Transgenic expression of TGF-beta on TECs inhibits development of spontaneous autoimmune thyroiditis and increases regulatory T cells in thyroids of NOD.H-2h4 mice. J Immunol. 2010;184:5352–5359. doi: 10.4049/jimmunol.0903620. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Generation of dnTβRII transgenic mice and expression of TβRII and FLAG on TECs. A: Map of transgene construct. B: Protein expression analysis by Western blot analysis in naïve wild-type and Tg+ thyroids. C: RNA expression by real-time PCR in naïve wild-type and Tg+ thyroids. Results are expressed as the mean relative ratio to actin ± SEM and are representative of two independent experiments. D: Representative IHC staining for TβRII and transgenic-specific FLAG.