Abstract
Kidney development is regulated by a coordinated reciprocal induction of metanephric mesenchymal (MM) and ureteric bud (UB) cells. Here, established MM and UB progenitor cell lines were recombined in three-dimensional Matrigel implants in SCID mice. Differentiation potential was examined for changes in phenotype, organization, and the presence of specialized proteins using immunofluorescence and bright-field and electron microscopy. Both cell types, when grown alone, did not develop into specialized structures. When combined, the cells organized into simple organoid structures of polarized epithelia with lumens surrounded by capillary-like structures. Tracker experiments indicated the UB cells formed the tubuloid structures, and the MM cells were the source of the capillary-like cells. The epithelial cells stained positive for pancytokeratin, the junctional complex protein ZO-1, collagen type IV, as well as UB and collecting duct markers, rearranged during transfection (RET), Dolichos biflorus lectin, EndoA cytokeratin, and aquaporin 2. The surrounding cells expressed α-smooth muscle actin, vimentin, platelet endothelial cell adhesion molecule 1 (PECAM), and aquaporin 1, a marker of vasculogenesis. The epithelium exhibited apical vacuoles, microvilli, junctional complexes, and linear basement membranes. Capillary-like structures showed endothelial features with occasional pericytes. UB cell epithelialization was augmented in the presence of MM cell–derived conditioned medium, glial-derived neurotrophic factor (GDNF), hepatocyte growth factor (HGF), or fibronectin. MM cells grown in the presence of UB-derived conditioned medium failed to undergo differentiation. However, UB cell–derived conditioned medium induced MM cell migration. These studies indicate that tubulogenesis and vasculogenesis can be partially recapitulated by recombining individual MM and UB cell lineages, providing a new model system to study organogenesis ex vivo.
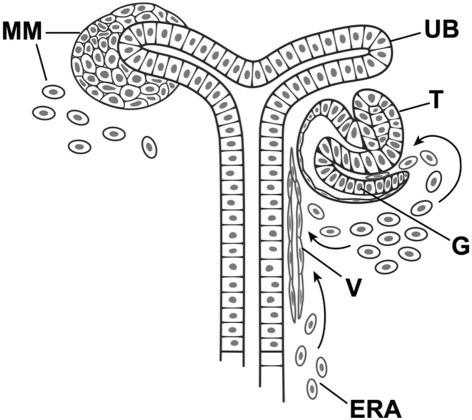
Development of the kidney is governed by a well-orchestrated series of reciprocal inductive events between the ureteric bud (UB) epithelium and the metanephric mesenchyme (MM)1–8 (Figure 1). The UB, an outgrowth of the Wolffian duct, invades and interacts with the MM.2–6 The MM induces UB branching morphogenesis, eventually giving rise to the collecting duct system, renal pelvis, and ureter.1,3 In turn, the mesenchyme is induced to form aggregates around the advancing tips of the UB, eventually forming the renal vesicle, committing the mesenchyme to epithelialize, and give rise to the visceral and parietal epithelial cells of the glomerulus, proximal tubule, loop of Henle, and distal tubule.1,7 MM cells may also differentiate into vascular and stromal structures throughout the developing kidney, including mesangial cells and endothelium of the developing glomerulus.4,8,9
Figure 1.
Reciprocal induction of metanephric mesenchymal (MM) and ureteric bud (UB) cells during nephrogenesis. Early nephrogenesis is distinguished by condensing MM cells around an elongating and branching ureteric bud destined to become the collecting duct system (shown on the left). Condensed metanephric mesenchyme differentiates into epithelium of the developing glomerulus (G), proximal and distal tubules (T) (right). MM may also contain or differentiate into angioblasts (arrows) destined to become the peritubular vasculature (V). Angioblasts destined to become the mesangium and capillary loops also migrate into the cleft of the developing glomerulus. Extrarenal angioblasts (ERA) may also contribute to vascular structures.
Based on data described by Saxen and Sariola,1 Abrahamson,7 and Ricono et al.8
Recent state-of-the-art methods such as targeted disruption of genes, in vivo delivery of test substances, and the examination of whole embryonic kidney explants have been especially informative in defining roles for growth factors, signaling pathways, and genes involved in inductive events during nephrogenesis.3,5,6,10 Also, developmental defects may result in death of transgenic animals before the onset of nephrogenesis, precluding the study of important developmental processes in vivo, making development of simple organotypic culture systems desirable.
In vitro experiments using intact MM or UB explants or isolated cells in monolayer or three-dimensional gels have been instrumental in examining the direct effect of soluble factors on the induction of differentiation. Factors known to induce MM cell differentiation include extracts of pituitary, nervous and salivary gland tissue, UB cell–conditioned media, as well as specific growth factors such as bone morphogenic protein-7 (BMP-7), epidermal growth factor (EGF), transforming growth factor α (TGF-α), basic fibroblast growth factor (bFGF), and hepatocyte growth factor (HGF).3,11–16 Similarly, UB branching can be induced by conditioned medium derived from MM cells and specifically with the growth factors glial-derived neurotrophic factor (GDNF) and HGF and extracellular matrix proteins, including fibronectin, collagen, and laminin,17–20 that are known to be abundant in the mesenchyme of the developing kidney.4,21
To date, in vitro studies have relied on isolated nephrogenic explants or growth of progenitor cells as single-cell cultures in monolayer or in three-dimensional matrices. The studies described herein were designed to mimic the conditions of nephrogenesis by co-culturing pre-existing mouse MM and UB cell lines in three-dimensional gels implanted in SCID mice. Such a format provides a microenvironment allowing for intermingling and direct cell–cell contact, reciprocal induction, and stimulation of morphogenesis in three-dimensional culture. Three-dimensional co-culture models have been widely used to emulate a more physiologically relevant microenvironment for the study of genes and signaling pathways in the induction of gliogenesis and neurogenesis,22 osteogenesis,23 intestinal epithelial differentiation,24 neovascularization,25 and stromal–epithelial interactions in endometrial26 and prostatic epithelial27 differentiation. Recent studies also indicate that adult kidney stem cells in Matrigel (BD Biosciences, Bedford, MA) differentiate into tubular profiles complete with lumens and junctional complexes,28 verifying an important tool in the study of kidney cell induction/differentiation.
In this study, we report that co-culture of established MM and UB cell lines in three-dimensional matrices results in the reciprocal induction of the cells to differentiate into simple organoid structures comprised of collecting duct–like epithelia with accompanying cells at their periphery in early stages of vasculogenesis and capillary differentiation.
Materials and Methods
Mouse MM and UB Cell Culture
Mouse MM cells and UB cells (Probetex, San Antonio, TX) were grown and maintained at 37°C in 5% CO2 in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum as originally described by Wagner et al29 and Ye et al.18 The cells were characterized according to cell type as described previously18,29 and further examined by Western blot analysis and immunohistochemistry for additional mesenchymal and ureteric bud or collecting duct markers. For co-culture experiments, MM and UB cells were then trypsinized, washed with Hanks' balanced salt solution, mixed in equal numbers, and then reseeded in monolayer and examined for alterations in structure using mesenchymal and ureteric bud markers by immunofluorescence microscopy (see below). Additionally, the cells were grown to confluency, trypsinized, and then washed for subsequent growth in three-dimensional Matrigel implants as described below.
Characterization of Cell Type
Western Blot Analysis
Immunoblotting was performed as previously described.29,30 Cells grown in monolayer to confluency were lysed in 0.5 mL of radioimmunoprecipitation assay (RIPA) buffer [50 mmol/L Tris-HCl (pH 7.5); 1 mmol/L EGTA; 140 mmol/L NaCl; 1.0% NP-40] containing 1 μg/mL leupeptin and aprotinin, 1 mmol/L sodium fluoride, 0.1 mmol/L sodium orthovanadate, and 1.0 mmol/L PMSF. Insoluble proteins were removed by centrifugation at 10,000 × g. Protein concentrations were determined using the Bio-Rad DC protein assay (Bio-Rad Laboratories, Hercules, CA). Protein lysates were boiled in sample buffer for 10 minutes, then equal amounts of samples were loaded onto 7.5% SDS-PAGE gels and electrophoretically separated. The proteins were transferred to polyvinylidene fluoride membranes using a Bio-Rad Trans-Blot cell followed by blocking with 5% nonfat dry milk in PBS containing 0.1% Tween 20 and incubated overnight in primary antibody diluted into ECL Advance Blocking Agent (Amersham Pharmacia Biotech, Piscataway, NJ). The antigens were detected and identified by enhanced chemiluminescence using standard enhanced chemiluminescence techniques as recommended by the manufacturer (Amersham). Signal was detected using a Syngene ChemiHR16 photo documentation system (Frederick, MD) or by film radiography. GAPDH or actin was used as loading control. Details of antibodies used for the identification of mesenchymal, endothelial, and tubular markers are listed in Table 1.
Table 1.
Differentiation Markers: Antibody Sources, Targets, Species, and Concentrations
| Marker | Primary antibody | Target cell | Source | Species/concentration |
|---|---|---|---|---|
| General epithelial | Pancytokeratin | Epithelial | Santa Cruz Biotechnology | Rabbit/10 μg/mL |
| ZO-1 (R26.4c) | Epithelial tight junctions | DSHB | Rat (1:5) | |
| Collagen IV | Epithelial basement membrane | Millipore | Rabbit/10 μg/mL | |
| UB | RET | UB | Santa Cruz Biotechnology | Rabbit/10 μg/mL |
| D. biflorus lectin | UB, collecting duct | Vector Laboratories | Lectin | |
| EndoA cytokeratin | UB, collecting duct | DSHB | Rat (1:50) | |
| Aquaporin 2 | Mature collecting duct | Santa Cruz Biotechnology | Goat/10 μg/mL | |
| MM | α-SMA (1A4) | MM, pericytes | Sigma-Aldrich | Mouse/10 μg/mL |
| Vimentin (V13.2) | MM, pericytes | Sigma-Aldrich | Mouse/10 μg/mL | |
| PDGFR-β | MM, pericytes | Santa Cruz Biotechnology | Rabbit/10 μg/mL | |
| PECAM | Endothelium | Santa Cruz Biotechnology | Rabbit/10 μg/mL | |
| Aquaporin 1 | Proximal tubule, limb of Henle, differentiating endothelium | Santa Cruz Biotechnology | Rabbit/10 μg/mL | |
| Aminopeptidase | Proximal tubule | Santa Cruz Biotechnology | Rabbit/10 μg/mL |
DSHB, Developmental Studies Hybridoma Bank; MM, metanephric mesenchyme; UB, ureteric bud.
Immunofluorescence Microscopy
Each cell line was grown to 50% to 70% confluence in multiwell plastic Lab-Tek chamber microscope slides (Nalge Nunc International, Naperville, IL) and examined for expression of the mesenchymal, endothelial, and epithelial markers listed in Table 1, using previously described immunohistochemical techniques.8,13,18,21,30,31 The cells were washed in PBS and fixed in cold (−20°C) methanol for 5 minutes, then briefly rinsed with 0.02 mol/L phosphate-buffered saline (pH 7.4). The slides were blocked with PBS containing 0.1% bovine serum albumin, and then the specific protein of interest was detected by indirect immunofluorescence using primary antibodies (Table 1) followed by a Cy3- or FITC-labeled secondary antibody appropriate for the primary antibody (Millipore, Billerica, MA). The sections were viewed and photographed under epifluorescence microscopy using band-pass filters optimal for red or green wavelengths using an Olympus BX51 Research microscope equipped with a DP-71 digital camera (Melville, NY). Paired digital images representing each fluorochrome were color balanced and merged using Image-Pro 4.5 software as previously described.8,13,18,30,31
Two-Dimensional Growth in Monolayer
To test for phenotypic changes of MM and UB cells grown in two-dimensional co-culture, initial experiments were conducted in chamber slides in which the cells were grown together and compared to each cell line grown alone. The cells were allowed to grow for sequential time periods of 1, 2, and 3 days, and then fixed and stained by dual-label immunohistochemistry. MM and UB cells were detected by staining for vimentin and EndoA cytokeratin, respectively, using dual-label immunohistochemistry methods as previously described.8,30
Three-Dimensional Growth in Matrigel
Differentiation potential of MM and UB progenitor cells in three-dimensional co-culture was conducted in a similar fashion as described for adult kidney stem cells by Bussolati et al.28 For homogeneous suspensions, 1 × 106 cells of each line were dispersed in 250 μL of medium, then combined with an equal volume of cold Matrigel, and immediately injected subcutaneously into the nape of the neck of 6-week-old ICR-SCID mice (Taconic Farms, Hudson, NY). Co-culture was performed by mixing an equal number of each of the cell lines, not exceeding a combined total of 1 × 106. Handling of cells, supplies, and Matrigel was conducted on ice to prevent gelling of the matrix before implantation. Once injected, the Matrigel solidifies, with cells dispersed throughout the three-dimensional gel. At the end of the incubation period, the implant was excised and frozen or fixed for subsequent histological analysis as described below. All animal protocols were performed in accordance with National Institutes of Health guidelines and reviewed by the University of Texas Health Science Center Institutional Animal Care and Use Committee.
Routine Histological Analysis
After removal, the implants were fixed in 10% neutral-buffered formalin overnight then processed for paraffin embedment. Three-micron-thick sections were cut and stained with hematoxylin and eosin (H&E), and then viewed and photographed using an Olympus BX51 research microscope and DP71 digital camera. Assessment of the differentiation potential of the cells grown in the three-dimensional matrix showed varying degrees of organization characterized by no organization, development of small round aggregates of cells without lumens (spheroids), tubuloid structures with lumens, or profiles showing one or more spheroid or tubuloid cross sections surrounded by capillary-like cells (organoid). The degree of organization of the cells in 10-day implants was quantified by counting the number of each type of profile in three random fields/slide (×20 objective magnification) of at least three experiments.
Cell Tracking Using PKH Fluorescent Cell Linkers
The MM and UB cells were labeled with PKH26 (red) or PKH67 (green) fluorescent linkers (Sigma Chemical Co., St Louis, MO) according to the manufacturer's instructions. In an additional experiment, the color labeling of the cells was reversed. Briefly, the cells were grown to confluence, detached with trypsin, and washed in serum-free medium using standard culture technique. A total of 2 × 107 cells were suspended in labeling diluent, then added to an equal volume of freshly prepared diluent containing PKH dye to make a final concentration of 2 × 10−6 mol/L at 25°C. The reaction was terminated by addition of buffer containing 1% bovine serum albumin followed by washing the cells in the same buffer. Finally the cells were resuspended in cold medium for incorporation into Matrigel matrices and injected into test animals as outlined above. At the end of the experiment, the implants were flash frozen in liquid nitrogen and 6-μm sections cut in a cryostat. The sections were dried for 30 minutes, fixed in formalin for 5 minutes, washed 3 times with PBS, and then mounted on glass slides in antifade Gold medium (Invitrogen, Life Technologies, Carlsbad, CA).
Identification of Cell Type in Matrigel Implants Using Differentiation Markers
The cells grown in implants were stained for specific differentiation markers by immunohistochemistry (Table 1). Frozen sections (6-μm thick) of the implants were allowed to air dry for 45 minutes, then fixed in cold acetone for 5 minutes. The slides were rehydrated in PBS, bovine serum albumin, then stained with primary antibody to the cell marker of interest (Table 1), followed by repetitive washes and FITC- or Cy3-labeled secondary antibody as described above. In some studies, dual-label immunofluorescence was used to assess the relative expression of the individual marker proteins in tubular epithelial cells and peritubular cells in the same section. Secondary antibodies, manufactured for dual-label applications, were obtained from Chemicon International (Temecula, CA).
Electron Microscopy
Matrigel implants containing kidney progenitor cells and differentiated structures at 10 days after implantation were examined by electron microscopy. Small portions of the implants were diced into <1-mm cubes and fixed with 4% paraformaldehyde, 1% glutaraldehyde at 4°C overnight. The tissue pieces were processed for plastic embedment using routine methods. Thin sections (60 to 70 nm) were stained with lead citrate and uranyl acetate. Differentiated features such as specialized epithelial structures, including tight junctions, vacuoles, microvilli, basement membranes, or vascular features, such as endothelium or pericytes, were assessed and photographed using a Jeol 100CX transmission electron microscope (Tokyo, Japan).
Growth of MM and UB Cells in Three-Dimensional Culture with Conditioned Medium Derived from the Reciprocal Cell Line, GDNF, HGF, or Fibronectin
Each cell line was grown in culture as above, then immediately before implantation suspended in conditioned medium derived from the reciprocal cell type. UB-conditioned medium (UB-CM) and MM-conditioned medium (MM-CM) were derived from 3-day cultures of the reciprocal cell type. The cells were mixed in an equal volume of Matrigel, then injected into SCID mice as above. In additional experiments, cells were re-suspended in medium containing GDNF, HGF (R&D Systems, Inc., Minneapolis, MN), or bovine fibronectin (Invitrogen, Life Technologies) (100 μg/mL) and mixed in equal volumes of Matrigel, then injected into SCID mice. Ten days later, the implants were harvested and fixed or frozen for subsequent histological analysis.
Migration in Response to Conditioned Medium
A scratch/wound assay32 was used to measure MM cell migration in response to UB-CM. Conditioned medium was collected from UB cells by growing the cells to near confluence, briefly rinsing and then incubating them in serum-free medium for 24 and 48 hours. The conditioned medium was collected, filtered, and then stored at −86°C until used in cell migration assays. For the assay, the cells were grown to near confluence and the surface of the monolayer scratched linearly using a 10-μL pipette tip. Digital images were taken at zero time and 8 hours after conditioned medium was added to each well. Controls consisted of serum-free medium in the absence of conditioned medium. The distance of migration from the initial scratch boundary to the plane of migration was measured by image analysis using the linear dimension tool of Image-Pro 4.5 software, and the distance of migration was reported as fold increase over control.
Statistical Analysis
No fewer than three replicates of each experiment were examined, and statistical comparisons were performed using analysis of variance with Bonferroni correction or Student's t-test for two-sample comparisons. Values were determined to be significant at P < 0.05.
Results
Characterization of MM and UB Cells Cultured in Monolayer
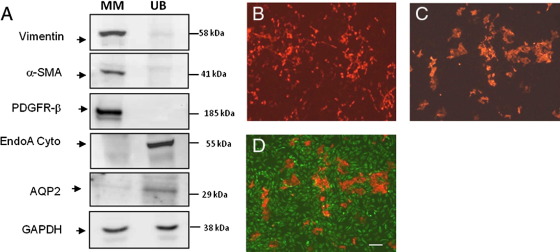
The MM and UB cells grown in monolayer showed the same phenotypic markers as previously described.18,29 MM cells expressed vimentin, α-smooth muscle actin (α-SMA), and platelet-derived growth factor receptor β (PDGFR-β) (Figure 2A). Ureteric bud cells were positive for specific markers for ureteric bud cells, including Dolichos biflorus lectin and EndoA cytokeratin (Figure 2, A and B). UB cells also stained weakly for aquaporin 2 (AQP2) by Western blot analysis (Figure 2A), but this protein was undetectable by immunohistochemistry. MM cells did not express UB markers, and conversely, UB cells did not express mesenchymal cell markers either by immunohistochemistry or Western blot analysis (Figure 2, A and B).
Figure 2.
Immunohistochemical and immunoblot characterization of MM and UB cells grown in monolayer. A: The MM cells express mesenchymal markers α-SMA, vimentin, and PDGFR-β, whereas UB cells express ureteric bud and collecting duct markers and EndoA cytokeratin and AQP2. UB cells (EndoA cytokeratin, red) 2 days in monolayer grow in dispersed formation with slight clustering (B). C and D: When grown in monolayer in co-culture with MM cells (C; vimentin, green), the UB cells form tight aggregates (merge, D) over the 2 day period. Scale bars: 10 μm.
Co-Culture of MM and UB Cells in Monolayer
To test for reciprocal induction, initial experiments were conducted to examine for phenotypic changes of MM and UB cells when grown in 2-dimensional co-culture. The cells were grown in mixed culture and compared microscopically to each cell line grown as a single homogeneous population. The results showed that each cell line formed a dispersed population of cells with some clustering when grown as a homogeneous populations (Figure 2B). When the cells were grown in co-culture, the UB cells, detected by EndoA cytokeratin staining, segregated over time, forming tight aggregates among large expanses of vimentin-positive MM cells (Figure 2, C and D). The aggregation of the UB cells in co-culture with MM cells suggests that these cells may release factors that lead inductive differentiation. To further define their differentiation potential, the cells were grown in three-dimensional co-culture in Matrigel implants in SCID mice (described below).
Three-Dimensional Co-Culture of MM and UB Cells in Matrigel Implants
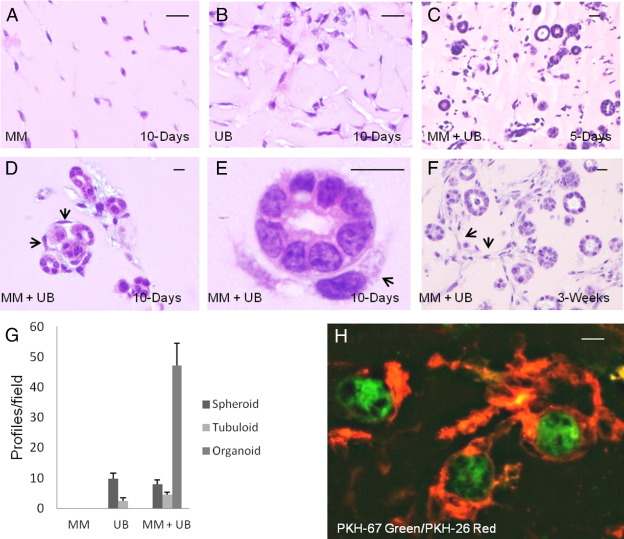
MM and UB cells were grown in three-dimensional co-culture in Matrigel implants in SCID mice for 3, 5, 10, 21, and 30 days. Each line was suspended as a homogeneous population of cells in Matrigel implants for the same duration. The homogeneous cell suspensions showed mainly monodispersed cells throughout the gel in H&E-stained sections (Figure 3, A and B). Additionally, UB cells showed infrequent small spheroid structures up to 3 weeks of growth. When both cell lines were grown in combination, they organized in spheroid and tubuloid structures beginning at 3 to 5 days and maturing over time to form larger organized profiles (Figure 3C). Most of the structures were circular or ovoid in cross section, measuring approximately 15 to 25 μm in diameter and displaying lumens. By 10 days, many of the spheroids and tubuloid structures formed “organoid” clusters associated with cells in capillary-like structures at their periphery (Figure 3, D and E). At 3 and 4 weeks, the capillary-like structures increased in mass, frequently forming anastomoses in a network (Figure 3F). Quantitative assessment of the organization of the cells when grown alone or in combination at 10 days revealed no differentiation of MM cells grown in homogeneous cell suspension, whereas UB cells underwent organization, showing 9.7 ± 1.8 SE spheroids and 2.5 ± 1.0 SE tubuloid profiles/×20 field (Figure 3G). When both cell types were grown in combination, most profiles were organized into epithelial structures in cross sections associated with capillary-like cells showing over 47 organoid profiles/field (Figure 3G).
Figure 3.
Three-dimensional co-culture of MM and UB cells leads to simple organogenesis. Three-dimensional growth of MM (A) and UB (B) cells in homogeneous suspensions in Matrigel implants results in little organization of the cells. When the two cell types are co-cultured, numerous epithelial spheroid and tubuloid structures with lumens develop by 5 days (C), progressing to simple organoid profiles in which epithelial structures are surrounded by cells (arrows) and capillary-like formations (D and E). Over time, the capillary-like structures anastomose, forming networks among spheroid and tubuloid structures (F). The cellular profiles observed per field were quantitated (G). Tracking experiments (H) reveal that the epithelial structures are derived from PKH-67-tagged UB cells (green), whereas the peripheral capillary-like structures are derived from PKH-26–tagged MM cells (red). A–E: H&E stain. F: Fluorescence microscopy. Scale bars: 10 μm.
Cellular Origin of Tubuloid and Vascular Structures
Conceptually, both MM and UB cells have the potential to differentiate into epithelial cells. In addition, the MM cells have the potential to differentiate into stroma, pericytes, or endothelium. Therefore, the origin of cells that ultimately form epithelial or vascular profiles in the Matrigel implants was investigated using tracker dyes to identify each cell type that ultimately form differentiated structures. Each cell line was pre-labeled with a different fluorescent marker (ie, UB cells-PKH67, green, and MM-PKH26, red) before co-culture in Matrigel (see Materials and Methods). The results showed that nearly all epithelial cells in spheroid, tubuloid, and organoid profiles were derived from the UB cell line and peritubular capillary-like cells were derived from the MM cell lineage (Figure 3H). Few isolated profiles co-expressed both dye trackers. An additional experiment switching the tracker dye on each cell line showed an identical outcome.
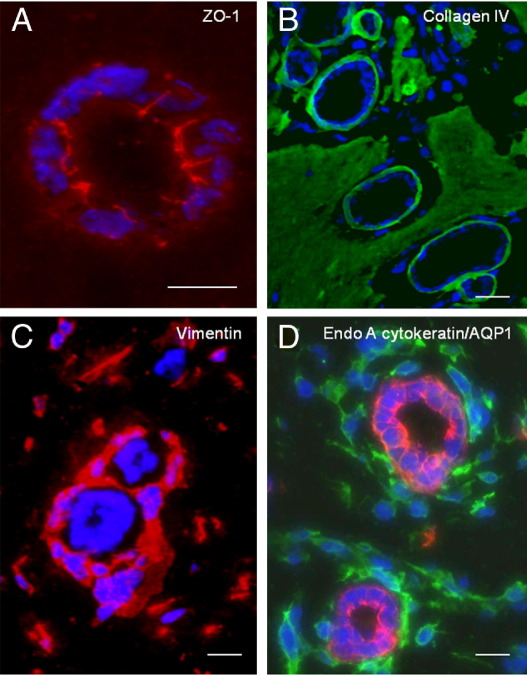
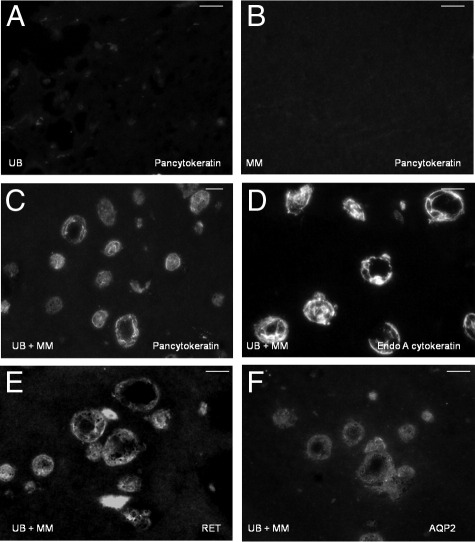
Tracking experiments in three-dimensional Matrigel implants determine the origin of cells that form various structures within Matrigel implants, but do not characterize cell type on the basis of differentiation markers. Therefore, immunohistochemical staining was performed to determine epithelial or mesenchymal characteristics of the cellular structures in the implants (Table 2). The results showed that spheroid and tubuloid structures stained for typical epithelial proteins including the apical junctional complex protein zonula occludens-1 (ZO-1) (Figure 4A), a basement membrane component collagen IV (Figure 4B), and pancytokeratin (Figure 5C). Cells grown in homogeneous suspension in the Matrigel implants showed negligible or no expression of these proteins (Figure 5, A and B; Table 2). In addition, capillary-like cells at the periphery of epithelial structures stained for mesenchymal cell markers including vimentin and α-smooth muscle actin as well as endothelial markers PECAM and AQP1 (Figure 4, C and D; Table 2). Biomarker analysis revealed that the epithelial cells were derived from UB and collecting duct phenotype expressing, RET, EndoA cytokeratin, D. biflorus lectin, and AQP2 (Figure 5). The epithelium was negative for AQP-1 indicating an absence of differentiated proximal tubule or limb of Henle cells (Figure 4D).
Table 2.
Staining for Differentiation Markers in Three-Dimensional Cell Growth
| Marker | Protein | Homogeneous |
Co-culture |
||
|---|---|---|---|---|---|
| MM cells | UB cells | Spheroids and tubuloids | Peritubular (endothelium, pericytes) | ||
| General epithelial | ZO-1 (tight junctions) | − | − | + | − |
| Collagen IV (basement membrane) | − | − | + | − | |
| UB and collecting duct | RET (UB, CD) | − | − | + | − |
| EndoA cytokeratin (UB, CD) | − | + | + | − | |
| D. biflorus lectin (UB, CD) | − | + | + | − | |
| AQP2 (CD) | − | ± | + | − | |
| Proximal tubule | AQP1 (PT⁎) | − | − | − | + |
| Aminopeptidase | − | − | − | − | |
| Mesenchymal | α-SMA | + | − | − | + |
| Vimentin | + | − | − | + | |
| PDGFR-β | + | − | − | + | |
| Endothelial | AQP1 (Endo⁎) | + | − | − | + |
| PECAM | − | − | − | + | |
A minus sign (−) indicates absence of the marker; a plus sign (+) indicates presence of the marker; a plus/minus sign (±) indicates trace staining.
AQP1 is a marker for proximal tubule and limb of Henle epithelia as well as differentiating endothelia. AQP1 localizes only to peritubular mesenchymal cells and early endothelial cells.
Figure 4.
Immunofluorescence characterization of organoid profiles in three-dimensional co-culture. Tubuloid epithelium expresses ZO-1 in apical and lateral membranes (A) and collagen type IV in linear basement membranes (B). Periepithelial and capillary-like cells stain for vimentin (C, red) and AQP-1 (D, green), a marker of developing vasculature. Epithelial cells in spheroids and tubuloid structures are immunoreactive for the UB and collecting duct marker EndoA cytokeratin (D, red). Indirect immunofluorescence using Cy3- (A, B, and D) and FITC- (B and D) labeled secondary antibodies. (D) Merged micrograph of dual-label immunofluorescence in the same section. Scale bars: 10 μm.
Figure 5.
Epithelial cells in organoid profiles express UB and collecting duct markers. UB cells (A), but not MM cells (B), express weak staining for pancytokeratin when grown as homogeneous cell suspensions in Matrigel implants. When MM and UB are co-cultured, staining of pancytokeratin (C) and EndoA cytokeratin (D) is increased in spheroid and tubuloid profiles. Similarly, UB and collecting duct marker proteins RET (E) and AQP-2 (F) are increased in epithelial cells in co-culture experiments. Cells at the periphery of epithelial structures are negative for these markers. Indirect immunofluorescence using Cy3-labeled second antibodies. Scale bars: 10 μm.
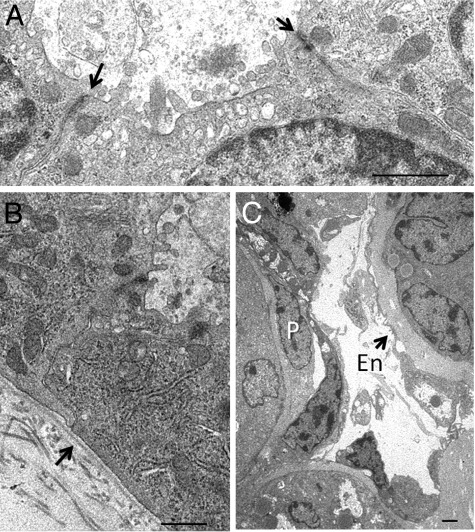
By electron microscopy, the tubuloid and organoid structures exhibited well-formed specialized epithelial features including apical vacuoles, few blunt microvilli, junctional complexes (Figure 6A), and linear basement membranes (Figures 6, B and C). An elaborate brush border typical of differentiated proximal tubular epithelium was not observed. The epithelial cells were surrounded by cells featuring a mesenchymal cell phenotype in capillary-like structures frequently with lumens lined by a thin layer of flat cells resembling endothelium. Cells resembling pericytes were also occasionally observed in locations between the endothelial-like cells and tubular basement membrane (Figure 6C).
Figure 6.
Ultrastructural features of organoid structures after MM and UB cell co-culture.: A: Electron micrographs of the epithelial cells in organoid structures in 10-day Matrigel implants illustrate luminal microvilli, junctional complexes (arrows), and a well-defined microvesicular apparatus. B: A linear basement membrane is also present (arrow). C: Peritubular structures resemble capillaries with lumens lined with flat endothelial-like cells (En) without fenestrae (arrows) and occasional cells resembling pericytes (P). Scale bars: 1 μm.
Differentiation of UB Cells by MM-Conditioned Medium and Defined Medium Containing GDNF, HGF, or Fibronectin
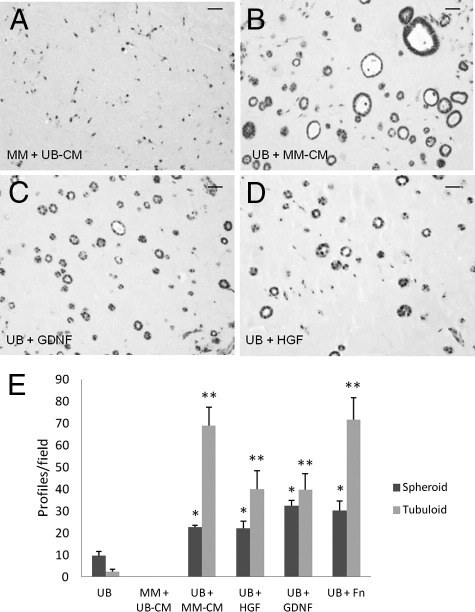
The above experiments show that early tubulogenesis and mesenchymal differentiation occur when MM and UB cell lines are combined in a Matrigel implant microenvironment. These studies indicate that substances are released from one or both cell types that have a direct effect on cell differentiation. It may be inferred that each cell type is “primed” for differentiation, but requires substance(s) released from the companion cell type to initiate differentiation. To test this hypothesis, each line was grown in three-dimensional culture with conditioned medium derived from the reciprocal cell line. The results showed that UB-derived conditioned medium did not lead to noticeable changes in MM cells assessed by H&E (Figure 7, A and E). In contrast, MM-conditioned medium induced organization of the UB cells to form spheroid and tubuloid profiles (Figure 7, B and E). These studies indicate that MM cell–conditioned medium contains substances that have a direct effect on UB epithelialization.
Figure 7.
MM-derived conditioned medium enhances UB epithelialization and organization. A: UB cell–derived conditioned medium (UB-CM) has no apparent effect on MM cell differentiation in homogeneous cell suspensions in Matrigel implants stained by H&E. B: Conversely, MM cell–derived conditioned medium (MM-CM) enhances UB cell epithelialization and tubuloid formation. Similarly, GDNF (C) and HGF (D) enhance UB cell epithelialization in spheroids and tubuloids in three-dimensional Matrigel implants. The data are quantitatively expressed in E. Scale bars: 10 μm. *P < 0.05 versus spheroid structures in UB-alone; **P < 0.05 versus tubuloid structures in UB-alone.
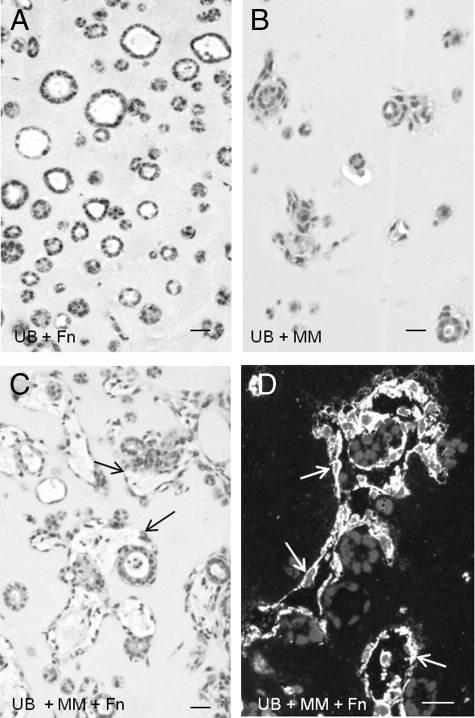
Because GDNF, HGF, and fibronectin are known to induce ureteric bud differentiation, and our previous studies showed that GDNF, HGF, and fibronectin induced UB cells to form cysts and cords when grown as homogeneous populations in three-dimensional collagen gels, additional studies were performed to examine the effect of these factors on UB cell organization in Matrigel implants. The results showed that both GDNF and HGF, each at a concentration of 100 ng/mL, induced a robust epithelialization by enhancement of the number of spheroid and tubuloid profiles at 10 days post implantation (Figure 7, C–E). Similarly, fibronectin stimulated UB cell tubulogenesis in homogeneous implants (Figures 7E and 8A). Fibronectin had no visible effect on MM cells grown as homogeneous population (not shown). However, both tubuloid and peritubular capillary-like structures were accentuated when the MM cells were combined in the presence of fibronectin in Matrigel implants (Figure 8, C and D) relative to cells combined in the absence of fibronectin (Figure 8B).
Figure 8.
Fibronectin accentuates UB and MM organogenesis. UB cell epithelialization and tubuloid formation is enhanced in homogeneous cell suspensions in the presence of fibronectin (A), but not in its absence (B). Conversely, when MM and UB cells are mixed with fibronectin in Matrigel, implants form elaborate peritubular capillary structures shown by H&E stain (C, arrows) and by vimentin immunohistochemistry (D), relative to both cell types grown in the absence of fibronectin (B). Scale bars: 10 μm.
Migration of MM Cells in Response to UB-Conditioned Medium
The above studies show that conditioned medium derived from MM cells stimulates UB organization. Such epithelial organization is further stimulated by GDNF, HGF, and fibronectin. Because UB cells may in turn release factors that lead to the attraction of MM cells and formation of peripheral capillary-like structures, studies were conducted using an in vitro scratch/wound assay (Materials and Methods) to examine the affect of UB-CM on MM cell migration. The results showed that MM cells migrated in a dose-dependent manner related to the duration of the collection of UB-CM (Figure 9). For example, MM cell migration in response to UB-CM collected over a 24-hour period stimulated a 1.7 ± 0.1 SEM fold increase in migration over control, P < 0.05. UB-CM collected over a duration of 48 hours stimulated a 2.1 ± 0.2 SEM fold increase in migration relative to controls, P < 0.05. These results indicate that the UB cells release substances that stimulate MM cell migration and may play a role in organoid formation.
Figure 9.
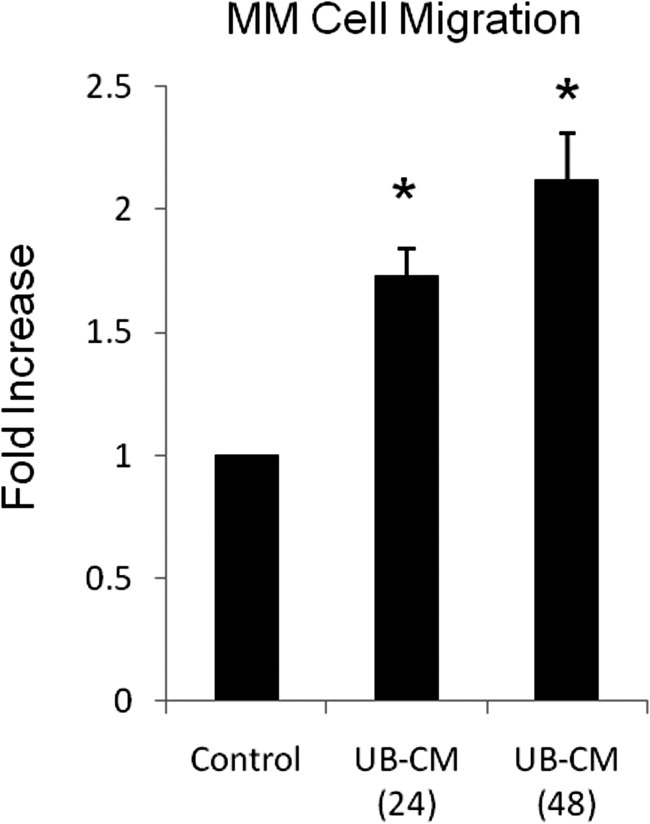
UB-derived conditioned medium stimulates MM cell migration. MM cell migration was incrementally dependent on the duration of time that conditioned medium (CM) was collected from UB cells. UB-CM stimulated a 1.7- and 2.1-fold increase in migration in response to CM collected over 24 and 48 hours, respectively. *P < 0.05 versus control (vehicle).
Discussion
These studies report that individual mouse UB and MM progenitor cell lines undergo a reciprocal induction of differentiation when co-cultured in three-dimensional Matrigel implants. These experiments suggest that the cells have a natural tendency to segregate into discrete structures forming collecting duct-like epithelia surrounded by vasculogenic structures. A variety of techniques have been developed to recreate nephrogenesis in vitro, ex vivo, or by cell or tissue grafting. These include explants from developing metanephric mesenchyme33; growth and propagation of organ rudiments of UB in vitro2,6,33–35; metanephric kidney implanted on chick chorioallontoic membrane36 or in rat mesentery,37,38 under the kidney capsule,39,40 into the anterior eye chamber,40 transplanted directly into the renal parenchyma,39,41 or seeded ex vivo into whole-kidney basement membrane scaffolds42; or by cellular dissociation and reaggregation on polycarbonate filters.43 The combination of established MM and UB cell lines in three-dimensional culture offers a new organotypic model to investigate MM and UB cell interactions, mutual inductive events, organization of cellular polarity, and epithelialization and renal vasculogenesis. Such a format lends itself to routine cell culture manipulations such as antibody neutralization, small-interfering RNA, gene knockout, knockin, and chemical inhibition studies currently used to examine a wide variety of cellular processes under controlled conditions.
Recombination of MM and UB cells in three-dimensional implants resulted in the formation of polarized epithelial cells with highly organized structures including microvilli, microvesicular apparatuses, tight junctions, and well-developed linear basement membranes. Tracking experiments verified that the epithelial cells were derived from the UB and not the MM cell population. The epithelial cells expressed typical epithelial cell markers including pancytokeratin, tight junctional protein zonula occludens-1, and collagen type IV in well-defined linear basement membranes. Additionally, the epithelial cells displayed specialized UB and collecting duct markers of principal cells such as D. biflorus lectin, EndoA cytokeratin, and AQP-2. AQP-2, a transporter expressed only in mature collecting ducts, was used as a marker of UB maturation; initial expression of AQP-2 is seen at approximately embryonic day 18 of rat metanephric kidney development.44
During nephrogenesis, mesenchymal cells are known to differentiate into epithelium of the proximal and distal tubules and limb of Henle (Figure 1). However, examination of the ultrastructure of the epithelial cells in tubuloid structures did not reveal specialized proximal tubule features such as a brush border, elongated, interdigitating lateral processes, or vertically oriented mitochondria. Also, the epithelial cells did not express AQP-1, known to be abundantly expressed in developing and mature proximal tubules and limb of Henle.45 Furthermore, the fluorescence tracker experiments indicated that all spheroid and tubular elements were comprised of UB cells without evidence of differentiation of MM cells into an epithelial fate. Rather, the MM cells were observed as cells at the periphery of tubules or in capillary-like structures displaying a continuous low-form cytoplasm with lumens resembling endothelium surrounding clustered elements.
As with epithelialization, renal vascularization during nephrogenesis requires a tightly regulated developmental program influenced by growth factors, cell membrane receptors, extracellular matrix components, and metalloproteinases.2,4,39,46 Classical studies with metanephroi grown in organ culture or on the chorioallantoic membrane suggested that kidney endothelium is derived via angiogenic process by in-growth of cells from an external source.36,47 Similarly, endothelial cells in developing pig metanephroi grafted into rats were determined to be derived from the host.37 However, studies using grafted metanephroi into the host anterior eye chamber indicate that peritubular vessels and glomeruli form in situ by vasculogenesis, whereby the majority of cells capable of forming the entire microvascular tree are already present in the early metanephric kidney.39,48,49 Nevertheless, host cells can form chimeric vessels through both processes,39 suggesting that both processes of angiogenesis and vasculogenesis probably participate in the formation of renal vessels.
Our studies show capillary-like vascular structures in the implants, many with lumens lined with continuous, flat endothelial-like cells and putative pericytes. The peripheral cells were of MM origin based on PKH cell tracking experiments and immunodetection of vimentin, smooth muscle actin, PECAM-1, and AQP-1. AQP-1 has traditionally been used as a marker in the kidney for proximal tubule and limb of Henle cells (see above). However, AQP-1 is also observed in endothelial cells and may be related to cell migration during vessel formation.50 Of interest are the observations by Kim et al51 showing AQP1 in differentiating renal vascular cells in embryonic kidney with strong expression particularly around the collecting duct system. These studies indicate that the formation of capillaries in three-dimensional co-culture occurs through a vasculogenic process involving an interaction between MM and UB cells, although a contribution from the host was not tested.
In vitro experiments with isolated intact metanephric mesenchyme indicate that various combinations of soluble factors can induce differentiation. These include extracts of pituitary, nervous, and salivary gland tissue, UB cell–conditioned medium and more specifically, growth factors such as bone morphogenic protein-7 (BMP-7), epidermal growth factor (EGF), transforming growth factor α (TGF-α), basic fibroblast growth factor (bFGF), and platelet-derived growth factor.2,5,12,13 UB differentiation requires GDNF, HGF, and FGF.2,3,15,52,53 Also, extracellular matrix proteins, such as fibronectin, that are abundant in the mesenchyme in the developing kidney21 are necessary for branching morphogenesis.4,17–19,54 Our current studies suggest that both cell lines secrete substances that initiate cell tropism and migration toward one another, cell–cell contact, and induction of differentiation into specialized epithelial and vascular structures. Differentiation of UB cells was potentiated when grown in three-dimensional Matrigel matrix in the presence of MM cell–derived conditioned medium, indicating a soluble substance or substances that initiate cell differentiation similar to nephrogenesis in the developing embryo. Furthermore, GDNF, HGF, and fibronectin, three growth substances that are known to initiate UB differentiation during nephrogenesis, as discussed above, induced a robust UB differentiation into epithelial structures in the absence of MM cells. Conversely, conditioned medium derived from UB cells did not appear to induce differentiation of MM cells into capillary structures in the implants.
These current studies also suggest that UB cells release soluble factors that are chemotactic to MM cells, suggesting that such factors are instrumental in forming the epithelial/capillary structures and that MM-UB cell contact may be required for vasculogenesis and formation of capillaries. Such a phenomenon is supported by the observation that both tubulogenesis and vasculogenesis were accentuated by fibronectin in UB and MM co-culture. These experiments form an in vitro corollary to studies by Abrahamson and colleagues48 in which nephrogenesis and microvessel assembly appeared to be tightly coupled in vivo in metanephric grafts, where the most advanced glomerulo- and tubulogenesis were observed when expression of endothelial cells was most abundant. Clearly, differentiation of both cell types is dependent on reciprocal cellular interactions. The specific factors involved in cell differentiation and migration in the implants are not known and are under further investigation. Three-dimensional co-culture of MM and UB cell types offers an opportunity to study fundamental processes of nephrogenesis under controlled conditions. This system may prove to be a useful tool in multiple disciplines including nephrogenesis, bioengineering, and regenerative medicine.
Acknowledgments
We thank Fredyne Springer for her assistance with sample processing. The EndoA cytokeratin (Troma) monoclonal antibody, developed by Philippe Brulet and Rolf Kemler, was obtained from the Developmental Studies Hybridoma Bank, which was developed under the auspices of the National Institute of Child Health and Human Development and maintained by The University of Iowa, Department of Biology (Iowa City, IA).
Footnotes
Supported by National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases Small Business Technology Transfer grant R41DK077436, Small Business Innovation Research grant R44 DK061834.
Disclosures: M.A. and H.E.A. receive patent royalties from Probetex, Inc, V.L.B. is owner and president of Probetex, Inc, and J.L.B. receives consulting fees from Probetex, Inc. None of the other authors disclosed any conflicts of interest.
References
- 1.Saxen L., Sariola H. Early organogenesis of the kidney. Pediatr Nephrol. 1987;1 doi: 10.1007/BF00849241. 385–192. [DOI] [PubMed] [Google Scholar]
- 2.Costantini F. Renal branching morphogenesis: concepts, questions, and recent advances. Differentiation. 2006;74:402–421. doi: 10.1111/j.1432-0436.2006.00106.x. [DOI] [PubMed] [Google Scholar]
- 3.Dressler G.R. The cellular basis of kidney development. Ann Rev Cell Dev Biol. 2006;22:509–529. doi: 10.1146/annurev.cellbio.22.010305.104340. [DOI] [PubMed] [Google Scholar]
- 4.Kanwar Y.S., Wada J., Lin S., Danesh F.R., Chugh S.S., Yang Q., Banerjee T., Lomasney J.W. Update of extracellular matrix, its receptors, and cell adhesion molecules in mammalian nephrogenesis. Am J Physiol Renal. 2004;286:F202–F215. doi: 10.1152/ajprenal.00157.2003. [DOI] [PubMed] [Google Scholar]
- 5.Monte J.C., Sakurai H., Bush K.T., Nigam S.K. The developmental nephrome: systems biology in the developing kidney. Curr Opin Nephrol Hypertens. 2007;16:3–9. doi: 10.1097/MNH.0b013e3280118a5a. [DOI] [PubMed] [Google Scholar]
- 6.Nigam S.K., Shah M.M. How does the ureteric bud branch. J Am Soc Nephrol. 2009;20:1465–1469. doi: 10.1681/ASN.2008020132. [DOI] [PubMed] [Google Scholar]
- 7.Abrahamson D.R. Development of kidney glomerular endothelial cells and their role in basement membrane assembly. Organogenesis. 2009;5:275–287. doi: 10.4161/org.7577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ricono J.M., Xu Y.C., Arar M., Jin D.C., Barnes J.L., Abboud H.E. Morphological insights into the origin of glomerular endothelial and mesangial cells and their precursors. J Histochem Cytochem. 2003;51:141–150. doi: 10.1177/002215540305100202. [DOI] [PubMed] [Google Scholar]
- 9.Levinson R., Mendelsohn C. Stromal progenitors are important for patterning epithelial and mesenchymal cell types in the embryonic kidney. Sem Cell Dev Biol. 2003;14:225–231. doi: 10.1016/s1084-9521(03)00025-9. [DOI] [PubMed] [Google Scholar]
- 10.Bouchard M. Transcriptional control of kidney development. Differentiation. 2004;72:295–306. doi: 10.1111/j.1432-0436.2004.07207001.x. [DOI] [PubMed] [Google Scholar]
- 11.Wallner E.I., Kumar A., Carone F.A., Kanwar Y.S. Growth factors in metanephric development. Ren Fail. 1998;20:331–341. doi: 10.3109/08860229809045119. [DOI] [PubMed] [Google Scholar]
- 12.Karavanova I.D., Dove L.F., Resau J.H., Perantoni A.O. Conditioned medium from a rat ureteric bud cell line in combination with bFGF induces complete differentiation of isolated metanephric mesenchyme. Development. 1996;122:4159–4167. doi: 10.1242/dev.122.12.4159. [DOI] [PubMed] [Google Scholar]
- 13.Simon M., Maresh J.G., Harris S.E., Hernandez J.D., Arar M., Olson M.S., Abboud H.E. Expression of bone morphogenetic protein-7 mRNA in normal and ischemic adult rat kidney. Am J Physiol Renal. 1999;276:F382–F389. doi: 10.1152/ajprenal.1999.276.3.F382. [DOI] [PubMed] [Google Scholar]
- 14.Vukicevic S., Kopp J.B., Luyten F.P., Sampath T.K. Induction of nephrogenic mesenchyme by osteogenic protein 1 (bone morphogenetic protein 7) Proc Natl Acad Sci U S A. 1996;93:9021–9026. doi: 10.1073/pnas.93.17.9021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woolf A.S., Kolatsi-Joannou M., Hardman P., Andermarcher E., Moorby C., Fine L.G., Jat P.S., Noble M.D., Gherardi E. Roles of hepatocyte growth factor/scatter factor and the met receptor in the early development of the metanephros. J Cell Biol. 1995;128:171–184. doi: 10.1083/jcb.128.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rogers S.A., Padanilam B.J., Hruska K.A., Giachelli C.M., Hammerman M.R. Metanephric osteopontin regulates nephrogenesis in vitro. Am J Physiol Renal. 1997;272:F469–F476. doi: 10.1152/ajprenal.1997.272.4.F469. [DOI] [PubMed] [Google Scholar]
- 17.Sakai T., Larsen M., Yamada K.M. Fibronectin requirement in branching morphogenesis. Nature. 2003;423:876–881. doi: 10.1038/nature01712. [DOI] [PubMed] [Google Scholar]
- 18.Ye P., Habib S.L., Ricono J.M., Kim N.H., Choudhury G.G., Barnes J.L., Abboud H.E., Arar M.Y. Fibronectin induces ureteric bud cells branching and cellular cord and tubule formation. Kidney Int. 2004;66:1356–1364. doi: 10.1111/j.1523-1755.2004.00897.x. [DOI] [PubMed] [Google Scholar]
- 19.George E.L., Georges-Labouesse E.N., Patel-King R.S., Rayburn H., Hynes R.O. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119:1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- 20.Zent R., Bush K.T., Pohl M.L., Quaranta V., Koshikawa N., Wang Z., Kreidberg J.A., Sakurai H., Stuart R.O., Nigam S.K. Involvement of laminin binding integrins and laminin-5 in branching morphogenesis of the ureteric bud during kidney development. Dev Biol. 2001;238:289–302. doi: 10.1006/dbio.2001.0391. [DOI] [PubMed] [Google Scholar]
- 21.Barnes V.L., Musa J., Mitchell R.J., Barnes J.L. Expression of embryonic fibronectin isoform EIIIA parallels alpha-smooth muscle actin in maturing and diseased kidney. J Histochem Cytochem. 1999;47:787–798. doi: 10.1177/002215549904700608. [DOI] [PubMed] [Google Scholar]
- 22.Yen B.L., Chien C.C., Chen Y.C., Chen J.T., Huang J.S., Lee F.K., Huang H.I. Placenta-derived multipotent cells differentiate into neuronal and glial cells in vitro. Tissue Eng Part A. 2008;14:9–17. doi: 10.1089/ten.a.2006.0352. [DOI] [PubMed] [Google Scholar]
- 23.Valarmathi M.T., Yost M.J., Goodwin R.L., Potts J.D. The influence of proepicardial cells on the osteogenic potential of marrow stromal cells in a three-dimensional tubular scaffold. Biomaterials. 2008;29:2203–2216. doi: 10.1016/j.biomaterials.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 24.Lussier C.R., Babeu J.P., Auclair B.A., Perreault N., Boudreau F. Hepatocyte nuclear factor-4alpha promotes differentiation of intestinal epithelial cells in a coculture system. Am J Physiol Gastr L. 2008;294:G418–G428. doi: 10.1152/ajpgi.00418.2007. [DOI] [PubMed] [Google Scholar]
- 25.Davie N.J., Gerasimovskaya E.V., Hofmeister S.E., Richman A.P., Jones P.L., Reeves J.T., Stenmark K.R. Pulmonary artery adventitial fibroblasts cooperate with vasa vasorum endothelial cells to regulate vasa vasorum neovascularization: a process mediated by hypoxia and endothelin-1. Am J Pathol. 2006;168:1793–1807. doi: 10.2353/ajpath.2006.050754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arnold J.T., Kaufman D.G., Seppala M., Lessey B.A. Endometrial stromal cells regulate epithelial cell growth in vitro: a new co-culture model. Hum Reprod. 2001;16:836–845. doi: 10.1093/humrep/16.5.836. [DOI] [PubMed] [Google Scholar]
- 27.Lang S.H., Stark M., Collins A., Paul A.B., Stower M.J., Maitland N.J. Experimental prostate epithelial morphogenesis in response to stroma and three-dimensional matrigel culture. Cell Growth Diff. 2001;12:631–640. [PubMed] [Google Scholar]
- 28.Bussolati B., Bruno S., Grange C., Buttiglieri S., Deregibus M.C., Cantino D., Camussi G. Isolation of renal progenitor cells from adult human kidney. Am J Pathol. 2005;166:545–555. doi: 10.1016/S0002-9440(10)62276-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wagner B., Ricono J.M., Gorin Y., Block K., Arar M., Riley D., Choudhury G.G., Abboud H.E. Mitogenic signaling via platelet-derived growth factor beta in metanephric mesenchymal cells. J Am Soc Nephrol. 2007;18:2903–2911. doi: 10.1681/ASN.2006111229. [DOI] [PubMed] [Google Scholar]
- 30.Faulkner J.L., Szcykalski L.M., Springer F., Barnes J.L. Origin of interstitial fibroblasts in an accelerated model of angiotensin II-induced renal fibrosis. Am J Pathol. 2005;167:1193–1205. doi: 10.1016/S0002-9440(10)61208-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arar M., Xu Y.C., Elshihabi I., Barnes J.L., Choudhury G.G., Abboud H.E. Platelet-derived growth factor receptor beta regulates migration and DNA synthesis in metanephric mesenchymal cells. J Biol Chem. 2000;275:9527–9533. doi: 10.1074/jbc.275.13.9527. [DOI] [PubMed] [Google Scholar]
- 32.Liang C.C., Park A.Y., Guan J.L. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2:329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- 33.Steer D.L., Bush K.T., Meyer T.N., Schwesinger C., Nigam S.K. A strategy for in vitro propagation of rat nephrons. Kidney Int. 2002;62:1958–1965. doi: 10.1046/j.1523-1755.2002.00694.x. [DOI] [PubMed] [Google Scholar]
- 34.Meyer T.N., Schwesinger C., Bush K.T., Stuart R.O., Rose D.W., Shah M.M., Vaughn D.A., Steer D.L., Nigam S.K. Spatiotemporal regulation of morphogenetic molecules during in vitro branching of the isolated ureteric bud: toward a model of branching through budding in the developing kidney. Dev Biol. 2004;275:44–67. doi: 10.1016/j.ydbio.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 35.Rosines E., Sampogna R.V., Johkura K., Vaughn D.A., Choi Y., Sakurai H., Shah M.M., Nigam S.K. Staged in vitro reconstitution and implantation of engineered rat kidney tissue. Proc Natl Acad Sci U S A. 2007;104:20938–20943. doi: 10.1073/pnas.0710428105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sariola H., Ekblom P., Lehtonen E., Saxen L. Differentiation and vascularization of the metanephric kidney grafted on the chorioallantoic membrane. Dev Biol. 1983;96:427–435. doi: 10.1016/0012-1606(83)90180-x. [DOI] [PubMed] [Google Scholar]
- 37.Rogers S.A., Lowell J.A., Hammerman N.A., Hammerman M.R. Transplantation of developing metanephroi into adult rats. Kidney Int. 1998;54:27–37. doi: 10.1046/j.1523-1755.1998.00971.x. [DOI] [PubMed] [Google Scholar]
- 38.Dekel B., Burakova T., Arditti F.D., Reich-Zeliger S., Milstein O., Aviel-Ronen S., Rechavi G., Friedman N., Kaminski N., Passwell J.H., Reisner Y. Human and porcine early kidney precursors as a new source for transplantation. Nat Med. 2003;9:53–60. doi: 10.1038/nm812. [DOI] [PubMed] [Google Scholar]
- 39.Abrahamson D.R., Robert B., Hyink D.P., St John P.L., Daniel T.O. Origins and formation of microvasculature in the developing kidney. Kidney Int Suppl. 1998;67:S7–S11. doi: 10.1046/j.1523-1755.1998.06702.x. [DOI] [PubMed] [Google Scholar]
- 40.Abrahamson D.R., St John P.L., Pillion D.J., Tucker D.C. Glomerular development in intraocular and intrarenal grafts of fetal kidneys. Lab Invest. 1991;64:629–639. [PubMed] [Google Scholar]
- 41.Woolf A.S., Hornbruch A., Fine L.G. Integration of new embryonic nephrons into the kidney. Am J Kid Dis. 1991;17:611–614. doi: 10.1016/s0272-6386(12)80332-5. [DOI] [PubMed] [Google Scholar]
- 42.Ross E.A., Williams M.J., Hamazaki T., Terada N., Clapp W.L., Adin C., Ellison G.W., Jorgensen M., Batich C.D. Embryonic stem cells proliferate and differentiate when seeded into kidney scaffolds. J Am Soc Nephrol. 2009;20:2338–2347. doi: 10.1681/ASN.2008111196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Unbekandt M., Davies J.A. Dissociation of embryonic kidneys followed by reaggregation allows the formation of renal tissues. Kidney Int. 2010;77:407–416. doi: 10.1038/ki.2009.482. [DOI] [PubMed] [Google Scholar]
- 44.Yamamoto T., Sasaki S., Fushimi K., Ishibashi K., Yaoita E., Kawashaki K., Fujinaka H., Marumo F., Kihara I. Expression of AQP family in rat kidneys during development and maturation. Am J Physiol Renal. 1997;272:F198–F204. doi: 10.1152/ajprenal.1997.272.2.F198. [DOI] [PubMed] [Google Scholar]
- 45.Knepper M.A., Wade J.B., Terris J., Ecelbarger C.A., Marples D., Mandon B., Chou C.L., Kishore B.K., Nielsen S. Renal aquaporins. Kidney Int. 1996;49:1712–1717. doi: 10.1038/ki.1996.253. [DOI] [PubMed] [Google Scholar]
- 46.Haas C.S., Gleason B., Lin S., Tramonti G., Kanwar Y.S. Matrix metalloproteinases in renal development. Connect Tiss Res. 2004;45:73–85. doi: 10.1080/03008200490442644. [DOI] [PubMed] [Google Scholar]
- 47.Sariola H., Peault B., LeDouarin N., Buck C., eterlen-Lievre F., Saxen L. Extracellular matrix and capillary ingrowth in interspecies chimeric kidneys. Cell Diff. 1984;15:43–51. doi: 10.1016/0045-6039(84)90028-9. [DOI] [PubMed] [Google Scholar]
- 48.Robert B., St John P.L., Abrahamson D.R. Direct visualization of renal vascular morphogenesis in Flk1 heterozygous mutant mice. Am J Physiol Renal. 1998;275:F164–F172. doi: 10.1152/ajprenal.1998.275.1.F164. [DOI] [PubMed] [Google Scholar]
- 49.Hyink D.P., Tucker D.C., St John P.L., Leardkamolkarn V., Accavitti M.A., Abrass C.K., Abrahamson D.R. Endogenous origin of glomerular endothelial and mesangial cells in grafts of embryonic kidneys. Am J Physiol Renal. 1996;270:F886–F899. doi: 10.1152/ajprenal.1996.270.5.F886. [DOI] [PubMed] [Google Scholar]
- 50.Saadoun S., Papadopoulos M.C., Hara-Chikuma M., Verkman A.S. Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nature. 2005;434:786–792. doi: 10.1038/nature03460. [DOI] [PubMed] [Google Scholar]
- 51.Kim J., Kim W.Y., Han K.H., Knepper M.A., Nielsen S., Madsen K.M. Developmental expression of aquaporin 1 in the rat renal vasculature. Am J Physiol Renal. 1999;276:F498–F509. doi: 10.1152/ajprenal.1999.276.4.F498. [DOI] [PubMed] [Google Scholar]
- 52.Costantini F., Shakya R. GDNF/Ret signaling and the development of the kidney. Bioessays. 2006;28:117–127. doi: 10.1002/bies.20357. [DOI] [PubMed] [Google Scholar]
- 53.Qiao J., Bush K.T., Steer D.L., Stuart R.O., Sakurai H., Wachsman W., Nigam S.K. Multiple fibroblast growth factors support growth of the ureteric bud but have different effects on branching morphogenesis. Mech Dev. 2001;109:123–135. doi: 10.1016/s0925-4773(01)00592-5. [DOI] [PubMed] [Google Scholar]
- 54.Larsen M., Wei C., Yamada K.M. Cell and fibronectin dynamics during branching morphogenesis. J Cell Sci. 2006;119:3376–3384. doi: 10.1242/jcs.03079. [DOI] [PubMed] [Google Scholar]