Abstract
Adenosine is generated in increased concentrations at sites of injury/hypoxia and mediates a variety of physiological and pharmacological effects via G protein–coupled receptors (A1, A2A, A2B, and A3). Because all adenosine receptors are expressed on osteoclasts, we determined the role of A2A receptor in the regulation of osteoclast differentiation. Differentiation and bone resorption were studied as the macrophage colony-stimulating factor-1–receptor activator of NF-κB ligand formation of multinucleated tartrate-resistant acid phosphatase (TRAP)–positive cells from primary murine bone marrow–derived precursors. A2A receptor and osteoclast marker expression levels were studied by RT-PCR. Cytokine secretion was assayed by enzyme-linked immunosorbent assay. In vivo examination of A2A knockout (KO)/control bones was determined by TRAP staining, micro–computed tomography, and electron microscopy. The A2A receptor agonist, CGS21680, inhibited osteoclast differentiation and function (half maximal inhibitory concentration, 50 nmol/L), increased the percentage of immature osteoclast precursors, and decreased IL-1β and tumor necrosis factor-α secretion, an effect that was reversed by the A2A antagonist, ZM241385. Cathepsin K and osteopontin mRNA expression increased in control and ZM241385-pretreated osteoclasts, and this was blocked by CGS21680. Micro–computed tomography of A2AKO mouse femurs showed a significantly decreased bone volume/trabecular bone volume ratio, decreased trabecular number, and increased trabecular space. A2AKO femurs showed an increased TRAP-positive osteoclast. Electron microscopy in A2AKO femurs showed marked osteoclast membrane folding and increased bone resorption. Thus, adenosine, acting via the A2A receptor, inhibits macrophage colony-stimulating factor-1–receptor activator of NF-κB ligand–stimulated osteoclast differentiation and may regulate bone turnover under conditions in which adenosine levels are elevated.
Communication between osteoclasts and osteoblasts is essential for bone modeling and remodeling. In bone remodeling, bone formation and resorption are at equilibrium. The principal mechanism for bone remodeling, between bone resorption and formation, lies in the sequential nature of the process by which osteoblasts refill resorption lacunae with an equivalent amount of osteoid.1,2 When this balance is disturbed in favor of bone resorption, the result is pathological bone destruction, as observed in osteoporosis3 or inflammatory diseases, such as rheumatoid arthritis.4
Osteoclasts degrade bone to initiate normal bone remodeling and mediate bone loss in pathological conditions by increasing their resorptive activity. Osteoclasts are multinucleated giant cells, derived from myeloid precursors belonging to the monocyte/macrophage family,5,6 that secret hydrochloric acid and proteases, such as cathepsin K and tartrate-resistant acid phosphatase (TRAP), into an extracellular lysosomal compartment, destroying and resorbing both the mineral and matrix components of bone simultaneously.7 The initial event associated with osteoclast commitment requires interaction with the hematopoietic growth factor, macrophage colony-stimulating factor (M-CSF)-1, which acts via its receptor. Further commitment, differentiation, and activation of osteoclasts are mediated by a complex network of regulatory factors, including systemic hormones, locally produced cytokines, and cell-cell and cell-matrix interactions that are required for transition of the osteoclast precursor (OCP) into a multinucleated and fully activated osteoclast.8,9 Among these factors, receptor activator of NF-κB ligand (RANKL) is critical for the stimulation of osteoclast differentiation and activation.10–14 RANKL binds to its receptor, RANK, on the surface of OCPs, activating signaling through NF-κB, c-Fos, phospholipase Cγ, and nuclear factor of activated T cells c1 (NFATc1), to induce differentiation of OCPs into osteoclasts.9
Adenosine, the metabolic product of adenine nucleotide dephosphorylation, is generated intracellularly and extracellularly from the catabolism of adenine nucleotides in response to stress, such as hypoxia and inflammatory injury. Ectonucleotidases, of which the apyrase CD39 and the 5′-nucleotidase CD73 are prominent examples, are present on the extracellular surface of many tissues and are crucially involved in numerous important functions, primarily via generation of adenosine.15 These enzymes rapidly and effectively shift signaling by releasing adenine nucleotides and their products to signaling through adenosine receptors. Extracellular adenosine regulates a variety of physiological processes via interaction with specific cell surface receptors. Adenosine receptors are members of the large superfamily of G protein–coupled receptors. Four subtypes are recognized: A1, A2A, A2B, and A3 receptors, each of which has a unique pharmacological profile and is present in virtually every tissue and cell type.16 The adenosine A1 and A2 receptors were initially subdivided on the basis of their effects on adenylyl cyclase activity, inhibition and stimulation, respectively,17,18 via coupling to Gi and Gs proteins, respectively; the A2B receptor is coupled to Gq, whereas the A3 receptor is Gi coupled. Recently, evidence was presented that the A2A receptor may be coupled to different G proteins in different areas and a variety of downstream signaling pathways, including cAMP-dependent, phospholipase C–dependent, and stimulation or inhibition of extracellular signal regulated kinase 1/2 via the cAMP-ras-MEK1 pathway have been implicated in signaling at this receptor.19–23
The study of the role of adenosine and adenosine receptors in the regulation of cells involved in bone metabolism and turnover has only recently begun. We have previously reported that both adenosine A1 and A2A receptors play an important role in promoting human monocyte fusion into giant cells in vitro.24 Moreover, we have found that deletion or blockade of adenosine A1Rs leads to increased bone density and prevents ovariectomy-induced bone loss without affecting bone formation,25 and we demonstrated that adenosine A1 receptor activation is required for appropriate formation and function of osteoclasts in vitro.26
Because we have previously reported that A2A receptor occupancy inhibits fusion of stimulated human monocytes to form giant cells in vitro, in this study, we determined whether there was a similar effect of A2A receptor occupancy on osteoclast formation and function both in vitro and in vivo and potentially identified novel approaches for the prevention of bone loss.
Materials and Methods
Reagents
Recombinant mouse RANKL and M-CSF were obtained from R&D Systems (Minneapolis, MN). CGS21680 and ZM241385 were obtained from Tocris (Ellisville, MO). α minimal essential medium (α-MEM; Gibco, Invitrogen, Carlsbad, CA) was used for all incubations, supplemented with 10% fetal bovine serum (Invitrogen), 100 IU/mL penicillin, and 100 μg/mL streptomycin (Invitrogen). Paraformaldehyde (PFA), sodium acetate, glacial acetic acid, naphthol AS MX phosphate disodium salt, Fast Red Violet LB, and Fluoroshield with DAPI were from Sigma-Aldrich (St. Louis, MO), and sodium tartrate was from Fisher Scientific (Pittsburgh, PA). The BD BioCoat Osteologic Bone Cell Culture System was from BD Biosciences (San Jose, CA). Silver nitrate and sodium carbonate were from Sigma-Aldrich, and sodium carbonate was obtained from Fisher Scientific. Alexa Fluor 555–phalloidin was obtained from Invitrogen.
Animals
Adenosine A2A receptor knockout (A2AKO) mice were a gift from Dr. Jiang Fan Chen (Boston University School of Medicine, Boston, MA).27 Female A2AKO mice were bred onto a C57BL/6 background (≥10 backcrosses) in the New York University School of Medicine Animal Facility. Mice described as wild type (WT) were all maintained on the C57BL/6 background by the breeder (Taconic Laboratories, Albany, NY). Genotyping was performed by PCR, as previously reported.28–30 The WT C57BL/6 mice were used as a control. All protocols were approved by the New York University School of Medicine Institutional Animal Care and Use Committee.
Osteoclast Differentiation from Bone Marrow Cells
Bone marrow cells were isolated from 6- to 8-week-old female C57BL/6 and A2AKO mice (n = 6 each). Femurs and tibiae were aseptically removed and dissected free of adherent soft tissues. The bone ends were cut, and the marrow cavity was flushed out with α-MEM from one end of the bone using a sterile 21-gauge needle. The bone marrow was carefully dispersed by pipetting and incubated overnight in α-MEM containing 10% fetal bovine serum to obtain a single-cell suspension. Nonadherent cells were collected and seeded at an appropriate density (200,000 cells) for assays in both 48-well plates and the BD BioCoat Osteologic Bone Cell Culture System in α-MEM with 10% fetal bovine serum and 30 ng/mL M-CSF for 2 days. On day 3 (day 0 of differentiation), 30 ng/mL RANKL was added to the culture, together with CGS21680 (10 μmol/L to 1 nmol/L), alone or in the presence of ZM241385 (10 μmol/L to 1 nmol/L). Cultures were fed every third day by replacing half of the culture medium with an equal quantity of fresh medium and reagents. To determine whether the effect of CGS21680 on osteoclast formation varied with amount of exposure time, the A2A receptor agonist was added at 10 μmol/L on consecutive days, starting on day 3 and ending 7 days later. To correlate the effects of A2A agonist with cytokine expression in osteoclast differentiation, TRAP staining was performed in M-CSF–RANKL–derived osteoclasts plated and differentiated over 7 days in the presence of IL-1β, 10 ng/mL, or tumor necrosis factor (TNF)-α, 50 ng/mL, together with CGS21680 alone or with ZM241385.
Characterization of Osteoclasts in Culture
After incubation for 7 days at 37°C in a humidified atmosphere of 5% CO2, wells were prepared for TRAP staining, to counteract osteoclast differentiation, or for Von Kossa staining, to study osteoclast function (n = 6 for each assay). Briefly, for TRAP staining, cells were washed with prewarmed PBS, fixed in 4% PFA for 10 minutes, and stained for acid phosphatase in the presence of 0.3 mol/L sodium tartrate, using naphthol AS-BI phosphate as a substrate. To determine osteoclast function, we cultured osteoclasts, as previously described, on the BD BioCoat Osteologic Bone Cell Culture System, a submicron synthetic mineralized calcium phosphate thin film coated onto culture vessels, as an alternative method to dentin slices, for direct assessment of osteoclast activity in vitro,31,32 following the manufacturer's recommendations, and stained the plates with Von Kossa stain. The number of TRAP-positive multinucleated giant cells containing three or more nuclei per cell was scored.33 The Von Kossa–stained images were analyzed with Matlab compiler software (Natick, MA). The Matlab compiler software used to analyze Von Kossa staining to quantify in vitro bone resorption takes an approach that is identical to that used by such commercial software packages as SigmaPlot. A program was developed using basic image analysis methods, such as edge, erode, and other imaging functions, to help extract the regions of interest and quantify the osteoclastic resorption. The edge function permitted transformation of color images into binary images, making it easier to identify and analyze the resorbed areas. After selecting the image and the region of interest, a basic threshold was applied to the images to obtain the optimum binary quality for analysis. The total area, which was equivalent to the total number of pixels in the selected image, was calculated via a simple counter. Then, the number of white pixels, which represents the resorbed areas, was also counted via a counter, and a percentage of the resorbed area was calculated. To further validate the software measurement of the area of resorption, we quantitated areas of known size and calculated the variance. The software performed these measurements in a reproducible fashion, and the results obtained accurately reflected the relative sizes of the areas measured.
Quantitative Real-Time RT-PCR
To validate the effect of A2A receptors in osteoclast differentiation, we measured the activation of the two osteoclast differentiation markers, cathepsin K and NFATc1, together with osteopontin, an extracellular structural protein that initiates the development of osteoclast ruffled borders, and A2A receptor by quantitative RT-PCR. WT osteoclasts derived from bone marrow were collected during the 7 days of differentiation, and total RNA was extracted using the RNeasy Mini Kit (Qiagen, Invitrogen), following the manufacturer's protocol, including sample homogenization with QIAshredder columns (Qiagen). To avoid genomic DNA contamination, we performed the on-column DNA digestion step. For first-strand cDNA synthesis, 20 μL of total RNA was retrotranscribed using the MuLV Reverse Transcriptase PCR kit (Applied Biosystems, Foster City, CA) at 2.5 U/μL, including the following reagents in the same reaction: RNase inhibitor, 1 U/μL; random hexamers, 2.5 U/μL; MgCl2, 5 mmol/L; PCR buffer II, one times; and deoxyribonucleotide triphosphates, 1 mmol/L (all from Applied Biosystems). Relative quantification of gene expression was performed using real-time RT-PCR on a Stratagene Mx3005P (Agilent Technologies, La Jolla, CA) with Brilliant SYBR Green Kit QPCR Master Mix (Stratagene, Agilent Technologies), according to the manufacturer's protocol. The following primers were used in real-time PCR amplification: A2A receptor, 5′-AGCCAGGGGTTACATCTGTG-3′ (forward) and 5′-TACAGACAGCCTCGACATGTG-3′ (reverse); cathepsin K, 5′-GCTGAACTCAGGACCTCTGG-3′ (forward) and 5′-GAAAAGGGAGGCATGAATGA-3′ (reverse); NFATc1, 5′-TCATCCTGTCCAACACCAAA-3′ (forward) and 5′-TCACCCTGGTGTTCTTCCTC-3′ (reverse); osteopontin, 5′-TCTGATGAGACCGTCACTGC-3′ (forward) and 5′-TCTCCTGGCTCTCTTTGGAA-3′ (reverse); and glyceraldehyde-3-phosphate dehydrogenase, 5′-CTACACTGAGGACCAGGTTGTCT-3′ (forward) and 5′-GGTCTGGGATGGAAATTGTG-3′ (reverse). The Pfaffl method34 was used for relative quantification of A2A receptor, cathepsin K, and NFATc1.
Morphological Characterization of Cultured Osteoclasts
Osteoclasts were generated from bone marrow cells extracted from the femurs and tibiae, as previously described. To differentiate osteoclasts, bone marrow cells were replated at 7500 cells/mL in fibronectin-coated glass coverslips in α-MEM containing 30 ng/mL M-CSF and 30 ng/mL RANKL, in the presence or absence of the adenosine A2A agonist, CGS21680. After 7 days in culture, cells were fixed with 4% PFA in PBS, blocked with PBS containing 1% bovine serum albumin and 0.1% Triton X-100 for 30 minutes, stained fluorescently with Alexa Fluor 555–phalloidin for 30 minutes, and counterstained with DAPI (Fluoroshield with DAPI mounting media), as previously described.35 To evaluate osteoclast morphological characteristics, the averages of 400 osteoclasts were examined in each sample using confocal microscopy (Leica SP5 confocal system, Buffalo Grove, IL).
Cytokine ELISAs
M-CSF–RANKL–derived osteoclasts were plated and differentiated over 7 days, as previously described. Cell supernatant was collected every day, centrifuged to remove any debris, and analyzed by Quantikine ELISA kits (R&D Systems) to determine the secretion of IL-1β and TNF-α. The manufacturer's protocol was strictly followed, and samples were run in duplicate with media-only controls and a standard curve correlation coefficient ≥0.98.
Bone Histological Features
The femurs from seven C57BL/6 (WT) and seven A2AKO mice were excised, cleaned of soft tissue, placed into 10% formaldehyde for 24 to 48 hours, and decalcified in EDTA. Paraffin-embedded histological sections were stained, using H&E and immunohistologic techniques, for type I procollagen or for TRAP activity. We measured the bone volume (BV) in a standard zone, situated at least 0.5 mm from the growth plate, excluding the primary spongiosa and trabeculae connected to the cortical bone, and enumerated the osteoclasts and trabecular area in the same zone as that used for assessing BV (original magnification, ×10), using BioQuant software (Nashville, TN).36 For measuring the osteoid, we used either Von Kossa staining (using calcified sections in methacrylate) or Goldner trichrome staining (also on calcified sections).
Electron Microscopic Examination of Osteoclasts
The femurs from five mice were fixed in 2.5% PFA plus 0.5% glutaraldehyde in 0.05 mol/L sodium cacodylate buffer (pH 7.4) for 12 hours at room temperature. After rinsing three times for 20 minutes in the same buffer, the material was postfixed for 1 hour in 1% osmium tetroxide (in 0.1 mol/L sodium cacodylate buffer), dehydrated in a graded ethyl alcohol series, and embedded in Epon (EMbed 812; Electron Microscopy Sciences, Hatfield, PA). Thin sections (80- to 90-nm thick) of calcified bone were collected in distilled water containing one drop of bromothymol blue (pH ≥8.0), to prevent mineral dissolution from the thin sections. Sections were stained with lead citrate and alcoholic uranyl acetate and examined using a Philips CM-12 electron microscope (Phillips, Mahwah, NJ).
Micro–X-Ray CT Analysis of Bone Mass
For measurements of the bone volume/trabecular volume (BV/TV), the femurs of seven WT and seven A2AKO mice were measured by micro–X-ray computed tomography (CT), as previously described,25,37 using an MS-8 scanner (GE Healthcare, London, UK) at 18-μm isotropic resolution. The scans were calibrated by air, water, and a mineral standard material phantom and the Parker's algorithm for digital reconstruction.25,38 Parameters were calculated using software supplied with the instrument.
Measurement of BMD
We assessed the bone mineral density (BMD; g/cm2) of the whole skeletons of 4-month-old mice, using a PIXImus bone densitometer (Lunar, Madison, WI). The instrument was calibrated before each scanning session, using a phantom with known BMD, according to the manufacturer's guidelines. There were seven WT and seven A2AKO mice anesthetized by i.p. injection of ketamine (100 μg/g of body weight) and xylazine (10 μg/g of body weight) and then placed in the prone position on the specimen tray to allow scanning of the entire skeleton.
Statistical Analysis
Statistical significance for differences between groups was determined by using analysis of variance or the Student's t-test. All statistics were calculated using GraphPad software (GraphPad, San Diego, CA).
Results
Effect of A2A Receptor Activation in Osteoclast Differentiation and Function in Vitro
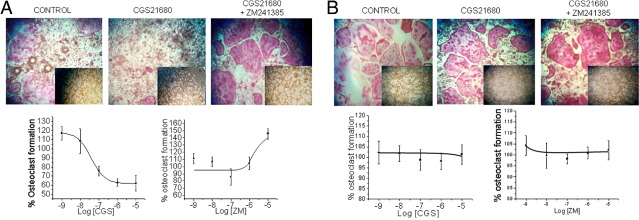
After 7 days of osteoclast differentiation, TRAP and Von Kossa staining were performed to counteract the role of A2A receptor activation in the differentiation process and in bone remodeling function in vitro. When we analyzed the osteoclast number in WT cell cultures, we observed a dose-dependent inhibition of osteoclastogenesis when cultures were treated with CGS21680 alone (Figure 1A; half maximal inhibitory concentration, 50 nmol/L; maximal decrease of 38% ± 8% inhibition; P < 0.05; n = 6). Interestingly, the A2A receptor agonist–mediated inhibition was more than completely reversed by pretreatment with the selective A2A antagonist ZM241385 in the presence of 10 μmol/L CGS21680 (Figure 1A). Moreover, there was dose-dependent activation of osteoclastogenesis by the A2A receptor antagonist (146% ± 8% of control in the presence of ZM241385, 10 μmol/L; P < 0.05; n = 6), suggesting that endogenous adenosine inhibits osteoclast formation in an autocrine fashion. When cells from A2AKO mice were studied, neither the agonist nor the antagonist, at any concentration, affected the number of osteoclasts that formed (Figure 1B).
Figure 1.
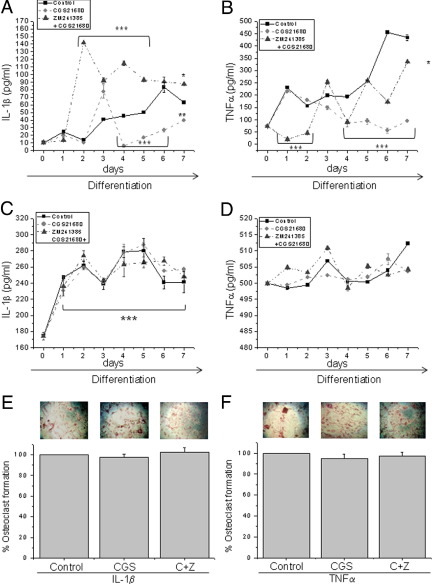
Effect of A2A receptor on osteoclast formation of mouse bone marrow cells and bone resorption. A: WT mouse osteoclast primary culture cells were fixed and stained for TRAP and Von Kossa after being cultured for 7 days, in 48- or 16-well culture plates, in the presence of CGS21680 (10 μmol/L to 1 nmol/L) alone or CGS21680 + ZM241385 (10 μmol/L to 1 nmol/L for the ZM241385 and 10 μmol/L for CGS21680). TRAP-positive cells containing three or more nuclei were counted as osteoclasts (red staining). Resorbed areas (inset on each TRAP staining image) appear in clear, with a contrasting brown background. The results were expressed as the means of four cultures. B: TRAP (red staining cells) and Von Kossa (inset on each TRAP staining image) staining analysis in A2A receptor KO mouse osteoclast primary culture cells and percentage of control in the presence of CGS21680 (10 μmol/L to 1 nmol/L) alone or pretreated with ZM241385 (10 μmol/L to 1 nmol/L for the ZM241385 and 10 μmol/L for CGS21680). The results were expressed as the means of four cultures. ZM241385 was added to cultures 30 minutes before CGS21680.
The effect of adenosine A2A receptor stimulation/antagonism on bone resorption paralleled the effects on osteoclast formation. CGS21680 treatment diminished osteoclast resorption in a dose-dependent fashion (half maximal inhibitory concentration, 0.5 μmol/L; maximal resorption of 31% ± 7%, where control resorption was 53% ± 3% of total osteoid; Figure 1A). The opposite effect was observed when cultures were pretreated with ZM241385 in the presence of CGS21680, 10 μmol/L, and resorption increased in a dose-dependent manner (Figure 1A). As with osteoclast formation, neither CGS21680 nor ZM241385 treatment affected osteoid resorption by cells from A2AKO mice (Figure 1B), confirming the results obtained with TRAP staining.
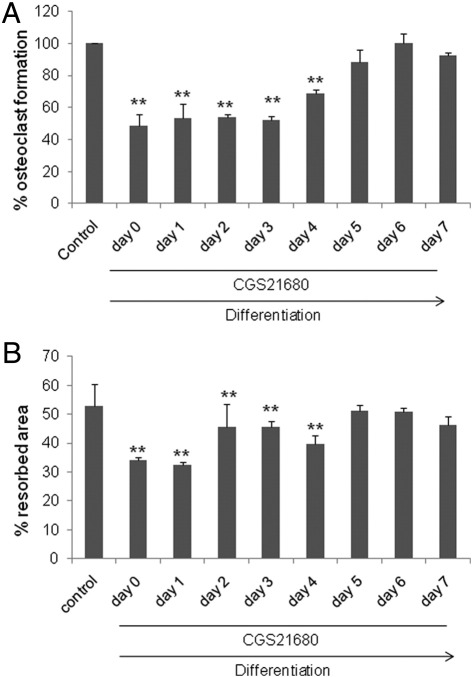
To determine the stage of osteoclast formation and function affected by A2A receptor stimulation, we began treatment of cultures with CGS21680 at various time points after the start of the cultures. We found that CGS21680 inhibited osteoclast differentiation (Figure 2A) and function (Figure 2B) when added to the culture at the beginning of differentiation (days 0 to 4) but had little or no effect when added after that time (Figure 2, A and B).
Figure 2.
Day response effect of CGS21680 on A2A receptor effect on osteoclast differentiation and function. A: WT mouse osteoclast primary culture cells stained with TRAP to counteract osteoclast differentiation being cultured for 7 days in the presence of CGS21680, 10 μmol/L, on different days. B: WT mouse osteoclast primary culture cells to study osteoclast function by Von Kossa staining being cultured for 7 days in the presence of CGS21680, 10 μmol/L, on different days. **P < 0.01.
Expression of Osteoclast Differentiation Markers in the Presence of CGS21680
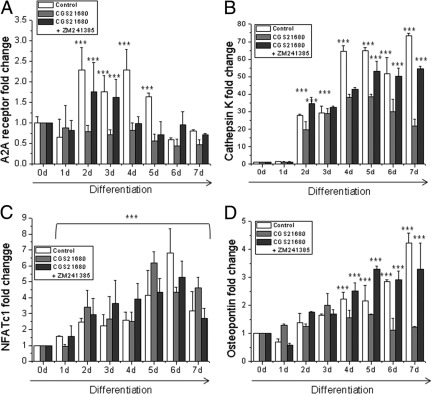
To confirm A2A receptor–mediated inhibition of osteoclast differentiation, M-CSF–RANKL–derived precursors were collected during the 7 days of osteoclast differentiation and RNA was extracted. We first analyzed the expression of the A2A receptor during M-CSF–RANKL–stimulated osteoclast formation. As shown in Figure 3A, the expression of A2A receptor changed over time, increasing in control cells (up to 2.2-fold on days 2 and 4 of differentiation, P < 0.005), an effect that was abrogated in A2A receptor–activated cells. Pretreatment with ZM241385 also reversed the effect of CGS21680 treatment on A2A receptor message expression. When we analyzed the change in mRNA expression for cathepsin K, we observed that, both in control and ZM241385-pretreated osteoclasts, cathepsin K was up-regulated during osteoclast differentiation (up to 80-fold on day 7 for control cells and 50-fold on day 7 of differentiation for ZM241385, P < 0.005), but CGS21680 reduced the increase (Figure 3B). NFATc1 mRNA expression was up-regulated during osteoclast differentiation (up to sixfold on day 6 of differentiation, P < 0.005), and neither A2A receptor activation nor blockade affected the M-CSF–RANKL–induced increase in expression (Figure 3C). Finally, mRNA expression for osteopontin was also up-regulated in the M-CSF–RANKL–stimulated osteoclast alone or pretreated with ZM241385, but CGS21680 inhibited the stimulated increase (Figure 3D). The observation that M-CSF–RANKL–induced up-regulation of NFATc1 was unaffected by the A2A agonist or its antagonist supports the hypothesis that A2A receptor activation selectively regulates cellular function and does not act as a general transcriptional inhibitor or cellular toxin.
Figure 3.
Expression of A2A receptor and osteoclast differentiation markers mRNA. A: Fold change in A2A receptor mRNA in M-CSF–RANKL OCPs during the 7 days of osteoclast differentiation in the presence of CGS21680 alone or with ZM241385. B: Fold change from control in cathepsin K mRNA in M-CSF–RANKL–treated OCPs during the 7 days of osteoclast differentiation in the presence of CGS21680 alone or with ZM241385. C: Fold change in NFATc1 mRNA in M-CSF–RANKL–treated OCPs during the 7 days of osteoclast differentiation in the presence of CGS21680 alone or with ZM241385. D: Fold change in osteopontin mRNA in M-CSF–RANKL–treated OCPs during the 7 days of osteoclast differentiation in the presence of the A2A agonist alone or with ZM241385. ***P < 0.005.
Morphological Characterization of Osteoclast Cultures
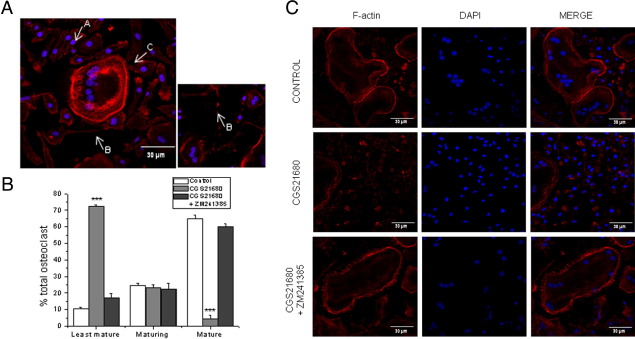
As we have previously reported,26 osteoclasts cultured on glass exhibit three distinct morphological features (Figure 4A). The least mature osteoclasts represent an early stage in osteoclast differentiation; are generally small, with fewer than five centrally located nuclei surrounded by a ring of F-actin; and are usually absent of podosomes. Maturing osteoclasts are variable in size, dendritic shaped, and contain more than five nuclei distributed throughout the cytoplasm, with podosomes located in patches at the edge of each pseudopod, and represent fusion intermediates because they are often connected with other maturing cells through cytoplasmic bridges. Finally, mature osteoclasts are large, with numerous nuclei located at the periphery near the peripheral podosome belt. We observed that the adenosine A2A agonist, CGS21680, increased (Figure 4, B and C) the percentage of least-differentiated osteoclasts (from 10.5% ± 1.0% to 72.3% ± 1.10% for CGS21680, P < 0.005). The A2A receptor–mediated increase in least-differentiated osteoclasts was reversed by the A2A antagonist, ZM241385 (to 17.3% ± 2.3%, P < 0.005). There were similar percentages of maturing cells (24.6% ± 1.2% for control, 23.3% ± 1.8% for CGS21680, and 22.6% ± 3.4% for CGS21680 + ZM241385) and an A2A receptor–mediated decrease in mature osteoclasts (64.9% ± 2.3% for control, 4.5% ± 2.0% for CGS21680, and 60.2% ± 1.7% for CGS21680 + ZM241385; P < 0.005). These data further confirm that the activation of A2A receptors plays an important regulatory role in osteoclast fusion and differentiation.
Figure 4.
Morphological characterization of osteoclast cultures. A: Morphological characteristics of the least mature (A), maturing (B), and mature (C) osteoclasts cultured on glass. B: Quantitative evaluation of the number of least mature, maturing, and mature osteoclasts in osteoclast cultures treated with CGS2160 alone or in the presence of ZM241385 compared with control cultures. C: F-actin was detected by Alexa 555–phalloidin staining in osteoclast cultures treated with CGS2160 alone or in the presence of ZM241385 compared with control cultures. Original magnification for all parts, ×63. ***P < 0.005.
Cytokine Secretion during Osteoclast Differentiation
CGS21680 treatment markedly decreased concentrations of IL-1β in culture supernatants during osteoclast differentiation (Figure 5A, P < 0.005), whereas pretreatment with ZM241385 produced a marked increase in IL-1β secretion starting on day 2 of differentiation and throughout differentiation (P < 0.005). In control and ZM241385 pretreated cells, there was an increase in TNF-α secretion over time, and treatment with CGS21680 diminished levels of this cytokine during differentiation (Figure 5B, P < 0.005). When we analyzed the levels of IL-1β and TNF-α in A2AKO cell culture supernatants, we observed an increase in cytokine levels compared with WT cell cultures. There was a detectable increase in IL-1β levels by day 1 of differentiation that remained stable during the 7 days of osteoclast differentiation and was unaffected by any of the treatments (Figure 5C, P < 0.005), whereas the secretion of TNF-α was not significantly changed in culture supernatants during osteoclast differentiation (Figure 5D). To determine whether the changes in TNF-α and IL-1β played a role in A2A receptor–mediated regulation of osteoclast formation, we determined the effect of A2A receptor stimulation on osteoclast formation in the presence of these two cytokines. Interestingly, both IL-1β and TNF-α completely abrogated the capacity of CGS21680 to inhibit osteoclast formation in vitro (2.5% ± 2.9% inhibition in the presence of IL-1β and 5.1% ± 4.4% inhibition in the presence of TNF-α; P = 0.15; Figure 5, E and F). These observations suggest that adenosine A2A receptor–mediated inhibition of IL-1β and TNF-α might play a role in the adenosine A2A receptor-mediated inhibition of osteoclast formation.
Figure 5.
Cytokine secretion changes during osteoclast differentiation. A: IL-1β–secreted values during the 7 days of osteoclast differentiation in WT M-CSF–RANKL cultures in the presence of CGS21680 alone or with ZM241385, compared with control. B: TNF-α–secreted values during the 7 days of osteoclast differentiation in WT M-CSF–RANKL cultures in the presence of CGS21680 alone or with ZM241385, compared with control. C: IL-1β–secreted values during the 7 days of osteoclast differentiation in A2AKO M-CSF–RANKL cultures in the presence of CGS21680 alone or with ZM241385, compared with control. D: TNF-α–secreted values during the 7 days of osteoclast differentiation in A2AKO M-CSF–RANKL cultures in the presence of CGS21680 alone or with ZM241385, compared with control. E: M-CSF–RANKL–derived osteoclast treated with CGS21680 alone or with ZM241385 in the presence of IL-1β were fixed and stained for TRAP. F: M-CSF–RANKL–derived osteoclasts treated with CGS21680 alone or with ZM241385 in the presence of TNF-α were fixed and stained for TRAP. ***P < 0.005.
Micro–X-Ray CT, Dual X-Ray Absorptiometry, and Histomorphometric Analysis of Bone in A2AKO Mice
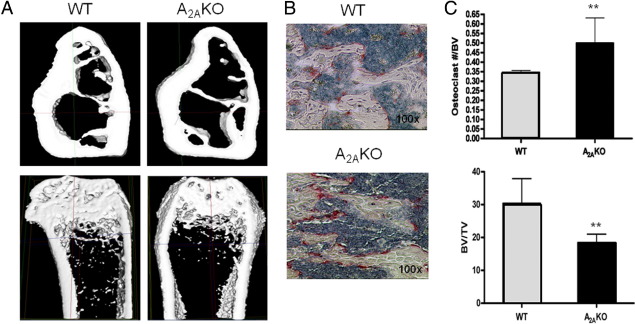
To correlate the in vitro A2A receptor–mediated effects on osteoclast differentiation and function with the in vivo effects, we studied the skeletons of A2AKO mice compared with those of WT mice. As previously described, A2AKO mice were similar in external appearance, body weight, and organ weight to WT controls (data not shown). Micro–X-ray CT analysis of femurs from WT and A2AKO mice showed a significantly decreased BV/total volume ratio and trabecular number in A2AKO mice (P < 0.05 and P < 0.001, respectively) and an increased trabecular space (P < 0.001) (Figure 6A and Table 1). Cortical bone, total area, and the outer perimeter in A2A receptor KO mice were similar to the WT mice (Table 1).
Figure 6.
Decreased bone mass in adenosine A2AKO mice. Histological and histomorphometric analysis of the femurs of A2AKO and WT mice. A: Representative high-resolution microfocal CT images showing significantly greater trabecular and cortical bone density in 4-month-old A2AKO mice compared with WT mice. Three-dimensional panel reconstruction of the femurs revealed increased bone mass in A1KO mice compared with their WT littermates. B: Representative histological sections obtained from the femurs of WT and A2AKO mice, stained for TRAP activity as a marker of osteoclasts and counterstained with hematoxylin. C: Osteoclast number/BV and BV/trabecular BV (TV) in WT and A2AKO mice. Data are from representative sections from transmission electron microscopy of femurs from WT and A2AKO mice after fixation in 2.5% PFA plus 0.5% glutaraldehyde in 0.05 mol/L sodium cacodylate buffer, postfixed in 1% osmium tetroxide and embedded in Epon, divided into sections, and stained with lead citrate and alcoholic uranyl acetate. **P < 0.01.
Table 1.
Histomorphometric Examination of Long Bones in 4-Month-Old WT and A2AKO Mice
| Variable | WT mice | A2AKO mice |
|---|---|---|
| BV/tissue volume ratio | 22.61 ± 2.154 | 17.49 ± 0.98⁎ |
| Trabecular no. (mm−1) | 5.8 ± 0.07 | 4.4 ± 0.15⁎⁎ |
| Trabecular separation (μm) | 0.13 ± 0.004 | 0.19 ± 0.006⁎⁎ |
| Cortical area (mm2) | 0.68 ± 0.04 | 0.6 ± 0.016⁎ |
| Total area (mm2) | 1.5 ± 0.03 | 1.3 ± 0.02⁎ |
| BMD (HA/cm3) | 0.053 ± 0.0025 | 0.049 ± 0.0016⁎⁎⁎ |
| BMC (g) | 0.48 ± 0.051 | 0.43 ± 0.028⁎⁎⁎ |
| Outer perimeter (mm) | 4.6 ± 0.04 | 4.4 ± 0.04⁎⁎⁎ |
| TMC (g) | 1.9 ± 0.12 | 1.7 ± 0.05⁎ |
| TRAP-positive osteoclasts (no./lpf) | 35 ± 1 | 50 ± 13⁎⁎⁎ |
Data are expressed as the mean ± SEM of three independent animals and related to control.
BMC, bone mineral content; HA, hydroxyapatite; lpf, low-power field; TMC, trabecular mineral content.
P < 0.5,
P < 0.005, and
P < 0.01.
To better characterize the bone phenotype of A2AKO mice, we examined the long bones of the mice histomorphometrically. Whole body dual X-ray absorptiometry scanning confirmed the decreased bone mineral content in the A2AKO mice (Figure 6A). We also observed a significant decrease in both BMD and bone mineral content (Table 1, P < 0.01). Interestingly, TRAP staining (Figure 6B) showed an increased number of TRAP-positive osteoclasts in the femoral metaphyses of A2AKO mice when compared with WT mice (six fields each from femurs of two different mice each) and a decrease in histomorphometrically determined BV/trabecular BV (Figure 6C, Table 1, P < 0.01), reflecting the bone loss.
Transmission electron microscopy of bone showed an apparent increase in osteoclast membrane folding and bone resorption in the femurs from A2AKO mice, compared with WT mice, consistent with our demonstration of enhanced bone resorption in the ZM241385-treated osteoclasts (data not shown).
Discussion
Previous results25,26 from our laboratory demonstrated that osteoclasts express all four adenosine receptor subtypes (A1, A2A, A2B, and A3). Because all four adenosine receptor subtypes are expressed on osteoclasts, we have examined the role of distinct adenosine receptors to determine their roles in regulating bone physiological and pathological characteristics. Our laboratory previously reported that deletion or blockade of adenosine A1 receptors diminished osteoclast formation and function and osteoclast-mediated bone loss in vitro. Adenosine A1 deletion or blockade led to osteopetrosis in KO mice and inhibition of post–ovariectomy-induced bone loss, a model for post-menopausal osteoporosis.25,26 These findings underlined the potential therapeutic importance of adenosine receptors in regulating bone physiological and pathological characteristics. Herein, we demonstrate that adenosine A2A receptors also regulate osteoclast formation and function in vitro and that deletion of these receptors leads to enhanced osteoclast formation and function both in vitro and in vivo, with a resulting decline in BMD.
Our in vitro studies reveal that adenosine A2A receptor stimulation diminished the number of differentiated TRAP-positive cells, bone resorption by these cells, and expression of osteoclast differentiation markers, such as cathepsin K and osteopontin. This effect is more notable when the agonist was added at early stages of culture. This observation correlates with the down-regulation in A2A receptor mRNA that occurred during differentiation. During differentiation, A2A receptor levels undergo no increase after CGS21680 treatment, whereas in RANKL alone or ZM241385-stimulated cells, the A2A receptor is overexpressed, a phenomenon consistent with the known effects of TNF-α stimulation and stimulation of NF-κB activation on adenosine A2A receptor expression.39–42 This, together with in vitro differentiation and function assays performed in A2AKO cells, suggests that the A2A receptor has to be either activated or blocked from the beginning of cell differentiation to exert its effect on osteoclast formation.
Although the KO animals studied were universal knockouts and not specific for osteoclasts or OCPs, it is likely that the primary effect on bone metabolism observed herein is primarily the result of specific loss of adenosine receptors on osteoclasts and their precursors. The studies of in vitro differentiation of osteoclasts studied herein start with a relatively pure population of myeloid cells containing precursors that will differentiate into osteoclasts in the presence of M-CSF and RANKL. As shown herein, an adenosine A2A-selective receptor agonist suppresses osteoclast formation in cells from WT mice (an effect completely reversed at pharmacologically relevant concentrations of selective antagonists) but not in the OCPs derived from A2A receptor KO mice. The increase in osteoclasts observed in vivo in the KO mice was clearly consistent with this in vitro effect, although it is possible that indirect effects of adenosine receptor deletion on other cells that regulate osteoclast formation could have been responsible for the increase in osteoclasts observed. Even if adenosine receptors on other cell types (eg, osteoblasts or inflammatory cells) play a role in the A2A receptor–mediated regulation of osteoclast formation in vivo, the direct effects of adenosine A2A receptor engagement on osteoclast differentiation remain critical to the observed changes in osteoclast number and bone resorption. The use of selective cell-specific knockouts could have more definitely demonstrated that the observed changes were only the result of the effects on OCPs.
The order of affinity of adenosine receptors for adenosine is A1>A2A≫A2B = A3; thus, as previously observed by Yang et al,43 endogenous adenosine levels are more potent stimuli for A1 and A2A receptors than A2B or A3 receptors. The potency of adenosine as an agonist for adenosine receptors also depends on the density of the receptors.44 Under physiological conditions, adenosine levels in most tissues are low but are sufficient to partially activate A1, A2A, and A3 receptors, which are abundantly expressed and highly sensitive. Based on the capacity of the A2A antagonist to increase osteoclast formation and function, it is likely that A2A receptors are activated by endogenous levels of adenosine (as are A1 receptors). Because A1 and A2A receptors have mutually antagonistic actions, it is likely that blockade of A2A receptors permits full activation and signaling of A1 receptors by endogenous adenosine. Similarly, blockade of A1 receptors may uncover endogenous activation of A2A receptors.
Pellegatti and colleagues45 recently reported that the purinergic axis plays a crucial role in osteoclast formation and confirms previous evidence advocating a key role for either ATP or adenosine receptors in multinucleated giant cell formation. Although a message for adenosine receptors was present in peripheral blood OCPs, an adenosine A1 receptor message was present at low levels. Surprisingly, Pellegatti and colleagues found that ATP, which activates P2X7 receptors, blocks osteoclast fusion and that catabolism of adenine nucleotides promotes osteoclast formation from precursors in peripheral blood, an effect they ascribed to A2A receptor activation by its selective agonist, CGS21680. However, in their work, blockade or stimulation of adenosine receptors regulates cellular fusion only when the P2X7 receptor is blocked or adenine nucleotides are catabolized. This work stands in contrast to prior work by Merrill and coworkers,24 in which adenosine A1 receptor stimulation promotes multinucleated giant cell formation and A2A receptor stimulation inhibits fusion of peripheral blood monocytes. The difference between their findings in human peripheral blood OCPs and the findings reported herein may be due to a species-dependent difference in response to A2A receptor stimulation. Another explanation may be that OCPs in peripheral blood differ from precursors in the marrow with respect to P2X7 or adenosine receptor expression or function.
During osteoclast maturation, proinflammatory cytokines cause an imbalance in bone metabolism favoring bone resorption.46 Indeed, even in the absence of RANKL-TNF–related activation-induced cytokine, TRAF6, or RANK, TNF can stimulate osteoclast formation.47 The well-documented inhibitory effects of adenosine A2A receptor stimulation on TNF-α and IL-1β secretion probably contribute, in our work, to inhibition of osteoclast formation and bone resorption in inflammatory diseases and help explain the therapeutic effects of methotrexate, the anti-inflammatory effects of which are mediated by adenosine acting at its receptors48 in the treatment of rheumatoid arthritis. Thus, it is likely that, under inflammatory conditions, the net effect of adenosine A2A receptor stimulation will be even further inhibition of osteoclast formation.
Adenosine is a potent biological mediator that affects numerous cell types, including neuronal cells, platelets, neutrophils, and smooth muscle cells, among others. Adenosine A2A receptors are coupled to GS and signal, primarily by activation of adenylate cyclase, accumulation of cAMP, and downstream activation of either protein kinase A or exchange protein activated by cAMP. Signaling downstream from protein kinase A proceeds, in part, through phosphorylation of the cAMP response element binding transcription factor, resulting in activation leading to either interaction of cAMP response element binding with specific promoters and gene expression or competition with NF-κB or other transcription factors. Prior reports49 had suggested that increases in cAMP inhibited osteoclast formation, but inhibition of protein kinase A had no effect on osteoclastogenesis. Similarly, Lerner et al50 detected the presence of functional A2 and P-site receptors, but not A1 receptors, in mouse calvaria and osteoblast-like cells; both receptors regulate cAMP, but the authors assumed they were not intimately linked to bone. Recently, Zhang et al51 linked the stimulation of adenylyl cyclase by A2A and A2B activation, which led to increased cAMP levels that, in turn, activated the canonical protein kinase A pathway and the exchange protein directly activated by cAMP52; in contrast, A1 and A3 activation diminishes cAMP.53 In addition, adenosine receptor signaling in mast cells has also been linked to phospholipase C and calcium mobilization (A2B and A3), phosphatidylinositol 3-kinase (A3), and protein kinase C and mitogen-activated protein kinases (A1, A2A, and A3 receptors).21 Future experiments will be directed at the dissection of adenosine receptor signaling in OCPs and the role of cAMP or other intermediates in the regulation of osteoclast formation.
In rheumatoid arthritis and other inflammatory diseases that affect the bone, there is inflammation of surrounding tissues with activation of osteoclasts and resorption of bone. Adenosine A2A receptors have diminished inflammation for a long time by directly inhibiting the inflammatory function of macrophages, dendritic cells, and neutrophils and by stimulating anti-inflammatory functions of macrophages and T cells.54 Moreover, methotrexate, which diminishes inflammation by increasing extracellular adenosine concentrations,55 diminishes bone erosions in rheumatoid arthritis, although not as well as anti-TNF agents.
Because adenosine mediates the anti-inflammatory effects of methotrexate,56 we further speculate that the capacity of methotrexate to inhibit bone erosion in patients with rheumatoid arthritis may be mediated by methotrexate-stimulated increases in adenosine concentration. In KO mouse models, the A2A receptor, together with the A3 receptor, mediated the anti-inflammatory effect of methotrexate, which is used as a treatment of arthritis.57,58 We first determined whether methotrexate induced an increase of extracellular adenosine via intracellular adenosine production or whether it was generated extracellularly from adenine nucleotides. The pathway leading to increased extracellular adenosine by methotrexate has been mostly delineated in studies56–58 that confirmed that low-dose methotrexate therapy increases tissue aminoimidazole carboxamide ribonucleotide (an intermediate in the generation of inosine monophosphate) levels in animal models of rheumatoid arthritis and urinary aminoimidazole carboxamide ribonucleotide levels in patients with psoriasis and rheumatoid arthritis.
Finally, downstream signaling from RANK activation includes activation of the NF-κB complex, NFATc1 transcription factor, C-Jun kinase, p38, and extracellular signal regulated kinase mitogen-activated protein kinases and the phosphatidylinositol 3-kinase–Akt axis.59,60 Each of these pathways plays a key role in osteoclast differentiation and/or function. Thus, mice lacking the p50 and p52 subunits of NF-κB or c-Fos, a member of the AP-1 family of transcription factors and a downstream target of stress-activated protein kinase/C-Jun kinase, generate no osteoclasts.61 Ectopic expression of NFATc1 in bone marrow macrophages induces the formation of multinuclear osteoclasts in the absence of known fusion-inducing factors, such as RANKL.62 In addition, adenosine A2A receptor activation increases mitogen-activated protein kinase phosphorylation and activation and activation of AKT, Cdc42, and other pathways.63–65 The interactions of adenosine A2A receptors with these signaling pathways in the formation and function of osteoclasts and in the final mechanism of the differentiating phenotype need further investigation.
In conclusion, these results indicate that adenosine A2A receptors inhibit M-CSF–RANKL–stimulated osteoclast differentiation and function and, thereby, regulate bone turnover.
Acknowledgments
We thank Maya Hawly and Elie Sellam for developing the Matlab compiler software used to quantify the bone resorption areas after Von Kossa staining, and we acknowledge the Microscopy Core at New York University Langone Medical Center for the use of and assistance with the core confocal microscope.
Footnotes
Supported by NIH grants (T32GM66704, AR56672, AR56672S1, and AR54897), the New York University (NYU)–Health & Hospital Corporations Clinical and Translational Science Institute (UL1RR029893), the NYU Applied Research Support Fund, and King Pharmaceuticals. The Microscopy Core at New York University Langone Medical Center is funded by NCRRS10 RR024708.
Disclosures: B.N.C. holds patents on the use of the following: i) adenosine A2A receptor agonists to promote wound healing and the use of A2A receptor antagonists to inhibit fibrosis, ii) adenosine A1 receptor antagonists to treat osteoporosis and other diseases of bone, iii) adenosine A1 and A2B receptor antagonists to treat fatty liver, and iv) adenosine A2A receptor agonists to prevent prosthesis loosening; has received consulting fees within the past 2 years from Bristol-Myers Squibb, Novartis, CanFite Biopharmaceuticals, Cypress Laboratories, Regeneron (Westat, DSMB), Endocyte, Protalex, Allos, Inc., Savient, Gismo Therapeutics, Antares Pharmaceutical, and Medivector; holds stock in CanFite Biopharmaceuticals; and has received research funding from King Pharmaceuticals, OSI Pharmaceuticals, URL Pharmaceuticals, Inc., and Gilead Pharmaceuticals.
References
- 1.Sims N.A., Gooi J.H. Bone remodeling: multiple cellular interactions required for coupling of bone formation and resorption. Semin Cell Dev Biol. 2008;19:444–451. doi: 10.1016/j.semcdb.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 2.Martin T.J., Seeman E. Bone remodelling: its local regulation and the emergence of bone fragility. Best Pract Res Clin Endocrinol Metab. 2008;22:701–722. doi: 10.1016/j.beem.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 3.Schett G. Cells of the synovium in rheumatoid arthritis: osteoclasts. Arthritis Res Ther. 2007;9:203. doi: 10.1186/ar2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ochi S., Shinohara M., Sato K., Gober H.J., Koga T., Kodama T., Takai T., Miyasaka N., Takayanagi H. Pathological role of osteoclast costimulation in arthritis-induced bone loss. Proc Natl Acad Sci U S A. 2007;104:11394–11399. doi: 10.1073/pnas.0701971104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suda T., Takahashi N., Udagawa N., Jimi E., Gillespie M.T., Martin T.J. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev. 1999;20:345–357. doi: 10.1210/edrv.20.3.0367. [DOI] [PubMed] [Google Scholar]
- 6.Manolagas S.C. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev. 2000;21:115–137. doi: 10.1210/edrv.21.2.0395. [DOI] [PubMed] [Google Scholar]
- 7.Teitelbaum S.L. Bone resorption by osteoclasts. Science. 2000;289:1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 8.McHugh K.P., Shen Z., Crotti T.N., Flannery M.R., Fajardo R., Bierbaum B.E., Goldring S.R. Role of cell-matrix interactions in osteoclast differentiation. Adv Exp Med Biol. 2007;602:107–111. doi: 10.1007/978-0-387-72009-8_14. [DOI] [PubMed] [Google Scholar]
- 9.Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol. 2007;7:292–304. doi: 10.1038/nri2062. [DOI] [PubMed] [Google Scholar]
- 10.Yoshida H., Hayashi S., Kunisada T., Ogawa M., Nishikawa S., Okamura H., Sudo T., Shultz L.D. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature. 1990;345:442–444. doi: 10.1038/345442a0. [DOI] [PubMed] [Google Scholar]
- 11.Yasuda H., Shima N., Nakagawa N., Yamaguchi K., Kinosaki M., Mochizuki S., Tomoyasu A., Yano K., Goto M., Murakami A., Tsuda E., Morinaga T., Higashio K., Udagawa N., Takahashi N., Suda T. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A. 1998;95:3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simonet W.S., Lacey D.L., Dunstan C.R., Kelley M., Chang M.S., Luthy R., Nguyen H.Q., Wooden S., Bennett L., Boone T., Shimamoto G., DeRose M., Elliott R., Colombero A., Tan H.L., Trail G., Sullivan J., Davy E., Bucay N., Renshaw-Gegg L., Hughes T.M., Hill D., Pattison W., Campbell P., Sander S., Van G., Tarpley J., Derby P., Lee R., Boyle W.J. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–319. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 13.Dougall W.C., Glaccum M., Charrier K., Rohrbach K., Brasel K., De Smedt T., Daro E., Smith J., Tometsko M.E., Maliszewski C.R., Armstrong A., Shen V., Bain S., Cosman D., Anderson D., Morrissey P.J., Peschon J.J., Schuh J. RANK is essential for osteoclast and lymph node development. Genes Dev. 1999;13:2412–2424. doi: 10.1101/gad.13.18.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J., Sarosi I., Yan X.Q., Morony S., Capparelli C., Tan H.L., McCabe S., Elliott R., Scully S., Van G., Kaufman S., Juan S.C., Sun Y., Tarpley J., Martin L., Christensen K., McCabe J., Kostenuik P., Hsu H., Fletcher F., Dunstan C.R., Lacey D.L., Boyle W.J. RANK is the intrinsic hematopoietic cell surface receptor that controls osteoclastogenesis and regulation of bone mass and calcium metabolism. Proc Natl Acad Sci U S A. 2000;97:1566–1571. doi: 10.1073/pnas.97.4.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zimmermann H. Extracellular metabolism of ATP and other nucleotides. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:299–309. doi: 10.1007/s002100000309. [DOI] [PubMed] [Google Scholar]
- 16.Fredholm B.B., Ijzerman A.P., Jacobson K.A., Linden J., Muller C.E. International Union of Basic and Clinical Pharmacology, LXXXI: nomenclature and classification of adenosine receptors–an update. Pharmacol Rev. 2011;63:1–34. doi: 10.1124/pr.110.003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Calker D., Muller M., Hamprecht B. Adenosine regulates via two different types of receptors, the accumulation of cyclic AMP in cultured brain cells. J Neurochem. 1979;33:999–1005. doi: 10.1111/j.1471-4159.1979.tb05236.x. [DOI] [PubMed] [Google Scholar]
- 18.Londos C., Cooper D.M., Wolff J. Subclasses of external adenosine receptors. Proc Natl Acad Sci U S A. 1980;77:2551–2554. doi: 10.1073/pnas.77.5.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kull B., Svenningsson P., Fredholm B.B. Adenosine A(2A) receptors are colocalized with and activate g(olf) in rat striatum. Mol Pharmacol. 2000;58:771–777. doi: 10.1124/mol.58.4.771. [DOI] [PubMed] [Google Scholar]
- 20.Fredholm B.B., Irenius E., Kull B., Schulte G. Comparison of the potency of adenosine as an agonist at human adenosine receptors expressed in Chinese hamster ovary cells. Biochem Pharmacol. 2001;61:443–448. doi: 10.1016/s0006-2952(00)00570-0. [DOI] [PubMed] [Google Scholar]
- 21.Jacobson K.A., Gao Z.G. Adenosine receptors as therapeutic targets. Nat Rev Drug Discov. 2006;5:247–264. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sexl V., Mancusi G., Holler C., Gloria-Maercker E., Schutz W., Freissmuth M. Stimulation of the mitogen-activated protein kinase via the A2A-adenosine receptor in primary human endothelial cells. J Biol Chem. 1997;272:5792–5799. doi: 10.1074/jbc.272.9.5792. [DOI] [PubMed] [Google Scholar]
- 23.Hirano D., Aoki Y., Ogasawara H., Kodama H., Waga I., Sakanaka C., Shimizu T., Nakamura M. Functional coupling of adenosine A2a receptor to inhibition of the mitogen-activated protein kinase cascade in Chinese hamster ovary cells. Biochem J. 1996;316(Pt 1):81–86. doi: 10.1042/bj3160081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merrill J.T., Shen C., Schreibman D., Coffey D., Zakharenko O., Fisher R., Lahita R.G., Salmon J., Cronstein B.N. Adenosine A1 receptor promotion of multinucleated giant cell formation by human monocytes: a mechanism for methotrexate-induced nodulosis in rheumatoid arthritis. Arthritis Rheum. 1997;40:1308–1315. doi: 10.1002/1529-0131(199707)40:7<1308::AID-ART16>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 25.Kara F.M., Doty S.B., Boskey A., Goldring S., Zaidi M., Fredholm B.B., Cronstein B.N. Adenosine A(1) receptors regulate bone resorption in mice: adenosine A(1) receptor blockade or deletion increases bone density and prevents ovariectomy-induced bone loss in adenosine A(1) receptor-knockout mice. Arthritis Rheum. 2010;62:534–541. doi: 10.1002/art.27219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kara F.M., Chitu V., Sloane J., Axelrod M., Fredholm B.B., Stanley E.R., Cronstein B.N. Adenosine A1 receptors (A1Rs) play a critical role in osteoclast formation and function. FASEB J. 2010;24:2325–2333. doi: 10.1096/fj.09-147447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen J.F., Huang Z., MA J., Zhu J., Moratalla R., Standaert D., Moskowitz M.A., Fink J.S., Schwarzschild M.A. A(2A) adenosine receptor deficiency attenuates brain injury induced by transient focal ischemia in mice. J Neurosci. 1999;19:9192–9200. doi: 10.1523/JNEUROSCI.19-21-09192.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montesinos M.C., Desai A., Cronstein B.N. Suppression of inflammation by low-dose methotrexate is mediated by adenosine A2A receptor but not A3 receptor activation in thioglycollate-induced peritonitis. Arthritis Res Ther. 2006;8:R53. doi: 10.1186/ar1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Montesinos M.C., Desai A., Chen J.F., Yee H., Schwarzschild M.A., Fink J.S., Cronstein B.N. Adenosine promotes wound healing and mediates angiogenesis in response to tissue injury via occupancy of A(2A) receptors. Am J Pathol. 2002;160:2009–2018. doi: 10.1016/S0002-9440(10)61151-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salvatore C.A., Tilley S.L., Latour A.M., Fletcher D.S., Koller B.H., Jacobson M.A. Disruption of the A(3) adenosine receptor gene in mice and its effect on stimulated inflammatory cells. J Biol Chem. 2000;275:4429–4434. doi: 10.1074/jbc.275.6.4429. [DOI] [PubMed] [Google Scholar]
- 31.Ariyoshi W., Takahashi T., Kanno T., Ichimiya H., Shinmyouzu K., Takano H., Koseki T., Nishihara T. Heparin inhibits osteoclastic differentiation and function. J Cell Biochem. 2008;103:1707–1717. doi: 10.1002/jcb.21559. [DOI] [PubMed] [Google Scholar]
- 32.Kurihara N., Tatsumi J., Arai F., Iwama A., Suda T. Macrophage-stimulating protein (MSP) and its receptor, RON, stimulate human osteoclast activity but not proliferation: effect of MSP distinct from that of hepatocyte growth factor. Exp Hematol. 1998;26:1080–1085. [PubMed] [Google Scholar]
- 33.Yasuda H., Shima N., Nakagawa N., Yamaguchi K., Kinosaki M., Goto M., Mochizuki S.I., Tsuda E., Morinaga T., Udagawa N., Takahashi N., Suda T., Higashio K. A novel molecular mechanism modulating osteoclast differentiation and function. Bone. 1999;25:109–113. doi: 10.1016/s8756-3282(99)00121-0. [DOI] [PubMed] [Google Scholar]
- 34.Pfaffl M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chitu V., Pixley F.J., Macaluso F., Larson D.R., Condeelis J., Yeung Y.G., Stanley E.R. The PCH family member MAYP/PSTPIP2 directly regulates F-actin bundling and enhances filopodia formation and motility in macrophages. Mol Biol Cell. 2005;16:2947–2959. doi: 10.1091/mbc.E04-10-0914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parfitt A.M., Drezner M.K., Glorieux F.H., Kanis J.A., Malluche H., Meunier P.J., Ott S.M., Recker R.R. Bone histomorphometry: standardization of nomenclature, symbols, and units: report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 37.Hildebrand T., Ruegsegger P. Quantification of bone microarchitecture with the Structure Model Index. Comput Methods Biomech Biomed Engin. 1997;1:15–23. doi: 10.1080/01495739708936692. [DOI] [PubMed] [Google Scholar]
- 38.Morgan S.L., Baggott J.E., Refsum H., Ueland P.M. Homocysteine levels in patients with rheumatoid arthritis treated with low-dose methotrexate. Clin Pharmacol Ther. 1991;50:547–556. doi: 10.1038/clpt.1991.180. [DOI] [PubMed] [Google Scholar]
- 39.Khoa N.D., Postow M., Danielsson J., Cronstein B.N. Tumor necrosis factor-alpha prevents desensitization of Galphas-coupled receptors by regulating GRK2 association with the plasma membrane. Mol Pharmacol. 2006;69:1311–1319. doi: 10.1124/mol.105.016857. [DOI] [PubMed] [Google Scholar]
- 40.Nguyen D.K., Montesinos M.C., Williams A.J., Kelly M., Cronstein B.N. Th1 cytokines regulate adenosine receptors and their downstream signaling elements in human microvascular endothelial cells. J Immunol. 2003;171:3991–3998. doi: 10.4049/jimmunol.171.8.3991. [DOI] [PubMed] [Google Scholar]
- 41.Khoa N.D., Montesinos M.C., Reiss A.B., Delano D., Awadallah N., Cronstein B.N. Inflammatory cytokines regulate function and expression of adenosine A2A receptors in human monocytic THP-1 cells. J Immunol. 2001;167:4026–4032. doi: 10.4049/jimmunol.167.7.4026. [DOI] [PubMed] [Google Scholar]
- 42.Morello S., Ito K., Yamamura S., Lee K.Y., Jazrawi E., Desouza P., Barnes P., Cicala C., Adcock I.M. IL-1 beta and TNF-alpha regulation of the adenosine receptor (A2A) expression: differential requirement for NF-kappaB binding to the proximal promoter. J Immunol. 2006;177:7173–7183. doi: 10.4049/jimmunol.177.10.7173. [DOI] [PubMed] [Google Scholar]
- 43.Yang J.N., Chen J.F., Fredholm B.B. Physiological roles of A1 and A2A adenosine receptors in regulating heart rate, body temperature, and locomotion as revealed using knockout mice and caffeine. Am J Physiol Heart Circ Physiol. 2009;296:H1141–H1149. doi: 10.1152/ajpheart.00754.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fredholm B.B. Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ. 2007;14:1315–1323. doi: 10.1038/sj.cdd.4402132. [DOI] [PubMed] [Google Scholar]
- 45.Pellegatti P., Falzoni S., Donvito G., Lemaire I., Di Virgilio F. P2X7 receptor drives osteoclast fusion by increasing the extracellular adenosine concentration. FASEB J. 2011;25:1264–1274. doi: 10.1096/fj.10-169854. [DOI] [PubMed] [Google Scholar]
- 46.Tanaka Y., Nakayamada S., Okada Y. Osteoblasts and osteoclasts in bone remodeling and inflammation. Curr Drug Targets Inflamm Allergy. 2005;4:325–328. doi: 10.2174/1568010054022015. [DOI] [PubMed] [Google Scholar]
- 47.Kim N., Kadono Y., Takami M., Lee J., Lee S.H., Okada F., Kim J.H., Kobayashi T., Odgren P.R., Nakano H., Yeh W.C., Lee S.K., Lorenzo J.A., Choi Y. Osteoclast differentiation independent of the TRANCE-RANK-TRAF6 axis. J Exp Med. 2005;202:589–595. doi: 10.1084/jem.20050978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chan E.S., Cronstein B.N. Methotrexate: how does it really work? Nat Rev Rheumatol. 2010;6:175–178. doi: 10.1038/nrrheum.2010.5. [DOI] [PubMed] [Google Scholar]
- 49.Yang D.C., Tsay H.J., Lin S.Y., Chiou S.H., Li M.J., Chang T.J., Hung S.C. cAMP/PKA regulates osteogenesis, adipogenesis and ratio of RANKL/OPG mRNA expression in mesenchymal stem cells by suppressing leptin. PLoS One. 2008;3:e1540. doi: 10.1371/journal.pone.0001540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lerner U.H., Sahlberg K., Fredholm B.B. Characterization of adenosine receptors in bone: studies on the effect of adenosine analogues on cyclic AMP formation and bone resorption in cultured mouse calvaria. Acta Physiol Scand. 1987;131:287–296. doi: 10.1111/j.1748-1716.1987.tb08239.x. [DOI] [PubMed] [Google Scholar]
- 51.Zhang L., Paine C., Dip R. Selective regulation of nuclear orphan receptors 4A by adenosine receptor subtypes in human mast cells. J Cell Commun Signal. 2010;4:173–183. doi: 10.1007/s12079-010-0104-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Palmer T.M., Trevethick M.A. Suppression of inflammatory and immune responses by the A(2A) adenosine receptor: an introduction. Br J Pharmacol. 2008;153(Suppl 1):S27–S34. doi: 10.1038/sj.bjp.0707524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou Q.Y., Li C., Olah M.E., Johnson R.A., Stiles G.L., Civelli O. Molecular cloning and characterization of an adenosine receptor: the A3 adenosine receptor. Proc Natl Acad Sci U S A. 1992;89:7432–7436. doi: 10.1073/pnas.89.16.7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hasko G., Linden J., Cronstein B., Pacher P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov. 2008;7:759–770. doi: 10.1038/nrd2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chan E.S., Cronstein B.N. Methotrexate: how does it really work? Nat Rev Rheumatol. 2010;6:175–178. doi: 10.1038/nrrheum.2010.5. [DOI] [PubMed] [Google Scholar]
- 56.Cronstein B.N., Naime D., Ostad E. The antiinflammatory mechanism of methotrexate: increased adenosine release at inflamed sites diminishes leukocyte accumulation in an in vivo model of inflammation. J Clin Invest. 1993;92:2675–2682. doi: 10.1172/JCI116884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Montesinos M.C., Desai A., Delano D., Chen J.F., Fink J.S., Jacobson M.A., Cronstein B.N. Adenosine A2A or A3 receptors are required for inhibition of inflammation by methotrexate and its analog MX-68. Arthritis Rheum. 2003;48:240–247. doi: 10.1002/art.10712. [DOI] [PubMed] [Google Scholar]
- 58.Morabito L., Montesinos M.C., Schreibman D.M., Balter L., Thompson L.F., Resta R., Carlin G., Huie M.A., Cronstein B.N. Methotrexate and sulfasalazine promote adenosine release by a mechanism that requires ecto-5′-nucleotidase-mediated conversion of adenine nucleotides. J Clin Invest. 1998;101:295–300. doi: 10.1172/JCI1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wei S., Wang M.W., Teitelbaum S.L., Ross F.P. Interleukin-4 reversibly inhibits osteoclastogenesis via inhibition of NF-kappa B and mitogen-activated protein kinase signaling. J Biol Chem. 2002;277:6622–6630. doi: 10.1074/jbc.M104957200. [DOI] [PubMed] [Google Scholar]
- 60.Hirata K., Taki H., Shinoda K., Hounoki H., Miyahara T., Tobe K., Ogawa H., Mori H., Sugiyama E. Inhibition of tumor progression locus 2 protein kinase suppresses receptor activator of nuclear factor-kappaB ligand-induced osteoclastogenesis through down-regulation of the c-Fos and nuclear factor of activated T cells c1 genes. Biol Pharm Bull. 2010;33:133–137. doi: 10.1248/bpb.33.133. [DOI] [PubMed] [Google Scholar]
- 61.Grigoriadis A.E., Wang Z.Q., Cecchini M.G., Hofstetter W., Felix R., Fleisch H.A., Wagner E.F. c-Fos: a key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science. 1994;266:443–448. doi: 10.1126/science.7939685. [DOI] [PubMed] [Google Scholar]
- 62.Kobayashi Y., Udagawa N., Takahashi N. Action of RANKL and OPG for osteoclastogenesis. Crit Rev Eukaryot Gene Expr. 2009;19:61–72. doi: 10.1615/critreveukargeneexpr.v19.i1.30. [DOI] [PubMed] [Google Scholar]
- 63.Feoktistov I., Goldstein A.E., Biaggioni I. Cyclic AMP and protein kinase A stimulate Cdc42: role of A(2) adenosine receptors in human mast cells. Mol Pharmacol. 2000;58:903–910. doi: 10.1124/mol.58.5.903. [DOI] [PubMed] [Google Scholar]
- 64.Mori Y., Higuchi M., Masuyama N., Gotoh Y. Adenosine A2A receptor facilitates calcium-dependent protein secretion through the activation of protein kinase A and phosphatidylinositol-3 kinase in PC12 cells. Cell Struct Funct. 2004;29:101–110. doi: 10.1247/csf.29.101. [DOI] [PubMed] [Google Scholar]
- 65.Thakur S., Du J., Hourani S., Ledent C., Li J.M. Inactivation of adenosine A2A receptor attenuates basal and angiotensin II-induced ROS production by Nox2 in endothelial cells. J Biol Chem. 2010;285:40104–40113. doi: 10.1074/jbc.M110.184606. [DOI] [PMC free article] [PubMed] [Google Scholar]